Abstract
Epidemiological studies suggest that Mediterranean diets rich in resveratrol are associated with reduced risk of coronary artery disease. Resveratrol was also shown to confer vasoprotection in animal models of type 2 diabetes and aging. However, the mechanisms by which resveratrol exerts its antioxidative vasculoprotective effects are not completely understood. Using a nuclear factor-E2-related factor-2 (Nrf2)/antioxidant response element-driven luciferase reporter gene assay, we found that in cultured coronary arterial endothelial cells, resveratrol, in a dose-dependent manner, significantly increases transcriptional activity of Nrf2. Accordingly, resveratrol significantly upregulates the expression of the Nrf2 target genes NAD(P)H:quinone oxidoreductase 1, γ-glutamylcysteine synthetase, and heme oxygenase-1. Resveratrol treatment also significantly attenuated high glucose (30 mM)-induced mitochondrial and cellular oxidative stress (assessed by flow cytometry using MitoSox and dihydroethidine staining). The aforementioned effects of resveratrol were significantly attenuated by the small interfering RNA downregulation of Nrf2 or the overexpression of Kelch-like erythroid cell-derived protein 1, which inactivates Nrf2. To test the effects of resveratrol in vivo, we used mice fed a high-fat diet (HFD), which exhibit increased vascular oxidative stress associated with an impaired endothelial function. In HFD-fed Nrf2+/+ mice, resveratrol treatment attenuates oxidative stress (assessed by the Amplex red assay), improves acetylcholine-induced vasodilation, and inhibits apoptosis (assessed by measuring caspase-3 activity and DNA fragmentation) in branches of the femoral artery. In contrast, the aforementioned endothelial protective effects of resveratrol were diminished in HFD-fed Nrf2−/− mice. Taken together, our results indicate that resveratrol both in vitro and in vivo confers endothelial protective effects which are mediated by the activation of Nrf2.
Keywords: endothelial cell, gracilis, resveratrol, nuclear factor-E2-related factor-2
recent studies provide strong evidence that the treatment of laboratory rodents with resveratrol (3,4′,5-trihydroxystilbene), a plant-derived polyphenolic compound, exerts significant vasoprotective effects both in type 2 diabetes and during aging (29, 44, 48). Recent studies also revealed that resveratrol improves the health and survival of mice with metabolic syndrome (1, 23). In addition, epidemiological studies suggest that Mediterranean diets which are rich in resveratrol are associated with a significantly reduced risk of cardiovascular disease in humans as well (16, 19). However, the molecular mechanisms that underlie the beneficial effects of resveratrol on cardiovascular function remain incompletely understood (35).
Nuclear factor-E2-related factor-2 (Nrf2) is a transcription factor that regulates the expression of numerous reactive oxygen species (ROS) detoxifying and antioxidant genes. Recent studies suggest that the Nrf2/antioxidant response element (ARE) pathway can be activated both pharmacologically and by dietary means (30). Under basal nonactivated conditions, Nrf2 interacts with Kelch-like erythroid cell-derived protein 1 (Keap-1), a cytosolic repressor protein, and limits Nrf2-mediated gene expression. Upon activation, the Keap-1-Nrf2 complex is dissociated and Nrf2 triggers the expression of genes mediated by the ARE to attenuate cellular oxidative stress. When Nrf2 is released from Keap-1 and translocates to the nucleus, it binds to ARE and activates ARE-dependent transcription of phase II and antioxidant defense enzymes, including NAD(P)H:quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (Hmox1). The Nrf2/ARE pathway also controls the expression of γ-glutamylcysteine synthetase (GCLC), the rate-limiting enzyme for glutathione (GSH) synthesis.
The present study was designed to determine whether the vasoprotective effects of resveratrol are mediated, at least in part, by the activation of Nrf2. We base this hypothesis on several lines of evidence. First, we have shown that resveratrol in cultured coronary arterial endothelial cells (CAECs) upregulates Nrf2/ARE-dependent antioxidant enzymes, including Hmox1 and glutathione peroxidase (40) and effectively decreases oxidative stress induced by inflammatory stimuli and metabolic stressors (40, 43). Second, resveratrol upregulates cellular antioxidant defense mechanisms protecting endothelial cells against oxidative stress in aging, diabetes (29, 48), and cigarette smoking (9, 21) in vivo. Third, resveratrol increases cellular GSH levels in cultured CAECs (43), as well as in other cell types (21). Fourth, resveratrol appears to act as a “caloric restriction mimetic” in model organisms (17, 46) as well as in mammalian systems (29). In that regard it is important that the antioxidant effects of caloric restriction are, at least in part, mediated by Nrf2 (30).
