Abstract
Forkhead L2 (FOXL2) is expressed in the ovary and acts as a transcriptional repressor of the steroidogenic acute regulatory (StAR) gene, a marker of granulosa cell differentiation. Human FOXL2 mutations that produce truncated proteins lacking the COOH terminus result in blepharophimosis/ptosis/epicanthus inversus (BPES) syndrome type I, which is associated with premature ovarian failure (POF). In this study, we investigated whether FOXL2's activity as a transcriptional repressor is regulated by phosphorylation. We found that FOXL2 is phosphorylated at a serine residue and, using yeast two-hybrid screening, identified LATS1 as a potential FOXL2-interacting protein. LATS1 is a serine/threonine kinase whose deletion in mice results in an ovarian phenotype similar to POF. Using coimmunoprecipitation and kinase assays, we confirmed that LATS1 binds to FOXL2 and demonstrated that LATS1 phosphorylates FOXL2 at a serine residue. Moreover, we found that FOXL2 and LATS1 are coexpressed in developing mouse gonads and in granulosa cells of small and medium follicles in the mouse ovary. Last, we demonstrated that coexpression with LATS1 enhances FOXL2's activity as a repressor of the StAR promoter, and this results from the kinase activity of LATS1. These results provide novel evidence that FOXL2 is phosphorylated by LATS1 and that this phosphorylation enhances the transcriptional repression of the StAR gene, a marker of granulosa cell differentiation. These data support our hypothesis that phosphorylation of FOXL2 may be a control mechanism regulating the rate of granulosa cell differentiation and hence, follicle maturation, and its dysregulation may contribute to accelerated follicular development and POF in BPES type I.
Keywords: large tumor suppressor gene 1 phosphorylation, transcriptional regulation, forkhead L2, granulosa cell, premature ovarian failure
premature ovarian failure (POF) is defined as a condition causing amenorrhea, hypoestrogenism, and elevated gonadotropins in women under 40 yr of age (1) and can be associated with failure to endow the follicle pool or an early loss of the fixed follicle pool following excess follicle recruitment and/or atresia. A genetic basis for selective cases of POF has been determined. Patients with blepharophimosis-ptosis-epicanthus inversus (BPES) syndrome type I exhibit POF in association with characteristic eyelid dysplasia, blepharophimosis, ptosis, and epicanthus inversus (60). Ovaries from BPES type I patients are histologically variable, ranging from the presence of some primordial follicles with atretic follicles to complete absence of follicles and scarring of the ovaries (23). The gene encoding the transcription factor forkhead L2 (FOXL2) (15) maps to the BPES locus on chromosome 3q22–3q23. FOXL2 is a member of the forkhead/hepatocyte nuclear factor 3 family of transcription factors (15), which is characterized by the presence of a conserved winged helix domain that is essential for DNA binding as well as more divergent transactivation or transrepression domains (12, 29, 33). FOXL2 mutations in individuals with BPES type I create premature stop codons that are predicted to generate truncated proteins lacking the carboxyl (COOH) terminus alanine/proline-rich domain (15–17, 44). Consistent with the BPES phenotype, FOXL2 is expressed selectively in the eyelids of developing mice (15) and in the mouse ovary (41). In addition, FOXL2 shows a highly specific expression pattern in undifferentiated granulosa cells of small and medium follicles in the mouse ovary but is not expressed in the other steroidogenic cells of the ovary, i.e., theca cells and luteal cells, nor is it expressed in nonovarian steroidogenic cells such as those of the testes or adrenal gland (15, 41).
We previously identified the COOH-terminal alanine/proline-rich region of FOXL2 as a transrepression domain (41). Furthermore, we showed that a human mutation that is associated with BPES type I produces a truncated FOXL2 protein that lacks this entire transrepression domain and loses its activity as a repressor of the StAR gene (41). StAR is a cholesterol transporter at the mitochondrial membrane and controls the rate-limiting step in steroidogenesis (14, 34, 49). It is present in the granulosa cell layer of large preovulatory and luteinized follicles and absent from immature follicles in several species (43, 46, 50). StAR activity is present in granulosa cells during follicular differentiation and after FOXL2 expression decreases, signaling early functional maturation of ovarian antral follicles (41, 46, 50). Thus, FOXL2 may inhibit premature differentiation of granulosa cells and control the number of primordial follicles that remain dormant, and mutations in FOXL2 may result in accelerated differentiation of granulosa cells, ultimately leading to POF.
A number of forkhead transcription factors have been shown to be regulated through phosphorylation, including members of the forkhead box O (FOXO) subfamily, which includes FOXO1, FOXO3, and FOXO4 (51). Outside the FOXO class, little is known about the regulation of other forkhead transcription factors via phosphorylation. Because premature follicle depletion is central to POF, we hypothesized that more refined regulatory processes may be involved in controlling FOXL2 activity. In this study, we tested whether FOXL2 is regulated through phosphorylation to begin to define the mechanisms underlying granulosa cell differentiation, follicle maturation, and premature ovarian failure.
MATERIALS AND METHODS
Yeast two-hybrid screening.
Yeast two-hybrid screening was performed using human FOXL2 as bait, and plasmids from positive colonies were isolated, purified, and sequenced (31). The specific interaction between wild-type FOXL2 and large tumor suppressor gene 1 (LATS1) was confirmed on the basis of activation of the GAL1-HIS3 reporter gene.
Plasmid construction.
A FLAG-tagged FOXL2 cDNA was generated as described previously (31). A 3.4-kb human LATS1 cDNA sequence was PCR amplified from human ovary cDNA (Ambion, Austin, TX), using the primers 5′-GTGGCGGCCGCATGAAGAGGAGTGAAAA-3′ and 5′-TCGCGATCTAGTATATGTTTAACTCGAGTGA-3′, and subcloned into pcDNA3 and pcDNA3.1-His-Xpress expression vectors using the NotI and XhoI restriction sites to generate LATS1 expression constructs. To generate the kinase-inactive LATS1 mutant D846A (13, 26), a residue in the LATS1 kinase domain, Asp 846, was mutated to Ala by performing site-directed mutagenesis using this pcDNA3.1-His-Xpress-LATS1 construct and the primer 5′-GGTCATATTAAATTGACTGCCTTTGGCCTCTGCACTGG-3′. The results were confirmed by sequencing.
