Introduction
External physical forces as well as internal constraints imposed by the microtubule, microfilament and intermediate filament cytoskeletal networks, junctional complexes and integrin-extracellular matrix (ECM) interactions are major determinants of cell structure and function [e.g., 1–3]. Indeed, several basic processes including cell cycle transit, DNA synthesis and apoptosis are profoundly influenced by changes in cellular structural organization [4–9]. The vasculature, for example, is constantly subjected to a continuum of hemodynamic stimuli (e.g., shear strain, flow disturbances, mechanical or pulsile stretch) that alter cytoskeletal dynamics, organization and associated signaling pathways. These same mechanical forces impact expression of genes that, in turn, modulate cell proliferation, migration and ECM synthesis/deposition resulting in the development of tissue-specific pathologies (e.g., focal atherosclerosis) [reviewed in 10–14]. Prominent among the repertoire of fibrosis-promoting proteins implicated in vascular fibroproliferative disease are the matricellular proteins plasminogen activator inhibitor inhibitor-1 (PAI-1, SERPINE1) and connective tissue growth factor (CTGF) [reviewed in 15,16]. Importantly, the transcriptional control networks for both genes are exquisitely sensitive to cytoskeletal perturbations [16]. The continued definition of pathways and mechanisms involved in vascular cell shape-deformation responses may well define new, translationally-relevant, targets for the treatment of vascular disorders.
Mechanosensitive Signaling: The Vascular Model
The available evidence suggests that, upon appropriate mechanical stimuli, integrins are mobilized to orchestrate cellular responses in coordination with (a) growth factor receptors (e.g., those that bind epidermal growth factor [EGFR], transforming growth factor-β [TGF-βR], vascular endothelial growth factor [VEGFR] family ligands), (b) cadherin junctional complexes and (c) clues from the ECM [10,17–21]. Integrins, in fact, are focal points for recruitment of signaling molecules (e.g., focal adhesion kinase [FAK]) to ECM contact sites in shear stress-induced endothelial cell migration [22]. The functional and spatial associations between non-receptor tyrosine kinases (e.g., pp125FAK, pp60c-src), moreover, increase with an applied mechanical load reminiscent of those induced by integrin-mediated cell adhesion [8,12,23,24]. Since these same signaling intermediates (pp125FAK, pp60c-src) also lie in the main path for mechanical force transfer (i.e., regions enriched in the cytostructural proteins paxcillin, actin and tensin), it is apparent that focal adhesion complexes can potentially translate load deformation stresses into specific biological responses as a consequence of this cytoskeletal “wiring” [8,25]. Indeed, mobilization of FAK, pp60c-src, and Grb2, to focal adhesions under conditions of varying cellular tensional forces engages downstream cascades (i.e., involving mitogen-activated protein [MAP] kinases and the small GTPases Ras, Rac, and Rho) similar to those stimulated by integrin-mediated matrix attachment [26]. Such mechanical forces may effectively cluster, or initiate conformational changes to, integrin receptors with recruitment of structural proteins at the focal adhesion complex. As part of this response, pp125FAK partitions to the cytoskeletal framework and is tyrosine phosphorylated at Tyr-397 leading to recruitment of pp60c-src, a key kinase in load-dependent signal transduction [27]. The pp60c-src kinase is also activated by mechanical deformation, albeit with different kinetics than that induced by growth factors such as EGF [28]. The association of pp60c-src with pp125FAK at focal adhesions further stimulates pp125FAK phosphorylation at Tyr-925, creating a binding site for Grb2. The adaptor protein Shc is tyrosine phosphorylated in endothelial cells in response to shear stress, binds to Grb2 by an SH2-dependent mechanism [29] facilitating, thereby, the assembly of a tripartite Shc/Grb2/Sos complex resulting in subsequent Ras GTPase activation. MAP kinase pathways in vascular smooth muscle cells (VSMC) similarly function via pp125FAK/pp60c-src/Grb2 interactions with Ras as a downstream target [27,30–32]. This has important adaptive consequences as both the extracellular signal-regulated kinase (ERK) and c-Jun-associated kinase (JNK) pathways are activated in a FAK-dependent manner, at least in the endothelium, in response to mechanical stimulation [27,29]. Cyclic stretch also rapidly activates p38 MAP kinase in VSMC which requires both the small GTPases Ras and Rac since expression of dominant-negative Ras or Rac constructs attenuates p38 phosphorylation as well as stretch-mediated VSMC migration/proliferation [33]. Stress-related ERK activation may further modulate cellular mechanical properties by regulating caldesmon, suggesting a direct effect on the contractile properties of the vascular wall [34].
Mechanical perturbation of cell structure may also activate small GTPases such as Rho, Rac or Cdc42 [35]. Indeed, the Rho kinases (Rho-associated coiled-coil forming kinases; ROCK1/2 which are the major immediate downstream targets of RhoA) and mDia are particularly important elements and impact critical functions including cytoskeletal organization, contractility, motility and gene expression [reviewed in 36] and may well be accessable targets for the clinical management of cardiovascular disease [e.g., 37]. Rho GTPases cycle between active GTP-bound and inactive GDP-bound states which are regulated by guanine nucleotide exchanges factors (GEF) and GTPases-activating proteins (GAPs) [38–41]. It appears that tight control of the temporal/spatial activation of Rho GTPases likely provides for the physiological adjustment to different cycles or amplitude of mechanical forces commonly encountered in the vascular system [e.g., 42]. The complex molecular details of mechanotransduction leading to Rho GTPase signaling, however, are only partially understood. While p190RhoGAP regulates capillary network formation by integrating mechanical and chemical signaling pathways, through the likely downstream intermediates Rho kinases, mDia, LIM-kinase, PAK1 and other small GTPases [36,43], it also functions as an integrator of Rac and RhoA cross-talk, which have opposing effects in the control of endothelial cell morphology [44]. The available evidence, in fact, supports a model wherein Rho→mDia signaling results in Rac activation via a Src-dependent mechanism with suppression of this pathway by ROCK activity [36].
