Abstract
Auditory transduction occurs by opening of Ca2+-permeable mechanotransducer (MT) channels in hair cell stereociliary bundles. Ca2+ clearance from bundles was followed in rat outer hair cells (OHCs) using fast imaging of fluorescent indicators. Bundle deflection caused a rapid rise in Ca2+ that decayed after the stimulus, with a time constant of about 50 ms. The time constant was increased by blocking Ca2+ uptake into the subcuticular plate mitochondria or by inhibiting the hair bundle plasma membrane Ca2+ ATPase (PMCA) pump. Such manipulations raised intracellular Ca2+ and desensitized the MT channels. Measurement of the electrogenic PMCA pump current, which saturated at 18 pA with increasing Ca2+ loads, indicated a maximum Ca2+ extrusion rate of 3.7 fmol·s−1. The amplitude of the Ca2+ transient decreased in proportion to the Ca2+ concentration bathing the bundle and in artificial endolymph (160 mM K+, 20 μM Ca2+), Ca2+ carried 0.2% of the MT current. Nevertheless, MT currents in endolymph displayed fast adaptation with a submillisecond time constant. In endolymph, roughly 40% of the MT current was activated at rest when using 1 mM intracellular BAPTA compared with 12% with 1 mM EGTA, which enabled estimation of the in vivo Ca2+ load as 3 pA at rest. The results were reproduced by a model of hair bundle Ca2+ diffusion, showing that the measured PMCA pump density could handle Ca2+ loads incurred from resting and maximal MT currents in endolymph. The model also indicated the endogenous mobile buffer was equivalent to 1 mM BAPTA.
INTRODUCTION
The sensory hair cells of the mammalian cochlea are responsible for sound transduction, amplifying and tuning the response in the outer hair cells (OHCs), and relaying the signal to the auditory nerve afferents via the inner hair cells (IHCs) (Fettiplace and Hackney 2006; Glowatzki et al. 2008). Ca2+ plays a prominent role in the performance of the OHCs, which possess large amounts of cytoplasmic calcium-binding proteins (Hackney et al. 2005) to buffer Ca2+ and pumps in the plasma membrane to extrude the ion (Ficarella et al. 2007; Yamoah et al. 1998). Mutation of the plasma membrane Ca2+ ATPase (PMCA) pump, a unique PMCA2a isoform in the stereociliary bundle (Dumont et al. 2001), causes deafness in mice (Kozel et al. 1998; Spiden et al. 2008; Street et al. 1998), implying that Ca2+ is a prime factor in hair cell pathology (Fridberger et al. 1998). The main persistent Ca2+ source is likely to be influx through mechanotransducer (MT) channels, which are about fivefold more permeable to Ca2+ than to Na+ (Beurg et al. 2006; Ohmori 1985) and partially open at rest. Ca2+ influx through the MT channels during stimulation controls the extent and rate of channel adaptation (Beurg et al. 2006; Ricci and Fettiplace 1998). Ca2+ may also modulate OHC electromotile behavior, both somatic contractility (Dallos et al. 1997; Frolenkov et al. 2000; Murugasu and Russell 1996) and hair bundle motility (Chan and Hudspeth 2005; Kennedy et al. 2005).
The time course and localization of cytoplasmic Ca2+ excursions are dependent on binding to diffusible calcium-binding proteins (Roberts 1994) and by uptake and release by organelles such as the mitochondria, which can act in a buffering capacity. OHCs have been previously shown to contain millimolar concentrations of the proteinaceous calcium buffer parvalbumin-β (Hackney et al. 2005; Sakaguchi et al. 1998), suggesting the occurrence of large somatic Ca2+ transients as in muscle fibers, which also contain a high concentration of a parvalbumin isoform (Celio et al. 1996). Mitochondria are conspicuously concentrated in OHCs not only in a band beneath the cuticular plate, the cytoskeletal anchor for the stereocilia bundle, but also along the lateral walls adjacent to the submembranous cisternae (Furness and Hackney 2006; Weaver and Schweitzer 1994). The mitochondria are known to affect Ca2+ balance not only by generating adenosine 5′-triphosphate (ATP) that fuels the PMCA pump but also by acting as a large-capacity calcium store (Babcock and Hille 1998; Gunter et al. 2000; Nicholls 2005; Xu et al. 1997). The aim of the present work was to quantify the time course and spread of Ca2+ transients originating from the MT channels using fast imaging of fluorescent calcium indicators and to assess the resting Ca2+ influx and mechanotransduction under in vivo like conditions, in which the hair bundles are bathed in endolymph containing 20 μM Ca2+ (Bosher and Warren 1978). Imaging data were complemented with a model of Ca2+ buffering and extrusion. The results suggest that correct Ca2+ balance is crucial for optimal performance of the MT channels, which may be deleteriously affected by mitochondrial or PMCA pump dysfunction.
METHODS
Preparation and recordings
Recordings were made from OHCs in the isolated organ of Corti of Sprague–Dawley rats between 6 and 14 days postnatal (P6–P14), as previously described (Beurg et al. 2006; Kennedy et al. 2003). Animals were killed by decapitation using methods approved by the Institutional Animal Care and Use Committee of the University of Wisconsin–Madison according to current National Institutes of Health guidelines. Excised apical turns were fixed in the experimental chamber with strands of dental floss and viewed through a long working distance water-immersion objective (Olympus ×60, numerical aperture [NA] = 1.1, or Zeiss ×63, NA 0.95) and a Hamamatsu charge-coupled device (CCD) camera on a Zeiss Axioskop FS microscope. The chamber was perfused with artificial perilymph of composition (in mM): 154 NaCl, 6 KCl, 1.5 CaCl2, 2 Na-pyruvate, 8 glucose, and 10 Na-HEPES (pH 7.4). The apical surface of the organ of Corti was separately superfused with artificial perilymph or with a saline of reduced Ca2+ concentration, either 0.15 or 0.02 mM, the latter being buffered with 4 mM HEDTA. In some experiments, the solution around the hair bundle was controlled by flow from a 10-μm pipette and was changed to artificial endolymph containing (in mM): 160 KCl, 0.02 CaCl2 (buffered with 4 HEDTA), 2 Na-pyruvate, 8 glucose, and 10 K-HEPES (pH 7.4). To block the PMCA pump, the concentration of H+ ions required for exchange during a pump cycle (Xu et al. 2000) was reduced by elevating the pH of the artificial perilymph to 9.0 by buffering with 10 mM TABS (Sigma–Aldrich, St. Louis, MO) rather than HEPES. Experiments to measure the electrogenic PMCA current were performed in salines in which Na+ was replaced by Tris(hydroxymethyl)aminomethane (Tris) with composition (in mM): 160 Tris-Cl, 6 KCl, 1.5 CaCl2, 10 glucose, and 10 HEPES (pH 7.4, control) or 235 Tris-Cl, 6 KCl, 1.5 CaCl2, 10 glucose, and 10 TABS (pH 9.0, to block the PMCA), both solutions having an osmolarity of 312 mOsm. The PMCA pump was also blocked with the membrane-permeant inhibitor carboxyeosin diacetate succinimidyl ester (CEDA; Invitrogen Life Sciences, Carlsbad, CA), which has been used as a specific inhibitor of PMCA pumps in rat myocytes (Choi and Eisner 1998). Ruthenium red (RR) was obtained from Fluka and carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) from Sigma–Aldrich, both agents being used to block Ca2+ uptake into mitochondria. All experiments were performed at room temperature (20–23°C).
Hair cell recordings were made using borosilicate patch electrodes introduced at the bottom of the apical turn, a location in vivo with a characteristic frequency range of 4 to 6 kHz (Müller 1991). Patch pipettes were filled with an intracellular solution of composition (in mM): 135 CsCl, 2.5 MgCl2, 1 EGTA, 2.5 Na2ATP, 10 creatine phosphate, and 10 HEPES (pH 7.2) and had starting resistances of 3–6 MΩ. In some experiments, K+ was substituted for Cs+ and 1 mM BAPTA was used instead of EGTA as the calcium buffer. Also included in the pipette solution was 0.25 mM of a calcium indicator dye, Fluo-4 or the low-affinity Fluo-4FF (Invitrogen Life Sciences), which have Ca2+ affinities of 0.5 and 10 μM, respectively. Mitochondrial Ca2+ was monitored with Rhod2, which was loaded by incubating the preparation with 20 μM of the membrane-permeant Rhod2AM (+0.5% DMSO) for 30 to 45 min prior to starting the recordings. The patch electrode was connected to an Axopatch 200A amplifier and MT currents were low-pass filtered at the amplifier output at 10 kHz. During imaging experiments hair bundles were deflected by a pressure-driven fluid jet from a pipette, tip diameter 5–10 μm, placed about 10 μm from the bundle so as not to interfere with its image. The pipette was normally filled with artificial perilymph solution. Fluid pressure and pipette location were adjusted to elicit a maximal MT current. In experiments to determine current–displacement (I–X) relationships, hair bundles were deflected with a glass stylus driven by a piezoelectric stack actuator (PA8/12; Piezosystems Jena, Hopedale, MA). The driving voltage to the actuator was filtered at 4 to 6 kHz, yielding a step displacement with a rise time of about 50 μs. The peak MT current in control (Na+, 1.5 mM Ca2+) extracellular solution ranged from about 600 to 1,000 pA (mean = 785 ± 182 pA; n = 27).
For comparing Ca2+ transients in normal and reduced extracellular Ca2+ concentrations, experiments used two puffer pipettes, one containing a control (1.5 mM Ca2+) and the other a test solution. The control puffer was first positioned near the bundle and the Ca2+ response to bundle deflection established; then it was moved away and the puffer with the test solution was brought up near the bundle and its response obtained; finally the control puffer was returned to its original position and a return control determined. This method was much cleaner than exposing the entire endolymphatic aspect of the preparation to reduced Ca2+, a procedure that was often poorly reversible and resulted in hair cell deterioration. To ensure the solution around the bundle was fully exchanged, the timing and duration of the puffer command and depolarizing voltage step were varied to give a constant outcome. To achieve sufficient sensitivity to measure calcium signals in very low extracellular Ca2+ concentrations, Fluo-4 was used as the fluorescent indicator.
Imaging
The cell apex and stereociliary bundle of a hair cell were imaged using a Photometrics Cascade 128 CCD camera (Photometrics, Tucson, AZ) at an acquisition rate of normally 500 frames/s, although this rate was reduced to 100 frames/s for measurements in low extracellular calcium. The fast camera facilitated quantification of the fast time constants of calcium accumulation and dissipation. Epifluorescence illumination was achieved with a 100 W Hg lamp through FITC or rhodamine filter sets (Chroma Technology, Brattleboro, VT). An intermediate magnification lens was inserted to accomplish a resolution at the CCD camera of 0.09 μm per pixel, about a third of the lateral resolution of the water-immersion objective. The axial resolution of the imaging system was determined from the axial intensity variation for 0.1 μm fluorescent beads, focused with a stepping motor at 0.5 μm intervals. The results were fitted with a Gaussian with SD of 1.0 μm, corresponding to a full width at half-maximum (FWHM) of 2.2 μm. This resolution is half that of the swept field confocal microscope (1 μm FWHM) used to localize stereociliary calcium transients in inner hair cells (Beurg et al. 2009). Images were captured with Metamorph (Molecular Devices, Downingtown, PA) and were analyzed with Metamorph or ImageJ software. The average intensity was calculated for small areas overlying the stereociliary bundle or cell apex and, in most cases, the fluorescence change was corrected by subtraction of the background fluorescence measured at a depolarized potential where there is no calcium influx. To allow the pipette and cytoplasmic solution to equilibrate, images were not captured until five minutes after achieving the whole cell condition. Results are expressed as ΔF/F, the change in fluorescence normalized to the background.
