Abstract
Osteoarthritis (OA) is a risk factor for physical inactivity and impaired mobility, but it is not well understood how these locomotor behaviors are affected by the age of onset of OA and disease severity. Male mice homozygous for a Col9a1 gene inactivation (Col9a1−/−) develop early onset knee OA, increased tactile pain sensitivity, and gait alterations by 9 mo of age. We hypothesized that aged Col9a1−/− mice would reduce joint pain by adopting locomotor behaviors that reduce both the magnitude and daily frequency of joint loading. We tested this hypothesis by evaluating gait and spontaneous locomotor activity in 15- to 17-mo-old male Col9a1−/− (n = 5) and Col9a1+/+(WT) (n = 5) mice using well-controlled measures of voluntary activity in overground and running wheel conditions, as well as studies of gait in a velocity-controlled treadmill. We found no difference due to genotype in freely chosen locomotor velocity, stride frequency, hindfoot duty factor, dark phase activity time, or dark-phase travel distance during overground, running wheel, or speed-matched treadmill locomotion. Interpretation of these findings is potentially confounded by the observation that WT mice have greater knee OA than Col9a1−/− mice in the lateral tibial plateau by 17 mo of age. When accounting for individual differences in knee OA, functional locomotor impairments in aged Col9a1−/− and WT mice are manifested as reductions in total locomotor activity levels (e.g., both distance traveled and time active), particularly for wheel running. These results support the concept that current disease status, rather than age of disease onset, is the primary determinant of impaired locomotor activity with aging.
Keywords: osteoarthritis, knee joint, mouse model, col9a1, gait, physical activity
osteoarthritis (OA) is a degenerative joint disease characterized by structural changes in the articular cartilage, menisci, and synovium in diarthrodial joints (15, 19). OA is associated with inflammation, pain, and impaired function in the affected joints, and is predicted to affect >25% of the adult US population by 2030 (24) with at least 9% of that population reporting activity limitations. OA has been linked to physical inactivity, presumably because weight-bearing activities such as walking and running cause joint pain (33). People with arthritis are less likely to meet recommended levels of moderate and vigorous activity; furthermore, they are active for shorter durations and are more likely to be inactive than nonarthritic peers (14, 25, 37). In addition, individuals with OA alter their gait mechanics in ways that appear to reduce joint stress (2, 23, 27, 30, 32). The relationships between gait mechanics, activity, and knee OA, however, are not well understood. In this regard, animal models of OA provide novel means of investigating mechanistic relationships between parameters of locomotion and disease severity.
In a previous study, we reported that mice homozygous for a Col9a1 gene inactivation (Col9a1−/−) develop early-onset knee OA, intervertebral disc degeneration (12, 26), and gait alterations that appear to reduce joint loads (5). Since the presence of functional type IX collagen is necessary for proper assembly and organization of type II collagen, the extracellular matrix of cartilaginous tissues exhibits an altered collagen fibrillar structure assembly. In particular, such changes in the extracellular matrix of articular cartilage contribute to altered tissue mechanical properties as well as disrupted chondrocyte-matrix interactions that regulate protease expression and activity. Previous studies suggest that these changes are ultimately responsible for the spontaneous development of premature cartilage degeneration in the intervertebral disc, knee, and temporomandibular joints (12, 26, 29).
In studies of animal function, 9-mo-old Col9a1−/− mice ambulated with slower velocities and used higher duty factors (i.e., the ratio of foot-ground contact time to stride time) and shorter stride lengths during unprompted locomotion conditions compared with wild-type (WT) controls (5). These compensatory gait changes in the Col9a1−/− mice were suggested to reduce the magnitude of joint loading, as animals may move at slower speeds and use higher duty factors to reduce the peak magnitude of vertical ground reaction force and thus attenuate weight-bearing induced joint pain (3). In addition, animals may reduce weight bearing-induced joint pain by reducing their voluntary activity patterns to lessen the frequency of joint loading, as observed for the lower magnitudes of self-selected gait velocity reported for these Col9a1−/− mice. While these gait patterns are consistent with factors that reduce both magnitude and frequency of joint loading, differences in the animals' self-selected gait velocity may confound gait parameter comparisons between WT and Col9a1−/− groups. Furthermore, comparison of daily spontaneous locomotor activity levels may reveal more substantial differences in joint loading frequency. Studies that report on activity levels (i.e., total activity time or travel distance) and make controlled use of running speed have great value in revealing the specific contributions of joint pathology to locomotor function in OA.
The goal of this study was to evaluate gait and activity in the Col9a1−/− mouse model using well-controlled measures of voluntary activity in overground and running wheel conditions, as well as studies of gait in a velocity-controlled treadmill. We hypothesized that animals with early-onset OA would adopt behavioral strategies that would alter both the magnitude and daily frequency of joint loading as assessed by well-controlled and velocity-independent measures of activity. To test this hypothesis, we chose to study the spontaneous locomotor patterns and gait of aged male Col9a1−/− mice that were previously shown to develop a moderate to severe knee joint OA phenotype. We studied 15- to 17-mo-old animals to better understand how an increased lifetime exposure to joint disease contributed to altered locomotor behaviors in aged animals. As spontaneous wheel running distances may exceed overground cage activity distances by an order of magnitude (4, 20, 38), data were obtained from both overground and wheel running conditions to isolate effects arising from differences in total activity amongst these two conditions. Furthermore, wheel running may allow the limbs to stay in contact with the surface for a longer period of time during stride (i.e., higher duty factor) and result in lower ground reaction forces than overground running. Thus, wheel running may be associated with not only higher frequency of activity, but also lower magnitude of joint loading compared with overground. For these reasons, the study design was developed to acquire data for both spontaneous overground and wheel running activities to test for an intrinsic ability for Col9a1−/− mice to alter their frequency or magnitude of joint loading, compared with WT controls. By comparing spontaneous activity and gait patterns during wheel and overground activity, these data reveal how animals with premature onset of knee joint OA cope by altering joint loading magnitude or frequency.