To test our hypotheses, we assessed Nrf2 activation in response to resveratrol treatment in cultured primary human CAECs. We characterized the effect of disruption of the Nrf2/ARE pathway on resveratrol-induced attenuation of endothelial ROS production and induction of antioxidant gene expression. We also determined whether resveratrol, via Nrf2, inhibits the induction of apoptosis by oxidative stress in cultured endothelial cells. For oxidative stressors we used high glucose, which generates large amounts of ROS in the mitochondria (25, 31), and TNF-α, which activates NAD(P)H oxidases (36). The relevance of the effects of resveratrol in vivo was tested on high-fat diet (HFD)-induced endothelial dysfunction, vascular oxidative stress, and apoptosis in wild-type and Nrf2−/− mice.
METHODS
Cell cultures, knockdown of Nrf2, and Keap-1 overexpression.
Cultured primary human CAECs (purchased from Cell Applications) were treated with resveratrol (purchased from Sigma-Aldrich) as previously described (6, 8, 12, 40). To disrupt Nrf2 signaling, Nrf2 was downregulated by RNA interference using proprietary small interfering RNA (siRNA) sequences (Origen) and the electroporation-bases Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (5, 6, 43). Cell density at transfection was 30%. Experiments were performed on day 2 after the transfection, when gene silencing was optimal. Keap-1 overexpression was achieved in CAECs by transfection with a Keap-1 full-length cDNA-encoding plasmid (Origen) as previously described (9).
Transient transfection and luciferase assays.
The effect of resveratrol on Nrf2 activity in CAECs was tested by a reporter gene assay. We used an ARE reporter comprised of tandem repeats of the ARE transcriptional response element upstream of firefly luciferase (SA Biosciences, Frederick, MD) and a renilla luciferase plasmid under the control of the cytomegalovirus promoter (as an internal control). Transfections in CAECs were performed using the Amaxa Nucleofector technology (Amaxa), as we have previously reported (5, 13, 15). Firefly and renilla luciferase activities were assessed after 24 h using the Dual Luciferase Reporter Assay Kit (Promega) and a Tecan Infinite M200 plate reader.
Quantitative real-time RT-PCR.
The quantitative real-time RT-PCR technique was used to analyze mRNA expression of the Nrf2/ARE target genes Nqo1, Gclc, and Hmox1 in resveratrol-treated CAECs, as previously reported (10, 14, 42, 44). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as previously described (12, 14). A real-time RT-PCR technique was used to analyze mRNA expression using the Strategen MX3000, as previously reported (12). Amplification efficiencies were determined using the dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes Gapdh, Hprt, and ACTB (β-actin) were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Oligonucleotides used for quantitative real-time RT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Table 1.
Oligonucleotides for real-time RT-PCR
| mRNA Targets | Description | Sense | Antisense |
|---|---|---|---|
| Nqo1 | NAD(P)H:quinone oxidoreductase 1 (NAD(P)H dehydrogenase, quinone 1), human | AGACCTTGTGATATTCCAGTTC | GGCAGCGTAAGTGTAAGC |
| Gclc | γ-glutamylcysteine synthetase (glutamate-cysteine ligase, catalytic subunit), human | CAGTGGTGGATGGTTGTG | ATTGATGATGGTGTCTATGC |
| Hmox1 | heme oxygenase-1, human | AAGTATCCTTGTTGACACG | TGAGCCAGGAACAGAGTG |
| Hprt | hypoxanthine phosphoribosyltransferase 1, human | CCGTGTGTTAGAAAAGTAAGAAGC | AACTGCTGACAAAGATTCACTGG |
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase, human | AACGAATTTGGCTACAGC | AGGGTACTTTATTGATGGTACAT |
| Actb | β-actin, human | CGGTGAAGGTGACAGCAG | TGTGTGGACTTGGGAGAGG |
Measurement of resveratrol-induced changes in mitochondrial and cellular ROS production in CAECs.