Immunofluorescence
Neonatal Sprague–Dawley rats 7–13 days old (P7–P10) were decapitated using methods approved by the Institutional Animal Care and Use Committee of the University of Wisconsin. The cochleae were freshly dissected in cold HBSS. After fixation in 4% paraformaldehyde in phosphate buffer (PB) for 90 min at room temperature, the the stria vascularis and tectorial membrane were removed and the cochlear coils were isolated. The apical and part of the middle turns, which included the region used for electrical recordings, was separated, permeabilized with 0.5% Triton X-100 for 30 min, washed in phosphate-buffered saline (PBS), blocked in 10% normal goat serum (Invitrogen Life Sciences) for 1 h at room temperature, then incubated with rabbit anti-PMCA2 polyclonal antibody (Affinity Bioreagents, Golden, CO) diluted 1:400 in blocking solution overnight at 4°C. The control groups were processed with blocking solution instead of primary antibody. After washing in PBS, the samples were incubated with Alexa Fluor 488 goat anti-rabbit IgG antibody (1:400; Invitrogen Life Sciences) diluted in blocking solution for 2 h at room temperature, washed in PBS, incubated with Alexa Fluor 594 or 568 phalloidin (1:200; Invitrogen Life Sciences) for 45 min at room temperature, and washed in PBS. The isolated cochlear coils were mounted on coverslips with Prolong Antifade mounting medium (Invitrogen Life Sciences), viewed under a ×60 objective (Nikon ELWD Planfluor; NA 1.4) oil-immersion objective using a Bio-Rad (Hemel Hempstead, UK) MRC-1024ES laser scanning microscope system operating in confocal mode.
Theory
GEOMETRY.
An OHC from the apical turn of the rat cochlea was simulated with three rows of stereocilia (4, 2, and 1.8 μm in height, all 0.25 μm in diameter) projecting from a 30 μm long cell body. Because of the repeated pattern of the OHC stereociliary columns, the three-dimensional problem was reduced to a two-dimensional one, a slice of the hair cell including a column of stereocilia and cell body being modeled. The width is the approximate distance from edge to edge along the column axis (x-axis). The thickness of the slice (y-axis) is the distance between the stereocilia columns. The depth (z-axis) is the primary direction of diffusion along the stereocilia and cell. The dimensions of the model cell body were 4.8 × 0.4 × 30 μm (width × thickness × depth). The diffusible space was divided into four sections along the z-axis: stereocilia (z ≥ 0), cuticular plate (0 > z ≥ −1), mitochondria belt (−1 > z ≥ −5), and deep cell body (z < −5). The grid size was 0.1 μm in the stereocilia and 0.2–1.0 μm in the cell body. The ratios of diffusible volume to total volume of the sections were 0.8, 0.6, 0.7, and 0.8 in the four compartments, with the smaller values in the cuticular plate and mitochondrial regions.
CALCIUM INFLUX AND REMOVAL.
Ca2+ dynamics were described by differential equations (Lumpkin and Hudspeth 1998; Wu et al. 1999) for the following processes: 1) Ca2+ entry through MT channels at the tips of the stereocilia and diffusion in the cytoplasm with coefficient DCa of 0.4 μm2·ms−1; 2) buffering by cytoplasmic calcium buffers, both diffusible (1 mM EGTA + 0.25 mM Fluo-4FF, 1 mM BAPTA, or 2 mM parvalbumin-β) and fixed buffers; 3) extrusion via PMCA pumps; and 4) uptake into the subcuticular plate mitochondria (see schematic in Fig. 10A). The Ca2+ influx through the channel was described by
| (1) |
where C is the free Ca2+ concentration, nC is the number of MT channels per stereocilia, iCa is the single-channel calcium current, z is calcium ion valence, F is Faraday's constant, and VC is the diffusible volume of the compartment. Two MT channels are assumed to be present at the bottom end of the tip links (i.e., at the tops of the second and third row stereocilia), each channel carrying 1.3 pA of calcium current (in 1.5 mM external Ca2+, apical OHCs have unitary MT channel currents of 8.5 pA, 15% of which is carried by Ca2+; Beurg et al. 2006). Other parameter values, with their sources, are given in Table 1. Ca2+ extrusion by PMCA pumps was combined with leakage through the cell membrane (Sala and Hernández-Cruz 1990)
| (2) |
where vmax,PMCA is the maximal velocity of transportation in mol·m−2·s−1, AC is the area of membrane of the compartment, KPMCA is the half-maximal activation concentration of the PMCA pump, and C0 is the equilibrium point established between the extrusion by PMCA pump and the leakage due to the calcium gradient through the cell membrane. An important feature of the model is that, in line with experimental observations (Apicella et al. 1997; Crouch and Schulte 1995; Dumont et al. 2001; Grati et al. 2006), the PMCA pumps were concentrated in the stereociliary membrane with a lower density in the somatic lateral membrane (here assumed to be a tenth of that in the stereociliary membrane). The properties of the fixed buffer have not been characterized experimentally but there are known to be significant concentrations of calcium-binding proteins such as calmodulin and L-plastin (fimbrin) in the stereocilia and cuticular plate (Furness and Hackney 2006). A fixed buffer dissociation constant of 10 μM was assumed, similar to that of fimbrin (Glenney Jr et al. 1981). Although the kinetic properties of the fixed buffer in hair cells are not known, the values assumed (Table 1) are close to those determined experimentally for chromaffin cells (Heinemann et al. 1994). Furthermore, these values gave good agreement between the experimental and theoretical fluorescence decay rates after a Ca2+ load.
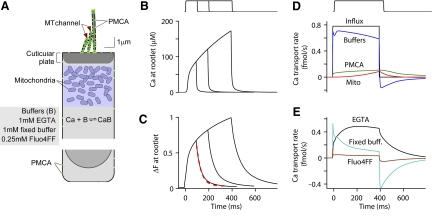
Fig. 10.
Model of Ca2+ homeostasis in an OHC. A: components of the model: Ca2+ influx through MT channels, binding to fixed and mobile buffers, absorption by mitochondria, and extrusion via PMCA pump (filled circles). Note the pumps are concentrated in the stereocilia and virtually absent from the cell body. See methods for more details. B: simulated change in Ca2+ at the base of the bundle. C: change in fluorescence at the base of the bundle. The time course is slowed relative to B due to the Fluo-4FF dye and the averaging over the focal volume; offset time constant = 40 ms. Compare with Fig. 1C. D: contribution of the different factors, buffers, PMCA pumps, and mitochondria to absorbing Ca2+ entering. E: contribution of the different buffers, the high-affinity EGTA (KCa = 0.2 μM) absorbing most compared with the lower-affinity fixed buffer and dye (KCa = 10 μM).
Table 1.
Model parameters
| Component | Property | Value | Reference |
|---|---|---|---|
| MT channel | Single-channel calcium current | 0.15 × 8.5 pA = 1.3 pA | Beurg et al. (2006) |
| Fixed buffer | Concentration (plastin) | 2 mM (stereocilia) | Furness and Hackney (2006) |
| Dissociation coefficient | 10 μM | Glenney et al. (1981); Heinemann et al. (1994) | |
| Forward binding rate | 107 M−1·s−1 | Heinemann et al. (1994) | |
| EGTA/BAPTA | Concentration | 1 mM; 1 mM | Experimental condition |
| EGTA/BAPTA | Dissociation coefficient | 0.2 μM; 0.2 μM | Naraghi (1997) |
| EGTA/BAPTA* | Forward binding rate | 3 × 106 M−1·s−1; 5 × 108 M−1·s−1 | Naraghi (1997) |
| Parvalbumin-β | Concentration | 4 mM binding sites | Hackney et al. (2005) |
| Dissociation coefficient | 0.5 μM | Henzl and Ndubuka (2007) | |
| Forward binding rate | 2 × 107 M−1·s−1 | Assumed | |
| Calbindin-D28k | Concentration | 0.5 mM binding sites | Hackney et al. (2005) |
| Dissociation coefficient | 0.35 μM | Nagerl et al. (2000) | |
| Forward binding rate | 4.5 × 107 M−1·s−1 | Nagerl et al. (2000) | |
| PMCA | Maximum transport velocity† | 0.34 μmol·m−2·s−1 | Yamoah et al. (1998) |
| Ca-ATPase | 1 μmol·m−2·s−1 | Measurements in text | |
| Dissociation coefficient | 0.5 μM | Kubitscheck et al. (1995) | |
| Mitochondrial uniporter | Transportation rate | 114 mM·s−1 | Wingrove et al. (1984) |
| Dissociation coefficient | 40 μM | Xu et al. (1997) | |
| Mitochondrial Na–Ca exchanger | Transportation rate | 1.8 mM·s−1 | Patterson et al. (2007) |
| Dissociation coefficient | 5 μM | Patterson et al. (2007) |
Fluo4FF in imaging had a concentration of 0.25 mM, a dissociation constant of 10 μM, and a forward rate constant identical to those in BAPTA.
0.34 μmol·m−2·s−1 = 2,000 pumps·μm−2, each transporting 100 ions·s−1; 1 μmol·m−2·s−1 = 6,000 pumps·μm−2, each transporting 100 ions·s−1. OHC soma were assumed to contain one tenth the PMCA density.
MITOCHONDRIA.
Transmission electron micrographs of the organ of Corti show a concentration of mitochondria beneath the cuticular plate (Weaver and Schweitzer 1994). Measurements on transmission electron micrographs provided by C. Hackney and S. Mahendrasingam (Keele University, Keele, UK) indicated that in prehearing rats, the mitochondrial layer in apical OHCs (mean lengths 32 μm) extended below the cuticular plate for 10.9 ± 0.6 μm and that the mitochondrial density (the ratio of mitochondrial volume to total cytosolic volume in a 5 μm band below the cuticular plate), determined from stereological measurements, was between 0.2 and 0.4 (mean = 0.32 ± 0.08; n = 7). In the modeling, the mitochondria were assumed to be confined to a belt between 1 and 5 μm below the apical surface of the cell (−1 > z ≥ −5 μm). Due to the steep Ca2+ gradient along the cell depth, the effects of sparser mitochondria <5 μm from the cell top are negligible and would minimally affect the results. The rate of change of cytosolic free Ca2+ due to mitochondrial Ca2+ transport was composed of the flux into the mitochondria by the calcium uniporter (Jm,uni) and transport out of the mitochondria by the sodium-calcium exchanger (Jm,NCX)
| (3) |
The rate of change of mitochondrial matrix free Ca2+ concentration (Cm) is
| (4) |
where κm is the free to fixed Ca2+ in the mitochondria (κm = 2.5 × 10−4; Babcock and Hille 1998) and γm is the ratio of mitochondrial volume to diffusible cytosolic volume. To assess the maximal mitochondrial contribution, a volume fraction of 0.3 was used: γm = 0.3/0.7 = 0.43. Second-order kinetics was assumed for the mitochondrial uniporter (Gunter et al. 2000; Nicholls 2005)
| (5) |
where vmax,uni is the maximum Ca2+ transportation rate of the uniporter in μM·ms−1 and Kuni is the half-maximal activation point (40 μM; Xu et al. 1997). The vmax value of 114 μM·ms−1 was derived from the in vitro mitochondrial calcium uptake rate of 4 nmol/min per mg of mitochondrial protein at C = 0.5 μM (Wingrove et al. 1984). Ca2+ extrusion from the mitochondria by the sodium-calcium exchanger (NCX) was assumed to have a first-order relationship (Patterson et al. 2007)
| (6) |
The maximal extrusion rate vmax,NCX of 1.81 μM/ms was chosen so that the mitochondrial Ca2+ transport is balanced at C = 0.5 μM, Cm = 0.05 μM, and KNCX = 5 μM. The half-activation point of the sodium-calcium exchanger KNCX = 5 μM (Patterson et al. 2007).