MATERIALS AND METHODS
Animals.
Ten 15-mo-old male mice from the strain C57Bl/6J were acquired from a colony housed at Duke University, which was bred from a colony at Harvard University (16, 26). Both wild-type (Col9a1+/+) and homozygous (Col9a1−/−) mice were studied (n = 5 for each genotype). Male mice were chosen because previous data have shown that knockout male mice of this strain develop more severe knee OA compared with female mice (5). Mice were given food and water ad libitum and were group and individually housed in a temperature (22° C)- and humidity-controlled (45%) room. There was no significant difference in body mass due to col9a1 deficiency (35.8 ± 1.5 vs. 33.0 ± 1.4 g, WT vs. Col9a1−/−, P = 0.20). All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
Voluntary locomotor activity levels.
Spontaneous locomotor activity measurements were made for both overground locomotion and wheel running. We first measured overground voluntary activity by placing mice in individual 21 × 21 × 30 cm open field chambers (Accuscan Instruments, Columbus, OH) with a 14:10-h light/dark cycle for three consecutive days (17). Horizontal activity (m) and speeds (m/min) were determined using the Accuscan analysis software, and average values were reported for each half hour time block of the testing period.
After open-field testing, mice were placed in individual cages (13 × 13 × 30 cm) containing 10.6 cm diameter running wheels (1) with a 10:14-h light/dark cycle. After a period of familiarization (3 days with wheels locked), mice were allowed to run freely on the wheels. Data were collected daily for a period of 2 wk. Voluntary wheel running activity levels were calculated through the use of a National Instruments Compact-DAQ, an NI 9411 module, and a custom-designed LabVIEW program, which converted wheel revolution data to daily running distances and speeds using a 10-s time block. By the final 3 days of the 2-wk running period, all of the mice had habituated to the wheels and were running consistent daily distances. Therefore, we compared average distances run on the wheel to average distances run overground using the final 3 days of wheel running data.
Gait kinematics.
We collected high-speed kinematic data of the mice during treadmill and wheel locomotion to compare joint loading parameters between genotypes and locomotor modes. High-speed digital video images were collected at 200 Hz (Phantom V4.2, Vision Research, Wayne, NJ) (5) to determine the following parameters: contact time (duration of time a limb is in contact with the substrate) (s), stride time (s), duty factor (contact time divided by stride time), stride frequency (Hz), and instantaneous speed (m/min). These parameters were calculated from the right hindlimb and forelimb foot strike and toe-off events for each animal using DLTdataviewer2 (21).
Wheel running gait kinematic data were collected at the start of the dark period on the 12th day of voluntary running. The camera was placed perpendicular to the wheel to collect a sagittal view, and a mirror angled at 45° below the running wheel cage provided a ventral view. Between 3 and 11 strides (median = 7) were recorded for each animal. Markers on the crossbar of the running wheel were tracked using DLTdataviewer2 to determine the angular velocity of the wheel (change in angle divided by change in time). Angular velocity was converted to linear velocity by multiplying by π*d/360°, where d is the diameter of the wheel.
We used a treadmill to collect overground data because it allowed us to make speed-matched kinematic comparisons between genotypes and to the running wheel. We habituated the mice to locomotion on a single lane mouse treadmill (Columbus Instruments, Columbus, OH) prior to data collection by allowing for a period of exploration with the treadmill turned off followed by repeated short periods (30–60 s) of the treadmill moving at a range of speeds (<10 m/min). On the day of data collection, mice were given warm-up bouts at 5 and 10 m/min. We then collected high-speed digital video during steady-speed locomotor bouts at three different speeds. Rest periods were provided for the mice between each trial, which lasted between 1–5 min. A soft brush was used to prompt the mice if they began to move toward the rear door of the treadmill during a trial. Treadmill speed was calibrated manually by recording the amount of time for 10 belt revolutions at a range of speeds that covered the entire range of speeds used in this experiment. Trials that provided the closest speed-matched comparisons for genotype and locomotor mode (i.e., treadmill vs. wheel) conditions were then used for analyses.
Histological evaluation.
All animals were killed at 17 mo of age. Left and right hind knee joints for each subject were harvested, fixed in 10% formalin, decalcified in Cal-Ex solution (Fisher Scientific), embedded in paraffin, and sectioned in a sagittal plane (6 μm thickness). Representative samples were graded for each subject from the cartilage load-bearing regions of both femur and tibia in both the medial and lateral compartments of the joint. The sections were stained with hematoxylin, Safranin-O, and fast green and then evaluated for degenerative changes by three blinded graders according to the modified Mankin scoring system for mouse articular cartilage (18). The scoring system included the categories of cartilage structure [0–11], tidemark duplication [0–3], loss of Safranin-O staining [0–8], fibrocartilage formation [0–2], chondrocyte cloning above the tidemark [0–3], presence of hypertrophic chondrocytes below the tidemark [0–3], and relative subchondral bone thickness [0–2]. Scores for each section were averaged between graders resulting in total scores between 0 and 30 for each location (medial femur, medial tibia, lateral femur, lateral tibia).
Statistical analysis.
