Abstract
Recent research suggests that ultraviolet radiation exposure (UVRE), our major source of vitamin D, is associated with reduced lymphoma risk. Animal and human studies support an association between vitamin D (vitD) insufficiency and increased risk of some malignancies. We conducted a clinic-based case-control study (140 lymphoma cases, 139 controls; 2002–2005, Rochester, NY) to evaluate UVRE and vitD insufficiency in relation to lymphoma risk. Subjects completed a survey and provided a blood sample. We used multivariable logistic regression to estimate lymphoma risk in relation to past (5–10 years prior) UVRE and current vitD insufficiency (determined by serum 25(OH)D). Possible differences in effect by lymphoma subtype were explored, but statistical power was limited. We confirmed the previously reported decrease in lymphoma risk with past UVRE, specifically sunbathing (>once/week versus never); adjusted odds ratio (ORadj), = 0.28, 95% confidence interval (CI): 0.10–0.79. Current vitD insufficiency was not associated with lymphoma risk (ORadj=0.89, 95% CI: 0.47–1.72). However, current sunbathing frequency was correlated with measured serum 25(OH)D values. Therefore, while our data do not support an association with current vitD status, development of accurate methods for past vitD assessment to further investigate its role in the association between past UVRE and lymphoma risk is warranted.
Keywords: case-control studies, epidemiology, lymphoma, ultraviolet radiation, vitamin D
Introduction
Lymphoma, specifically the non-Hodgkin lymphoma (NHL) subgroup, is the 5th most common cancer overall in the United States, among both men and women, with an estimated incidence rate of 19.3 per 100,000 [1]. The etiology of most lymphoma subtypes remains largely unknown [2]. There has been marked increase in lymphoma incidence over the past 30 years (estimated 82% increase overall) affecting almost all histologic categories [3].
Several recent studies have found an inverse relationship between individual ultraviolet radiation (UVR) exposure and lymphoma, suggesting that increased UVR exposure is protective against lymphoma [4–9]. While the majority of the studies to date have investigated the association between lymphoma and UVR exposure, two studies did demonstrate similar inverse associations between sun exposure and HL risk specifically [6, 8]. Additionally, a pooled analysis recently conducted by the InterLymph consortium reported that increased recreational sun exposure was reported to be significantly associated with a decreased risk of NHL [10]. This observation is particularly intriguing in light of evidence from earlier ecological studies indicating, if anything, a possibly detrimental impact of UVR exposure on lymphoma risk [9]. UVR from the sun provides nearly 90% of the needed vitamin D for most people [11], and evidence of a decrease in serum 25(OH)D levels in the United States population have been recently published [12]. Ongoing animal and human research in many cancers provides support for a protective effect of vitamin D status related to malignancy [13]. As such, one proposed explanation for these unexpected reports of reduced risk of lymphoma with increased UVR exposure in the literature is that UVR exposure measures are actually proxy measurements of vitamin D status, and that vitamin D sufficiency is protective against lymphoma [14].
Vitamin D, obtained through UVR exposure, diet, and/or diet supplement intake, is metabolized in the liver to 25-hydroxyvitamin D (25(OH)D), the major circulating form of vitamin D [15]. Upon stimulation by parathyroid hormone, 25(OH)D is further metabolized in the kidney to its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D), which plays a major role in calcium homeostasis [16]. However, it is the extra-renal 1-α-hydroxylation of 25(OH)D to 1,25(OH)2D that appears to be central to chronic disease prevention, including cancer [15]. The autocrine and paracrine effects of extra-renal 25(OH)D metabolism include regulation of cell proliferation, apoptosis induction, and increased cell differentiation [17–19]. Evidence supporting an effect of 1,25(OH)D on lymphoma cells in particular has been demonstrated both in vitro, with evidence of vitamin D promotion of differentiation and antiproliferative effects on a variety of lymphoma cell lines [20], and in vivo with a early study demonstrating tumor response to alfacalcidol, a synthetic vitamin D analog, in 24% of 36 low grade follicular, small-cleaved cell type, lymphoma [21]. Recent literature supports ≥30ng/ml circulating 25(OH)D as the threshold for vitamin D sufficiency in order to maximize the health benefit given the many conditions for which vitamin D is currently hypothesized to be protective [15].
Nine published studies have evaluated the association between vitamin D and lymphoma risk [4, 5, 22–28]. Overall, the published estimates of association between dietary vitamin D intake or serum 25(OH)D and lymphoma risk are largely weak or null. However, it is critical to note that vitamin D status in the majority of these studies was determined by self-report dietary consumption on food frequency questionnaires, a method vulnerable to potential dietary recall inaccuracy, and more importantly, limited by the variability of vitamin D content in both the few naturally occurring and fortified sources [29, 30]. Potential inaccuracy of vitamin D insufficiency exposure assessment may be obscuring the true influence of vitamin D on lymphoma risk. These studies have all been limited to NHL subtypes to date.
We conducted a case-control study to evaluate both UVR exposure and vitamin D insufficiency in relation to lymphoma risk.
Materials and Methods
Cases and controls were enrolled from the James P. Wilmot Cancer Center (JPWCC) Lymphoma Clinic and General Neurology Clinic, respectively, both outpatient clinics at the University of Rochester Medical Center, Rochester, New York. This study was approved by the University of Rochester Institutional Review Board, and all patients provided written informed consent. Study participation consisted of one study visit during which a serum sample and self-administered survey were collected.
Subject Recruitment
Eligible cases were adult (age ≥21) patients with a pathologically confirmed diagnosis of malignant lymphoma (inclusive of all WHO classification of malignant lymphoma [31] subtypes) who were newly diagnosed (within 6 months), previously untreated, and without evident CNS involvement. As two studies have demonstrated an association between UVR and HL risk specifically [6, 8], we did include the patients with HL in our study, but have provided estimates with all lymphomas included, and for NHL alone. Controls were adult patients, free of a known lymphoma diagnosis, recruited from the General Neurology outpatient clinic. The General Neurology outpatient clinic has a similar referral pattern to the James P. Wilmot Cancer Center, from which the cases were recruited, and thus was thought to be a valid representation of the base population from which the cases came with respect to exposure distribution. Cases or controls with a history of significant immunosuppressive condition, clinically evident hypercalcemia, corticosteroid or immunosuppressive drug use within 1 month of study visit (≥10 mg/d prednisone or equivalent), or unable to speak and read English were excluded. Additionally, those patients with any history of anticonvulsant medication use were excluded, given the impact of these medications on 25(OH)D [32].