COMPUTATION.
Finite difference equations were derived from the partial differential equations and were integrated using the Crank–Nicholson method with a time step of 0.2 μs. The code was validated by comparing the Ca2+ influx rate calculated analytically to the sum of the changes in free, buffered, and extruded Ca2+ in the simulation. The error did not exceed 1%. Initial conditions were as follows: the initial resting Ca2+ concentration was C = 0.1 μM, which is determined by the set point of the PMCA pump-leakage equation (Eq. 2); the initial concentrations of the buffers were obtained by static equilibrium with the 0.1 μM free calcium. Because the Ca2+ equilibrium point of the mitochondria (C ≅ 0.5 μM by Eq. 3; Nicholls 2005) is higher than that of the PMCA pump (C = 0.1 μM), in the initial state with the channel closed, the mitochondrial free calcium should be depleted and was set to zero.
DYE FLUORESCENCE.
The fluorescence of the calcium indicator dye is intended to indicate the concentration of free Ca2+ in the focal plane, but it is an indirect measure because the fluorescent signal reflects Ca2+ binding to a dye and is integrated over the focal volume of the object defined by the radial and axial resolution of the microscopic system, 0.22 and 2.2 μm (see preceding text), respectively, which are comparable to the diameter and height of the stereocilia. Therefore it is inappropriate to equate the experimentally measured fluorescence in the stereocilia with the concentration of the calcium or calcium-bound dye in the simulation. For a better comparison of the experiment and the model, the simulated results were postprocessed to obtain the fluorescent intensity. The fluorescent intensity f(x, y, z) was calculated by integrating the concentration of Ca2+-bound dye, g(x, y, z), weighted by the Airy function w(x, y, z) over the focal space
| (7) |
In our case, the space is two-dimensional and the integration along the y-axis can be simplified by assuming uniform dye concentration in the y-direction
| (8) |
Instead of the computationally intractable Airy function, a Gaussian distribution that well matches the large central region of the Airy function was used
| (9) |
where xm and zm are the coordinates of the focal point. The SD terms σx and σz were obtained by dividing the measured radial and axial resolution by 2.355. The term q(x, z) represents the integration of the Gaussian function along the y-axis
| (10) |
where r is the half-thickness of stereocilia at point (x, z).
RESULTS
Hair bundle calcium signals
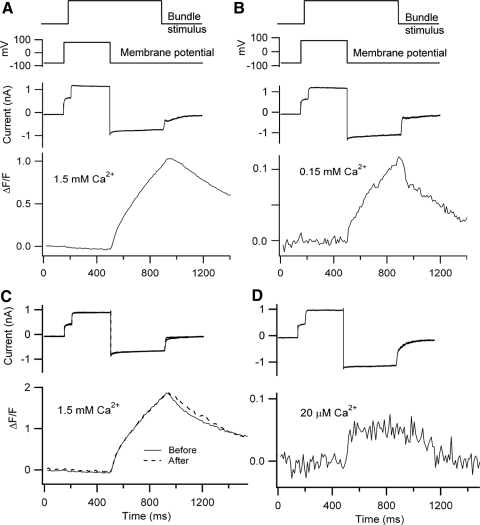
Hair bundles of OHCs were orientated vertically along the optical axis and were imaged by focusing in the middle or base of the bundle. Mechanical stimulation evoked a rapid rise in intracellular calcium that was initially confined to the bundle (Fig. 1A). A rapid onset to the calcium flux was ensured by using a protocol in which the bundle was deflected while the cell was held at a positive potential so that there was no calcium influx. The cell was then repolarized to −80 mV initiating calcium entry. With this paradigm, the average calcium in the hair bundles accumulated and dissipated rapidly for brief stimuli. Although there was often a progressive increase in background fluorescence throughout the experiment, probably reflecting calcium loading, the time course of the Ca2+ (ΔF/F) was stable for the first 15 to 20 min (Fig. 1B). The bundle signal developed with two kinetic components, which could be most clearly distinguished when the duration of the bundle displacement was varied (Fig. 1C). For stimuli lasting 50 to 200 ms, the calcium decayed quickly after the stimulus, with a time constant of 25 to 70 ms (mean = 54 ± 11 ms, n = 9), but for longer stimuli, a secondary component of removal with a time constant of 100 to 300 ms appeared. These time constants are not limited by the off rate of the low-affinity dye, which will be a fraction of a millisecond. The fluorescent signals were abolished by depolarization and also by blocking the MT channels with streptomycin (Beurg et al. 2009), indicating they reflect influx via the MT channels.
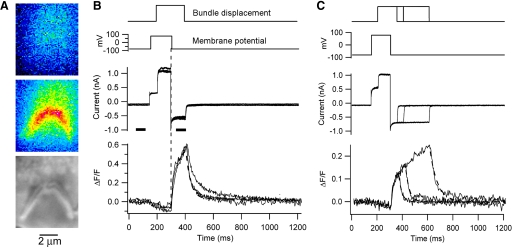
Fig. 1.
Hair bundle calcium signals during transduction in outer hair cells (OHCs). A: hair bundle images showing average calcium fluorescence before (top) and during (middle) mechanotransducer (MT) channel opening; Nomarski view of bundle shown at bottom. B: hair bundle calcium signal for OHC in A. The stimulus was a depolarization followed by a hair bundle displacement evoked by a water jet stimulus, upon which calcium influx occurs when the cell was repolarized to −80 mV. Superimposed membrane currents and fluorescence responses (plotted as ΔF/F) are shown for 3 times (5, 10, and 15 min) after achieving the whole cell condition. Offsets for the 5 and 10 min delays are fitted with an exponential τOFF = 35 ms. τOFF was slightly slowed for a delay of 15 min. The fluorescence images in A acquired during times indicated by the horizontal bars below current traces. C: bundle calcium response vs. stimulus duration in another OHC repolarized to −80 mV. Note the fast and slow components of the response onset. Offset time constants (dashed lines) τOFF = 25 ms, 30 ms, and 53 ms. For both cells, extracellular solution contained 154 mM Na+, 1.5 mM calcium, and calcium indicator was 0.25 mM Fluo-4FF.
Mitochondria and PMCA pumps in the clearance of hair bundle calcium
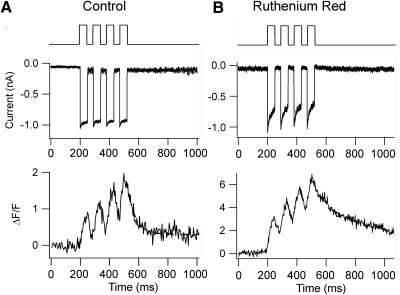
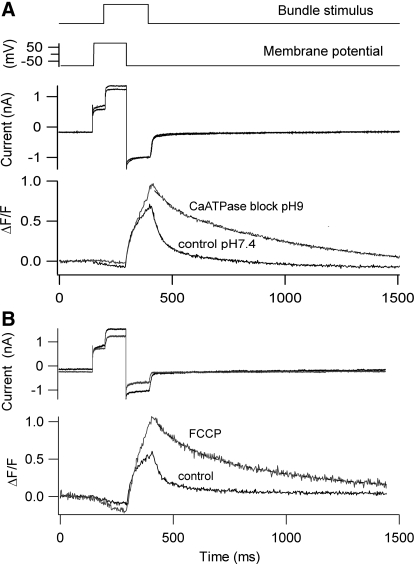
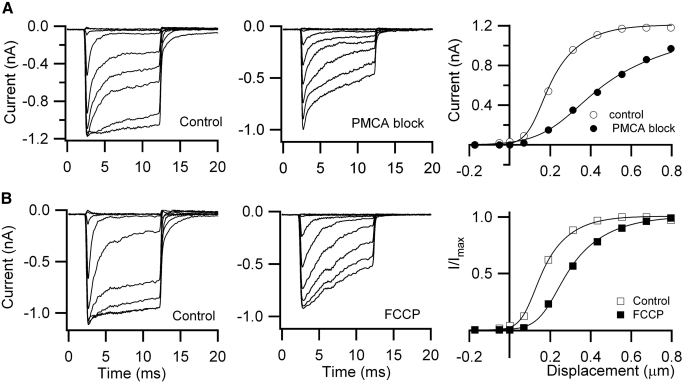
Deflection of the bundle with a train of stimuli delivered with a stiff piezoelectric probe produced fast transients superimposed on a secondary buildup of calcium (Fig. 2A) that was cleared with a slower time constant over 100 ms (mean = 115 ± 90 ms, n = 6). This secondary component was prolonged and the Ca2+ transient was augmented if the mitochondrial uptake via the calcium uniporter was blocked by addition of 1 μM Ruthenium red (RR) to the intracellular medium (Fig. 2B). The mean time constant of the decay of the calcium transient was increased over fivefold to 607 ± 92 ms (n = 5). The increase in ΔF/F and slowing of the decay time constant with RR probably reflect saturation of the calcium buffer, EGTA, due to Ca2+ loading because the removal mechanisms are too slow to contribute to fast clearance (see Modeling of calcium transients). RR, which is a polyvalent cation, has other actions including blocking the ryanodine receptor and, when present extracellularly, abolishing the MT current (Farris et al. 2004). However, there was no evidence of an effect on the MT current following intracellular perfusion of RR. Another argument against a nonspecific effect of RR is that more than a 10-fold prolongation of the Ca2+ transients also occurred with the protonophore, FCCP (2 μM; Fig. 3B), which by eliminating the mitochondrial membrane potential also prevents Ca2+ uptake. The effects cannot be due to loss of ATP because the nucleotide was present at 2.5 mM in the pipette solution. Furthermore, the slowing of the Ca2+ decay occurred over a period during which the kinetics were stable in control measurements (Fig. 1B). A similar prolongation (Fig. 3A) was seen when the plasma membrane PMCA pumps were blocked by lowering the extracellular H+ concentration (Duman et al. 2008; Xu et al. 2000). Comparable effects of alkalinization on the Ca2+ transient, increasing background Ca2+, and slowing the responses to bundle stimulation were seen in two other cells, although this maneuver had no effect on the maximum MT current in OHCs (mean current 830 ± 21 pA, pH 7.4; 846 ± 20 pA, pH 9; n = 3). For both the FCCP treatment and increasing pH, the effects of changing the extracellular perfusion medium took some time to develop. It seems likely that the slow onset of the effect (∼10 min) is due in part to the time to saturate the cytoplasmic calcium buffers because, in all cases, the change in the kinetics of Ca2+ clearance did not occur immediately after blocking the pump but only later when the background fluorescence had increased significantly.
Fig. 2.
Effects of Ruthenium red (RR), a blocker of the mitochondrial uniporter, on OHC hair bundle Ca2+ signals. A: response to a train of four 50-ms bundle deflections of nearly 0.5 μm amplitude. Note the 2 components of the response, a series of fast transients with τON and τOFF of about 20 ms, superimposed on a slower calcium increase. Slow offset fitted with a single exponential (dashed line) of time constant 67 ms. B: after block of the uniporter with 1 μM RR, introduced via the patch pipette, the magnitude of the calcium responses, expressed as ΔF/F, was threefold larger and slower, indicating saturation of mobile calcium buffers. Offset fitted with a single exponential (dashed line) of time constant 313 ms. In both experiments, the camera was focused near the bottom of the hair bundle encompassing all 3 rows of stereocilia.
Fig. 3.