We tested for statistical differences between genotypes (KO and WT) and activity type (overground and wheel) using a two-factor analysis of variance (ANOVA) (JMP® 7; SAS Institute, Cary, NC). Variables tested were average daily running distance, voluntary speed, stride frequency, stride duration, contact time (fore and hindlimbs), stride time (fore and hind), and duty factor (fore and hind). Post hoc analysis was performed using Tukey's HSD test for significance (P < 0.05). To compare within genotype variation among these locomotor variables, we calculated the coefficient of variation (CV) as a percentage of the ratio of the standard deviation to the mean. We also compared histopathological OA scores to the spontaneous activity and gait kinematic data using Pearson product-moment correlations.
RESULTS
Histological assessment of knee OA.
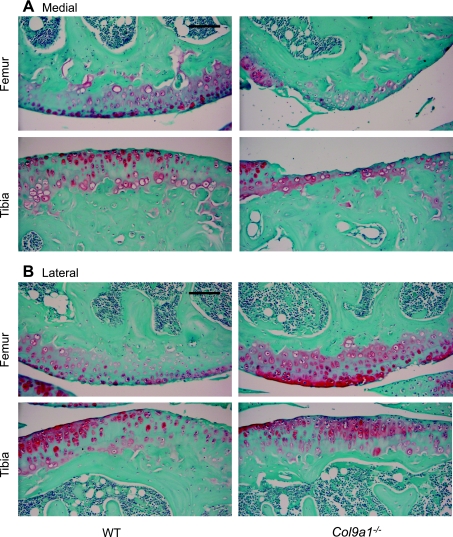
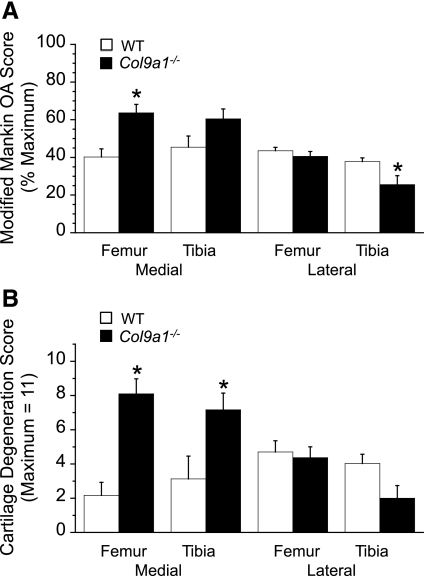
The greatest osteoarthritic changes in the knee joint of 17-mo-old Col9a1−/− mice occurred in the medial femoral condyle (Figs. 1, 2A). Here, the modified Mankin OA score for Col9a1−/− mice was significantly higher than that of the WT mice (Col9a1−/−: 63.7 ± 4.5% total possible score vs. WT: 40.2 ± 4.3%; P < 0.01). The higher OA score of the Col9a1−/− mice was due primarily to an increase in cartilage structural damage (Fig. 2B). Col9a1−/− mice had an average structural score of 73.6% maximum, which corresponds to structural changes that include “severe fibrillation, clefts, and/or loss involving greater than two-thirds of the depth of the noncalcified articular cartilage thickness in less than one-half of the condyle.” In contrast, the average WT medial femoral structural score was 20% maximum, which corresponds to “mild superficial fibrillation involving less than one-half the condyle.” Although the overall modified Mankin OA score for the medial tibial plateau was not statistically different between the Col9a1−/− mice and WT controls (60.4 ± 5.3 vs. 45.4 ± 5.9%, Col9a1−/− vs. WT, P = 0.096, Fig. 2A), Col9a1−/− mice had significantly greater structural damage than WT mice. The average structural score in the medial tibial compartment was 65.2% of maximum for Col9a1−/− mice vs. 28.4% for WT mice (Fig. 2B).
Fig. 1.
Sagittal sections of the femoral and tibial articular surfaces from 17-mo-old wild-type (WT) and Col9a1−/− mice. Representative sections are shown for the medial (A) and lateral (B) compartments of the knee. A: in the medial compartment, Col9a1−/− mice have significant cartilage loss, whereas, only mild superficial fibrilation is observed in WT mice. B: in the lateral compartment, both Col9a1−/− and WT mice show minor cartilage structural changes and loss of Safranin-O staining. Sections were stained with hematoxylin, fast green, and Safranin-O. Images are ×200 magnification (scale bars = 100 μm).
Fig. 2.
Histomorphometric knee osteoarthritis (OA) scores for 17-mo-old Col9a1−/− mice and WT mice. A: modified Mankin OA score, expressed as a percent of the maximum score, for each graded site in the knee joint. Col9a1−/− scores were significantly greater than WT scores in the medial femur and lower in the lateral tibia. B: cartilage structural degeneration score, a subcomponent of the Modified Mankin score, for each graded site in the knee joint. Col9a1−/− mice had significantly greater cartilage degeneration than WT mice in the medial compartment, while there was no significant difference in degeneration in the lateral compartment. Values are means ± SE.