Cell Shape-Dependent Metabolic Controls: Genomic Responses to Cytoskeletal Deformation
Experimental approaches that specifically perturb cell structure (e.g., multidirectional force application, cadherin- or integrin-interfering antibodies, cytoskeletal-active drugs, expression of mutant or cell type “unrelated” cytoskeletal elements, substrate-modulation of cell morphology by plating on poly-HEMA-coated surfaces or on complex micro-patterned adhesive substrates) provide accessible models to probe deformation-sensitive signaling pathways and their target genes [e.g., 2,3,24,25,45–55]. Such studies highlight the effects of cell shape on transcriptional outputs as well as the resultant phenotypic response. The use of rather novel “phosphotyrosine reporters”, that measure fluorescence of YFP fused to multiple SH2 domains derived from the c-Src tyrosine kinase, and specific microtubule disrupting agents, moreover, confirmed the dynamic nature of cytoskeletal framework-anchored signaling events (e.g., involving pp125FAK, paxillin, vinculin, pp60c-src) [56].
Distortion of cell morphology with cytoskeleton-targeting drugs has emerged as an important approach to the identification of cell shape-responsive genes as well as, in some cases, the involved signaling pathways [57–61]. Protein-protein interaction mapping revealed that the cellular signaling apparatus is networked with the cytoskeletal framework and, therefore, is highly sensitive to shape perturbation [e.g., 3]. It has become apparent in recent years, moreover, that the expression of certain genes is particularly responsive to changes in cellular configuration regardless of the basis for the cell deformation event. Thus, actin and microtubule cytoskeletal networks, key regulators of cell morphology, integrate and transduce intracellular signals provided by cues from the extracellular matrix, cell-cell interactions and growth factors [1,24,62,63]. Disruption of either the microtubule network (e.g., with colchicine or nocodazole) or cellular microfilaments (e.g., with cytochalasin D or latrunculin B), however, also constitutes effective inductive stimuli leading to changes in gene transcription largely as a consequence of altered signaling events [16,61,64–67]. Transcriptional outputs induced by cell deformation, quite unexpectedly, actually proved to be rather limited when compared to the global reprogramming of gene expression that typically accompanies exposure to serum or individual growth factors [64,65,68–77]. Microarray analyses, in fact, identified only several dozen shape deformation-sensitive genes in endothelial cells and VSMC exposed to steady laminar or turbulent shear stress, among the most prominent of which were PAI-1 and CTGF [e.g., 78,79]. When endothelial cells are exposed to non-uniform shear stress, CTGF is upregulated in a RhoA-dependent manner [80]. However, comparison of the partial inhibition by Rho kinase inhibitors compared to the much stronger effect of statins, which interfere with isoprenylation of multiple signaling molecules among them different small GTPases, indicates involvement of additional signaling molecules in shear stress-mediated induction of CTGF. Remodeling of static cultures of HUVEC to adopt to laminar flow as observed in straight areas of vessels promotes down-regulation of CTGF [80,81]. Downregulation was attributed to the transcription factor KLF2 (Kruppel-like factor 2) [81], which is also involved in the rearrangement of actin fibers in the presence of laminar shear stress [82] The latter effects were linked to an activation of RhoA. The apparent discrepancy of activation of RhoA and downregulation of CTGF needs further investigation and may be resolved by a closer analysis of time-dependent effects of KLF2.
Induced PAI-1 gene expression in each instance, furthermore, correlated with specific stress-associated restructuring within the cellular microfilament system [45,53,54,79,83]. While uncertainties regarding the threshold of expression change, cell type universality of response, kinetics of induced changes and the actual number of sequences assessed, the transcription of genes that encode proteins involved in tissue remodeling processes (e.g., CTGF, PAI-1, several metalloproteinases, urokinase plasminogen activator [uPA], vascular endothelial growth factor, cyclooxygenase, fibronectin, collagen-1) is closely associated with dynamic changes in cellular morphology and shape-altering physiologic processes (Figure 1) [53,60,65,72–77,78,84–86]. Indeed, targeted reorganization of cell morphology with cytoskeletal-disrupting drugs does, in fact, transcriptionally impact several genes in this subset implicating the cytoskeleton in the signaling apparatus [60,64]. Analysis of dose response, a critical aspect in such assessments, has implicated a “threshold” CD concentration required for dramatic increases in PAI-1 expression that appears different for individual cells types [e.g., 61,83]. Since CD also effectively increased PAI-1 synthesis in suspended cells and colchicine significantly induced PAI-1 expression in adherent cells without the same effect on cellular aborization [45,61,83], it would appear that PAI-1 gene control is more closely associated with changes in cytoskeletal structure than with overall cell shape perturbation. This interpretation is consistent with the realization that cytoskeletal modifications may relieve growth state-dependent constraints on particular signaling events (i.e., Rho-GEFs, SMADs) that impact downstream transcriptional-level controls as these particular effectors are normally microtubule-anchored, at least in unstimulated cells [3,39,63,87]. Disruption of the microtubule network with colchicine or nocodazole, in fact, stimulates several pathways and activates Rho-GEFs leading to Rho-GTP loading with subsequent effects on PAI-1 and CTGF expression [39,65,88,89] as well as SMAD phosphorylation [16,90] (Figure 2). Interestingly, the Rho-ROCK pathway utilizes SMAD3 as a transcriptional activator for PAI-1 (but not for CTGF) induction in colchicine-treated cells while SMAD3 is required for both PAI-1 and CTGF expression in TGF-β1-stimulated VSMC [16]. These findings highlight what appears to be the stimulus-specific engagement of signaling pathways that regulate the expression of these two, clinically-important, profibrotic genes. In microvascular endothelial cells, RhoA-ROCK signaling is essential for upregulation of CTGF by various microtubule disrupting agents, among them combretastatin A4, a tumor vessel-targeting drug [91]. Activation of FoxO transcription factors by inhibition of phosphatidyl inositol 3-kinase further increased the stimulating effect of microtubule disruption. The genomic effects of cytoskeletal deformation are, thus, modulated by additional factors. Indeed, microtubule disruption by combretastatin A4 induced CTGF expression more strongly when endothelial cells were cultured under hypoxic conditions compared to normoxic conditions [92].