Effects of Ca2+ uptake and extrusion blockers on OHC hair bundle Ca2+ signals. A: effects of increasing the pH of the extracellular solution to 9.0 to block the plasma membrane Ca2+ ATPase (PMCA) pump, which requires H+ influx to exchange for Ca2+ efflux. This caused an increase in cytoplasmic calcium and a slowing of the recovery from bundle stimulation. B: extracellular perfusion of 1 μM carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP), a protonophore that abolishes the mitochondrial membrane potential, increases cytoplasmic calcium, and slows the response to a bundle stimulus. In both A and B, the stimulus was a depolarization from −80 to +80 mV followed by a bundle displacement.
These experiments suggest that both the PMCA pumps and mitochondrial uptake contribute to clearance of calcium from the hair bundle during mechanical stimulation. Impairment of either process results in an elevation and a protracted elimination of the bundle Ca2+. Since the MT current is regulated by changes in stereociliary Ca2+, hindering Ca2+ removal would be expected to affect the MT currents. Blocking the PMCA pump had more pronounced effects on the current–displacement (I–X) relationship, both shifting it in the positive direction and substantially reducing its slope (Fig. 4A). Similar effects on the I–X relationship in OHCs have been reported by mutation or knock out of the PMCA2 isoform of the plasma membrane Ca-ATPase (Ficarella et al. 2007). The effects of FCCP (Fig. 4B) and RR (not shown) were similar to those with block of the PMCA pump, a translation of the I–X relationship, and a decrease in its slope. The control MT currents in these types of experiment were comparable to those previously measured for apical rat OHCs (Beurg et al. 2006, 2008; Kennedy et al. 2003; Waguespack et al. 2007). In eight control measurements, the maximum MT current was 1.09 ± 0.09 nA, the mean adaptation time constant was 0.32 ± 0.08 ms, and the activation range for the I–X relationships was 200 to 300 nm. For the treatments intended to elevate intracellular Ca2+ there was a small reduction in the maximum current, a significant reduction in the extent and speed of fast adaptation, and an increase in the activation range to 500 to 1,000 nm. Maximum currents and adaptation time constants under the different conditions were: 1.07 ± 0.11 nA, 0.98 ± 0.12 ms (n = 3; pH 9); 0.80 ± 0.17 nA, 0.95 ± 0.12 ms (n = 3; FCCP); 0.99 ± 0.17 nA, 0.98 ± 0.13 ms (n = 3; RR). Although the shift in the activation relationship resembles that during adaptation and would be expected simply by elevating stereociliary Ca2+, the slowing of adaptation and the broadening of the I–X relationship imply that other factors may be involved.
Fig. 4.
Factors affecting Ca2+ homeostasis also alter the current–displacement (I–X) relationship of the OHC transducer. A: mechanotransducer currents in response to bundle deflection, control (left), and high pH to block the PMCA pump (middle). Elevation of intracellular calcium due to block of the Ca-ATPase shifted the I–X relationship to the right and reduced its slope (sensitivity). B: mechanotransducer currents in response to bundle deflection, control (left), and 1 μM FCCP to block Ca2+ uptake into the mitochondria (middle). Elevation of intracellular Ca2+ due to block of the mitochondrial Ca2+ uptake shifted the I–X relationship to the right. In both A and B, the holding potential was −80 mV. I–X results were fitted with 2 Boltzmann components: I = IMAX/〈 1 + {exp[a1(X1 − X)]} × {1 + exp[a2(X1 − X)]}〉, where IMAX = 1.22 nA, a1 = 8.5 μm−1, X1 = 0.155 μm, a2 = 19 μm−1 (A, control); IMAX = 1.2 nA, a1 = 2.7 μm−1, X1 = 0.35 μm, a2 = 8 μm−1 (A, high pH); IMAX = 1.1 nA, a1 = 9.5 μm−1, X1 = 0.12 μm, a2 = 21 μm−1 (B, control); IMAX = 0.92 nA, a1 = 6.8 μm−1, X1 = 0.23 μm, a2 = 15 μm−1 (B, FCCP).
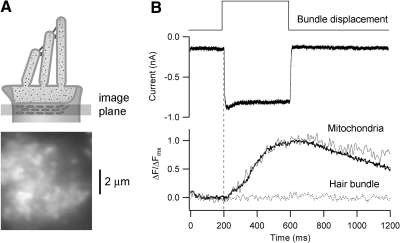
To confirm that the mitochondria in the belt beneath the cuticular plate contributed to uptake of Ca2+ originating from the MT channels, mitochondrial Ca2+ was monitored with Rhod2 during bundle stimulation (Fig. 5A). Hair cells had been previously loaded with the esterified form, Rhod2AM, and after 30 min incubation, it was possible to visualize a series of bright spots at the cell apex just beneath the cuticular plate that were interpreted as being the mitochondria (Duchen et al. 2001). After attaining whole cell recording, we waited 5 min to wash out any deesterified dye remaining in the cytoplasm. On bundle deflection, an increase in the mitochondrial signal was obtained in all cells studied (Fig. 5B). When we focused on the hair bundle, no fluorescence signal was measurable, indicating that the signal does not originate from Ca2+ binding to cytoplasmic Rhod2, which should have been washed out after attaining the whole cell configuration. The intramitochondrial Ca2+ increase began with a diffusional delay of about 20 ms and in one cell was sustained but in the others showed some decline after the stimulus. The decay time constant, probably reflecting rerelease of Ca2+ by the mitochondrial sodium/calcium exchanger averaged over five cells, was 1.20 ± 0.75 s (Fig. 5B).
Fig. 5.
Ca2+ accumulation in mitochondria during hair bundle stimulation. A: imaging of mitochondrial belt after loading an OHC with Rhod2-AM. Fluorescence image shows region of cell viewed from above during patch recording. B: MT current and mitochondrial Ca2+ during bundle deflection. The thin trace is the response in a single cell; the thick trace is the average response over 5 different cells, scaled to the maximum increase in fluorescence. The dashed line represents imaging of the hair bundle where no fluorescence signal was detected.
PMCA exchange current
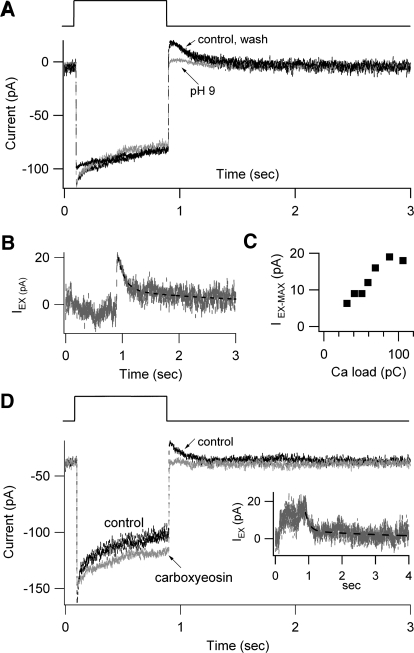
A crucial factor in maintaining long-term Ca2+ balance is the number of PMCA pumps. The operation of the pump is electrogenic, one Ca2+ being extruded in exchange for one H+ during each ATPase cycle (Hao et al. 1994). The resulting current, a small outward current following Ca2+ loading, has been recorded in frog saccular hair cells (Yamoah et al. 1998). We used a similar approach to estimate the exchange current in OHCs, by comparing MT currents evoked by prolonged bundle deflections in the absence and the presence of a pump inhibitor (Fig. 6). Measurements were made in an extracellular saline in which the Na+ had been replaced by the relatively impermeant Tris (see methods), so the MT current was carried largely by Ca2+. At the offset of the bundle stimulus, there was a small slow outward current that could be reversibly blocked by increasing the pH around the hair bundle to 9.0 by a brief 10-s perfusion from a nearby puffer pipette (Fig. 6A). The exchange current IEX was derived by subtracting the currents with and without pump block (Fig. 6B). It was recorded in all seven cells studied, decayed back toward the baseline with a principal time constant of 143 ± 23 ms, and had an initial amplitude that grew with the Ca2+ load and saturated at close to 18 pA (Fig. 6C), corresponding to a Ca2+ efflux of 3.7 fmol·s−1. The Ca2+ load was determined by integrating the current during the bundle deflection. In some but not all cells growth of a small outward current occurred during the stimulus (see Fig. 6D).
Fig. 6.
Quantification of the PMCA pump rate from the exchange current IEX. A: MT currents to 0.6 μm hair bundle displacements before, during, and after increasing the pH of the saline around the bundle from 7.4 to 9.0 to block the PMCA pump. Three responses are superimposed: the pH 7.4 (control and wash) as black lines and the pH 9.0 (pump block) as gray. B: IEX, obtained by subtracting the pH 9.0 from the pH 7.4 in A. The decline in IEX after termination of the stimulus is fitted with 2 time constants of 128 ms and 2.2 s (dashed line). C: the IEX in 7 cells plotted against the Ca2+ load, obtained by integrating the MT current during the stimulus. For the 2 smallest Ca2+ loads, the hair bundle stimulus was 400 ms in duration, whereas that for the rest it was 800 ms. D: MT currents to 0.6 μm hair bundle displacements before (control) and several minutes after replacing the bath saline, with one containing 50 μM carboxyeosin diacetate succinimidyl ester to block the PMCA. Inset shows IEX, obtained by subtracting the test from the control in D. The decline in IEX is fitted with 2 time constants of 125 ms and 3.3 s (dashed line). All experiments were performed on P7 to P10 rats in salines in which the Na+ had been replaced by Tris so the MT current is carried mainly by Ca2+.
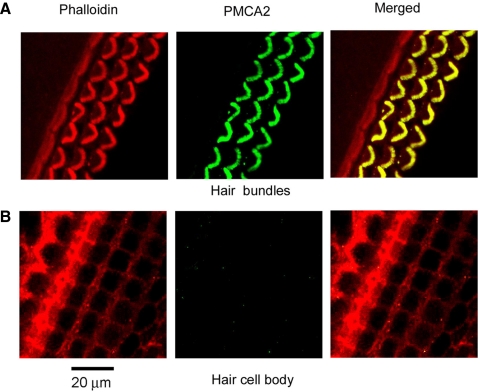
Several arguments have been previously advanced that the current is not an artifact of a change in transduction (Yamoah et al. 1998). In our experiments, the puffer pipette was placed about 50 μm away from the bundle and the flow did not mechanically stimulate the bundle, as indicated by the fact that the I–X relationships were superimposable with and without the blocker. IEX therefore seems unlikely to be attributable to a change in adaptation. Second, IEX cannot be an exchange current due to either the Na pump (Na+ omitted from the extracellular medium) or proton extrusion (which in the hair bundle is exchanged for K+ and is electroneutral in the presence of some extracellular K+; Hill et al. 2006). Third, a quantitatively similar IEX could be observed on blocking the PMCA by incubation in the membrane-permeant form of the inhibitor eosin (CEDA; 50 μM), although this treatment was not reversible (Fig. 6D). An important conclusion can be reached by comparing block of the pump by alkalinization or with CEDA. The change in pH was achieved by local 10-s perfusion, which would have predominantly affected the PMCA in the hair bundle. The positioning of the puffer pipette coupled with the direction of flow would have minimized access to the basolateral aspect of the OHC because the reticular lamina was intact around the cell from which the recording was taken. In contrast, extended perfusion with CEDA, which was taken up by the cell, would have blocked all PMCA pumps in both the bundle and the soma. This suggests that the majority of the PMCA pumps are localized to the OHC hair bundle. Immunofluorescence labeling of P7 to P10 rats in the same region as that used in the electrophysiological experiment showed strong labeling for the PMCA2 isoform in OHC bundles but not in the cell body (Fig. 7). No significant difference in intensity was seen between the P7 and P10 preparations. The PMCA2 distribution in the neonatal animals was similar to that reported for adult rat cochleas (Dumont et al. 2001).
Fig. 7.
