Abstract
Infection of humans by the human immunodeficiency virus (HIV) causes a progressive, multifactorial impairment of the immune system eventually leading to the acquired immunodeficiency syndrome (AIDS). No cure or vaccine exists yet against HIV infection. More worrisome is the fact that despite having identified HIV as the cause of the AIDS, we still do not understand what pathogenic mechanisms lead to the debacle of the immune system. In this review we consider the extent and the limits of our knowledge of HIV pathogenesis, and how this knowledge may be used to design preventive and therapeutic approaches.
Keywords: acquired immunodeficiency syndrome, human immunodeficiency virus, pathogenesis
Introduction
For approximately 25 years, the human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) problem has played a dominant role in biomedical science. Due to the worldwide urgency of this problem, pressure has mounted to promise cures and efficacious vaccines without adequate understanding of the complex orchestration of multiple, time-sensitive interactions between virus and host. The early optimistic predictions of how the ‘AIDS problem’ could have been solved have consistently fallen short of their objectives, resulting in discouragement within the research community. Although AIDS is the obvious consequence of HIV-1 infection, our understanding of the mode by which the virus interacts with the immune system and the consequences of their relation are still limited. This review presents the aspects of the HIV-1-associated immune dysregulation that we consider critical for pathogenesis. We also discuss our opinion on how some of these discoveries may suggest new directions for immune-based therapy and vaccines. This review is by no means complete and reflects the acknowledged biases of the authors. A better understanding of the immunopathogenic mechanisms of HIV-1 infection (‘know it’) must be achieved before successful therapeutic (‘fix it’) and prophylactic (‘prevent it’) approaches are found.
Immune dysregulation during HIV-1 infection
The defining features of the acquired immunodeficiency are the ‘persistent and profound selective decrease in the function as well as number of T lymphocytes of the helper/inducer subset and a possible activation of the suppressor/cytotoxic subset’, as described in 1982 [1]. At present, pathogenesis of the disease is considered multifactorial, in that no unique immune alteration has been identified that can fully explain the plethora of dysregulations described so far. We first review some historical and recent findings on innate and adaptive immune mechanisms that are dysfunctional during HIV-1 infection.
Dysregulation of innate immunity
Activation of natural killer (NK) cells and production of type I interferon (IFN-α/β) by plasmacytoid dendritic cells (pDC) are the main effector arms of innate antiviral responses [2]. Both mechanisms are severely affected during HIV-1 infection, with potential consequences for pathogenesis and generation of an efficient vaccine-induced response.
Dysregulation of NK cells
Natural killer cells contribute to eliminating infected cells and produce immunostimulatory factors which support the generation of antigen-specific adaptive responses [3]. Activation of NK cells is regulated by a balance between positive and negative signals delivered through ligand receptor systems. Inhibitory NK cell receptors (iNKR) recognize major histocompatibility complex (MHC) class I molecules expressed on target cells, preventing NK cell-mediated cytolysis [3]. Most viruses, including HIV, escape CD8+ T-cell recognition by downregulating MHC class I on the infected cells, which favour NK cell activation [4]. Surprisingly, HIV-infected cells appear to be resistant to NK cell-mediated killing despite markedly reduced expression of MHC class I [5–7]. The reasons for this dysfunction are not fully understood, but probably depend on a combination of increased expression of iNKR and concomitant low expression of receptors with activating function (reviewed in [8]). Both cytolytic activity and cytokine production of NK cells are affected possibly as a consequence of HIV-mediated signalling through CCR5 and CXCR4 and of general immune activation of the host, rather than infection of a small subset of CD4-expressing NK cells [8–11].
Dysregulation of pDC
The second major component of innate immune responses triggered by viral infections is the production of type I IFN by pDC [12, 13]. pDC are activated upon recognition of common structural patterns of viruses, particularly single-stranded viral RNA and unmethylated CpG-rich DNA which trigger Toll-like receptors (TLR) 7 and 9, respectively [14, 15]. Type I IFN create a cellular environment that is hostile to the virus by limiting the uptake of nutrients from the extracellular environment, increasing the degradation of RNA, arresting progression through the cell cycle and eventually favouring the apoptotic death of the target cell [12, 16]. Furthermore, type I IFN directly induces the expression of MHC and co-stimulatory molecules on antigen-presenting cells, thus promoting efficient antigen presentation and the priming of adaptive immune responses [15].
Although early studies showed reduced in vitro type I IFN production by peripheral leucocytes from HIV-infected patients [17–22], recent evidence suggest otherwise. HIV-1 efficiently activates pDC in vitro, inducing both type I IFN production and expression of CCR7, which is associated with migration to lymph nodes [23, 24]. Increased pDC frequency was observed in lymph nodes of asymptomatic HIV-infected patients [25]. Furthermore, increased levels of type I IFN are detected in both plasma and tonsils of HIV-infected patients with progressive disease, in association with increased expression of IFN-inducible genes [26–29]. These findings suggest that the decrease in circulating pDC in HIV-infected patients may reflect their relocation to lymphoid tissues, rather than numerical depletion [20]. A recent study provides evidence supporting the hypothesis that pDC are chronically stimulated by replicating HIV-1 and that the continuous production of type I IFN renders pDC refractory to in vitro stimulation [30].
Activation of pDC by HIV-1 requires endocytosis of the virion in order to expose the viral RNA to the endosomes that contain TLR7 [23]. Although the numerous molecular interaction that stabilize the virus cell synapse may facilitate engulfment of HIV-1, the binding of gp120 to CD4 on the surface of pDC appears to be required for initiating the endocytotic process, as soluble CD4 molecules or anti-CD4 antibodies inhibit HIV-mediated pDC activation in vitro [23, 24, 31].
Production of type I IFN is not the only consequence of pDC activation. pDC are one of the main sources of the enzyme indoleamine (2,3)-dioxygenase (IDO), which initiates catabolism of the essential amino acid tryptophan into the kynurenine pathway [32]. The rate of tryptophan catabolism is increased in HIV-infected patients (reviewed in [33]), and increased IDO expression was observed in both peripheral blood and lymphoid tissues of HIV-infected humans and simian immunodeficiency virus (SIV)-infected macaques at different stages of the infection [34–39]. Direct HIV-mediated activation of pDC could be the primary cause of increased IDO and tryptophan catabolism during HIV-1 infection [35]. The depletion of tryptophan from the extracellular environment and the simultaneous accumulation of potentially cytotoxic bioproducts was suggested to contribute to the control of different microbes whose life cycle depends on this essential amino acid (reviewed in [40]). However, more recently, IDO has been described as a central player in the downregulation of T-cell responses, by the same mechanisms of tryptophan depletion and production of biologically active catabolites (reviewed in [41]).
Dysregulation of adaptive immunity
Defective CD4 T-cell function
CD4+ T-cell responses, in particular anti-HIV-1 CD4+ T-cell responses are profoundly altered in HIV-infected patients. Underlying mechanisms include both cellular depletion and functional abnormalities. HIV-1 is cytopathic to CD4+ T cells infected in vitro. Intestinal CD45RO+CCR5+CD4+ T cells are rapidly depleted in the intestines of HIV-infected patients, similar to that reported in the SIV/macaque model [42]. Impaired production of new cells may also be implicated, as evidenced by defective thymocyte proliferation [43].
Functional CD4+ T-cells abnormalities are also well described in HIV-infected individuals. These defects occur early in the course of HIV-1 infection, prior to the decline of circulating CD4+ T-cell numbers [44–46]. Moreover, loss of in vitro CD4+ T-cell responses and of in vivo delayed-type hypersensitivity is predictive of disease progression and time to death in untreated HIV-1 infection [47, 48]. Several abnormalities have been defined ex vivo and can be summarized as follows: (i) decreased polyfunctionality, particularly of HIV-specific T cells, in that activated CD4+ T cells exhibit decreased proliferation and production of interleukin (IL)-2, but not IFN-γ and tumour necrosis factor (TNF)-α [49, 50], and defective upregulation of the activation marker CD40 ligand, but not CD69 or OX40 [51]; (ii) upregulation of the inhibitory molecules cytotoxic T lymphocyte antigen (CTLA-4) [52] and programmed death-1 (PD-1) [53, 54]; (iii) increased expression of apoptosis markers and increased percentage of CD4+ T cells entering apoptosis [55]; (iv) blunted T-cell signalling induced by T-cell receptor (TCR) cross-linking, IL-2 or phorbol esters/ionomycin [56–58]. Interestingly, there is a discrepancy between the ex vivo picture, in which CD4+ T cells from HIV-infected patients exhibit increased expression of many activation markers, including increased basal level of kinase phosphorylation and CD40L, and the in vitro picture, in which CD4+ T cells from patients respond poorly to stimulation [51, 58]. These findings suggest a model of chronic ‘tickling’ of the adaptive immune system, which precludes its full response to pathogens.