Cell culture and DNA transfection.
Chinese hamster ovary (CHO) cells and FOXL2 stable CHO cells were grown in culture and transfected as described previously (31). The cells were then lysed using RIPA buffer, Tris buffer (50 mM Tris·HCl, 150 mM sodium chloride, pH 7.4), immunoprecipitation buffer, or reporter lysis buffer (Promega, Madison, WI) for subsequent experiments.
Alkaline phosphatase and phosphatase inhibitor treatments.
Cells were lysed with Tris buffer and heated at 95°C for 5 min to denature the proteins, and the cell lysates (60 μg) were resuspended in dephosphorylation buffer (50 mM Tris·HCl, 0.1 mM EDTA, pH 8.5). The cell lysates were then treated with either 1) Halt Phosphatase Inhibitor cocktail (Pierce, Rockford, IL), 2) Halt Phosphatase Inhibitor cocktail and 5 U alkaline phosphatase (AP; Roche, Indianapolis, IN), or 3) 5 U AP alone. The cell lysates were then incubated at 37°C for 1 h, heated at 95°C for 15 min to inactivate the AP, and either analyzed directly by Western blotting or immunoprecipitated (see below) and then analyzed by Western blotting.
Immunoprecipitation.
For FOXL2, CHO cells were transfected with the pFLAG-FOXL2 expression construct for 24 h in serum-free or normal culture media and then lysed and immunoprecipitated, as described previously (31). For negative controls, the cell lysates were immunoprecipitated with protein A (Millipore, Billerica, MA) coated with mouse IgG (Sigma, St. Louis, MO). The eluted samples or cell lysates were added to 4× SDS sample buffer and heated at 95°C for 5 min to denature the proteins. The proteins were then analyzed by Western blotting.
For LATS1, CHO cells were transfected with the pcDNA3.1-His-Xpress-LATS1 expression construct for 24 h and then lysed in immunoprecipitation buffer containing 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, and 0.2 mM PMSF. The cell lysates were incubated with protein A (Millipore) coated with LATS1 antibody (Bethyl Laboratories, Montgomery, TX) or Xpress antibody (Invitrogen, Carlsbad, CA) at 4°C overnight. The gel was washed with TBS (50 mM Tris·HCl, pH 7.4, and 150 mM NaCl) to eliminate nonspecific binding. The bound LATS1 was then eluted by incubating with 0.1 M glycine HCl at pH 3.5 for 5 min. The supernatants were neutralized by adding 10× TBS and then used in the kinase assays.
Kinase assays.
The pFLAG-FOXL2 expression construct was transfected into CHO cells. The lysates were treated with AP, and the resulting dephosphorylated FLAG-tagged FOXL2 proteins were purified by immunoprecipitation, as described above. The pcDNA3.1-His-Xpress-LATS1 expression construct was transfected into CHO cells in normal culture media and purified using protein A coated with LATS1 antibody or Xpress antibody, also as described above. The purified FLAG-tagged FOXL2 proteins were then combined with the immunoprecipitates containing wild-type or mutant His-Xpress-LATS1, and the mixtures were adjusted to 50 mM Tris·HCl, 150 mM sodium chloride, 2 mM ATP, 10 mM magnesium chloride, and 10 mM manganese chloride and incubated at 30°C for 4 h. The protein-gel mixtures were then added to 4× SDS sample buffer and heated at 95°C for 5 min to denature the proteins. The assay products were then analyzed by Western blotting.
Western blotting.
Cell lysates in RIPA buffer, immunoprecipitation products, or kinase assay products were added to 4× SDS sample buffer and heated at 95°C for 5 min to denature the proteins. The proteins were then separated on 7.5 or 12% SDS-PAGE gels and transferred to PVDF membranes. The membranes were incubated with a custom-made FOXL2 antibody (Zymed Laboratories, San Francisco, CA), which was raised against amino acids 20–33 of FOXL2, or with antibodies to LATS1 (Bethyl Laboratories) or phosphoserine (Sigma), washed, and then incubated with HRP-conjugated secondary antibodies. Chemiluminescent detection was performed using enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ).
RNA extraction and reverse transcriptase PCR.
Mouse ovaries from fetal (17.5 days postconception), immature (days 13 and 23), and adult (7 wk) mice were harvested from Swiss Webster outbred female mice under an approved Cedars-Sinai Medical Center Institutional Animal Care and Use Committee (IACUC) protocol. Total RNA from whole ovaries was isolated using an RNeasy mini kit (Qiagen, Valencia, CA) and reverse transcribed to generate ovary cDNAs using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). A LATS1 cDNA sequence was then PCR amplified from these ovary cDNAs using the primers 5′-TGCAGGAAATTCGAAACTCTC TGCTTC-3′ and 5′-GTGAGTTGGGACTCTCAGAATGATAGGCCA-3′. FOXL2 and GAPDH primers were used as described previously (41). Human Ovary PCR-Ready cDNA (Ambion) was used as a positive control, and water was used as a negative control. After PCR, the amplified products were subjected to electrophoresis on 1.5% agarose gels, which were then stained with ethidium bromide to visualize the anticipated fragments. Bands of the expected sizes were eluted, purified using a QIAquick gel extraction kit (Qiagen), and confirmed by sequencing.
Immunohistochemistry.
Ovaries were dissected from immature Swiss Webster outbred female mice at 21 days of age (under an approved IACUC protocol), and slides were prepared, pretreated, and blocked as described previously (31). The slides were then hybridized without primary antibody as a control or with a custom-made FOXL2 antibody (Zymed Laboratories) or with a LATS1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h, followed by incubation with a biotinylated anti-rabbit secondary antibody (Vector) for another 30 min. The slides were then incubated with avidin/biotin complex solution (Vector) for 30 min and stained by adding fresh 3,3-diaminobenzidine tetrahydrochloride solution for 2 min. They were then counterstained with hematoxylin (Sigma) and mounted with Vectamount AQ (Vector).