CAECs were treated with high glucose (30 mM for 24 h) to assess the protective effect of resveratrol on mitochondrial ROS production. Mitochondrial O2·− production in endothelial cells was assessed by flow cytometry (Millipore/Guava Easycyte) using MitoSox red (Invitrogen, Carlsbad CA), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (22, 43). Cell debris (low forward and side scatter), dead cells (Sytox Green and annexin V positive), and apoptotic cells (annexin V positive) were gated out for analysis (25, 26). The effects of Nrf2 knockdown or Keap-1 overexpression on resveratrol-induced attenuation of high glucose-induced mitochondrial O2·− production were also determined. The data are presented as the fold change in the median intensity of MitoSox fluorescence when compared with the respective controls.
In other experiments oxidative stress was induced in CAECs by TNF-α treatment (10 ng/ml for 24 h). To assess the effects of resveratrol on TNF-α-induced cellular ROS production, we used the cell-permeant oxidative fluorescent indicator dye dihydroethidine (DHE, Invitrogen) as we previously reported (7). In brief, the cells were washed with warm PBS and incubated with DHE (3 μM at 37°C for 30 min). The buildup of DHE fluorescence over time was compared by flow cytometry (Guava Easycyte). The effects Nrf2 knockdown or Keap-1 overexpression on resveratrol-induced attenuation of TNF-α-induced cellular ROS production were also determined.
Assessment of resveratrol-induced inhibition of apoptotic cell death in CAECs.
To determine the efficacy of resveratrol to protect against oxidative stress-induced apoptosis, CAECs grown in 96-well plates were treated with high glucose (30 mM for 72 h). After the treatment period, the ratio of terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL)-positive cells, a marker of apoptosis, was determined by flow cytometry (Guava Easycyte) using the Guava TUNEL Assay (Millipore) according to the manufacturer's guidelines. The effects of Nrf2 knockdown or Keap-1 overexpression on resveratrol-induced attenuation of high glucose-induced apoptosis were determined.
Animal studies.
Male ICR wild-type mice (Nrf2+/+) were purchased from Taconic, and male Nrf2 knockout mice on an ICR background (Nrf2−/−) were obtained from a well-characterized colony (30) at the National Institute on Aging. In this study only male mice were studied to exclude the possible confounding effects of the estrous cycle in females. The mice were housed in an environmentally controlled vivarium with unlimited access to water and a controlled photoperiod (12-h light:12-h dark). Body weight was recorded biweekly. All mice were maintained according to National Institutes of Health's guidelines, and all animal use protocols were approved by the Institutional Animal Care and Use Committees of the participating institutions. At 20 wk of age, mice were assigned to three groups and were fed a standard AIN-93G diet or AIN-93G modified to provide 60% of calories from fat (HFD) or a HFD plus resveratrol (2.4 g resveratrol per kg diet), as described previously (29). The Nrf2+/+ (n = 10) and Nrf2−/− (n = 16) mice fed the HFD gained 43.0 ± 3.0% and 42.0 ± 4.6% of their starting weight, respectively. The Nrf2+/+ (n = 10) and Nrf2−/− (n = 15) mice fed HFD + resveratrol trended toward a decrease with weight gains of 33.0 ± 4.3% and 31.8 ± 4.3%, respectively. Resveratrol significantly decreased body weight gain percentage when the genotypes were pooled (P < 0.05). There was considerable variation in weight gain in the mice, and the intent of the studies was to determine the influence of resveratrol and Nrf2 on endothelial function, independent of body weight gain differences. Therefore, we selected a cohort of mice (n = 6) from the larger population with a mean body weight percent gain of ∼40%. The mean body weight percent gain of the cohort analyzed in Figs. 3 and 4 was 40.6 ± 4.7% and 40.3 ± 4.6% for Nrf2+/+ mice fed the HFD and HFD + resveratrol diets, respectively, whereas the HFD-fed Nrf2−/− mice gained 42.3 ± 4.1% and resveratrol-fed Nrf2−/− mice gained 41.8 ± 4.6%. There were no significant differences in body weight percentage gained for this cohort (P = 0.987), which indicates that the results in Figs. 3 and 4 are independent of body weight gain differences.