Compared with ‘conventional’ CD4+ T-helper (Th) cells, it is less established how HIV-1 infection affects the newly described CD4+ T-cell subsets, Th17 and regulatory T cells (Treg). However, some notable findings have already emerged. IL-17 production induced by Salmonella typhimurium infection in the ileum was inhibited in SIV-infected macaques [59]. HIV-infected children with detectable viral load exhibited decreased IL-17 production [60]. Early depletion of Th17 cells is observed in the gut mucosa of HIV-infected patients and SIV-infected macaques, but not in non-pathogenic SIV-infection of sooty mangabeys [61, 62], and may contribute to the disruption of the mucosal barrier during pathogenic SIV/HIV-1 infection. These results imply viral-mediated destruction or impairment of Th17 cells.
More is known about HIV/Treg interactions. Studies of HIV-infected patient and SIV-infected rhesus macaque, particularly the comparison between progressive and nonprogressive infection, showed an association between Treg accumulation in lymphoid tissues and high viral loads and reduced cytotoxic T-cell activity [34, 36–39]. Furthermore, suppressive antiretroviral therapy (ART) was associated with low Treg levels in lymphoid tissues [34]. However, decreased Treg number was found in tissues, particularly in the gut, during the highly pathogenic SIV infection of nemestrina and fascicularis macaques [63, 64], suggesting either interspecies variations, or different Treg-virus interactions in the case of fast progression. Treg inhibitory function appears to be preserved in HIV-infected patients and SIV-infected macaques [65, 66]. Thus, Tregs probably play a complex balancing role during HIV-1 infection. They probably limit the nonspecific immune activation [63, 67, 68], but at the price of dampened antiviral immune responses, particularly in the lymphoid tissues [65, 66]. Moreover, Tregs are a major source of tissue transforming growth factor (TGF)-β, which promotes tissue fibrosis and limits immune reconstitution [69].
Defective CD8 T-cell function
Effector CD8+ T cells are essential for the control of HIV-1 infection, something revealed by in vitro studies as well as through experimental deletion of such cells in the SIV/macaque model (reviewed in [70, 71]). Studies of long-term nonprogressors (LTNP) support this hypothesis. Viral loads in LTNP are orders of magnitude lower than those typically found in progressors [72–74]. Many LTNP maintain vigorous HIV-specific CTL activity and have greater CD8+ T-cell-mediated HIV-1 suppressive activity compared with that seen in progressors [72–74]. CTL in LTNP also exhibit broad specificity and polyfunctionality, whereas narrowly directed and monofunctional CTL responses are found in progressors [75–78]. Low perforin levels have also been detected in situ in lymph nodes of infected individuals [79]. Recent studies conducted in HIV-infected adults demonstrated that the increased expression of PD-1 molecule on HIV-specific CD8+ T cells associates with exhaustion of these cells and disease progression [53, 54, 80, 81]. In vitro treatment with anti-PD-1 or PD-1 ligand 1 (PD-L1) blocking antibodies enhances HIV-specific CD4+ and CD8+ T-cell responses [53, 54, 80].
Importantly, a suboptimal CD8 response could facilitate selection of virus escape mutants that are no longer recognized by effector CD8+ T cells. Such mutants have been identified in HIV+ patients and in SIV+ macaques (reviewed in [71]). Of note, studies in several experimental systems have shown a link between weak CD4+ T cell and suboptimal CD8+ T cell responses (reviewed in [82, 83]).
Nature/causes of immune dysregulation
The key events leading to the immune dysregulation observed during HIV-1 infection are still unknown. Although several hypotheses have been formulated through the years, the main challenge in identifying pivotal alterations in the pathogenesis of HIV-1 infection is our inability to distinguish them from ‘normal’ immune response to a chronic viral infection. What are the relevant pathogenic alterations that are directly caused by HIV-1 infection or exposure? Are these events specifically triggered by HIV-1 or are they also observed in other chronic infections? What cellular and molecular features of HIV-1 infection drive these alterations? These and other questions are considered in the next section of this review.
Direct effects of HIV/SIV infection and exposure: what have in vivo and in vitro experiments using HIV/SIV taught us?
We summarize below experimental approaches that investigate the effects of in vivo infection of macaques with SIV and in vitro exposure of human leucocytes to replication-competent or -incompetent HIV-1, as well as to HIV-1 proteins, which have helped to identify events that are directly caused by the virus.
Gut-associated lymphoid tissue immunopathogenesis
Studies performed on both HIV-infected humans and SIV-infected macaques revealed that rapid kinetics of CD4+ T-cell depletion is observed at the gut mucosal level, where the vast majority of CD4+ T lymphocytes express CCR5 [84]. The fact that transmission of both SIV and HIV-1 is primarily driven by CCR5-tropic strains has led to the hypothesis that the depletion of these cells is a direct consequence of the cytopathic infection of the target cells [84]. The mechanisms underlying this cytopathic effect are still to be clarified, but the interaction of envelope proteins with CD4 (reviewed in [85]) and the production of cytopathic viral proteins such as Vpr [86] may independently contribute to the depletion of gut CD4+ T cells. Depletion of uninfected ‘bystander’ CD4+ T cells most probably also contributes to the extensive depletion occurring in the gut. The large amount of virus present during acute infection may result in increased apoptotic death of CD4+ T cells by cross-linking of CD4 on the cell membrane [87]. Furthermore, we recently observed that CCR5+ CD4+ T cells preferentially expressed the receptor for IFN-α/β, and may therefore be particularly susceptible to the pro-apoptotic effect of type I IFN [88]. ART efficiently inhibits HIV-1 replication, potentially preventing both the direct and indirect mechanisms of CD4+ T-cell depletion, allowing for partial reconstitution of CD4+ T cells (reviewed in [89]). However, in primary SIV infection of rhesus macaques, early ART treatment did not prevent the rapid loss of gut CD4+ T cells from the gut mucosa [90]. Instead, early inhibition of viral replication positively affected the repopulation of the mucosal T-cell pool by the central memory CD4+ T-cell subset. Thus, the initial burst of viral replication may not only cause the unavoidable depletion of mucosal CD4+ T cells, but also alter the general dynamics of different T-cell subsets migrating to and from the gut.
However, severe and acute gut-associated lymphoid tissue (GALT) depletion is a feature shared by pathogenic and nonpathogenic infections, as shown in SIV-infected African green monkeys and sooty mangabeys [91, 92]. Furthermore, in a few chronically infected sooty mangabeys, persistent and generalized loss of CD4+ T cells did not lead to AIDS and increased immune activation [93]. The compromised function of the gastrointestinal mucosa due to the infection and depletion of CCR5+ CD4+ T cells may result in leakage of microbial bioproducts from the gut to the circulation, thereby contributing to chronic activation and exhaustion of the immune system [94]. Of interest, despite suffering a similar pattern of mucosal CD4 T-cell depletion, SIV-infected African green monkeys and sooty mangabeys do not show signs of microbial translocation [94]. Taken together, these findings suggest that CD4 depletion in the GALT is not intrinsically sufficient for the development of pathogenic HIV/SIV infection.
Direct effects of HIV-cell interactions: gp120-CD4
The peculiar lymphotropic nature of HIV-1 not only accounts for its ability to infect immune cells, but also favours its interaction with surface molecules that subsequently signal through their normal transduction pathway, exerting a direct effect on leucocytes without productively infecting them.