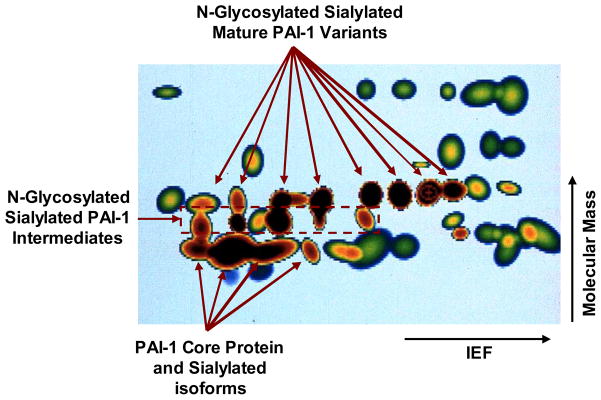
Figure 1. Proteomic mapping of cytochalasin D-induced PAI-1 expression in rat kidney epithelial cells.
The 35S-methionine-labeled, saponin-resistant fraction, of control and CD-stimulated rat kidney epithelial cells was separated by 2-D gel electrophoresis and the constitutent proteins visualized by fluorography. Computer-generated spot profiling was used to superimposition common control/CD-treated cellular proteins (green or green with red core spots) with proteins unique to CD-stimulated cells (dark brown spots). Combined 2-D electrophoresis/immunoblotting identified the CD-induced (dark brown microheterogeneous protein group) as the various glycosylated isoforms of PAI-1 [51]. Directions of isoelectric focusing (IEF) and molecular mass separations are indicated.
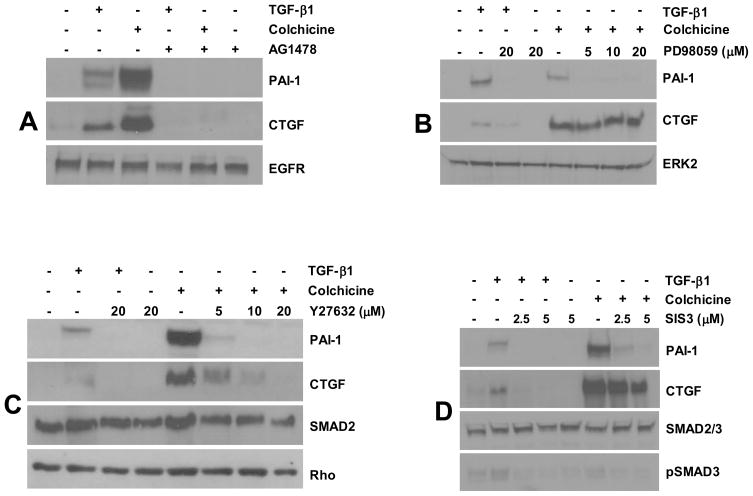
Figure 2. Signaling pathways involved in PAI-1 and CTGF expression in vascular smooth muscle cells.
PAI-1 and CTGF expression in response to colchicine as well as TGF-β1 (serving as a classic “inducer”) is virtually eliminated by AG1478 pretreatment (at a concentration of 2.5 μM) implicating EGFR in cell deformation induced signaling pathway (A). While PAI-1 induction by microtubule deformation or TGF-β1 is sensitive to the MEK inhibitor PD98059 at the lowest concentration tested (5 μM), CTGF expression, in contrast, is unaffected by even the highest concentration of PD98059 (20 μM) (B). To investigate the signaling role of ROCK, a major downstream effector of RhoA, vascular smooth muscle cells were pretreated with Y-27632 prior to colchicine or growth factor stimulation. PAI-1 and CTGF induction in response to both stimuli is dose-dependently blocked by Y-27632 with virtual ablation of expression at concentrations of 10–20 μM (C). Pretreatment with SIS3, a specific SMAD3 inhibitor, eliminated PAI-1 expression (but not CTGF) upon microtubule disruption and, as expected, addition of TGF-β1 (D). In all instances, treatment with colchicine or TGF-β1 utilized concentrations of 10 μM and 1 ng/ml, respectively.
