Abstract
Thiazide and thiazide-like diuretics are among the most commonly used antihypertensives and have been available for over 50 years. However, the mechanism by which these drugs chronically lower blood pressure is poorly understood. Possible mechanisms include direct endothelial- or vascular smooth muscle-mediated vasodilation and indirect compensation to acute decreases in cardiac output. In addition, thiazides are associated with adverse metabolic effects, particularly hyperglycemia, and the mechanistic underpinnings of these effects are also poorly understood. Thiazide-induced hypokalemia, as well as other theories to explain these metabolic disturbances, including increased visceral adiposity, hyperuricemia, decreased glucose metabolism and pancreatic β-cell hyperpolarization, may play a role. Understanding genetic variants with differential responses to thiazides could reveal new mechanistic candidates for future research to provide a more complete understanding of the blood pressure and metabolic response to thiazide diuretics.
Keywords: chlorthalidone, epithelial sodium channel, hydrochlorothiazide, hypertension, metabolic syndrome, pharmacogenetics, potassium, sodium–chloride cotransporter, thiazide
In the USA, approximately one in three adults suffers from hypertension [1]. The lifetime risk for developing hypertension is very high, estimated at almost 90% in those who live to 80–85 years of age [2]. Hypertension is a known risk factor for both heart disease and stroke. In fact, 69% of people who have their first heart attack and 77% who have their first stroke have previously been diagnosed with hypertension [1]. Hypertension is also a major economic concern; an estimated US$73.4 billion was spent in 2009 on direct and indirect costs relating to hypertension [1].
The thiazide diuretic hydrochlorothiazide (HCTZ) is the second most commonly prescribed antihypertensive in the USA, with approximately 47.5 million prescriptions dispensed in 2008 [101]. Because most hypertensives require multidrug regimens for blood pressure control, many more patients are exposed to HCTZ through the use of combination antihypertensive products. Other less prescribed thiazide or thiazide-like diuretics include chlorthalidone, indapamide, metolazone, bendroflumethiazide and chlorothiazide. These drugs’ low cost and antihypertensive effectiveness have made them attractive choices for the management of hypertension for almost half a century. In addition, current hypertension treatment guidelines in the USA recommend thiazide diuretics as a first-line treatment for most patients with essential hypertension [2].
Despite the widespread use of this drug class, the mechanism by which thiazide diuretics lower blood pressure remains poorly understood. In addition, while many agree that thiazides adversely affect glucose and lipid homeostasis, only hypotheses currently exist as to why and how these metabolic effects occur. The aim of this article is to review the existing knowledge surrounding the mechanisms by which thiazides lower blood pressure and cause adverse metabolic effects. In addition, phenotypes of genetic variants will be discussed to provide insights into possible mechanistic candidates that warrant further research.
Antihypertensive effects
Thiazides achieve their diuretic action via inhibition of the Na+/Cl− cotransporter (NCC) in the renal distal convoluted tubule [3–5]. The NCC facilitates the absorption of sodium from the distal tubules back to the interstitium and accounts for approximately 7% of total sodium reabsorption [6]. By decreasing sodium reabsorption, thiazide use acutely results in an increase in fluid loss to urine, which leads to decreased extracellular fluid (ECF) and plasma volume. This volume loss results in diminished venous return, increased renin release, reduced cardiac output and decreased blood pressure [7]. Within days, the reduction in cardiac output increases total peripheral resistance (TPR), which stems mostly from activation of the sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS) [8,9]. This acute effect is evidenced by the fact that an infusion of dextran, a volume expander, during the acute thiazide treatment phase restores blood pressure to pretreatment levels [10].
Chronically, however, thiazides must lower blood pressure via some other mechanism. Plasma and ECF volumes almost fully recover within 4–6 weeks of thiazide initiation, yet blood pressure reduction is maintained [8,11]. After chronic administration, discontinuation of thiazides result in a decrease in renin levels and rapid volume replenishment, although the rise in blood pressure is much slower [12]. In addition, when given a dextran infusion, patients taking thiazides over longer periods of time (>2 months) experience an expansion of body fluid volume but blood pressure does not increase to baseline [12]. In addition, if diuretics simply lowered blood pressure by reducing plasma volume, then one would expect loop diuretics (such as furosemide, torsemide and bumetanide), which are superior diuretics to thiazides, to be superior antihypertensives. However, loop diuretics do not lower blood pressure to the degree thiazides do (although some difficulty exists in establishing equivalent doses) [13,14]. The aforementioned evidence indicates that the chronic thiazide antihypertensive effect is not exclusively due to a loss of blood volume.
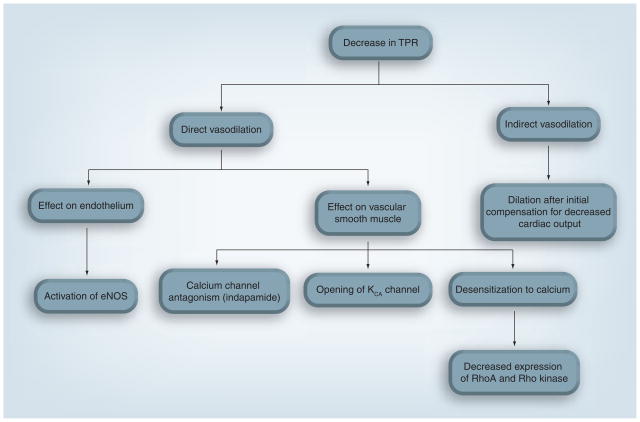
Research has shown for decades that a decrease in TPR plays an important role in the antihypertensive effect of thiazide diuretics over the long term [7,8,10]. However, the mechanism of this decrease in TPR has not been fully elucidated. Many hypotheses exist to describe this mechanism (Figure 1), with varying amounts of evidence to support them.
Figure 1. Theoretical mechanisms of thiazide-induced chronic blood pressure lowering.
eNOS: Endothelial nitric oxide synthase; KCA: Calcium-activated potassium; TPR: Total peripheral resistance.
Direct vasodilatory effects
Many have hypothesized that the thiazide-associated decrease in TPR could be the result of a direct vasodilatory effect, perhaps separate from the diuretic effect [15–18]. Multiple reports suggest a reduced vasoconstrictive effect of various pressor agents in animals and humans pretreated with thiazides [17,18]. However, these findings do not specify whether the effect was by direct vasodilation or stemming from an indirect action, such as volume depletion [15].
