Abstract
We studied the genetic and clinical features of diabetic subjects in a five-generation Michigan-Kentucky pedigree ascertained through a proband with pancreatic agenesis and homozygous for the IPF1 mutation Pro63fsx60. Diabetic and nondiabetic family members were genotyped and phenotyped. We also carried out genetic studies to determine the history of the IPF1 mutation in the Michigan-Kentucky family and a Virginia family with the same mutation. We identified 110 individuals. Thirty-four are currently being treated for diabetes and ten of these are Pro63fsX60 carriers (i.e. MODY4). Subjects with MODY as well as those with type 2 diabetes are characterized by obesity and hyperinsulinemia. Genetic studies suggest that the IPF1 mutation was inherited from an ancestor common to both the Michigan-Kentucky and Virginia families. MODY4 and type 2 diabetes in the Michigan-Kentucky pedigree are associated with obesity and hyperinsulinemia. Obesity and hyperinsulinemia have been observed occasionally in other subtypes of MODY suggesting that hyperinsulinemia may be a general phenomenon when obesity occurs in MODY subjects. Hypoinsulinemia in non-obese MODY subjects appears to be due to a functional defect in the beta cell. Genetic testing should be considered in multigenerational obese diabetic subjects, particularly when such families contain young diabetic members.
Keywords: Diabetes Mellitus, MODY4, IPF1, Obesity, Hyperinsulinemia, Insulin Resistance
INTRODUCTION
Maturity-onset diabetes of the young (MODY) is a heterogeneous group of disorders characterized by diabetes mellitus, an autosomal dominant mode of inheritance, diagnosis usually before the age of 25 years and frequently in childhood or adolescence, and a primary defect in beta-cell function. In contrast to subjects with type 2 diabetes, obesity occurs infrequently in MODY subjects [1]. MODY can result from mutations in any one of at least eight different genes: glucokinase (MODY2) [2,3], hepatocyte nuclear factor (HNF)-4α (MODY1) [4], HNF-1α (MODY3) [5], insulin promoter factor-1 (IPF1, MODY4) [6,7], HNF-1β (MODY5) [8], neurogenic differentiation factor 1 (MODY6) [9], carboxyl-ester lipase (MODY7) [10] and insulin (MODY8) [11].
MODY4 is a rare form of MODY resulting from mutations in IPF1, a transcription factor that plays a critical role in pancreatic and beta-cell development and function [12, 13]. The largest and best described MODY4 family is a 5-generation pedigree with the IPF1 mutation Pro63fsdelC (Pro63fsX60; c.188delC) [6,7]. This Virginia family was ascertained through a female infant with permanent neonatal diabetes and severe exocrine pancreatic insufficiency due to pancreatic agenesis [6,7,14]. The infant was homozygous for the mutation Pro63fsX60 [6,7]. The parents were heterozygous carriers. The father had diabetes whereas the mother did not. A consanguineous loop was observed in generation I linking the two families [6,7]. Eight diabetic members were heterozygous carriers. Nonobese diabetic carriers in this pedigree had a greatly decreased insulin response to glucose [15]. A second subject with permanent neonatal diabetes, exocrine pancreatic insufficiency and pancreatic agenesis was described in Switzerland [16]. This subject was a compound heterozygote: IPF1, Glu164Asp and Glu178Lys. The infant’s mother carried the Glu164Asp mutation and the father the Glu178Lys mutation. Both parents had normal oral glucose tolerance tests but were noted to have high normal fasting plasma glucose levels (101 and 102 mg/dl, respectively). The mother had a history of gestational diabetes. There was a two-generation family history of diabetes in each parent. The studies, to date, of heterozygous carriers of IPF1 mutations suggest that MODY4 is a relatively mild disorder of glucose intolerance characterized by diminished insulin secretion.
We recently described a third case of permanent neonatal diabetes, exocrine pancreatic insufficiency and pancreatic agenesis due to an IPF1 mutation [17]. Interestingly, this infant was homozygous for the same mutation (Pro63fsX60) as the first case from Virginia described above [6,7]. Presentation of this infant initiated a study of the second extended 5-generation pedigree (Michigan-Kentucky; R-T), a pedigree with permanent neonatal diabetes, obese MODY4 and obese type 2 diabetes.