The average OA score for the lateral knee compartment of Col9a1−/− mice was significantly lower compared with the medial compartment (33.1 ± 3.6 vs. 62.0 ± 3.3%, respectively, P < 0.001; Fig. 2A). Correspondingly, and in contrast to the findings for the medial compartment, the average modified Mankin OA score of the femur and tibia in the lateral knee compartments of Col9a1−/− and WT mice were not significantly different (33.1 ± 3.6 vs. 40.7 ± 1.6%, respectively, P = 0.07; Fig. 2A). The reduced lateral OA scores of the Col9a1−/− mice were associated with a significantly lower lateral tibial plateau OA score than WT mice (25.6 ± 4.7 vs. 37.8 ± 2.0%, P < 0.05; Fig. 2A). This difference in OA scores can be attributed to a significantly greater proteoglycan loss in WT mice (as indicated by a loss of Safranin-O staining intensity) but not cartilage structural damage (Fig. 2B). These differences were not observed in the lateral femoral condyles where the total OA scores were similar for the Col9a1−/− mice and the WT mice (40.6 ± 2.6 vs. 43.6 ± 1.8%, respectively, P = 0.37; Fig. 2A).
Gait comparisons for overground locomotion.
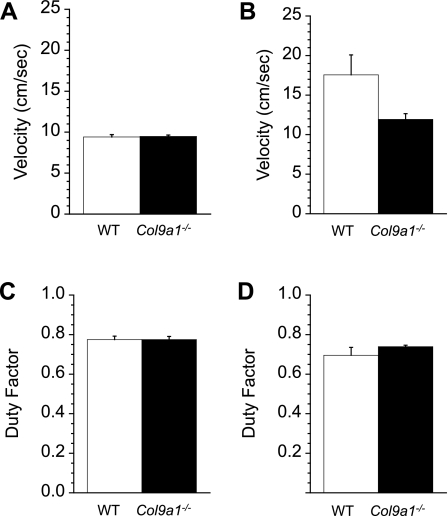
Freely chosen locomotor speeds were measured over three consecutive nights (i.e., dark phases) during spontaneous overground locomotion in open field chambers. During this period, WT mice moved at nearly the same speeds as Col9a1−/− mice (WT = 9.42 ± 0.33 vs. Col9a1−/− = 9.48 ± 0.18 cm/s; dark period activity only; P = 0.88 Fig. 3A). To compare hindlimb duty factors, mice ran on a treadmill at speeds that were similar for WT and Col9a1−/− mice and were slightly faster than the freely chosen open field speeds (WT = 12.43 ± 1.77 vs. Col9a1−/− = 11.80 ± 1.33 cm/s). Although we hypothesized that Col9a1−/− mice would use greater duty factors compared with WT mice to reduce peak vertical ground reaction forces, there was no difference in hindlimb duty factors (WT = 0.78 ± 0.02 vs. Col9a1−/− = 0.78 ± 0.02; P = 0.99, Fig. 3C).
Fig. 3.
Kinematics of locomotion related to the magnitude of ground force generation. Freely chosen average speeds over a 72-h time period for “open field” overground locomotion (A) and wheel running (B). Spontaneous wheel running speeds were significantly faster than those used overground and tended to be slower in Col9a1−/− mice. C: hindlimb duty factors during speed-matched treadmill locomotion were not significantly different between Col9a1−/− mice and WT mice. D: hindlimb duty factors during voluntary wheel running were not significantly different between Col9a1−/− and WT mice. Duty factors were compared at 10 cm/s for both Col9a1−/− and WT mice from strides collected at the beginning of the dark cycle. Values are means ± SE.
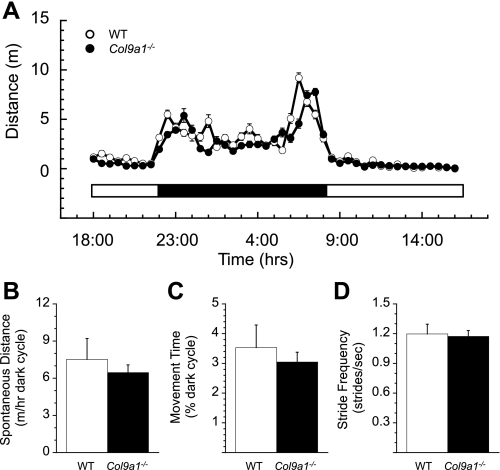
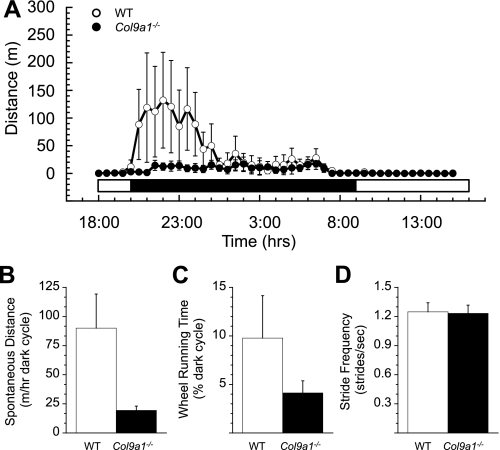
The pattern and amount of spontaneous overground locomotion, averaged over a 72-h time period, was also similar between WT and Col9a1−/− mice (Fig. 4A). The average distance traveled by WT mice per dark cycle hour was ∼15% further than Col9a1−/− mice (WT = 7.5 ± 1.7 m h−1 vs. Col9a1−/− = 6.5 ± 0.6 m h−1; Fig. 4B), although the difference was not significant (P = 0.57). As would be expected from the comparable locomotor speeds between WT and Col9a1−/− mice, the greater horizontal distance traveled by WT mice corresponded to a greater amount of time active during the dark cycle (Fig. 4C), although again, this difference was not significant (WT = 3.53 ± 0.76% of dark period vs. Col9a1−/− = 3.04 ± 0.33; P = 0.57). The average stride frequencies, calculated during speed-matched treadmill locomotion as described previously, were not significantly different between the WT and Col9a1−/− mice (WT = 1.20 ± 0.10 vs. Col9a1−/− = 1.17 ± 0.06 Hz; P = 0.83, Fig. 4D).
