accumulating evidence suggests that Mediterranean diets rich in plant-derived polyphenols may be one of the factors responsible for the lower incidence of coronary heart disease among Mediterranean populations (16, 21). Resveratrol (3,4′,5-trihydroxystilbene), a plant-derived polyphenolic compound belonging to a class of stilbenes (abundantly found in some roots, grapes, berries, peanuts, etc.) received rekindled scientific attention following its identification in red wine almost two decades ago (29). It has been speculated that resveratrol might be the red wine constituent to provide an explanation for the phenomenon known as the “French paradox” (French people suffer a relatively low incidence of coronary heart disease presumably because of the consumption of red wine) (14). Reports on the potential for resveratrol to extend life span in cell culture and in lower model organisms (18, 39, 41) and to inhibit the development of cancer (19) have continued to generate tremendous interest to further investigate the mechanisms and/or the potential therapeutic benefits of this natural compound both in vitro as well as in different preclinical disease models (4, 15). Resveratrol attenuated myocardial ischemic-reperfusion injury and atherosclerosis (14) as well as was shown to confer vasoprotection in rodent models of metabolic diseases (26, 28, 32, 34, 43) and in aged mice without extending life span (26, 37). The available evidence has suggested that it can mimic, at least in part, the antiaging effects of caloric restriction in rodents (2, 3, 30).
Despite the growing evidence that resveratrol confers cardiac and vascular protective effects in preclinical disease models, the precise molecular and cellular mechanisms of its action remain elusive. From the recent literature the view emerges that resveratrol elicits complex cellular responses by promoting cell survival, maintaining cellular energetics, and attenuating proinflammatory phenotypic changes induced by oxidative stressors.
In this issue of the American Journal of Physiology-Heart and Circulatory Physiology, Ungvari et al. (35) provide evidence that the activation of NF-E2-related factor-2 (Nrf2) is an important mechanism by which resveratrol exerts its beneficial effects in the vascular endothelium. Nrf2 is a transcription factor that regulates coordinated key antioxidant responses in cells, and its activation is therefore capable of protecting in a wide variety of animal models of oxidative stress-related injury and inflammatory disease (22). Ungvari et al. (35) demonstrate that in cultured human coronary arterial endothelial cells, resveratrol increases the transcriptional activity of Nrf2, which is associated with the upregulation of several Nrf2 target genes such as NAD(P)H:quinone oxidoreductase 1, γ-glutamylcysteine synthetase, and heme oxygenase-1. Many of these Nrf2 targets [e.g., NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1] have been implicated in promoting endothelial health under conditions of metabolic stress (37). Indeed, resveratrol treatment was shown to attenuate mitochondrial and cellular oxidative stress induced by hyperglycemia in endothelial cells in vitro, and these effects were significantly reversed by the small interfering RNA-mediated knockdown of Nrf2 or by the overexpression of the protein that inactivates Nrf2 (Kelch-like erythroid cell-derived protein 1) (35). Importantly, the in vivo relevance of resveratrol-induced Nrf2 activation was supported by using Nrf2 knockout mice fed on a high-fat diet, in which the endothelial protective effects of resveratrol were largely diminished compared with those of their wild-type littermates fed on the same diet.
Recent studies provide compelling evidence that resveratrol treatment can improve endothelial function in rodents models of type 2 diabetes (26, 36, 44), as well as in aged mice and rats (26, 37), by attenuating reactive oxygen/nitrogen species generation and vascular inflammation and by decreasing endothelial apoptosis. Since resveratrol can activate Nrf2-driven pathways at concentrations that were likely achieved in these rodent models, one can envision that Nrf2 activation may also contribute to the vasoprotective effects of resveratrol observed in diabetic and aging animals. As mentioned above, resveratrol can mimic some of the molecular events characteristic of caloric restriction (30, 38), which can also induce Nrf2 activation in rodents (27).
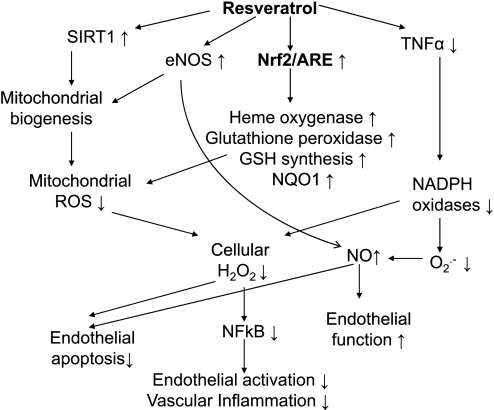
There are most likely multiple synergistic effects of resveratrol that are responsible for its vasoprotective potential (Fig. 1). These may include an upregulation of endothelial nitric oxide synthase causing an increased nitric oxide bioavailability (5, 33, 44), a promotion of mitochondrial biogenesis (8), an inhibition of NF-κB (9, 10, 42), a downregulation of TNF-α, an inhibition of NADPH oxidases (44), and a proliferation of vascular smooth muscle cells (7, 40), an attenuation of mitochondrial reactive oxygen species generation (36), and an inhibition of platelet aggregation (31).