Transactivation of Growth Factor Receptors and Downstream Signaling in Response to Cytoskeletal Deformation
EGFR activation and engagement of downstream MAP kinases (e.g., ERK1/2) is a common response to microtubule destabilizing drugs resulting in specific changes in gene expression [16,60,61,64,77,93,94] (Figure 2). EGFR phosphorylation upon microtubule disruption requires generation of reactive oxygen species (ROS) [e.g., 16], in sharp contrast to EGFR activation by native ligands [reviewed in 95]. Vascular cell shape perturbation by cytoskeletal deformation, moreover, involves engagement of at least a subset of receptor and non-receptor tyrosine kinase cascades (e.g., EGFR, Src) leading to gene reprogramming (e.g., in the case of PAI-1 and CTGF) [16,64]. Src kinase involvement, furthermore, is necessary for ERK1/2 activation as well as for PAI-1 and uPA transcription suggesting that cellular deformation-initiated Src signaling is a critical element in the expression of cell shape-sensitive genes [16,60,61,64,74,96–98]. ERK1/2 activation downstream of EGFR/Src induction, however, does not play a major role in CTGF expression (unlike PAI-1 and uPA induction) by microtubule disruption in VSMC indicating that further bifurcation of the signaling pathway downstream of the EGFR [16]. Despite reports that fibroblasts produce TGF-β transcripts upon cytoskeletal disruption [99], colchicine-induced PAI-1 and CTGF expression in VSMC is independent of autocrine release of TGF-β ligands or TGF-βR activity [16,61]. Indeed, microtubule disruption initiates SMAD2/3 phosphorylation, albeit in a delayed manner (2 hours) and in sharp contrast to the rapid (within 15 minutes) and robust SMAD2/3 activation following TGF-β1 stimulation This is consistent with findings that SMAD2/3 can be activated by TGF-βR-independent mechanisms (e.g., via MSP1; a component of the mitotic check point kinase) [16,90,100,101]. SMAD3, moreover, is essential for PAI-1 expression in both TGF-β1- and colchicine-treated VSMC [16] and, in most cells, for CTGF induction upon TGF-β1 addition [reviewed in 15]. Nevertheless, while there is evidence for ligand-independent activation of growth factor receptors as a consequence of cell deformation, the release of soluble ligands in response to mechanical forces can also activate signaling events in the vasculature [102,103] further complicating delineation of the underlying mechanisms.
Molecular Mechanisms of Gene Control in Response to Cell Shape Perturbation
While there is ample evidence that members of the Rho family impact gene expression, the underlying molecular mechanisms, particularly those involving interplay with cellular structural elements, are only partially understood. The linkage between cytoskeletal remodeling and gene regulation, moreover, largely focus on RhoA signaling and its downstream effectors ROCK and mDia leading to increases in cellular F-actin structures and a corresponding decrease in monomeric actin. Monomeric or G-actin levels profoundly affect the activity of certain serum response factor (SRF)-responsive genes. In this regard, target genes activated by RhoA→actin→MAL→SRF signaling (e.g. CTGF or vinculin) differ from those which are activated via the MEK→ERK→TCF→SRF pathway [104,105]. The PAI-1 and CTGF genes display an interesting, albeit complex, dichotomy in expression control mechanisms by agents that alter actin/microfilament dynamics. Changes in the ratio of G- to F-actin affect CTGF expression as illustrated by the induction of CTGF by jasplakinolide, a drug that recruits monomeric G-actin into higher order F-actin structures, and the reduction in CTGF levels by latrunculin-mediated disruption of F-actin microfilaments [65]. Monomeric actin binds to and sequesters (in the cytoplasm) MAL/MRTF-A/MKL-1, a required cofactor of SRF, and in doing so interferes with the transcription of a subset of genes among them CTGF [67,106–108]. CD and swinholide A actually disrupt actin-MAL interactions [57], thus, stimulating SRF transcriptional activity and, thereby, leading to the induction of SRF-responsive genes (e.g., CTGF) (Figure 3). SRF targets genes with single or multiple copies of the SRF-binding element (the CArG box) and a CArG-like motif at position −3791 is present in the CTGF promoter [67]. CArG box-containing SRF response genes frequently have an adjacent ETS motif that is recognized by the ELK family of SRF co-factors. Two distinct subsets of co-activators, thus, modulate SRF activity. The MAL-like proteins, that are regulated by Rho GTPases, and monomeric actin and the TCF (ternary complex factor) family of Ets domain proteins (e.g., ELK-1, SAP-1, NET), that are activated by MAP kinases. Actin-regulated SRF-dependent gene expression is also subject to negative controls by MAP kinase (ERK) signaling [109,110] and high nuclear levels of monomeric actin [reviewed in 57]. While nuclear G-actin sequesters MAL, making this co-factor unavailable to SRF, ERK-mediated MAL phosphorylation, in contrast, promotes its nuclear export [109]. The PAI-1 gene, however, does not possess a CArG motif and is, thus, unlikely to be a direct SRF target. While TCFs regulate immediate early genes, including c-fos, egr-1, or junB, in association with SRF, PAI-1 expression is apparently SRF independent [111]. Indeed, SRF does not bind to the PAI-1 promoter region that recruits the TCF member Net and there are no obvious consensus SRF motifs in a 300 bp scan region either upstream or downstream of the Net site. Whether SRF binds to a more distal site with subsequent interaction with Net through extended spatial flexibility, however, cannot be excluded although the evidence for SRF independence of PAI-1 expression includes siRNA and ChIP [111].