A murine model showed in 1990 that cross-linking of CD4 on the surface of CD4+ T lymphocytes primes the target cells to activation-induced apoptosis [95]. Similar results were observed for gp120-mediated cross-linking of CD4 in human T lymphocytes [87]. These apoptotic events appear to be mediated by ligand receptor systems of the tumour necrosis factor superfamily [87, 95]. In addition, CD4 cross-linking induces FasL expression not only on T cells but also on monocytes/macrophages, which could indirectly contribute to the apoptotic death of uninfected CD4+ T cells [96].
Binding and cross-linking of CD4 may have different effect on different subsets of CD4+ T cells. Thus, although CD4+ Th cells show increased susceptibility to apoptosis upon CD4 cross-linking, the same molecular event promotes survival of the suppressive Treg subset, which appear to increase or accumulate in lymphoid organs during HIV/SIV infection, particularly in tissues exhibiting active viral replication [34, 36–39]. Interestingly, an increase in Treg frequency in the duodenum was observed in HIV-infected patients, but not in patients with norovirus intestinal infection [37], suggesting that the accumulation of Treg at this site may be characteristic of chronic HIV infection, not a common consequence of any viral intestinal infection. However, as noted above, Tregs were depleted in mucosal areas in a highly pathogenic model of SIV infection [63], which suggests that differences may be found depending on the severity of the infection.
Binding and cross-linking of CD4 affects the function of conventional CD4+ Th cells in normal uninfected individuals, inhibiting the upregulation of the activation marker, CD40L [51] and the formation of the immunological synapse (A. Nyakeriga, C.J. Fichtenbaum, J. Goebel, S. Nicolaou, L. Conforti and C. Chougnet, unpublished data). In contrast, Treg suppressive function is not inhibited by in vitro exposure to HIV-1 [39]. These in vitro data suggest that CD4 engagement by HIV-1 may have a synergistic suppressive effect, by inhibiting the effector function of CD4+ Th cells whilst promoting the suppressor activity of Treg.
Human immunodeficiency virus-induced activation of pDC also requires the binding of HIV-1 to CD4 via gp120 [23, 35]. The turnover of CD4 on the surface of pDC is more rapid than on CD4 T lymphocytes due to the lack of the CD4-associated protein kinase p56lck in pDC, which allows for efficient and rapid clathrin-dependent endocytosis of CD4 molecules [97, 98]. Thus, the peculiar ability of HIV-1 to bind CD4 may also promote the endocytotic process and therefore the activation of pDC [99]. In vitro studies reported that type I IFN protect CD4+ T cells and monocytes/macrophages against HIV-1 infection, and limit replication in chronically infected cell lines [100]. IFNs block an early step in the HIV-1 replication cycle, and inhibit viral assembly and release, depending on the target cell and the type of infection (reviewed in [101]). To what extent the production of type I IFN is protective or pathogenic in vivo is complex to understand, and needs to consider the fact that the antiproliferative and proapoptotic effects of IFN-α/β are expected to affect uninfected cells, the number of which largely exceeds that of infected cells (reviewed in [27]).
Direct effects of HIV-cell interactions: gp120-CCR5/CXCR4
The HIV-1 envelope also triggers signal transduction through binding to chemokine receptors on the surface of the target cell [102]. CCR5 or CXCR4 engagement by HIV envelope results in altered chemotaxis and trafficking as well as alterations of cell cycle [103, 104]. CCR5 cross-linking on monocytes and macrophages triggers production of IL-1β, TNF-α and CCL2, and maturation into DC [102, 105–108]. Interestingly, CCR5-gp120 interactions activate nuclear factor kB via phosphatidylcholine-specific phospholipase C, whereas the interaction of CCR5 with its natural ligand CCL4 (macrophage inflammatory protein-1β) does not induce such effect [106]. This finding suggests that the signalling pathways activated by HIV-1 differ from those physiologically associated with CCR5 function, rendering this potentially pathogenic interaction unique to HIV-1 [106].
Direct effects of HIV-cell interactions: gp120-α4β7
Human immunodeficiency virus-cell interaction also involves an activated form of the heterodimeric integrin, α4β7, normally associated with mucosal localization of lymphocytes, inducing increased lymphocyte function-associated antigen-1 (LFA-1) expression on CD4+ T cells, which favours the formation of the virus-cell synapse [109]. However, whether the α4β7-gp120 interaction and subsequent signalling play direct role in the dysfunction of CD4+ T cells or of other immune cells is still unknown.
Direct effects of HIV-cell interactions: gp120-mannose C-type lectin receptor
HIV-1 gp120s interact with lectin receptors on DCs. Depending on the gp120 mannose moieties, such interactions will induce or not IL-10 production. In addition, gp120-treated DCs respond poorly to maturation stimuli and these adverse reactions are mannose C-type lectin receptor-dependent but independent of IL-10 production [110].
Immunological effects of other HIV-1 proteins
Nef and Tat are viral proteins that regulate HIV-1 replication. They are expressed by infected cells, and they can be released in the extracellular environment and penetrate bystander cells [111, 112]. Results from the Sydney Cohort as well as data on infection of rhesus macaques with Nef-deleted SIV support an immune-suppressive role for Nef [113, 114]. Nef-induced downmodulation of MHC class I expression by infected cells reduce their susceptibility to CTL-mediated killing [115]. Nef can directly inhibit CD4+ T-cell function by disturbing tyrosine phosphorylation at the immunological synapse [116] and by inducing CD4+ T-cell apoptosis through membrane permeabilization [117]. Downregulation of DC antigen-capture function and suppression of immunoglobulin class switch are other Nef-mediated immune-suppressive mechanisms (reviewed in [112, 118]). However, recent studies showed that in natural nonpathogenic SIV infection, Nef contributes to protect against disease progression by reducing CTL-mediated lysis of infected CD4+ T cells, preventing activation-induced cell death and suppressing PD-1 induction [119]. Similar to Nef, Tat was shown to repress MHC-I gene promoter activity. In addition, Tat induces upregulation of several immune suppressive cytokines, such as TGF-β and IL-10 in vitro, and impairs the production of IL-12 (reviewed in [111]).
HIV-induced dysregulation: what other chronic infections have taught us?
Many infections become chronic in human hosts. Studying how the immune system responds to other chronic infections could help illuminate the respective contribution of chronicity versus HIV-specific pathways during HIV-1 infection. However, it is difficult to draw strong conclusions from the comparison of different infections because each microbe comes with its specific arsenal of immune-modulatory molecules and its peculiar survival strategy. Nevertheless, the following review highlights several characteristics of selected chronic human infections that do not induce severe immune deficiency.
Persistent viral infections with low viral loads
Many human viruses persist for life, but with low levels of viral replication that in normal circumstances remain below the detection threshold. Those viruses do not cause immune suppression. Amongst them, cytomegalovirus (CMV) and Epstein-Barr virus have been extensively studied. The most notable aspect of the immune response to these viruses is the size of the T cell response, particularly the CD8+ T-cell responses, which increase throughout life and can dominate the entire repertoire in the elderly (reviewed in [120]). Interestingly, specific antiviral T cells from CMV viraemic patients exhibit defective function, notably PD-1 upregulation and low cytokine production, suggesting that chronic exposure to high viral loads induce defective CD8 function, whatever virus is involved [121]. Amongst the low viral load persistent viruses, human T-cell lymphotropic virus (HTLV-1) is of particular interest for HIV-1 research because, despite its cell tropism (mainly CD4+ T cells) and its integration into the DNA of host cells, the immense majority of HTLV-1-infected persons remain lifelong asymptomatic carriers. One of the major differences with HIV-1 is that heparan sulfate proteoglycans, not CD4 or chemokine receptors, mediate entry of HTLV-1 in primary CD4+ T cells [122]. Another distinguishing feature of HTLV-1 infection is that HTLV-1 exists in infected hosts predominantly as a provirus; most infected cells are produced by division of cells harbouring proviruses rather than de novo infections. The current model is that HTLV-1 Tax drives mitosis of infected CD4+ T cells but most HTLV-1-infected CD4+ T cells are killed by specific CTL, thus establishing a dynamic equilibrium between HTLV-1 and the immune system [123, 124]. HTLV-1 regulation of Tregs is controversial. One group reported that HTLV-1 associated myelopathy/tropical spastic parapesia (HAM/TSP) is associated with a low percentage of circulating CD4+CD25+ that express FoxP3 [125]. Moreover, HTLV-1 Tax was shown to inhibit FoxP3 expression and Treg function [126], suggesting that impaired FoxP3 expression contributes to the development of inflammatory HTLV-1 associated disease. However, a recent study reported a high percentage of FoxP3+CD4+ cells (independent of their CD25 expression) in HAM/TSP patients, and FoxP3 expression was negatively correlated with CTL-mediated lysis of HTLV-1-infected cells ex vivo [127]. Some evidence also suggests high expression of IDO in HTLV-1-associated adult T-cell leukaemia cells [128].