Direct action on the endothelium has been implicated in the acute vascular actions of thiazides. Methaclothiazide inhibited norepinephrine-induced vasoconstriction in the aortas of spontaneously hypertensive rats, but not in Wistar–Kyoto (non-hypertensive) rats [19]. Removal of the endothelium abolished this response in spontaneously hypertensive rat aortas, as did treatment with Nω-nitro-L-arginine, a nitric oxide synthesis inhibitor [19]. In addition, HCTZ and indapamide were found to dilate guinea pig mesentery arteries in vitro [20,21]. When arteries were cotreated with charybdotoxin, an inhibitor of the large conductance calcium-activated potassium (KCA) channel, HCTZ caused a reduced vasorelaxant effect, suggesting that HCTZ has direct vascular relaxant effects via opening of the KCA channel [21]. Clinically, when infused in the brachial artery, HCTZ caused a local vasodilation in hypertensive subjects [22]. The addition channel, of tetraethylammonium, another inhibitor of the KCA channel in the abolished this effect, further implicating the KCA mechanism of dilation [22]. It is important to note that the thiazide doses used in these infusions resulted in plasma concentrations of 11 μg/ml, which is approximately 10–20 times the plasma concentration found in patients clinically treated with thiazides [23,24]. Moreover, these findings were contrary to previous studies, which did not find any vasoactivity in the human forearm, although such studies used doses more comparable to those found in clinical practice [25,26]. Last, the difference in thiazide-induced vasodilation did not appear to vary between hypertensives and normotensives [22]. Because thiazides generally have a negligible blood pressure-lowering effect in normotensives, the clinical impact of this vasodilation is debatable.
Since the KCA channel is pH activated, another hypothesis explaining the chronic antihypertensive effect of thiazides is that their carbonic anhydrase-inhibiting ability affects KCA channel activity [27]. This hypothesis is supported by data indicating that bendroflumethiazide, which possesses minimal carbonic anhydrase-inhibiting activity, has only negligible effects on nore-pinephrine-induced vasoconstriction, which is not influenced by charybdotoxin [27]. In addition, the rise in intracellular pH from carbonic anhydrase inhibition was not inhibited by charybdotoxin [27]. If this hypothesis were correct, then bendroflumethiazide would have to reduce TPR by a mechanism different from all other thiazides. However, no evidence exists to support this concept.
Unlike HCTZ, indapamide was not shown to have a reduced vasorelaxant effect in the brachial arteries of human subjects [22], or guinea pig mesentery arteries cotreated with charybdotoxin [21]. Thus, it may be that indapamide chronically lowers blood pressure via a different mechanism than HCTZ. Reports have shown that indapamide inhibits norepinephrine-induced Ca2+ influx in various animal artery types [28,29]. Thus, unlike other thiazides, indapamide may have some calcium antagonist-like activities that could contribute to its chronic blood pressure-lowering ability.
Hydrochlorothiazide and chlorthalidone were shown to exhibit weak vasorelaxant effects in multiple rat vessel types, but only in the presence of plasma [30]. The key cofactor in the plasma required for the vasorelaxant effect was later shown to be albumin [31]. However, thiazides have shown a high affinity for albumin binding, which would cause a reduction in free thiazide concentration. The mechanism of this thiazide–albumin-dependent vasodilation remains unclear and requires further research for elucidation.
Recently, clinically used concentrations of HCTZ and chlorthalidone were shown to significantly reduce both angiotensin II and norepinephrine-induced vasoconstriction in rat aortic rings [32]. The magnitude of reduction was similar to that of a specific Rho kinase inhibitor [32]. This reduction in vasoconstriction occurred without changes in cellular calcium levels, suggesting calcium desensitization as a possible mechanism for thiazides’ chronic antihypertensive effect. Furthermore, RhoA and Rho kinase expression were decreased in vascular smooth muscle cells when treated with HCTZ or chlorthalidone [32]. This change in expression was endothelium independent, suggesting that thiazide action occurs directly on the smooth muscle. This latter finding is contrary to data mentioned previously, which indicate that the vasodilatory effect of thiazides is an endothelial-dependent mechanism [19]. Thus, the source of a direct thiazide-induced vasodilatory effect remains unclear.
Indirect vasodilatory effects
Rather than a direct action on the vasculature, the thiazide-mediated reduction in TPR could be the result of a systemic loss of fluid or electrolytes, which cause a reaction from the vasculature [16]. One long-standing indirect vasodilation hypothesis is reverse whole-body regulation [33]. Specifically, this hypothesis states that blood vessels adapt to the initial thiazide-induced plasma volume loss and decrease in cardiac output by constricting [33]. Then, over time, vessels dilate to increase cardiac output back toward baseline levels [33]. According to this hypothesis, the thiazide-induced vasodilation would originate from sodium-induced fluid loss, via inhibition of the NCC. In support of this mechanism, some evidence indicates that sodium balance controls the blood pressure response to thiazides. The addition of 20 g of salt daily to HCTZ-treated hypertensives practically abolishes the antihypertensive effect of the drug [12]. In addition, if sodium loss was the major mechanism of TPR reduction, one would expect kidney function to be necessary for thiazide-induced antihypertensive effects, as the kidneys are the primary organ responsible for sodium excretion. In fact, HCTZ and metolazone have little blood pressure-lowering effect in patients undergoing hemodialysis [34]. On the other hand, evidence also exists to contradict reverse whole-body regulation. Primarily, a poor correlation exists between acute volume loss and chronic antihypertensive effect [35], which would not be expected if fluid loss (via loss of sodium) drove the decrease in TPR.
