Abstract
Leber congenital amaurosis (LCA) is a rare hereditary retinal degeneration caused by mutations in more than a dozen genes. RPE65, one of these mutated genes, is highly expressed in the retinal pigment epithelium where it encodes the retinoid isomerase enzyme essential for the production of chromophore which forms the visual pigment in rod and cone photoreceptors of the retina. Congenital loss of chromophore production due to RPE65-deficiency together with progressive photoreceptor degeneration cause severe and progressive loss of vision. RPE65-associated LCA recently gained recognition outside of specialty ophthalmic circles due to early success achieved by three clinical trials of gene therapy using recombinant adeno-associated virus (AAV) vectors. The trials were built on multitude of basic, pre-clinical and clinical research defining the pathophysiology of the disease in human subjects and animal models, and demonstrating the proof-of-concept of gene (augmentation) therapy. Substantial gains in visual function of clinical trial participants provided evidence for physiologically relevant biological activity resulting from a newly introduced gene. This article reviews the current knowledge on retinal degeneration and visual dysfunction in animal models and human patients with RPE65 disease, and examines the consequences of gene therapy in terms of improvement of vision reported.
Keywords: rod and cone photoreceptors, RPE65, visual (retinoid) cycle, retinal degeneration, electroretinogram (ERG), retinal pigment epithelium (RPE), optical coherence tomography (OCT), transient pupillary light reflex (TPLR), cortical plasticity, perimetry, functional MRI
1. Introduction
A critical mass of knowledge reached after decades of basic, pre-clinical and clinical research recently culminated in three independent clinical trials of ocular gene therapy in patients with a rare hereditary blindness caused by mutations in the RPE65 gene (NCT00481546, NCT00516477, NCT00643747, clinicaltrials.gov). Results so far attest not only to the safety of the procedure but also detectable improvements in vision (Bainbridge et al., 2008; Cideciyan et al., 2008, 2009a, 2009b; Hauswirth et al., 2008; Maguire et al., 2008, 2009; Simonelli et al., 2010). Although there have been previous attempts of gene therapy in human ocular disease (Campochiaro et al., 2006; Chevez-Barrios et al., 2005), recent RPE65 gene therapy trials provide exciting evidence of biological activity resulting from a newly introduced gene in human patients. The expression of the new gene was physiologically relevant causing up to 4.8 log unit improvement in vision corresponding to the retinal location of gene introduction, was detectable by 30 days, and lasted unabated for at least 1 year (Cideciyan et al., 2008, 2009a). These findings bode well for current and future plans of gene therapy approaches to many other rare hereditary retinal conditions as well as more common ocular diseases.
This review summarizes the literature on the retinal disease caused by RPE65 mutations in murine and canine models as well as human patients with particular emphasis on the resulting degeneration of retinal cells and loss of function. Also reviewed are the improvements of the visual function achieved with gene therapy in pre-clinical experiments as well as results of the human clinical trials. The reader is directed to other recent reviews for in depth coverage of phototransduction, retinoid cycle of vision and RPE65 biology (Cai et al., 2009; McBee et al., 2001; Lamb & Pugh, 2004; Rando, 2001; Redmond, 2009; Travis et al., 2007), Leber congenital amaurosis and related retinal degenerative conditions (Henderson et al. 2006; Koenekoop, 2007; den Hollander et al., 2008; Moradi and Moore, 2007; Stone, 2007; Wright et al., 2010), treatment strategies for retinal degenerative diseases (Stone, 2009; Thompson & Lottery, 2009), and ocular gene therapy (Alexander and Hauswirth, 2008; Chung et al., 2009; Colella et al., 2009; Conley et al., 2008; Rex, 2007; Smith et al., 2009). Not covered in this review are the studies demonstrating the successful use of substitute chromophores in RPE65-deficiency (Moise et al., 2007; Travis et al., 2007; van Hooser et al., 2000) which have led to the recent initiation of a clinical trial (NCT01014052, clinicaltrials.gov).
2. Biology of RPE65
Vertebrate vision is signaled by the activation of the phototransduction cascade in rod and cone photoreceptor cells of the retina when light quanta are absorbed by the ubiquitous chromophore 11-cis-retinal and converted to its all-trans isomer. Continued function of photoreceptors requires removal of the all-trans-retinal and resupply with chromophore. Sources of 11-cis retinoids are provided by two visual (retinoid) cycles of vision taking place in cells neighboring photoreceptors: the retinal pigment epithelium (RPE) and the Muller glia (Bok, 1993; Lamb & Pugh, 2004; McBee et al., 2001; Rando, 2001; Travis et al., 2007; Wang & Kefalov, 2009). The canonical (classical) visual cycle takes place in the RPE and uses a key enzyme, termed retinoid isomerase, to produce 11-cis-retinal for both rod and cone photoreceptors using all-trans retinoid substrates either recycled from photoreceptors as vision byproducts or originating from the choroidal blood supply and ultimately from dietary vitamin A. The alternative (retinal) visual cycle involves the Muller glial cells to regenerate chromophore for the cones (Travis et al., 2007; Wang & Kefalov, 2009). RPE65 is the indispensible retinoid isomerase of the canonical RPE visual cycle (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005, 2010) and it is highly and preferentially expressed in the RPE cells (Redmond, 2009). Crystal structure of the RPE65 molecule has recently been solved (Kiser et al., 2009). The isomerase of the alternative visual cycle is not yet known.
3. Human retinopathy caused by RPE65 mutations
Mutations in the RPE65 gene cause a devastating blindness with an autosomal recessive inheritance (Gu et al., 1997; Henderson et al., 2006; den Hollander et al., 2008; Koenekoop, 2007; Lotery et al., 2000; Marlhens et al., 1997; Moradi and Moore, 2007; Morimura et al., 1998; Perrault et al., 1999). Either shortly after birth or in the first few years of life, a child is noted by parents to be less visually responsive than normal. Eventually, a diagnosis such as Leber congenital amaurosis (LCA), early-onset severe retinal dystrophy or early-onset retinitis pigmentosa is given depending on the age the patient is first seen, the clinical impression of the ophthalmic practitioner involved, the timing and type of tests performed, and the variability of the disease expression. This review will refer to all RPE65-associated human disease as RPE65-LCA for simplicity and uniformity.
LCA is a rare disease with an estimated prevalence of about 1:80,000 (Stone, 2007); mutations in more than a dozen genes can cause LCA and RPE65-LCA is thought to represent about 6% of all LCA cases (den Hollander et al., 2008). Severely abnormal electroretinograms (ERGs) in RPE65-LCA point to retina-wide involvement of photoreceptors, and abnormal visual acuity and nystagmus suggest compromise of foveal vision congenitally or during early development. Opthalmoscopic appearance of the ocular fundus often show signs of retinal degeneration such as attenuated retinal blood vessels, bone-spicule pigmentation, and localized regions of RPE atrophy. A quantitative relationship between phenotypic disease severity and genotype has not been definitively established. In vitro experiments suggest that different RPE65 mutations may result in a continuum from a complete lack of 11-cis retinoid production to very low but detectable levels (Bereta et al., 2008; Jin et al., 2005; Lorenz et al., 2008; Nikolaeva et al., 2010; Philp et al., 2009; Redmond et al., 2005; Takahashi et al., 2006) but available in vivo results from a knockin mouse appear to contradict the conclusions from in vitro analyses (Samardzija et al., 2008). Reproducible and quantifiable measures of disease severity in human patients in combination with physiologically-relevant predictions of biochemical defects for specific mutations will be necessary to ultimately understand the relationship between genotype and phenotype.
4. Retinal degeneration resulting from RPE65-deficiency
4.1 Murine models
Three murine models of RPE65-deficiency have been reported. Rpe65−/− knockout model was created by homologous recombination involving replacement of the first three exons and intervening introns of the mouse Rpe65 gene by a neomycin resistance cassette (Redmond et al., 1998). A naturally occurring mouse retinopathy in rd12 mice was shown to be based on a homozygous mutation in exon 3 of the Rpe65 gene resulting in a premature stop codon at codon 44 (Pang et al., 2005). And a third mouse model was generated by targeting exon 4 of the Rpe65 gene and modifying codon 91 from a CGA to TGG (Samardzija et al., 2008). The retinal degeneration and RPE disease resulting from these three models is summarized below.
4.1.1 Rpe65−/− knockout mouse
Rpe65−/− knockout mice develop a slowly progressive retinal degeneration as measured by the thickness of the outer nuclear layer (ONL) of the retina where the photoreceptor cell nuclei reside. In the mouse, the ONL mainly represents rod photoreceptors which form ~97% of the total photoreceptor population (Carter-Dawson & LaVail, 1979; Jeon et al., 1998). Between the ages of 1 and 2 months, the ONL of Rpe65−/− knockout mice is of near-normal thickness (Gouras et al., 2002; Redmond et al., 1998; Rohrer et al., 2003; Samardzija et al., 2008) but there are abnormalities visible at the OS (Redmond et al., 1998). By 6 to 7 months of age, photoreceptor loss has caused the ONL to become ~70% of normal thickness (Gouras et al., 2002; Redmond et al., 1998; Rohrer et al., 2003; Samardzija et al., 2008; Woodruff et al., 2003). By 12-18 months of age, ONL layer may be reduced to ~50% of normal thickness (Fan et al., 2005; Gouras et al., 2002; Rohrer et al., 2003; Samardzija et al., 2008; Woodruff et al., 2003) and by 24 months of age, the ONL thickness reduces to 30% or less of wildtype (Jacobson et al., 2005). Spatial distribution of the rate of rod photoreceptor degeneration across the Rpe65−/− retina has not been defined to date.
The rate of degeneration of Rpe65−/− photoreceptors could depend on environmental and genetic variables. Cyclic-light rearing in normal laboratory lighting conditions causes a greater rate of photoreceptor degeneration than dark-rearing when evaluated at 11 months (Fan et al., 2005). This result is counterintuitive because of the protection from bright light damage in the short term afforded by the lack of Rpe65 (Grimm et al., 2000); however, it is important to note that the long term consequences of bright light exposure in Rpe65−/− mice are unknown. Among genetic variables, coat color (and ocular melanin) may interact with the environmental light load controlling the rate of retinal degeneration (Fan et al., 2006). Specifically, morphological differences between dark-reared and cyclic-light reared conditions are greater in tan colored mice than in agouti mice (Fan et al., 2006).
Unlike rod photoreceptors, mouse cones show a fast rate of degeneration consequent to Rpe65-deficiency. In the central area of Rpe65−/− mouse retinas, massive degeneration of cones is observed before 1 month of age (Znoiko et al., 2005). This initial loss of cells affects preferentially short-wavelength sensitive cones of the inferior (ventral) retina (Znoiko et al., 2005). Examination of cone-only retinas of Nrl−/−Rpe65−/− and Rho−/−Rpe65−/− double knockout mice confirmed the degeneration of cones upon ablation of Rpe65 (Feathers et al., 2008; Kunchithapautham et al., 2009; Wenzel et al., 2007). Hypersensitivity of Rpe65−/− cones to degeneration has been initially speculated (Znoiko et al., 2005) to relate to the function of Rpe65 expressed directly in cones, which remains controversial (Hemati et al., 2005; Znoiko et al., 2002). Follow-up studies suggest that at least a subset of the cones require 11-cis-retinal for the proper transport of cone opsins and several other membrane-associated cone phototransduction polypeptides. The resulting cone opsin mislocalization and/or cone outer segment instability causes increased rates of apoptotic cone cell loss in Rpe65−/− retinas (Rohrer et al., 2005; Zhang et al., 2008). Interestingly, apoptotic pathways triggered in cone photoreceptors is different than that in rod photoreceptors (Hamann et al., 2009). Administration of exogenous chromophore rescues the cone photoreceptors in experimental paradigms involving Rpe65-deficient retinas (Kunchithapautham et al., 2009; Maeda et al., 2009; Rohrer et al., 2005; Zhang et al., 2008).
Consequences of RPE65 disease on the RPE is of interest because Rpe65 is primarily and abundantly expressed within the RPE and the primary pathology in Rpe65-deficiency is the block within the RPE of the metabolic transformation from all-trans-retinyl esters to 11-cis-retinol. Biochemically, there is a massive accumulation of all-trans-retinyl esters which appear as lipid droplets in Rpe65−/− RPE at the electron microscope level (Katz and Redmond, 2001; Redmond et al., 1998; van Hooser et al., 2000, 2002). Use of two-photon imaging has shown that retinosomes, which are thought to be distinct cellular organelles corresponding to retinyl ester storage particles, are abnormally increased in Rpe65−/− RPE as early as 3 weeks (Imanishi et al., 2004). The contribution of these abnormal structures to RPE degeneration remains unexplored, but they do not appear to be the primary reason for rod photoreceptor degeneration (Woodruff et al., 2003). In contrast to the massive over-accumulation of retinyl esters, RPE cells show a dramatic absence of lipofuscin granules consistent with the hypothesis that cycling between all-trans- and 11-cis-retinal is necessary for the accumulation of this pigment (Katz and Redmond, 2001).
4.1.2 Rpe65rd12 naturally occurring mouse
The ONL layer in Rpe65rd12 mouse shows near normal thickness for at least the 8 weeks of age (Pang et al., 2005; Thompson et al., 2008). During this time, there are increasing numbers of voids seen in the rod outer segments (Pang et al., 2005) likely representing reduced density of discs. By 7 months of age, the ONL is reduced to 6 to 7 rows of nuclei compared to 10 to 11 rows in wildtype animals; and by 27 months, the ONL is reduced to 3-4 layers with the OS nearly absent (Pang et al., 2005, 2006). The rate of degeneration does not appear to be substantially different than the Rpe65−/− mouse although a direct comparison has not been performed. Rpe65rd12 mouse also shows a massive degeneration of cones similar to Rpe65−/− mice within the first month (Pang et al., 2010).
In parallel with the progression of photoreceptor disease, RPE cells in Rpe65rd12 mouse appear normal at 3 weeks with occasional lipid-like droplets. The frequency and size of lipidlike droplets increase slowly with age and by 27 months, RPE cells have become atrophied and hypopigmented (Pang et al., 2005). Levels of retinyl esters in Rpe65rd12 are similar to normal at 3 weeks of age but increase dramatically with age reaching levels 10 fold higher than normal by 5 months (Pang et al., 2005). This rate of accumulation does not appear to be substantially different than in the Rpe65−/− mouse (Redmond et al., 1998; Samardzija et al., 2008).
4.1.3 Rpe65R91W knockin mouse
Rpe65R91W knockin mice were generated (Samardzija et al., 2008) to model the disease caused by one of the common missense mutations observed in RPE65-LCA patients (El Matri et al., 2006; Jacobson et al., 2009; Lorenz et al., 2000; Thompson et al., 2000). Rpe65R91W knockin mice show partial expression of the mutant RPE65 protein in the RPE and generation of 11-cis-retinal and rhodopsin reaching levels up to 10% of normal (Samardzija et al., 2008). Retinal morphology of knockin mice were compared to wildtype and Rpe65−/− mice between the ages of 4 weeks to 1 year. In terms of the thickness of the ONL, knockin and knockout mice are similar with about five rows of nuclei remaining in the central area at 1 year compared with 9-10 rows in wildtype eyes (Samardzija et al., 2008). These results suggest that minimal production of rhodopsin is not adequate to stop progression of rod degeneration. In terms of OS disorganization however, knockin mice appear to show a better preservation than age-matched knockout mice (Samardzija et al., 2008).
Cone degeneration in Rpe65R91W knockin mice proceeds more slowly than in Rpe65−/− knock-out mice (Samardzija et al., 2008, 2009). In both models, short-wavelength sensitive cones of the ventral (inferior) retina are most affected but the extent of cone degeneration is greater in Rpe65−/− knock-out mice as compared to Rpe65R91W knockin mice. Data from double and triple mutant animals support a hypothesis of competition between rod and cone opsins in conditions of limited chromophore supply (Samardzija et al., 2009).
4.2 Canine models
The Briard dog breed had been known to be affected with a recessively inherited retinopathy characterized by blindness under dim light conditions and with various degrees of visual impairment under bright light conditions ranging from normal to complete blindness (Narfstrom et al., 1989; Riis & Aguirre, 1983; Veske et al., 1999; Wrigstad et al., 1994). Molecular genetic studies demonstrated that the disease in American and Swedish strains of dogs is caused by the same 4-bp deletion designated variably as representing nucleotides 487-490 or 486-489 of the wildtype RPE65 sequence (Aguirre et al., 1998; Gal et al., 1998; Veske et al., 1999).
The retinal degeneration in RPE65-mutant dogs is slowly progressive such that there is no evidence of loss of photoreceptors for up to 1.5 years of age (Hernandez et al., 2010; Wrigstad et al., 1992, 1994). At four months of age, there are prominent lipid inclusions within the RPE mostly at central and midperipheral tapetal areas (Wrigstad et al., 1992). The lipid inclusions increase in number and spread towards the periphery with increasing age (Wrigstad et al., 1994). Photoreceptor degeneration is increasingly detectable with age with a possible difference in severity between the different strains (Aguirre et al., 1998; Acland et al., 2005; Hernandez et al., 2010; Le Meur et al., 2007; Wrigstad et al., 1994) but comparative studies have not been performed. Cone or rod numbers show no changes for up to 1.5 years of age, but molecular changes occur in photoreceptors, bipolar and amacrine cells (Hernandez et al., 2010). The end stage of the disease in the Swedish strain has been shown to involve complete degeneration of photoreceptors in the peripheral retina by 5-7 years of age (Wrigstad et al., 1994; Narfstrom et al., 2008).
4.3 Human patients
Current knowledge of the structural phenotype of the human retina and the RPE resulting from RPE65 mutations comes from a single case of post-mortem histology, extensive analyses using retinal microstructural imaging in dozens of molecularly clarified patients, and investigation of RPE lipofuscin autofluorescence imaging.
4.3.1 Histology
Descriptions of donor retinas from patients diagnosed with LCA in the premolecular era show considerable diversity of observation with severe outer retinal degeneration found in some retinas whereas minimal outer retinal abnormalities found in others (Horsten & Winkleman, 1960; Kroll and Kuwabara, 1964; Mizuno et al., 1977; Noble and Carr, 1978). In retrospect, these findings are not inconsistent with what is now known to be a diversity of molecular causes for LCA (den Hollander et al., 2008). Histological evaluation of RPE65-mutant human retina has been reported in a case of a male fetus voluntarily aborted at 33 weeks of gestation (Porto et al., 2002, 2003). Prenatal genetic testing showed homozygous Cys330Tyr mutations in the RPE65 gene which was carried by the affected sister. Histology showed loss of retinal cells centrally and retained ONL numbers more peripherally. Immunohistochemistry with anti-rhodopsin showed stunted rod OS and reduced rhodopsin labeling with extensive cell loss in the outer and inner nuclear layers. Immunolabeling with peanut agglutinin suggested loss of cone OS. Ultrastructural studies revealed vesicular structures within the RPE interpreted as lipid droplets containing all-trans-retinyl esters. These results suggest embryonic retinal degeneration occurring in some cases of human RPE65 disease; however, the interpretation of these findings have been challenged by others (Ben-Arie-Weintrob et al, 2005).
4.3.2 Optical coherence tomography
Optical coherence tomography (OCT) is a modern cross-sectional imaging technique that allows detection of the small scattering differences within the retinal tissue previously thought to be transparent (Huang et al., 1991). Evaluation of clinically well-established retinal diseases set the stage for OCT becoming a transformative method for ophthalmology (Puliafito et al., 1996). Demonstration of a major OCT signal component originating from the junction of photoreceptor inner and outer segments and disappearance of this signal with the loss of photoreceptors defined the importance of the OCT for hereditary retinal degenerations (Huang et al., 1998, 2000; Jacobson et al., 1998). Improvements in hardware and software for the next decade have made OCT the de facto technique for in vivo microscopy of human eyes (Drexler and Fujimoto, 2008).
First publication of the OCT in a patient with RPE65-LCA due to a homozygous null mutation and severe vision loss showed a well defined foveal contour and nearly normal retinal thickness (van Hooser et al., 2000). Even though these results were obtained using first generation OCT system with limited resolution, images showed a detectable photoreceptor layer that was thinner than normal and a reduction of the signal originating from photoreceptor inner-outer segments (van Hooser et al., 2000). Qualitative studies published by other investigators in RPE65-mutant patients have generally shown results that were consistent (Lorenz et al., 2004, 2008; Pasadhika et al., 2010; Simonelli et al., 2007).
A series of quantitative OCT studies in a large number of RPE65-LCA patients at different ages has provided exquisite details of the spatial distribution and extent of retinal degeneration in this disease (Jacobson et al., 2005, 2007a, 2007b, 2008a; Maeda et al., 2009). An easily accessible OCT measure is overall retinal thickness referring to the distance from the vitreoretinal interface to the RPE. Spatial topography of overall retinal thickness in RPE65-LCA can be within normal limits or show a preserved central island surrounded by a ring of thinning or demonstrate retina-wide thinning; within the first three decades of life, there is no simple relationship of age and retinal thickness (Jacobson et al., 2005). The thickness of the ONL, apparent as a hyporeflective band on OCT scans, can define directly the extent of photoreceptor loss. Along the horizontal and vertical meridians, RPE65-LCA retinas show regions of identifiable ONL (Jacobson et al., 2005). At the fovea, ONL thickness can be normal or reduced; immediately adjacent to the fovea, ONL is abnormally thinned in nearly all patients (Jacobson et al., 2005). Greatest non-foveal preservation of the photoreceptors corresponds to the region 3- to 5 mm from the fovea in the superior retina which is also the region of highest rod density in normal eyes (Jacobson et al., 2005).