Fig. 4.
Spontaneous overground locomotor behavior and treadmill kinematics related to the frequency of joint loading. A: no significant differences were observed between Col9a1−/− and WT mice in the pattern of spontaneous overground activity averaged over a 72-h time period. Data are plotted for the total distance moved for each half hour of the day (bar beneath the plot indicates the light/dark periods). B: Col9a1−/− mice moved slightly shorter distances during the dark cycle compared with WT mice, although this difference was not significant. C: there was also a nonsignificant trend for reduced activity time, expressed as a percent of the dark period, in Col9a1−/− mice compared with WT mice. D: stride frequencies recorded during speed-matched treadmill locomotion were not significantly altered in Col9a1−/− mice. Values are means ± SE.
Gait comparisons for wheel locomotion.
Freely chosen wheel running speeds, compared over three dark cycles, were ∼50% faster in WT compared with Col9a1−/− mice (WT = 17.57 ± 4.70 cm/s vs. Col9a1−/− = 11.93 ± 1.33 cm/s; Fig. 3B). Despite these apparently large differences in average speed, they were not significantly different (P = 0.28) primarily due to a large amount of variation within the WT animals. The coefficient of variation (CV) was 60% for WT mice compared with 25% for Col9a1−/−. Duty factors were compared during voluntary running trials recorded with high-speed video at the beginning of the dark cycle. The per-stride speeds for these trials were similar for WT and Col9a1−/− mice (WT = 9.95 ± 0.55 cm/s vs. Col9a1−/− = 10.00 ± 0.46 cm/s). Thus duty factor comparisons for wheel locomotion, like those for treadmill locomotion, were essentially speed-matched between the two genotypes. Duty factors measured at these speeds were not significantly different between genotypes (WT = 0.72 ± 0.02 and KO = 0.74 ± 0.01; P = 0.40; Fig. 3D).
Spontaneous running wheel activity was much greater in WT vs. Col9a1−/− mice in the first, but not the second, half of the dark cycle (Fig. 5A). These differences in dark cycle locomotion corresponded to an almost five-fold difference in average daily running distance between the two genotypes (Fig. 5B; WT = 90.0 ± 54.6 m/h vs. Col9a1−/− = 19.3 ± 6.7 m/h). As for the difference in average wheel running speeds, the difference in running distances were not statistically different (P = 0.23), in part due to the greater than expected variation in running distance for WT mice compared with Col9a1−/− mice (CV = 136% for WT vs. 77% for Col9a1−/−). We also observed that WT mice ran on the wheel for more than twice as much time during the dark cycle as Col9a1−/− mice (Fig. 5C; WT = 9.7 ± 4.4% vs. Col9a1−/− = 4.1 ± 1.2%; P = 0.46). Consistent with the previous findings, variation was greater in WT compared with KO mice (CV = 101% vs. 68% for WT and Col9a1−/−, respectively). The average stride frequencies, calculated for similar-speed strides as described previously, were not significantly different between the WT and Col9a1−/− mice (WT = 1.25 ± 0.09 Hz vs. Col9a1−/− = 1.23 ± 0.08 Hz; P = 0.90; Fig. 5D). Coefficients of variation were smaller for stride frequency than for the other running wheel kinematics and were the same for WT and Col9a1−/− mice (CV = 17%).
Fig. 5.
Spontaneous wheel running behavior and kinematics related to the frequency of joint loading. A: WT mice showed increase wheel running activity during the first half of the dark cycle compared with Col9a1−/− mice, but there was no difference in wheel activity during the second half of the dark cycle. Average data for a 72-h time period are plotted for the total distance moved for each half hour of the day (bar beneath the plot indicates the light/dark periods). B: Col9a1−/− mice ran shorter distances during the dark cycle compared with WT mice, although this difference was not significant. C: there was also a nonsignificant trend for reduced activity time, expressed as a percent of the dark period, in Col9a1−/− mice compared with WT mice. D: stride frequencies recorded during approximately speed-matched wheel locomotion were similar in Col9a1−/− and WT mice. Values are means ± SE.
Gait comparisons for overground vs. wheel locomotion.
To gain further insight into the potential differences in the biomechanics of running on a wheel vs. overground, we compared results related to the magnitude of joint loading (i.e., speed and duty factor) and the frequency of joint loading (e.g., spontaneous nightly distance, activity time, and stride frequency). On the basis of results from a 2-factor ANOVA, locomotor type (i.e., overground vs. wheel) had a significant effect on factors related to the magnitude of joint loading: freely chosen speeds and duty factors. Wheel running speeds were significantly faster than those used overground (wheel = 14.75 ± 2.48 cm/s vs. overground = 9.45 ± 0.18; P < 0.05) and duty factor during wheel locomotion was significantly lower than those used overground (wheel = 0.72 ± 0.02 vs. overground = 0.78 ± 0.01; P = 0.01). Although this duty factor comparison was associated with a slightly faster average treadmill speed (wheel = 9.98 ± 0.34 cm/s and treadmill = 12.12 ± 1.04), this difference was not statistically significant and is not expected to explain the differences in duty factor since slower speeds are typically associated with higher duty factors. In this case, the wheel running speeds were slower than the treadmill speeds and the wheel running duty factors were also lower than those for treadmill locomotion.