Analysis of genetic variants
Gitelman’s syndrome is an autosomal recessive renal disorder characterized by hypokalemia, hypomagnesmia, hypocalciuria, metabolic alkalosis and low blood pressure [36]. Gitelman’s syndrome has been traced to inactivating mutations in SLC12A3, the gene that encodes NCC. Lack of functioning NCC should mimic the effects observed when someone with functional NCC is treated with a thiazide diuretic, making patients with the disease useful mechanistic models for study. Supporting this concept, a study of SLC12A3 mutations in an Amish population found those with variant alleles had systolic and diastolic blood pressures approximately 8 mmHg lower than those without variant alleles [37]. Findings from the Framingham Heart Study show that carriers of renal ion channel (including SLC12A3) mutations averaged 6.3 mmHg lower systolic and 3.4 mmHg lower diastolic blood pressure compared with those without mutations [38]. The decrease in blood pressure seen by these groups is similar to what is seen following treatment with thiazides. However, vasodilation appears to play a role in the hypotension exhibited by those with Gitelman’s syndrome, at least in part by the upregulation of endothelial nitric oxide synthase [39]. This finding is surprizing because SLC12A3 is predominantly expressed in the kidney and is not known to be expressed in the endothelium or vascular smooth muscle [40]. In addition, tetraethylammonium was shown to abolish the vasodilatory effect of HCTZ in vivo in patients with Gitelman’s syndrome [22]. This evidence in patients with Gitelman’s syndrome suggests thiazide blood pressure lowering may chronically involve the KCA channel rather than NCC.
Furthermore, variation in genes involved in thiazide response could provide a link associating these genes with thiazide blood pressure-lowering mechanisms. One such candidate gene, SCNN1G (the gene encoding the γ-subunit of the epithelial sodium channel [ENaC]), has previously been implicated in the thiazide blood pressure response. Those with polymorphisms in SCNN1G experienced a larger blood pressure response to HCTZ than those without [41,42]. Since thiazides may indirectly influence ENaC activation via the decrease in luminal calcium concentrations [43], ENaC’s role in the thiazide-induced blood pressure response should be investigated further.
Another gene, WNK1 (the gene encoding without lysine kinase 1), has also been associated with the blood pressure response to thiazides. Patients with polymorphisms in WNK1 achieved greater blood pressure lowering from HCTZ than those without WNK1 polymorphisms [42]. This finding is potentially important because WNK1 has been associated with an increase in NCC activity [44], which perhaps strengthens the evidence linking NCC to thiazide chronic blood pressure lowering. Thus, the exploration of WNK1 as a mechanistic candidate gene for thiazide response is warranted.
Another mechanistic candidate gene is STK39, the gene encoding ste20/SPS1-related proline/alanine-rich kinase. While not yet tested for pharmacogenetic association, STK39 has been associated with increased blood pressure in humans and was suggested as a possible thiazide response gene [45]. This kinase is a member of the MAPK-like superfamily and activates other ion channels, including NCC by direct phosphorylation [46]. The study of this gene could help further clarify the role of NCC in chronic thiazide-induced blood pressure lowering.
Metabolic effects
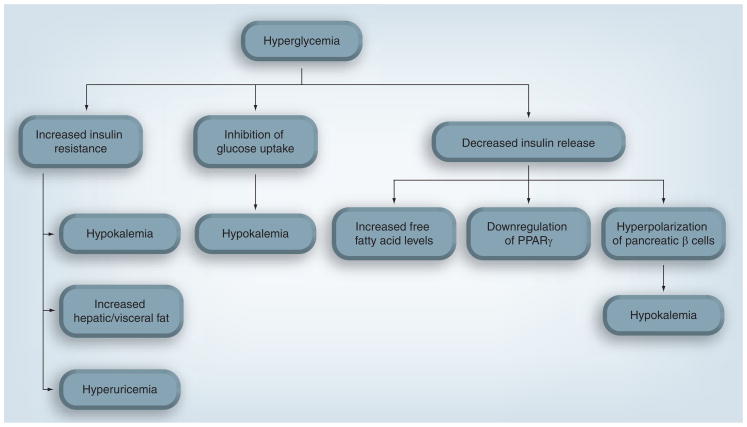
Thiazides have been associated with hyperlipidemia, hyperglycemia (Figure 2), new-onset diabetes, hypokalemia, hyperuricemia and stimulation of the RAAS [47–52]. While many large randomized, prospective clinical trials show an association between thiazide use and increased blood glucose, findings are mixed regarding the association with new-onset diabetes [48–50,53,54]. However, many issues must be considered when evaluating these associations in these trials, including:
Figure 2.
Theoretical mechanisms of thiazide-induced glycemic effects.
Most are post hoc findings and were not adequately powered to assess this association
New-onset diabetes was defined differently in many studies
Many studies had follow-up durations of only a few years, which may not be long enough to fully assess prolonged hyperglycemia
Comparing antihypertensive drug classes is difficult owing to differing study designs [55]
In an effort to better interpret the findings of these studies, a recent network meta-analysis of antihypertensive trials was published [51]. This analysis found that thiazides are associated with higher risk of diabetes than placebo and, along with β-blockers, had the highest risk of all major classes of antihypertensives [51].
In addition to diabetes, alterations in LDL, HDL and triglycerides have also been attributed to thiazides [50,56,57]. In one study, total cholesterol and apolipoprotein B were elevated in patients treated with HCTZ for 8 weeks, although these changes were not found at 1 year [57]. However, following 1 year of treatment with HCTZ, an increase in the ratio of LDL to HDL, as well as increases in the ratio of apolipoprotein B to A1, were observed in another study [50]. A high LDL to HDL ratio is a predictor of increased risk of cardiovascular disease [58]. In addition, a recent clinical trial observed significantly increased triglycerides in abdominally obese patients treated with HCTZ and atenolol, compared with non-obese patients [52]. At this time, the mechanism by which thiazides alter lipid profiles is poorly understood.
Mechanisms involving potassium
Evidence shows that large potassium infusions (causing >1–1.5 mEq/l elevation in plasma potassium) enhance insulin release by two to three times basal levels [59]. Insulin can cause increased cellular uptake of potassium, which is an important mechanism of potassium disposal [60,61]. However, smaller increases in potassium (<1 mEq/l) do not appear to induce this insulin-secreting effect [61,62]. In one study, a moderate potassium infusion given together with somatostatin (an inhibitor of insulin) decreased insulin levels and significantly elevated potassium levels [62]. Since potassium excretion is not affected by somatostatin, the authors concluded that this decrease in potassium tolerance must be due to decreased insulin levels [62]. While the association between increases in potassium and increases in insulin appears strong, less evidence exists supporting the concept that decreased potassium results in decreased insulin levels. Deprivation of potassium in the diet has been shown to decrease plasma insulin levels [63], but has also been shown to be ineffective in modulating glucose uptake [64]. Definitive evidence does not yet exist to answer whether decreases in potassium on the order of those seen in thiazide-treated patients cause decreases in insulin release [65].