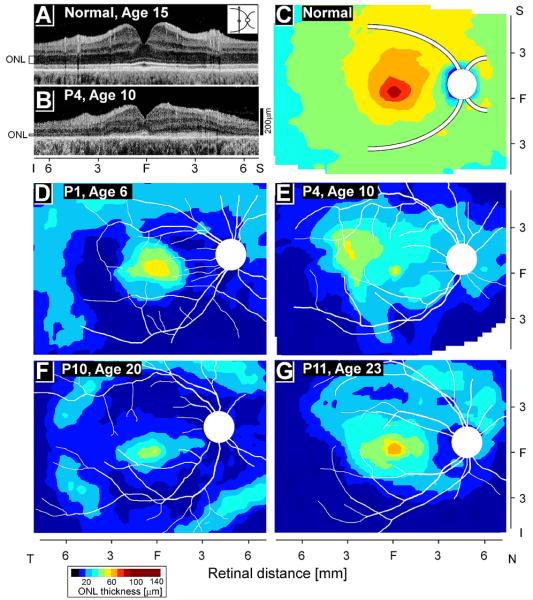
Topography of photoreceptor loss across a wide region of central retinas has also been defined in RPE65-LCA eyes (Jacobson et al., 2008a). On average, young patients between the ages of 6 and 17 years, show retained ONL thickness centrally and into the supero-temporal and temporal retinas. But there can be wide interindividual differences that are not explained by age (Jacobson et al., 2008a). For example, some patients in their early 20s have greater regions of retained photoreceptors compared to patients in the first decade of life (Fig.1). Potential contributions of remnant retinoid isomerase activity, genetic background or environmental influences on the natural history of RPE65-LCA disease remain mostly unexplored.
Figure 1.
Photoreceptor layer thickness topography in patients with RPE65-LCA. (A, B) Cross-sectional scans along the vertical meridian in a 15-year-old normal subject and a 10-year-old patient (P4) with RPE65-LCA. Hyporeflective layer corresponding to the outer nuclear layer (ONL) is shown. (C) Normal topography of ONL thickness as an average map based on a group of six normal subjects. (D, E) Patients at 6 and 10 years of age compared with (F, G) two patients in their early 20s. Traces of major blood vessels and location of the optic nerve head are overlaid on each map (depicted as right eyes). T, temporal; N, nasal; S, superior; I, inferior. Bottom left: color scale for ONL thickness. Reprinted from Jacobson et al., 2008, copyright © held by the Association for Research in Vision and Ophthalmology.
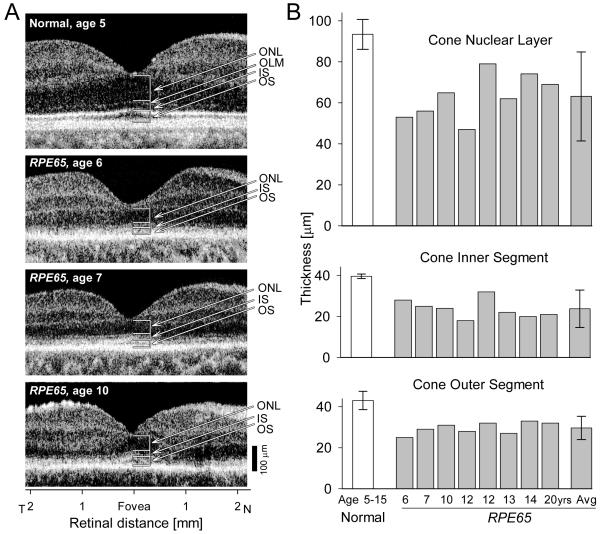
Even though human retinas are dominated by rod photoreceptors, the minority cone photoreceptors are of critical importance because they provide high resolution spatial vision and color perception. Across most of the retina, rod and cone nuclei colocalize to ONL where OCT imaging cannot currently differentiate between them. There is an exception, however. Human and nonhuman primate eyes have a cone-only fovea where the effects of RPE65 mutations on cones can be directly evaluated (Jacobson et al., 2005; 2007a; 2007b; Maeda et al., 2009). ONL thickness at the fovea was evaluated in 23 RPE65-LCA patients over an age range of 3 to 52 years (Jacobson et al., 2007a). On average, there was little difference between 3-14 year old and 18-28 year old groups of patients; both retaining 60-65% of normal thickness. The group of patients older than 30 years showed significantly greater ONL loss, retaining on average 37% of normal thickness. More recently, use of an ultra-high speed spectral-domain OCT system has confirmed partial loss of cones at the fovea at early ages in RPE65-LCA (Fig.2). Furthermore, analysis of the cone inner and outer segment lengths at the fovea have shown the details of the structural abnormalities occurring in young patients due to RPE65 disease (Maeda et al., 2009).
Figure 2.
Foveal cone morphology of young RPE65-LCA patients. (A) Cross-sectional scans along the horizontal meridian of the central retina of a normal child (upper panel) and three children with LCA due to RPE65 mutations (lower panels). Arrows and brackets indicate ONL, outer nuclear layer; OLM, outer limiting membrane; IS, inner segment; OS, outer segment. (B) Mean foveal ONL, IS and OS thickness in a group of young subjects with normal vision (ages 5–15 years) and in eight young patients with RPE65–LCA (ages 6–20 years). Mean values for the parameters from the RPE65–LCA group are also shown (Avg). Error bars represent +/−2 SD. Reprinted from Maeda et al., 2009, by permission from Oxford University Press.
4.3.3 Lipofuscin autofluorescence
Lipofuscin is an RPE pigment known to accumulate with normal aging, and it consists of many distinct fluorophores formed upon phagocytosis of photoreceptor outer segments containing remnant all-trans-retinal molecules (Eldred & Lasky, 1993; Mata et al., 2000; Parish et al., 1998; Wassell & Boulton, 1997). Since all-trans-retinal is produced from 11-cis-retinal upon photon absorption during phototransduction, environmental and dietary manipulations that either block all-trans-retinal generation in the photoreceptors or block 11-cis-retinal synthesis in the RPE result in prevention of lipofuscin formation (Katz et al., 1993, 1999). Similarly, Rpe65−/− mice lacking detectable levels of 11-cis-retinal, show a dramatic absence of lipofuscin granules within the RPE (Katz and Redmond, 2001).
Non-invasive methods can be used to image the intrinsic autofluorescence of the lipofuscin distribution (Delori et al., 1995; von Ruckmann et al., 1995) and quantify its abnormalities (Cideciyan et al., 2004) in human eyes. In several patients with RPE65 mutations, autofluorescence imaging showed signal levels that are either not detectable or severely reduced (Lorenz et al., 2004; Wabbels et al., 2006). There have been two notable exceptions with evidence for low but detectable levels of lipofuscin autofluorescence. One of these is a young patient homozygous for P25L mutation in the RPE65 gene (Lorenz et al., 2008) and another one is a compound heterozygote with R91W/Y368H mutations (Lorenz et al., 2004). Contributing to the apparent variability of the results would be the difficulty of autofluorescence imaging in LCA patients with nystagmus, and wide difference observed in the retinal locations of RPE atrophy as well as differences due to RPE65 genotype. Taking currently available in vitro and in vivo analyses together, both P25L and R91W in the two patients with detectable autofluorescence may represent hypomorphic mutations where there may be partial protein expression and a low level of chromophore production (Lorenz et al., 2008; Samardzija et al., 2008; but also see, Chen et al., 2006; Takahashi et al., 2006).
4.4 Summary
Species comparisons of rates of photoreceptor degeneration can be made to a first approximation by use of allometric scaling with maximum lifespan; by this analysis, the age range of 1 to 6 months in the mouse would approximately correspond to 0.5 to 3 years for the dog, and 3 to 17 years for the human, respectively (Wright et al., 2004). In the mouse versions of Rpe65-deficiency, ONL layer thickness is near-normal at 1 month of age and becomes detectably abnormal by 6 months of age; there is a massive loss of central cones before 1 month. In RPE65-deficient dogs, the rod degeneration appears comparable to the mouse disease but there is substantially greater retention of cones. The reason for the greater vulnerability of mouse cones compared to dog cones remains unknown. Human patients have a more severe natural history of ONL loss compared to the murine and canine models. Specifically, all RPE65-LCA patients published to date in the 3-17 year age range have shown retinal regions with severe ONL loss as well as regions with partially retained ONL. RPE65-LCA cones at 3 years of age were only partially lost at the fovea and may be intermediate between the canine and mouse cone degeneration rates.
5. Loss of visual function resulting from RPE65-deficiency
It is now accepted that loss of Rpe65 isomerase function results in a substantial loss of visual function in patients with RPE65-LCA and murine and canine models of this disease. The interpretation of the details, sources and natural history of remnant vision has evolved over the last decade.
5.1 Murine models
5.1.1 Rpe65−/− knockout mouse
Initial ERG recordings performed in Rpe65−/− knockout animals at 10- to 14-weeks age showed a clear retinal function phenotype resulting from ablation of Rpe65 activity (Redmond et al., 1998). Under dark-adapted conditions, when rod photoreceptor driven responses normally dominate the wildtype response, there were no responses evoked with dim flashes and only small responses evoked with higher intensity flashes (Redmond et al., 1998). The intensity-response curve was desensitized by about 4.5 log units and the maximum amplitude was reduced to ~25% of normal. Under increasing levels of light-adaptation, when wildtype rods get desensitized and responses are increasingly driven by cone photoreceptor driven activity, Rpe65−/− ERGs did not change. These results were interpreted as being consistent with abolished rod function and retained cone function (Redmond et al., 1998). Follow-up studies led to progressive modifications of this interpretation.
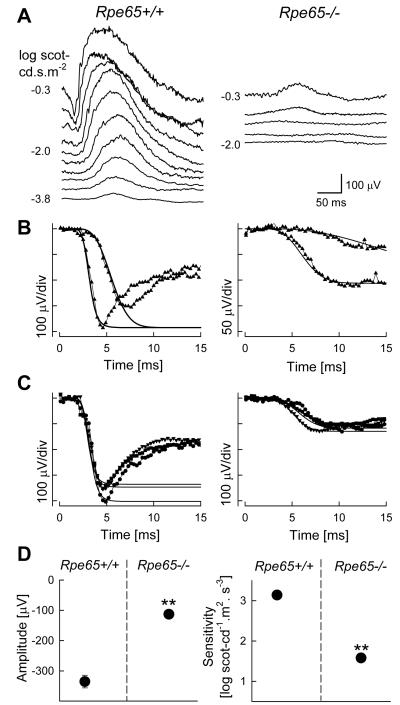
In a study investigating restoration of visual pigment using an oral retinoid, it was shown for the first time that Rpe65−/− mice have an insensitive but detectably functional rod system (van Hooser et al., 2000). ERG b-wave luminance response functions representing bipolar cell activity showed thresholds elevated by ~3 log units with small responses to brighter stimuli consistent with previous work (Fig.3). ERG photoresponses, that normally track the activation phase of rod photoreceptors, were slow but unexpectedly large in Rpe65−/− animals (Fig.3); use of a physiologically-based model of rod phototransduction fit to the leading edges of the photoresponses showed Rpe65−/− rods to have 1.7 log unit reduction in sensitivity (S) and a reduction in maximum amplitude (Rmax) to ~30% of wildtype (van Hooser et al., 2000). The maximum amplitude was substantially larger than that produced by normal cone photoreceptors (Lyubarsky et al., 1999) and thus the data pointed to the existence of a dominant contribution to the Rpe65−/− ERG by an insensitive rod system (van Hooser et al., 2000). This was later confirmed by the use of Cnga3−/−Rpe65−/− and Rho−/−Rpe65−/− double knockout mice designed to genetically ablate the cone and rod contributions to the ERG, respectively (Seeliger et al., 2001).
Figure 3.
Retinal function in the Rpe65−/− mouse model of RPE65-LCA. (A) Dark-adapted ERGs evoked by increasing intensities of blue light stimuli (shown to the left of the traces) in a representative Rpe65−/− mouse show an elevated b-wave threshold compared to Rpe65+/+. (B) A physiologically based model (smooth lines) is fit to the leading edges (initial 4–15 ms depending on response) of dark-adapted ERG photoresponses (symbols) evoked by 3.6 and 2.2 log scot-cd.s.m−2 flashes to quantify the activation phase of rod phototransduction. Rpe65−/− mouse photoresponses show smaller saturated amplitude and a slower initial slope. (C) Photoresponses in three Rpe65+/+ mice are compared to a group of Rpe65−/− mice. Lines are the model of rod phototransduction activation fitted to a pair of photoresponses; only maximal responses are shown for clarity. (D) Maximum amplitude and sensitivity parameters of ERG photoresponses in dark-adapted Rpe65−/− mice are significantly (**) different than the results in Rpe65+/+ mice. Error bars (1 SEM) are smaller than symbols for some data. Modified from van Hooser et al., 2000, copyright © by the National Academy of Sciences.
Reconsideration of the ERGs in Rho−/−Rpe65−/− double knockout mice suggested the existence of a small cone-photoreceptor driven component in addition to the larger rod-driven component (Rohrer et al., 2005). Further studies in Nrl−/−Rpe65−/− (Feathers et al., 2008; Kunchithapautham et al., 2009; Wenzel et al., 2007) and in a Gnat1−/−Rpe65−/− (Maeda et al., 2009) double knockout confirmed the existence of cone-driven ERGs. Studies to date, taken together, suggest that severe chromophore deficiency in Rpe65−/− animals results in strong desensitization of rod and cone photoreceptor function.
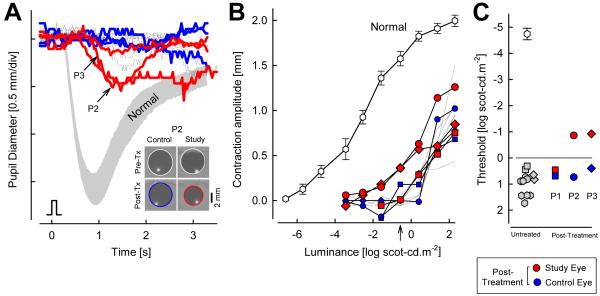
Transient pupillary light reflex (TPLR) refers to the light-induced contraction of the iris muscle and thus it can be used an objective test of the transmission of retinal activity to the brainstem (Aleman et al., 2004). In Rpe65+/+ mice, dark-adapted pupil diameters averaged 2.1 mm and short flashes with luminance energies near −4.9 log scot-cd.m−2 produced a criterion (>0.1 mm) TPLR response. Luminance-response functions performed over a 9 log unit range showed a steep rise followed by shallow saturation. In Rpe65−/− mice (ages 2-4 months), dark-adapted pupil diameters were not significantly different from Rpe65+/+. TPLR response thresholds were elevated by 5 log units to near 0.1 log scot-cd.m−2 and the luminance-response function was right-shifted by ~5 log units also (Aleman et al., 2004). The TPLR waveforms in Rpe65−/− mice were similar to those evoked by ~5 log units dimmer stimuli in Rpe65+/+ mice. Thus it was concluded that the neural circuitry driving the TPLR in Rpe65−/− mice is acting as if experiencing the ambient light levels through a ~5 log unit neutral density filter (Aleman et al., 2004).
5.1.2 Rpe65rd12 naturally occurring mouse
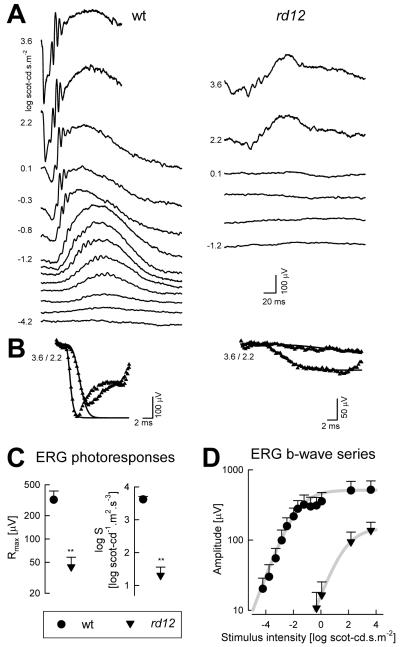
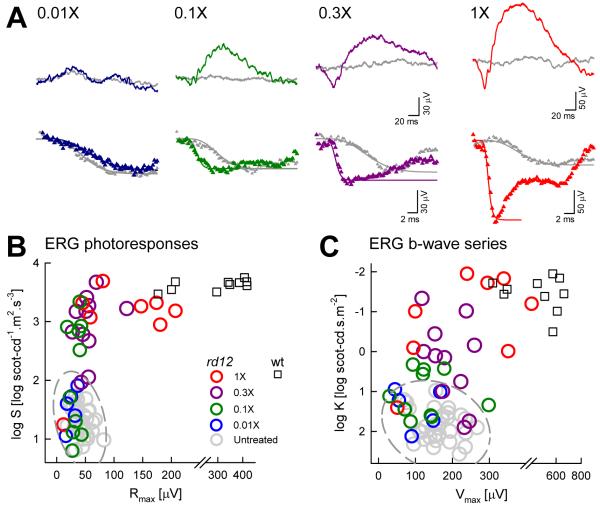
ERG responses are profoundly abnormal in Rpe65rd12 mice as early as 3 weeks of age (Pang et al., 2005). Responses to dimmer range of flashes under dark-adapted conditions are not detectable in Rpe65rd12 mice (Pang et al., 2005; Roman et al., 2007b) suggesting substantial abnormality of rod photoreceptor driven function. Intermediate flash intensities evoke detectable ERG waveforms in Rpe65rd12 mice that are smaller than wildtype animals (Pang et al., 2005; Roman et al., 2007b) and these responses may not change substantially between 1 and 8 months of age (Pang et al., 2005). ERG b-wave amplitudes were analyzed with Naka-Rushton functions to obtain estimates of maximum amplitude (Vmax) and sensitivity (K) at the level of bipolar cells. At the ages of 2 to 4 months, Rpe65rd12 mice showed 3.3 log unit loss of light sensitivity in addition to a pronounced reduction in amplitude (Fig.4). A quantitative measure of overall post-receptoral function as estimated with a log(Vmax/K) parameter showed 3.8 log unit difference from wildtype (Roman et al., 2007b).
Figure 4.
Retinal function in the Rpe65rd12 mouse model of RPE65-LCA. (A) Dark adapted ERGs evoked by increasing stimulus intensities (shown to the left of key traces) for representative 2-month-old wt and Rpe65rd12 mice. Blue flashes were used for all intensities except the highest, which were evoked by white flashes. Traces start at stimulus onset. (B) A physiologically based model of phototransduction (smooth lines) is fit as an ensemble to the leading edges of ERG photoresponses (symbols) evoked by 3.6 and 2.2 log scot-cd.s.m−2 flashes. The response from the mutant shows reduced amplitude and a slower initial slope. (C) Summary statistics of maximum amplitude (Rmax) and sensitivity (log S) parameters obtained from photoresponse modeling in Rpe65rd12 mice are significantly (**) different than wt. (D) Luminance-response functions derived from ERG b-wave series show diminished light sensitivity in mutant animals indicated by a shift to the right of the curves. Mutant animals also show a reduction in maximum amplitude. Error bars are 1SD. Reprinted from Roman et al., 2007b, copyright © by Molecular Vision.
Use of high intensity stimulation allows for recording of ERG photoresponses with maximal (saturated) components originating from rod photoreceptors (Roman et al., 2007b). Leading edges of these ERG a-waves can assess photoreceptor activation kinetics when fit with a model of phototransduction activation (Aleman et al., 2001). At the ages of 2-4 months, maximum amplitude (Rmax) was reduced to 12.5% of normal and sensitivity (S) was reduced by 2.3 log units (Fig.4). In general, the maximum amplitude would be expected to scale with the length and number of rod photoreceptor outer segments as well as changes in the photoreceptor dark current, and the sensitivity would be proportional to the photon catch by the remnant endogenous chromophore. The overall photoreceptor function as estimated with a log (Rmax*S) parameter showed a 3.2 log unit difference from wildtype (Roman et al., 2007b). The significance of the relatively small difference between the extent of photoreceptor dysfunction and post-receptoral dysfunction is not known at this time.
5.1.3 Rpe65R91W knockin mouse
Similar to the other mouse models of Rpe65-deficiency, Rpe65R91W knockin mice show abnormal ERGs from an early age (Samardzija et al., 2008). At 8 weeks of age, luminance-response functions of ERG b-waves under dark-adapted conditions show elevated thresholds and a sensitivity loss of 2.5 log units. It is thought that detectable levels of 11-cis-retinal produced by Rpe65R91W knockin mice make them about 1 log unit more sensitive than Rpe65−/− knockout mice (Samardzija et al., 2008). With age over 40 weeks, the dark-adapted ERG b-waves retain sensitivity but progressively reduce in maximum amplitude. ERGs recorded under light-adapted conditions that are normally driven by cone function showed no difference in threshold, sensitivity or amplitude compared to wild-type animals. The authors hypothesize that the light-adapted ERGs in Rpe65R91W knockin mice originate from a combination of cone and incompletely adapted rod systems (Samardzija et al., 2008) and this hypothesis is consistent with the ERG recordings in Rpe65R91WGnat1a−/− animals with blocked rod transducin signaling (Samardzija et al., 2009). However, other researchers have previously found that blocking of transducin signaling in Rpe65−/−Gnat1a−/− double knockout mice reduces the overall rate of retinal degeneration (Woodruff et al., 2003) thus possibly complicating the interpretation of the cone component ERG function in Rpe65R91W knockin mice.