Between October 31, 2005 and September 26, 2007, 157 eligible cases and 190 eligible controls were invited to participate. All consecutive new patients seen in the JPWCC Lymphoma Clinic were screened for potential eligibility. Likewise, consecutive patients seen in the General Neurology Clinic were concurrently screened for potential eligibility. Seven cases and 41 controls declined participation during the consent process (96 and 78% response rate for cases and controls, respectively). Twenty subjects (10 of the 150 consented cases and 10 of the 149 consented controls were excluded from further analysis due to discovered ineligibility (14 patients; 9 cases, 5 controls) or failure to return the study survey (6 patients; 1 case, 5 controls). Among the 279 consented and evaluable subjects, 8 of 140 cases and 7 of 139 controls did not have a blood sample drawn for 25(OH)D measurement.
Largely, the distribution of lymphoma cases by histology was as expected; 92% NHL, 85% B cell lymphomas; 23% (n=32) diffuse large B cell lymphoma, 32% (n=45) follicular lymphoma, 17% (n=24) marginal zone lymphoma, 8% (n=11) Hodgkin lymphoma, 7% (n=10) T cell lymphoma, 5% (n=7) CLL/SLL, 5% (n=7) mantle cell lymphoma, and 3% (n=4) other histologic subtypes. A majority of cases (64%) presented with advanced stage disease (stage III/IV), though 79% were asymptomatic (no documented B symptoms at time of study visit). Median time between diagnosis and study consent was 21 days; maximum 4.9 months.
The enrolled control patients represent a heterogeneous group of neurological diagnoses and symptoms; the most frequent visit indications were stroke (31%), headache (24%), and numbness/pain (17%). Other control diagnoses included: mild cognitive impairment (1), suspected or newly diagnosed MS (2), transient global amnesia (1), weakness (1), pernicious anemia (1), right 6th cranial nerve palsy (1), aphasia (1), cervical dystonia (1), concentration disturbances (2), and hereditary spastic paraparesis (1).
UVR and diet survey
Participants were asked to complete a self-administered survey during their scheduled clinic visit, and study staff was available to answer any questions. Usual UVR exposure habits during the time periods 5–10 years (past exposure) and 4 weeks prior to study participation were assessed using questions and categorical response options similar to those used by Smedby et al. [8], including sunbathing with the intention to tan (never, once a week or less, more than once a week), occurrence of sore and/or blistering sunburn (yes/no), tanning bed use (yes/no), sunscreen use (most of the time, about half of the time, once in a while, never), and holding a job that involved being outdoors at least 2 days a week (yes/no). One additional sun exposure question, assessing average hours outdoor per week (0–2, 3–5, 6–8, >8), was adapted from a case-control study of sun exposure and lymphoma risk [4]. Subjects were specifically asked to report their usual UVR exposure habits during summer months for the set of questions regarding the study period 5–10 years (past exposure) prior to study participation.
Questions assessing dietary vitamin D intake (weekly servings of fish and milk) and nutrient intake (multivitamins, vitamin D supplements and cod liver oil supplements) were similar to those published by Holick et al. in a study to assess prevalence and predictors of vitamin D inadequacy [33]. Questions on smoking and alcohol have been modified from surveys used in studies published by Brown et al. investigating the role of alcohol and tobacco in squamous-cell esophageal cancer and multiple myeloma [34, 35]. The survey also collected data on other variables thought to influence the circulating pool of 25(OH)D, including BMI (at time of study participation), age, liver disease, and other co-morbidities.
Serum 25(OH)D measurement
Serum 25(OH)D was quantified by radioimmunoassay (RIA; Heartland Assays Inc., Ames, IA) [36]. Blood was collected (prior to initiation of therapy) and available for analysis from 132 cases and 132 controls. Assays were run in two batches, with approximately equal numbers of cases and controls per batch. Median storage time, from collection date to shipment for analysis, was 11.7 months (range 2.5–18.0 months). Heartland Assays Inc. was blinded to case-control status. A recently published estimate of overall (intrabatch andinterbatch) coefficient of variation for the 25(OH)Dassay as performed by the Heartland Assay, Inc. laboratory was 4.7% [37].
Of the 264 patients from whom both a completed survey and blood sample were received, the majority of patients (84%) were able to complete and return the survey on the same day as the blood draw. Of those who returned their survey on a different day from the date blood was drawn, the median gap between survey collection and blood collection was 5 days, and there were only 4 subjects with a gap between survey collection and blood collection >14 days.
Data analysis and statistical considerations
Cases and controls were compared with regard to distribution of the following potentially confounding variables: gender, race, age, family history of lymphoma or other cancer, medical history (including, previous skin cancer diagnosis, diabetes, rheumatoid arthritis, lupus, and eczema; yes/no to each condition), smoking (ever/never, 5–10 years ago), alcohol (ever/never, 5–10 years ago), BMI, highest level of education, and season of blood draw (Winter: December–February; Spring//Fall: March–May, September–November; Summer: June–August). Factors differentially distributed by case status, as determined by a conservative p< 0.20 by chi-square test (categorical measures) or t-test (continuous measures), were included in each multivariable logistic regression [38]. Due to the well-documented association of age, race, and gender with lymphoma risk, these variables were included in the final multivariable logistic models regardless of distribution by case status.