The relationship between potassium and glucose homeostasis is important because many believe that thiazide-induced potassium depletion drives hyperglycemia [65]. Following thiazide treatment, serum potassium levels decrease 0.2–0.6 mEq/l in a dose-dependent manner [48,49,57]. Since muscle blood flow markedly decreases in potassium-depleted dogs [66], one of the simplest hypotheses explaining the role of hypokalemia in hyperglycemia attributes the hyperglycemia to decreased perfusion to skeletal muscle [67]. Decreasing glucose access to the skeletal muscle, which metabolizes large amounts of glucose, would theoretically leave more glucose circulating in the blood [67]. Another hypothesis involves the hyperpolarization of pancreatic β cells by the thiazide-induced opening of KCA channels [68]. Because thiazides have been shown to open KCA channels in vascular smooth muscle [21], thiazides could also open these channels in pancreatic β cells, decreasing intracellular potassium levels and hyperpolarizing the cells [68]. This hyperpolarization would then inhibit calcium influx and decrease insulin release, which is calcium dependent [68]. This hypothesis would explain the reduction in calcium uptake found when mouse islets were treated with HCTZ [69].
Clinically, conflicting data also exist regarding thiazide-induced hypokalemia and diabetes. A meta-analysis of 59 clinical studies showed a significant correlation between thiazide-induced potassium depletion and increased glucose levels in the blood, as well as a correlation between potassium supplementation (or concomitant use of potassium-sparing agents) and attenuation of hyperglycemia [70]. Of note, the authors derived these correlations from the aggregate mean changes from several studies, rather than data from individual patients. In addition, a secondary analysis of the Systolic Hypertension in Elderly Patients (SHEP) trial investigated the relationship between serum potassium and thiazide-induced diabetes [71]. In support of the meta-analysis findings, risk for developing diabetes was increased in the first year of thiazide treatment, but was not associated over the long term [71]. In addition, independent of drug treatment, each 0.5-mEq/l decrease in serum potassium was associated with a 45% increased risk for developing diabetes throughout the course of the study [71]. Contrasting these findings, a prospective study found no significant correlation between changes in serum potassium and serum glucose in HCTZ-treated patients [72]. A similar lack of correlation was found between changes in serum potassium and plasma insulin levels [72]. While these findings do not rule out the possibility that intracellular potassium is correlated with hyperglycemia or diabetes risk, measuring intracellular potassium is not usual clinical practice and these data suggest limited usefulness of serum potassium monitoring for the prevention of thiazide-associated hyperglycemia or new-onset diabetes.
Other mechanisms
Alteration in fat composition is another possible mechanism for thiazide-induced dysglycemia. Free fatty acids have been shown to decrease insulin secretion of pancreatic β cells in response to glucose [73]. Recently, a clinical trial found patients treated with 25–50 mg of HCTZ had significant reductions in insulin sensitivity, compared with those treated with candesartan or placebo [74]. Serum potassium levels were significantly lower in patients taking HCTZ, but levels in all groups remained within normal limits and potassium supplementation was allowed [74]. While on HCTZ, patients also developed a significantly higher hepatic fat content, and a significant correlation was found between hepatic fat content and decrease in insulin sensitivity [74]. Whether decreased insulin sensitivity was a result of this visceral fat accumulation, or vice versa, has not been established. Furthermore, C-reactive protein was also significantly increased with HCTZ treatment [74], indicating a possible role for inflammation in the development of insulin resistance. In support of this hypothesis, a recent prospective clinical trial reported that patients with abdominal obesity were more likely to experience new-onset diabetes with HCTZ treatment than those without abdominal obesity [52].
Hyperuricemia has also been implicated in thiazide-induced metabolic effects, as thiazides can increase serum urate levels by up to 35% [55]. In rats with fructose-induced metabolic syndrome given HCTZ, correcting hyperuricemia improved insulin resistance and hypertriglyceridemia to similar levels before HCTZ treatment [75]. In addition, patients treated with HCTZ experience significantly increased levels of serum uric acid [52]. Importantly, this increase in uric acid was associated with a greater than threefold increased risk of developing diabetes [52].
A recent hypothesis involves direct downregulation of PPARγ by thiazides, which would decrease insulin release. As mentioned previously, thiazides decrease cardiac output and reduce plasma and extracellular fluid volume. This change in plasma volume induces an elevation in plasma renin activity, which is hypothesized to be a pathway by which thiazides may cause hyperglycemia [65]. The RAAS interacts with the PPARγ signaling pathway [76], and increased plasma renin activity results in an increase in angiotensin II, which has been shown in mice to downregulate PPARγ [77].
Analysis of genetic variants
In an Amish cohort, patients with Gitelman’s syndrome were reported to have decreased potassium levels compared with age-matched family members, but did not have an increased incidence of hyperglycemia [37]. In addition, despite their hypokalemic state, it has been reported that these Gitelman’s patients do not have worse lipid profiles [43]. Because patients with Gitelman’s syndrome mimic thiazide-treated patients only to the extent of NCC impairment, these data may suggest thiazides induce dysglycemia via a mechanism other than their NCC-antagonizing effect.
The clinical opposite of Gitelman’s syndrome, pseudohypoaldosteronism type II (PHAII), is characterized by hyperkalemia, metabolic acidosis and high blood pressure [78]. Administration of a thiazide diuretic often corrects these abnormalities. Mutations in the genes encoding WNK1 and WNK4 have been traced to the cause of this disease. A loss-of-function mutation in WNK4 (which inhibits NCC) causes overactivation of NCC, while a gain-of-function mutation in WNK1 (which can reduce WNK4 inhibition) also causes overactivation [78]. When treated with a thiazide diuretic, patients with PHAII have potassium levels that drop from an average of 5.7 to 4.6 mEq/l [79]. However, these patients still experience increased plasma glucose, which suggests a lack of association between hypokalemia and thiazide-induced hyperglycemia [79]. However, it may be the magnitude of decrease in potassium, rather than actual potassium level, that influences the increase in glucose levels.