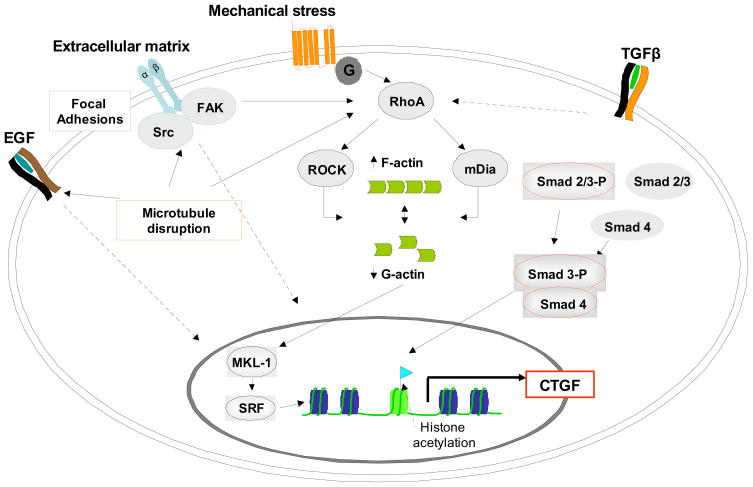
Figure 3. Simplified model of signaling pathways involved in the regulation of CTGF expression by cell shape deformation.
Pathways are based on data obtained in different cell types (refer to text) and may not always occur simultanously. For the sake of clarity, several kinase pathways, e.g. p38 MAPK, PI-3K/AKT or PKC, have been omitted, although they contribute to the network of interacting pathways translating morphological alterations into gene expression.
Summary and Significance
Mechanosensory signaling pathways play a crucial role in vascular cell migration, proliferation and differentiation as well as disease progression [10,13,14,21]. The “tensegrity model”, for example, suggests that the plasma membrane is hardwired to the nucleus via cytoskeletal networks facilitating signal propagation in response to mechanical stimuli originating from either ECM modifications or changes in tensional forces due to alterations of blood flow [reviewed in 8,14]. Recent in vivo studies suggest that not all stress responses result in equivalent outcomes. Vascular endothelial cells can distinguish between laminar shear and disturbed flow; these stimuli initiate two different biological responses. Laminar shear-induced mechanisms are associated with less inflammation and oxidative stress leading to an atheroprotective phenotype; disturbed flow, in contrast, results in significantly greater inflammation and increased oxidative stress, thereby, acerbating the atherosclerotic phenotype [21,112,113]. PAI-1 expression is augmented in response to mechanical forces such as shear stress. In terms of gene expression, CTGF belongs to a select group of genes which are suppressed by laminar flow but increased in areas of non-uniform shear stress [80], whereas others (e.g., endothelial NO synthetase) are regulated in the opposite direction. Since PAI-1 and CTGF contribute to the pathogenesis of cardiovascular disease and fibrosis, clarifying mechanisms associated with the disturbed flow leading to genomic reprogramming are crucial for identification of novel therapeutic targets. Moreover, the potential importance of morphology-linked controls on the transcription of profibrotic genes such as PAI-1 and CTGF are underscored by their marked induction in mechanical- and hypoxia-stressed, as well as growth factor-stimulated, cells [e.g.,15,69,78,85] and the obvious changes in cytoskeletal dynamics associated with these shape-altering physiological processes. It is increasingly apparent that microtubule and microfilament networks integrate signaling originating from integrin and growth factor receptors (largely due to direct interactions) and activate kinase cascades leading to alterations in gene expression. Several of the involved intermediates (e.g., SMADs, Rho-GEFs), moreover, are sequestered on the cytoskeleton in an “inactive state” and cell shape modifications due to fluctuating mechanical forces could dissociate some of these from their cytoskeletal anchors thus alleviating the “repressive state” leading to the downstream signaling. Not surprisingly, drug-based actin and tubulin cytoskeletal modifications lead cell deformation-sensitive genetic changes including PAI-1 and CTGF induction. Interestingly, comparative analysis of PAI-1 and CTGF expression in response to microtubule disruption in vascular smooth muscle cells highlights a complex network of both unique (e.g., SRF, ERK, SMAD) and common (e.g., Rho, EGFR) signaling elements in gene transcription. Given the emerging importance of these profibrogenic factors, further clarification of the involved pathways could yield novel therapeutic tools and targets to modulate the pahtophysiology of cardiovascular disease.
Acknowledgments
Supported by NIH grant GM57242
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuchs E, Karakesisoglou I. Bridging cytoskeletal intersections. Genes Dev. 2001;15:1–14. doi: 10.1101/gad.861501. [DOI] [PubMed] [Google Scholar]
- 2.Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun. 2001;285:1077–1083. doi: 10.1006/bbrc.2001.5177. [DOI] [PubMed] [Google Scholar]
- 3.Forgacs G, Yook SH, Janmey PA, Jeong H, Burd CG. Role of the cytoskeleton in signaling networks. J Cell Sci. 2004;117:2769–2775. doi: 10.1242/jcs.01122. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ze’ev A, Farmer SR, Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980;21:365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- 6.Crossin KL, Carney DH. Evidence that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis. Cell. 1981;23:61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- 7.Chou IN, Zeiger J, Solomon JA, Black PH. Stimulation of plasminogen activator expression and induction of DNA synthesis by microtubule-disruptive drugs. Biochem Biophys Res Comm. 1981;101:1266–1273. doi: 10.1016/0006-291x(81)91584-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 9.Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 10.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 11.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 12.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 13.Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36:631–643. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- 14.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009;35:200–208. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- 16.Samarakoon R, Higgins CE, Higgins SP, Higgins PJ. Differential requirement for MEK/ERK and SMAD signaling in PAI-1 and CTGF expression in response to microtubule disruption. Cell Signal. 2009;21:986–995. doi: 10.1016/j.cellsig.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 18.