Unlike the results for speed and duty factor, variables related to the frequency of joint loading were not significantly different between activity modes. While the average spontaneous nightly running distances tended to be greater for wheel running compared with overground locomotion, substantial variation in wheel running distances resulted in nonsignificant differences (wheel = 54.7 ± 28.5 m/h dark cycle vs. overground = 6.99 ± 0.87; P = 0.14). Furthermore, no differences were observed in either the amount of active time (%dark cycle) that mice spent running on the wheel vs. overground (wheel = 6.92 ± 2.34% of dark cycle vs. overground = 3.29 ± 0.40%; P = 0.15) or the speed-matched stride frequency used on the wheel vs. overground (wheel = 1.24 ± 0.06 Hz vs. overground = 1.18 ± 0.05 Hz; P = 0.52).
Association between knee OA and locomotor variables.
Correlation analyses reveal several significant relationships between locomotor performance and joint damage (Table 1). The most striking finding is that the overground distance traveled during the dark phase and the percent of the dark phase that the animals were active were negatively related to the modified Mankin knee OA scores. These locomotor indexes were also compared with the average and most severe levels of cartilage structural damage, based on the upper 95% confidence interval, to evaluate the potential relationship between high levels of focal cartilage damage and altered locomotor performance. Cartilage damage was also negatively related to nightly overground travel distance and activity time. In addition, there was a negative trend between cartilage damage and nightly wheel running distance and time spent running. Unexpectedly, there was a positive relationship between the average overground locomotor speed and knee OA, although the relationship was not associated with cartilage damage. A significant relationship was not observed between knee OA and duty factor or stride frequency for either over ground or wheel locomotion. Thus reduced activity time is the primary locomotor behavior in aged mice that is negatively impacted by knee OA and cartilage structural damage.
Table 1.
Locomotor and knee OA correlations within WT and Col9a1−/− mice
| Modified Mankin Knee OA | Cartilage Structural Damage | Upper 95% Cartilage Structural Damage | |
|---|---|---|---|
| Overground speed | 0.65* | ns | ns |
| Overground duty factor (treadmill) | ns | ns | ns |
| Overground distance | −0.67* | −0.69* | −0.65* |
| Overground time of locomotor activity | −0.70* | −0.69* | −0.65* |
| Overground stride frequency (treadmill) | ns | ns | ns |
| Wheel speed | ns | ns | −0.59† |
| Wheel duty factor | ns | ns | ns |
| Wheel distance | ns | −0.62† | −0.64* |
| Wheel time of locomotor activity | ns | −0.59† | −0.62† |
| Wheel stride frequency | ns | ns | ns |
Pearson product-moment correlation coefficients.
P < 0.05.
Near-significant correlations (0.5 < P ≤ 0.07).
DISCUSSION
OA is a major cause of physical disability and often results in gait adaptations in people with OA (2, 6, 23, 27, 30, 32). Adults with arthritis are less likely to engage in recommended amounts of daily physical activity (25), which may facilitate the development of other chronic diseases such as type II diabetes (9), heart disease (10), and hypertension, among others (11). Furthermore, physical activity limitations due to joint pain may reduce adherence to exercise-based lifestyle modifications for the management of chronic diseases, including OA itself (28). In the current study, we sought to determine how aged Col9a1−/− mice alter their locomotor activity and gait patterns following an elevated lifetime of exposure to joint disease. We hypothesized that Col9a1−/− mice would decrease both the magnitude and frequency of joint loading during daily activity both on a running wheel and overground compared with their WT littermates. Furthermore, because wheel running is associated with a higher frequency of joint loading in terms of the number of steps taken per day, we also hypothesized that Col9a1−/− mice would decrease their spontaneous locomotor activity to a greater extent during wheel running compared with overground activity.
We found that the average dark-phase locomotor speeds and duty factors were similar between Col9a1−/− and WT mice during overground and speed-matched treadmill locomotion, respectively. These results were unexpected based on our earlier findings by Allen and coworkers that male Col9a1−/− mice used higher duty factors compared with WT mice over a comparable range of speeds (5). In the current study, however, the animals were ∼6 mo older than those examined by Allen and coworkers (5), a difference of likely great importance. By 17 mo of age, WT mice had developed significant levels of knee OA, even exceeding values for Col9a1−/− mice in the lateral tibial plateau. C57BL/6 strains are well known to develop spontaneous knee OA with aging, which may contribute to the development of protective locomotor behaviors in both WT and KO mice. Thus a significant increase in spontaneous knee OA within WT mice, as observed in the lateral tibial plateau, may also reduce the number of statistically significant differences in locomotor behavior between aged Col9a1−/− and WT mice. A similar scenario may also explain the findings for a study involving Col2a1+/− mice. Col2a1+/− mice have an increased risk of developing knee OA and, compared with WT mice, exhibit lower levels of spontaneous wheel running activity. However, WT mice have a greater age-dependent decline in spontaneous wheel running activity such that by 15 mo of age, the difference in spontaneous running activity is greatly reduced between Col2a1+/− and WT mice (31).
In the current study, several gait parameters, including speed, distance traveled, and percent of the dark phase active, appear to be lower in Col9a1−/− mice but were not statistically different. The higher degree of variation in locomotor parameters among WT mice parallels a greater degree of variation in cartilage structural damage compared with Col9a1−/− mice (CV = 36% vs. 15%, WT vs. Col9a1−/−, respectively). When gait and locomotor activity parameters are compared with knee OA and cartilage damage scores, a significant pattern is observed. Namely, knee OA and cartilage degeneration are strongly associated with a reduction in both nightly locomotor distance and time spent in activity. This finding is observed in two independent locomotor modes, overground activity and wheel running, and it suggests that animals with chronic knee OA primarily alter locomotor activity levels rather than gait kinematics. These results are consistent with those in the literature for other species. Bliven and colleagues (8) found that after induction of arthritis in hamsters, the primary cause of the animals' reduced daily running distance was a decrease in the amount of time spent in activity. Humans with OA also show reduced physical activity levels in the form of shorter durations of activity and increased incidence of inactivity compared with nonarthritic peers (14, 25, 33, 37).