After descriptive analysis, we first estimated the association between past UVR exposure (5–10 years prior to study participation) and lymphoma risk using unconditional multivariable logistic regression analysis. In subsequent multivariable logistic regression analyses we evaluated the association between current measured vitamin D and lymphoma risk. Odds Ratios (OR) were estimated using vitamin D as a dichotomous variable (vitamin D insufficient: 25(OH)D < 30 ng/mL), by tertiles defined according to 25(OH)D distribution among the controls (tertile 1: 25(OH)D < 20.9 ng/ml; tertile 2: 25(OH)D 20.9–28.5 ng/ml; tertile 3, reference: 25(OH)D >28.6 ng/ml), and using serum 25(OH)D as a continuous variable. The above multivariable models were run both with all of the lymphoma cases and with the subset of only NHL cases. Stratified analyses by gender, dichotomous age (divided at the median, age 57), and BMI category were performed to evaluate whether the observed associations between UVR or vitamin D insufficiency and lymphoma risk differed within any of these subgroups. In secondary analyses, the association between vitamin D insufficiency and lymphoma risk was further assessed in 2 of the major lymphoma subtypes: diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL). Polytomous logistic regression was used to formally test the null hypothesis that there is no heterogeneity of the estimated association between serum 25(OH)D and lymphoma between these two lymphoma subtypes [38]. These secondary analyses are purely exploratory due to limited power.
Finally, in order to explore whether or not vitamin D production could function as an intermediate variable in the association between past UVR exposure and reduced lymphoma risk, we assessed whether the UVR exposure variables associated with lymphoma were in fact predictive of serum 25(OH)D values. The half-life of 25(OH)D is approximately 2–4 weeks [15]. Therefore, we used multivariate linear regression to determine whether levels of UVR exposure variables during the 4 weeks prior to study participation, which were associated with lymphoma risk at levels reported for the time period 5–10 years prior, were predictive of current measured 25(OH)D levels after adjustment for the other UVR exposure variables as well as other potential vitamin D predictor variables. This analysis was conducted first with all cases and controls with available serum samples for 25(OH)D analysis (n=264), and subsequently with just the controls.
SAS statistical software was used for all statistical analysis (SAS Institute, Cary, N.C.), and all p-values are two-sided.
Results
Patient characteristics
Cases and controls were both recruited throughout four seasons, and there was no statistically significant differential distribution of cases and controls by season of recruitment or blood draw (Table 1). The racial distribution was balanced between cases and controls with approximately 89, 5, and 6% in the white, black, and other racial categories, respectively. However, the cases were older, included more males, more skin cancer diagnoses, a lower proportion of obesity, and had increased proportion of family history of lymphoma and other cancers when compared to the controls. The effects of differential distribution between cases and controls with regard to gender, age, skin cancer diagnosis, family history of lymphoma, family history of other cancer, and BMI were controlled for in the multivariable analyses.
Table 1.
Demographic and clinical characteristics of cases and controls, Rochester, New York, 2005–2007. Demographic characteristics for the entire study population, n=279.
| Cases |
Controls |
p valuea | |||
|---|---|---|---|---|---|
| n=140 | % | n=139 | % | ||
| Gender | |||||
| Male | 89 | 63.6 | 61 | 43.9 | 0.001 |
| Race | |||||
| White | 124 | 88.6 | 123 | 88.5 | 0.78 |
| Black | 7 | 5.0 | 9 | 6.5 | |
| Other | 9 | 6.4 | 7 | 5.0 | |
| Age | |||||
| 21–40 | 20 | 14.3 | 43 | 30.9 | 0.0004 |
| 41–60 | 51 | 36.4 | 59 | 42.5 | |
| 61–80 | 61 | 43.6 | 28 | 20.1 | |
| 80+ | 8 | 5.7 | 9 | 6.5 | |
| Medical history | |||||
| Skin cancer | 27 | 19.3 | 8 | 5.8 | 0.0006 |
| Diabetes | 16 | 11.4 | 20 | 14.4 | 0.46 |
| Rheumatoid arthritis | 6 | 4.3 | 11 | 7.9 | 0.21 |
| Lupus | 0 | 0.0 | 2 | 1.4 | 0.25 |
| Eczema | 10 | 7.1 | 10 | 7.2 | 0.99 |
| Family history | |||||
| Lymphomab | 20 | 14.3 | 2 | 1.4 | <0.0001 |
| Skin Cancerb | 20 | 14.3 | 18 | 13.0 | 0.74 |
| Other Cancerb | 76 | 54.3 | 59 | 42.5 | 0.05 |
| Smoking history | |||||
| Ever | 80 | 57.1 | 76 | 54.7 | 0.68 |
| Alcohol use | |||||
| Ever | 105 | 75.0 | 96 | 69.1 | 0.27 |
| BMIc | |||||
| Normal weight (<25.0) | 50 | 35.7 | 42 | 30.4 | 0.03 |
| Overweight (25.0–29.9) | 57 | 40.7 | 47 | 34.1 | |
| Obese (≥30) | 33 | 23.6 | 49 | 35.5 | |
| Educationd | |||||
| <High school | 11 | 7.9 | 14 | 10.5 | 0.93 |
| Completed high school | 42 | 30.0 | 41 | 30.6 | |
| Some college | 36 | 25.7 | 35 | 26.1 | |
| Completed 4 years of college | 24 | 17.1 | 22 | 16.4 | |
| Graduate school | 27 | 19.3 | 22 | 16.4 | |
| Season of Blood Drawe | |||||
| Winter (Dec–Feb) | 40 | 30.3 | 28 | 21.2 | 0.17 |
| Spring/fall (Mar–May; Sep–Nov) | 60 | 45.5 | 62 | 47.0 | |
| Summer (Jun–Aug) | 32 | 24.2 | 42 | 31.8 | |
Two-sided p-values determined by χ2 (categorical variables) and t-test (continuous variables, BMI and age)
Known family history of lymphoma, skin cancer, or other cancer, self-reported on study survey. “Don’t know” and missing responses were considered “no known history” for each variable;
Height and weight data missing for 0 (0%) cases, 1 (0.7%) of controls;
Education data missing for 0 (0%) cases, 5 (3.6%) of controls;
n=262 patients with blood for serum 25(OH)D assessment.