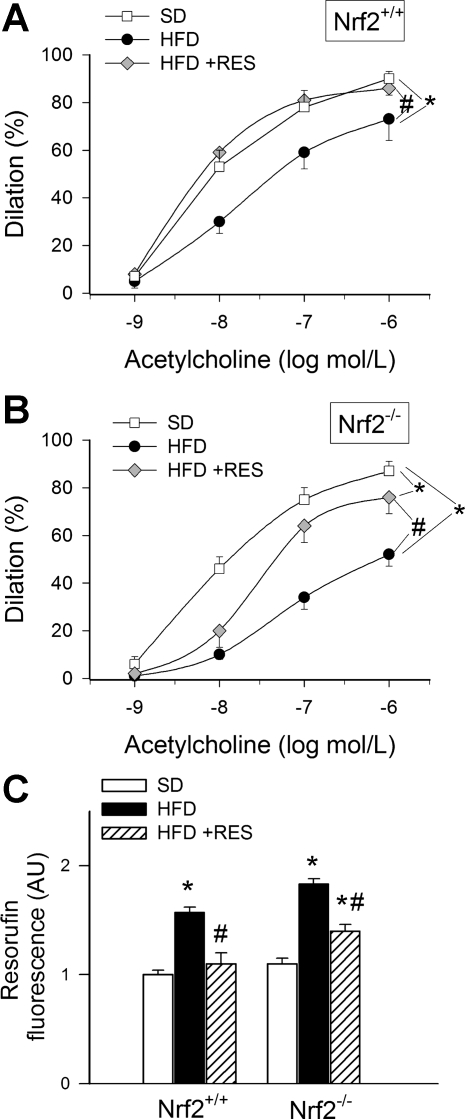
Fig. 3.
Dilation of skeletal muscle arterioles isolated from Nrf2+/+ mice (A) and Nrf2−/− mice (B) in response to increasing concentrations of acetylcholine. Mice were fed a standard diet (SD), high-fat diet (HFD), or HFD enriched with Res. Data are means ± SE; n = 5 in each group. C: results from Amplex red/horseradish peroxidase assay. Data are normalized resorufin fluorescences, representing ROS production by segments of the femoral arteries of Nrf2+/+ mice and Nrf2−/− mice, fed a SD, HFD or HFD enriched with Res. AU, arbitrary units. *P < 0.05 vs. untreated; #P < 0.05 vs. no Res.
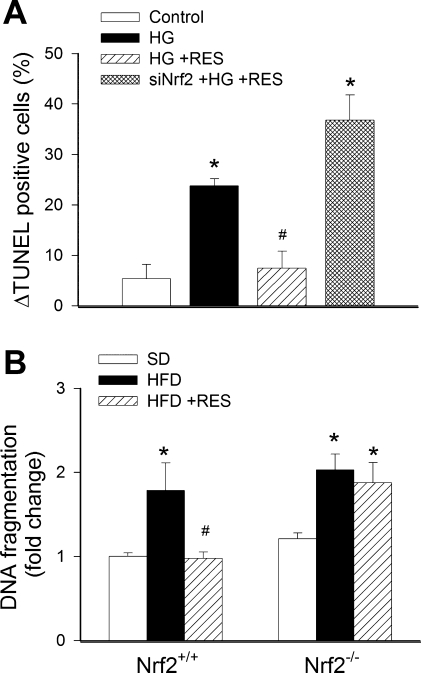
Fig. 4.
A: in primary human coronary arterial endothelial cells, HG significantly (P < 0. 05 vs. control) increased apoptotic cell death as shown by the increased terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) positivity (flow cytometry). Treatment with Res prevented HG-induced endothelial apoptosis (#P < 0. 05 vs. HG only). By contrast, in endothelial cells with siNrf2, Res failed to inhibit HG-induced endothelial apoptosis. Data are means ± SE; n = 6 for each group. B: DNA fragmentation in femoral arterial branches of Nrf2+/+ mice and Nrf2−/− mice fed a SD, HFD, or HFD enriched with Res. Data are means ± SE; n = 6 for each group. *P < 0.05 vs. untreated; #P < 0.05 vs. no Res.
Assessment of the effect of resveratrol treatment on microvascular endothelial function.