As Col9a1−/− mice develop OA earlier than WT mice, they will experience an increased lifetime exposure to joint disease, despite the observation that both genotypes develop OA by age 17 mo. Our findings suggest that the current knee OA status of the mice appears to be a better indicator of changes in locomotor behavior than this overall lifetime exposure to disease. Previous research has shown that Col9a1−/− mice develop histological evidence of degenerative joint disease as young as 4 mo of age (16, 26). The degeneration presents itself as loss of proteoglycan staining, severe fibrillation of the tibial plateau, and complete loss of articular knee cartilage by 9 mo to 1 year of age (16, 26). Col9a1−/− mice also have increased vertebral body degeneration at 3 mo of age, although there is no evidence of differences in vertebral body degeneration between WT and Col9a1−/− mice at 12 mo of age (12). In the current study, Col9a1−/− mice had greater levels of knee OA in the medial compartment, WT animals had greater knee OA levels in the lateral tibial plateau, and WT animals had greater variation in the degree of knee OA compared with Col9a1−/− mice. We found that the current level of knee OA in both WT and Col9a1−/− mice was a better predictor of changes in locomotor behavior than the earlier development of knee OA and vertebral body degeneration caused by the absence of collagen IX. These data suggest that the age of onset of joint degeneration is not a critical determinant of future locomotor impairment and that strategies that retard the progression of OA may be an effective means of maintaining physical function with aging.
A limitation of the current study is that it does not address other potential factors that may contribute to altered locomotor behaviors in aged WT and Col9a1−/− mice. Col9a1−/− mice develop progressive hearing loss due to degeneration of the tectorial membrane in the inner ear (7, 39). Age-dependent hearing loss also occurs in WT C57BL/6 mice (22), albeit independent of vestibular impairment (36). While severe vestibular loss significantly alters posture and locomotion in mice (40), it is not known to what extent hearing loss, independent of vestibular impairment, alters locomotor behavior. In a previous study, we reported that 9-mo-old Col9a1−/− and WT mice startle in response to a 100-dB pulse (5). While this test ensures that animals were not deaf at 9 mo of age, it does not verify normal inner ear structure or that balance and coordination were unaffected by an inner ear malformation. Aged (≥1.5 yr) Col9a1+/− mice have also recently been shown to develop osteoporosis and thoracic kyphosis (41), factors that may contribute to altered musculoskeletal function. Wang and colleagues (41) also reported that aged Col9a1+/− mice developed reduced body weight and fat mass compared with WT controls. Although we did not observe a significant difference in body weights between Col9a1−/− and WT animals, a reduction in body fat may contribute to reduced locomotor activity levels in Col9a1−/− mice via mechanisms that regulate energy storage and expenditure levels (13, 35).
In conclusion, the development of functional locomotor impairments in aged osteoarthritic Col9a1−/− and WT mice is primarily manifested as reductions in total locomotor activity levels (e.g., both distance traveled and time active). These impairments in total activity are most clearly observed during wheel running, suggesting that spontaneous running wheel activity can serve as an important measurement for evaluating functional and behavioral phenotypes in mouse models of musculoskeletal disease. Our findings support using mouse models of OA in translational studies that seek to identify the mechanisms by which genetic mutations contribute to reduced locomotor activity or that seek to identify interventions that slow the progression of OA or maintain physical function with OA in aging.
GRANTS
Funding was provided by grants from the National Institutes of Health (AR-047442, AR-050245, AG-015768, AR-048182, EB-001630, and EB-002263) and an Arthritis Foundation Arthritis Investigator Award.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank the following individuals for their assistance in the completion of this study: Kyle Allen, Bridgette Furman, Stephen Johnson, Holly Leddy, Tim Lightfoot, Roger Nightingale, Ramona Rodriguiz, William Wetsel, and Zhen Yan.
Current addresses: K. Costello, Virginia Tech-Wake Forest University School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061; T. Griffin, Program in Free Radical Biology and Aging, Oklahoma Medical Research Foundation, and Department of Biochemistry and Molecular Biology, University of Oklahoma Health Science Center, Oklahoma City, OK 73104.