Immunofluorescence labeling for PMCA2 in rat neonatal organ of Corti. A: confocal section through the hair bundles labeled with phalloidin (left), anti-PMCA2 antibody (middle), and merged (right). Bundles of OHCs but not inner hair cells (IHCs) are strongly labeled. B: confocal section through cell bodies showing no PMCA2 labeling in P7 rat, from the base of the apical turn, the same cochlear region where the electrophysiological measurements were performed.
The exchange current can be used to estimate the pump density, assuming all pumps are operating at their maximum rate and are located in the hair bundle. If the membrane area of the hair bundle is taken as approximately 200 μm2, a peak exchange current of 18 pA would require a pump density of roughly 6,000/μm2, assuming a pump turnover rate of 100 s−1, the upper limit of that measured for the erythrocyte PMCA pump (Kubitscheck et al. 1995). Such a high pump density is several times greater than that inferred for frog saccular hair cells (2,000/μm2; Lumpkin and Hudspeth 1998; Yamoah et al. 1998).
Calcium influx in endolymph solutions
The experiments described so far were conducted in a saline containing high (1.5 mM) Ca2+ bathing the hair bundle similar to perilymph, whereas rat endolymph has only 20 μM Ca2+ (Bosher and Warren 1978). Problems with maintaining homeostasis depend critically on the size of the Ca2+ influx under in vivo conditions and there are suggestions for hair cells of both turtle (Ricci and Fettiplace 1998) and frog (Lumpkin and Hudspeth 1998) that the Ca2+ influx in vivo might be substantial. To reexamine this point in mammalian cochlear hair cells, bundle Ca2+ transients were measured for reduced extracellular Ca2+ concentrations using a pair of puffer pipettes containing control (1.5 mM Ca2+) and test solutions (see methods). Because the transduction-related Ca2+ signals were smaller than expected, it was necessary to use the high-affinity indicator Fluo-4. Although lowering the Ca2+ augmented the MT current due to relief of external block by the divalent cation (Kennedy et al. 2003), the size of the Ca2+ transient decreased substantially (Fig. 8). Thus the ratio IR of the current amplitude in 20 μM Ca2+, compared with control, was 1.55 ± 0.14 (n = 6), but the peak fluorescence change decreased by a factor ΔFR of 53 ± 0.28. The product of these values (IR·ΔFR) was 82 ± 8, yielding the reduction in Ca2+ carried by the MT current for a 75-fold lowering of Ca2+ from 1.5 to 0.02 mM. When Ca2+ was reduced 10-fold to 0.15 mM, IR was 1.49 ± 0.09 (n = 4), ΔFR was 7.4 ± 2.1, and (IR·ΔFR) was 11 ± 3. The measurements in the two Ca2+ concentrations indicate that the fraction of MT current carried by Ca2+ decreases directly in proportion to its external concentration.
Fig. 8.
Hair bundle Ca2+ signals in reduced extracellular Ca2+ concentration. A: measurements in control 1.5 mM Ca2+ solution. B: responses in the same cell with 0.15 mM Ca2+ puffed on the bundle. C: measurements in another cell in control 1.5 mM Ca2+ solution before and after the test. D: responses with test solution containing 0.02 mM Ca2+. In both cells, the stimulus was a depolarization from −80 to +80 mV followed by a bundle displacement elicited by a fluid jet from a puffer pipette containing the requisite (1.5 mM Ca2+ or reduced Ca2+) solution. All fluorescence measurements were made with Fluo-4 as the calcium indicator. The timing of the bundle stimulus and depolarization apply to both cells. The Ca2+ signals in the lowest Ca2+ concentration were substantially attenuated despite an increase in the MT current.
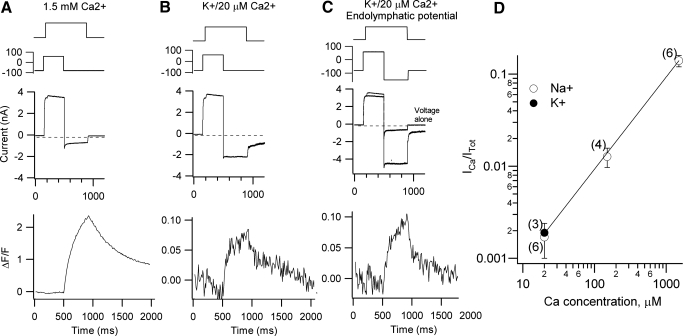
In the intact cochlea, the hair bundles are exposed to endolymph, which is not only low in calcium but also has K+ as the major monovalent cation. To more accurately reproduce the in vivo condition, the pipette solution was changed from a Cs+-based to a K+-based intracellular and the bundle was perfused with an artificial endolymph, 160 mM K, 20 μM Ca2+ (Fig. 9, A and B). In control measurements (1.5 mM Ca2+) using a K+-based intracellular, the maximum MT current was 800 ± 190 pA (n = 9). When Ca2+ was reduced from the control 1.5 mM to the artificial endolymph, the current ratio IR was 2.1 ± 0.4 (n = 3), the fluorescence ratio ΔFR was 30.7 ± 3.3, and (IR·ΔFR) was 65 ± 5. Thus although the increase in current size was somewhat larger than that in the measurements in Na+, the fraction of current carried by Ca2+ still decreased roughly in proportion to its extracellular concentration, which was 75-fold. Collected measurements from the low-Ca2+ imaging experiments are given in Fig. 9D and are well described by a linear fit. Absolute values for the fraction of current carried by Ca2+ were calculated using a control value of 0.15 for a 1.5 mM Ca2+ perilymph solution. This value was derived from experiments in which the extracellular monovalent ions Na+ and K+ had been replaced by the relatively impermeant N-methylglucamine, so the MT current was carried solely by Ca2+ (Beurg et al. 2006). The conclusion from Fig. 9D is that Ca2+ accounts for only about 0.2% of the transduction current in a saline comparable to endolymph.
Fig. 9.
Hair bundle Ca2+ signals in artificial endolymph (160 mM K+, 0.02 mM Ca2+). A: measurements in control (Na+, 1.5 mM Ca2+) solution. B: responses in artificial endolymph (160 mM K+, 0.02 mM Ca2+). C: responses in artificial endolymph (160 mM K+, 0.02 mM Ca2+), with the addition of a hyperpolarization to −150 mV to simulate the endolymphatic potential. The current response without the bundle stimulus is superimposed on that with the bundle deflection. Note the MT current amplitude is about 4 nA. The stimulus was a depolarization from −80 to +80 mV followed by a bundle displacement elicited by a fluid jet from a puffer pipette containing either Na+, 1.5 mM Ca2+ or K+, 0.02 mM Ca2+ solution. D: collected measurements of the fraction of MT current carried by Ca2+ (ICa/ITot) evaluated as described in the text and plotted against the extracellular Ca2+ concentration. Open symbols, extracellular Na+, intracellular Cs+; filled symbols with extracellular and intracellular K+. Numbers of experiments are given beside each point. The line is a straight line fit to the points.
One further feature of the intact cochlea is the potential difference between endolymph and perilymph, known as the endolymphatic potential, which is about 90 mV in rats (Bosher 1979). This endolymphatic potential sums with the resting potential of −60 mV to augment the electrical driving force across the MT channels in the hair bundle. Resting potential measurements in P8 to P14 rats using EGTA as the intracellular calcium buffer gave a mean value of −60 ± 6 mV (n = 7), implying a driving force in vivo of 150 mV. This condition was simulated by increasing the holding potential to −150 mV and measuring the MT current amplitude and calcium transient in K+ endolymph (Fig. 9C). When Ca2+ was reduced from the control 1.5 mM to that in artificial endolymph, the current ratio IR was 3.7 ± 0.9 (n = 3), the fluorescence ratio ΔFR was 19.7, and (IR·ΔFR) was 73 ± 18, again similar to the 75-fold reduction in Ca2+ in the apical perfusate. The amplitude of the MT current under the in vivo like conditions, at a holding potential of −150 mV with the hair bundle exposed to 160 K+, 20 μM Ca2+ endolymph, was 3.29 ± 0.7 nA (n = 4), with the largest value observed being 4.2 nA. Assuming 0.2% of this current is carried by Ca2+ (Fig. 9D), a peak Ca2+ current of about 7 pA is predicted to flow in vivo.
Modeling of calcium transients
Hair bundle and somatic Ca2+ transients were simulated using a model incorporating Ca2+ influx via the MT channels, diffusion, and binding to both mobile and fixed buffers, extrusion by PMCA pumps, and uptake into mitochondria (Fig. 10A; see methods). The model incorporated several new features compared with previous simulations of hair bundle Ca2+ (Lumpkin and Hudspeth 1998; Wu et al. 1999), including diffusion into the cell body, the contribution of the mitochondria, and correction for the limited resolution of the imaging system. For a single stimulus in 1.5 mM extracellular Ca2+, Ca2+ rapidly accumulated in the stereocilia, rising after a small delay then increasing with two phases as observed experimentally (Fig. 1B). The speed of the first phase was inversely proportional to distance from the channel, whereas the second was dominated by the dimensions of the cell body, increasing as the cell width decreased. The time constant of decay of the Ca2+ (Fig. 10B) was as fast as the stimulus offset until its concentration approached the equilibrium constant of the fixed buffer (10 μM), after which it was slowed. However, if the time course of the Ca2+ transient was expressed in terms of the integrated fluorescence intensity (Fig. 10C), both the onset and decay of the fluorescence signal were slower than those of the Ca2+ itself and were similar to the experimental results. The simulation agreed with the experiments if there was ≥2 mM of fixed buffer with dissociation coefficient (KCa) of 10 μM, in which case the decay time constant of the fluorescence was 40 ms, similar to the measurements. This decay time constant was dependent on the concentration and KCa of the fixed buffer and, when there was no fixed buffer, the time constant decreased from 40 to 10 ms.
In the first 100 ms, the transient Ca2+ profile was dominated by the mobile and fixed buffers rather than PMCA pumps and mitochondria (Fig. 10D). The PMCA pump could transport <10% of the entering calcium. The mitochondrial Ca2+ uptake was negligible within the first 100 ms of stimulation but, as the free Ca2+ concentration in the cell body increased, the mitochondrial contribution became comparable to that of the PMCA pump. The high-affinity mobile buffer (EGTA) had the greatest effect on the Ca2+ concentration at the stereociliary tips. At the termination of the stimulus, the buffering system returned most of the Ca2+ it had absorbed within a fraction of a second (Fig. 10, D and E). The Ca2+ was shuttled along the chain from low-affinity to high-affinity binding sites, from fixed buffer (KCa = 10 μM) to mitochondria (equilibrium point near 0.5 μM), EGTA (KCa = 0.2 μM), and finally extruded by PMCA pumps. An important conclusion from this modeling is that the kinetics of the Ca2+ response to a single bundle stimulus is scarcely affected by the pumping mechanisms.