The animals were euthanized at 36 wk of age, and skeletal muscle arterioles were prepared as previously described (37, 38, 41). In brief, the gracilis muscle was removed and arterioles were isolated using microsurgery instruments. The arterioles were mounted onto two glass micropipettes in a vessel chamber and pressurized to 60 mmHg. The inner arteriolar diameter was measured with a video micrometer system and continuously recorded using a computerized data acquisition system. All vessels were allowed to stabilize for 60 min in oxygenated (21% O2-5% CO2, balanced with N2) Krebs buffer (at 37°C). After the equilibration period, during which spontaneous myogenic tone (∼50%) developed, the arteriolar dilation to cumulative doses of acetylcholine (10−9 to 10−6 mol/l) was measured.
Measurement of vascular ROS production.
Cellular ROS production was measured fluorometrically in femoral arterial segments using the Amplex red/horseradish peroxidase assay in the presence of PEG-SOD as previously described (11). The H2O2 generation rate, normalized to tissue mass, was compared by measuring the time course of the buildup of resorufin fluorescence for 60 min by a Tecan Infinite M200 plate reader.
Assessment of apoptotic cell death in vascular tissue.
To determine the effect of Nrf2 depletion on the antiapoptotic effects of resveratrol in vivo, segments of the femoral arteries were lysed and cytoplasmic histone-associated DNA fragments, which indicate apoptotic cell death, were quantified by the Cell Death Detection ELISAPlus kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol as previously described (10, 40). The results are reported as arbitrary optical density units normalized to protein concentration.
Caspase-3/7 activities in tissue lysates were measured using Caspase-Glo 3/7 assay kits according to the manufacturer's instruction (Promega, Madison, WI) as previously reported (10, 40). In 96-well plates, a 50-μl sample was gently mixed for 30 s with 50 μl Caspase-Glo-3/7 reagent and incubated for 2 h at room temperature. The lysis buffer with the reagent served as blank. Luminescence of the samples was measured using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC). Luminescent intensity values were normalized to the sample protein concentration.
Data analysis.
Gene expression data were normalized to the respective control mean values. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey's post hoc test, as appropriate. P < 0.05 was considered statistically significant. Data are expressed as means ± SE.
RESULTS
Resveratrol increases transcriptional activity of Nrf2 and upregulates Nrf2/ARE-driven genes in CAECs.
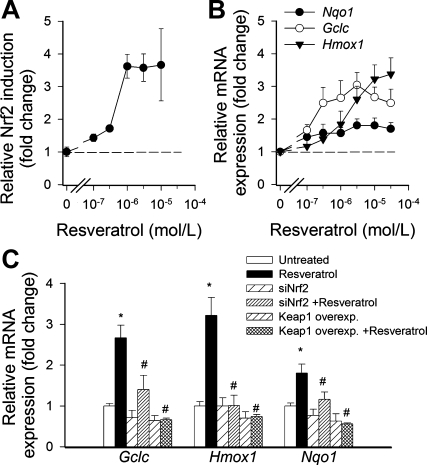
To determine the effect of resveratrol on Nrf2 activation, we transiently transfected CAECs with a Nrf2/ARE-driven reporter gene construct and then treated the cells with resveratrol. A significant, concentration-dependent increase in luciferase activity over the vector control was noted upon stimulation with resveratrol (Fig. 1A). Resveratrol, in a concentration-dependent manner, also significantly increased mRNA expression of the known Nrf2 targets Nqo1, Gclc, and Hmox1 (Fig. 1B). Overexpression of Keap-1 or siRNA knockdown of Nrf2 prevented resveratrol-induced upregulation of Nqo1, Gclc, and Hmox1 (Fig. 1C).
Fig. 1.
A: reporter gene assay showing the effects of resveratrol on nuclear factor-E2-related factor-2 (Nrf2)/antioxidant response element reporter activity in cultured primary human coronary arterial endothelial cells. Cells were transiently cotransfected with antioxidant response element-driven firefly luciferase and cytomegalovirus-driven renilla luciferase constructs followed by resveratrol (Res) treatment. Cells were then lysed and subjected to luciferase activity assay. After normalization, relative luciferase activity was obtained from 4 to 6 independent transfections. Data are means ± SE. The effect of Res was significant (P < 0.05) at each concentration used. B: effect of Res on mRNA expression of NAD(P)H:quinone-oxidoreductase 1 (Nqo1), γ-glutamylcysteine synthetase (GCLC), and heme oxygenase-1 (Hmox1) in cultured primary human coronary arterial endothelial cells. Data are means ± SE; n = 5 in each group. The effect of Res was significant (P < 0.05) at each concentration used. C: the effects of small interfering RNA (siRNA) downregulation of Nrf2 (siNrf2) or overexpression (Overexp) of Kelch-like erythroid cell-derived protein 1 (Keap-1) on Res (10 μmol/l)-induced mRNA expression of Nqo1, Gclc, and Hmox1 in cultured primary human coronary arterial endothelial cells. Data are means ± SE; n = 5 in each group. *P < 0.05 vs. control; #P < 0.05 vs. Res only.