Serum 25(OH)D Assessment
Among the cases, serum 25(OH)D values ranged from 2.5 ng/ml to 45.6 ng/ml (Shapiro-Wilk test for normality p = 0.31), with a mean value of 23.8 ng/ml. Among the controls, serum 25(OH)D values ranged from 5.4 ng/ml to 45.5 ng/ml (Shapiro-Wilk test for normality p= 0.48), with a mean value of 24.5 ng/ml. Notably, nearly 74% of the study population was vitamin D insufficient, (25(OH)D <30 ng/ml), with 74 and 73% insufficiency among the cases and controls, respectively.
Past (5–10 years ago) UVR exposure and lymphoma risk
Table 2 presents the results from a multivariable analysis of the 7 self-reported measures of UVR exposure 5–10 years ago, and their estimated association with lymphoma risk. Covariates included in this model were all variables unbalanced between cases and controls (p<0.20), as previously described. Sunbathing with the intention to tan was significantly associated with lymphoma risk. Increased sunbathing frequency 5–10 years ago was associated with decreased lymphoma risk (ptrend=0.02), with a 72% reduction in lymphoma risk among those who sunbathed with the intention to tan >1/week versus never (OR=0.28, 95% CI: 0.10–0.79). After adjustment for sunbathing with the intention to tan, none of the other measured UVR exposure variables were significantly associated with lymphoma risk. These results were unchanged when this analysis was limited to just the NHL subtypes. Likewise, though education level was not differentially distributed between the cases and the controls, additional adjustment for this factor as an attempt to control for potential confounding by socioeconomic status did not affect these estimates. We found no evidence of interaction or differential association between sunbathing frequency 5–10 years ago and lymphoma risk by gender, BMI category, or dichotomous age.
Table 2.
Odds ratio for lymphoma according to measures of sun exposure 5–10 years ago, Rochester, New York, 2005–2007.
| Sun variable measure |
Controls n=139 | All lymphoma cases |
Only NHL cases |
|||
|---|---|---|---|---|---|---|
| Variable | Level | Cases n=140 | Adjusted odds ratio (95% confidence interval)a,b | Cases n=129 | Adjusted odds ratio (95% confidence interval)b,c | |
| Sun exposure hours | 0–2 hrs/week | 6 | 7 | 1.0 (reference) | 7 | 1.0 (reference) |
| 3–5 hrs/week | 20 | 20 | 0.48 (0.11–2.14) | 19 | 0.44 (0.10–2.01) | |
| 6–8 hrs/week | 29 | 33 | 0.75 (0.18–3.24) | 31 | 0.76 (0.17–3.35) | |
| >8 hrs/week | 84 | 80 | 0.47 (0.12–1.90) | 72 | 0.44 (0.11–1.84) | |
| ptrend | 0.38 | 0.36 | ||||
| Sunbathing with the intention to tan | Never | 85 | 104 | 1.0 (reference) | 98 | 1.0 (reference) |
| Once/wk or less | 28 | 25 | 0.64 (0.30–1.39) | 21 | 0.52 (0.23–1.19) | |
| >1/week | 26 | 11 | 0.28 (0.10–0.79) | 10 | 0.27 (0.09–0.78) | |
| ptrend | 0.02 | 0.01 | ||||
| Sore sunburnd | No | 73 | 89 | 1.0 (reference) | 85 | 1.0 (reference) |
| Yes | 66 | 50 | 0.88 (0.42–1.84) | 43 | 0.85 (0.39–1.82) | |
| Blistering sunburnd | No | 106 | 121 | 1.0 (reference) | 113 | 1.0 (reference) |
| Yes | 33 | 18 | 0.68 (0.28–1.65) | 15 | 0.71 (0.28–1.81) | |
| Tanning bed use | No | 115 | 124 | 1.0 (reference) | 115 | 1.0 (reference) |
| Yes | 24 | 16 | 1.85 (0.67–5.10) | 14 | 1.81 (0.63–5.21) | |
| Outdoor occupatione | 98 | |||||
| No | 92 | 108 | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 42 | 32 | 0.52 (0.27–1.01) | 31 | 0.62 (0.31, 1.23) | |
| Sunscreen use | Most of the time | 19 | 17 | 1.0 (reference) | 15 | 1.0 (reference) |
| About 1/2 the time | 20 | 23 | 1.25 (0.44–3.56) | 21 | 1.22 (0.41–3.62) | |
| Once in a while | 44 | 56 | 0.93 (0.36–2.37) | 53 | 0.98 (0.37–2.60) | |
| Never | 56 | 44 | 0.66 (0.26–1.69) | 40 | 0.65 (0.24–1.74) | |
| ptrend | 0.22 | 0.25 | ||||
n=273 for the multivariable analysis (all lymphoma cases);
Adjusted for gender, age 5–10 yrs ago (continuous), race (black, white, other), skin cancer diagnosis, known family history of lymphoma, known family history of other cancer, BMI (normal weight, overweight, obese), alcohol use as of 5–10 yrs ago (ever/never) and all of the other sun exposure variables in the table;
n=262 for the multivariate analysis (NHL cases only);
Numbers do not sum to 140 cases due to missing data for 1 (0.3%) case in each of these variables;
Numbers do not sum to 139 controls due to missing data for 5 (1.8%) controls in this variable
Vitamin D insufficiency and lymphoma risk
Figure 1 presents the distribution of serum 25(OH)D for cases and controls, according to season of blood draw. As expected, measured serum 25(OH)D did increase as the amount of seasonal sun exposure increased, though visual inspection does not reveal a differential pattern of 25(OH)D between the cases and controls by season. The multivariable adjusted odds ratio (OR) and 95% confidence interval (CI) estimate of the association between vitamin D insufficiency and lymphoma risk was 0.89 (95% CI: 0.47–1.72) (Table 3). Similar results were found with vitamin D defined as both a three level ordinal variable (tertiles defined by 25(OH)D distribution among the controls) and as a continuous variable. Furthermore, no significant evidence of an association between vitamin D insufficiency and lymphoma risk was found when the analysis was limited to the NHL cases or when the analysis was repeated with cases presenting with B symptoms excluded. Stratified analyses by gender, dichotomous age (divided at the median, age 57), and BMI category were performed and no evidence of interaction or differential association between vitamin D insufficiency and lymphoma risk was found within any of these subgroups. Exploratory analyses within 2 of the major lymphoma subtypes did not reveal an association with DLBCL (n=30) or FL (n=44).