An interesting case of chronic infection with low viral load is that of HIV-2. HIV-2 has very similar characteristics to HIV-1, in that it infects CD4+ cells, and can cause AIDS with symptoms identical to the disease caused by HIV-1. However, HIV-2 infection rarely progresses to AIDS and the viral load remains low for prolonged period of time. Progression of HIV-2 infection to AIDS can occur many years after infection and is probably associated with the weakening of the immune system during ageing. In patients who develop AIDS, plasma HIV-2 levels are comparable to those observed in HIV-1-infected AIDS patients, and the symptoms of the disease are undistinguishable [129–131]. The reasons for the difference in disease progression between HIV-1 and HIV-2 reside clearly in the different level of viral replication, but why HIV-2 does not replicate as efficiently as HIV-1 in humans is not clearly established [130]. The finding that stronger T-cell responses are mounted against HIV-2 compared with those against HIV-1 suggest that similar to HIV-1-infected LTNP, HIV-2-infected patients develop an efficient T-cell-mediated immune response [130, 132]. However, the current knowledge of HIV-2 infection, primarily based on comparisons between HIV-1-and HIV-2-infected patients does not discriminate between whether the reduced viral replication is due to the stronger immune response or this apparently efficient immune response is a mere consequence of the low levels of virus, and therefore of the lack of virus-induced pathogenesis.
Persistent viral infections with high viral loads
Chronic hepatitis induced by hepatitis B or C viruses are persistent viral infections with high viral loads. They are therefore of great interest in comparison to HIV. One main distinguishing feature from HIV-1 is that hepatitis C virus (HCV) and hepatitis B virus (HBV) preferentially infect hepatocytes. Although there is evidence for infection of peripheral mono-nuclear cells, these extrahepatic targets are thought to contribute little to the overall viral load (reviewed in [133]). Many T-cell defects described in chronic HIV-1 infection also affect virus-specific T cells during chronic hepatitis C (reviewed in [134, 135]). Patients with chronic hepatitis C exhibit weak and narrowly focused HCV-specific CD4+ T-cell responses. Specific CD8+ T cells, particularly intrahepatic ones exhibit reduced cytotoxicity, low capacity to proliferate, low production of cytokines and profile of ‘exhausted’ cells (high CTLA-4, high PD-1, low CD27 and low CD28 expression), similar to HIV-specific CD8+ T cells. However, in contrast to chronic HIV-1 infection, only HCV-specific responses are affected, and responses against other antigens are maintained [136–138]. Patients with chronic hepatitis B and C exhibit high frequencies of suppressive Tregs in the circulating blood as well as in the liver. Expansion of other regulatory subsets such as IL-10-producers or FoxP3+ CD8+ T cells was also described (reviewed in [139]). These regulatory subsets were found in liver areas exhibiting low cellular apoptosis and fibrosis, supporting a complex role for regulatory subsets. In chronic hepatitis C, IDO expression was upregulated in the liver and was associated with increased serum kynurenine/tryptophan ratio [140]. Of particular interest is the fact that increases in death molecules (TRAIL, TNF-R2 and FasL) in the plasma were found only during acute HIV and not during acute HBV/HCV [141].
Nonviral chronic infections with high antigenic loads: the example of chronic helminth infections
Helminths are long-lived organisms that induce chronic infections in humans. Although they vary greatly in their biology, helminths induce relatively similar immune responses in their human hosts, which are characterized by a skewing towards a type-2 profile as well as switching towards IgE antibody production (reviewed in [142]). Chronic helminth infections induce immune suppressive mechanisms, such as production of TGF-β and IL-10 or suppression of T-cell activation by ‘nematode-induced macrophages’ (reviewed in [143]). Although chronic helminth infections do not cause severe immune deficiency, in vitro findings as well as in vivo experimental models suggest that the immune system chronically exposed to high parasite burdens becomes progressively hyporesponsive, first to parasite antigens, then to bystander antigens. However, the relevance of such experimental models is not clear because epidemiological studies have not clearly documented an impact of chronic helminth infections on the course of other diseases, such as malaria, tuberculosis or HIV/AIDS (reviewed in [142, 144]).
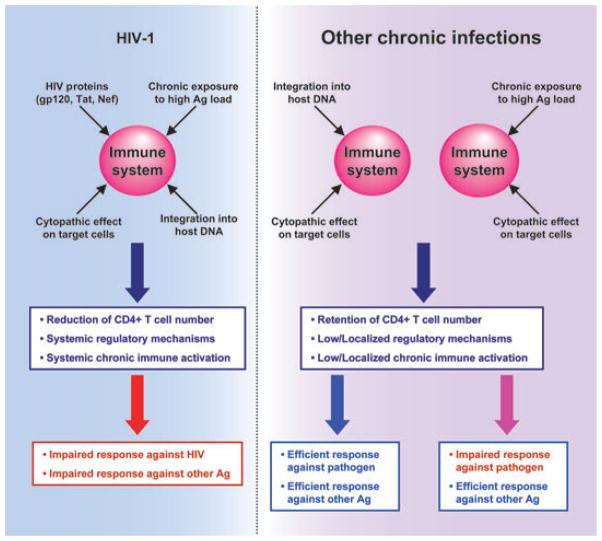
In conclusion, certain chronic infections persist at very low levels and do not induce significant damage to the immune system, even if caused by microbes that target CD4+ T cells and can integrate into the host’s DNA (the case of HTLV-1 or HIV-2). In contrast, chronic exposure to high antigenic loads induces ‘exhaustion’ of CD4+ and CD8+ T cells, similar to the exhausted anti-HIV-1 responses. However, in contrast to what occurs during chronic HIV-1 infection, such defects affect almost exclusively, the responses specific to the persistent microbe and rarely extend to bystander antigens (Fig. 1). Two main potentially synergistic reasons may underlie such a major difference: (i) the main target cells of those persistent microbes are not immune cells; and (ii) the infection is anatomically compartmentalized (HCV/HBV in the liver, helminth infections in the liver, gut or urinary tract). Interestingly, defective bystander responses have been described in the case of filariasis, which are not compartmentalized (reviewed in [145]). Of note, most chronic infections induce local expansion of regulatory subsets and production of immune-modulatory cytokines. These pathways play a complex role, protecting the host against the effect of immune-mediated damage on one hand, but dampening protective immune responses, and thus favouring microbe persistence on the other hand.
Fig. 1.
Impairment of immune function by human immunodeficiency virus (HIV)-1. In contrast to other chronic infections, HIV-1 attacks the immune system via multiple mechanisms, some of which are specific (direct deleterious effect of HIV-1 proteins and destruction of immune cells), and some of which are nonspecific (chronic exposure to high Ag load or cytopathic effect). Limiting the specific deleterious effects of HIV-1 may represent a better approach for immune-based therapies than trying to interfere with feedback mechanisms induced by chronic exposure to high Ag load. These specific deleterious effects of HIV also constitute challenges for the development of protective vaccines.
Potential clinical interventions
The realization that HIV-1 infection activates different immune regulatory mechanisms (see above) presents HIV/AIDS immunologists with a spectrum of seemingly formidable challenges that impact efforts to develop effective strategies for both immune-based therapy and AIDS vaccines. At present, neither attempts at reversing HIV-induced immune dysfunction nor vaccine-induced prevention of infection have been successful. Concerning the three questions indicated in the title of this review, we are still at the stage of learning to know it, despite more than two decades of ongoing attempts to fix it and prevent it. A critical issue is whether we yet know it well enough to begin considering logical approaches that might allow us to effectively fix it and/or prevent it. This section will consider some such issues from a clinical perspective.