Nrf2 contributes to the antioxidative effects of resveratrol in CAECs.
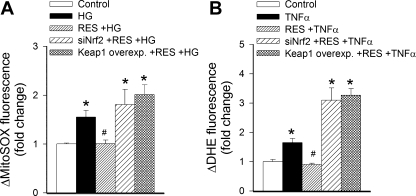
A series of experiments was conducted to examine the role of Nrf2 in the effects of resveratrol on the production of ROS in CAECs. First, MitoSox fluorescence intensities in CAECs were compared using flow cytometry (Fig. 2A). We found that high-glucose treatment elicited substantial increases in MitoSox fluorescence in CAECs (Fig. 2A). Pretreatment of cells with resveratrol (for 24 h) significantly attenuated MitoSox fluorescence in CAECs treated with high glucose (Fig. 2A). In contrast, in CAECs with an overexpression of Keap-1 or siRNA knockdown of Nrf2, resveratrol failed to significantly attenuate high glucose-induced increases in MitoSox fluorescence (Fig. 2A). Similar results were obtained when DHE fluorescence was compared in CAECs (Fig. 2B). We found that treatment with TNF-α elicited substantial increases in DHE fluorescence in CAECs (Fig. 2B). Pretreatment of CAECs with resveratrol prevented TNF-α-induced increases in DHE fluorescence (Fig. 2B). In contrast, in CAECs with an overexpression of Keap-1 or siRNA knockdown of Nrf2, resveratrol failed to attenuate significantly TNF-α-induced increases in DHE fluorescence (Fig. 2B).
Fig. 2.
In primary human coronary arterial endothelial cells, high glucose (HG, 30 mmol/l; A) and TNF-α (10 ng/ml; B) induce mitochondrial and cellular oxidative stress, as shown by the significant increases in the mean fluorescence intensity of oxidized MitoSox (A) and dihydroethidine (DHE; B), respectively. Res treatment significantly attenuates both HG-induced mitochondrial oxidative stress (A) and decreases TNF-α-induced cellular O2·− levels (B). Both of these effects are prevented by siRNA downregulation of Nrf2 and overexpression of Keap-1. Data are means ± SE; n = 6 in each group. *P < 0.05 vs. baseline; #P < 0.05 vs. no Res.
Nrf2 contributes to the vasoprotective effects of resveratrol in HFD-fed mice.
We compared male Nrf2+/+ and Nrf2−/− mice (n = 6 in each group) with similar initial body weights after 16 wk of the HFD. Fasting blood glucose levels significantly differed between the two strains on the control diet (Nrf2+/+, 5.5 ± 0.6 mmol/l; and Nrf2−/−, 2.8 ± 0.2 mmol/l), yet both strains developed comparable relative hyperglycemia on the HFD (Nrf2+/+, 8.6 ± 0.4 mmol/l; and Nrf2−/−, 5.7 ± 0.8 mmol/l). Resveratrol treatment did not result in significant differences in fasting blood glucose levels in Nrf2+/+ and Nrf2−/− mice on HFD (Nrf2+/+, 8.6 ± 0.9 mmol/l; and Nrf2−/−, 5.7 ± 0.3 mmol/l, not significant vs. HFD alone), but glucose tolerance was not assayed.
The HFD elicited significant vasodilator dysfunction in arteriolar branches of the femoral artery in both Nrf2+/+ and Nrf2−/− mice, as shown by the impaired dilator responses to acetylcholine (Fig. 3, A and B). Resveratrol treatment restored acetylcholine-induced dilations to control levels in arterioles of HFD-fed Nrf2+/+ mice (Fig. 3A). In contrast, resveratrol treatment resulted only in a partial improvement of acetylcholine-induced dilations in arterioles of HFD-fed Nrf2−/− mice, and the difference between acetylcholine-induced responses in these vessels and those from control diet-fed Nrf2−/− mice remained significant (Fig. 3B).