Promoter activity assays.
CHO cells or FOXL2 stable cells (31) were transfected with 100 ng of the human pcDNA3.1-His-Xpress-LATS1 expression construct (wild-type or D846A mutant) or the pcDNA3.1-His vector backbone with 0.5 μg of the 95-bp human StAR promoter luciferase construct (41) for 24 h. The indicator plasmid pCMV-β-galactosidase was used to estimate transfection efficiency. The total DNA concentration was maintained at 1 μg/well in a 24-well plate through the inclusion of empty pFLAG-CMV-2. Twenty-four hours after transfection, the cells were lysed using reporter lysis buffer and repeated freeze-thaw cycles. The luciferase activities in the cell lysates were determined as described previously (31). Results are reported as relative luminescence units and normalized with β-galactosidase activity.
Statistical analysis.
For promoter assay studies, experiments were performed in quadruplicate, and the standard error (± SE) was shown. Significant differences between groups were analyzed by ANOVA followed by the Neuman-Keuls test using GraphPad Prism software (San Diego, CA). P values <0.05 were considered significant.
RESULTS
FOXL2 interacts with the serine/threonine kinase LATS1.
To identify FOXL2-interacting proteins, we performed a yeast two-hybrid screen using human FOXL2. LATS1, a serine/threonine kinase, was found to interact strongly with FOXL2, which suggested that LATS1 might be involved in phosphorylating FOXL2.
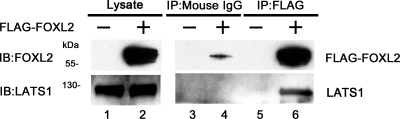
To characterize the interaction between FOXL2 and LATS1 in mammalian cells, CHO cells were transfected with FLAG-FOXL2 expression construct or an empty FLAG-CMV-2 expression vector, and the cells were subsequently lysed and immunoprecipitated with an antibody to FLAG or with mouse IgG as a control. The cell lysates and immunoprecipitates were then analyzed by immunoblotting with antibodies to FOXL2 and LATS1. LATS1 is expressed endogenously in CHO cells, whereas FOXL2 is not. When the empty pFLAG-CMV-2 vector was used as a template for protein synthesis, no FOXL2 was synthesized, but when the pcDNA3-FOXL2 construct was used as a template, FOXL2 was synthesized in the CHO cells (lysates; Fig. 1). Some endogenous LATS1 expression was also detected in the CHO cell lysates (Fig. 1). When the lysates from these cells were immunoprecipitated with the control mouse IgG, a faint band was obtained for FLAG-FOXL2 in the immunoprecipitates from cells expressing FLAG-FOXL2 but not in immunoprecipitates from cells expressing the empty pFLAG-CMV-2 vector, and no band was obtained for LATS1 (IP:mouse IgG; Fig. 1). In contrast, when the same lysates were immunoprecipitated with an antibody to FLAG, both FLAG-FOXL2 and LATS1 were identified in the immunoprecipitates from cells expressing FLAG-FOXL2 but not in immunoprecipitates from cells expressing the empty pFLAG-CMV-2 vector (IP:FLAG; Fig. 1). These results confirm that FOXL2 and LATS1 interact with each other, as suggested by the results of the yeast two-hybrid screening.
Fig. 1.
Large tumor suppressor gene 1 (LATS1) is coimmunoprecipitated with forkhead L2 (FOXL2). Mammalian Chinese hamster ovary (CHO) cells were transfected with an empty expression vector (−) or FLAG-FOXL2 expression construct (+). Twenty-four hours after transfection, the cells were lysed, and the lysates were immunoprecipitated with a control mouse IgG or an antibody to FLAG. The lysates (Lysate) and immunoprecipitates (IP) were analyzed by immunoblotting with FOXL2 and LATS1 antibodies. When the empty pFLAG-CMV-2 vector was used as a template, FOXL2 was not synthesized (Lysate, −), but when the pFLAG-CMV-2-FOXL2 construct was used as a template, FOXL2 was synthesized (Lysate, +). Some endogenous LATS1 expression was also detected in the lysates prior to immunoprecipitation. When these lysates were immunoprecipitated with mouse IgG, a faint band was obtained for FLAG-FOXL2 in immunoprecipitates from cells expressing FLAG-FOXL2, and no band was obtained for LATS1 (IP:mouse IgG). When the lysates were immunoprecipitated with an antibody to FLAG, endogenous LATS1 was coimmunoprecipitated in cells expressing FLAG-FOXL2 (IP:FLAG, +) but not in cells expressing the empty expression vector (IP:FLAG, −).
FOXL2 is phosphorylated in CHO cells.
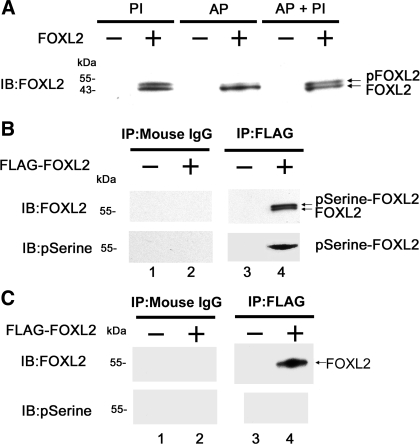
To test whether FOXL2 is phosphorylated in mammalian cells, we transfected CHO cells (which do not express endogenous FOXL2) with the pcDNA3-FOXL2 expression construct or with an empty pcDNA3 expression vector, lysed the cells under various conditions, and analyzed the resulting proteins by immunoblotting with the FOXL2 antibody (Fig. 2A). When FOXL2-transfected cells were lysed in the presence of a phosphatase inhibitor (PI) to eliminate phosphatase activity, two bands were detected (Fig. 2A). The lower band was the same size as the FOXL2 band detected previously (41), and the larger band was presumed to be phosphorylated FOXL2. To confirm that this larger band was in fact phosphorylated FOXL2, FOXL2-transfected cell lysates were treated with AP to remove phosphogroups. After phosphatase treatment, the larger FOXL2 band was eliminated (AP; Fig. 2A). When the cell lysates were treated with both AP and PI, the larger band was preserved (AP + PI; Fig. 2A).