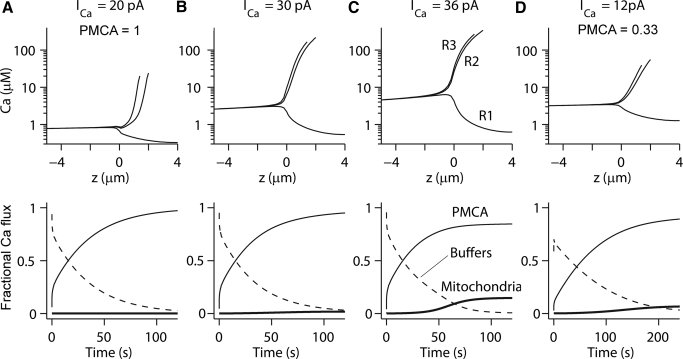
The simulations in Fig. 10 were for a single stimulus starting from a condition in which the MT channels were closed. However, in reality there is a persistent calcium influx attributable to the MT channels being partially open at rest. For rat OHCs in vitro, bathed in 1.5 mM extracellular calcium, about 5% of the total MT current (IMAX ≈ 1,000 pA) is activated at rest (Kennedy et al. 2003), equivalent to a standing Ca2+ current of about 8 pA. To avoid continual accumulation of intracellular Ca2+, the entire Ca2+ load must be extruded solely by the PMCA pumps. To examine the importance of the PMCA pumps, two different densities in the stereociliary membrane were simulated: 2,000 PMCA pumps·μm−2, as suggested for amphibian hair cells (Yamoah et al. 1998) and 6,000 pumps·μm−2, as implied from our OHC measurements (Fig. 7), in each case assuming a maximum transport rate of 100 ions/s per pump (Kubitschek et al. 1995). When there were 6,000 PMCA pumps·μm−2 in the stereociliary membrane, the simulated hair cell could marginally sustain 30 pA of constant Ca2+ current (Fig. 11B). Although the mitochondria were still accumulating calcium, the uptake rate was decreasing (Fig. 11B). Furthermore, with this resting Ca2+ influx, about 99% of the mobile buffer was depleted at the tips of the stereocilia. For a constant current of 20 pA, all the Ca2+ was extruded by the PMCA pumps without loading the mitochondria and this therefore represents the safe rate. When the resting Ca2+ current was increased to 36 pA, the PMCA pumps could not extrude the Ca2+, which accumulated within the mitochondria (Fig. 11C). As the resting Ca2+ current was increased from 20 to 36 pA, the maximum Ca2+ concentration grew nearly 10-fold and the free cytoplasmic Ca2+ concentration in the mitochondrial region, 4 μm below the stereociliary rootlets, increased from 0.8 to 4.5 μM. When the PMCA density was reduced to 2,000 pumps·μm−2, equivalent to the nonmammalian density, the simulated hair cell could marginally sustain a constant Ca2+ current of 12 pA (Fig. 11D) and could cope safely with a Ca2+ influx of only 8 pA (not shown). As expected, the time course of Ca2+ clearance was threefold slower at the lower pump rate.
Fig. 11.
Theoretical assessment of sustainable Ca2+ current through the MT channels. A: resting Ca2+ influx of 20 pA. Top: Ca2+ concentration along the 3 stereociliary rows as a function of distance z, from the base of the bundle (z = 0). Bottom: time course of contribution of the PMCA pump (thin line), buffers (dashed line), and mitochondria (thick line). B: for a resting calcium influx of 30 pA, almost all the Ca2+ was extruded, but there was a small accumulation in the mitochondria. This case is marginal. C: for a resting Ca2+ influx of 36 pA, only about 75% was extruded, the remainder accumulating in the mitochondria, which over prolonged timescale will become saturated. Simulations in A–C performed with a PMCA density of 6,000/μm2 (PMCA = 1). D: when the PMCA density was reduced to 2,000/μm2 (PMCA = 0.33), a sustained Ca2+ influx of 12 pA was now marginal and resulted in Ca2+ accumulation in the mitochondria. Individual stereociliary rows: R1, R2, and R3. All simulations with endogenous calcium buffer, 2 mM parvalbumin-β, and 0.25 mM calbindin-28K.
Thus with 6,000 PMCA pumps·μm−2, the OHCs should be able to cope with sustained Ca2+ influxes in both 1.5 and 0.02 mM external Ca2+, with resting currents three- to tenfold smaller than could be safely extruded. However, an important caveat is the contribution of the PMCA pumps in the soma. Although in the simulations, the lateral somatic membrane contained only one tenth the density of PMCA pumps, compared with that of the stereociliary membrane, the role of the somatic PMCA pumps was not negligible. If these were removed entirely, the OHC could handle a resting Ca2+ influx through the MT channels of <15 pA, even with the higher PMCA pump density in the stereociliary membrane.
Effects of intracellular calcium buffer
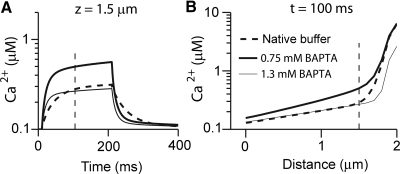
The model was also useful for comparing the efficacy of the endogenous mobile calcium buffer, 2 mM parvalbumin-β (oncomodulin), and 0.25 mM calbindin (Hackney et al. 2005), with the extrinsic buffers used in the pipette solution. Because parvalbumin-β binds two Ca2+ ions and calbindin binds four Ca2+ ions, the effective concentration of Ca2+ binding sites is 5 mM. EGTA has been the principal buffer used experimentally in our studies of rat cochlear hair cells but at 1 mM, and with a slow Ca2+ binding rate, it was more than 10-fold less effective in buffering stereociliary Ca2+ than parvalbumin-β. The endogenous buffer was therefore compared with BAPTA (Fig. 12). Based on fits to the Ca2+ gradient along the stereocilia, the endogenous buffer was roughly equivalent to between 0.75 and 1.3 mM BAPTA (Fig. 12B), the lower concentration providing the best match to the distal part of the gradient near the stereociliary tip and the higher concentration to the proximal gradient around the rootlet. It was not possible to obtain a perfect match to the kinetics (Fig. 12A), which was faster with BAPTA due to its more rapid Ca2+ binding rate (Table 1). EGTA was much less effective as a buffer and ≥10 mM was needed to give a match to the endogenous buffer at the stereocilia tip (not shown). Modeling showed that the PMCA pumps contributed little to the stereociliary Ca2+ gradient because their inhibition had no effect in the short term until the buffers became saturated. To assess transduction under in vivo conditions, an intracellular solution containing 1 mM BAPTA was used to approximate the endogenous mobile buffer in OHC. Stereociliary Ca2+ gradients for different concentrations of exogenous buffers were also computed for inner hair cells. These cells in posthearing animals contain one tenth the mobile endogenous buffer of OHCs: about 0.5 mM Ca2+ binding sites, composed of parvalbumin and calretinin (Hackney et al. 2005). Assigning binding properties for calretinin similar to those for calbindin, stereociliary Ca2+ gradients at long times could be well matched to 0.5 mM EGTA (not shown). A low concentration of endogenous calcium buffer has previously been inferred for IHCs from effects on exocytosis (Moser and Beutner 2000).
Fig. 12.
Theoretical comparison of native OHC calcium buffer with BAPTA. A: time courses of Ca2+ transient with different concentrations of BAPTA and with the native buffer as the mobile buffer at a position z = 1.5 μm from rootlet. B: Ca2+ gradients along the middle row stereocilium in the 3 buffers 100 ms after stimulus onset. The Ca2+ source, the MT channels, located at 2 μm. The mobile Ca2+ buffer in OHCs of posthearing rats is assumed to be 2 mM parvalbumin-β and 0.25 mM calbindin (Hackney et al. 2005). Based on the matches to the Ca2+ kinetics and the gradients along stereocilia, the native buffer is equivalent to 0.75 to 1.3 mM BAPTA.
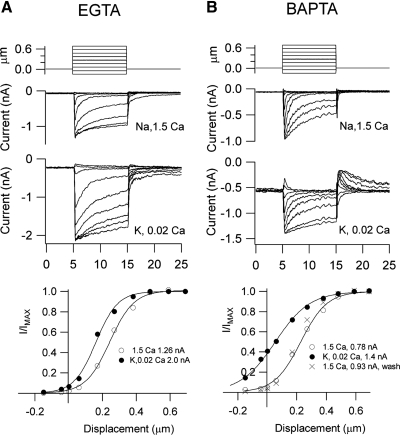
The nature and concentration of the cytoplasmic calcium buffers contribute to the transient responses and to the gradients away from the source (Roberts 1993). As a consequence, they influence several aspects of the MT currents, including the fraction of current activated at rest and the kinetics of adaptation (Ricci et al. 1998). These parameters were measured in OHCs by hair bundle deflection with a glass probe attached to a piezoelectric actuator. With an intracellular solution containing K+ and 1 mM EGTA as the calcium buffer, changing the hair bundle perfusate from control (Na+, 1.5 mM Ca2+) to artificial endolymph (K+, 0.02 mM Ca2+) increased the maximum MT current from 989 ± 195 to 1,680 ± 190 pA and the fraction of current activated at rest from 0.04 ± 0.01 to 0.12 ± 0.02 (n = 5; Fig. 13A). Thus a mean sustained current of 200 pA flowed at rest. The artificial endolymph also slowed the fast adaptation time constant from 0.20 ± 0.05 to 0.58 ± 0.03 ms as reported previously (Ricci et al. 2005). When 1 mM BAPTA was used instead of EGTA, during perfusion with artificial endolymph a standing inward current was evident in all cells examined. Since the standing current could be blocked by 0.2 mM dihydrostreptomycin, this suggests a large resting probability of opening of the MT channels. On switching from control to artificial endolymph in OHCs recorded with 1 mM BAPTA, the peak current in five cells increased from 924 ± 128 to 1,334 ± 152 pA; the fast adaptation time constant slowed from 0.24 ± 0.08 to 0.5 ± 0.05 ms; and the fractional current turned on at rest increased from 0.07 ± 0.01 to 0.40 ± 0.08 (n = 5), the leftward shift in the I–X relationship being fully reversible (Fig. 13B). In this case, the resting current through the MT channels in endolymph amounted to 550 pA (mean in five OHCs, 523 ± 50 pA). As a consequence, the OHC depolarized from a resting potential of −49 mV (control) to −29 mV (endolymph), returning to −46 mV (wash).
Fig. 13.
MT currents in control and endolymph solutions with different exogenous calcium buffers. A: calcium buffer 1 mM EGTA. MT currents in response to bundle deflections with a stiff probe, current records (top), and I–X relationships (bottom) for control (Na+, 1.5 mM Ca2+) and endolymph (K+, 0.02 mM Ca2+). B: calcium buffer 1 mM BAPTA. MT currents in response to bundle deflections with a stiff probe, current records (top), and I–X relationships (bottom) for control (Na+, 1.5 mM Ca2+) and endolymph (K+, 0.02 mM Ca2+). Note that the endolymph solution increases the maximum current and shifts the I–X relationship in the negative direction. The shift is much larger with BAPTA, in which 40% of the current is activated at rest. In B, controls before (○) and after (X) low calcium perfusion (●). I–X results were fitted with a single Boltzmann: I = IMAX/〈1 + {exp[a1(X1 − X)]}〉, where IMAX = 1.26 nA, a1 = 14.1 μm−1, X1 = 0.24 μm (A, control); IMAX = 2.0 nA, a1 = 15.4 μm−1, X1 = 0.16 μm (A, endolymph); IMAX = 0.85 nA, a1 = 11.2 μm−1, X1 = 0.23 μm (B, control); IMAX = 1.4 nA, a1 = 9 μm−1, X1 = 0.05 μm (B, endolymph). Abscissa for current responses is time in milliseconds.