5.2 Canine models
The recessively inherited retinopathy in the Briard dogs that was molecularly clarified as an RPE65-disease was long known to cause severe retinal dysfunction (Narfstrom et al., 1989; Nilsson et al., 1992; Aguirre et al., 1998). Specifically, ERG waves associated with normal rod photoreceptor activity were reported to be extremely reduced suggesting severe abnormalities with the night vision system (Narfstrom et al., 1989; Nilsson et al., 1992; Aguirre et al., 1998). The ERG waves normally associated with cone photoreceptor activity could be more retained. Visual behavior data demonstrating greater difficulty navigating through dimly-lit obstacle courses as compared to bright light illumination conditions (Bennicelli et al., 2008; Gearhart et al., 2008; Narfstrom et al., 1989), appeared to be consistent with a disease process affecting night vision system more than the day vision system.
More recently, modern ERG methods were applied to 45 eyes of 23 RPE65-mutant dogs at ages 2-11 months to evaluate rod- and cone-mediated retinal function (Acland et al., 2005). ERGs evoked by standard (0.4 log scot-cd.s.m−2) white flashes were not recordable (or substantially reduced) in the RPE65-mutant dogs both in the dark- and light-adapted states (Fig.5). In normal dogs, these flashes evoke ERGs dominated by rod and cone-driven postreceptoral activity in dark- and light-adapted states, respectively. Further, normally cone-dominated ERGs evoked by flashes presented at a rate (29 Hz), too fast for the sluggish rod system to follow, were also not recordable or reduced to <1% of the normal amplitude (Fig.5). These results taken together implied substantial rod and cone dysfunction in the canine RPE65 disease (Acland et al., 2005).
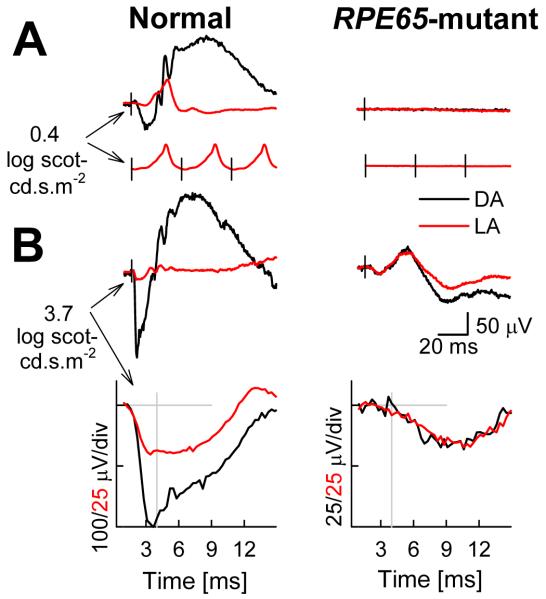
Figure 5.
Electroretinographic (ERG) abnormalities in a representative RPE65-mutant dog. (A) ERGs evoked by standard white flashes (0.4 log scot-cd.s.m−2) presented under dark-adapted (DA, black traces) and light-adapted (LA, red traces) conditions. DA traces are single flashes, LA traces are averages obtained at repetition frequencies of 1 (top) and 29 Hz (bottom). Black vertical lines show the timing of the flashes. (B) ERG photoresponses evoked by white flashes of high energy (3.7 log scot-cd.s.m−2) under DA and LA conditions, same data are shown on slow (top) and fast (bottom) time scales to allow interpretation of late and early components, respectively. Gray lines show the baseline and the 4-ms time point at which rod and cone photoreceptor responses were measured. Modified from Acland et al., 2005.
Function in RPE65-mutant dogs at the level of rod and cone photoreceptors was more precisely studied (Acland et al., 2005) using higher energy (3.7 log scot-cd.s.m−2) flashes that saturate the leading edge of the ERG (Cideciyan & Jacobson, 1996). At the earliest phases of the waveform, dark-adapted responses are dominated by rod photoreceptor activity, whereas light-adapted responses are dominated by the cone system as normal dog rod photoreceptors are strongly desensitized (Kijas et al. 2002). RPE65-mutant dogs under dark-adapted conditions showed small but detectable ERG waveforms (Fig.5). Light-adapted waveforms were identical to those recorded under dark-adapted conditions. Unexpectedly, light-adapted ERG b-waves of RPE65-mutant dogs were larger than the b-waves recorded in normal dogs under the same conditions (Acland et al., 2005). The most parsimonious interpretation of these ERGs would include contributions from rod- and cone-derived signals with significantly reduced sensitivity. Consistent with this hypothesis are the exceedingly slow leading edges of the a-waves in RPE65-mutant dogs (Fig.5) suggesting a substantially reduced rate of activation reactions occurring within the phototransduction cascade.
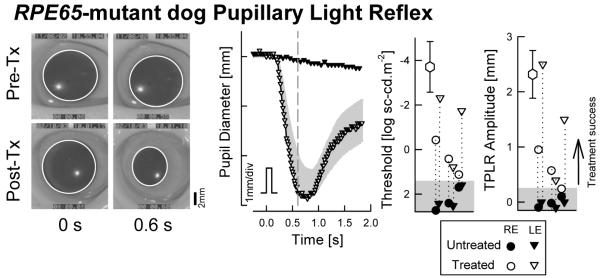
TPLR is an objective test of the transmission of retinal activity to the brainstem; the same test can be applied to humans as well as experimental animals (Aleman et al., 2004). In RPE65-mutant dogs, pupil responses were variably reported to be normal (Narfstrom et al., 1989) versus severely abnormal by at least 4 log units (Acland et al., 2001). A recent study utilized 100 ms transient stimuli spanning a dynamic range of ~9 log units to quantitatively evaluate the TPLR properties in RPE65-mutant dogs in the dark-adapted state (Aguirre et al., 2007). The threshold stimulus to produce a criterion pupillary contraction in RPE65-mutant dogs was found to be ~6 log units elevated compared to normal dogs; the amplitude of the pupillary contraction was abnormal also (Aguirre et al., 2007). Thus retinal activity produced by sufficiently bright stimuli is transferred to the brainstem.
To evaluate the responsiveness of the visual brain in RPE65-mutant dogs more directly, fMRI responses to light were recorded (Aguirre et al., 2007). Visual stimulation was 21 s of an 18-degree high-contrast reversing (5 Hz) annular checkerboard (0.2-0.6 cycles per degree) with a maximum luminance of 2.8 log cd.m−2. In normal dogs, the anatomical site of activity included the lateral gyrus, as well as a smaller response within the more laterally located ectomarginal and suprasylvian areas. Using conventional statistical thresholds, RPE65-mutant dogs showed no significant cortical or subcortical responses to light stimulation. Upon lowering statistical thresholds however, there was a weak response eccentrically located within the lateral gyrus which was confirmed statistically by group analysis (Aguirre et al., 2007).
5.3 Human patients
5.3.1 Electroretinogram
An abnormal ERG is implied by the LCA diagnosis. Therefore, not surprisingly, many reports in the literature have stated that the RPE65-LCA patients had severely reduced ERGs (e.g. El Matri et al., 2006; Hamel et al., 2001; Mamatha et al., 2008; Marlhens et al 1997; Morimura et al., 1998; Thompson et al., 2002). Several reports have provided further detail on the type of ERG abnormalities in RPE65-LCA to allow understanding of the photoreceptor origin of the remnant function. The rod ERGs were not detectable but there can be detectable cone ERGs with reduced amplitude and/or delayed timing in some patients (e.g. Felius et al., 2002; Gu et al., 1997; Lorenz et al., 2000, 2008; Marlhens et al., 1998; Paunescu et al., 2005; Poehner et al., 2000; Thompson et al., 2000; Van Hooser et al., 2000; Yzer et al., 2003). Some investigators have questioned the existence of cone function in human RPE65-LCA and have hypothesized that all of the remnant function originates from light-insensitive rod photoreceptors responding under recording conditions that optimized for cone function (Seeliger et al., 2001). Detailed studies of the residual function were recently performed to attempt to clarify the ERG abnormalities (Jacobson et al., 2009).
Full-field ERGs were performed in 29 RPE65-LCA patients with a standard protocol (Fig.6). The normal ERG to a dim blue light flash in the dark-adapted state represents a rod-mediated b-wave. A white light flash, dark-adapted, elicits a faster and larger waveform with both rod and cone (i.e., mixed) contributions, but is rod dominated. Cone ERGs are recordable in the light-adapted state to single flashes of light or to flickering light at 29 Hz. ERGs were abnormal in all patients with RPE65-LCA tested. None of the patients showed ERG responses to the dim blue light stimuli that normally evoke rod b-waves. Twelve patients (41%) had no detectable ERGs to any stimuli; eight patients (28%) had only small amplitude (range, 2–4 μV) flicker ERGs; and nine patients (31%) showed similar responses to dark- and light-adapted white stimuli and flicker waveforms with amplitudes ranging from 4 up to 35 μV (Jacobson et al., 2009). Remnant ERGs in RPE65-LCA were comparable, in shape and magnitude, to an early-onset rod disease, autosomal dominant retinitis pigmentosa (adRP) caused by an R135L mutation in the rhodopsin gene, (Fig.6) that has been previously shown to retain residual (but abnormal) cone function only (Cideciyan et al., 1998b).
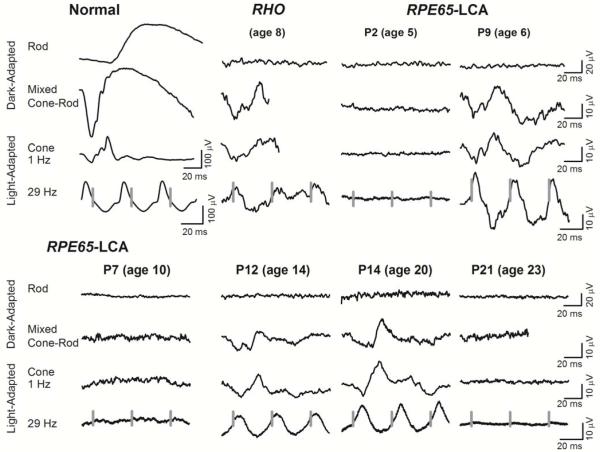
Figure 6.
Standard ERGs in patients with RPE65-LCA. Rod, mixed cone–rod, and cone ERGs from six representative patients with RPE65–LCA (age range, 5-23 years) were compared with ERGs of a patient with a rhodopsin (R135L) gene mutation showing residual, severely abnormal, cone-mediated function. A normal subject (age 20 years) is shown for comparison (left). Traces start at stimulus onset except for 29 Hz where the timing of stimuli is shown with vertical gray lines; calibrations are to the right and below waveforms. ERGs can be undetectable, even at early ages. Detectable ERGs show a pattern similar to that of the patient with a RHO mutation and severe rod dysfunction but residual and abnormal cone function. Modified from Jacobson et al., 2009, copyright © held by the Association for Research in Vision and Ophthalmology.
In animal models of RPE65 disease, severe loss of quantum catch due to the visual cycle block could be bypassed with the use of bright flashes which could evoke unexpectedly large ERG waveforms originating from near-normal numbers of retained photoreceptors (van Hooser et al., 2000; Acland et al., 2001; Roman et al., 2007b). The same approach was used in four RPE65-LCA patients in whom bright flashes were used to evoke ERG photoresponses in search of latent photoreceptor function but none was found (Jacobson et al., 2009). ERG evidence is thus consistent with results with other modalities confirming the existence of a retina-wide degenerative component that is much greater in human patients compared to animal models.
5.3.2 Perception of lights
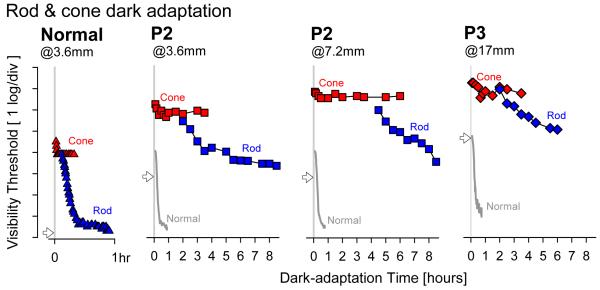
The photoreceptor origin, retinal distribution and inter-individual variation of the substantial loss of light sensitivity in RPE65-LCA have been methodically defined by one group of investigators (Aguirre et al., 2007; Cideciyan et al., 2008, 2009a, 2009b; Hauswirth et al., 2008; Jacobson et al., 2005, 2007a, 2007b, 2009; van Hooser et al., 2000); there have been supporting studies from other investigators (Felius et al., 2002; Lorenz et al., 2000, 2008; Paunescu et al. 2005). One of the techniques used was the full-field stimulus test (FST) which was designed to evaluate the most-sensitive retinal locus in degenerative conditions independent of its location or the patient’s ability to stably fixate (Roman et al., 2005, 2007a). The wide dynamic range of the FST has afforded testing of RPE65-LCA patients (ages 5 to 55) at varying disease stages who can demonstrate sensitivity losses of at least 3 log units and as much as 7 log units (Aguirre et al., 2007; Hauswirth et al., 2008; Jacobson et al., 2009). Comparison of FST sensitivities to different wavelength stimuli allows for estimation of the photoreceptor type mediating the perception (Roman et al., 2005, 2007a). Chromatic measurements in RPE65-LCA showed that ~60% of the patients had remnant rod and cone function; the remaining patients had cone only vision. Notably, there was no clear relationship of patient age to presence of residual rod function (Jacobson et al., 2009).
Retinal distribution of the retained vision can be estimated in a subset of RPE65-LCA patients with traditional kinetic visual fields (Cideciyan et al., 2008; Felius et al., 2002; Jacobson et al., 2009; Lorenz et al., 2000; Paunescu et al. 2005; van Hooser et al., 2000). With the standard large bright target (V4e), kinetic fields were measurable in most patients, and there was a sizable extent in many patients. At later disease stages, kinetic fields showed either a residual central island, with or without peripheral islands, or only a far peripheral island (Felius et al., 2002; Jacobson et al., 2009; Lorenz et al., 2000; Paunescu et al. 2005). The available cross-sectional and longitudinal data indicates that there can be a wide variation in kinetic visual field extent in the first two decades of life and limited islands of vision after the third decade of life (Jacobson et al., 2009).
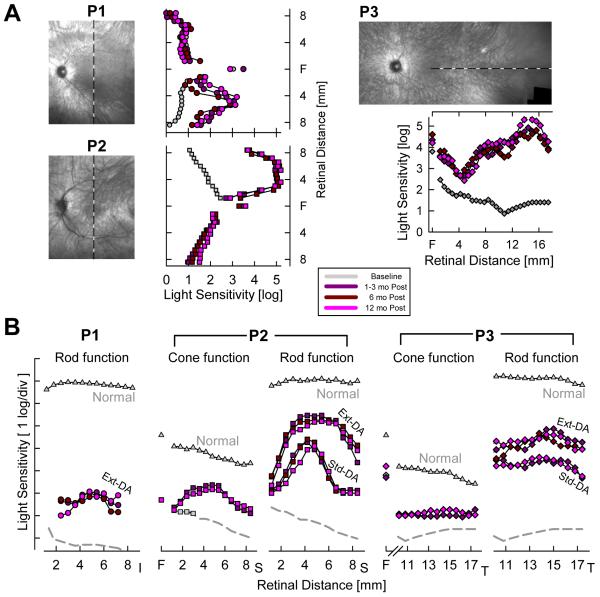
Static threshold perimetry has 1 log unit higher stimulus luminance available compared to the traditional kinetic perimeter, and together with use of testing in the dark-adapted state, static perimetry allows for much wider range of disease stages to be examined. Earliest results with achromatic perimetry in an 11 year old homozygote for a 20 bp deletion at codon 97 (presumed null mutation) demonstrated unusual properties of the visual sensitivity across the visual field in RPE65-LCA (van Hooser et al., 2000). Under dark-adapted conditions, RPE65-LCA sensitivity was reduced by ~6 log units whereas under light-adapted conditions that sensitivity reduction was ~2 log units (van Hooser et al., 2000). In normal vision, these two conditions would assay rod- and cone-mediates vision, respectively, but in RPE65-LCA interpretation of photoreceptor origins were not simple. Light adaptation did not change the visual sensitivity of the patient whereas it elevated normal thresholds by 4 log units. The results were consistent with a minimum of 4 log unit loss for the cone system and a minimum of 6 log unit loss for the rod system in this patient; the exact extent of the respective sensitivity losses could not be estimated (van Hooser et al., 2000). To better understand the effect of RPE65 disease on cone vision, studies were performed with chromatic dark-adapted perimetry in 15 patients with RPE65-LCA (Jacobson et al., 2005, 2007a, 2007b). At the cone-only fovea, sensitivity losses could range from a minimum of 1.5 log units to greater than 3 log units. In two patients, extra-foveal macular regions showed cone-only mediation of light sensitivity with losses in the range of 2.5 log units (Fig.7). Similar results were found in a young patient by different investigators (Lorenz et al., 2008). In most patients, sensitivity in extrafoveal retinal regions was too reduced to allow chromatic discrimination of the type of photoreceptors mediating the response (Jacobson et al., 2005).
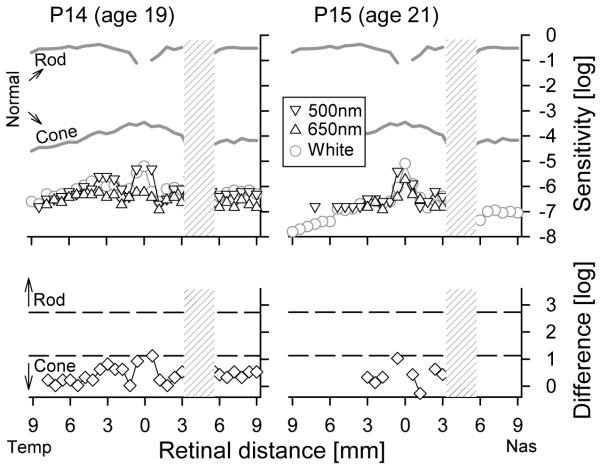
Figure 7.
Cone-photoreceptor mediated visual function in RPE65-LCA. (Upper) Dark-adapted psychophysical sensitivities to chromatic and achromatic stimuli presented across the horizontal meridian in two young adult RPE65-LCA patients (P14, P15). Sensitivities to chromatic stimuli (500 nm blue, 650 nm red) are shown on a common axis of radiometric equivalence, and sensitivities to achromatic (white) stimuli are vertically shifted to match the 500 nm stimulus results. Mean normal dark adapted rod sensitivity to the 500 nm stimulus and dark-adapted cone sensitivity to 650-nm stimulus obtained during the cone plateau are shown (gray lines labeled Rod and Cone). (Lower) Comparison of the difference in chromatic sensitivity at each locus (symbols) to predicted difference for rod or cone mediation (dashed lines) based on spectral sensitivities of normal rod- and cone-mediated vision. Physiological blind spot is shown as a hatched bar. Temp, temporal; Nas, nasal. Modified from Jacobson et al., 2007a, copyright © by the National Academy of Sciences.
Retina-wide distribution of visual dysfunction was recently determined with achromatic static perimetry in 17 RPE65-LCA patients (Jacobson et al., 2009). Patients with the least visual dysfunction showed some decrement in peripheral sensitivity with relative central and mid-peripheral field preservation. More advanced stages showed losses in the mid-peripheral field but with residual islands of central and peripheral function or extensive mid-peripheral scotomas separating central from peripheral vision. Static perimetry results could not always be predicted from the traditional kinetic perimetry result because of the greater dynamic range afforded by the former. For example, patients at advanced disease stages with only central islands by kinetic perimetry showed detectable peripheral islands by dark-adapted static perimetry. Quantifying sensitivity as a function of eccentricity supported the notion that widespread loss of sensitivity occurs early in the disease and this may progress to patchy loss involving the midperipheral region which then increases in severity as well as extent (Jacobson et al., 2009).
5.3.3 Visual acuity
Best corrected visual acuity in RPE65-LCA can vary substantially in the first three decades of life. Between the ages of 1 to 29 years, milder losses in the range of 20/32 to 20/60 as well as severe losses in the range of 20/200 to light perception (LP) and values in between have been reported by many investigators (Al-Khayer et al., 2004; Felius et al., 2002; Jacobson et al., 2009; Lorenz et al., 2000, 2008; Lotery et al., 2000; Paunescu et al., 2005; Thompson et al., 2000; Walia et al., 2010; Yzer et al., 2003). After the third decade of life, most patients present with acuities of 20/200 or worse (Jacobson et al., 2009; Paunescu et al., 2005; Walia et al., 2010). Rare longitudinal follow-up studies at ages where reliable acuity estimates can be obtained have shown stable acuities or progressive decline as would be expected from all retinal degenerative conditions (Al-Khayer et al., 2004; Jacobson et al., 2009; Paunescu et al., 2005). For the subset of RPE65-LCA patients with foveal fixation, the interaction between oculomotor instability, foveal sensitivity and visual acuity is worthy of study especially considering the loss of photon catch in RPE65 disease and the effect of reduced illumination conditions on visual acuity (Shlaer et al., 1942).