Fig. 2.
FOXL2 is phosphorylated in vitro. A: mammalian CHO cells, which do not express endogenous FOXL2, were transfected with the pcDNA3-FOXL2 expression construct (+) or with an empty pcDNA3 expression vector (−). Twenty-four hours after transfection, the cells were lysed and treated with phosphatase inhibitor alone (PI), alkaline phosphatase alone (AP), or AP and PI (AP + PI). The proteins were then analyzed by immunoblotting (IB). Two FOXL2 bands are seen in lysates treated with phosphatase inhibitor (PI), the lower of which corresponds in size to FOXL2 (41). The upper band was eliminated in lysates treated with AP but was preserved in lysates treated with AP + PI. B: CHO cells were transfected with pFLAG-FOXL2 expression construct (+) or an empty expression vector (−). Twenty-four hours after transfection, the cells were lysed and the resulting proteins immunoprecipitated with FLAG or mouse IgG (control) antibodies and analyzed by IB with FOXL2 and phosphoserine antibodies. Two FOXL2 bands were visualized using the FOXL2 antibody in the FLAG immunoprecipitates but not in the mouse IgG precipitates, the larger of which was also detected by the phosphoserine antibody (pSerine-FOXL2). C: lysates treated with AP in A were immunoblotted with the FOXL2 and phosphoserine antibodies. A single FOXL2 band was seen in the FLAG immunoprecipitates but not in the mouse IgG precipitates. No bands were detected using the phosphoserine antibody.
To determine whether FOXL2 is phosphorylated at a serine or a threonine residue, CHO cells were transiently transfected with the pFLAG-FOXL2 expression construct or an empty pFLAG-CMV-2 vector backbone and lysed, and cell lysates were immunoprecipitated using an antibody to FLAG or mouse IgG. The immunoprecipitates were then analyzed by immunoblotting with the FOXL2 antibody and a phosphoserine antibody (Fig. 2B). When the immunoprecipitates were probed with the FOXL2 antibody, the two bands we previously identified corresponding to FOXL2 and phosphorylated FOXL2 (Fig. 2A) were again seen in the FLAG immunoprecipitates but not in the mouse IgG precipitates (Fig. 2B). When this blot was stripped and reprobed with a phosphoserine antibody, the upper band was again identified, suggesting that this band is phosphoserine FOXL2 (pSerine-FOXL2) (Fig. 2B). To further confirm these results, immunoprecipitates from cell lysates treated with AP were also immunoblotted with the FOXL2 and phosphoserine antibodies. When the immunoprecipitates were probed with the FOXL2 antibody, a single band corresponding to FOXL2 was seen in the FLAG immunoprecipitates but not in the mouse IgG precipitates (Fig. 2C). When this blot was stripped and reprobed with the phosphoserine antibody, no bands were observed (Fig. 2C). No bands were detected when the immunoprecipitates were probed with an anti-phospho-threonine antibody (data not shown). Taken together, these results suggest that FOXL2 is phosphorylated at a serine residue in mammalian cells.
LATS1 phosphorylates FOXL2 in vitro.
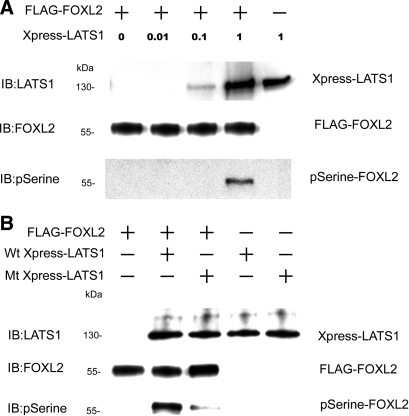
To determine whether FOXL2 is phosphorylated by LATS1, we performed a kinase assay in which we used dephosphorylated FOXL2 as a substrate for LATS1. The immunoprecipitates containing LATS1 were then incubated with the dephosphorylated FOXL2 for 4 h at 30°C. The products of the kinase reactions were then analyzed by immunoblotting with antibodies to phosphoserine, FOXL2, and LATS1. As shown in Fig. 3A, in the absence of LATS1, FOXL2 was not phosphorylated at a serine residue. However, when dephosphorylated FOXL2 was incubated with increasing amounts of LATS1, a phosphoserine-FOXL2 band was observed in the presence of LATS1 at the highest concentration (Fig. 3A), indicating that incubation with LATS1 results in phosphorylation of FOXL2. These results suggest that LATS1 can act as a kinase for FOXL2 in vitro.
Fig. 3.
FOXL2 is phosphorylated by LATS1. A: CHO cells were transfected with the pcDNA3-His-Xpress LATS1 construct and lysed, and Xpress-tagged LATS1 was purified using a LATS1 antibody for use as the kinase. Dephosphorylated FLAG-tagged FOXL2 proteins were used as substrates in kinase assays. Different amounts of Xpress-tagged LATS1 were mixed with FLAG-tagged FOXL2 for kinase phosphorylation, and the reaction products were analyzed by IB with antibodies to FOXL2, LATS1, and phosphoserine. In the absence of Xpress-LATS1, FOXL2 was not phosphorylated at a serine residue. In the presence of increasing concentrations of Xpress-LATS1, the amount of phosphoserine-FOXL2 (pSerine-FOXL2) increased. B: to determine whether LATS1 is a kinase for FOXL2, CHO cells were transfected with pcDNA3-His-Xpress LATS1 constructs, either the wild-type (Wt Xpress-LATS1) or the kinase-inactive D846A mutant (Mt Xpress-LATS1), lysed, and purified using the Xpress antibody for use as kinases. Xpress-tagged wild-type LATS1 or Xpress-tagged mutant LATS1 D846A was mixed with dephosphorylated FLAG-tagged FOXL2 for kinase phosphorylation. A strong phosphoserine-FOXL2 band was observed when wild-type LATS1 was used as the kinase, but only a very faint band was observed when the same concentration of the D846A mutant LATS1 was used.