Genetic predictors of metabolic adverse effects were analyzed in a population of hypertensives treated with HCTZ [80]. Significant predictors for thiazide-induced increase in total cholesterol included polymorphisms in the genes encoding renin (REN) and the β-1 adrenergic receptor (ADRB1). Predictors for thiazide-induced triglyceride elevation included polymorphisms in the genes encoding angiotensinogen (AGT), endothelial nitric oxide synthase (NOS3) and WNK1. Furthermore, the genes encoding the potassium inwardly rectifying channel (KCNJ1) and the β-2 adrenergic receptor (ADRB2) were genetic predictors for elevated glucose. Perhaps polymorphisms in these genes (and likely others) collectively contribute toward a clinically significant rise in blood glucose.
Recently, a pharmacogenetic case–control study implicated genes in the RAAS system as playing a role in thiazide-induced diabetes. Patients with polymorphisms in the guanine nucleotide binding protein, β-polypeptide 3 gene (GNB3) displayed decreased risk of diabetes associated with thiazide use, and those with polymorphisms in ACE displayed increased risk [81]. While this study was retrospective and thus subject to important limitations, these findings help our understanding of the complex thiazide–hyperglycemia association and warrant further investigation.
Expert commentary
Despite decades of use and study, much of the data regarding thiazide mechanisms of action are in conflict. Most of the blood pressure-lowering mechanistic studies are small, in vitro projects using animal rather than human tissue, and many are more than 20 years old. In many of these older studies, the diuretic doses (or equivalent in vitro concentrations) used were 50 mg of HCTZ or more. Current guidelines recommend 50 mg as the maximum dose of HCTZ for the treatment of hypertension [2], and today clinicians do not often prescribe doses greater than 25 mg of HCTZ or its equivalent for hypertension. It is uncertain whether many noted effects witnessed in these older studies would be observed using lower doses. In addition, modern techniques and larger sample sizes could lead to the creation of data that are more reliable and reproducible than in these older studies. Another explanation of why findings vary in many of these in vitro studies is that if, in fact, vasodilatory effects only fully manifest themselves after chronic use, such effects would fail to be detected in many of these short-term studies.
Recently, there has been controversy over the long-held notion that all thiazides act similarly. The theory that all thiazides do not have a similar efficacy or mechanism of action would help explain the conflicting findings in many studies investigating their blood pressure-lowering and metabolic effects, and has been addressed previously by others [15]. Evidence already exists documenting the differences between chlorthalidone and HCTZ, although whether they exhibit differential effects on clinical outcomes is unclear [82]. Regardless, the mechanisms by which thiazides exert blood pressure-lowering and metabolic effects remain incompletely understood. From the current evidence, thiazides appear to exert their effects via multiple complex and interacting mechanisms.
Studying functional variants of NCC function, such as those with Gitelman’s syndrome, is useful in better understanding the effects of thiazide diuretics. While nonfunctional NCC seems to mimic the effect seen in patients taking thiazides, differences exist that make this model imperfect. In addition to examples given in previous sections, some component of vascular inflammation likely exists in hypertensives that is not seen in those with Gitelman’s syndrome. In addition, pharmacogenetic studies can provide mechanistic leads that have not been previously investigated. However, as pharmacogenetic findings can often fail to replicate [83], replication and functional studies must be provided to confirm true mechanistic candidates.
Five-year view
A recent call for research by the NIH National Heart, Lung and Blood Institute emphasizes the need for a better understanding of the mechanisms of action of thiazide and thiazide-like diuretics [65]. Based on the call for research and issues raised in this article, future research is needed help to better elucidate the mechanisms of thiazides’ desired and undesired effects. With additional knowledge, it may be possible to better identify which patients are most likely to experience robust blood pressure-lowering effects, but minimal metabolic effects.
While thiazide diuretics will likely remain an important component of hypertension therapy, particularly for patients requiring multiple medications, future guidelines will probably be more conservative with regard to which patients thiazides are recommended for. Avoidance will probably be suggested, when possible, in those at greatest risk for adverse metabolic effects. The apparent absence of metabolic effects and increasing generic availability of ARBs, calcium channel blockers and ACE inhibitors also provide alternative antihypertensive options for clinicians. Nevertheless, the efficacy of thiazides and low cost will keep them in use as a potent medication option for many patients with hypertension.
Key issues.
Some of the most commonly prescribed antihypertensives, thiazide and thiazide-like diuretics are thought to chronically lower blood pressure primarily by reducing total peripheral resistance.
How thiazides reduce total peripheral resistance is unclear. One hypothesis involves thiazides exerting a direct action on blood vessels. Another hypothesis involves an indirect mechanism, perhaps via a chronic vascular response to acute decreases in plasma volume.
Thiazides are also thought to increase the risk of metabolic disturbances, particularly hyperglycemia and hyperlipidemia. Thiazide-associated hypokalemia is a popular but controversial theory as a cause of hyperglycemia. Other theoretical mechanisms include hyperuricemia, alterations in fat distribution, and PPARγ downregulation.