METHODS
Proband and family
The details of the presentation, diagnosis, treatment, and course of the male proband with permanent neonatal diabetes, exocrine pancreatic insufficiency and pancreatic agenesis have been presented [17]. Genetic testing showed that he was homozygous for the IPF1 mutation Pro63fsX60.
The families of the proband’s father and mother came to Michigan independently from the same town in eastern Kentucky in the early 1980s. The families were not known to be related. Other family members remain in Kentucky. Most of those who came to Michigan have subsequently moved to Florida, Kentucky, and Tennessee. We were able to construct a 5-generation pedigree through interviews. The Michigan-Kentucky pedigree includes 110 individuals and 34 diabetic subjects (Fig. 1 and 2). Subjects II-8 and II-9 were reported to be third cousins and further consanguinity is possible as both families came from the same town. The surnames of members of the Michigan-Kentucky pedigree were different from those of the Virginia pedigree having the same IPF1 mutation (W.L. Clarke, personal communication). This study was approved by the University of Michigan Medical Center Institutional Review Board. All subjects or their parents provided written informed consent. The research was carried out according to the principles of the Declarations of Helsinki.
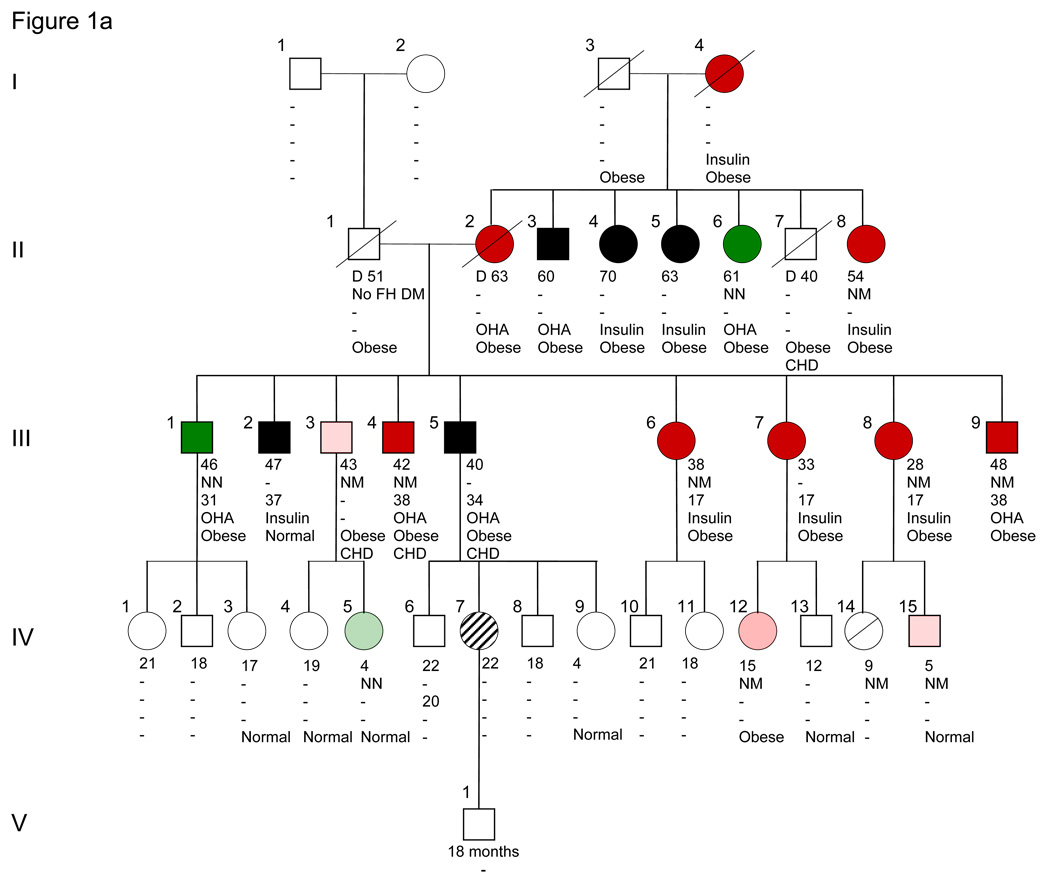
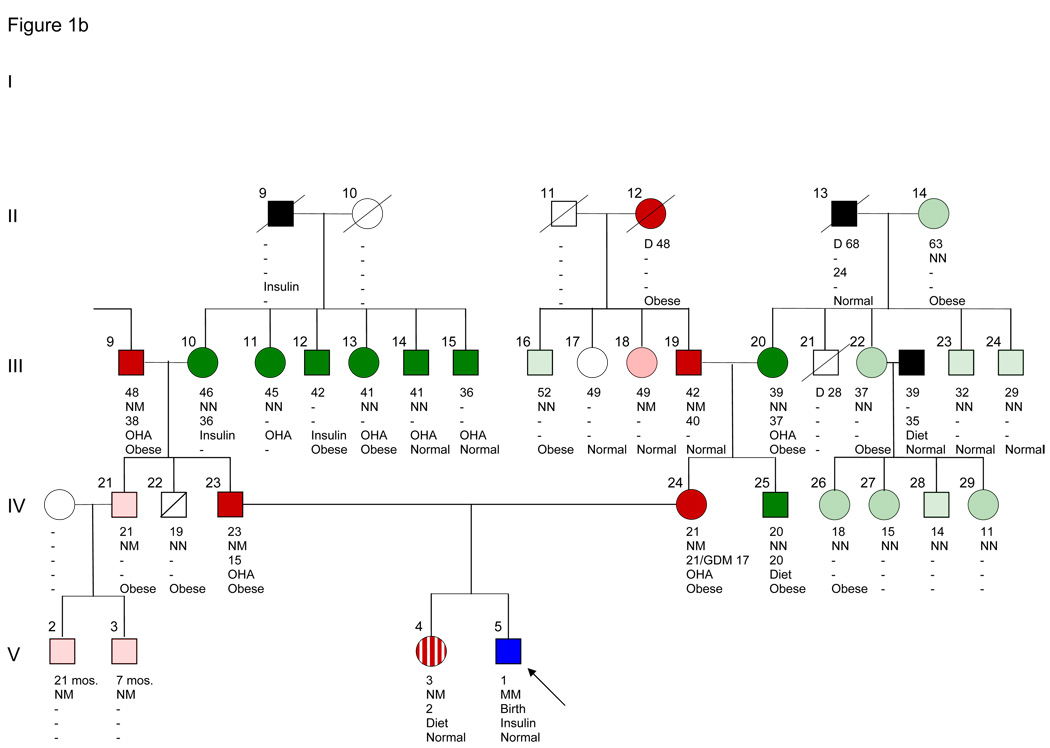
Figure 1.