To confirm that phosphorylation of FOXL2 is due primarily to the kinase activity of LATS1 and not another protein kinase that copurifies with LATS1, we repeated the kinase assays with the inclusion of a kinase-inactive LATS1 mutant, D846A, that cannot bind ATP (13, 26). CHO cells were transfected with pcDNA3.1-His-Xpress-LATS1 (wild type or mutant) expression constructs, and lysates from these cells were purified using protein A coated with Xpress antibody. The immunoprecipitates containing wild-type LATS1 or mutant LATS1 D846A were used to perform the kinase assays. A strong phosphoserine-FOXL2 band was observed when wild-type LATS1 was used as the kinase, but only a very faint band was observed when the D846A mutant LATS1 was used (Fig. 3B). These results indicate that FOXL2 is phosphorylated primarily by LATS1.
The LATS1 gene is expressed in developing mouse gonads.
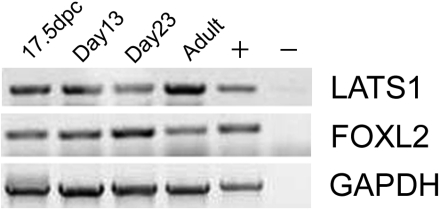
Our previous studies showed that FOXL2 is expressed in the mouse ovary from ≥17.5 days postconception (dpc) to adulthood (41). Because our results indicated that LATS1 can act as a kinase for FOXL2 in vitro, we hypothesized that, if LATS1 also phosphorylates FOXL2 in vivo, LATS1 and FOXL2 should have overlapping expression patterns in the mouse ovary. Therefore, we used RT-PCR to determine LATS1 expression in fetal (17.5 dpc), immature (days 13 and 23), and adult (7 wk) mouse ovaries. As shown in Fig. 4, both FOXL2 and LATS1 are expressed in the ovaries of fetal mice at 17.5 dpc, in the ovaries of immature mice at days 13 and 23 of life, and in the ovaries of adult mice.
Fig. 4.
LATS1 gene is expressed in developing mouse ovary. Total RNA was extracted from whole ovaries of fetal [17.5 days postconception (dpc)], immature (days 13 and 23), and adult mice, reverse transcribed, PCR amplified with LATS1 primers, and analyzed by electrophoresis. Human Ovary PCR-Ready cDNA (Ambion, Austin, TX) was used as a positive control (+), and water was used as a negative control (−). The identities of the LATS1 PCR products were confirmed by sequencing. FOXL2 expression in the same cDNAs (41) is shown for comparison.
FOXL2 and LATS1 are coexpressed in granulosa cells in the mouse ovary.
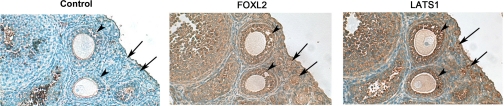
The FOXL2 transcript has been shown to be expressed specifically in the granulosa cells of small and medium follicles in the mouse ovary (41). To further investigate the respective locations and expression levels of FOXL2 and LATS1 in the ovary, immunohistochemical analysis was performed using mouse ovarian tissue sections. Because the FOXL2 transcript is known to be expressed in the less differentiated granulosa cells of small and medium follicles, serial sections of ovaries from immature (21-day-old) mice, which do not contain mature (steroidogenic) follicles, were used for these studies. As shown in Fig. 5, FOXL2 and LATS1 were found to have overlapping patterns of expression in the granulosa cells of small (indicated by arrows) and medium (indicated by arrowheads) follicles in the immature mouse ovary.
Fig. 5.
FOXL2 and LATS1 are coexpressed in mouse granulosa cells. Immunohistochemical staining was performed on serial sections of ovaries from immature (21-day-old) mice, which do not contain mature (steroidogenic) follicles, incubating without primary antibody (as a control) or with antibodies to FOXL2 or LATS1, followed by counterstaining with hematoxylin. Both FOXL2 and LATS1 were expressed in granulosa cells of small (arrow) and medium (arrowhead) follicles.
Coexpression with LATS1 enhances the repressive effect of FOXL2 on the StAR gene promoter.
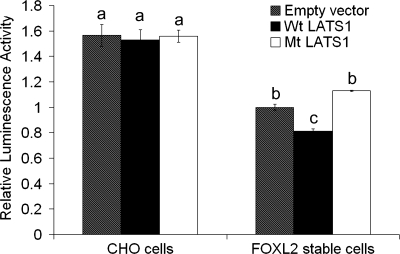
FOXL2 acts as a key transcriptional repressor during follicle development and is known to interact directly with the promoter of the StAR gene. In a previous study, we showed that FOXL2 can repress the activity of a −95-bp human StAR promoter-luciferase construct in CHO cells (41). Therefore, we used this construct to examine whether phosphorylation by LATS1 is involved in regulating the repression of the StAR gene promoter by FOXL2. Wild-type CHO cells and CHO cells expressing FOXL2 (31) were transfected with 100 ng of the pcDNA3.1-His-Xpress-LATS1 expression construct, 100 ng of the kinase-inactive D846A mutant LATS1 expression construct, or 100 ng of the empty pcDNA3.1-His vector backbone for 24 h, followed by cotransfection with the −95-bp human StAR promoter-luciferase reporter plasmid. Twenty-four hours after transfection, the activity of the StAR promoter was assessed by measuring luciferase activity in the cell lysates. As shown in Fig. 6, in the absence of FOXL2, luciferase activities in cells transfected with wild-type or mutant LATS1 were not significantly different from cells transfected with the control vector (CHO cells), indicating that LATS1 itself does not affect StAR promoter activity. In contrast, in cells expressing FOXL2 (empty vector, FOXL2 stable cells), the basal level of StAR promoter activity was decreased significantly (P < 0.05), indicating that FOXL2 represses the activity of the StAR promoter, as expected. When FOXL2-expressing cells were transfected with wild-type LATS1 (Wt LATS1; FOXL2 stable cells), a further significant reduction in luciferase activity was observed (P < 0.05), indicating that StAR promoter activity was further repressed in the presence of both FOXL2 and LATS1. In contrast, when FOXL2-expressing CHO cells were transfected with the kinase-inactive LATS1 mutant, luciferase activity was restored to the levels seen in cells expressing FOXL2 alone (Mt LATS1; FOXL2 stable cells). These results suggest that coexpression with LATS1 enhances the activity of FOXL2 as a transcriptional repressor of the StAR gene and that this effect is due to the kinase activity of LATS1.