Therapeutic approaches
Early assays at correcting the immunological ravages resulting from HIV-1 infection involved attempting to restore CD4+ T-cell numbers and function without knowing the causes of their depletion and loss. More recent studies of HIV-induced pathogenesis suggest that much of the immune suppression associated with HIV-1 infection is due to HIV’s remarkable ability to efficiently hijack pre-existing, physiological mechanisms that normally function to: (i) inhibit infection by different viruses [146]; (ii) prevent autoimmune diseases or excessive inflammatory responses [27, 88, 147]; and (iii) prevent rejection of allogeneic foetuses [148]. As discussed above, to activate some of these pathways, HIV-1 is required to only bind cellular receptors, and not to reverse transcribe and productively infect CD4+ target cells.
Because several regulatory pathways are functionally altered, it is hard to establish which of them may be the most suitable target for immunotherapy. For example, interventions against the PD-1/PDL-1 system may not correct the Treg-induced dysfunction, whilst inhibition of CTLA-4-mediated negative signalling would not protect from HIV-induced apoptosis. Antibodies against type I IFN would potentially block HIV-induced TRAIL and PDL-1 [31, 55, 88], but would not be expected to control HIV-induced IDO expression or Treg function [149]. 1-Methyl tryptophan (1MT) is effective in blocking IDO enzymatic activity in vitro, even in the setting of HIV-1 infection [35, 149] and has the potential for also influencing Treg function, similar to that shown in other systems [150], but would not affect HIV-induced apoptosis. An example of the failed attempts made to block one of the immune regulatory mechanisms usurped by HIV-1 is the use of anti-CTLA-4 blocking antibodies in SIV-infected macaques. Treatment of chronically SIV-infected, ART-treated macaques with a single dose of anti-CTLA-4 appeared to have marginal beneficial effects on antiviral immune responses [65]. However, administration of three doses of anti-CTLA-4 to SIV-infected macaques during primary or chronic infection increased T-cell activation and viral replication, reduced responsiveness to ART and prevented therapeutic vaccine reduction of the viral set-point [151]. More critically, none of the above strategies would discriminate between HIV-induced and physiologically controlled immune regulation. Therefore, the emergence of autoimmune diseases is a great concern when these approaches are considered for the treatment of HIV-infected patients. In this context, it should be noted that treatment of melanoma patients with anti-CTLA-4 antibody resulted in the induction of autoimmune symptoms [152, 153].
Therefore, alternative strategies that selectively target the HIV-induced components of these immune-regulatory mechanisms need to be developed. Approaches aimed at inhibiting the activity of viral protein involved in HIV-1 pathogenesis such as Nef and Tat represent intriguing possibilities to correct some of the specific deleterious effects of the virus. Another such selective approach, with a potentially broad ‘correcting’ ability, would be to block the binding of HIV-1 gp120 to cellular CD4 with soluble CD4-IgG (sCD4-IgG). This molecule blocks in vitro HIV-induced immune defects, such as pDC activation, CD4+ helper T-cell dysfunction and increased Treg survival [35, 39, 51, 87, 154]. Furthermore, by interfering with the attachment of HIV-1 to the target CD4+ T cells, sCD4-IgG should directly limit the infection of new cells and indirectly inhibit the immunopathogenic mechanisms caused by de novo synthesized Nef and Tat. Of interest is the fact that sCD4-IgG partly restored Th cell function when administered to AIDS patients [155]. sCD4-IgG could constitute an effective immunotherapy agent because it might not compromise mechanisms critical for the regulation of self-reactive T cells, in contrast to 1-MT or blocking antibodies such as anti-CTLA-4 or anti-PD1/PD-L1. However, the requirement for intravenous administration of sCD4-IgG renders it an inconvenient therapeutic agent, and nothing is known about how chronic administration of sCD4-IgG might alter the physiological function of cellular CD4.
Reports indicating that HIV-induced immune activation and expression of immune-regulatory molecules depend on viral endocytosis by pDC [23, 24, 88] suggest another strategy to reduce HIV-induced immunopathogenesis. Endocytosis of HIV-1 by pDC can be blocked using endosomal acidification inhibitors such as chloroquine [23, 24, 88]. This approach should prevent HIV-1 from activating the immune downregulatory components that depend on pDC activation (such as IFN-α/β-regulated genes and IDO). Chloroquine decreases proinflammarory cytokines in mice treated with CpG oligodeoxynucleotides and lipopolysaccharide (LPS) [156], and it has direct antiviral activity [157, 158]. Chloroquine is inexpensive and has been administrated to millions of people worldwide, and it will soon be tested in a NIAID-sponsored phase II trial for inhibition of HIV-1 replication and immune activation in HIV-infected patients. However, this approach is expected to be ineffective against immune alterations that do not depend on pDC activation. Furthermore, no prediction can be made on its potential effects on antigen presentation by other DCs.
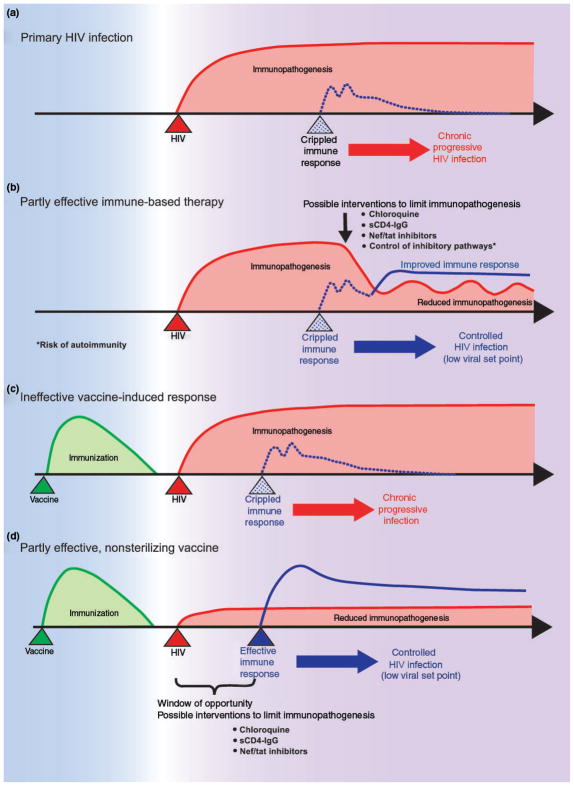
In conclusion, the twin questions that we, as many HIV/AIDS immunologists, struggle to resolve are: first, how much intervention is enough to transitorily or permanently turn chronically infected individuals into LTNPs? The different approaches tried so far have failed to achieve this goal. Extensive effort is currently directed in blocking the PD-1/PD-L1 pathway and may prove successful, although this strategy affects only the effector arm and not the causes of immune suppression. Second, how much damage might a broad approach inflict on chronically infected patients? Several general immune-based approaches used in the past in other fields had devastating consequences, which serve as a cautionary reminder that the immune system is a very complex machine that cannot be easily altered to the overall advantage of the patient. However, it is our belief that ‘knowing’ better how HIV-1 specifically interacts with immune cells, and how the regulatory pathways are linked together, will make it possible to find ways to stay on the narrow road of ‘fixing it’ (Fig. 2a,b).
Fig. 2.
Human immunodeficiency virus (HIV) immunopathogenesis, its influence on vaccine-induced responses and possible interventions to limit it. a) HIV-induced immunopathogenesis cripples immune responses thus resulting in chronic progressive infection. b) Immune-based therapy aimed at limiting HIV-induced immunopathogenesis may allow a partial recovery of immune responses which control viral replication and disease progression. These potential therapeutic approaches include blockade of HIV interaction with the immune system and manipulation of physiological inhibitory pathways, which carry the risk of inducing autoimmune reactions. c) Vaccine-induced anti-HIV immune responses may also be suppressed by HIV-induced immunopathogenesis since the early stages of infection. d) Rapid therapeutic interventions aimed at inhibiting the interaction of HIV with the immune system may allow the vaccine-induced response to develop normally and to effectively control viral replication and disease progression.