5.3.4 Eye movements, fixation and nystagmus
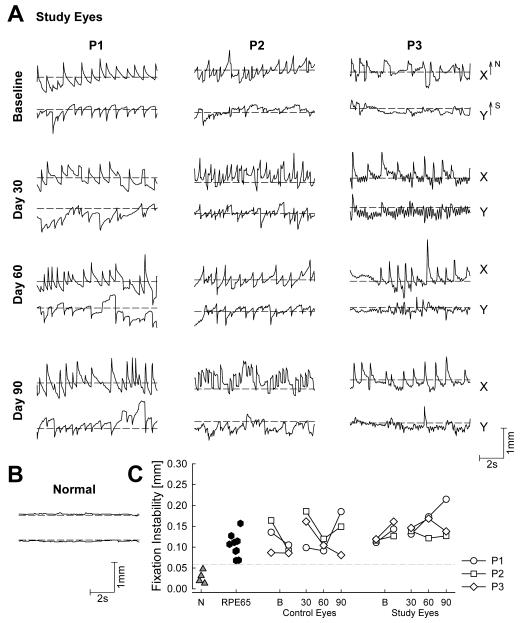
Many patients with childhood onset retinal degeneration or dysfunction, including those with LCA, demonstrate abnormal oculomotor behavior that may include slower wondering eye movements or faster oscillations generally included under the clinical rubric of sensory nystagmus (Hall et al., 2000; Leigh & Zee, 1980). The amplitude, direction and frequency of these eye movements can vary depending on many factors that may include, but not be limited to, visual experience in early childhood, attention and mood of the subject, retinal location of remaining vision while attending to a visible cue, binocular or uniocular viewing, and direction of the gaze. The patients often have no voluntary control over the eye movements although nonvisual external feedback can partially stabilize gaze in some patients (Hall & Ciuffreda, 2002). Quantitative recordings of the retinal location of fixation and instability of eye movements have been published in RPE65-LCA patients (Jacobson et al., 2007a). Recordings were made uniocularly, in fully-dark-adapted eyes, and in a darkened room with the subject viewing a target with an illuminance adjusted to be easily visible. Continuous video image of the retina was obtained under near-infrared light invisible to the subject. Movement of each video frame was recorded with respect to a reference image over time, and calibrated in terms of absolute distances from the anatomical fovea (Jacobson et al., 2007a; Cideciyan et al., 2008). In eight RPE65-LCA patients between the ages of 13 and 23, average fixation instability ranged from 0.19° to 0.45° and was linearly related to logMAR visual acuity. Patients with more severe disease stages (in their fourth decade of life or older) exhibited wandering eye movements without a specific locus of fixation (Jacobson et al., 2007a).
5.3.5 Pupillary light reflex
TPLR may be used as a noninvasive and quantifiable indicator of rod- or cone-based photoreceptor function assuming a lack of conduction abnormalities along the afferent and efferent pathways, and a normal iris muscle (Loewenfeld, 1993). In order to minimize contributions from sluggish photosensitive ganglion cells (Hatter et al., 2003; Lucas et al., 2003; Tu et al., 2006; Wilhelm, 2008), short duration stimuli can be used and quantitation limited to the early transient portion of the TPLR (Aleman et al., 2004). Clinically, pupillary responses can appear to be normal or sluggish or nonresponsive in LCA (Heher et al., 1992; Lambert et al., 1989); differences likely reflect different levels of retinal function, underlying molecular causes, as well as observation conditions. Using a full-field stimulus to take advantage of the area summation capabilities (Schweitzer & Bouman, 1958), TPLR was recorded in 18 LCA patients of unknown genotype (Aleman et al., 2004). Eyes were fully dark-adapted and uniocular luminance-response functions were recorded over a ~9 log unit range of stimulus intensity and compared to normal results. There was a wide range of abnormalities with elevated response thresholds and smaller amplitudes; in most patients, pupil response kinetics, once adjusted for the threshold elevation, were similar to those of normal subjects suggesting a primary role of loss of quantum catch at the image-forming photoreceptors as the main underlying TPLR abnormality in many LCA patients (Aleman et al., 2004).
In patients with RPE65-LCA, TPLR recordings were also successfully performed (Aguirre et al., 2007; Cideciyan et al., 2008). Abnormal elevations of TPLR threshold could range from ~4 to 6 log units (Aguirre et al., 2007; Cideciyan et al., 2008). Time course of pupillary constriction with high intensity stimuli in RPE65-LCA closely resembled normal responses to dimmer stimuli when adjusted for the threshold difference (Aguirre et al., 2007; Cideciyan et al., 2008). These results suggest that TPLR under dark-adapted conditions with short duration stimuli (but not necessarily other methods of pupillary reflex recording) provide a quantitative readout of retina-wide photoreceptor function and can be used as a adjunct to psychophysical methods of visual function in RPE65-LCA.
5.3.6 Cortical function
Reports of early-onset blind subjects have shown altered visual pathway anatomy, including atrophy of the optic nerves and thinning of occipital lobe gray and white matter (Fine et al., 2003; Levin et al., 2010; Noppeney et al., 2005; Shimony et al., 2006). If such abnormalities were to exist in RPE65-LCA, they would be expected to limit the potential improvements in perception achievable by gene therapy at the retina. Thus, the receptivity of cortical substrates for retinal input was evaluated by examining the function and anatomical structure of the visual pathways from retina to cortex in RPE65-LCA (Aguirre et al., 2007).
High-resolution MRI images of the intra-orbital optic nerves showed RPE65-LCA patients to have normal optic nerve diameters (Aguirre et al., 2007). To examine alterations in cerebral anatomy, whole-brain anatomical images obtained from RPE65-LCA were compared to a population of age-matched normal subjects. The analysis included both cortical gray and white matter as well as sub-cortical structures, and no significant differences were found between the two populations at the map-wise level (Aguirre et al., 2007). To rule out the possibility of focal anatomical differences between the two populations alterations of the structure of the LGN was evaluated and RPE65-LCA patients were found not to differ from controls (Aguirre et al., 2007). When the white matter within the occipital lobe underlying early visual areas was considered, a small degree of atrophy was found in the patients as compared to controls (Aguirre et al., 2007), but this change was subtle compared to the marked reduction seen in patients with early blindness from other causes (Shimony et al., 2006).
The volume of RPE65-LCA visual cortex responsive to visual stimulation was determined with fMRI (Aguirre et al., 2007). Using a dimmer range of stimuli estimated to be about 1 log unit brighter than visual threshold in RPE65-LCA, there was a greatly attenuated response, both in extent and intensity, compared to control subjects (Fig.8). However, when a higher intensity stimulus was used, patients demonstrated markedly increased cortical activation. Notably, the cortical area responsive to visual stimulation was comparable to that in controls (Aguirre et al., 2007). To characterize more precisely the cortical responses to visual input, fMRI measures of neural activity were obtained in response to a range of different stimulus intensities following dark adaptation. For control subjects, the lowest light stimulus presented was associated with a small, but significant neural response within visual areas. The volume of cortical tissue with a substantial response to stimulation increased monotonically with stimulus intensity. In contrast, RPE65–LCA showed no measurable neural response to lower intensity stimuli that evoked neural responses in control subjects. At 3 log units below maximum intensity, RPE65-LCA showed a measurable response, although it was on average one-third the size of control subjects. With increasing intensity of light stimulation, the cortical response from RPE65–LCA grew, reaching similar volumes as in normal controls at the maximum intensities used in the study (Fig.8). At the maximum level of stimulation achieved in each group, there was no difference in the extent of cortical response (Aguirre et al., 2007). The findings indicate that the visual pathway in RPE65-LCA remains anatomically intact despite years of disuse and that the visual cortex can be activated even though the patients have limited visual experience.
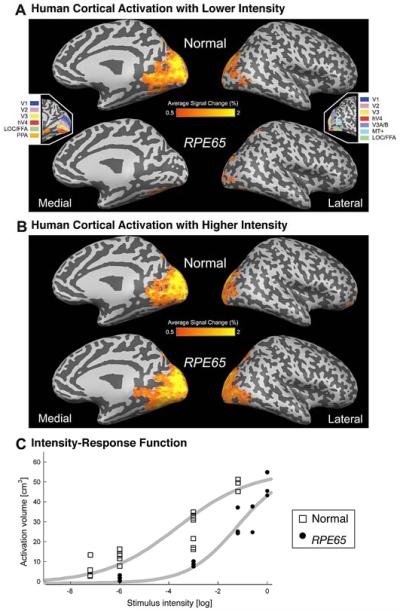
Figure 8.
Mean cortical signal change in response to visual stimulation in human RPE65-LCA (n=6) and normal control populations (n=8). (A and B) The BOLD fMRI response is shown for each population at two stimulus intensities: (A) −3 log and (B) at/near maximum (between −1.2 log and 0 log). The areas of response are displayed upon a digitally inflated right hemisphere. Sulci are indicated in dark gray and gyri in light gray. (Insets) The general position of several retinotopic and higherorder visual areas, derived from data from control participants, is shown. (C) Cortical activation as a function of stimulus luminance is presented. The volume of posterior cortical tissue demonstrating a substantial (>2%) response shows a sigmoidal relationship to the strength of visual stimulation in normal controls and in patients. A Hill function (gray smooth lines) is fit by eye to the data points corresponding to each participant. Reprinted from Aguirre et al., 2007.
5.4 Summary
Common among murine, canine and human versions of RPE65 disease was a marked decrease in residual function at all disease stages. Differences could be apparent, however, upon quantitative comparisons between and within species. Electroretinography (ERG) and transient pupillary light reflexes (TPLR) were two uniformly applied methods that allowed direct comparison. With standard recording conditions, murine, canine and human ERGs showed non-detectable or very small ERGs. Specialized ERG photoresponse recordings in Rpe65-deficient mice and dogs at early disease stages showed unexpectedly large waveforms dominated by the activity of light-insensitive rod photoreceptors. Specialized ERG photoresponses in human RPE65-LCA could not evoke any waveforms above noise. Small cone ERG signals are likely recordable in all three species but their sensitivity has not been quantitatively defined.
TPLR recordings showed sensitivity losses due to RPE65 disease of nearly ~5 log units in mice, dogs and humans. In humans in whom visual perceptual tests could be performed, loss of sensitivity to full-field stimuli was comparable to the loss calculated from TPLR measures. Perceptual perimetric tests allowed determination of the spatial distribution of visual dysfunction in human RPE65 disease. Generally foveal location retained the best sensitivity whereas extra-foveal locations showed 4-6 log unit sensitivity losses comparable to the full-field sensitivity and TPLR measures.
6. Disproportional loss of function compared to retinal degeneration in RPE65-deficiency
In hereditary progressive retinopathies, degeneration of photoreceptors with age generally coincides with a predictable loss of photoreceptor function (Machida et al., 2000). Young RPE65-deficient mice and dogs do not fit this predictive relationship as their near-normal retinal architecture is associated with a severe loss of function. This dysfunction is dominated by reduced quantum catch and reduced dark current within photoreceptors secondary to the block of the normal visual cycle at the RPE. As retinal degeneration sets in, an additional component of vision loss is expected due to photoreceptor apoptosis, shortening of surviving photoreceptor OS, and possible modifications of the OS/RPE interface. Thus, for the majority of the life span of the human, mouse and dog species involved, RPE65 disease is a complex retinopathy involving components with degeneration and dysfunction. It is important to understand the relative proportion of these components in order to predict which treatments are most applicable at different disease stages.
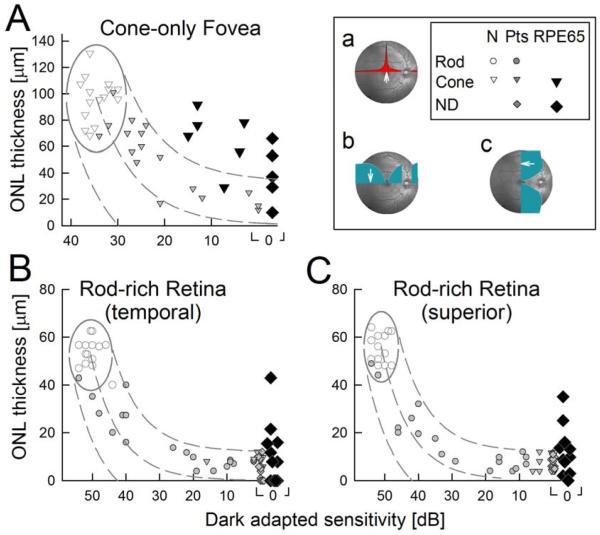
In human patients, the relationship between retinal architecture and co-localized visual function was examined using in vivo measures of photoreceptor (ONL) structure and function (dark-adapted visual sensitivity) at selected locations known to have the highest densities of cones or rods in normal retinas (Curcio et al., 1990). Data from a cohort of human retinal degeneration patients known to not carry RPE65 mutations established the relationship of visual loss to cell loss (Fig.9). Patient results were compared to an idealized model of the expected relationship in photoreceptor degenerations (Jacobson et al., 2005). The model assumes that photoreceptor function is proportional to the product of the number of surviving photoreceptors and the length of their outer segments; both of these parameters are proportional to ONL thickness (Machida et al., 2000). Thus, to a first approximation, loss of light sensitivity (in linear units) would be expected to be proportional to the square of ONL thinning (Jacobson et al., 2005). The model has been subsequently tested on patients with retinopathy due to mutations in CACNA1F, GPR98, MYO7A, NYX, PCDH15, RDH12, RHO, or USH2A genes (Jacobson et al., 2007b, 2008b) and ungenotyped patients with retinitis pigmentosa (Rangaswamy et al., 2010).
Figure 9.
Retinas of patients with RPE65-LCA can have more photoreceptor nuclear layer than predicted from vision. (A) Foveal outer nuclear layer (ONL) thickness as a function of dark-adapted cone-mediated sensitivity (650 nm). (B and C) ONL thickness as a function of dark-adapted sensitivity (500 nm) at 3.6 mm in temporal (B) and superior (C) retina. Rod, rod-mediated sensitivity; Cone, cone-mediated sensitivity; Pts, patients without RPE65 mutations. Normal variability is described by the ellipses encircling the 95% confidence interval of a bivariate Gaussian distribution. Dotted lines define the idealized model of the relationship between retinal structure and function in pure photoreceptor degenerations and the region of uncertainty that results by translating the normal variability along the idealized model. (Inset) Retinal location (white arrow on fundus image) of colocalized measures of structure and function. Overlaid onto the fundus image are cone density (a) and rod density (b and c) along horizontal and vertical meridia. Modified from Jacobson et al., 2005, copyright © by the National Academy of Sciences.
Once the non-RPE65 relationship between retinal architecture and visual function was defined, RPE65-LCA could be examined quantitatively (Fig.9). At the fovea, in non-RPE65 patients, ONL thickness reduction was predictably related to central visual function over a 3 log unit range from normal to severely abnormal vision. In 8 of 11 RPE65-LCA patients, ONL thickness was greater than expected for the level of dysfunction. At two rod-rich regions, 3.6 mm temporal or superior to the fovea, non-RPE65 patients showed ONL thickness reductions predictably related to dark-adapted vision over a 5 log unit range from normal to severely abnormal. Whereas in RPE65-LCA, 5 of 11 patients showed a substantially greater amount of ONL thickness preservation for their severity of visual loss. These data provide quantitative evidence on the complex but interpretable relationship between retinal architecture and visual function in human RPE65-LCA. Visual loss greater than expected from the corresponding co-localized photoreceptor loss is a necessary prerequisite for therapeutic options aiming to restore vision.
7. Treatment of RPE65-deficiency with gene (augmentation) therapy
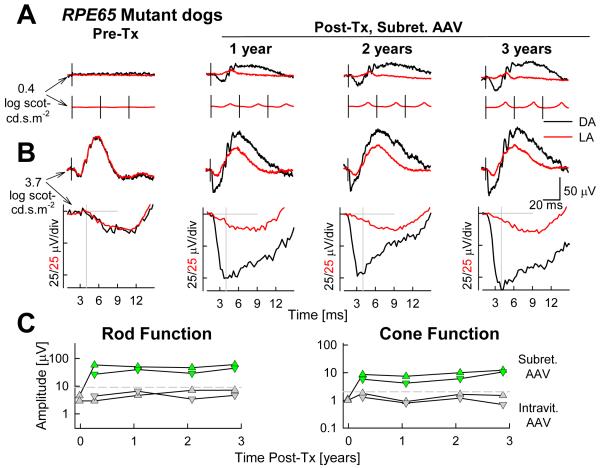
Ideal targets for gene (augmentation or replacement) therapy are genetic diseases resulting from the reduction or absence of a protein with an essential function. Expression of the normal gene encoding this protein at a physiologically appropriate level in the target cell could result in a “cure” of the disease - assuming a lack of secondary changes. RPE65 disease with severe loss of retinoid isomerase function and with relatively retained photoreceptors and relatively healthy RPE cells was thus considered to be an attractive target for gene therapy. Initial proof of principle in the canine model was followed by studies in further dogs, mice and more recently in human patients.
7.1 Murine models
Gene therapy using adeno-associated virus (AAV), adenovirus (Ad) and lentiviral vectors have been performed in both the Rpe65−/− knockout mouse model as well as the naturally occurring Rpe65rd12 mouse model.
7.1.1 Rpe65−/− knockout mouse
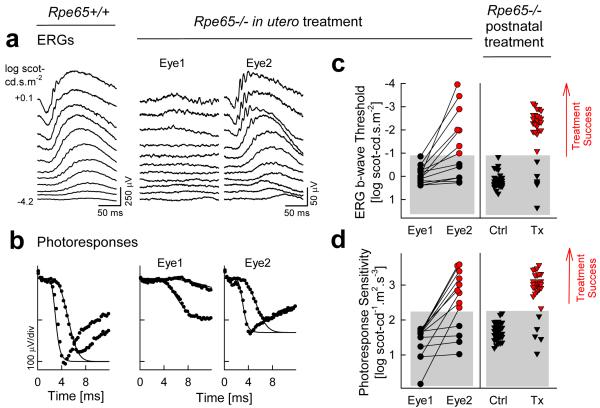
Subretinal delivery of the human RPE65 cDNA to Rpe65−/− mice with a serotype 2 AAV packaged within a serotype 1 AAV capsid (AAV2/1 or AAV1) carrying a CMV promoter resulted in efficient transduction of the RPE, restoration of visual function, and production of measurable rhodopsin (Dejneka et al., 2004). Gene therapy was equally successful when delivered to fetuses at embryonic day 14 or to adult mice at 1-2.5 months of age (Fig.10). Specifically, Rpe65 expression was detectable immunohistochemically in injected eyes at least through 6 months. Retinal function as measured by ERG photoresponses showed highly significant treatment success in ~80% of the post-natally injected eyes and ~70% of the in utero-treated eyes (Fig.10). Additionally, significant levels of rhodopsin were found in both groups of animals (Dejneka et al., 2004). When 24-month-old Rpe65−/− mice with advanced retinal degeneration were treated with the same AAV1 vector, gene therapy could also be effective but in a smaller percentage of eyes (Jacobson et al., 2005). Significant improvements were observed in ~16% of the older animals using ERG criteria and ~29% of the animals using biochemical analysis of rhodopsin content (Jacobson et al., 2005).
Figure 10.
Improvement of retinal function by gene therapy in Rpe65−/− mice performed either in utero or at the post natal age of 1-2.5 months. (a) Dark-adapted electroretinograms (ERGs) from the two eyes (Eye 1, Eye 2) of a representative Rpe65−/− mouse 2 months after a monocular in utero subretinal injection of AAV2/1-CMV-hRPE65. ERGs were evoked by increasing intensities of blue light stimuli (stimulus luminance is to the left of key traces). Traces start at stimulus onset. ERG waveforms from Eye 1 are severely abnormal (elevated bwave threshold of 3-4 log units) and resemble those from uninjected Rpe65−/− mice. Eye 2 responses are dramatically different and more like those of an age matched normal mouse (left column, for comparison). The b-wave threshold is near normal and there are sizeable but abnormal amplitudes; an a-wave can be detected at the brighter intensities. (b) A physiologically-based model of rod phototransduction activation is fit as an ensemble to the leading edges (first 4-12 ms after stimulus) of dark-adapted photoresponses (symbols) evoked in the eyes of the Rpe65−/− mouse shown in (a); normal photoresponses to the same stimuli are shown on the left. (c and d) Retinal function results from all animals in all groups. For in utero-injected animals (left), Eye 1 is defined as the eye with the lower photoresponse sensitivity. Red-filled symbols (“treatment success”) represent those results falling beyond the 99% confidence interval limit (upper boundary of the gray bars) for each parameter determined from uninjected age-matched Rpe65−/− mice. Lines connect data obtained from the two eyes of each animal. Many of the Eye 2 group show retinal function that is as good as or better than that in eyes treated postnatally (Tx, right). Reprinted from Dejneka et al., 2004.
To directly evaluate the retinoid isomerase activity, human RPE65 cDNA was delivered subretinally with an Ad vector under the CMV promoter to 2-week-old Rpe65−/− mice (Chen et al., 2006). At 2 weeks after injection, there was robust Rpe65 gene expression and generation of significant amounts of 11-cis-retinal together with reconstitution of isomerase activity comparable to that of wildtype mice. It was noteworthy that the gene therapy prevented the massive cone degeneration seen at early ages of Rpe65−/− mice (Chen et al., 2006).