Figure 1.
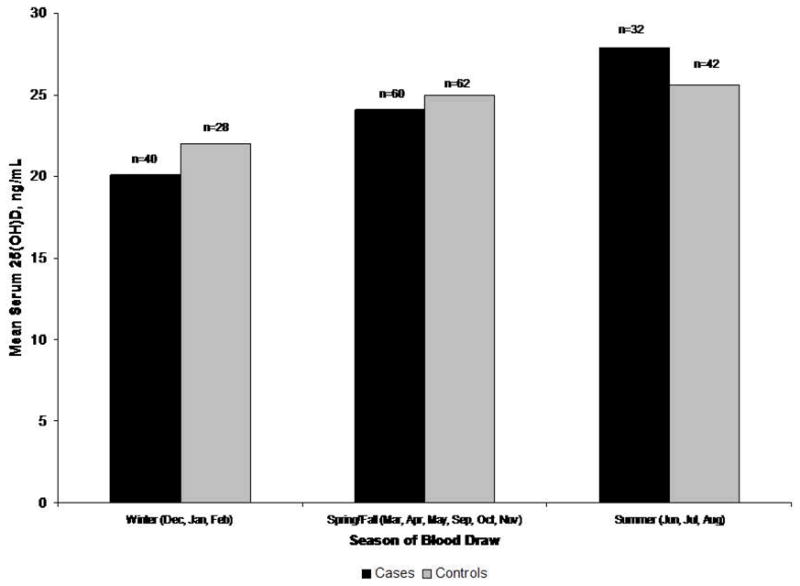
Mean serum 25(OH)D (ng/ml) for cases and controls by season of blood draw, Rochester, New York, 2005–2007.
Table 3.
Odds ratio for lymphoma according to current measured 25(OH)D, Rochester, New York, 2005–2007.
| Vitamin D |
Controls n=132 | All Lymphoma Cases |
Only NHL Cases |
||
|---|---|---|---|---|---|
| 25(OH)D range (ng/ml) | Cases n=132 | Adjusteda odds ratio (95% confidence interval) | Cases n=121 | Adjusteda odds ratio (95% confidence interval) | |
| Insufficient (<30) | 97 | 98 | 0.89 (0.47–1.72) | 88 | 0.86 (0.44–1.68) |
| Sufficient (≥30) | 35 | 34 | 1.0 (reference) | 34 | 1.0 (reference) |
| Tertileb 1 (<20.9) | 42 | 55 | 1.32 (0.66–2.66) | 50 | 1.38 (0.67–2.85) |
| Tertile 2 (20.9–28.5) | 45 | 37 | 0.79 (0.39–1.63) | 33 | 0.79 (0.38–1.68) |
| Tertile 3 (≥28.6) | 45 | 40 | 1.0 (reference) | 38 | 1.0 (reference) |
| ptrendc | 0.39 | 0.35 | |||
| Continuous | 132 | 132 | 0.99 (0.96–1.03) | 121 | 0.99 (0.96–1.03) |
NHL, non-Hodgkin lymphoma;
ORs adjusted for age (continuous), gender, race (white, black, other), prior skin cancer diagnosis (BCC, SCC, melanoma), known family history of lymphoma, known family history of other cancer, BMI (continuous), and season (Winter: December–February; Spring/Fall: March–May, September–November; Summer: June–August);
Tertiles set according to distribution of serum 25(OH)D levels among the controls;
ptrend is based on ordinal score.
Association between UVR exposure variables and serum 25(OH)D
Sunbathing with the intention to tan in the 4 weeks immediately prior to study participation was significantly associated with measured serum 25(OH)D, after adjustment for the other measured sun exposure variables as well as season of blood draw, dietary intake, and demographic factors associated with serum 25(OH)D levels (Table 4). Repeat analyses limited to the controls and limited to those who completed and returned their survey on the same day as their blood draw (data not shown) did not materially alter these results. Furthermore, we observed that tanning bed use, daily vitamin D intake, and seasons with greater sun, and white race were all associated with significantly increased serum 25(OH)D levels, as was expected.
Table 4.
Association between current levels of sun exposure variables and measured serum 25(OH)D, controlling for the contribution of other dietary and demographic predictors of serum 25(OH)D, Rochester, New York, 2005–2007.
| Variable | Variable type | Level | All subjects |
Controls only |
||
|---|---|---|---|---|---|---|
| β | p valuea | β | p valuea | |||
| Intercept | -- | -- | 15.4 | 15.5 | ||
| Sunbathing, past 4 wks | ordinal | -- | 2.8 | 0.01 | 2.7 | 0.04 |
| Sun exposure, past 4 wks | categorical | 0–2 hrs/wk | ref | 0.01 | ref | 0.02 |
| 3–5 hrs/wk | −2.8 | −4.3 | ||||
| 6–8 hrs/wk | −1.4 | −3.7 | ||||
| >8 hrs/wk | 1.2 | −0.02 | ||||
| Tanning bed use, past 4 wks | yes/no | Yes | 13.9 | 0.0004 | 11.7 | 0.006 |
| Current daily multivitamin | yes/no | Yes | 5.9 | <0.0001 | 5.3 | 0.0002 |
| Current milk servings per wk | ordinal | -- | 1.31 | 0.02 | 1.7 | 0.02 |
| Current fatty fish servings per wk | categorical | 0/wk | ref | 0.02 | ref | 0.07 |
| <1/wk | 3.8 | 3.5 | ||||
| 1–2/wk | −1.3 | −2.6 | ||||
| ≥3/wk | 1.9 | −0.9 | ||||
| Current BMI | continuous | -- | −0.2 | 0.005 | −0.2 | 0.02 |
| Race | categorical | White | ref | <0.0001 | ref | 0.03 |
| Black | −9.5 | −7.5 | ||||
| Other | −2.5 | −1.3 | ||||
| Age | continuous | -- | 0.06 | 0.07 | 0.1 | 0.02 |
| Season of blood draw | Ordinal (winter, ref; spring/fall, summer) | -- | 2.8 | <0.0001 | 2.7 | 0.002 |
The p values in the table above are the overall global p-value for each variable from multivariable linear regression models including all variables shown.