Vascular ROS generation was measured by the Amplex red/horseradish peroxidase method. We found that a HFD significantly increased ROS production in the femoral arteries of both Nrf2+/+ mice and Nrf2−/− mice. Resveratrol treatment restored vascular ROS production to control levels in Nrf2+/+ mice (Fig. 3C). In contrast, resveratrol treatment resulted only in a partial attenuation of ROS production in vessels of HFD-fed Nrf2−/− mice, and the difference between ROS production in these vessels and those from control diet-fed Nrf2−/− mice remained significant (Fig. 3C).
Nrf2 contributes to the antiapoptotic effects of resveratrol.
In CAECs, high-glucose treatment elicited substantial increases in the rate of apoptotic cell death, as indicated by the increased number of TUNEL-positive cells (Fig. 4A). Resveratrol treatment significantly decreased the number of apoptotic cells, an effect that was prevented by siRNA knockdown of Nrf2 (Fig. 4A). To assess the in vivo relevance of our findings, we assessed markers of apoptosis in homogenates of femoral arteries from mice in each group. We found that the HFD promoted apoptosis, indicated by the increased rate of DNA fragmentation (Fig. 4B) and caspase-3 activity (not shown) in vessels of both Nrf2+/+ mice and Nrf2−/− mice. Resveratrol treatment prevented vascular apoptotic cell death in Nrf2+/+ mice, indicated by the normal levels of DNA fragmentation (Fig. 4B) and caspase-3 activity (not shown). In contrast, resveratrol treatment failed to prevent apoptosis in vessels of HFD-fed Nrf2−/− mice (Fig. 4B).
DISCUSSION
Resveratrol exerts significant vasoprotective effects in pathophysiological conditions associated with the increased production of ROS (4, 9, 27, 29, 39, 48). However, the mechanisms that contribute to the endothelial protective effects of resveratrol are only partially understood. Here we provide evidence that in human CAECs, resveratrol increases the transcriptional activity of Nrf2 and upregulates several ARE-regulated genes involved in free radical metabolism in an Nrf2-dependent manner (Fig. 1). The concentrations of resveratrol, which elicit significant Nrf2 activation (Fig. 1) and Nrf2-dependent induction of major cellular antioxidant enzymes (39, 40), effectively attenuate cellular and mitochondrial oxidative stress in cultured endothelial cells (Fig. 2). The findings that the knockdown of Nrf2 or the overexpression of Keap-1 abrogate the protective effects of resveratrol against high glucose- and TNF-α-induced oxidative stress (Fig. 2, A and B) provide direct evidence that in vascular endothelial cells, Nrf2 contributes to the antioxidative action of resveratrol. The concentration range of resveratrol in which it activates Nrf2 is physiologically relevant. Although such levels are not generally achieved through the consumption of Mediterranean diets or red wine, when resveratrol is used as a dietary supplement, it can achieve micromolar levels in the plasma (2). It is significant that in endothelial cells and other cell types (3, 18, 24, 32), resveratrol activates Nrf2 and attenuates oxidative stress at lower concentrations than the resveratrol levels needed for activation/induction of silent information regulator 2/sirtuin 1 (SIRT1) in vitro (17, 46). Interestingly, recent studies called into question the ability of resveratrol to directly activate SIRT1, raising the possibility that resveratrol acts on a target upstream from SIRT1 (28). Because the interaction between Nrf2 and SIRT1 in endothelial cells is not well understood, future studies are warranted to elucidate whether Nrf2 contributes to the resveratrol-induced upregulation of SIRT1 in endothelial cells.