In a study concentrating on the functional and structural rescue of cones, mouse RPE65 cDNA was delivered subretinally with a lentiviral vector and an RPE-specific (R0.8) promoter to 5-day-old Rpe65−/− mice (Bemelmans et al., 2006). The transgene was expressed for up to 4 months when assayed immunohistologically. Immunofluorescence labelling of the cone-specific proteins showed retained cones for up to 4 months and restored cone ERG function as confirmed by the use of Gnat1−/−Rpe65−/− double knockout mice (Bemelmans et al., 2006). Of interest, the investigators demonstrated the existence of a transitional penumbral area surrounding the injection bleb wherein cones are rescued without apparent expression of the transgene in the RPE. It is speculated that either the lateral diffusion of 11-cis-retinal promotes cone survival or the sensitivity of immunocytochemical detection of RPE expression is below that of cone survival markers (Bemelmans et al., 2006).
Unlike the success of AAV1, initial investigation with an AAV2 vector had achieved limited and transient functional rescue when expressing mouse RPE65 cDNA under the CMV promoter in 3 week-old Rpe65−/− mice (Lai et al., 2004). In a more recent study, the AAV2 vector was used to subretinally deliver human RPE65 cDNA under the CMV/CBA promoter to 1–2 month-old Rpe65−/− mice and retinal function was assayed with ERGs 3-4 weeks after the injection (Bennicelli et al., 2008). RPE65 expression was identifiable immunohistochemically in the RPE but only 4 of 10 injected eyes were reported to show improvements (Bennicelli et al., 2008). The reason for the failure of functional improvement in the majority of the injected eyes is not clear at this time.
7.1.2 Rpe65rd12 naturally occurring mouse
Human RPE65 cDNA was delivered subretinally to Rpe65rd12 mice at 2 weeks of age with an AAV5 vector with the CBA promoter (Pang et al., 2006). At about 6.5 months post-injection, there was evidence for detectable levels of rhodopsin biosynthesis, reduced accumulation of all-trans-retinyl esters, rescue from photoreceptor degeneration and lipid-like inclusions in the RPE, restored ERG function under dark- and light-adapted conditions, and improvement in visually guided behavior (Pang et al., 2006). It was later shown that the efficiency of subretinal AAV5 vector transduction is not altered by a previous intravitreal injection in the partner eye (Li et al., 2009).
In a related series of experiments evaluating the functional integrity of the visual pathway, Rpe65rd12 mice were injected at P18, at 6 months and at 13 months of age (Nusinowitz et al., 2006). Investigators compared retinally-derived ERG parameters to cortically-derived visually evoked potential (VEP) parameters to test the integrity of the visual pathway after gene replacement therapy. Their observations suggest that a relatively intact visual pathway for mice treated at P18 or at 6 months of age for the channels carrying high contrast and low temporal frequency luminance information (Nusinowitz et al., 2006). However, there was evidence for a systematic loss of function for visual pathways carrying high temporal frequency luminance information. Consistent with other studies, early gene replacement therapy resulted in better rescue of cone-mediated function. Surprising was the observation of detectable cone responses in the mice for which treatment was delayed until 6 months supporting the existence of a subset of preserved cones in Rpe65rd12 mice that degenerate slower than the initial phase of massive loss (Nusinowitz et al. 2006). More recently, a self-complementary AAV5 vector with a CMV/CBA promoter was used in Rpe65rd12 mice at P14 and P35 (Pang et al., 2010). Evaluated at 5 or 6 months, rod function was restored with either injection time point but cone function was restorable only with the P14 injection.
Gene therapy efficiency of an integration-deficient lentiviral vector carrying human RPE65 transgene with various promoters was evaluated in Rpe65rd12 mice (Yanez-Munoz et al., 2006). Subretinal delivery at 5 weeks of age resulted in expression of RPE65 in the transduced RPE. Significant improvements in ERG b-wave amplitudes were observed at 3 weeks after the injection and the improvements were stable for at least 8 weeks after the injection (Yanez-Munoz et al., 2006).
Therapeutic efficiency of gene therapy was evaluated in Rpe65rd12 mice (Roman et al., 2007b) in order to develop an in vivo bioassay of the biological activity of human grade AAV2 vector (Jacobson et al., 2006a, 2006b). This in vivo bioassay was later used successfully for stability testing during human clinical trials of gene therapy (Cideciyan et al., 2008). Rpe65rd12 mice were injected subretinally between 2 to 5 weeks of age and evaluated between 2 to 4 months of age with an extensive ERG protocol that included quantification of the luminance-response function over an ~8 log unit range and of the photoresponses with a phototransduction model (Roman et al., 2007b). Efficacy of treatment was evaluated statistically with stringent 99% confidence intervals using overall photoreceptor and post-receptoral ERG function parameters. Results of the complex efficacy analysis were compared to a simpler and shorter methodology based on the inter-ocular difference of the ERG b-wave amplitude evoked by a single appropriately chosen flash intensity. A dose range spanning 2 log units from 108 to 1010 vg/μl demonstrated an orderly relationship with increasing therapeutic efficacy: 108 vg/μl (0.01X) was inefficient; 109 and 3×109 vg/μl (0.1X and 0.3X, respectively) showed increases in photoreceptor and postreceptoral sensitivity but no increase in amplitude; and 1010 vg/μl (1X) showed increased sensitivity and amplitude of both photoreceptoral and postreceptoral function (Fig.11).
Figure 11.
Electroretinography parameters of Rpe65rd12 mice to different doses of subretinal AAV2-hRPE65. (A) ERGs evoked by 0.1 log scot-cd.s.m−2 flashes (upper row) and by 3.6 log scot-cd.m.s−2 flashes (lower row) in treated (colors) and untreated (gray) eyes of one Rpe65rd12 mouse from each dose group (0.01X-1X). As vector dose increases, responses become asymmetric with treated retinas showing increasing amplitude of b-waves and faster photoresponses. Photoreceptor activation models (smooth lines) fit to the photoresponses are shown. All traces start at stimulus onset. (B) Photoresponse parameters in Rpe65rd12 eyes treated with a range of vector doses. As dosage increases above 0.01X, parameter pairs drift outside of the 99% confidence region (dashed ellipse) defined by the untreated eyes of Rpe65rd12 animals and start approaching wt levels. (C) Luminance response parameters in treated Rpe65rd12 eyes similarly show a dose-related progression from the region corresponding to untreated eyes to the region corresponding to wt eyes. Reprinted from Roman et al., 2007, copyright © by Molecular Vision.
7.2 Canine models
An important stepping stone en route to the initiation of human clinical trials in 2007 was a proof-of-concept study that showed subretinal gene therapy can improve visual function in three RPE65-mutant dogs (Acland et al., 2001). In this study, a recombinant AAV2 vector carrying wildtype dog RPE65 cDNA with a CMV/CBA promoter was injected (~4×1010 infectious particles) either subretinally or intravitreally at ages of ~4 months and visual function was assayed ~3 months after the injection. These initial proof-of-concept experiments were confirmed and extended in many additional studies from the same investigators as well as from other laboratories (Acland et al., 2005; Aguirre et al., 2007; Bennicelli et al., 2008; Ford et al., 2003; Jacobson et al., 2006; Le Meur et al., 2007; Narfstrom et al., 2003, 2005, 2007, 2008). The original study of gene therapy in the RPE65-mutant dog and the other studies are reviewed below with respect to the visual function improvements measured at retinal, subcortical and cortical levels, as well as biochemical, morphological, and ultrastructural observations.
7.2.1 Improvement of retinal photoreceptor-driven function
ERG recordings were performed to evaluate rod- and cone-photoreceptor driven retinal activity before and after gene therapy in RPE65-mutant dogs and compared to control animals. In the first study (Acland et al., 2001), the threshold to dimmest lights was assayed with luminance-series of ERG b-wave responses which are dominated by the activity of rod bipolar cells summing signals from presynaptic rod photoreceptor cells within their dendritic fields. The photoreceptor function was assayed using ERG photoresponses. And, cone-photoreceptor driven retinal function was assayed with 29 Hz flicker ERG recordings. For uninjected and intravitreally injected RPE65 mutant dog eyes, thresholds were elevated by over 4.5 log units compared to wildtype dogs. After subretinal injection, the thresholds were restored to near normal levels. Photoresponse and flicker amplitudes recovered to 10-25% of wildtype compared to being nonrecordable in uninjected and intravitreally injected RPE65 mutant dog eyes. These results were strongly suggestive of restoration of retinal function following subretinal delivery of gene therapy in RPE65-mutant dogs (Acland et al., 2001).
Other short-term studies generally confirmed the initial experiments. In one study, 11 dogs at varying ages between 4 and 24 months were injected subretinally with an AAV-RPE65 vector carrying a CMV promoter (Narfstrom et al., 2003). At 3 months after the injection, 10 of 11 dogs showed an increase in ERG signal amplitudes consistent with an improvement of retinal function. In the largest study of its kind todate, 26 eyes of 17 RPE65-mutant dogs were injected subretinally with AAV2, AAV1, AAV5 vector pseudotypes carrying human or canine RPE65 cDNA with various promoters (Acland et al., 2005). Ages at injection were 2-11 months. ERGs in treated eyes were evaluated 1 to 3 months after the injection and compared to results from 45 untreated RPE65-mutant eyes using a conservative statistical criterion encompassing 3SD from the mean result. The results demonstrated a significant improvement in rod- and cone-photoreceptor ERG measures in 23 of 26 eyes. Importantly, these series of experiments demonstrated for the first time, the restoration of near-normal sensitivity to both rod as well as cone photoreceptors in RPE65-mutant dogs with the use of the earliest components of ERG photoresponses evoked with high luminance lights under different adapting conditions. There were no obvious differences in terms of age of injection, vector serotype or promoter but superior retinal injections showed greater ERG response per area transduced compared to inferior injections (Acland et al., 2005). In a fourth study, 13 RPE65-mutant dogs received subretinal injections of AAV2 gene therapy with a CMV/CBA promoter to estimate the treatment response at 2-3 months post-injection as a function of >4 log unit variation in dose (Jacobson et al., 2006a). Using the same stringent 3SD statistical criterion, ERG improvement was seen in 94% of eyes injected with >= 15×1010 vg whereas only 7% showed evidence of efficacy when injected with =<4.5×1010 vg (Jacobson et al., 2006a). In a fifth study, 8 eyes of 4 RPE65-mutant dogs treated with therapeutic levels of AAV gene therapy with the CMV/CBA promoter showed a significant improvement in ERG thresholds and amplitudes at 3 months after injections (Aguirre et al., 2007). In a sixth study, 8 RPE65-mutant dogs were injected subretinally at ages ranging from 8-30 months with AAV pseudotypes 2 and 4 carrying the RPE65 promoter (LeMeur et al., 2007). The investigators found ERG improvements within 15 to 30 days after injection in 7 of the 8 dogs; the non-responding dog was the oldest dog tested. In other studies however, older dogs were successfully treated with gene therapy (Aguirre et al., 2007; Narfstrom et al., 2003, 2007). In a seventh study, 3 RPE65-mutant dogs were treated at an age of 3.5 months with AAV2 carrying the CMV/CBA promoter and followed up to 3 months (Bennicelli et al., 2008). ERG improvements were found in 4 of 5 subretinally injected eyes but there was a diminution of the ERG amplitudes between 5 weeks and 3 months after the injection (Bennicelli et al., 2008) as opposed to stability or improvement observed over the same period in another study (Le Meur et al., 2007). In summary, multiple laboratories using a variety of AAV pseudotypes, human or dog RPE65 cDNAs, promoter sequences and doses showed that the retinal function in RPE65-mutant dogs can be improved substantially in the short term (~2 weeks to ~3 months) with subretinal delivery of gene therapy.
A small subset of studies at several laboratories investigated the longevity of the retinal improvements due to gene therapy and found mostly positive results over the longer term. The first long-term studies were in the two animals injected at an age of 4 months in the proof-of-concept studies (Acland et al., 2001); these animals were followed for three years (Acland et al., 2005). Early improvements seen after subretinal gene therapy in ERG responses were found to be stable through three years (Fig.12). In a different study, mostly preserved ERG responses were also found in two dogs treated at an age of 1 year and followed for 2 and 4 years, respectively (Narfstrom et al., 2005, 2008). A third group of investigators found stable ERG responses at 1 year post injection in two dogs treated at 8 months of age (Le Meur et al., 2007). These results taken together strongly suggest that ERG improvements measured after subretinal gene therapy in RPE65-mutant dogs can remain stable from ~3 months to up to ~3 years; the longevity of the functional improvement beyond this period is not known.
Figure 12.
Long term restoration of vision in RPE65-mutant dogs by gene therapy. (A) ERGs evoked by standard white flashes in the right eye of an RPE65-mutant dog before treatment (Pre-Tx) and over a 3-year interval after treatment. Flashes presented under dark-adapted (DA) and light-adapted (LA) conditions. DA traces are single waveforms, LA traces are averages obtained at repetition frequencies of 1 (top) and 29 Hz (bottom). Black vertical lines show the timing of the flashes. (B) ERG photoresponses evoked with white flashes of high energy over the same 3-year interval in the same eye as in A. Waveforms displayed as in A. (C) Two eyes with subretinal AAV-RPE65 (green symbols) show stable level of partial restoration of retinal rod and cone function, whereas two eyes with intravitreal AAV-RPE65 (gray symbols) show amplitudes similar to those of untreated eyes. Horizontal dashed lines represent the upper limit (mean + 3 SD) of the respective measurement in the group of control RPE65-mutant affected eyes, which had not received treatment. Modified from Acland et al., 2005.
7.2.2 Improvement of subcortical and cortical function
Restoration of retinal function with gene therapy would be of little practical consequence unless the retinal information is transmitted along visual pathways to the primary visual cortex for interpretation. Behavioral studies in treated dogs have suggested that some information from the retina reaches the cortex (Acland et al., 2001; Bennicelli et al., 2008; Narfstrom et al., 2003) but the extent and quality of this information is not clear. Deafferentation resulting from long standing retinal degeneration could result in abnormalities in post-receptoral neurons within the eye as well neurons along the visual pathway located extraocularly. The normal reflexive contraction of the pupil in response to light – the pupillary light reflex, PLR – was used to evaluate subcortical function in the initial proof-of-concept study (Acland et al., 2001). In the two dogs treated with subretinal injections, the criterion PLR response threshold improved on the average by ~0.8 log units. In another study, six eyes of three dogs treated with subretinal injections showed significant improvements in PLR response thresholds ranging from ~0.5 to ~4 log units (Fig.13) supporting the hypothesis that improved sensitivity at the retina was being transmitted to the level of the brainstem (Aguirre et al., 2007).
Figure 13.
Brainstem responses in the dog using the pupillary light reflex show gene therapy treatment effect. (Left panel) A representative RPE65-mutant dog pre- and 1 month post-treatment (video frames show the pupil before and 0.6 s after a 0.6 log scot-cd.m−2 stimulus; pupillary margin delineated). (Middle panel) Pupillary contraction amplitude and timing in this eye post-treatment (unfilled triangles) was within normal limits (gray band); there was no response pre-treatment (filled triangles). (Right panel) Threshold and amplitude parameters show treatment success in RPE65-mutant eyes after gene therapy compared to untreated/pretreatment results. Modified from Aguirre et al., 2007.
To more directly evaluate the activity of the visual cortex, visual cortical potentials were recorded in the proof-of-concept studies (Acland et al., 2001). Consistent with ERGs and PLRs, visual cortical potentials were recordable in the subretinally-treated eyes. Evaluation at the cortex was extended with the use of blood oxygen level-dependent (BOLD) functional MRI (fMRI) before and after treatment in RPE65-mutant dogs as compared to controls (Aguirre et al., 2007). Pretreatment, there were weak responses within the lateral gyrus whereas after successful treatment in five animals, significant cortical activation was observed within the lateral gyrus. Surprisingly in two of the animals, the extent of the recovered response approached that seen in wild-type controls, despite the limited area of the retina that underwent treatment. Recovery of cortical responses was observed as soon as 1 month after therapy ruling out any substantial delay between the retinal and cortical recoveries.
7.2.3 Expression, morphology and biochemistry
In the initial proof-of-concept study, one eye of an RPE65-mutant dog subretinally treated at 4 months was enucleated 3 months later and ocular tissues were harvested (Acland et al., 2001). When evaluated by genomic PCR, there was persistence of the transferred wildtype DNA restricted to the injected quadrant; both the RPE and the retina showed expression. In a second study, eyes of two treated dogs were examined morphologically (Narfstrom et al., 2003). RPE65 was immunolocalized to the RPE, but not the retina, within the area of the injection. One dog treated at 8 months of age and sacrificed 3 months later, showed apparent disappearance of the accumulation of lipid droplets within the RPE corresponding to the injected region; the other dog treated also at 8 months and sacrificed 6 months later, did not show this reversal. The photoreceptor morphology was abnormal in both animals both within and outside the injected regions.
In the largest study of its kind, 18 eyes of RPE65-mutant dogs having received subretinal injections were studied morphologically as compared to 19 control eyes (Acland et al, 2005). Post-treatment intervals ranged from 3.5 months to 2 years. In eyes with subretinal injections, RPE65 labeling was limited to the RPE cells of the treated area. Natural variability of the size and number of lipid inclusions across RPE65-mutant retinas precluded analysis of this feature as a function of treatment (Acland et al., 2005). Photoreceptor morphology was normal and there were no appreciable differences in the thickness of the nuclear layers for comparable regions of normal, affected, and treated affected retinas reflecting the very slow course of retinal degeneration in RPE65-mutant dogs (Acland et al., 2005). In a fourth study, morphological study of the treated eye of a dog 30 months after successful gene therapy showed normal appearing photoreceptor outer segments within and outside the injection bleb area (Narfstrom et al., 2005).
In a study examining safety of the RPE65-AAV vector in a large number of dogs injected with different doses, retinal morphometry was performed (Jacobson et al., 2006a). ONL thickness was quantified by sampling along the vertical meridian in 27 eyes with subretinal injections. At dose levels up to 30×1010 vector genomes delivered, focal lesions mostly attributable to the surgical trauma of the retinotomy were apparent but there was no regional thinning of the ONL. In most eyes injected with doses 150×1010 vector genomes or higher, there was a substantial regional thinning generally corresponding to the bleb formed by the injection (Jacobson et al., 2006a).
In a sixth study, a subretinally treated eye of an RPE65-mutant dog was assessed with immunocytochemical studies at 6 months post-treatment (Le Meur et al., 2007). Within the treated area, there was distinct RPE65 labeling of the RPE; there was no labeling of photoreceptor cells. The study found no appreciable differences from normal in terms of the thickness of the nuclear layers. The morphological study of an eye enucleated 4 years following gene transfer is the longest followup available todate (Narfstrom et al., 2008). This study showed photoreceptors retained near the injection site as compared to complete photoreceptor degeneration observed in the peripheral retina (Narfstrom et al., 2008). In an eighth study, histopathology performed at 5 weeks and 3 months after subretinal gene therapy revealed mostly normal retinas both within and outside treated regions (Bennicelli et al., 2008).
Retinoids in treated eyes of RPE65-mutant dogs have been analyzed biochemically and compared to control eyes (Acland et al., 2005). In three eyes with subretinal injections there were detectable levels of 11-cis-retinal which was restricted to the retinal region of the injection. There were no dramatic differences in retinyl ester levels in the subretinally injected retinal regions compared to surrounding regions over 6 to 14 months post-treatment (Acland et al., 2005).
7.3 Human patients
Three independent clinical trials of RPE65 gene therapy were initiated nearly contemporaneously in 2007 (NCT00481546, NCT00516477, NCT00643747, clinicaltrials.gov) and results in 18 patients covering maximum follow up periods ranging from 90 days to 1.5 years have been published to date (Bainbridge et al., 2008; Cideciyan et al., 2008, 2009a, 2009b; Hauswirth et al., 2008; Maguire et al., 2008, 2009; Simonelli et al., 2010). Common among the three trials was the use of replication deficient recombinant AAV2 vectors carrying the wildtype human RPE65 cDNA delivered surgically to the subretinal space of the worst seeing eye of each patient. Even though there were many differences such as manufacturing, regulatory sequences, concentration and volume, use of steroids and anesthesia, and patient characteristics, general conclusions can still be made. The primary conclusion supported by these studies is that subretinal gene delivery to the eye appears to be generally safe without vector-related serious adverse events, toxicity, or a major immune response. Second, there is improvement in visual function within a short period of days to weeks following gene delivery and persistence of these improvements for up to 1.5 years supporting physiologically relevant levels of gene expression and durability of the gene product. The details of the findings related to visual function improvement are reviewed below.