Prophylactic approaches
The failure of AIDS vaccine trials was acknowledged at the recent NIAID-sponsored summit on AIDS vaccines (Bethesda, MD, 25 March 2008). Why have none of these AIDS vaccine trials been protective? Possible reasons include: (i) sterilizing immunity may be necessary but not achievable, at least with our current knowledge; (ii) correlates of protective immunity have not yet been identified or refined; (iii) integration of HIV-1 genome into host CD4+ cells may result in the early creation of a ‘reservoir’ of virus, hidden from the immune system; and (iv) the rapid rate of infection and activation of immune-suppressive mechanisms at the site of entry may block even potentially efficacious vaccines. Because this review is focused on mechanisms of HIV-induced immune suppression, we will limit this brief vaccine-related discussion to the last above-mentioned reason for AIDS vaccine failure.
Human immunodeficiency virus-1 vaccination itself might result in suboptimal immunization due to vaccine- and/or adjuvant-induced activation of the above-noted immune-regulatory mechanisms. Treg induction/expansion as well as induction of PD-1 is a potential concern. AIDS vaccine trials should include screening for activation of such mechanisms, including changes in the number and activity of different regulatory subsets. Each antigen/vaccine combination strategy needs to be individually tested. As an example, DNA/modified vaccinia virus Ankara delivery of one Leishmania antigen drove an IL-10-secreting Treg response, whereas the similar delivery of another Leishmania antigen induced protective IFN-γ responses [159]. Due to the in vitro immune suppressive properties of HIV-1 gp120/gp160, its inclusion in a vaccine may be detrimental, although it did not appear to be the case in an SIV vaccine/chimeric simian-human immunodeficiency virus challenge setting [160]. If a vaccine activates these immune-suppressive mechanisms, it could be followed by a compound that prevents activation of the immunesuppressive effects. However, strategies of regulation/counter-regulation are fraught with difficulties due to the presence of interacting factors, such as age, concurrent infections and genetic variability that render it arduous to establish the correct timing and doses.
Human immunodeficiency virus-1 prophylactic vaccines that optimally reduce the viral set-point could be envisioned, although the social and economic implications of such a strategy are so complex that the discussion about this approach must go well beyond scientific discussion. However, HIV-mediated activation of immune-suppressive mechanisms might also be induced by HIV-1 exposure or infection at the time of challenge and ruin such a strategy [161]. If the memory immune response is not activated with sufficient speed and strength to prevent infection upon challenge, activated memory CD4+ T cells, including those specific for HIV-1, will provide the virus with a pool of targets at the site of entry. Concurrently, HIV-1 will activate multiple mechanisms of immune suppression. The sum of these events will probably compromise a primed immune system that might otherwise have been effective. Consistent with this concern is a recent study of acutely infected patients, which concluded that the speed with which HIV-1 could activate immune-suppressive mechanisms could place critical time restraints on the window of opportunity that an AIDS vaccine has to block infection [141]. In support of this critical time issue, a recent study by Estes and Haase (presented in this symposium) indicates that SIV activates type I IFN expression in the endocervical epithelium, within 3 h of intravaginal SIV inoculation. One potential intervention to counteract such deleterious effect would be that vaccine participants could receive immune-based therapy immediately after detection of infection, using a drug such as chloroquine that would potentially block activation of HIV-induced immune-suppressive mechanisms. This additional therapeutic step might ‘rescue’ the remaining primed immune system to continue the fight against HIV-1 and convert infected vaccine participants into LTNPs (Fig. 2c,d).
We believe that the most difficult obstacle to overcome resides in our incomplete comprehension of the complex virus-host interactions, which eventually lead to the disarray of the immune function. This unawareness prevents us from identifying the ideal targets for immune-based therapies, and decreases our chances of generating a protective vaccine-induced immune response, which will survive the deleterious effect of being challenged by HIV. Therefore, research efforts should now be primarily directed in supporting basic research in HIV-1 pathogenesis in order to make significant progress in the future.
Acknowledgments
A.B. was supported by the Wellcome Trust (value in people award DQAD-P04191) and by the Westminster Medical School Research Trust (PMS/MMS-07/08-10124); G.M.S. was supported by the Intramural research Program of the CCR, NCI and by the Intramural AIDS Targeted Antiviral Program (IATAP); C.C. was supported by grants from the National Institutes of Health (AI 056927 and AI068524).
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Mildvan D, Mathur U, Enlow RW, et al. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982;96:700–4. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- 2.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–26. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 4.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853–85. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 5.Bonaparte MI, Barker E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS. 2003;17:487–94. doi: 10.1097/00002030-200303070-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–94. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 7.Tasca S, Tambussi G, Nozza S, et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS. 2003;17:2291–8. doi: 10.1097/00002030-200311070-00003. [DOI] [PubMed] [Google Scholar]
- 8.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 9.Kottilil S, Chun TW, Moir S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–45. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 10.Kottilil S, Shin K, Planta M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004;189:1193–8. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 11.Valentin A, Rosati M, Patenaude DJ, et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2002;99:7015–20. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandvaux N, tenOever BR, Servant MJ, Hiscott J. The interferon antiviral response: from viral invasion to evasion. Curr Opin Infect Dis. 2002;15:259–67. doi: 10.1097/00001432-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–81. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Wang FS. Plasmacytoid dendritic cells act as the most competent cell type in linking antiviral innate and adaptive immune responses. Cell Mol Immunol. 2005;2:411–7. [PubMed] [Google Scholar]
- 16.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman S, Stein D, Amrute S, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–10. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 18.Hosmalin A, Lebon P. Type I interferon production in HIV-infected patients. J Leukoc Biol. 2006;80:984–93. doi: 10.1189/jlb.0306154. [DOI] [PubMed] [Google Scholar]
- 19.Martinson JA, Tenorio AR, Montoya CJ, et al. Impact of class A, B and C CpG-oligodeoxynucleotides on in vitro activation of innate immune cells in human immunodeficiency virus-1 infected individuals. Immunology. 2007;120:526–35. doi: 10.1111/j.1365-2567.2007.02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–12. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 21.Feldman SB, Milone MC, Kloser P, Fitzgerald-Bocarsly P. Functional deficiencies in two distinct interferon alpha-producing cell populations in peripheral blood mononuclear cells from human immunodeficiency virus seropositive patients. J Leukoc Biol. 1995;57:214–20. doi: 10.1002/jlb.57.2.214. [DOI] [PubMed] [Google Scholar]
- 22.Howell DM, Feldman SB, Kloser P, Fitzgerald-Bocarsly P. Decreased frequency of functional natural interferon-producing cells in peripheral blood of patients with the acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1994;71:223–30. doi: 10.1006/clin.1994.1076. [DOI] [PubMed] [Google Scholar]
- 23.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–75. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci USA. 2007;104:17453–8. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foussat A, Bouchet-Delbos L, Berrebi D, et al. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1-infected patients. Blood. 2001;98:1678–86. doi: 10.1182/blood.v98.6.1678. [DOI] [PubMed] [Google Scholar]
- 26.Herbeuval JP, Nilsson J, Boasso A, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci USA. 2006;103:7000–5. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–8. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE. 2007;2:e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–99. [PubMed] [Google Scholar]
- 30.Tilton JC, Manion MM, Luskin MR, et al. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol. 2008;82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbeuval JP, Hardy AW, Boasso A, et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2005;102:13974–9. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallarino F, Gizzi S, Mosci P, Grohmann U, Puccetti P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab. 2007;8:209–16. doi: 10.2174/138920007780362581. [DOI] [PubMed] [Google Scholar]
- 33.Schroecksnadel K, Zangerle R, Bellmann-Weiler R, Garimorth K, Weiss G, Fuchs D. Indoleamine-2, 3-dioxygenase and other interferon-gamma-mediated pathways in patients with human immunodeficiency virus infection. Curr Drug Metab. 2007;8:225–36. doi: 10.2174/138920007780362608. [DOI] [PubMed] [Google Scholar]