The evidence for many proposed mechanisms are conflicting, raising the prospect that thiazides may have many complex and interacting mechanisms, or even slightly different mechanisms of action.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work is supported in part by NIH grants T32DK007518, K23HL086558 and GM074492. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Ellison DH, Velazquez H, Wright FS. Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol. 1987;253(3 Pt 2):F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann S, Velazquez H, Obermuller N, Reilly RF, Moser D, Ellison DH. Expression of the thiazide-sensitive Na–Cl cotransporter by rabbit distal convoluted tubule cells. J Clin Invest. 1995;96(5):2510–2514. doi: 10.1172/JCI118311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obermuller N, Bernstein P, Velazquez H, et al. Expression of the thiazide-sensitive Na–Cl cotransporter in rat and human kidney. Am J Physiol. 1995;269(6 Pt 2):F900–F910. doi: 10.1152/ajprenal.1995.269.6.F900. [DOI] [PubMed] [Google Scholar]
- 6.Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 7.Conway J, Lauwers P. Hemodynamic and hypotensive effects of long-term therapy with chlorothiazide. Circulation. 1960;21:21–27. doi: 10.1161/01.cir.21.1.21. [DOI] [PubMed] [Google Scholar]
- 8.van Brummelen P, Man in ‘t Veld AJ, Schalekamp MA. Hemodynamic changes during long-term thiazide treatment of essential hypertension in responders and nonresponders. Clin Pharmacol Ther. 1980;27(3):328–336. doi: 10.1038/clpt.1980.44. [DOI] [PubMed] [Google Scholar]
- 9.Lake CR, Ziegler MG, Coleman MD, Kopin IJ. Hydrochlorothiazide-induced sympathetic hyperactivity in hypertensive patients. Clin Pharmacol Ther. 1979;26(4):428–432. doi: 10.1002/cpt1979264428. [DOI] [PubMed] [Google Scholar]
- 10.Wilson IM, Freis ED. Relationship between plasma and extracellular fluid volume depletion and the antihypertensive effect of chlorothiazide. Circulation. 1959;20:1028–1036. doi: 10.1161/01.cir.20.6.1028. [DOI] [PubMed] [Google Scholar]
- 11.Tarazi RC, Dustan HP, Frohlich ED. Long-term thiazide therapy in essential hypertension. Evidence for persistent alteration in plasma volume and renin activity. Circulation. 1970;41(4):709–717. doi: 10.1161/01.cir.41.4.709. [DOI] [PubMed] [Google Scholar]
- 12.Winer BM. The antihypertensive actions of benzothiadiazines. Circulation. 1961;23:211–218. doi: 10.1161/01.cir.23.2.211. [DOI] [PubMed] [Google Scholar]
- 13.Anderson J, Godfrey BE, Hill DM, Munro-Faure AD, Sheldon J. A comparison of the effects of hydrochlorothiazide and of frusemide in the treatment of hypertensive patients. Q J Med. 1971;40(160):541–560. [PubMed] [Google Scholar]
- 14.Holland OB, Gomez-Sanchez CE, Kuhnert LV, Poindexter C, Pak CY. Antihypertensive comparison of furosemide with hydrochlorothiazide for black patients. Arch Intern Med. 1979;139(9):1015–1021. [PubMed] [Google Scholar]
- 15•.Hughes AD. How do thiazide and thiazide-like diuretics lower blood pressure? J Renin Angiotensin Aldosterone Syst. 2004;5(4):155–160. doi: 10.3317/jraas.2004.034. Comprehensive review of thiazide blood pressure-lowering mechanisms. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Khatri I, Freis ED. Mechanism of antihypertensive effect of thiazide diuretics. Am Heart J. 1978;95(5):611–618. doi: 10.1016/0002-8703(78)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Aleksandrow D, Wysznacka W, Gajewski J. Influence of chlorothiazide upon arterial responsiveness to nor-epinephrine in hypertensive subjects. N Engl J Med. 1959;261:1052–1055. doi: 10.1056/NEJM195911192612103. [DOI] [PubMed] [Google Scholar]
- 18.Freis ED, Wanko A, Schnaper HW, Frohlich ED. Mechanism of the altered blood pressure responsiveness produced by chlorothiazide. J Clin Invest. 1960;39:1277–1281. doi: 10.1172/JCI104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colas B, Slama M, Collin T, Safar M, Andrejak M. Mechanisms of methyclothiazide-induced inhibition of contractile responses in rat aorta. Eur J Pharmacol. 2000;408(1):63–67. doi: 10.1016/s0014-2999(00)00704-4. [DOI] [PubMed] [Google Scholar]
- 20.Calder JA, Schachter M, Sever PS. Direct vascular actions of hydrochlorothiazide and indapamide in isolated small vessels. Eur J Pharmacol. 1992;220(1):19–26. doi: 10.1016/0014-2999(92)90006-p. [DOI] [PubMed] [Google Scholar]
- 21.Calder JA, Schachter M, Sever PS. Potassium channel opening properties of thiazide diuretics in isolated guinea pig resistance arteries. J Cardiovasc Pharmacol. 1994;24(1):158–164. doi: 10.1097/00005344-199407000-00024. [DOI] [PubMed] [Google Scholar]
- 22•.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension. 1998;32(6):1071–1076. doi: 10.1161/01.hyp.32.6.1071. Studies local direct vasodilation in humans, and calcium-activated potassium channels are implicated as a mechanism. [DOI] [PubMed] [Google Scholar]
- 23.Beermann B, Groschinsky-Grind M. Pharmacokinetics of hydrochlorothiazide in man. Eur J Clin Pharmacol. 1977;12(4):297–303. doi: 10.1007/BF00607430. [DOI] [PubMed] [Google Scholar]
- 24.Niemeyer C, Hasenfuss G, Wais U, Knauf H, Schafer-Korting M, Mutschler E. Pharmacokinetics of hydrochlorothiazide in relation to renal function. Eur J Clin Pharmacol. 1983;24(5):661–665. doi: 10.1007/BF00542218. [DOI] [PubMed] [Google Scholar]
- 25.Kreeft JH, Langlois S, Ogilvie RI. Comparative trial of indapamide and hydrochlorothiazide in essential hypertension, with forearm plethysmography. J Cardiovasc Pharmacol. 1984;6(4):622–626. doi: 10.1097/00005344-198407000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Pickkers P, Russel FG, Hughes AD, Thien T, Smits P. Hydrochlorothiazide exerts no direct vasoactivity in the human forearm. J Hypertens. 1995;13(12 Pt 2):1833–1836. [PubMed] [Google Scholar]
- 27.Pickkers P, Garcha RS, Schachter M, Smits P, Hughes AD. Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension. 1999;33(4):1043–1048. doi: 10.1161/01.hyp.33.4.1043. [DOI] [PubMed] [Google Scholar]
- 28.Mironneau J, Savineau JP, Mironneau C. Compared effects of indapamide, hydrochlorothiazide and chlorthalidone on electrical and mechanical activities in vascular smooth muscle. Eur J Pharmacol. 1981;75(2–3):109–113. doi: 10.1016/0014-2999(81)90068-6. [DOI] [PubMed] [Google Scholar]
- 29.Del Rio M, Chulia T, Gonzalez P, Tejerina T. Effects of indapamide on contractile responses and 45Ca2+ movements in various isolated blood vessels. Eur J Pharmacol. 1993;250(1):133–139. doi: 10.1016/0014-2999(93)90630-z. [DOI] [PubMed] [Google Scholar]