Discussion
A growing number of epidemiologic studies report a reduced lymphoma risk in relation to increased levels of a variety of personal UVR exposure indicators [4–10]. Similarly, we found that people who reported a history (i.e., 5–10 years ago) of sunbathing with the intention to tan (>1 time per week compared to never) had a 72% lower risk of lymphoma. These findings are consistent with the overall findings reported from the InterLymph pooled analysis of sun exposure and NHL risk, the most comprehensive analysis of this association to date, which observed that increased recreational sun exposure was significantly associated with a decreased risk of NHL, yet the observed decreased risk with increasing overall sun exposure failed to reach statistical significance [10]. In light of both the role of UVR in vitamin D production [11] and published research to suggest a protective effect of vitamin D with regard to malignancy [13], we subsequently evaluated the association between vitamin D levels and lymphoma risk.
Of the 9 previously published studies investigating the association between vitamin D and NHL, 6 have relied exclusively on recall of dietary intake on a food frequency questionnaire for exposure assessment [4, 5, 22–24, 28]. However, there are very limited dietary sources of vitamin D, and significant variability of vitamin D content in both the naturally occurring and fortified sources has been well established [29, 30]. Circulating 25(OH)D concentrations represent the combined contributions of both UVR and dietary (D2 and D3) sources of vitamin D [16], and the long half-life of this metabolite makes 25(OH)D the major circulating form of vitamin D [15], and the preferred biomarker for determining vitamin D sufficiency. As such, we measured current serum 25(OH)D to evaluate the association between vitamin D and lymphoma risk. The OR estimate of the association between vitamin D insufficiency (serum 25(OH)D <30 ng/ml) and lymphoma risk indicates that vitamin D insufficiency was not an independent marker of lymphoma risk. Furthermore, secondary evaluation of the association using a three level ordinal vitamin D variable (tertiles defined by 25(OH)D distribution among the controls) did not demonstrate a dose-response, and the odds ratio for 25(OH)D as a continuous variable was near unity.
Our findings are consistent with the literature to date, which, overall, provides limited support for an association between vitamin D status and lymphoma [4, 5, 22–27]. With the exception of the findings by Polesel et al. [23], and Lim et al. [27], the published estimates of association with dietary vitamin D intake or serum 25(OH)D and lymphoma risk are largely weak or null. Limitations in retrospective vitamin D insufficiency exposure assessment in epidemiologic research have been discussed in the literature [39], and may be obscuring a true influence of vitamin D status on lymphoma risk in the current study and the previously published literature. In this study, we were limited to assessing current 25(OH)D with the assumption that this level was consistent with average adult vitamin D status. However, we were able to demonstrate an association between past (5–10 years prior) sunbathing frequency and lymphoma risk. Additionally, we confirmed that current sunbathing frequency (within the 4 weeks prior to study visit) was in fact independently predictive of serum 25(OH)D after adjustment for other dietary and demographic factors as well as season of blood draw, an observation consistent with published data indicating that active sunbathing was the strongest determinant of serum 25(OH)D in healthy Danish women [40]. Together, these observations raise the possibility that vitamin D could have had a role in the association between past UVR exposure and lymphoma risk if there had been a change in sun exposure behaviors over the 5–10 prior to study participation. As current sun exposure variables were reported for only the 4 weeks prior to study participation, and are thus limited to a particular season, we are not able to assess this directly with our data. Although there is evidence of stability of serum 25(OH)D levels within individuals over several years [41], our findings suggest the importance of more direct vitamin D status assessment at an etiologically relevant time period prior to diagnosis in order to determine the role of vitamin D in the reported relationship between UVR exposure and lymphoma risk.
A limitation of the case-control design is that we must evaluate whether the malignant disease process among the lymphoma cases may be affecting vitamin D status as opposed to vitamin D being related to lymphoma etiology. Lymphoma symptoms could possibly impact vitamin D levels by changing UVR exposure behaviors. However, strengths in our case selection and recruitment methods minimize this concern. Our study includes case patients who were recruited very soon after diagnosis (median time between diagnosis and study consent was 21 days), minimizing the likelihood that their diagnoses changed behaviors that would impact serum 25(OH)D. In addition, the case population was largely asymptomatic (79%), and limiting the analysis of the association between current measured 25(OH)D and lymphoma risk to patients without documented B symptoms at study participation did not change the results. Furthermore, patients with hypercalcemia, likely related to their hematologic condition if present [42], were ineligible for study participation.
Control selection is a critical factor in case-control study design. In clinic-based studies such as this, defining the base population from which the cases originated becomes difficult. The vast majority of the previously untreated lymphoma cases seen in the James P. Wilmot Cancer Center Lymphoma Clinic come from 17 Western New York counties. However, due to referral patterns to this academic clinic, not all cases from this geographic area had a chance of inclusion in the case set. As such, clinic-based controls are more appropriate, even over randomly selected healthy controls from the base population. The General Neurology Clinic has available an adequate patient base, and a similar referral pattern due to academic medical center affiliation, such that a sufficient number of controls with diseases unrelated to the exposure of interest could be identified. With the rapidly growing list of chronic diseases for which vitamin D is reported to possibly play an etiologic role, it is difficult to choose a clinic with a patient base having diagnoses unrelated to vitamin D. However, the enrolled control group was recruited in the outpatient setting, represents a heterogeneous group of neurological diagnoses and symptoms, and does not include patients with diseases (or on medications; e.g., seizure disorders and medications) known to impact serum 25(OH)D. Moreover, the prevalence of vitamin D insufficiency among the controls (73%) is consistent with the recently reported prevalence of vitamin D insufficiency (77%) among the healthy general adult population in the United States [12], providing some confidence that the conditions among the controls are not associated with differential UVR or vitamin D exposure as compared to the general population. However, we do acknowledge that this comparison may be confounded by a wide variety of covariates. As such, we cannot fully rule out a decrease in 25(OH)D as a result of diagnoses, either directly or as a result of a change in behavior, among the controls.