Fig. 6.
LATS1 enhances the repressive effect of FOXL2 on the steroidogenic acute regulatory (StAR) promoter. Wild-type CHO cells and CHO cells expressing FOXL2 (FOXL2 stable cells) were transfected with 100 ng of the pcDNA3.1-His-Xpress-LATS1 expression construct (Wt LATS1), 100 ng of the kinase-inactive D846A mutant LATS1 expression construct (Mt LATS1), or 100 ng of the empty pcDNA3.1-His vector backbone (empty vector) and the −95-bp StAR promoter-luciferase plasmid. The resulting luciferase activities were determined 24 h after cotransfection. Luciferase activity values were normalized against activity levels in FOXL2 stable cells transfected with the StAR promoter plasmid in the presence of the empty vectors (value = 1). One-way ANOVA revealed that CHO cells were transfected with wild-type or mutant LATS1 in the absence of FOXL2, and luciferase activities were similar to the control (a). In the presence of FOXL2 (FOXL2 stable cells; b) luciferase activity was reduced significantly compared with that in CHO cells without FOXL2 (P < 0.05; a). When FOXL2 stable cells were transfected with wild-type LATS1 (c), the luciferase activity of the StAR promoter was reduced significantly compared with control-transfected stable cells (b) (P < 0.05). When FOXL2 stable cells were transfected with the kinase-inactive LATS1 mutant (MtLATS1), luciferase activity was restored to levels in control-transfected stable cells (b).
DISCUSSION
In this article, we have shown that the serine/threonine kinase LATS1 phosphorylates FOXL2 in vitro and that this phosphorylation occurs at a serine residue. Furthermore, we have demonstrated that LATS1 is expressed in the mouse ovary from ≥17.5 dpc to adulthood and have localized this expression to the less differentiated granulosa cells of small and medium follicles in the immature mouse ovary, where LATS1 is coexpressed with FOXL2. Moreover, our data indicate that phosphorylation by LATS1 enhances, whereas a kinase-inactive LATS1 mutant fails to enhance, FOXL2's activity as a transcriptional repressor of the StAR gene, which controls a rate-limiting step in steroidogenesis and is a marker of granulosa cell differentiation. Taken together, these data support our hypothesis that phosphorylation may be one of the mechanisms controlling the activity of FOXL2 as a transcriptional repressor during follicular development and suggest that LATS1 may be involved in mediating this regulation.
Regulation through phosphorylation has been shown to be an important mechanism for controlling the activities of transcription factors (28, 37), including other forkhead transcription factors (38) expressed in both human and rodent granulosa cells (42, 45). Protein kinase B/Akt phosphorylates the FOXO subfamily, leading to export from the nucleus and inhibition of transcriptional activities (6, 9, 30), whereas activation of the Ras-Ral pathway leads to phosphorylation of FOXO members and induces transcriptional activity (18, 30). In rodent granulosa cells, FOXO1 is phosphorylated through the phosphatidylinositol 3-kinase pathway in response to follicle-stimulating hormone (FSH) and insulin-like growth factor I, leading to nuclear exclusion (45). Moreover, key genes in the lipid, sterol, and steroidogenic biosynthetic pathways that are induced by FSH are suppressed by constitutively active FOXO1, indicating that FOXO1 functions as a transcriptional repressor (35). Similar to FOXO1, we have found that FOXL2 also plays a key role in transcriptional repression of the rate-limiting step of the steroidogenic pathway (41). However, in contrast to the FOXO family, little has been identified regarding the regulation of FOXL2 and other forkhead transcription factors via phosphorylation. However, a study by Benayoun et al. (2), which was performed using the granulosa-like KGN cell line, suggested that FOXL2 was highly modified posttranslationally and that multiple phosphorylation events were likely involved in its modification. Our results demonstrate for the first time that FOXL2 is indeed phosphorylated and that LATS1 is at least one kinase that is involved in its phosphorylation and subsequent functional activity, as indicated by the increase in FOXL2's activity as a transcriptional repressor of the StAR gene in the presence of wild-type, but not mutant, LATS1.
We have demonstrated previously that FOXL2 is expressed in granulosa cells, where it may be involved in regulating the early stages of folliculogenesis (41). We demonstrated that FOXL2 is expressed in granulosa cells of small and medium follicles in the immature mouse ovary and also in a few type 6 follicles (41). In larger follicles, FOXL2 is expressed in the less differentiated cumulus cells but shows much lower expression in mural granulosa cells (41). In contrast to FOXL2, the StAR gene is not expressed in immature follicles (43, 46, 50) but is present in granulosa cells of large preovulatory and luteinized follicles in humans, mural granulosa cells in the rodent ovary following treatment with pregnant mare's serum gonadotropin, and granulosa cells of periovulatory follicles (43, 46, 50). Therefore, FOXL2 appears to repress StAR expression during follicle development, and it is only with loss of this transcriptional repression that StAR begins to be expressed in granulosa cells, signaling early functional maturation of ovarian antral follicles. Furthermore, FOXL2 may also work globally as a transcriptional repressor of granulosa cell proliferation and differentiation, as demonstrated by its effects on the aromatase, P450scc, cyclin D2 promoters (4), and the CYP17 promoter (39). Interestingly, anti-Müllerian hormone (AMH) (20, 27, 54) and activin A (22, 55) are also expressed in granulosa cells of primary and growing follicles and may function as inhibitory factors for the primordial to primary follicle transition (8, 11, 53). AMH and activin A are downregulated specifically in the ovaries of FOXL2-knockout mice (47), which exhibit early follicular depletion and undergo primary ovarian failure (47). Taken together, these data suggest that FOXL2 may function as a determinant of tissue differentiation by inhibiting premature differentiation of granulosa cells, possibly acting through AMH and/or activin A. With the inhibition of granulosa cell differentiation, FOXL2 may control the number of primordial follicles that remain dormant and prevent the premature depletion of ovarian follicles.