- 34.Andersson J, Boasso A, Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–7. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 35.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boasso A, Vaccari M, Hryniewicz A, et al. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J Virol. 2007;81:11593–603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epple HJ, Loddenkemper C, Kunkel D, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072–8. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 38.Estes JD, Li Q, Reynolds MR, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–12. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–17. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–44. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 41.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–4. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 42.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 43.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–68. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 45.Shearer GM. HIV-induced immunopathogenesis. Immunity. 1998;9:587–93. doi: 10.1016/s1074-7613(00)80656-1. [DOI] [PubMed] [Google Scholar]
- 46.Shearer GM, Clerici M. Early T-helper cell defects in HIV infection. AIDS. 1991;5:245–53. doi: 10.1097/00002030-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Dolan MJ, Clerici M, Blatt SP, et al. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 48.Miedema F, Meyaard L, Koot M, et al. Changing virus-host interactions in the course of HIV-1 infection. Immunol Rev. 1994;140:35–72. doi: 10.1111/j.1600-065x.1994.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 49.Palmer BE, Boritz E, Wilson CC. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J Immunol. 2004;172:3337–47. doi: 10.4049/jimmunol.172.5.3337. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Schmitz JE, Acierno PM, et al. Dysfunction of simian immunodeficiency virus/simian human immunodeficiency virus-induced IL-2 expression by central memory CD4+ T lymphocytes. J Immunol. 2005;174:4753–60. doi: 10.4049/jimmunol.174.8.4753. [DOI] [PubMed] [Google Scholar]
- 51.Zhang R, Fichtenbaum CJ, Hildeman DA, Lifson JD, Chougnet C. CD40 ligand dysregulation in HIV infection: HIV glycoprotein 120 inhibits signaling cascades upstream of CD40 ligand transcription. J Immunol. 2004;172:2678–86. doi: 10.4049/jimmunol.172.4.2678. [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 53.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 54.D’Souza M, Fontenot AP, Mack DG, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–87. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 55.Herbeuval JP, Grivel JC, Boasso A, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–31. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bostik P, Wu P, Dodd GL, et al. Identification of protein kinases dysregulated in CD4(+) T cells in pathogenic versus apathogenic simian immunodeficiency virus infection. J Virol. 2001;75:11298–306. doi: 10.1128/JVI.75.23.11298-11306.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cayota A, Vuillier F, Siciliano J, Dighiero G. Defective protein tyrosine phosphorylation and altered levels of p59fyn and p56lck in CD4 T cells from HIV-1 infected patients. Int Immunol. 1994;6:611–21. doi: 10.1093/intimm/6.4.611. [DOI] [PubMed] [Google Scholar]
- 58.Schweneker M, Favre D, Martin JN, Deeks SG, McCune JM. HIV-induced changes in T cell signaling pathways. J Immunol. 2008;180:6490–500. doi: 10.4049/jimmunol.180.10.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndhlovu LC, Chapman JM, Jha AR, et al. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS. 2008;22:990–2. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cecchinato V, Trindade CJ, Laurence A, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunology. 2008;1:279–88. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chase AJ, Sedaghat AR, German JR, et al. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2007;81:12748–57. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin S, Sui Y, Soloff AC, et al. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J Immunol. 2008;180:5530–6. doi: 10.4049/jimmunol.180.8.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hryniewicz A, Boasso A, Edghill-Smith Y, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–42. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA. 2007;104:3390–5. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eggena MP, Barugahare B, Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–14. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 68.Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J Leukoc Biol. 2008;83:254–62. doi: 10.1189/jlb.0507281. [DOI] [PubMed] [Google Scholar]
- 69.Estes JD, Wietgrefe S, Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–61. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 70.Levy JP. Questions about CD8+ anti-HIV lymphocytes in the control of HIV infection. Antibiot Chemother. 1996;48:13–20. doi: 10.1159/000425153. [DOI] [PubMed] [Google Scholar]
- 71.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–7. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 72.Cao Y, Ho DD, Todd J, et al. Clinical evaluation of branched DNA signal amplification for quantifying HIV type 1 in human plasma. AIDS Res Hum Retroviruses. 1995;11:353–61. doi: 10.1089/aid.1995.11.353. [DOI] [PubMed] [Google Scholar]
- 73.Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 74.Rinaldo C, Huang XL, Fan ZF, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–42. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Betts MR, Exley B, Price DA, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci USA. 2005;102:4512–7. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrer T, Harrer E, Kalams SA, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–92. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 77.Hay CM, Ruhl DJ, Basgoz NO, et al. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J Virol. 1999;73:5509–19. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masemola A, Mashishi T, Khoury G, et al. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J Virol. 2004;78:3233–43. doi: 10.1128/JVI.78.7.3233-3243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersson J, Kinloch S, Sonnerborg A, et al. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J Infect Dis. 2002;185:1355–8. doi: 10.1086/340124. [DOI] [PubMed] [Google Scholar]
- 80.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 81.Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–8. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 82.Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol. 2000;67:607–14. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- 83.Kaech SM, Ahmed R. Immunology. CD8 T cells remember with a little help. Science. 2003;300:263–5. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 84.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 85.Siliciano RF. The role of CD4 in HIV envelope-mediated pathogenesis. Curr Top Microbiol Immunol. 1996;205:159–79. doi: 10.1007/978-3-642-79798-9_8. [DOI] [PubMed] [Google Scholar]
- 86.Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci USA. 2006;103:3369–74. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banda NK, Bernier J, Kurahara DK, et al. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boasso A, Hardy AW, Landay AL, et al. PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin Immunol. 2008;129:132–44. doi: 10.1016/j.clim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soria A, Lazzarin A. Antiretroviral treatment strategies and immune reconstitution in treatment-naive HIV-infected patients with advanced disease. J Acquir Immune Defic Syndr. 2007;46:S19–30. doi: 10.1097/01.qai.0000286598.00313.a6. [DOI] [PubMed] [Google Scholar]
- 90.Verhoeven D, Sankaran S, Silvey M, Dandekar S. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J Virol. 2008;82:4016–27. doi: 10.1128/JVI.02164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gordon SN, Klatt NR, Bosinger SE, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–34. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pandrea IV, Gautam R, Ribeiro RM, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–46. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milush JM, Reeves JD, Gordon SN, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol. 2007;179:3047–56. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 94.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 95.Newell MK, Haughn LJ, Maroun CR, Julius MH. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990;347:286–9. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- 96.Oyaizu N, Adachi Y, Hashimoto F, et al. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis: a possible mechanism of bystander cell death in HIV infection. J Immunol. 1997;158:2456–63. [PubMed] [Google Scholar]
- 97.Pelchen-Matthews A, da Silva RP, Bijlmakers MJ, Signoret N, Gordon S, Marsh M. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur J Immunol. 1998;28:3639–47. doi: 10.1002/(SICI)1521-4141(199811)28:11<3639::AID-IMMU3639>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 98.Pitcher C, Honing S, Fingerhut A, Bowers K, Marsh M. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol Biol Cell. 1999;10:677–91. doi: 10.1091/mbc.10.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamamoto JK, Barre-Sinoussi F, Bolton V, Pedersen NC, Gardner MB. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986;6:143–52. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]
- 101.Pitha PM. Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antiviral Res. 1994;24:205–19. doi: 10.1016/0166-3542(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 102.Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, Collman RG. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol. 2003;74:676–82. doi: 10.1189/jlb.0503206. [DOI] [PubMed] [Google Scholar]