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009;104:995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol. 2009;296:H550–558. doi: 10.1152/ajpheart.01176.2008. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A. 2002;99:3546–3551. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiger B. A role for p130Cas in mechanotransduction. Cell. 2006;127:879–881. doi: 10.1016/j.cell.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Banes AJ, Tsuzaki M, Yamamoto J, Fisher T, Brigman B, Brown T, Millar L. Mechanoreception at the cellular level. Biochem Cell Biol. 1995;73:349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 25.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 26.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chien S, Shyy JY. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 28.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 30.Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertension. 1998;16:1921–1929. doi: 10.1097/00004872-199816121-00011. [DOI] [PubMed] [Google Scholar]
- 31.Rao GN. Hydrogen peroxide induces complex formation of SHC-grb2-SOS with receptor tyrosine kinase and activate signal-related protein kinases group of mitogen-activated protein kinases. Oncogene. 1996;13:713–719. [PubMed] [Google Scholar]
- 32.Iwasaki H, Shichiri M, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol. 2000;278:H521–529. doi: 10.1152/ajpheart.2000.278.2.H521. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Hu Y, Strum G, Wick G, Xu Q. Ras/Rac-dependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arterioscler Thromb vasc Biol. 2000;20:E1–9. doi: 10.1161/01.atv.20.3.e1. [DOI] [PubMed] [Google Scholar]
- 34.Adam LP, Franklin MT, Raff GJ, Hathaway DR. Activation of mitogen-activated protein kinase in porcine carotid arteries. Circ Res. 1995;76:183–90. doi: 10.1161/01.res.76.2.183. [DOI] [PubMed] [Google Scholar]
- 35.Birukov KG. Small GTPases in mechanosensitive regulation of endothelial barrier. Microvasc Res. 2009;77:46–52. doi: 10.1016/j.mvr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narumiya S, Tanji M, Ishizaki T. Rho signalling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 37.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular disease. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Arthur WT, Petch LA, Burridge K. Integrin engagement suppesses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 39.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J Cell Sci. 2007;120:456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 43.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–23918. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 45.Providence KM, Kutz SM, Higgins PJ. perturbation of the actin cytoskeleton induces PAI-1 gene expresión in cultured epithelial cells independent of substrate anchorage. Cell Motil Cytoskeleton. 1999;42:218–229. doi: 10.1002/(SICI)1097-0169(1999)42:3<218::AID-CM5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 2000;98:159–69. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Patel K, Harding P, Sorokin A, Glass WF. Regulation of TGF-β1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cells. Exp Cell Res. 2007;313:1240–1250. doi: 10.1016/j.yexcr.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charest JL, Jennings JM, King WP, Kowalczyk AP, Garcia AJ. Cadherin-mediated cell-cell-contact regulates keratinocyte differentiation. J Invest Dermatol. 2009;129:564–572. doi: 10.1038/jid.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T-H, Wang HS, Ichijo H, Giannakakou P, Foster JS, Fojo T, Wimalasena J. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J Biol Chem. 1998;273:4928–4936. doi: 10.1074/jbc.273.9.4928. [DOI] [PubMed] [Google Scholar]
- 50.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 51.Higgins PJ, Ryan MP. Identification of the 52 kDa cytoskeletal-like protein of cytochalasin D-stimulated normal rat kidney (NRK/CD) cells as substrate-associated glycoprotein p52 [plasminogen –activator inhibitor type-1 (PAI-1)]. Expression of p52(PAI-1) in NRK/CD cells is regulated at the level of mRNA abundance. Biochem J. 1992;284:433–439. doi: 10.1042/bj2840433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, Succar L, Rangan GK, Hu M, Henderson BR, Alexander SI, harris DC. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-β1 in renal tubular epithelial cells. Am J Path. 2009;175:580–591. doi: 10.2353/ajpath.2009.080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higgins PJ, Ryan MP, Ahmed A. Cell shape-associated transcriptional activation of the p52(PAI-1) gene in rat kidney cells. Biochem J. 1992;288:1017–1024. doi: 10.1042/bj2881017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thakar RG, Cheng Q, Patel S, Chu J, Nasir M, Liepmann D, Komvopoulos K. Cell-shape regulation of smooth muscle cell proliferation. Biophys J. 2009;96:3423–3432. doi: 10.1016/j.bpj.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JY, Jones C, Zern MA, Revzin A. Analysis of local tissue-specific gene expression in cellular micropatterns. Anal Chem. 2006;78:8305–8312. doi: 10.1021/ac0613333. [DOI] [PubMed] [Google Scholar]
- 56.Kirchner J, Kam Z, Tzur G, Bershadsky AD, Geiger B. Live-cell monitoring of tyrosine phosphorylation in focal adhesions following microtubule disruption. J Cell Sci. 2003;116:975–986. doi: 10.1242/jcs.00284. [DOI] [PubMed] [Google Scholar]
- 57.Kustermans G, Piette J, Legrand-Poels S. Actin-targeting natural compounds as tools to study the role of actin cytoskeleton in signal transduction. Biochem Pharmacol. 2008;76:1310–1322. doi: 10.1016/j.bcp.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 58.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 59.Downing KH. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu Rev Cell Dev Biol. 2000;16:89–111. doi: 10.1146/annurev.cellbio.16.1.89. [DOI] [PubMed] [Google Scholar]