As noted previously, we have demonstrated that resveratrol effectively attenuates vascular oxidative stress and improves endothelial function in animal models of type 2 diabetes and the metabolic syndrome (29, 48). Results from the present study show for the first time that the genetic depletion of Nrf2 abrogates the ability of resveratrol to improve endothelial function (Fig. 3, A and B) and prevent oxidative stress in HFD-fed mice (Fig. 3C). These findings demonstrate that Nrf2 is a physiologically important mediator of the vasoprotective effects of resveratrol. Previous studies have attributed some of the vascular effects of resveratrol to the activation of estrogen receptors (20), but their role in resveratrol-induced Nrf2 activation is not understood. Recent studies suggest that resveratrol can recapitulate many of the molecular events downstream of caloric restriction in vivo (29, 33). In that context, it is significant that caloric restriction also induces Nrf2-driven genes in various tissues (30). It should be noted, however, that resveratrol is also likely to exert vasoprotective effects, which are likely to be independent of the direct induction of Nrf2 in the vascular wall. For example, resveratrol was reported to induce endothelial nitric oxide synthase, increasing the synthesis and bioavailability of nitric oxide (8, 45, 48), and to downregulate NAD(P)H oxidases, which likely also contribute to the vasoprotective effects of resveratrol in type 2 diabetes (34, 48) and aging (29). In addition, resveratrol may also regulate microvascular function indirectly through the modulation of the paracrine/endocrine function of adipose tissue (47, 49).
In addition to clarifying the role of Nrf2 with respect to the attenuation of endothelial oxidative stress and the improvement of endothelial function by resveratrol, the results of our studies provide strong evidence that Nrf2-dependent mechanisms also contribute to the prevention of programmed endothelial cell death by resveratrol treatment. Several lines of evidence support this intriguing point of view. First, the molecular disruption of Nrf2 signaling abrogates the antiapoptotic effects of resveratrol in cultured endothelial cells (Fig. 4A). Second, our previous studies demonstrated that in human CAECs, resveratrol induces the Nrf2 targets catalase, glutathione peroxidase, and heme oxygenase and the pharmacological inhibition of these enzymes also significantly attenuates resveratrol-mediated protection against oxidative stress-induced apoptosis (40). In keeping with this point of view, we previously found that resveratrol treatment in vivo also attenuates endothelial apoptosis in HFD-fed mice, which is associated with a reduction of oxidative stress (29). The findings that the genetic depletion of Nrf2 abrogates the antiapoptotic effects of resveratrol in vascular tissues of HFD-fed mice (Fig. 4B) provide further evidence that resveratrol exerts its vasoprotective effects, at least in part, via Nrf2-dependent pathways.
Conclusion.
The results of this study suggest that the activation of the Nrf2/ARE pathway has a critical role in the endothelial protective effects of resveratrol both in vitro and in laboratory animals. Resveratrol concentrations sufficient to activate Nrf2 in vitro are achievable in humans by the consumption of dietary supplements containing resveratrol (2). Thus we posit that the activation of Nrf2-driven pathways by resveratrol treatment can importantly contribute to an intervention strategy for the prevention of cardiovascular diseases in humans as well.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-077256 and P01-AG-11370 and grants from the American Diabetes Association (to Z. Ungvari), the American Federation for Aging Research (to A. Csiszar), and the Intramural Research Program of the NIH (to R. de Cabo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun 331: 993–1000, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol 102: 1520–1527, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 168: 629–638, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNF-alpha treatment in aging. Am J Pathol 170: 388–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111: 2364–2372, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 16.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99: 779–785, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hsieh TC, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem 2: 275–285, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, Kromhout D, Nedeljkovic S, Punsar S, Seccareccia F, Toshima H. The diet and 15-year death rate in the seven countries study. Am J Epidemiol 124: 903–915, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22: 2185–2197, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L478–L488, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946–H956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res 53: 6–15, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay P, Rajesh M, Haskó G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson SK, Tucker GA, Brameld JM. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc 67: 42–47, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA 105: 2325–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 293: H610–H619, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol 591: 66–72, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 3: 31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol 60, Suppl 4: 111–116, 2009 [PubMed] [Google Scholar]
- 35.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 290: E1339–E1346, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 23: 418–424, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 165: 219–226, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungvari Z, Koller A. Endothelin and PGH2/TXA2 enhances myogenic constricton in hypertension by increasing Ca2+ sensitivity of arteriolar smooth muscle. Hypertension 36: 856–861, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ungvari Z, Pacher P, Rischak K, Szollar L, Koller A. Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler Thromb Vasc Biol 19: 1899–1904, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and type 2 diabetes: evidence of an adipose-vascular loop. Am J Biomed Sci 1: 133–142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Yong W, Wu X, Yu Y, Lv J, Liu C, Mao X, Zhu Y, Xu K, Han X, Liu C. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun 369: 471–477, 2008 [DOI] [PubMed] [Google Scholar]