- 103.Cicala C, Arthos J, Martinelli E, et al. R5 and X4 HIV envelopes induce distinct gene expression profiles in primary peripheral blood mononuclear cells. Proc Natl Acad Sci USA. 2006;103:3746–51. doi: 10.1073/pnas.0511237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cicala C, Arthos J, Ruiz M, et al. Induction of phosphorylation and intracellular association of CC chemokine receptor 5 and focal adhesion kinase in primary human CD4+ T cells by macrophage-tropic HIV envelope. J Immunol. 1999;163:420–6. [PubMed] [Google Scholar]
- 105.Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG. Signaling mechanism of HIV-1 gp120 and virion-induced IL-1beta release in primary human macrophages. J Immunol. 2008;180:6675–84. doi: 10.4049/jimmunol.180.10.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fantuzzi L, Spadaro F, Purificato C, et al. Phosphatidylcholine-specific phospholipase C activation is required for CCR5-dependent, NF-kB-driven CCL2 secretion elicited in response to HIV-1 gp120 in human primary macrophages. Blood. 2008;111:3355–63. doi: 10.1182/blood-2007-08-104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nimura F, Zhang LF, Okuma K, et al. Cross-linking cell surface chemokine receptors leads to isolation, activation, and differentiation of monocytes into potent dendritic cells. Exp Biol Med (Maywood) 2006;231:431–43. doi: 10.1177/153537020623100409. [DOI] [PubMed] [Google Scholar]
- 108.Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol. 2005;78:1016–23. doi: 10.1189/jlb.0105056. [DOI] [PubMed] [Google Scholar]
- 109.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–9. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 110.Shan M, Klasse PJ, Banerjee K, et al. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huigen MC, Kamp W, Nottet HS. Multiple effects of HIV-1 transactivator protein on the pathogenesis of HIV-1 infection. Eur J Clin Invest. 2004;34:57–66. doi: 10.1111/j.1365-2362.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 112.Quaranta MG, Mattioli B, Giordani L, Viora M. The immuno-regulatory effects of HIV-1 Nef on dendritic cells and the pathogenesis of AIDS. FASEB J. 2006;20:2198–208. doi: 10.1096/fj.06-6260rev. [DOI] [PubMed] [Google Scholar]
- 113.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the Nef gene. Science. 1992;258:1938–41. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 114.Dyer WB, Geczy AF, Kent SJ, et al. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS. 1997;11:1565–74. doi: 10.1097/00002030-199713000-00004. [DOI] [PubMed] [Google Scholar]
- 115.Lewis MJ, Balamurugan A, Ohno A, Kilpatrick S, Ng HL, Yang OO. Functional adaptation of Nef to the immune milieu of HIV-1 infection in vivo. J Immunol. 2008;180:4075–81. doi: 10.4049/jimmunol.180.6.4075. [DOI] [PubMed] [Google Scholar]
- 116.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24:547–61. doi: 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 117.Laforge M, Petit F, Estaquier J, Senik A. Commitment to apoptosis in CD4(+) T lymphocytes productively infected with human immunodeficiency virus type 1 is initiated by lysosomal membrane permeabilization, itself induced by the isolated expression of the viral protein Nef. J Virol. 2007;81:11426–40. doi: 10.1128/JVI.00597-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–10. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 119.Schindler M, Schmokel J, Specht A, et al. Inefficient Nef-mediated downmodulation of CD3 and MHC-I correlates with loss of CD4+T cells in natural SIV infection. PLoS Pathog. 2008;4:e1000107. doi: 10.1371/journal.ppat.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koch S, Larbi A, Ozcelik D, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 121.Heintel T, Sester M, Rodriguez MM, et al. The fraction of perforin-expressing HIV-specific CD8 T cells is a marker for disease progression in HIV infection. AIDS. 2002;16:1497–501. doi: 10.1097/00002030-200207260-00006. [DOI] [PubMed] [Google Scholar]
- 122.Jones KS, Petrow-Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J Virol. 2005;79:12692–702. doi: 10.1128/JVI.79.20.12692-12702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene. 2005;24:6035–46. doi: 10.1038/sj.onc.1208970. [DOI] [PubMed] [Google Scholar]
- 124.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–81. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 125.Oh U, Grant C, Griffith C, Fugo K, Takenouchi N, Jacobson S. Reduced Foxp3 protein expression is associated with inflammatory disease during human t lymphotropic virus type 1 infection. J Infect Dis. 2006;193:1557–66. doi: 10.1086/503874. [DOI] [PubMed] [Google Scholar]
- 126.Yamano Y, Takenouchi N, Li HC, et al. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I-associated neuroimmunological disease. J Clin Invest. 2005;115:1361–8. doi: 10.1172/JCI23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Toulza F, Heaps A, Tanaka Y, Taylor GP, Bangham CR. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood. 2008;111:5047–53. doi: 10.1182/blood-2007-10-118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoshi M, Ito H, Fujigaki H, et al. Indoleamine 2,3-dioxygenase is highly expressed in human adult T-cell leukemia/lymphoma and chemotherapy changes tryptophan catabolism in serum and reduced activity. Leuk Res. 2008 Jul 16; doi: 10.1016/j.leukres.2008.05.023. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 129.Jaleco AC, Covas MJ, Victorino RM. Analysis of lymphocyte cell death and apoptosis in HIV-2-infected patients. Clin Exp Immunol. 1994;98:185–9. doi: 10.1111/j.1365-2249.1994.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rowland-Jones SL, Whittle HC. Out of Africa: what can we learn from HIV-2 about protective immunity to HIV-1? Nat Immunol. 2007;8:329–31. doi: 10.1038/ni0407-329. [DOI] [PubMed] [Google Scholar]
- 131.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–6. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 132.Leligdowicz A, Yindom LM, Onyango C, et al. Robust Gag-specific T cell responses characterize viremia control in HIV-2 infection. J Clin Invest. 2007;117:3067–74. doi: 10.1172/JCI32380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 134.Ishii S, Koziel MJ. Immune responses during acute and chronic infection with hepatitis C virus. Clin Immunol. 2008;128:133–47. doi: 10.1016/j.clim.2008.03.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis. 2007;27:152–60. doi: 10.1055/s-2007-979468. [DOI] [PubMed] [Google Scholar]
- 136.Chang KM, Thimme R, Melpolder JJ, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–76. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 137.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37, 1937. e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 139.Billerbeck E, Bottler T, Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13:4858–64. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Larrea E, Riezu-Boj JI, Gil-Guerrero L, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J Virol. 2007;81:3662–6. doi: 10.1128/JVI.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gasper-Smith N, Crossman DM, Whitesides JF, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma micro-particles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–10. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–90. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 143.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 144.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–84. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 145.Harnett W, Harnett MM. What causes lymphocyte hyporesponsiveness during filarial nematode infection? Trends Parasitol. 2006;22:105–10. doi: 10.1016/j.pt.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 146.Vilcek J. Boosting p53 with interferon and viruses. Nat Immunol. 2003;4:825–6. doi: 10.1038/ni0903-825. [DOI] [PubMed] [Google Scholar]
- 147.Boasso A, Vaccari M, Nilsson J, et al. Do regulatory T-cells play a role in AIDS pathogenesis? AIDS Rev. 2006;8:141–7. [PubMed] [Google Scholar]
- 148.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 149.Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS ONE. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor {zeta}-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 151.Cecchinato V, Tryniszewska E, Ma ZM, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–47. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 154.Herbeuval JP, Shearer GM. Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis? AIDS Rev. 2006;8:3–8. [PubMed] [Google Scholar]
- 155.Clerici M, Yarchoan R, Blatt S, et al. Effect of a recombinant CD4-IgG on in vitro T helper cell function: data from a phase I/II study of patients with AIDS. J Infect Dis. 1993;168:1012–6. doi: 10.1093/infdis/168.4.1012. [DOI] [PubMed] [Google Scholar]
- 156.Hong Z, Jiang Z, Liangxi W, et al. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol. 2004;4:223–34. doi: 10.1016/j.intimp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 157.Tsai WP, Nara PL, Kung HF, Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res Hum Retroviruses. 1990;6:481–9. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- 158.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–7. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J Immunol. 2005;175:2517–24. doi: 10.4049/jimmunol.175.4.2517. [DOI] [PubMed] [Google Scholar]
- 160.Liang X, Casimiro DR, Schleif WA, et al. Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys. J Virol. 2005;79:12321–31. doi: 10.1128/JVI.79.19.12321-12331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boasso A, Shearer GM, Clerici M. The hunt for an HIV vaccine: time to rethink recent failures. Lancet. 2008;371:1897–8. doi: 10.1016/S0140-6736(08)60812-0. [DOI] [PubMed] [Google Scholar]