REFERENCES
- 1. Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol 287: C1311– C1319, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Al-Zahrani KS, Bakheit AM. A study of the gait characteristics of patients with chronic osteoarthritis of the knee. Disabil Rehabil 24: 275– 280, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Alexander RM, Jayes AS. Fourier analysis of forces exerted in walking and running. J Biomech 13: 383– 390, 1980 [DOI] [PubMed] [Google Scholar]
- 4. Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90: 1900– 1908, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Allen KD, Griffin TM, Rodriguiz RM, Wetsel WC, Kraus VB, Huebner JL, Boyd LM, Setton LA. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum 60: 2684– 2693, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng 32: 447– 457, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Asamura K, Abe S, Imamura Y, Aszodi A, Suzuki N, Hashimoto S, Takumi Y, Hayashi T, Fassler R, Nakamura Y, Usami S. Type IX collagen is crucial for normal hearing. Neuroscience 132: 493– 500, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Bliven ML, Eskra JD, Otterness IG. Limitation of activity in an acute model of arthritis: effect of drug treatment. Inflamm Res 46: 491– 495, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Bolen J, Hootman JM, Helmick CG, Murphy L, Langmaid G, Caspersen CJ. Arthritis as a potential barrier to physical activity among adults with diabetes–United States, 2005 and 2007. MMWR Morb Mortal Wkly Rep 57: 486– 489, 2008 [PubMed] [Google Scholar]
- 10. Bolen J, Murphy L, Greenlund K, Helmick CG, Hootman JM, Brady TJ, Langmaid G. Arthritis as a potential barrier to physical activity among adults with heart disease—United States, 2005 and 2007. MMWR Morb Mortal Wkly Rep 58: 165– 169, 2009 [PubMed] [Google Scholar]
- 11. Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 88: 774– 787, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Boyd LM, Richardson WJ, Allen KD, Flahiff C, Jing L, Li Y, Chen J, Setton LA. Early-onset degeneration of the intervertebral disc and vertebral end plate in mice deficient in type IX collagen. Arthritis Rheum 58: 164– 171, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Choi YH, Li C, Hartzell DL, Little DE, Della-Fera MA, Baile CA. ICV leptin effects on spontaneous physical activity and feeding behavior in rats. Behav Brain Res 188: 100– 108, 2008 [DOI] [PubMed] [Google Scholar]
- 14. de Groot IB, Bussmann JB, Stam HJ, Verhaar JA. Actual everyday physical activity in patients with end-stage hip or knee osteoarthritis compared with healthy controls. Osteoarthritis Cartilage 16: 436– 442, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 365: 965– 973, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Fassler R, Schnegelsberg PN, Dausman J, Shinya T, Muragaki Y, McCarthy MT, Olsen BR, Jaenisch R. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci USA 91: 5070– 5074, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci 27: 10520– 10529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res 25: 578– 592, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Guilak F, Setton LA, Kraus VB. Structure and function of articular cartilage. In: Principles and Practice of Orthopaedic Sports Medicine, edited by Garrett WEJ, Speer KP, Kirkendall DT. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 53– 73 [Google Scholar]
- 20. Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol 92: 313– 322, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hedrick TL. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir Biomim 3: 34001, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse. Audiology 19: 369– 383, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Hinman RS, Bennell KL, Metcalf BR, Crossley KM. Delayed onset of quadriceps activity and altered knee joint kinematics during stair stepping in individuals with knee osteoarthritis. Arch Phys Med Rehabil 83: 1080– 1086, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 54: 226– 229, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Hootman JM, Macera CA, Ham SA, Helmick CG, Sniezek JE. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum 49: 129– 135, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum 54: 2891– 2900, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Hubley-Kozey CL, Deluzio KJ, Landry SC, McNutt JS, Stanish WD. Neuromuscular alterations during walking in persons with moderate knee osteoarthritis. J Electromyogr Kinesiol 16: 365– 378, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Jordan JL, Holden MA, Mason EE, Foster NE. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 20: CD005956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lam NP, Li Y, Waldman AB, Brussiau J, Lee PL, Olsen BR, Xu L. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch Oral Biol 52: 579– 584, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landry SC, McKean KA, Hubley-Kozey CL, Stanish WD, Deluzio KJ. Knee biomechanics of moderate OA patients measured during gait at a self-selected and fast walking speed. J Biomech 40: 1754– 1761, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Lapvetelainen T, Hyttinen M, Lindblom J, Langsjo TK, Sironen R, Li SW, Arita M, Prockop DJ, Puustjarvi K, Helminen HJ. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage 9: 152– 160, 2001 [DOI] [PubMed] [Google Scholar]
- 32. McGibbon CA, Krebs DE. Compensatory gait mechanics in patients with unilateral knee arthritis. J Rheumatol 29: 2410– 2419, 2002 [PubMed] [Google Scholar]
- 33. Minor MA. Impact of exercise on osteoarthritis outcomes. J Rheumatol Suppl 70: 81– 86, 2004 [PubMed] [Google Scholar]
- 34. Paassilta P, Pihlajamaa T, Annunen S, Brewton RG, Wood BM, Johnson CC, Liu J, Gong Y, Warman ML, Prockop DJ, Mayne R, Ala-Kokko L. Complete sequence of the 23-kilobase human COL9A3 gene Detection of Gly-X-Y triplet deletions that represent neutral variants. J Biol Chem 274: 22469– 22475, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Salbe AD, Nicolson M, Ravussin E. Total energy expenditure and the level of physical activity correlate with plasma leptin concentrations in five-year-old children. J Clin Invest 99: 592– 595, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiga A, Nakagawa T, Nakayama M, Endo T, Iguchi F, Kim TS, Naito Y, Ito J. Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol Neurootol 10: 97– 104, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis National Health Interview Survey, 2002. Am J Prev Med 30: 385– 393, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Spink AJ, Tegelenbosch RA, Buma MO, Noldus LP. The EthoVision video tracking system—a tool for behavioral phenotyping of transgenic mice. Physiol Behav 73: 731– 744, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki N, Asamura K, Kikuchi Y, Takumi Y, Abe S, Imamura Y, Hayashi T, Aszodi A, Fassler R, Usami S. Type IX collagen knock-out mouse shows progressive hearing loss. Neurosci Res 51: 293– 298, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J. Inner ear defects induced by null mutation of the isk gene. Neuron 17: 1251– 1264, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Wang CJ, Iida K, Egusa H, Hokugo A, Jewett A, Nishimura I. Trabecular bone deterioration in col9a1+/− mice associated with enlarged osteoclasts adhered to collagen IX-deficient bone. J Bone Miner Res 23: 837– 849, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]