DISCUSSION
Calcium homeostasis in vivo
We have used fast millisecond imaging to monitor calcium changes in the hair bundle and cell apex of OHCs during MT channel activation. For brief stimuli the time course of the hair bundle transient was dictated largely by diffusion and binding to cytoplasmic buffers and not by extrusion mechanisms, which operate on a slow timescale of seconds. However, significant perturbation of the Ca2+ dynamics occurred during prolonged inhibition of the PMCA pumps or the mitochondrial uptake system as the calcium buffers became saturated. Quantification of the PMCA pump rate indicated that it was at least threefold larger than that in nonmammalian hair cells, which should allow the OHCs to cope with large Ca2+ loads incurred during periods of high stimulation. Initial results were obtained in isolated preparations of the organ of Corti that differ in several respects from the intact cochlea. Ca2+ influx through the MT channels will be altered because of a low endolymph Ca2+ concentration, an endolymphatic potential, and an elevated body temperature. This led us to examine the Ca2+ load under conditions more closely resembling those in vivo in which the solution enveloping the hair bundles had a reduced Ca2+ concentration (20 μM), resulting in a smaller Ca2+ influx. Our measurements indicated that the fraction of current carried by Ca2+ decreased in proportion to the extracellular concentration of the divalent cation and, in 20 μM external calcium, only about 0.2% of the current was due to Ca2+. This result differs from previous conclusions that in nonmammalian vertebrates, such as frogs or turtles (Jørgensen and Kroese 1995; Ricci and Fettiplace 1998), a much larger fraction of current (≤10%) is carried by calcium in endolymph. The reasons for the discrepancy are unclear, although the results carry implications for the resting Ca2+ load in vivo and for the role of Ca2+ in fast adaptation.
The much reduced Ca2+ influx in endolymph is puzzling in light of the proposed role of Ca2+ in adaptation, which is only weakly sensitive to extracellular Ca2+. Lowering Ca2+ from 1.5 to 0.02 mM slows the fast adaptation time constant by no more than a factor of three to four (Ricci et al. 2005; present study). One explanation for this result is that the adaptation time constant in high Ca2+ is overestimated because of a limitation in the speed of stimulus delivery. Alternatively, the time constant, when it gets briefer, may become independent of intracellular Ca2+ because other factors, such as conformational transitions in the MT channel underlying adaptation, become rate limiting.
Hair bundle calcium load in vivo
Our results allow a determination of the Ca2+ load under in vivo like conditions. With K+ as the monovalent cation, as in endolymph, and with the addition of an endolymphatic potential, we estimate that the maximum Ca2+ load in apical OHCs is 7 pA. Using an intracellular calcium buffer (1 mM BAPTA) roughly equivalent to the endogenous mobile buffer, about 40% of the MT current is activated at rest, so the sustained calcium load is 3 pA under ionic conditions resembling those in vivo. These currents can be compared with the ability of the OHC to extrude the accumulated Ca2+ via the PMCA pumps in the stereocilia. Simulations with brief calcium loads showed that the pump can safely clear a sustained influx of 20 pA (Fig. 11A), several times larger than either the resting or maximum MT currents, thus providing a reasonable safety margin. This safety margin arises because of a larger than expected PMCA pump density inferred from measurements of the electrogenic pump current that are greater than those recorded in bullfrog saccular hair cells (Yamoah et al. 1998). The electrogenic exchange current does not directly yield the pump density but requires assumptions about the turnover rate and the membrane area. We assumed a turnover rate of 100 ions/s, equivalent to the maximum measured in biochemical experiments on erythrocytes (Kubitscheck et al. 1995), but the turnover rate in the PMCA2 isoform, which is regarded as a fast neuronal pump (Brini et al. 2003), may be larger than the PMCA1 and -4 isoforms in erythrocytes. The PMCA pumps in OHCs were assumed to be largely confined to the hair bundle because they were inhibited by brief perfusion with a high pH saline that enveloped the hair bundles, but was unlikely to gain access to the OHC basolateral membrane. However, some PMCA pumps must occur in the soma to extrude Ca2+ that has entered as the voltage-dependent Ca2+ current, although this current is small in rats older than P8 (Beurg et al. 2008).
The present conclusions apply only to the apical low-frequency OHCs. High-frequency OHCs may be more vulnerable to becoming Ca2+ loaded because of larger MT currents (Ricci et al. 2005) and shorter stereocilia (Roth and Bruns 1992). Because of tonotopic gradients in MT channel size and numbers of stereocilia (Beurg et al. 2006; Ricci et al. 2003, 2005), there could be up to a fivefold greater MT current flowing into hair bundles of high-frequency OHCs compared with low-frequency ones. As a consequence, the Ca2+ load during transduction will be substantially larger than that in low-frequency OHCs. The measured pump density may then be only marginal to extrude the larger load and it will be important to establish whether there is a concomitant increase in the PMCA pump rate in high-frequency cells to deal with this load. One factor not accounted for in the present experiments is the raised body temperature (37°C) in vivo, which will increase both Ca2+ influx and efflux. However, the effect of temperature on the Ca-ATPase rate (Q10 ≈ 3; e.g., Kratje et al. 1983) will be larger than that on the MT channel conductance (Q10 ≈ 1.5 by analogy with other ion channel conductances; Hille 2001), which therefore widens the safety margin between the maximum rates of influx and removal.
Inner and outer hair cells
These effects of the calcium buffer on the resting open probability recall similar measurements in turtle auditory hair cells. In those cells, recording with a 0.5 mM BAPTA electrode equivalent to the endogenous calcium buffer and exposing the hair bundles to low-calcium endolymph yielded a fraction of MT current activated at rest as 28% (Ricci et al. 1998). In turtle, as in rat OHCs, the high resting open probability of the MT channels caused a sustained inward current that depolarized the resting potential (Farris et al. 2006). The differing calcium buffer concentrations in OHCs (≅1 mM BAPTA) and IHCs (≅0.5 mM EGTA) suggests that larger fractions of MT channels will be activated at rest in OHCs than those in IHCs. This implies that, other things being equal, the receptor potentials in vivo will be more symmetrical and the resting potentials more depolarized in OHCs than those in IHCs. This conclusion differs from what has been reported with sharp electrode recordings in vivo (Cody and Russell 1987; Dallos 1985), in which the resting potentials of OHCs were very negative, about −70 mV. We suggest that such negative resting potentials are unphysiological and reflect compromise of the MT apparatus, indicated by small receptor potentials <10 mV peak to peak (Cody and Russell 1987; Dallos 1985), and therefore reduction of the large inward standing current via MT channels open at rest.
Role of organelles
Although Ca2+ signals from the mitochondrial belt below the cuticular plate were detectable only during transduction 1.5 mM external calcium, such results demonstrate a potential contribution of these organelles in acting as a fixed buffer to block Ca2+ diffusion into the cell body. A role for mitochondria in Ca2+ homeostasis has been demonstrated experimentally in other cell types (Babcock and Hille 1998; Brini 2003; Duchen 2000). Furthermore, by clearing submembranous calcium, mitochondria can influence the inactivation of voltage-dependent Ca2+ channels in both chromaffin cells and heart muscle (Hernández-Guijo et al. 2001; Sánchez et al. 2001). Here, we demonstrated that block of the Ca2+ removal systems, both mitochondria and PMCA pumps, shifted the working range of the MT channels in OHCs by causing Ca2+ accumulation in the stereocilia. This is consistent with the proposed role of stereociliary Ca2+ in regulating adaptation.
It might be argued that the main function of mitochondria in hair cells is to supply energy for the PMCA pump, but this role is intimately linked to the hair cell Ca2+ homeostasis. In other neurons, the majority of ATP produced is known to be expended in fueling ATPase ion pumps (Nicholls 2008). However, with even a modest decline in mitochondrial oxidative metabolism (as may occur in aging; Beal 2005) so that the ATP supply is diminished, the PMCA pumps may no longer cope with the calcium influx through MT channels open at rest; the extra calcium load will accumulate in the mitochondria and may further impair their ability to supply energy. Thus there is likely to be positive feedback between mitochondrial insult and loss of PMCA activity. Moreover, the problems will be exacerbated in high-frequency OHCs in which the Ca2+ load is larger and may account for their preferential vulnerability.
Several cytoplasmic factors were not considered in the present experiments, including the endoplasmic reticulum and the mitochondria and submembranous cisternae that line the OHC lateral wall (Lim 1986). Extracellular ATP is known to increase cytoplasmic Ca2+ in the OHC by influx via P2X receptors and by release from intracellular compartments such as Hensen's body (Ashmore and Ohmori 1990; Mammano et al. 1999). The physiological significance of this ATP pathway is unclear and its link to OHC mechanotransduction is unknown. It is conceivably a pathological response to extracellular ATP released by cellular damage. We found no evidence of a Ca2+-induced Ca2+ release secondary to hair bundle stimulation, as indicated by a larger or regenerative signal in the subcuticular plate region compared with the hair bundle. Nevertheless, it is possible that a compartment such as Hensen's body in the apical cytoplasm could assist mitochondria in absorbing and buffering calcium entering through the MT channels and hindering its access to the soma. The role of the lateral cisternae in Ca2+ homeostasis is also controversial, although they may be involved in Ca2+-induced Ca2+ release secondary to acetylcholine discharge from the efferent terminals at the OHC base (Dallos et al. 1997; Lioudyno et al. 2004; Murugasu and Russell 1996). The Ca2+ released may decrease axial compliance of OHCs, thereby modulating their motor activity (Dallos et al. 1997; Frolenkov et al. 2003). However, a larger Ca2+ load emanating from the hair bundle would activate proteases and irreversibly digest the somatic cytoskeleton. It will be important in future experiments to explore the factors dictating Ca2+ balance in the cell soma and whether there is any interaction with Ca2+ signals in the hair bundle.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01 DC-01362 to R. Fettiplace and Chinese Scholarship Council Grant 2009616042 to Q. Chen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank M. Evans for participating in initial experiments, T. Ricci for helpful discussions, and C. Hackney and S. Mahendrasingam for quantifying outer hair cell mitochondrial densities. Information about the MatLab code for the Ca2+ simulations may be accessed at jnam@wisc.edu.