- 60.Irigoyen JP, Besser D, Nagamine Y. Cytoskeleton reorganization induces the urokinase-type plasminogen activator gene via the Ras/extracellular signal-regulated kinase (ERK) signaling pathway. J Biol Chem. 1997;272:1904–1909. doi: 10.1074/jbc.272.3.1904. [DOI] [PubMed] [Google Scholar]
- 61.Samarakoon R, Higgins PJ. MEK/ERK pathway mediates cell-shape-dependent plasminogen activator inhibitor type 1 gene expression upon drug-induced disruption of the microfilament and microtubule networks. J Cell Sci. 2002;115:3093–3103. doi: 10.1242/jcs.115.15.3093. [DOI] [PubMed] [Google Scholar]
- 62.Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 63.Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 64.Samrakoon R, Higgins PJ. pp60c-src mediates ERK activation/nuclear localization and PAI-1 gene expression in response to cellular deformation. J Cell Physiol. 2003;195:411–420. doi: 10.1002/jcp.10247. [DOI] [PubMed] [Google Scholar]
- 65.Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M. Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem. 2003;278:44305–44311. doi: 10.1074/jbc.M309140200. [DOI] [PubMed] [Google Scholar]
- 66.Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem. 2006;281:20608–20622. doi: 10.1074/jbc.M600214200. [DOI] [PubMed] [Google Scholar]
- 67.Muehlich S, Cicha I, Garlichs CD, Krueger B, Posern G, Goppelt-Struebe M. Actin-dependent regulation of connective tissue growth factor. Am J Physiol Cell Physiol. 2007;292:C1732–1738. doi: 10.1152/ajpcell.00552.2006. [DOI] [PubMed] [Google Scholar]
- 68.Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 69.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic Programs of epithelial plasticity directed by transforming growth factor-β. Proc Natl Acad Sci USA. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi L, Higgins SP, Lu Q, Samarakoon R, Wilkins-Port CE, Ye Q, Higgins CE, Staiano-Coico L, Higgins PJ. SERPINE1 (PAI-1) is a prominent member of the early G0→G1 transition “wound repair“ transcriptome in p53 mutant human keratinocytes. J Invest Dermatol. 2008;128:749–753. doi: 10.1038/sj.jid.5701068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb. 2003;10:304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- 72.Aggeler J, Frisch SM, Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol. 1984;98:1662–1671. doi: 10.1083/jcb.98.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Unemori EN, Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986;103:1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irigoyen JP, Nagamine Y. Cytoskeletal reorganization leads to induction of the urokinase-type plasminogen activator gene by activating FAK and Src and subsequently the Ras/Erk signaling pathway. Biochem Biophys Res Commun. 1999;262:666–670. doi: 10.1006/bbrc.1999.1202. [DOI] [PubMed] [Google Scholar]
- 75.Bayraktutan U, Jones P. Expression of the human gene encoding urokinase plasminogen activator receptor is activated by disruption of the cytoskeleton. Exp Cell Res. 1996;221:486–495. doi: 10.1006/excr.1995.1400. [DOI] [PubMed] [Google Scholar]
- 76.Ailenberg M, Silverman M. Cellular activation of mesangial gelatinase by cytochalasin D is accompanied by enhanced mRNA expression of both gelatinase A and its membrane-associated gelatinase A activator (MT-MMP) Biochem J. 1996;313:879–884. doi: 10.1042/bj3130879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase cascades. J Biol Chem. 2000;275:14838–14845. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 78.Feng Y, Yang J-H, Huang H, Kennedy SP, Turi TG, Thompson JF, Libby P, Lee RT. Transcriptional profile of mechanically induced genes in human vascular smooth muscle cells. Circ Res. 1999;85:1118–1123. doi: 10.1161/01.res.85.12.1118. [DOI] [PubMed] [Google Scholar]
- 79.Garcia-Cardena G, Comander JI, Blackman BR, Anderson KR, Gimbrone MA. Mechanosensitive endothelial gene expression profiles: script for the role of hemodynamics in athrogenesis? Ann NY Acad Sci. 2001;947:1–6. [PubMed] [Google Scholar]
- 80.Cicha I, Goppelt-Struebe M, Muehlich S, Yilmaz A, Raaz D, Daniel WG, Garlich CD. Pharmacological inhibition of RhoA signaling prevents connective tissue growth factor induction in endothelial cells exposed to non-uniform shear stress. Atherosclerosis. 2008;196:136–145. doi: 10.1016/j.atherosclerosis.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 81.Mack PJ, Zhang Y, Chung S, Vickerman V, Kamm RD, Garcia-Cardena G. Biomechanical Regulation of Endothelium-dependent Events Critical for Adaptive Remodeling. J Biol Chem. 2009;284:8412–8420. doi: 10.1074/jbc.M804524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boon RA, Leyen TA, Fontijin RD, Fledderus JO, Baggen JM, Volger OL, van Nieuw Amerongen GP, Horrevoets AJ. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood. 2009 doi: 10.1182/blood-2009-06-228726. in press. [DOI] [PubMed] [Google Scholar]
- 83.Hawks K, Higgins PJ. Cell shape-dependent pathway of plasminogen activator inhibitor type-1 gene expression requires cytoskeletal reorganization. J Cell Physiol. 1998;176:293–302. doi: 10.1002/(SICI)1097-4652(199808)176:2<293::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 84.Ryan MP, Higgins PJ. Growth state-regulated expression of p52(PAI-1) in normal rat kidney cells. J Cell Physiol. 1993;155:376–384. doi: 10.1002/jcp.1041550219. [DOI] [PubMed] [Google Scholar]