FOXL2 in the ovary is one of the first sexually dimorphic genes expressed and a determinant of tissue differentiation. Likewise, FOXL2 in the pituitary also precedes the expression of gonadotrope-specific markers and has been suggested to be involved in the differentiation of this lineage (7, 21, 52). In the gonadotrope, FOXL2 functions as a Smad3 partner and drives transcription in αT3–1 cells treated with activin (7). Lamba et al. (32) also demonstrated recently that FOXL2 is involved in mediating activin A-regulated murine FSHβ (Fshb) transcription in pituitary gonadotropes, likely in association with Smad3. These findings suggest that FOXL2 functions as a transcriptional regulator and a coordinator of Smad3 targets in pituitary gonadotropes (7), and differentiation may be regulated by interaction of the forkhead and Smad family of transcription factors under the control of activin.
Activin, in conjunction with FSH, also plays a significant role in granulosa cell proliferation and differentiation through phosphorylation. Phosphorylation of FOXO1 and Smad3 by FSH and activin leads to stimulation of granulosa cell differentiation and proliferation (40). Similarly, we have demonstrated that FOXL2 is also phosphorylated through LATS1, which is present in granulosa cells of immature follicles, and this phosphorylation may enhance the activity of FOXL2 as a transcriptional repressor that may regulate differentiation, perhaps with the Smad family of transcription factors, as demonstrated in other cell types of the reproductive pathway, such as the gonadotropes (7, 32).
LATS1 is part of the Hippo pathway, which was originally defined in Drosophila and is conserved in mammals. LATS1 is a serine/threonine kinase with demonstrated tumor suppressor activity in Drosophila (57) and has been shown to inhibit cell proliferation in vitro via its kinase activity (56). Studies have shown that the Hippo pathway suppresses tumor growth by regulating cell proliferation, growth, and death (10, 19, 58, 59). The component proteins in Drosophila, Hippo, Sav, Wts, and Mats, are conserved in mammals as Mst1/2, WW45, LATS1/2, and Mob1, respectively. In mammals, LATS1 is regulated by the MOB superfamily proteins; MOB1 associates with LATS1 and activates it, and MST2 phosphorylates and activates LATS1 (5, 25). LATS1 in turn binds to and phosphorylates the transcriptional activator YAP (24). LATS1 is highly expressed in the ovary (48), and we found LATS1 to be highly expressed in the granulosa cells of small and medium follicles, cells that have the potential for rapid proliferation. LATS1-knockout mice have severe fertility defects; their ovaries contain fewer follicles than wild types, with only primary or secondary follicles present. Although some of the fertility defects are attributed to an isolated LH-hypogonadotropic hypogonadism, some LATS1-deficient females are able to give birth to one to three litters before becoming infertile, and those females who are initially fertile appear to undergo premature ovarian failure (48). These fertility defects are reminiscent of those seen in women with BPES type I and heterozygous mutations of FOXL2, who exhibit a complete sequence of follicle development to the ovulatory stage with early depletion of the follicle pool and premature ovarian failure (23). In contrast, FOXL2-null female mice exhibit a more severe ovarian phenotype, with follicular arrest between the primordial and primary stages followed by follicle degeneration (47). They fail to undergo sexual maturation and undergo primary ovarian failure; thus, unlike the heterozygous mutation in humans, the complete loss of FOXL2 in these mice may result in this more severe phenotype and hence, primary ovarian failure and not secondary failure, as seen in BPES type I.
Xia et al. (56) demonstrated that a kinase-inactive LATS1 mutant protein fails to induce apoptosis of MCF-7 cells and loses its ability to inhibit cell growth, suggesting that the kinase activity of LATS1 is required for the induction of apoptosis and inhibition of cell proliferation. Therefore, we hypothesize that LATS1 and FOXL2 may function in a common pathway inhibiting proliferation and differentiation until follicle development is signaled. In this article, we have shown that coexpression with LATS1 enhances the ability of FOXL2 to repress transcription of the StAR promoter, whereas coexpression with the kinase-inactive LATS1D846A mutant failed to enhance FOXL2's activity as a transcriptional repressor. These results suggest that the kinase activity of LATS1 is involved in regulating FOXL2's functional activity as a transcriptional repressor.
We found that LATS1 and FOXL2 are coexpressed in less differentiated granulosa cells of small and medium ovarian follicles in the mouse ovary and that LATS1 phosphorylates FOXL2 and enhances its transcriptional repression of the StAR gene in vitro. These data are consistent with our hypothesis that LATS1 may phosphorylate FOXL2 during follicle development. We propose that this phosphorylation may be one of the mechanisms by which FOXL2's activity as a transcriptional repressor is regulated in vivo and that release of repression may allow the StAR gene to be expressed in granulosa cells, signaling granulosa cell differentiation and proliferation and subsequent functional maturation of ovarian follicles. FOXL2 is also sumoylated (3, 36), and sumoylation is also necessary for transcriptional regulation (31). Thus, it appears that there are multiple fine-tuning mechanisms necessary for FOXL2 as a transcriptional regulator, including its potential role with the Smad family of transcription factors, as demonstrated in other cell types (7). Whether there is a mechanism similar to FOXO1 cooperativity with the Smad family of transcription factors in granulosa cells (40) that leads to granulosa cell proliferation and differentiation remains to be determined.
GRANTS
This work was supported by R01-HD-047603 from the National Institute of Child Health and Human Development and the Office of Research on Women's Health (M. D. Pisarska) and by a grant from the Helping Hands of Los Angeles (M. D. Pisarska).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank J. F. Strauss III for the StAR promoter construct.