REFERENCES
- Apicella et al., 1997.Apicella S, Chen S, Bing R, Penniston JT, Llinás R, Hillman DE. Plasmalemmal ATPase calcium pump localizes to inner and outer hair bundles. Neuroscience 79: 1145–1151, 1997 [DOI] [PubMed] [Google Scholar]
- Ashmore and Ohmori, 1990.Ashmore JF, Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol 428: 109–131, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock and Hille, 1998.Babcock DF, Hille B. Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol 8: 398–404, 1998 [DOI] [PubMed] [Google Scholar]
- Beal, 2005.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58: 495–505, 2005 [DOI] [PubMed] [Google Scholar]
- Beurg et al., 2006.Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26: 10992–11000, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg et al., 2009.Beurg M, Fettiplace R, Nam J-H, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12: 553–558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg et al., 2008.Beurg M, Nam J-H, Crawford AC, Fettiplace R. The effects of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94: 2639–2653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg et al., 2008.Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci 28: 1798–1803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher, 1979.Bosher SK. The nature of the negative endocochlear potentials produced by anoxia and ethacrynic acid in the rat and guinea-pig. J Physiol 293: 329–345, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher and Warren, 1978.Bosher SK, Warren RL. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature 273: 377–378, 1978 [DOI] [PubMed] [Google Scholar]
- Brini, 2003.Brini M. Ca2+ signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium 34: 399–405, 2003 [DOI] [PubMed] [Google Scholar]
- Brini et al., 2003.Brini M, Coletto L, Pierobon N, Kraev N, Guerini D, Carafoli E. A comparative functional analysis of plasma membrane Ca2+ pump isoforms in intact cells. J Biol Chem 278: 24500–24508, 2003 [DOI] [PubMed] [Google Scholar]
- Celio et al., 1996.Celio MR, Pauls T, Schwaller B. Guidebook to Calcium-Binding Proteins. New York: Oxford Univ. Press, 1996 [Google Scholar]
- Chan and Hudspeth, 2005.Chan DK, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci 8: 149–155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi and Eisner, 1999.Choi HS, Eisner DA. The role of sarcolemmal Ca2+-ATPase in the regulation of resting calcium concentration in rat ventricular myocytes. J Physiol 515: 109–118, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody and Russell, 1987.Cody AR, Russell IJ. The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol 383: 551–569, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrove et al., 2000.Colegrove SL, Albrecht MA, Friel DD. Dissection of mitochondrial Ca2+ uptake and release fluxes in situ after depolarization-evoked [Ca2+](i) elevations in sympathetic neurons. J Gen Physiol 115: 351–370, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton et al., 1978.Crompton M, Moser R, Lüdi H, Carafoli E. The interrelations between the transport of sodium and calcium in mitochondria of various mammalian tissues. Eur J Biochem 82: 25–31, 1978 [DOI] [PubMed] [Google Scholar]
- Crouch and Schulte, 1995.Crouch JJ, Schulte BA. Expression of plasma membrane Ca-ATPase in the adult and developing gerbil cochlea. Hear Res 92: 112–119, 1995 [DOI] [PubMed] [Google Scholar]
- Dallos, 1985.Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci 5: 1591–1608, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos et al., 1997.Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci 17: 2212–2226, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen, 2000.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol 529: 57–68, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen et al., 2001.Duchen MR, Jakobson J, Keelan J, Mojet MH, Vergun O. Functional imaging of mitochondria within cells. In: Methods in Cellular Imaging, edited by Periasamy A. Oxford, UK: Oxford Univ. Press, 2001, p. 88–111 [Google Scholar]
- Duman et al., 2008.Duman JG, Chen L, Hille B. Calcium transport mechanisms of PC12 cells. J Gen Physiol 131: 307–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont et al., 2001.Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci 21: 5066–5078, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris et al., 2004.Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558: 769–792, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris et al., 2006.Farris HE, Wells GB, Ricci AJ. Steady-state adaptation of mechanotransduction modulates the resting potential of auditory hair cells, providing an assay for endolymph [Ca2+]. J Neurosci 26: 12526–12536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace and Hackney, 2006.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci 7: 19–29, 2006 [DOI] [PubMed] [Google Scholar]
- Fettiplace and Ricci, 2006.Fettiplace R, Ricci AJ. Mechanoelectrical transduction in auditory hair cells. In: Springer Handbook of Auditory Research: Hair Cells, edited by Eatock RA, Popper A, Fay RR. Berlin: Springer-Verlag, 2006, p. 154–203 [Google Scholar]
- Ficarella et al., 2007.Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci USA 104: 1516–1521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberger et al., 1998.Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci USA 95: 7127–7132, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov et al., 2000.Frolenkov GI, Mammano F, Belyantseva IA, Coling D, Kachar B. Two distinct Ca2+-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci 20: 5940–5948, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov et al., 2003.Frolenkov GI, Mammano F, Kachar B. Regulation of outer hair cell cytoskeletal stiffness by intracellular Ca2+: underlying mechanism and implications for cochlear mechanics. Cell Calcium 33: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- Furness and Hackney, 2006.Furness DN, Hackney CM. The structure and composition of the stereociliary bundle in vertebrate hair cells. In: Springer Handbook of Auditory Research: Hair Cells, edited by Eatock RA, Popper A, Fay RR. Berlin: Springer-Verlag, 2006, p. 95–153 [Google Scholar]
- Glenney et al., 1981.Glenney, Kaulfus P, Matsudaira P, Weber K. F-actin binding and bundling properties of fimbrin, a major cytoskeletal protein of microvillus core filaments. J Biol Chem 256: 9283–9288, 1981 [PubMed] [Google Scholar]
- Glowatzki et al., 2008.Glowatzki E, Grant L, Fuchs P. Hair cell afferent synapses. Curr Opin Neurobiol 18: 389–395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati et al., 2006.Grati M, Schneider ME, Lipkow K, Strehler EE, Wenthold RJ, Kachar B. Rapid turnover of stereocilia membrane proteins: evidence from the trafficking and mobility of plasma membrane Ca2+-ATPase 2. J Neurosci 26: 6386–6395, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter et al., 2000.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium 28: 285–296, 2000 [DOI] [PubMed] [Google Scholar]
- Hackney et al., 2005.Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci 25: 7867–7886, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao et al., 1994.Hao L, Rigaud JL, Inesi G. Ca2+/H+ countertransport and electrogenicity in proteoliposomes containing erythrocyte plasma membrane Ca-ATPase and exogenous lipids. J Biol Chem 269: 14268–14275, 1994 [PubMed] [Google Scholar]
- Heinemann et al., 1994.Heinemann C, Chow RH, Neher E, Zucker RS. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J 67: 2546–2557, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzl and Ndubuka, 2007.Henzl MT, Ndubuka K. Low-affinity signature of the rat beta-parvalbumin CD site: evidence for remote determinants. Biochemistry 46: 23–35, 2007 [DOI] [PubMed] [Google Scholar]
- Hernández-Guijo et al., 2001.Hernández-Guijo JM, Maneu-Flores VE, Ruiz-Nuño A, Villarroya M, García AG, Gandía L. Calcium-dependent inhibition of L, N, and P/Q Ca2+ channels in chromaffin cells: role of mitochondria. J Neurosci 21: 2553–2560, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill et al., 2006.Hill JK, Brett CL, Chyou A, Kallay LM, Sakaguchi M, Rao R, Gillespie PG. Vestibular hair bundles control pH with (Na+, K+)/H+ exchangers NHE6 and NHE9. J Neurosci 26: 9944–9955, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, 2001.Hille B. Ion Channels of Excitable Membranes (3rd ed.). Sunderland, MA: Sinauer, 2001 [Google Scholar]
- Jørgensen and Kroese, 1995.Jørgensen F, Kroese ABA. Ca selectivity of the transduction channels in hair cell of the frog sacculus. Acta Physiol Scand 155: 363–376, 1995 [DOI] [PubMed] [Google Scholar]
- Kennedy et al., 2005.Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433: 880–883, 2005 [DOI] [PubMed] [Google Scholar]
- Kennedy et al., 2003.Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6: 832–836, 2003 [DOI] [PubMed] [Google Scholar]
- Kozel et al., 1998.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem 273: 18693–18696, 1998 [DOI] [PubMed] [Google Scholar]
- Kratje et al., 1983.Kratje RB, Garrahan PJ, Rega AF. The effects of alkali metal ions on active Ca2+ transport in reconstituted ghosts from human red cells. Biochim Biophys Acta 731: 40–46, 1983 [DOI] [PubMed] [Google Scholar]
- Kubitscheck et al., 1995.Kubitscheck U, Pratsch L, Passow H, Peters R. Calcium pump kinetics determined in single erythrocyte ghosts by microphotolysis and confocal imaging. Biophys J 69: 30–41, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, 1986.Lim DJ. Functional structure of the organ of Corti: a review. Hear Res 22: 117–146, 1986 [DOI] [PubMed] [Google Scholar]
- Lioudyno et al., 2004.Lioudyno M, Hiel H, Kong JH, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E, Fuchs PA. A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci 24: 11160–11164, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin and Hudspeth, 1998.Lumpkin EA, Hudspeth AJ. Regulation of free Ca2+ concentration in hair-cell stereocilia. J Neurosci 18: 6300–6318, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammano et al., 1999.Mammano F, Frolenkov GI, Lagostena L, Belyantseva IA, Kurc M, Dodane V, Colavita A, Kachar B. ATP-induced Ca2+ release in cochlear outer hair cells: localization of an inositol triphosphate-gated Ca2+ store to the base of the sensory hair bundle. J Neurosci 19: 6918–6929, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser and Beutner, 2000.Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA 97: 883–888, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugasu and Russell, 1996.Murugasu E, Russell IJ. The role of calcium on the effects of intracochlear acetylcholine perfusion on basilar membrane displacement in the basal turn of the guinea pig cochlea. Aud Neurosci 2: 363–376, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, 1991.Müller M. Frequency representation in the rat cochlea. Hear Res 51: 247–254, 1991 [DOI] [PubMed] [Google Scholar]
- Nägerl et al., 2000.Nägerl UV, Novo D, Mody I, Vergara JL. Binding kinetics of calbindin-D(28k) determined by flash photolysis of caged Ca2+. Biophys J 79: 3009–3018, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi, 1997.Naraghi M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium 22: 255–268, 1997 [DOI] [PubMed] [Google Scholar]
- Nicholls, 2005.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium 38: 311–317, 2005 [DOI] [PubMed] [Google Scholar]
- Nicholls, 2008.Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann NY Acad Sci 1147: 53–60, 2008 [DOI] [PubMed] [Google Scholar]
- Ohmori, 1985.Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359: 189–217, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson et al., 2007.Patterson M, Sneyd J, Friel DD. Depolarization-induced calcium responses in sympathetic neurons: relative contributions from Ca2+ entry, extrusion, ER/mitochondrial Ca2+ uptake and release, and Ca2+ buffering.J Gen Physiol 129: 29–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci et al., 2003.Ricci AJ, Crawford AC, Fettiplace R. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40: 983–990, 2003 [DOI] [PubMed] [Google Scholar]
- Ricci and Fettiplace, 1998.Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol 506: 159–173, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci et al., 2005.Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R. The transduction channel filter in auditory hair cells. J Neurosci 25: 7831–7839, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci et al., 1998.Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18: 8261–8277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, 1994.Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci 14: 3246–3262, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth and Bruns, 1992.Roth B, Bruns V. Postnatal development of the rat organ of Corti. II. Hair cell receptors and their supporting elements. Anat Embryol (Berl) 185: 571–581, 1992 [DOI] [PubMed] [Google Scholar]
- Sakaguchi et al., 1998.Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA. Oncomodulin is expressed exclusively in outer hair cells in the organ of Corti. J Histochem Cytochem 46: 29–39, 1998 [DOI] [PubMed] [Google Scholar]
- Sala and Hernández-Cruz, 1990.Sala F, Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J 57: 313–324, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez et al., 2001.Sánchez JA, García MC, Sharma VK, Young KC, Matlib MA, Sheu SS. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. J Physiol 536: 387–396, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiden et al., 2008.Spiden SL, Bortolozzi M, Di Leva F, Hrabe de Angelis M, Fuchs H, Lim D, Ortolano S, Ingham NJ, Brini M, Carafoli E, Mammano F, Steel KP. The novel mouse mutation Oblivion inactivates PMCA2 pump and causes progressive hearing loss. PLoS Genet 4: 1–12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street et al., 1998.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in plasma membrane Ca2+-ATPase gene causes deafness in deafwaddler mice. Nat Genet 19: 390–394, 1998 [DOI] [PubMed] [Google Scholar]
- Waguespack et al., 2007.Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27: 13890–13902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver and Schweitzer, 1994.Weaver SP, Schweitzer L. Development of gerbil outer hair cells after the onset of cochlear function: an ultrastructural study. Hear Res 72: 44–52, 1994 [DOI] [PubMed] [Google Scholar]
- Wingrove et al., 1984.Wingrove DE, Amatruda JM, Gunter TE. Glucagon effects on the membrane potential and calcium uptake rate of rat liver mitochondria. J Biol Chem 259: 9390–9394, 1984 [PubMed] [Google Scholar]
- Wu et al., 1999.Wu YC, Ricci AJ, Fettiplace R. Two components of transducer adaptation in auditory hair cells. J Neurophysiol 82: 2171–2181, 1999 [DOI] [PubMed] [Google Scholar]
- Xu et al., 1997.Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytoplasm of adrenal chromaffin cells. Biophys J 73: 532–545, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al., 2000.Xu W, Wilson BJ, Huang L, Parkinson EL, Hill BJ, Milanick MA. Probing the extracellular release site of the plasma membrane calcium pump. Am J Physiol Cell Physiol 278: C965–C972, 2000 [DOI] [PubMed] [Google Scholar]
- Yamoah et al., 1998.Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci 18: 610–624, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]