- 85.Providence KM, Kutz SM, Staiano-Coico L, Higgins PJ. PAI-1 gene expression is regionally induced in wounded epithelial cell monolayers and required for injury repair. J Cell Physiol. 2000;182:269–280. doi: 10.1002/(SICI)1097-4652(200002)182:2<269::AID-JCP16>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 86.Cicha I, Beronov K, Ramirez EL, Osterode K, Goppelt-Struebe M, Raaz D, Yilmaz A, Daniel WG, Garlichs CD. Shear stress preconditioning modulates endothelial susceptibility to circulating TNF-alpha and monocytic cell recruitment in a simplified model of arterial bifurcations. Atherosclerosis. 2009;207:93–102. doi: 10.1016/j.atherosclerosis.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 87.van Horck FP, Ahmadian MR, Haeusler LC, Moolenaar WH, Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J Biol Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- 88.Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19:2147–2153. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nature Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 90.Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGF β activity. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- 91.Samarin J, Cicha I, Goppelt-Struebe M. Cell type-specific regulation of CCN2 protein expression by PI3K-AKT-FoxO signaling. J Cell Commun Signal. 2009;3:79–84. doi: 10.1007/s12079-009-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Samarin J, Wessel J, Cicha I, Kroening S, Warnecke C, Goppelt-Struebe M. FOXO proteins mediate hypoxic induction of connective tissue growth factor (CTGF) in endothelial cells. J Biol Chem. 2010;285:4328–4336. doi: 10.1074/jbc.M109.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rijken PJ, van Hal GJ, van der Heyden MA, Verkleij AJ, Boonstra J. Actin polymerization is required for negative feedback regulation of epidermal growth factor signal transduction. Exp Cell Res. 1998;243:254–262. doi: 10.1006/excr.1998.4142. [DOI] [PubMed] [Google Scholar]
- 94.Yujiri T, Fanger GR, Garrington TP, Schlesinger TK, Gibson S, Johnson GL. MEK kinase 1 (MEKK1) transduces c-Jun NH2-terminal kinase activation in response to changes in the microtubule cytoskeleton. J Biol Chem. 1999;274:12605–12610. doi: 10.1074/jbc.274.18.12605. [DOI] [PubMed] [Google Scholar]
- 95.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013–2023. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 96.Faisal A, Kleiner S, Nagamine Y. Non-redundant role of Shc in Erk activation by cytoskeletal reorganization. J Biol Chem. 2004;279:3202–3211. doi: 10.1074/jbc.M310010200. [DOI] [PubMed] [Google Scholar]
- 97.Schmid-Alliana A, Menou L, Manie S, Schmid-Antomarchi H, Millet MA, Giuriato S, Ferrua B, Rossi B. Microtubule integrity regulates src-like and extracellular signal-regulated kinase activities in human pro-monocytic cells. Importance for interleukin-1 production. J Biol Chem. 1998;273:3394–3400. doi: 10.1074/jbc.273.6.3394. [DOI] [PubMed] [Google Scholar]
- 98.Graness A, Cicha I, Goppelt-Struebe M. Contribution of Src-FAK signaling to the induction of connective tissue growth factor (CTGF) in renal fibroblasts. Kidney Int. 2006;69:1341–1349. doi: 10.1038/sj.ki.5000296. [DOI] [PubMed] [Google Scholar]
- 99.Varedi M, Ghahary A, Scott PG, Tredget EE. Cytoskeleton regulates expression of genes for transforming growth factor-β1 and extracellular matrix proteins in dermal fibroblasts. J Cell Physiol. 1997;172:192–199. doi: 10.1002/(SICI)1097-4652(199708)172:2<192::AID-JCP6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 100.Zhu S, Wang W, Clarke DC, Liu X. Activation of Mps1 promotes transforming growth factor-beta-independent Smad signaling. J Biol Chem. 2007;282:18327–18338. doi: 10.1074/jbc.M700636200. [DOI] [PubMed] [Google Scholar]
- 101.Liu X, Zhu S, Wang T, Hummers L, Wigley FM, Goldschmidt-Clermont PJ, Dong C. Paclitaxel modulates TGFβ signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med. 2005;2:e354. doi: 10.1371/journal.pmed.0020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adam RM, Eaton SH, Estrada C, Nimgaonkar A, Shih SC, Smithe LE, Kohane IS, Bagli D, Freeman MR. Mechanical stretch is a highly selective regulator of gene expression in human bladder smooth muscle cells. Physiol Genomics. 2004;20:36–44. doi: 10.1152/physiolgenomics.00181.2004. [DOI] [PubMed] [Google Scholar]
- 103.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PT, Lauffenburger DA, Kamm RD, Drazen JM. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gineitis D, Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J Biol Chem. 2001;276:24531–24539. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- 105.Selvaraj A, Prywes R. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol Biol. 2004;5:13–28. doi: 10.1186/1471-2199-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Descot A, Hoffmann R, Shaposhnikov D, Reschke M, Ullrich A, Posern G. Negative regulation of the EGFR-MAPK cascade by actin-MAL-mediated Mig6/Errfi-1 induction. Mol Cell. 2009;35:291–304. doi: 10.1016/j.molcel.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 107.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 108.Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Muehlich S, Wang R, Lee SM, Lewis TC, Dai C, Prywes R. Serum-induced phosphorylation of the SRF coactivator MKL1 by the ERK1/2 pathway inhibits its nuclear localization. Mol Cell Biol. 2008;28:6302–6313. doi: 10.1128/MCB.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Samarin J, Rehm M, Krueger B, Waschke J, Goppelt-Struebe M. Up-regulation of connective tissue growth factor in endothelial cells by the microtubule destabilizing agent combretastatin A-4. Mol Cancer Res. 2009;7:180–188. doi: 10.1158/1541-7786.MCR-08-0292. [DOI] [PubMed] [Google Scholar]
- 111.Buchwalter G, Gross CH, Wasylyk B. The ternary complex factor Net regulates cell migration through inhibition of PAI-1 expression. Mol Cell Biol. 2005;25:10853–10862. doi: 10.1128/MCB.25.24.10853-10862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 113.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]