Despite the considerable clinical heterogeneity of the lymphoma subtypes [3, 43], the studies to date that have evaluated the association between vitamin D status and lymphoma risk, current study included, have been designed to evaluate this association with all lymphoma subtypes combined as the primary hypothesis. As discussed recently by Evens and Chiu, evaluation of distinct etiologic processes within the NHL subtypes is one of the major and ongoing challenges in epidemiologic research [44]. In exploratory analyses, we found no evidence of heterogeneity in effects by subtype, although we evaluated only the two most common lymphoma subtypes and statistical power was limited. It is possible that any potential association between vitamin D status and individual lymphoma subtypes could be masked when the subtypes are combined.
Furthermore, the true dose-response relationships between vitamin D (dietary or serum 25(OH)D) and cancer is unknown [45]. We defined vitamin D insufficiency using a clinically relevant 25(OH)D threshold (30ng/ml), currently thought to be minimum level required for maximizing the vitamin D health benefit [15]. However, there is evidence in the literature to suggest that the threshold levels for an effect of 25(OH)D may vary by cancer type, and preventive effects may be limited to higher levels of 25(OH)D than anticipated [45, 46]. As such, the range of serum 25(OH)D measurements in our study may be too low to observe a protective effect with regard to lymphoma risk.
Finally, the relevant etiologic period of exposure for lymphoma is not clear, as the complete natural history of lymphoma prior to onset of symptoms is still undefined. With particular regard to cancer, many steps are necessary for malignant transformation [47]. Most recently, Lim et al. demonstrated a differential association between 25(OH)D and lymphoma risk by length of follow-up, with a statistically significant protective effective of higher serum 25(OH)D on lymphoma risk observed only in the subgroup of subjects with less than 7 years of follow-up [27], a finding consistent with the evidence presented by Smedby [8] and Hartge [4], emphasizing the importance of exposure assessment timing in investigations of lymphoma etiology.
In conclusion, we observed a decrease in lymphoma risk with increased UVR exposure consistent with previous reports, thereby providing further evidence of a true association. With growing consistency in the literature to suggest a protective role for UVR exposure in lymphoma, the null association between measured serum vitamin D and lymphoma risk demonstrated in our study may suggest the need to explore possible alternative explanations of association between UVR and lymphoma that are independent of the role of vitamin D. For example, there is evidence from experimental studies of immune system modulation by UVR, resulting in decreased immune challenge responses to antigens applied to even non-UVR exposed areas [48–50]. In light of ongoing investigation into antigenic stimulation with regard to lymphoma risk [2, 51, 52], one could hypothesize that this is suggestive of a role for direct influence of UVR, through immune modulation, without the impact of an intermediary such as vitamin D. Additionally, another hypothesis is that folate and folate derivatives, on which cell replication is dependent, are degraded by UVR [53]. Decreased folate availability via UVR degradation could limit DNA replication, and thus slow the cell cycle, particularly among cells that are rapidly dividing (such as pre-malignant and malignant cells) [53]. These two hypotheses, and certainly countless others, indicate the need for further critical evaluation of the association, either direct or indirect, between UVR and lymphoma risk and potential mechanisms for pathogenesis.
Alternatively, the limited evidence of an association between vitamin D status and lymphoma risk to date may very well be due to methodological limitations, particularly in accurate assessment of serum 25(OH)D levels during etiologically relevant periods, and further investigation of this potential association while addressing these methodological difficulties is warranted.
Acknowledgments
This work was supported by a hematology training grant award from the National Institutes of Health, National Heart Lung and Blood Institute [T32 HL007152]; the Leukemia and Lymphoma Society Clinical Scholar Program; the National Institutes of Health, National Center for Research Resources [UL 1 RR024160]; and the National Institutes of Health, National Cancer Institute [K23 CA102216 and P50 CA130805]. The authors declare no competing financial interests, corporate involvement or patent holdings. The authors alone are responsible for the content and writing of the paper.