7.3.1 Improvement in perception of lights
In RPE65 disease, photoreceptors have substantially reduced sensitivity to visual stimulation with lights due to at least three causes: a) loss of photoreceptor cells, b) lack of visual chromophore 11-cis-retinal to absorb photons, and c) increased thermal activation of the phototransduction cascade by chromophore-free opsin molecules (van Hooser et al., 2000, 2002; Fan et al., 2005). Successful treatment of the disease with gene therapy could not bring back lost cells but could result in availability of greater amounts of chromophore, increased capture of photons and decreased thermal activation by chromophore-free opsin. The result would be an improvement in light sensitivity such as previously observed in small and large animal models (van Hooser et al., 2000, 2002; Acland et al., 2005; Roman et al., 2007b). To the delight of the investigators involved as well as others in the field of retinal degenerations, many patients undergoing gene therapy have reported an increase in sensitivity to dim lights (Cideciyan et al., 2008, 2009a, 2009b; Hauswirth et al., 2008; Maguire et al., 2008, 2009). To quantify the patients’ observations, sensitivity was measured with a full-field sensitivity test (FST), a psychophysical method designed specifically for subjects with severe visual loss independent of their fixation ability (Roman et al., 2005, 2007b; Aguirre et al., 2007). There were statistically significant improvements from baseline in the study eyes at 30, 60 and 90 days after gene therapy; control eyes did not show changes (Hauswirth et al., 2008). Another study using a similar FST method evaluated the interocular sensitivity difference and concluded that 5 of 7 patients showed a response to treatment (Maguire et al., 2009), but it is not known whether the interocular differences were driven by improvements in injected eyes or reductions in control eyes or a combination of both.
In order to determine the spatial co-localization of the gene therapy injection and the resulting biological activity, computerized perimetry methods were used. One group of investigators used combinations of achromatic, short- and long-wavelength stimuli (Cideciyan et al., 2008, 2009a) and another group used short-wavelength stimuli (Bainbridge et al., 2008) to investigate sensitivity to lights under dark-adapted conditions. In one study, all three patients showed statistically significant improvements ranging from 2 to 5 log units over baseline and co-localizing to the injection area; the improvements were apparent by 30 days and remained unchanged for at least 1 year (Fig.14). With the use of chromatic stimuli and specialized testing methods, it was determined that gene therapy improved the sensitivity of both rod and cone photoreceptors within retinal regions of peak biological activity in two of the patients; only restored rod function could be unambiguously measured in the third patient (Cideciyan et al., 2008, 2009a). In another study, one of three patients showed statistically significant improvements of 2 log units during a 6 month follow-up period; photoreceptor source mediating the response was indeterminate (Bainbridge et al., 2008). Fundus-controlled microperimetry which tracks eye movements in real time in patients with unstable fixation was able to be used in two patients undergoing gene therapy; the results were consistent with perimetry performed under free viewing conditions (Bainbridge et al., 2008; Cideciyan et al., 2009b).
Figure 14.
Visual function improvement in RPE65-LCA patients after gene therapy is stable up to 12 months after treatment. (A) Light sensitivity to achromatic stimuli in study eyes along vertical (P1 and P2) and horizontal (P3) meridians after allowing for an extended (3–8 hr) period of dark adaptation. Sensitivity values recorded post-treatment (colored symbols), are compared with the mean baseline values recorded before treatment (gray symbols). Images of the ocular fundus of the study eyes obtained at 12 months after treatment. All images are shown as left retina for clarity and comparability. F, fovea. (B) Light sensitivity measures with chromatic stimuli support stability of rod and cone photoreceptor-based vision 12 months after treatment in retinal regions of peak response to gene therapy. Rod function measured with blue stimuli after standard (Std) or extended dark adaptation (Ext-DA) conditions. Cone function measured with red stimuli after Ext-DA conditions (at the fovea) or during the cone plateau period in the dark after light adaptation (at extrafoveal locations). The sensitivity axes are shifted vertically to match red and blue stimuli for cone-mediated detection in patients. I, inferior; S, superior; T, temporal retina. Reprinted from Cideciyan et al. 2009a, copyright © by Mary Ann Liebert, Inc..
7.3.2 Investigation of visual acuity
Visual acuity is the historical gold standard outcome measure in ocular studies (Ferris et al., 1982); in normal eyes, visual acuity quantifies the identification of high-contrast letters projected onto the foveal cones, but in retinal diseases the retinal location or the photoreceptor source of vision contributing to the detection task is rarely known (Fishman et al., 2005). All three gene therapy studies recorded and reported visual acuity by standard methods but the conclusions differed (Bainbridge et al., 2008; Cideciyan et al., 2009a; Hauswirth et al., 2008; Maguire et al., 2008, 2009; Simonelli et al., 2010). For two of the groups, changes in visual acuity were considered not to be statistically significant in any of the 6 patients followed for up to 1 year after gene therapy (Bainbridge et al., 2008; Cideciyan et al., 2009a; Hauswirth et al., 2008). Two of the patients whose foveas were included within the injection bleb showed no evidence of an improvement of light sensitivity at the fovea even though there was significant and substantial improvement in extrafoveal light sensitivity in the same eyes (Bainbridge et al., 2008; Cideciyan et al., 2008, 2009a). The third group of investigators reported significant improvements in visual acuity in most of their patients (Maguire et al., 2008, 2009; Simonelli et al., 2010). There was a tendency for the patients with the worst baseline acuities to show the greatest improvement (Maguire et al., 2009) despite the greater variability expected under such low vision conditions (Miller, 2008). It is possible that these acuities were driven by extrafoveal retinal loci. One of the largest acuity changes observed was of interest and counterintuitive – the uninjected control eye in one of the patients demonstrated six ETDRS lines of improvement from 20/500 to 20/126 over a 1.5 year follow-up period post-injection (Simonelli et al., 2010). It was speculated that the treatment in the study eye reduced nystagmus amplitude in both eyes and resulted in an improved acuity in the control eye (Maguire et al., 2008, 2009; Simonelli et al., 2010).
7.3.3 Investigation of the electroretinogram
The electroretinogram (ERG) can provide an objective means to evaluate the function of the retina by measuring the electrical response evoked in response to light stimulation. The ERG was recorded with standard full-field flash stimulation and post-injection results were reported to be not different than pre-injection (Bainbridge et al., 2008; Maguire et al., 2008, 2009; Simonelli et al., 2010). More specialized ERG techniques were also used. Pattern ERG is a technique for the assessment of central retinal function and showed no differences related to gene therapy (Bainbridge et al., 2008). Multifocal ERG is another technique where the electrical activity originating from many retinal locations can be recorded simultaneously. In one of the studies, multifocal ERGs were recorded before and after gene therapy in one 11 year old patient. The results were reported to show an increase at the center of the macula after treatment (Maguire et al., 2009) but the significance of this result is difficult to evaluate without data on variability of this response in the RPE65-LCA population.
7.3.4 Investigation of eye movements, fixation and nystagmus
Retinal location of fixation and instability of eye movements were recorded in three patients taking part in one of the clinical trials (Cideciyan et al., 2008) using retinal imaging methodology where the anatomical fovea could be directly localized (Jacobson et al., 2007a). All three patients fixated to a stimulus at or near their anatomical fovea at baseline and through 90 days of follow-up both in control and treated eyes (Fig.15). All patients showed abnormal instability of fixational eye movements at baseline and there were no consistent increases or decreases in the extent of this instability for the treated or control eyes through 90 days of follow-up (Fig.15). In contrast, another study used corneal imaging and found decreased nystagmus frequency and amplitude in treated and control eyes of many patients undergoing gene therapy (Maguire et al., 2008, 2009; Simonelli et al., 2010). Contributing to the apparent discrepancy between the results could be methodological differences, patient characteristics and the conditions of the recording environment such as existence of visible targets of the same luminosity at baseline and follow-up.
Figure 15.
Fixation location and fixation stability of control and study eyes in RPE65-LCA patients do not change after gene therapy treatment. (A) Pairs of traces show the x- and y-axis deflections of the location of the fovea recorded with infrared retinal imaging over 10-s epochs in the study eyes of three patients at baseline and days 30, 60, and 90 after treatment. Control eyes showed similar results (not shown). Horizontal dashed lines are the reference location of the fovea at the start of the recording session 15–35 s preceding the selected epoch. All patients show abnormal eye movements with horizontal and vertical components of instability and larger amplitude jerk nystagmus occurring at frequencies of ~1–2 Hz. All plots are shown as equivalent left eyes for clarity. (B) Traces from a representative normal subject. (C) Summary of fixation instability in all subjects. Horizontal dashed line is a conservative (+3 SD) upper limit of normal (N) from the mean value. Study and control eyes of patients 1, 2, and 3 (P1, P2, P3) at two baseline (B) visits show fixation instability similar to other patients with RPE65-LCA (dark hexagons). At posttreatment days 30, 60, and 90, there are no consistent increases or decreases in fixation instability for the study and control eyes. Reprinted from Cideciyan et al. 2008, copyright © by the National Academy of Sciences.
7.3.5 Investigation of dark adaptation kinetics
Subjects taking part in one of the trials reported noticeably increased brightness in their treated eye when they awoke from sleep in their darkened room (Cideciyan et al., 2008). These observations were reminiscent of those from other patients with conditions that cause slowing of the rate of their visual adaptation to darkness (Cideciyan et al., 1997, 1998a, 2000; Jacobson et al., 1995, 2001) and thus dark adaptation kinetics were investigated in two of the gene therapy patients (Cideciyan et al., 2008). Chromatic sensitivities measured repetitively over 8 hours after a single desensitizing flash showed that cone photoreceptor function reconstituted with gene therapy progressed with fast dark adaptation kinetics (Fig.16). Recovery of rod function, on the other hand, progressed very slowly lasting at least 8 hours to reach the level of sensitivity attained before the desensitizing flash; the dominant time constants of recovery appeared to be at least 13 to 25 times longer than normal (Fig.16). The abnormality could be even greater accounting for the reduced photon capture likely to exist in RPE65-LCA photoreceptors. The dark adaptation kinetic abnormality has remained unabated through 1 year after treatment (Cideciyan et al., 2009a).
Figure 16.
Dark adaptation kinetics across the retinal region of study eyes in RPE65-LCA patients (P2 and P3) after gene therapy. Rod- and cone-photoreceptor-mediated visual function is measured with chromatic stimuli after a 7 log scot-td.s yellow adapting flash (presented at time 0) in patient 2 (at 3.6 and 7.2 mm inferior loci) and patient 3 (at 17 mm temporal locus). Also shown are detailed results from one normal (N) subject at 3.6 mm inferior (Left) and mean results from normal subjects at each location (gray lines). Cone adaptation kinetics (red symbols) are fast and do not show a difference from healthy cones. Rod adaptation kinetics (blue symbols) are extremely slow compared with healthy rods, and in patient 2 there is evidence for a large intraretinal difference in recovery rate. Additionally, absolute thresholds (arrow along the ordinate) of both rod and cone systems are abnormally elevated. Modified from Cideciyan et al. 2008, copyright © by the National Academy of Sciences.
A slow rate of delivery of 11-cis-retinal chromophore from the RPE to the rod photoreceptors either due to a reduced rate of its synthesis, or due to increased obstruction to its inter- or intra-cellular transport could help explain the findings. Reduced expression of RPE65 can result in slowed rate of rhodopsin regeneration (Lyubarsky et al., 2005; Samardzija et al., 2008; Wenzel et al., 2001) and a limited expression of RPE65 through gene therapy could have caused such an enzymatic bottleneck. If so, gene therapy driven RPE65 expression would have to be substantially lower than 50% of the normal amount since heterozygote carriers for null mutations in two key retinoid cycle enzymes RPE65 and RDH5 show normal rates of dark adaptation (Cideciyan et al., 2000; Poehner et al., 2000). Alternatively, RPE65 disease could have caused an increase in the natural “resistive barrier” to 11-cis-retinal movement between RPE cells and rod photoreceptors. The accumulation of all-trans-retinyl esters and lipid droplets, disorganized rod outer segments, or altered RPE/photoreceptor interface due to the surgical detachment may have contributed to such a barrier (Acland et al., 2001; Geller et al., 2001; Pang et al., 2006; Porto et al., 2002; Redmond et al., 1998).
7.3.6 Improvement in pupillary light reflex
The pupillary light reflex (PLR) may be used as a noninvasive and quantifiable indicator of rod- or cone-based photoreceptor function but only under carefully designed experimental paradigms in which it would be valid to assume a lack of input from photosensitive ganglion cells (Wilhelm, 2008), a lack of conduction abnormalities along the afferent and efferent pathways of the reflex, and a normal iris muscle (Loewenfeld, 1993). In one of the gene therapy trials, a purpose-built instrument (Aleman et al., 2004) was used to record transient PLRs (TPLRs) in order to augment and corroborate psychophysical results (Cideciyan et al., 2008). To avoid contributions from sluggish photosensitive ganglion cells (Hatter et al., 2003; Lucas et al., 2003), short duration stimuli were used and the early portion of the PLR was quantified (Aguirre et al., 2007; Aleman et al., 2004; Cideciyan et al., 2008). The stimulus was full-field to take advantage of the area summation capabilities of the PLR (Schweitzer & Bouman, 1958); luminance-response functions were recorded in fully dark-adapted eyes uniocularly over a ~9 log unit range. Two of the three patients showed a shift of their luminance response functions to lower stimulus intensities in their injected eye, signifying a better sensitivity; there was no change in the control eyes or the injected eye of a third patient (Fig.17). In the two treated eyes that did change with treatment, the threshold stimulus intensity that would produce a criterion TPLR response was significantly improved by 1 month after the injection compared to baseline (Cideciyan et al., 2008).
Figure 17.
Pupillary responsivity of RPE65-LCA patients increases after gene therapy. (A) Change in pupil diameter evoked by a short duration light stimulus (0.1 s, −0.6 log scot-cd.m−2, green, dark-adapted) in study and control eyes of the three RPE65-LCA patients at 18 months before treatment (thin black traces) and 1 month after treatment (red, study eye; blue, control eye) compared with results from normal subjects (gray band, mean+/−2 SEM; n=13). Pretreatment eyes do not show changes in pupil diameter that are distinguishable in magnitude (<0.3 mm) from spontaneous oscillations of the pupil diameter of the dark-adapted eye. Posttreatment (red) traces show sizable pupil contractions in the study eyes of two of the patients (patients 2 and 3) but not in patient 1. Pupils from control eyes (blue traces) after treatment do not contract in response to this stimulus. Stimulus monitor trace at the bottom left. (Inset) Video frames of pupils of control and study eyes of patient 2 at 0.9 s after the light stimulus; pupillary margin is outlined in white (pretreatment), red (post-treatment, study eye), or blue (posttreatment, control eye) for visibility; calibration bar at the bottom right. (B) Luminance response functions measured at 0.9 s after stimulus onset in both eyes of each patient before treatment (thin black lines) and after treatment in the study eye (red) compared with the control eye (blue) and with normal data (mean+/−SEM, white symbols). Before treatment (thin black lines), pupil contractions were observed only with the two highest luminance stimuli. After treatment (red symbols), there were contractions in the study eye of patients 2 and 3 within a range of stimuli and there was a shift of the luminance response function to the left; no such responding was recorded in patient 1 or in the control eyes. Arrow points to stimulus luminance of the waveforms shown in A. (C) Pupillary response thresholds in patients with untreated RPE65-LCA (gray hexagons, other RPE65-LCA patients; gray squares, patient 1; gray circles, patient 2; gray diamonds, patient 3) show abnormalities in excess of 5 log units compared with normal (white hexagon, mean+/−2 SD). Thresholds in the study subjects before treatment are similar to each other and to other patients with RPE65-LCA (gray hexagons, n=3; gray line, mean+/−2 SD for untreated RPE65-LCA). The treated eye of patient 2 improved by 1.6 log units, from 0.74 log scot-cd.m−2 before treatment to −0.86 log scot-cd.m−2 after treatment (red). Patient 3 improved by 1.8 log units from 0.83 log scot-cd.m−2 before treatment to −0.92 log scot-cd.m−2 after treatment. The untreated eyes did not change (blue symbols). Patient 1 showed no detectable changes, which may be attributed to the smaller increase in posttreatment visual sensitivity compared with patients 2 and 3. Reprinted from Cideciyan et al. 2008, copyright © by the National Academy of Sciences.
Another group used a binocular pupillography method designed to detect relative afferent pupillary defects (Volpe et al., 2009) in order to evaluate their gene therapy patients (Maguire et al., 2008, 2009; Simonelli et al., 2010). Using three stimulus intensities covering a limited dynamic range of 2.4 log units, sensitivity was defined as the intensity at which the PLR was eliminated from the test eye before injection and at which the relative afferent pupillary defect developed after injection (Maguire et al., 2009). Before treatment, most patient eyes had sensitivity losses >4 log units, and after the treatment all patients show significant improvements in PLR sensitivity (Maguire et al., 2008, 2009; Simonelli et al., 2010).
PLR based methods can be an important adjunct to psychophysical methods for the evaluation of treatment efficacy. In the youngest patients, or others who cannot perform psychophysical testing, PLR based methods may be the only alternative to the traditionally-used ERG methods which are predictably insensitive to the improvements achieved by gene therapy.
7.3.7 Slow emergence of further visual improvement
Within 5 to 30 days after RPE65 gene therapy there was improved vision whether self-reported by the patients or measured with perimetric methods evaluating sensitivity to small transient stimuli presented across the visual field (Cideciyan et al., 2008). Biological plausibility of such a fast time course of visual improvement in human subjects was confirmed with studies in Rpe65rd12 mice that showed robust ERG improvements within 10 days of injection (Cideciyan et al., 2008). Up to 1 year follow-up showed no further changes in visual sensitivity values obtained at the 30 day time point (Cideciyan et al., 2009a) consistent with the ERG stability seen in treated dogs between 3 months and 3 years post-injection (Acland et al., 2005). Unexpectedly at the 1 year time point but not before, one of the study patients reported that she could read (with her treated eye only) the illuminated numerical clock display on the dashboard of the family vehicle while sitting in the front seat (Cideciyan et al., 2009b). The simplest explanation would have been an improvement in visual acuity but the numerals of the clock subtended a visual angle equivalent to a visual acuity of 20/200 which was not different than the patient’s acuity at baseline or at 1 year after gene therapy measured by the standard ETDRS method. Another simple explanation would have been an increased visual sensitivity either at the untreated fovea or in the treated region of the superior temporal retina. Although the patient had a substantial gain in visual sensitivity within 30 days after gene therapy, these parameters were unchanged between 30 days and one year. And a third possibility involved an expansion of the central boundary of the treated region towards the fovea, but microperimetry demonstrated no changes to boundaries of native and treatment-associated visual islands between 30 days and one year (Fig.18). Apparent stability of all recordable visual function measures between 30 days and one year appeared incompatible with the self-report of newly found visual ability at one year (Cideciyan et al., 2009b).
Figure 18.
Slow emergence of a pseudo-fovea in an RPE65-LCA patient within the treated retinal region and perception of previously unseen stimuli. Panel (a) shows the eye (upper image) and the patient’s retina (lower image). Overlaid contours of constant sensitivity (measured by means of microperimetry) show no change in visual sensitivity between 1 and 12 months after treatment. F denotes the fovea, and ST the superotemporal retina that received treatment. The circular pattern is a standard grid centered on the fovea. Retinal distance calibration corresponding to 5 degrees of visual angle is shown. Panel (b) shows fixation clouds (scatter plots) in the study eye of the patient at baseline and the statistics of fixation dwell time (bar graphs) along the diagonal meridian as a function of the target luminance. All three luminances were perceived by the patient at all visits. At the 2.7- and 2.4-log10 luminances, fixation was within 3 degrees of the fovea more than 99% of the time at all visits except at the 12-month visit for 2.4-log10 luminance, when 68% of fixation time dwelled in an ST retinal region 4 to 9 degrees from the fovea. At 2.1-log10 luminance, fixations showed increasingly greater excursions into the ST retina between 2 and 9 months after treatment. At 12 months, 89% of fixation time dwelled in the ST region 4 to 9 degrees from the fovea. Thin vertical lines represent the foveal location. Red bars indicate significant (>3 degrees) excursions from the fovea. Panel (c) shows that a dimmer target (1.8 log10) was not perceived by the patient’s study eye during baseline though 9 months after treatment. At 12 months, this stimulus was perceived for the first time with a coincident shift of fixation into the ST retinal region. Reprinted from Cideciyan et al., 2009b. Copyright © 2009 Massachusetts Medical Society. All rights reserved.
To understand the basis of this patient’s observation, fixation of her gaze to dim targets was quantified over a range of luminances straddling perception (Cideciyan et al., 2009b). Recordings were monocular and performed during a fixation task involving visualization of a deep red target the luminance of which was progressively lower at each sequential recording until it could not be perceived by the subject. The target subtended 1 degree in the visual field and had a sustained luminance varied between 2.7 to 1.8 log units higher than normal perceptual threshold; recordings were made in control and study eyes at baseline and at 1, 2, 3, 6, 9, 12 months post-injection. Initial clues to understanding the patient’s observations came from consideration of the intermediate 2.4 log target. At baseline for this target, the patient had foveal fixation in both eyes whereas at 1 year post-treatment, the treated eye showed distinct excursions of fixation from the fovea into the supero-temporal treated retina (Fig.18). Quantitation of fixation dwell time recorded with a range of target luminances provided further evidence supporting greater fixational use of the treated retina may have been slowly emerging over many months and this was particularly evident at lower luminances (Cideciyan et al., 2009b). A further surprise was the lowest luminance target (1.8 log) which was never perceived by the patient at baseline or through 9 months post-treatment in either control or study eyes. By 12 months, the patient reported perception of this target with her treated eye for the first time. Perception was accompanied by a distinct shift in fixation from fovea centralis to the treated supero-temporal retina.