REFERENCES
- 1.Adashi EY. Immune modulators in the context of the ovulatory process: a role for interleukin-1. Am J Reprod Immunol 35: 190–194, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Benayoun BA, Auer J, Caburet S, Veitia RA. The post-translational modification profile of the forkhead transcription factor FOXL2 suggests the existence of parallel processive/concerted modification pathways. Proteomics 8: 3118–3123, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Benayoun BA, Caburet S, Dipietromaria A, Georges A, D'Haene B, Pandaranayaka PJ, L'Hôte D, Todeschini AL, Krishnaswamy S, Fellous M, De Baere E, Veitia RA. Functional exploration of the adult ovarian granulosa cell tumor-associated somatic FOXL2 mutation p.Cys134Trp (c.402C>G). PloS One 5: e8789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentsi-Barnes IK, Kuo FT, Barlow GM, Pisarska MD. Human forkhead L2 represses key genes in granulosa cell differentiation including aromatase, P450scc, and cyclin D2. Fertil Steril. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bichsel SJ, Tamaskovic R, Stegert MR, Hemmings BA. Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J Biol Chem 279: 35228–35235, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96: 7421–7426, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem 284: 7631–7645, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braw-Tal R. The initiation of follicle growth: the oocyte or the somatic cells? Mol Cell Endocrinol 187: 11–18, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod 21: 2223–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol 250: 1–23, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269: 28314–28322, 1994 [PubMed] [Google Scholar]
- 15.Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27: 159–166, 2001 [DOI] [PubMed] [Google Scholar]
- 16.De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, Devriendt K, Dixon M, Fellous M, Fryns JP, Garza A, Jonsrud C, Koivisto PA, Krause A, Leroy BP, Meire F, Plomp A, Van Maldergem L, De Paepe A, Veitia R, Messiaen L. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet 72: 478–487, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Baere E, Dixon MJ, Small KW, Jabs EW, Leroy BP, Devriendt K, Gillerot Y, Mortier G, Meire F, Van Maldergem L, Courtens W, Hjalgrim H, Huang S, Liebaers I, Van Regemorter N, Touraine P, Praphanphoj V, Verloes A, Udar N, Yellore V, Chalukya M, Yelchits S, De Paepe A, Kuttenn F, Fellous M, Veitia R, Messiaen L. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype-phenotype correlation. Hum Mol Genet 10: 1591–1600, 2001 [DOI] [PubMed] [Google Scholar]
- 18.De Ruiter ND, Burgering BM, Bos JL. Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451. Mol Cell Biol 21: 8225–8235, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143: 1076–1084, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol 20: 2796–2805, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Findlay JK. An update on the roles of inhibin, activin, and follistatin as local regulators of folliculogenesis. Biol Reprod 48: 15–23, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Fraser IS, Shearman RP, Smith A, Russell P. An association among blepharophimosis, resistant ovary syndrome, and true premature menopause. Fertil Steril 50: 747–751, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 283: 5496–5509, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hergovich A, Bichsel SJ, Hemmings BA. Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol Cell Biol 25: 8259–8272, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun 345: 50–58, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hirobe S, He WW, Lee MM, Donahoe PK. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology 131: 854–862, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol 5: 747–757, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann E, Knöchel W. Five years on the wings of fork head. Mech Dev 57: 3–20, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398: 630–634, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Kuo FT, Bentsi-Barnes IK, Barlow GM, Bae J, Pisarska MD. Sumoylation of forkhead L2 by Ubc9 is required for its activity as a transcriptional repressor of the Steroidogenic Acute Regulatory gene. Cell Signal 21: 1935–1944, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol Endocrinol 23: 1001–1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. Fox's in development and disease. Trends Genet 19: 339–344, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267: 1828–1831, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, Richards JS. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol 23: 649–661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marongiu M, Deiana M, Meloni A, Marcia L, Puddu A, Cao A, Schlessinger D, Crisponi L. The forkhead transcription factor Foxl2 is sumoylated in both human and mouse: sumoylation affects its stability, localization, and activity. PloS One 5: e9477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montminy M. Transcriptional activation. Something new to hang your HAT on. Nature 387: 654–655, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 27: 2263–2275, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Park M, Shin E, Won M, Kim JH, Go H, Kim HL, Ko JJ, Lee K, Bae J. FOXL2 Interacts with Steroidogenic Factor-1 (SF-1) and Represses SF-1-Induced CYP17 Transcription in Granulosa Cells. Mol Endocrinol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem 280: 9135–9148, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisarska MD, Bae J, Klein C, Hsueh AJ. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology 145: 3424–3433, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Pisarska MD, Kuo FT, Tang D, Zarrini P, Khan S, Ketefian A. Expression of forkhead transcription factors in human granulosa cells. Fertil Steril 91: 1392–1394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, Strauss JF., 3rd Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metab 82: 4243–4251, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Ramírez-Castro JL, Pineda-Trujillo N, Valencia AV, Muñetón CM, Botero O, Trujillo O, Vásquez G, Mora BE, Durango N, Bedoya G, Ruiz-Linares A. Mutations in FOXL2 underlying BPES (types 1 and 2) in Colombian families. Am J Med Genet 113: 47–51, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 16: 580–599, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 139: 303–315, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131: 933–942, 2004 [DOI] [PubMed] [Google Scholar]
- 48.St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, Parlow AF, McGrath J, Xu T. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet 21: 182–186, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63: 193–213, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Thompson WE, Powell J, Thomas KH, Whittaker JA. Immunolocalization and expression of the steroidogenic acute regulatory protein during the transitional stages of rat follicular differentiation. J Histochem Cytochem 47: 769–776, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE 2003: RE5, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12: 1691–1704, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med 27: 14–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10: 77–83, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Woodruff TK, D'Agostino J, Schwartz NB, Mayo KE. Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science 239: 1296–1299, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Xia H, Qi H, Li Y, Pei J, Barton J, Blackstad M, Xu T, Tao W. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene 21: 1233–1241, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13: 188–192, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zlotogora J, Sagi M, Cohen T. The blepharophimosis, ptosis, and epicanthus inversus syndrome: delineation of two types. Am J Hum Genet 35: 1020–1027, 1983 [PMC free article] [PubMed] [Google Scholar]