- 30.Abrahams Z, Tan LL, Pang MY, Abrahams B, Tan MM, Wright JM. Demonstration of an in vitro direct vascular relaxant effect of diuretics in the presence of plasma. J Hypertens. 1996;14(3):381–388. doi: 10.1097/00004872-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Abrahams Z, Pang MY, Lam EK, Wright JM. What is the plasma cofactor required by diuretics for direct vascular relaxant effect in vitro? J Hypertens. 1998;16(6):801–809. doi: 10.1097/00004872-199816060-00011. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Z, Zhu S, Liu D, Cao T, Wang L, Tepel M. Thiazide-like diuretics attenuate agonist-induced vasoconstriction by calcium desensitization linked to Rho kinase. Hypertension. 2005;45(2):233–239. doi: 10.1161/01.HYP.0000152701.97426.5f. [DOI] [PubMed] [Google Scholar]
- 33•.Tobian L. Why do thiazide diuretics lower blood pressure in essential hypertension? Annu Rev Pharmacol. 1967;7:399–408. doi: 10.1146/annurev.pa.07.040167.002151. First instance of reverse whole-body regulation theory presented. [DOI] [PubMed] [Google Scholar]
- 34.Bennett WM, McDonald WJ, Kuehnel E, Hartnett MN, Porter GA. Do diuretics have antihypertensive properties independent of natriuresis? Clin Pharmacol Ther. 1977;22(5 Pt 1):499–504. [PubMed] [Google Scholar]
- 35.Roos JC, Boer P, Koomans HA, Geyskes GG, Dorhout Mees EJ. Haemodynamic and hormonal changes during acute and chronic diuretic treatment in essential hypertension. Eur J Clin Pharmacol. 1981;19(2):107–112. doi: 10.1007/BF00568396. [DOI] [PubMed] [Google Scholar]
- 36.Roser M, Eibl N, Eisenhaber B, et al. Gitelman syndrome. Hypertension. 2009;53(6):893–897. doi: 10.1161/HYPERTENSIONAHA.108.127993. [DOI] [PubMed] [Google Scholar]
- 37.Cruz DN, Simon DB, Nelson-Williams C, et al. Mutations in the Na–Cl cotransporter reduce blood pressure in humans. Hypertension. 2001;37(6):1458–1464. doi: 10.1161/01.hyp.37.6.1458. [DOI] [PubMed] [Google Scholar]
- 38.Ji W, Foo JN, O’Roak BJ, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40(5):592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calo L, Ceolotto G, Milani M, et al. Abnormalities of Gq-mediated cell signaling in Bartter and Gitelman syndromes. Kidney Int. 2001;60(3):882–889. doi: 10.1046/j.1523-1755.2001.060003882.x. [DOI] [PubMed] [Google Scholar]
- 40.Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflugers Arch. 2004;447(5):580–593. doi: 10.1007/s00424-003-1066-3. [DOI] [PubMed] [Google Scholar]
- 41.Maitland-van der Zee AH, Turner ST, Schwartz GL, Chapman AB, Klungel OH, Boerwinkle E. A multilocus approach to the antihypertensive pharmacogenetics of hydrochlorothiazide. Pharmacogenet Genomics. 2005;15(5):287–293. doi: 10.1097/01213011-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Turner ST, Schwartz GL, Chapman AB, Boerwinkle E. WNK1 kinase polymorphism and blood pressure response to a thiazide diuretic. Hypertension. 2005;46(4):758–765. doi: 10.1161/01.HYP.0000186240.81996.57. [DOI] [PubMed] [Google Scholar]
- 43.Ellison DH, Loffing J. Thiazide effects and adverse effects. Insights from molecular genetics. Hypertension. 2009;54(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.129171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriguchi T, Urushiyama S, Hisamoto N, et al. WNK1 regulates phosphorylation of cation–chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280(52):42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, O’Connell JR, McArdle PF, et al. From the cover: whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA. 2009;106(1):226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson C, Rafiqi FH, Karlsson HK, et al. Activation of the thiazide-sensitive Na+–Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121(Pt 5):675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 47.Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470–476. doi: 10.1097/MNH.0b013e328305b9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savage PJ, Pressel SL, Curb JD, et al. Influence of long-term, low-dose, diuretic-based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: the Systolic Hypertension in the Elderly Program. SHEP Cooperative Research Group. Arch Intern Med. 1998;158(7):741–751. doi: 10.1001/archinte.158.7.741. [DOI] [PubMed] [Google Scholar]
- 49••.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. Landmark trial that resulted in thiazides being suggested by many guidelines as first-line antihypertensive therapy. [DOI] [PubMed] [Google Scholar]
- 50.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study) J Hypertens. 2003;21(8):1563–1574. doi: 10.1097/01.hjh.0000084723.53355.76. [DOI] [PubMed] [Google Scholar]
- 51•.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369(9557):201–207. doi: 10.1016/S0140-6736(07)60108-1. Meta-analysis of clinical trials showing of all antihypertensives analyzed, β-blockers and thiazide diuretics are associated with the highest risk of diabetes. [DOI] [PubMed] [Google Scholar]
- 52.Cooper-Dehoff RM, Wen S, Beitelshees AL, et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2009;55(1):61–68. doi: 10.1161/HYPERTENSIONAHA.109.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil–Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290(21):2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 54.Barzilay JI, Davis BR, Cutler JA, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006;166(20):2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 55.Sica DA. Diuretic-related side effects: development and treatment. J Clin Hypertens (Greenwich) 2004;6(9):532–540. doi: 10.1111/j.1524-6175.2004.03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasiske BL, Ma JZ, Kalil RS, Louis TA. Effects of antihypertensive therapy on serum lipids. Ann Intern Med. 1995;122(2):133–141. doi: 10.7326/0003-4819-122-2-199501150-00010. [DOI] [PubMed] [Google Scholar]
- 57.Lakshman MR, Reda DJ, Materson BJ, Cushman WC, Freis ED. Diuretics and β-blockers do not have adverse effects at 1 year on plasma lipid and lipoprotein profiles in men with hypertension. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Arch Intern Med. 1999;159(6):551–558. doi: 10.1001/archinte.159.6.551. [DOI] [PubMed] [Google Scholar]