Fig. 1.
Potential targets of beneficial effects of resveratrol in cardiovascular disease. SIRT1, silent information regulator 2/sirtuin 1; eNOS, endothelial nitric oxide (NO) synthase; Nrf2, NF-E2-related factor-2; ARE, antioxidant response element; ROS, reactive oxygen species; NQO1, NAD(P)H:quinone-oxidoreductase 1.
Because vascular oxidative-nitrosative stress, endothelial activation, and an increased rate of endothelial cell death are hallmarks of cardiovascular aging and are known to be involved in the pathophysiology of multiple diabetic complications (1, 11–13, 20, 23, 24), it is desirable to develop pharmacological treatment approaches that can simultaneously target several of these pathophysiological processes. Another potential interesting, but much more risky, avenue (in terms of unexpected side effects) is the targeting of master regulators of cellular programs such as Nrf2. Nevertheless, based on the results of the preclinical studies discussed above, together with the absence of the significant adverse effects of resveratrol in humans (4), resveratrol may offer a safe way to pharmacologically target several simultaneous pathways involved in the development of cardiovascular diseases in high-risk patients. However, to achieve this goal, the poor oral absorption and bioavailability of resveratrol in humans should be resolved by the optimization of formulation and delivery, and the promising animal data should be confirmed in controlled human trials.
Until very recently, it was widely held that resveratrol is a direct activator of the silent information regulator 2/sirtuin 1 (SIRT1) pathway that was proposed to be the major effector for many of the biological actions of resveratrol (4). SIRT1 and other sirtuins catalyze NAD+-dependent protein deacetylation and are critical regulators of transcription, apoptosis, and metabolism (17). Even though recent studies using cell-free assays question that resveratrol activates SIRT1 directly (25), it is very likely that resveratrol (or its metabolites) in vivo or ex vivo can promote SIRT1 activation. Importantly, the overexpression of SIRT1 can mimic many of the effects of resveratrol in endothelial cells (36), whereas the depletion of SIRT1 tends to attenuate resveratrol-induced cellular effects (6–9). On the basis of the available evidence, it is possible that SIRT1 acts as a permissive factor, modulating Nrf2-driven responses in the vasculature. Further studies are also warranted to test the possibility that the expression of SIRT1 is regulated by Nrf2 and that downstream pathways regulated by SIRT1 and Nrf2 might act synergistically.
Collectively, the present study of Ungvari et al. (35) describes some exciting novel findings that contribute to our understanding of the multifaceted cardiovascular protective effects of resveratrol treatment in mammals, and it also raises new questions on the possibility of the interaction between Nrf2 and SIRT1, which remains to be seen in upcoming studies.
GRANTS
P. Pacher was supported by the Intramural Program of the National Institute on Alcohol Abuse and Alcoholism. G. Haskó was supported by National Institute of General Medical Sciences Grant R01-GM-66189.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 3: e2264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, Beemster P, De Jong JW. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol 465: 115–123, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol 105: 1333–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv 6: 36–47, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Das DK, Mukherjee S, Ray D. Resveratrol and red wine, healthy heart and longevity. Heart Fail Rev. In press [DOI] [PubMed] [Google Scholar]
- 16.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99: 779–785, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218–220, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med 40: 183–192, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 169: 659–669, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev 25: 235–260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacholec M, Chrunyk BA, Cunningham D, Flynn D, Griffith DA, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA 105: 2325–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 76: 69–75, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic 43: 49–52, 1992 [Google Scholar]
- 30.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 3: 31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stef G, Csiszar A, Lerea K, Ungvari Z, Veress G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J Cardiovasc Pharmacol 48: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 290: E1339–E1346, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Taubert D, Berkels R. Upregulation and activation of eNOS by resveratrol. Circulation 107: e78–e79, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med 43: 720–729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ungvari Z, Bagi Z, Feher A, Recchia F, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol (April23, 2010). doi:10.1152/ajpheart.00260.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 16: 296–300, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem Biophys Res Commun 346: 367–376, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]