These data support the slow (over ~1 year) emergence of two interrelated visual gains in the treated retina under certain favorable lighting conditions: a change in fixational use and an improvement in perception of stationary targets. A primary driver for these changes may have been the fast (<30 days) emergence of treatment-created extrafoveal cone vision with better sensitivity (to transient targets) than the untreated foveal region (Cideciyan et al., 2009b). In macular degeneration, retinal damage to the fovea centralis commonly provokes a slow fixational change involving recalibration of the oculomotor system to position the object of interest into a better-functioning extrafoveal retina (Cheung and Legge, 2005; Crossland et al., 2005; Deruaz et al., 2002, 2006; Lei and Schuchard, 1997). In the treated eye of the RPE65 patient, the time course of change in fixation behavior was similarly slow but it was not associated with a change in foveal function or structure. Instead there were faster and slower phases of extrafoveal functional improvement not unlike two phases of auditory function restoration after cochlear implantation in neonatally-deaf animals and congenitally-deaf children (Kral et al., 2006).
The new visual experiences originating from the treated retinal area in the gene therapy patient likely drive cortical adaptations (Butz et al., 2009). This form of plasticity based on added input of visual information from an extrafoveal retinal location to a congenitally-deprived cortex may partially overlap with recovery of retina-wide input following long-term blindness (Fine et al., 2003; Maurer et al., 2006; Sjostrand et al., 2010), experience-dependent changes seen during visual perceptual learning (Gilbert et al., 2009; Holtmaat & Svoboda, 2009; Sasaki et al., 2010), and deafferentation-dependent changes seen with retinal lesions (Baker et al., 2005; Little et al., 2008; Sunness et al., 2004). Many details of cortical reorganization remain to be fully elucidated (Cheung and Legge, 2005; Giannikopoulos and Eysel, 2006; Hubel & Wiesel, 1959; Kleim et al., 2006; Smirnakis et al., 2005; Yamahachi et al., 2009) but the faster and slower phases of functional recovery support differential involvement of parallel cortical visual pathways (Ellemberg et al., 1999a, 1999b, 2000; Hammarrenger et al., 2007; McCloskey, 2004; Mechler et al., 1998; Milner & Goodale, 2008) in RPE65-LCA and its treatment with gene therapy. Independent of the underlying causes, the plasticity of the adult visual system observed widens the prospect for gene therapy to improve visual function over the longer term and this process may well be accelerated with visual training (Deruaz et al., 2002; Levi & Li, 2009).
8. Conclusions and future directions
RPE65-LCA is probably one of the most intensively studied and best understood among all rare molecular forms of human hereditary retinopathies, but much uncertainty about the human disease, as well as its animal counterparts remains. For example, a predictive relation between a pair of mutant RPE65 alleles and resulting disease severity is currently unknown. Part of the difficulty originates from the quantification of disease severity which ideally must include intraretinal variation of retinal dysfunction, degeneration and RPE health, and their respective natural histories – a doable but difficult proposition. In terms of retinal dysfunction, the key question is not why there is loss of vision but why there is any remnant vision at all. This is especially true in patients (and animal models) with no predicted RPE65 expression. The chromophore mediating the remnant vision and the biochemical pathway of its generation is not known; neither is it known whether there are different pathways for rods and cones. Human results suggest that there may even be differences between foveal and extra-foveal cones and this may explain the lack of foveal functional improvement demonstrable with gene therapy to date. The exact causes of photoreceptor degeneration and substantial interspecies differences for cone demise are not known. Very little information exists on the health of the RPE and how it is related to the degeneration of photoreceptors that it supports.
There are some common misconceptions about RPE65-LCA. It is thought for example that children with RPE65-LCA have a better preservation of photoreceptors compared to adult patients. This proposition is not supported in any given cross-sectional sample of RPE65-LCA patients in the first three decades of life since substantial inter-patient variability of the human disease allows no reliable predictions of disease severity with age. Better understanding of the contribution of specific genotypes to disease severity may allow such predictions in the future but until then there are no short-cuts to measuring the remaining photoreceptors that are potentially treatable in each candidate patient.
Existence of a multitude of independent clinical trials of gene therapy in a rare but molecularly-homogenous group of patients provides unprecedented opportunity for scientific scrutiny and development of new knowledge. Many differences in the details of AAV preparation, surgical/medical intervention, testing methodology, and details of reporting complicate interpretation of the results. However, the bigger picture appears clear: what was only imaginable a few short years ago has been proven possible. It is indeed possible to introduce a new gene vectored by AAV into the RPE cells in order to improve human vision in a matter of days in patients with severe and life-long visual impairment. There are many caveats, much unknown, and room for improvement remains expansive. One of the major caveats is the rarity of the RPE65-LCA disease, one of the most important unknowns is whether the partial reversal of the biochemical defect will change the course of progressive retinal degeneration, and one of the key improvements would involve methods to introduce the genetic material without causing a retinal detachment. Appropriate to the transformative nature of the initial gene therapy investigations, there are many more questions about RPE65-LCA and its treatment than answers, but the future looks bright.
Acknowledgments
The author would like to thank Samuel G. Jacobson, MD, PhD, for many insightful discussions and critical reading of the manuscript, and the National Eye Institute, Foundation Fighting Blindness, National Neurovision Research Institute, Macula Vision Research Foundation, and Hope for Vision for research funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GD, Baldwin V, Pearce-Kelling S, Narfström K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- Aguirre GK, Komáromy AM, Cideciyan AV, Brainard DH, Aleman TS, Roman AJ, Avants BB, Gee JC, Korczykowski M, Hauswirth WW, et al. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med. 2007;4:e230. doi: 10.1371/journal.pmed.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman TS, LaVail MM, Montemayor R, Ying G, Maguire MM, Laties AM, Jacobson SG, Cideciyan AV. Augmented rod bipolar cell function in partial receptor loss: an ERG study in P23H rhodopsin transgenic and aging normal rats. Vision Res. 2001;41:2779–2797. doi: 10.1016/s0042-6989(01)00157-2. [DOI] [PubMed] [Google Scholar]
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EAM, Furushima M, Redmond TM, Bennett J, Palczewski K, et al. Impairment of the transient pupillary light reflex in Rpe65−/− mice and humans with Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Hauswirth WW. Adeno-associated viral vectors and the retina. Adv. Exp. Med. Biol. 2008;613:121–128. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- Al-Khayer K, Hagstrom S, Pauer G, Zegarra H, Sears J, Traboulsi EI. Thirty-year follow-up of a patient with leber congenital amaurosis and novel RPE65 mutations. Am. J. Ophthalmol. 2004;137:375–377. doi: 10.1016/S0002-9394(03)00913-9. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J. Neurosci. 2005;25:614–618. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL, Seeliger MW, Wenzel A, Arsenijevic Y. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie-Weintrob Y, Berson EL, Dryja TP. Histopathologic-genotypic correlations in retinitis pigmentosa and allied diseases. Ophthalmic Genet. 2005;26:91–100. doi: 10.1080/13816810590968032. [DOI] [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereta G, Kiser PD, Golczak M, Sun W, Heon E, Saperstein DA, Palczewski K. Impact of retinal disease-associated RPE65 mutations on retinoid isomerization. Biochemistry. 2008;47:9856–9865. doi: 10.1021/bi800905v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J. Cell Sci. Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Butz M, Wörgötter F, van Ooyen A. Activity-dependent structural plasticity. Brain Res. Rev. 2009;60:287–305. doi: 10.1016/j.brainresrev.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley SM, Naash MI. RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009;30:57–62. doi: 10.1080/13816810802626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum. Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol. Vis. Sci. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- Chévez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C, Hurwitz MY, Hurwitz RL. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J. Clin. Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- Cheung SH, Legge GE. Functional and cortical adaptations to central vision loss. Vis. Neurosci. 2005;22:187–201. doi: 10.1017/S0952523805222071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DC, Lee V, Maguire AM. Recent advances in ocular gene therapy. Curr. Opin. Ophthalmol. 2009;20:377–381. doi: 10.1097/ICU.0b013e32832f802a. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG. An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vis. Res. 1996;36:2609–2621. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Pugh EN, Jr, Lamb TD, Huang Y, Jacobson SG. Rod plateaux during dark adaptation in Sorsby’s fundus dystrophy and vitamin A deficiency. Invest. Ophthalmol. Vis. Sci. 1997;38:1786–1794. [PubMed] [Google Scholar]
- Cideciyan AV, Zhao X, Nielsen L, Khani SC, Jacobson SG, Palczewski K. Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc Natl Acad Sci U S A. 1998a;95:328–33. doi: 10.1073/pnas.95.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hood DC, Huang Y, Banin E, Li ZY, Stone EM, Milam AH, Jacobson SG. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc. Natl. Acad. Sci. U S A. 1998b;95:1703–1708. doi: 10.1073/pnas.95.12.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis Neurosci. 2000;17:667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Swider M, Schwartz SB, Steinberg JD, Brucker AJ, Maguire AM, Bennett J, Stone EM, Jacobson SG. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum. Mol. Genet. 2004;13:525–534. doi: 10.1093/hmg/ddh048. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U S A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum. Gene. Ther. 2009a;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, et al. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N. Engl. J. Med. 2009b;361:725–727. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Cotugno G, Auricchio A. Ocular gene therapy: current progress and future prospects. Trends Mol. Med. 2009;15:23–31. doi: 10.1016/j.molmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Conley SM, Cai X, Naash MI. Nonviral ocular gene therapy: assessment and future directions. Curr. Opin. Mol. Ther. 2008;10:456–463. [PMC free article] [PubMed] [Google Scholar]
- Crossland MD, Culham LE, Kabanarou SA, Rubin GS. Preferred retinal locus development in patients with macular disease. Ophthalmology. 2005;112:1579–1585. doi: 10.1016/j.ophtha.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, Redmond TM, Tang W, Wei Z, Rex TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol. Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest. Opthalmol. Vis. Sci. 1995;36:718–729. [PubMed] [Google Scholar]
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Deruaz A, Whatham AR, Mermoud C, Safran AB. Reading with multiple preferred retinal loci: implications for training a more efficient reading strategy. Vision Res. 2002;42:2947–2957. doi: 10.1016/s0042-6989(02)00354-1. [DOI] [PubMed] [Google Scholar]
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog. Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- El Matri L, Ambresin A, Schorderet DF, Kawasaki A, Seeliger MW, Wenzel A, Arsenijevic Y, Borruat FX, Munier FL. Phenotype of three consanguineous Tunisian families with early-onset retinal degeneration caused by an R91W homozygous mutation in the RPE65 gene. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:1104–1112. doi: 10.1007/s00417-005-0096-2. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Liu CH, Maurer D. Development of spatial and temporal vision during childhood. Vision Res. 1999a;39:2325–33. doi: 10.1016/s0042-6989(98)00280-6. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Lui CH, Brent HP. Spatial and temporal vision in patients treated for bilateral congenital cataracts. Vision Res. 1999b;39:3480–9. doi: 10.1016/s0042-6989(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Brent HP. Influence of monocular deprivation during infancy on the later development of spatial and temporal vision. Vision Res. 2000;40:3283–3295. doi: 10.1016/s0042-6989(00)00165-6. [DOI] [PubMed] [Google Scholar]
- Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain GL. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J. Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Wu BX, Sarna T, Rohrer B, Redmond TM, Crouch RK. 9-cis Retinal increased in retina of RPE65 knockout mice with decrease in coat pigmentation. Photochem. Photobiol. 2006;82:1461–1467. doi: 10.1562/2006-02-02-RA-793. [DOI] [PubMed] [Google Scholar]
- Feathers KL, Lyubarsky AL, Khan NW, Teofilo K, Swaroop A, Williams DS, Pugh EN, Jr., Thompson DA. Nrl-knockout mice deficient in Rpe65 fail to synthesize 11-cis retinal and cone outer segments. Invest. Ophthalmol. Vis. Sci. 2008;49:1126–1135. doi: 10.1167/iovs.07-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felius J, Thompson DA, Khan NW, Bingham EL, Jamison JA, Kemp JA, Sieving PA. Clinical course and visual function in a family with mutations in the RPE65 gene. Arch. Ophthalmol. 2002;120:55–61. doi: 10.1001/archopht.120.1.55. [DOI] [PubMed] [Google Scholar]
- Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am. J. Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- Fine I, Wade AR, Brewer AA, May MG, Goodman DF, Boynton GM, Wandell BA, MacLeod DI. Long-term deprivation affects visual perception and cortex. Nat. Neurosci. 2003;6:915–916. doi: 10.1038/nn1102. [DOI] [PubMed] [Google Scholar]
- Fishman GA, Jacobson SG, Alexander KR, Cideciyan AV, Birch DG, Weleber RG, Hood DC. Outcome measures and their application in clinical trials for retinal degenerative diseases: outline, review, and perspective. Retina. 2005;25:772–777. doi: 10.1097/00006982-200509000-00014. [DOI] [PubMed] [Google Scholar]
- Ford M, Bragadóttir R, Rakoczy PE, Narfström K. Gene transfer in the RPE65 null mutation dog: relationship between construct volume, visual behavior and electroretinographic (ERG) results. Doc. Ophthalmol. 2003;107:79–86. doi: 10.1023/a:1024431827812. [DOI] [PubMed] [Google Scholar]
- Gal A, Gu S, Bremser D, Lorenz B, Apfelstedt-Sylla E, Zrenner E, Gerding H, Denton MJ, Thompson DA. Mutation spectrum of RPE65 in autosomal recessive childhood-onset severe retinal dystrophy. Invest. Ophthalmol. Vis. Sci. 1998;39:S901. [Google Scholar]
- Gearhart PM, Gearhart CC, Petersen-Jones SM. A novel method for objective vision testing in canine models of inherited retinal disease. Invest. Ophthalmol. Vis. Sci. 2008;49:3568–3576. doi: 10.1167/iovs.07-0625. [DOI] [PubMed] [Google Scholar]
- Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: reactive Müller and RPE cells. Invest. Ophthalmol. Vis. Sci. 2001;42:1363–1369. [PubMed] [Google Scholar]
- Giannikopoulos DV, Eysel UT. Dynamics and specificity of cortical map reorganization after retinal lesions. Proc. Natl. Acad. Sci. U S A. 2006;103:10805–10810. doi: 10.1073/pnas.0604539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Pirogovsky E, Brushfield AM, Luu TT, Tolentino JC, Renteria AF. Age-related changes in associative learning for olfactory and visual stimuli in rodents. Ann. N. Y. Acad. Sci. 2009;1170:718–724. doi: 10.1111/j.1749-6632.2009.03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Kong J, Tsang SH. Retinal degeneration and RPE transplantation in Rpe65(−/−) mice. Invest. Ophthalmol. Vis. Sci. 2002;43:3307–3311. [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Remé CE. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat. Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- Hall EC, Gordon J, Hainline L, Abramov I, Engber K. Childhood visual experience affects adult voluntary ocular motor control. Optom. Vis. Sci. 2000;77:511–523. doi: 10.1097/00006324-200010000-00005. [DOI] [PubMed] [Google Scholar]
- Hall EC, Ciuffreda KJ. Fixational ocular motor control is plastic despite visual deprivation. Vis. Neurosci. 2002;19:475–481. doi: 10.1017/s0952523802194090. [DOI] [PubMed] [Google Scholar]
- Hamann S, Schorderet DF, Cottet S. Bax-induced apoptosis in Leber’s congenital amaurosis: a dual role in rod and cone degeneration. PLoS One. 2009;4:e6616. doi: 10.1371/journal.pone.0006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel CP, Griffoin JM, Lasquellec L, Bazalgette C, Arnaud B. Retinal dystrophies caused by mutations in RPE65: assessment of visual functions. Br. J. Ophthalmol. 2001;85:424–427. doi: 10.1136/bjo.85.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarrenger B, Roy MS, Ellemberg D, Labrosse M, Orquin J, Lippe S, Lepore F. Developmental delay and magnocellular visual pathway function in very-low-birthweight preterm infants. Dev. Med. Child. Neurol. 2007;49:28–33. doi: 10.1017/s0012162207000084.x. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene. Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heher KL, Traboulsi EI, Maumenee IH. The natural history of Leber’s congenital amaurosis. Age-related findings in 35 patients. Ophthalmology. 1992;99:241–245. doi: 10.1016/s0161-6420(92)31985-2. [DOI] [PubMed] [Google Scholar]
- Hemati N, Feathers KL, Chrispell JD, Reed DM, Carlson TJ, Thompson DA. RPE65 surface epitopes, protein interactions, and expression in rod- and cone-dominant species. Mol. Vis. 2005;11:1151–1165. [PubMed] [Google Scholar]
- Henderson R, Lorenz B, Moore AT. Clinical and molecular genetic aspects of Leber’s congenital amaurosis. In: Lorenz B, Moore AT, editors. Pediatric Ophthalmology, Neuro-Ophthalmology, Genetics. Springer; Berlin: 2006. pp. 157–177. [Google Scholar]
- Hernández M, Pearce-Kelling SE, Rodriguez FD, Aguirre GD, Vecino E. Selective alteration of the expression of retinal molecular markers in the canine RPE65 model of Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2010 doi: 10.1167/iovs.10-5213. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Horsten GPM, Winkleman JE. Development of the ERG in relation to histological differentiation of the retina in man and animals. Arch. Ophthalmol. 1960;63:232–242. doi: 10.1001/archopht.1960.00950020234005. [DOI] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Cideciyan AV, Papastergiou GI, Banin E, Semple-Rowland SL, Milam AH, Jacobson SG. Relation of optical coherence tomography to microanatomy in normal and rd chickens. Invest. Ophthalmol. Vis. Sci. 1998;39:2405–2416. [PubMed] [Google Scholar]
- Huang Y, Cideciyan AV, Alemán TS, Banin E, Huang J, Syed NA, Petters RM, Wong F, Milam AH, Jacobson SG. Optical coherence tomography (OCT) abnormalities in rhodopsin mutant transgenic swine with retinal degeneration. Exp. Eye Res. 2000;70:247–251. doi: 10.1006/exer.1999.0793. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J. Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Regunath G, Rodriguez FJ, Vandenburgh K, Sheffield VC, Stone EM. Night blindness in Sorsby’s fundus dystrophy reversed by vitamin A. Nat. Genet. 1995;11:27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Huang Y, Hanna DB, Freund CL, Affatigato LM, Carr RE, Zack DJ, Stone EM, McInnes RR. Retinal degenerations with truncation mutations in the cone-rod homeobox (CRX) gene. Invest. Ophthalmol. Vis. Sci. 1998;39:2417–2426. [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Wright E, Wright AF. Phenotypic marker for early disease detection in dominant late-onset retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2001;42:1882–1890. [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA, Traboulsi EI, Heon E, Pittler SJ, Milam AH, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc. Natl. Acad. Sci. U S A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, Zeiss CJ, Komaromy AM, Kaushal S, Roman AJ, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol. Ther. 2006a;13:1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Boye SL, Aleman TS, Conlon TJ, Zeiss CJ, Roman AJ, Cideciyan AV, Schwartz SB, Komaromy AM, Doobrajh M, et al. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006b;17:845–58. doi: 10.1089/hum.2006.17.845. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl. Acad. Sci. U S A. 2007a;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Schwartz SB, Windsor EA, Roman AJ, Heon E, Stone EM, Thompson DA. RDH12 and RPE65, visual cycle genes causing leber congenital amaurosis, differ in disease expression. Invest. Ophthalmol. Vis. Sci. 2007b;48:332–338. doi: 10.1167/iovs.06-0599. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Windsor EA, Schwartz SB, Heon E, Stone EM. Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2008a;49:4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Gardner LM, Prosser HM, Mishra M, Bech-Hansen NT, Herrera W, et al. Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum. Mol. Genet. 2008b;17:2405–2415. doi: 10.1093/hmg/ddn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Roman AJ, Sumaroka A, Windsor EA, Schwartz SB, Heon E, Stone EM. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2009;50:2368–2375. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J. Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, White HA, Gao CL, Roth GS, Knapka JJ, Ingram DK. Dietary restriction slows age pigment accumulation in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1993;34:3297–3302. [PubMed] [Google Scholar]
- Katz ML, Gao CL, Rice LM. Long-term variations in cyclic light intensity and dietary vitamin A intake modulate lipofuscin content of the retinal pigment epithelium. J. Neurosci. Res. 1999;57:106–116. doi: 10.1002/(SICI)1097-4547(19990701)57:1<106::AID-JNR11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2001;42:3023–3030. [PubMed] [Google Scholar]