- 58.Williams K, Sniderman AD, Sattar N, D’Agostino R, Jr, Wagenknecht LE, Haffner SM. Comparison of the associations of apolipoprotein B and low-density lipoprotein cholesterol with other cardiovascular risk factors in the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2003;108(19):2312–2316. doi: 10.1161/01.CIR.0000097113.11419.9E. [DOI] [PubMed] [Google Scholar]
- 59.Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol. 1981;240(4):F257–F268. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 60.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238(5):E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 61.Sterns RH, Feig PU, Pring M, Guzzo J, Singer I. Disposition of intravenous potassium in anuric man: a kinetic analysis. Kidney Int. 1979;15(6):651–660. doi: 10.1038/ki.1979.85. [DOI] [PubMed] [Google Scholar]
- 62.DeFronzo RA, Sherwin RS, Dillingham M, Hendler R, Tamborlane WV, Felig P. Influence of basal insulin and glucagon secretion on potassium and sodium metabolism. Studies with somatostatin in normal dogs and in normal and diabetic human beings. J Clin Invest. 1978;61(2):472–479. doi: 10.1172/JCI108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism. 1980;29(6):498–502. doi: 10.1016/0026-0495(80)90074-8. [DOI] [PubMed] [Google Scholar]
- 64.Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH. Short-term K(+) deprivation provokes insulin resistance of cellular K(+) uptake revealed with the K(+) clamp. Am J Physiol Renal Physiol. 2001;280(1):F95–F102. doi: 10.1152/ajprenal.2001.280.1.F95. [DOI] [PubMed] [Google Scholar]
- 65••.Carter BL, Einhorn PT, Brands M, et al. Thiazide-induced dysglycemia: call for research from a working group from the National Heart, Lung, and Blood Institute. Hypertension. 2008;52(1):30–36. doi: 10.1161/HYPERTENSIONAHA.108.114389. National Heart, Lung and Blood Institute calls for research that underlines the importance of further research regarding the mechanism of thiazide-associated dysglycemia. Also a good review of the role of potassium in dysglycemia. [DOI] [PubMed] [Google Scholar]
- 66.Knochel JP, Schlein EM. On the mechanism of rhabdomyolysis in potassium depletion. J Clin Invest. 1972;51(7):1750–1758. doi: 10.1172/JCI106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal R. Hypertension, hypokalemia, and thiazide-induced diabetes: a 3-way connection. Hypertension. 2008;52(6):1012–1013. doi: 10.1161/HYPERTENSIONAHA.108.121970. [DOI] [PubMed] [Google Scholar]
- 68.Pickkers P, Schachter M, Hughes AD, Feher MD, Sever PS. Thiazide-induced hyperglycaemia: a role for calcium-activated potassium channels? Diabetologia. 1996;39(7):861–864. doi: 10.1007/s001250050522. [DOI] [PubMed] [Google Scholar]
- 69.Sandstrom PE. Inhibition by hydrochlorothiazide of insulin release and calcium influx in mouse pancreatic β-cells. Br J Pharmacol. 1993;110(4):1359–1362. doi: 10.1111/j.1476-5381.1993.tb13969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48(2):219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. Meta-analysis of clinical trials associating thiazide-induced hypokalemia with hyperglycemia and attenuation of hyperglycemia with potassium treatment. [DOI] [PubMed] [Google Scholar]
- 71.Shafi T, Appel LJ, Miller ER, 3rd, Klag MJ, Parekh RS. Changes in serum potassium mediate thiazide-induced diabetes. Hypertension. 2008;52(6):1022–1029. doi: 10.1161/HYPERTENSIONAHA.108.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Smith SM, Anderson SD, Wen S, et al. Lack of correlation between thiazide-induced hyperglycemia and hypokalemia: subgroup analysis of results from the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Pharmacotherapy. 2009;29(10):1157–1165. doi: 10.1592/phco.29.10.1157. Prospective clinical trial that contrasts with the previous belief that thiazide-induced serum potassium decreases are correlated with hyperglycemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ayvaz G, Balos Törüner F, Karakoç A, Yetkin I, Cakir N, Arslan M. Acute and chronic effects of different concentrations of free fatty acids on the insulin secreting function of islets. Diabetes Metab. 2002;28(6 Pt 2):3S7–3S12. [PubMed] [Google Scholar]
- 74••.Eriksson JW, Jansson PA, Carlberg B, et al. Hydrochlorothiazide, but not candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the Mechanisms For the Diabetes Preventing Effect of Candesartan (MEDICA) Study. Hypertension. 2008;52(6):1030–1037. doi: 10.1161/HYPERTENSIONAHA.108.119404. Clinical evidence associating visceral fat with thiazide-induced insulin resistance. [DOI] [PubMed] [Google Scholar]
- 75•.Reungjui S, Roncal CA, Mu W, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. 2007;18(10):2724–2731. doi: 10.1681/ASN.2007040416. Shows an improvement of thiazide-induced insulin resistance by correcting hyperuricemia or hypokalemia in rats. [DOI] [PubMed] [Google Scholar]
- 76.Tikellis C, Cooper ME, Thomas MC. Role of the renin–angiotensin system in the endocrine pancreas: implications for the development of diabetes. Int J Biochem Cell Biol. 2006;38(5–6):737–751. doi: 10.1016/j.biocel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Tham DM, Martin-McNulty B, Wang YX, et al. Angiotensin II is associated with activation of NF-κB-mediated genes and downregulation of PPARs. Physiol Genomics. 2002;11(1):21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 78.Hadchouel J, Delaloy C, Faure S, Achard JM, Jeunemaitre X. Familial hyperkalemic hypertension. J Am Soc Nephrol. 2006;17(1):208–217. doi: 10.1681/ASN.2005030314. [DOI] [PubMed] [Google Scholar]
- 79.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87(7):3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 80.Maitland-van der Zee AH, Turner ST, Schwartz GL, Chapman AB, Klungel OH, Boerwinkle E. Demographic, environmental, and genetic predictors of metabolic side effects of hydrochlorothiazide treatment in hypertensive subjects. Am J Hypertens. 2005;18(8):1077–1083. doi: 10.1016/j.amjhyper.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Bozkurt O, de Boer A, Grobbee DE, et al. Variation in renin–angiotensin system and salt-sensitivity genes and the risk of diabetes mellitus associated with the use of thiazide diuretics. Am J Hypertens. 2009;22(5):545–551. doi: 10.1038/ajh.2009.38. [DOI] [PubMed] [Google Scholar]
- 82.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4–9. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 83.Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype–phenotype associations. Nature. 2007;447(7145):655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]