Abbreviations
- 25(OH)D
25-Hydroxyvitamin D
- CI
Confidence interval
- DLBCL
Diffuse large B cell lymphoma
- FL
Follicular lymphoma
- NHL
Non-Hodgkin lymphoma
- OR
Odds ratio
- UVR
Ultraviolet radiation
References
- 1.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2005. http://seer.cancer.gov/csr/1973_2004/. Vol. based on November 2006 SEER data submission, posted to the SEER website, 2007. [Google Scholar]
- 2.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin’s lymphoma. Oncogene. 2004;23(38):6524–34. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 3.Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84(1):1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 4.Hartge P, Lim U, Freedman DM, et al. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2006;17(8):1045–52. doi: 10.1007/s10552-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 5.Soni LK, Hou L, Gapstur SM, et al. Sun exposure and non-Hodgkin lymphoma: A population-based, case-control study. Eur J Cancer. 2007;43(16):2388–95. doi: 10.1016/j.ejca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Weihkopf T, Becker N, Nieters A, et al. Sun exposure and malignant lymphoma: a population-based case-control study in Germany. Int J Cancer. 2007;120(11):2445–51. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AM, Armstrong BK, Vajdic CM, et al. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;112(5):865–71. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 8.Smedby KE, Hjalgrim H, Melbye M, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong BK, Kricker A. Sun Exposure and Non-Hodgkin Lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):396–400. doi: 10.1158/1055-9965.EPI-06-1068. [DOI] [PubMed] [Google Scholar]
- 10.Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–54. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 12.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Curr Opin Clin Nutr Metab Care. 2007;10(1):6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- 14.Egan KM, Sosman JA, Blot WJ. Sunlight and reduced risk of cancer: is the real story vitamin D? J Natl Cancer Inst. 2005;97(3):161–3. doi: 10.1093/jnci/dji047. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiol. 2008;19(2):73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 18.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs--a brief overview. Mol Cell Biochem. 2003;253(1–2):247–54. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 20.Hickish T, Cunningham D, Colston K, et al. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68(4):668–72. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raina V, Cunningham D, Gilchrist N, Soukop M. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63(3):463–5. doi: 10.1038/bjc.1991.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang ET, Balter KM, Torrang A, et al. Nutrient intake and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2006;164(12):1222–32. doi: 10.1093/aje/kwj330. [DOI] [PubMed] [Google Scholar]
- 23.Polesel J, Talamini R, Montella M, et al. Linoleic acid, vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol. 2006;17(4):713–8. doi: 10.1093/annonc/mdl054. [DOI] [PubMed] [Google Scholar]
- 24.Purdue MP, Hartge P, Davis S, et al. Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2007;18(9):989–99. doi: 10.1007/s10552-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99(21):1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 27.Lim U, Freedman DM, Hollis BW, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–86. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erber E, Maskarinec G, Lim U, Kolonel LN. Dietary vitamin D and risk of non-Hodgkin lymphoma: the multiethnic cohort. Br J Nutr. 2009;103(4):581–4. doi: 10.1017/S0007114509992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 30.Tanner JT, Smith J, Defibaugh P, et al. Survey of vitamin content of fortified milk. J Assoc Off Anal Chem. 1988;71(3):607–10. [PubMed] [Google Scholar]
- 31.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 32.Kulak CA, V, Borba Z, Bilezikian JP, et al. Bone mineral density and serum levels of 25 OH vitamin D in chronic users of antiepileptic drugs. Arq Neuropsiquiatr. 2004;62(4):940–8. doi: 10.1590/s0004-282x2004000600003. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D Inadequacy among Postmenopausal North American Women Receiving Osteoporosis Therapy. J Clin Endocrinol Metab. 2005;90(6):3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 34.Brown LM, Hoover R, Gridley G, et al. Drinking practices and risk of squamous-cell esophageal cancer among Black and White men in the United States. Cancer Causes Control. 1997;8(4):605–9. doi: 10.1023/a:1018446430228. [DOI] [PubMed] [Google Scholar]
- 35.Brown LM, Pottern LM, Silverman DT, et al. Multiple myeloma among Blacks and Whites in the United States: role of cigarettes and alcoholic beverages. Cancer Causes Control. 1997;8(4):610–4. doi: 10.1023/a:1018498414298. [DOI] [PubMed] [Google Scholar]
- 36.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39(3):529–33. [PubMed] [Google Scholar]
- 37.Stolzenberg-Solomon RZ, Hayes RB, Horst RL, et al. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res. 2009;69(4):1439–47. doi: 10.1158/0008-5472.CAN-08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia, PA: Lipincott-Raven Publishers; 1998. [Google Scholar]
- 39.Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87(4):1102S–5S. doi: 10.1093/ajcn/87.4.1102S. [DOI] [PubMed] [Google Scholar]
- 40.Brot C, Vestergaard P, Kolthoff N, et al. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 41.Al-Delaimy WK, Jansen EH, Peeters PH, et al. Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers. 2006;11(4):370–82. doi: 10.1080/13547500600799748. [DOI] [PubMed] [Google Scholar]
- 42.Davies M, Hayes ME, Yin JA, Berry JL, Mawer EB. Abnormal synthesis of 1,25-dihydroxyvitamin D in patients with malignant lymphoma. J Clin Endocrinol Metab. 1994;78(5):1202–7. doi: 10.1210/jcem.78.5.8175979. [DOI] [PubMed] [Google Scholar]
- 43.Morton LM, Wang SS, Cozen W, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112(13):5150–60. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evens AM, Chiu BC. The Challenges of Epidemiologic Research in Non-Hodgkin Lymphoma. JAMA. 2008;300(17):2059–2061. doi: 10.1001/jama.2008.589. [DOI] [PubMed] [Google Scholar]
- 45.Garland CF, Grant WB, Mohr SB, Gorham ED, Garland FC. What is the dose-response relationship between vitamin D and cancer risk? Nutr Rev. 2007;65(8 Pt 2):S91–5. doi: 10.1111/j.1753-4887.2007.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 46.Crew KD, Gammon MD, Steck SE, et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila Pa) 2009;2(6):598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss RA. Multistage carcinogenesis. Br J Cancer. 2004;91(12):1981–2. doi: 10.1038/sj.bjc.6602318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vermeer M, Schmieder GJ, Yoshikawa T, et al. Effects of ultraviolet B light on cutaneous immune responses of humans with deeply pigmented skin. J Invest Dermatol. 1991;97(4):729–34. doi: 10.1111/1523-1747.ep12484259. [DOI] [PubMed] [Google Scholar]
- 49.Hersey P, Bradley M, Hasic E, et al. Immunological effects of solarium exposure. Lancet. 1983;1(8324):545–8. doi: 10.1016/s0140-6736(83)92808-8. [DOI] [PubMed] [Google Scholar]
- 50.Melbye M, Adami HO, Hjalgrim H, Glimelius B. Ultraviolet light and non-Hodgkin’s lymphoma. Acta Oncol. 1996;35(6):655–7. doi: 10.3109/02841869609083994. [DOI] [PubMed] [Google Scholar]
- 51.Fisher SG, Fisher RI. The emerging concept of antigen-driven lymphomas: epidemiology and treatment implications. Curr Opin Oncol. 2006;18(5):417–24. doi: 10.1097/01.cco.0000239878.31463.0b. [DOI] [PubMed] [Google Scholar]
- 52.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):401–4. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 53.Steindal AH, Porojnicu AC, Moan J. Is the seasonal variation in cancer prognosis caused by sun-induced folate degradation? Med Hypotheses. 2007;69(1):182–5. doi: 10.1016/j.mehy.2006.07.063. [DOI] [PubMed] [Google Scholar]