- Kijas JW, Cideciyan AV, Aleman TS, Pianta MJ, Pearce-Kelling SE, Miller BJ, Jacobson SG, Aguirre GD, Acland GM. Naturally occurring rhodopsin mutation in the dog causes retinal dysfunction and degeneration mimicking human dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2002;99:6328–6333. doi: 10.1073/pnas.082714499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc. Natl. Acad. Sci. U S A. 2009;106:17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Koenekoop RK. Leber Congenital Amaurosis. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology, Diagnostics, and Therapeutics. Humana Press; New Jersey: 2007. pp. 61–90. [Google Scholar]
- Kral A, Tillein J, Heid S, Klinke R, Hartmann R. Cochlear implants: cortical plasticity in congenital deprivation. Prog. Brain Res. 2006;157:283–313. doi: 10.1016/s0079-6123(06)57018-9. [DOI] [PubMed] [Google Scholar]
- Kroll AJ, Kuwabara T. Electron microscopy of a retinal abiotrophy. Arch. Ophthalmol. 1964;71:683–690. doi: 10.1001/archopht.1964.00970010699016. [DOI] [PubMed] [Google Scholar]
- Kunchithapautham K, Coughlin B, Crouch RK, Rohrer B. Cone outer segment morphology and cone function in the Rpe65−/− Nrl−/− mouse retina are amenable to retinoid replacement. Invest. Ophthalmol. Vis. Sci. 2009;50:4858–4864. doi: 10.1167/iovs.08-3008. [DOI] [PubMed] [Google Scholar]
- Lai CM, Yu MJ, Brankov M, Barnett NL, Zhou X, Redmond TM, Narfstrom K, Rakoczy PE. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet. Vaccines Ther. 2004;2:3. doi: 10.1186/1479-0556-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr. Dark adaptation and the retinoid cycle of vision. Prog. Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lambert SR, Taylor D, Kriss A. The infant with nystagmus, normal appearing fundi, but an abnormal ERG. Surv. Ophthalmol. 1989;34:173–186. doi: 10.1016/0039-6257(89)90101-x. [DOI] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Péréon Y, Cherel Y, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- Lei H, Schuchard RA. Using two preferred retinal loci for different lighting conditions in patients with central scotomas. Invest. Ophthalmol. Vis. Sci. 1997;38:1812–1818. [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. Eye movements of the blind. Invest. Ophthalmol. Vis. Sci. 1980;19:328–331. [PubMed] [Google Scholar]
- Levi DM, Li RW. Improving the performance of the amblyopic visual system. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin N, Dumoulin SO, Winawer J, Dougherty RF, Wandell BA. Cortical maps and white matter tracts following long period of visual deprivation and retinal image restoration. Neuron. 2010;65:21–31. doi: 10.1016/j.neuron.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kong F, Li X, Dai X, Liu X, Zheng Q, Wu R, Zhou X, Lü F, Chang B, et al. Gene therapy following subretinal AAV5 vector delivery is not affected by a previous intravitreal AAV5 vector administration in the partner eye. Mol. Vis. 2009;15:267–75. [PMC free article] [PubMed] [Google Scholar]
- Little DM, Thulborn KR, Szlyk JP. An FMRI study of saccadic and smooth-pursuit eye movement control in patients with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2008;49:1728–1735. doi: 10.1167/iovs.07-0372. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. The light reflex. In: Loewenfeld IE, editor. The Pupil: Anatomy, Physiology and Clinical Applications. Iowa State University Press, Ames; Iowa: 1993. pp. 193–267. [Google Scholar]
- Lorenz B, Gyürüs P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004;111:1585–1594. doi: 10.1016/j.ophtha.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Lorenz B, Poliakov E, Schambeck M, Friedburg C, Preising MN, Redmond TM. A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation. Invest. Ophthalmol. Vis. Sci. 2008;49:5235–5242. doi: 10.1167/iovs.07-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Namperumalsamy P, Jacobson SG, Weleber RG, Fishman GA, Musarella MA, Hoyt CS, Héon E, Levin A, Jan J, et al. Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch. Ophthalmol. 2000;118:538–543. doi: 10.1001/archopht.118.4.538. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsinknockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN., Jr. UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J. Neurosci. 1999;19:442–55. doi: 10.1523/JNEUROSCI.19-01-00442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Savchenko AB, Morocco SB, Daniele LL, Redmond TM, Pugh EN., Jr. Mole quantity of RPE65 and its productivity in the generation of 11-cis-retinal from retinyl esters in the living mouse eye. Biochemistry. 2005;44:9880–9888. doi: 10.1021/bi0505363. [DOI] [PubMed] [Google Scholar]
- Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, Bush RA, Sieving PA. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest. Ophthalmol. Vis. Sci. 2000;41:3200–3209. [PubMed] [Google Scholar]
- Maeda T, Cideciyan AV, Maeda A, Golczak M, Aleman TS, Jacobson SG, Palczewski K. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum. Mol. Genet. 2009;18:2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamatha G, Srilekha S, Meenakshi S, Kumaramanickavel G. Screening of the RPE65 gene in the Asian Indian patients with leber congenital amaurosis. Ophthalmic Genet. 2008;29:73–78. doi: 10.1080/13816810802008259. [DOI] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat. Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- Marlhens F, Griffoin JM, Bareil C, Arnaud B, Claustres M, Hamel CP. Autosomal recessive retinal dystrophy associated with two novel mutations in the RPE65 gene. Eur. J. Hum. Genet. 1998;6:527–531. doi: 10.1038/sj.ejhg.5200205. [DOI] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. U S A. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Ellemberg D, Lewis TL. Repeated measurements of contrast sensitivity reveal limits to visual plasticity after early binocular deprivation in humans. Neuropsychologia. 2006;44:2104–2112. doi: 10.1016/j.neuropsychologia.2005.10.008. [DOI] [PubMed] [Google Scholar]
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog. Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- McCloskey M. Spatial representations and multiple-visual-systems hypotheses: evidence from a developmental deficit in visual location and orientation processing. Cortex. 2004;40:677–694. doi: 10.1016/s0010-9452(08)70164-3. 2004. [DOI] [PubMed] [Google Scholar]
- Mechler F, Victor JD, Purpura KP, Shapley R. Robust temporal coding of contrast by V1 neurons for transient but not for steady-state stimuli. J. Neurosci. 1998;18:6583–6598. doi: 10.1523/JNEUROSCI.18-16-06583.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW. Preliminary results of gene therapy for retinal degeneration. N. Engl. J. Med. 2008;358:2282–2284. doi: 10.1056/NEJMe0803081. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Takei Y, Sears ML, Peterson WS, Carr RE, Jampol LM. Leber’s congenital amaurosis. Am. J. Ophthalmol. 1977;83:32–42. doi: 10.1016/0002-9394(77)90188-x. [DOI] [PubMed] [Google Scholar]
- Moise AR, Noy N, Palczewski K, Blaner WS. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46:4449–4458. doi: 10.1021/bi7003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. Rpe65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi P, Moore AT. Molecular genetics of infantile-onset retinal dystrophies. Eye (Lond) 2007;21:1344–1351. doi: 10.1038/sj.eye.6702843. [DOI] [PubMed] [Google Scholar]
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc. Natl. Acad. Sci. U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfström K, Wrigstad A, Nilsson SE. The Briard dog: a new animal model of congenital stationary night blindness. Br. J. Ophthalmol. 1989;73:750–756. doi: 10.1136/bjo.73.9.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfström K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest. Ophthalmol. Vis. Sci. 2003;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- Narfström K, Vaegan, Katz M, Bragadottir R, Rakoczy EP, Seeliger M. Assessment of structure and function over a 3-year period after gene transfer in RPE65−/− dogs. Doc. Ophthalmol. 2005;111:39–48. doi: 10.1007/s10633-005-3159-0. [DOI] [PubMed] [Google Scholar]
- Narfström K, Tullis GE, Seeliger M. Effective Treatment for the Canine RPE65 Null Mutation, a Hereditary Retinal Dystrophy Comparable to Human Leber’s Congenital Amaurosis. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology, Diagnostics, and Therapeutics. Humana Press; New Jersey: 2007. pp. 415–431. [Google Scholar]
- Narfström K, Seeliger M, Lai CM, Vaegan, Katz M, Rakoczy EP, Remé C. Morphological aspects related to long-term functional improvement of the retina in the 4 years following rAAV-mediated gene transfer in the RPE65 null mutation dog. Adv. Exp. Med. Biol. 2008;613:139–146. doi: 10.1007/978-0-387-74904-4_15. [DOI] [PubMed] [Google Scholar]
- Nikolaeva O, Takahashi Y, Moiseyev G, Ma JX. Negative charge of the glutamic acid 417 residue is crucial for isomerohydrolase activity of RPE65. Biochem. Biophys. Res. Commun. 2010 doi: 10.1016/j.bbrc.2009.12.149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SE, Wrigstad A, Narfström K. Changes in the DC electroretinogram in Briard dogs with hereditary congenital night blindness and partial day blindness. Exp. Eye Res. 1992;54:291–296. doi: 10.1016/s0014-4835(05)80218-0. [DOI] [PubMed] [Google Scholar]
- Noble KG, Carr RE. Leber’s congenital amaurosis. Arch. Ophthalmol. 1978;96:818–821. doi: 10.1001/archopht.1978.03910050424004. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Friston KJ, Ashburner J, Frackowiak R, Price CJ. Early visual deprivation induces structural plasticity in gray and white matter. Curr. Biol. 2005;15:R488–R490. doi: 10.1016/j.cub.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Nusinowitz S, Ridder WH, 3rd, Pang JJ, Chang B, Noorwez SM, Kaushal S, Hauswirth WW, Heckenlively JR. Cortical visual function in the rd12 mouse model of Leber Congenital Amarousis (LCA) after gene replacement therapy to restore retinal function. Vision Res. 2006;46:3926–3934. doi: 10.1016/j.visres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol. Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- Pang JJ, Chang B, Kumar A, Nusinowitz S, Noorwez SM, Li J, Rani A, Foster TC, Chiodo VA, Doyle T, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol. Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pang J, Boye SE, Lei B, Boye SL, Everhart D, Ryals R, Umino Y, Rohrer B, Alexander J, Li J, et al. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 2010 doi: 10.1038/gt.2010.29. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. U S A. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasadhika S, Fishman GA, Stone EM, Lindeman M, Zelkha R, Lopez I, Koenekoop RK, Shahidi M. Differential macular morphology in patients with RPE65, CEP290, GUCY2D and AIPL1 related Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2010 doi: 10.1167/iovs.09-3734. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu K, Wabbels B, Preising MN, Lorenz B. Longitudinal and cross-sectional study of patients with early-onset severe retinal dystrophy associated with RPE65 mutations. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243:417–426. doi: 10.1007/s00417-004-1020-x. [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet JM, Ghazi I, Leowski C, Bonnemaison M, Gerber S, Ducroq D, Cabot A, Souied E, Dufier JL, et al. Different functional outcome of RetGC1 and RPE65 gene mutations in Leber congenital amaurosis. Am. J. Hum. Genet. 1999;64:1225–1228. doi: 10.1086/302335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp AR, Jin M, Li S, Schindler EI, Iannaccone A, Lam BL, Weleber RG, Fishman GA, Jacobson SG, Mullins RF, et al. Predicting the pathogenicity of RPE65 mutations. Hum Mutat. 2009;30:1183–8. doi: 10.1002/humu.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehner WJ, Fossarello M, Rapoport AL, Aleman TS, Cideciyan AV, Jacobson SG, Wright AF, Danciger M, Farber DB. A homozygous deletion in RPE65 in a small Sardinian family with autosomal recessive retinal dystrophy. Mol. Vis. 2000;6:192–198. [PubMed] [Google Scholar]
- Porto FB, Perrault I, Hicks D, Rozet JM, Hanoteau N, Hanein S, Kaplan J, Sahel JA. Prenatal human ocular degeneration occurs in Leber’s congenital amaurosis (LCA2) J. Gene Med. 2002;4:390–396. doi: 10.1002/jgm.278. [DOI] [PubMed] [Google Scholar]
- Porto FB, Perrault I, Hicks D, Rozet JM, Hanoteau N, Hanein S, Kaplan J, Sahel JA. Prenatal human ocular degeneration occurs in Leber’s Congenital Amaurosis (LCA1 and 2) Adv. Exp. Med. Biol. 2003;533:59–68. [PubMed] [Google Scholar]
- Puliafito CA, Fujimoto JG, Schuman JS, Hee MR. Optical Coherence Tomography of Ocular Diseases. Slack Inc, Thorofare; New Jersey: 1996. [Google Scholar]
- Rangaswamy NV, Patel HM, Locke KG, Hood DC, Birch DG. A comparison of visual field sensitivity to photoreceptor thickness in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2010 doi: 10.1167/iovs.09-4945. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR. The biochemistry of the visual cycle. Chem. Rev. 2001;101:1881–1896. doi: 10.1021/cr960141c. [DOI] [PubMed] [Google Scholar]
- Redmond TM. Focus on Molecules: RPE65, the visual cycle retinol isomerase. Exp. Eye Res. 2009;88:846–7. doi: 10.1016/j.exer.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of Rpe65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, Kuo S, Chander P, Gentleman S. RPE65, visual cycle retinol isomerase, is not inherently 11-cis-specific: support for a carbocation mechanism of retinol isomerization. J. Biol. Chem. 2010;285:1919–1927. doi: 10.1074/jbc.M109.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex TS. Rescue of sight by gene therapy - closer than it may appear. Ophthalmic Genet. 2007;28:127–133. doi: 10.1080/13816810701503707. [DOI] [PubMed] [Google Scholar]
- Riis RC, Aguirre GD. The briard problem. Proc. Am. Coll. Vet. Ophthalmol. 1983:62–75. [Google Scholar]
- Rohrer B, Goletz P, Znoiko S, Ablonczy Z, Ma JX, Redmond TM, Crouch RK. Correlation of regenerable opsin with rod ERG signal in Rpe65−/− mice during development and aging. Invest. Ophthalmol. Vis. Sci. 2003;44:310–315. doi: 10.1167/iovs.02-0567. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest. Ophthalmol. Vis. Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- Roman AJ, Schwartz SB, Aleman TS, Cideciyan AV, Chico JD, Windsor EA, Gardner LM, Ying GS, Smilko EE, Maguire MG, et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp. Eye Res. 2005;80:259–272. doi: 10.1016/j.exer.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Roman AJ, Cideciyan AV, Aleman TS, Jacobson SG. Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol. Meas. 2007a;28:N51–N56. doi: 10.1088/0967-3334/28/8/N02. [DOI] [PubMed] [Google Scholar]
- Roman AJ, Boye SL, Aleman TS, Pang JJ, McDowell JH, Boye SE, Cideciyan AV, Jacobson SG, Hauswirth WW. Electroretinographic analyses of Rpe65-mutant rd12 mice: developing an in vivo bioassay for human gene therapy trials of Leber congenital amaurosis. Mol. Vis. 2007b;13:1701–1710. [PubMed] [Google Scholar]
- Samardzija M, von Lintig J, Tanimoto N, Oberhauser V, Thiersch M, Remé CE, Seeliger M, Grimm C, Wenzel A. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum. Mol. Genet. 2008;17:281–292. doi: 10.1093/hmg/ddm304. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Tanimoto N, Kostic C, Beck S, Oberhauser V, Joly S, Thiersch M, Fahl E, Arsenijevic Y, von Lintig J, et al. In conditions of limited chromophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Hum. Mol. Genet. 2009;18:1266–1275. doi: 10.1093/hmg/ddp026. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer NM, Bouman MA. Differential threshold measurements on the light reflex of the human pupil. A.M.A. Arch. Ophthalmol. 1958;59:541–550. doi: 10.1001/archopht.1958.00940050097012. [DOI] [PubMed] [Google Scholar]
- Seeliger MW, Grimm C, Ståhlberg F, Friedburg C, Jaissle G, Zrenner E, Guo H, Remé CE, Humphries P, Hofmann F, et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat. Genet. 2001;29:70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb. Cortex. 2006;16:1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaer S, Smith EL, Chase AM. Visual acuity and illumination in different spectral regions. J. Gen. Physiol. 1942;25:553–569. doi: 10.1085/jgp.25.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE, Fossarello M, Signorini S, Bertone C, Galantuomo S, et al. Clinical and molecular genetics of Leber’s congenital amaurosis: a multicenter study of Italian patients. Invest. Ophthalmol. Vis. Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010 doi: 10.1038/mt.2009.277. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand J, Magnusson G, Nyström A, Jonsson R. Stability of visual outcome from 7 years in children treated surgically for bilateral dense congenital cataracts before 37 weeks of age. Acta Ophthalmol. 2010 doi: 10.1111/j.1755-3768.2009.01618.x. in press. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Brewer AA, Schmid MC, Tolias AS, Schüz A, Augath M, Inhoffen W, Wandell BA, Logothetis NK. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–307. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Bainbridge JW, Ali RR. Prospects for retinal gene replacement therapy. Trends Genet. 2009;25:156–165. doi: 10.1016/j.tig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Stone EM. Leber congenital amaurosis-a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2007;144:791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Stone EM. Progress toward effective treatments for human photoreceptor degenerations. Curr. Opin. Genet. Dev. 2009;19:283–289. doi: 10.1016/j.gde.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–1598. doi: 10.1016/j.ophtha.2003.12.050. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chen Y, Moiseyev G, Ma JX. Two point mutations of RPE65 from patients with retinal dystrophies decrease the stability of RPE65 protein and abolish its isomerohydrolase activity. J. Biol. Chem. 2006;281:21820–21826. doi: 10.1074/jbc.M603725200. [DOI] [PubMed] [Google Scholar]
- Thomson H, Lotery A. The promise of nanomedicine for ocular disease. Nanomed. 2009;4:599–604. doi: 10.2217/nnm.09.43. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Gyürüs P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, Zrenner E, Lorenz B, Richards JE, Jacobson SG, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2000;41:4293–4299. [PubMed] [Google Scholar]
- Thompson DA, McHenry CL, Li Y, Richards JE, Othman MI, Schwinger E, Vollrath D, Jacobson SG, Gal A. Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively. Am. J. Hum. Genet. 2002;70:224–229. doi: 10.1086/338455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Mullins RF, Philp AR, Stone EM, Mrosovsky N. Divergent phenotypes of vision and accessory visual function in mice with visual cycle dysfunction (Rpe65 rd12) or retinal degeneration (rd/rd) Invest. Ophthalmol. Vis. Sci. 2008;49:2737–2742. doi: 10.1167/iovs.07-1546. [DOI] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Owens LA, Anderson L, Golczak M, Doyle SE, McCall M, Menaker M, Palczewski K, Van Gelder RN. Inner retinal photoreception independent of the visual retinoid cycle. Proc. Natl. Acad. Sci. U S A. 2006;103:10426–10431. doi: 10.1073/pnas.0600917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser JP, Aleman TS, He YG, Cideciyan AV, Kuksa V, Pittler SJ, Stone EM, Jacobson SG, Palczewski K. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc. Natl. Acad. Sci. U S A. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser JP, Liang Y, Maeda T, Kuksa V, Jang GF, He YG, Rieke F, Fong HK, Detwiler PB, Palczewski K. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J. Biol. Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veske A, Nilsson SE, Narfström K, Gal A. Retinal dystrophy of Swedish briard/briard-beagle dogs is due to a 4-bp deletion in RPE65. Genomics. 1999;57:57–61. doi: 10.1006/geno.1999.5754. [DOI] [PubMed] [Google Scholar]
- Volpe NJ, Dadvand L, Kim SK, Maguire MG, Ying GS, Moster ML, Galetta SL. Computerized binocular pupillography of the swinging flashlight test detects afferent pupillary defects. Curr. Eye Res. 2009;34:606–613. doi: 10.1080/02713680902993891. [DOI] [PubMed] [Google Scholar]
- von Rückmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br. J. Ophthalmol. 1995;79:407–12. doi: 10.1136/bjo.79.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabbels B, Demmler A, Paunescu K, Wegscheider E, Preising MN, Lorenz B. Fundus autofluorescence in children and teenagers with hereditary retinal diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:36–45. doi: 10.1007/s00417-005-0043-2. [DOI] [PubMed] [Google Scholar]
- Walia S, Fishman GA, Jacobson SG, Aleman TS, Koenekoop RK, Traboulsi EI, Weleber RG, Pennesi ME, Heon E, Drack A, et al. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010 doi: 10.1016/j.ophtha.2009.09.056. in press. [DOI] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr. Biol. 2009;19:1665–1669. doi: 10.1016/j.cub.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassell J, Boulton M. A role for vitamin A in the formation of ocular lipofuscin. Br. J. Ophthalmol. 1997;81:911–918. doi: 10.1136/bjo.81.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J. Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, von Lintig J, Oberhauser V, Tanimoto N, Grimm C, Seeliger MW. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest. Ophthalmol. Vis. Sci. 2007;48:534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- Wilhelm H. The pupil. Curr. Opin. Neurol. 2008;21:36–42. doi: 10.1097/WCO.0b013e3282f39173. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat. Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- Wright AF, Jacobson SG, Cideciyan AV, Roman AJ, Shu X, Vlachantoni D, McInnes RR, Riemersma RA. Lifespan and mitochondrial control of neurodegeneration. Nat. Genet. 2004;36:153–158. doi: 10.1038/ng1448. [DOI] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- Wrigstad A, Nilsson SE, Narfström K. Ultrastructural changes of the retina and the retinal pigment epithelium in Briard dogs with hereditary congenital night blindness and partial day blindness. Exp. Eye Res. 1992;55:805–818. doi: 10.1016/0014-4835(92)90007-f. [DOI] [PubMed] [Google Scholar]
- Wrigstad A, Narfström K, Nilsson SE. Slowly progressive changes of the retina and retinal pigment epithelium in Briard dogs with hereditary retinal dystrophy. A morphological study. Doc. Ophthalmol. 1994;87:337–354. doi: 10.1007/BF01203343. [DOI] [PubMed] [Google Scholar]
- Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Yzer S, van den Born LI, Schuil J, Kroes HY, van Genderen MM, Boonstra FN, van den Helm B, Brunner HG, Koenekoop RK, Cremers FP. A Tyr368His RPE65 founder mutation is associated with variable expression and progression of early onset retinal dystrophy in 10 families of a genetically isolated population. J. Med. Genet. 2003;40:709–713. doi: 10.1136/jmg.40.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K, Frederick JM, Crouch RK, Baehr W. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J. Neurosci. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znoiko SL, Crouch RK, Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest. Ophthalmol. Vis. Sci. 2002;43:1604–1609. [PubMed] [Google Scholar]
- Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest. Ophthalmol. Vis. Sci. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]