Michigan-Kentucky family with IPF1 mutation Pro63fsX60 and permanent neonatal diabetes, MODY4, type 2 diabetes, obesity and hyperinsulinemia. Because of its large size we show the pedigree in two panels, Figure 1A and 1B. Note that III-9 is shown in both Fig. 1A and B. Subject III-9, the grandfather of proband V-5, had three wives, two of whom with their offspring IV-16–20 are omitted from the figures. II-8 and II-9 were said to be third cousins. Squares indicate male and circles female subjects. The proband is shown in blue. Dark red denotes diabetic IPF-1 carrier (NM) or diabetic presumptive IPF1 carriers (I-4, II-2, II-12, III-7). Dark green denotes type 2 diabetes (NN) or presumptive type 2 diabetes (III-12, III-15). Black denotes non-genotyped diabetic subjects. Light red denotes untested (for carbohydrate intolerance) IPF1 carriers (NM) and light green untested IPF1 non-carriers (NN). A diagonal line through the symbol indicates normal glucose tolerance (IV-14 and -22). An open symbol indicates untested for carbohydrate intolerance and non-genotyped. White vertical stripe (V-4) indicates intermittent diabetes. A circle with slashes denotes gestational diabetes (IV-7). A slash extending beyond symbol indicates that the subject is deceased. Generation number is given on the left. The number to the upper left of individual subject gives individual numbers within each generation. Number immediately below subject is present age, followed by genotype (N = normal sequence allele, M – allele with Pro63fsX60 mutation). Number below genotype is age at diagnosis of diabetes. OHA = oral hypoglycemic agent; insulin = insulin therapy. Body weight: Obese, Normal = normal weight or thin.
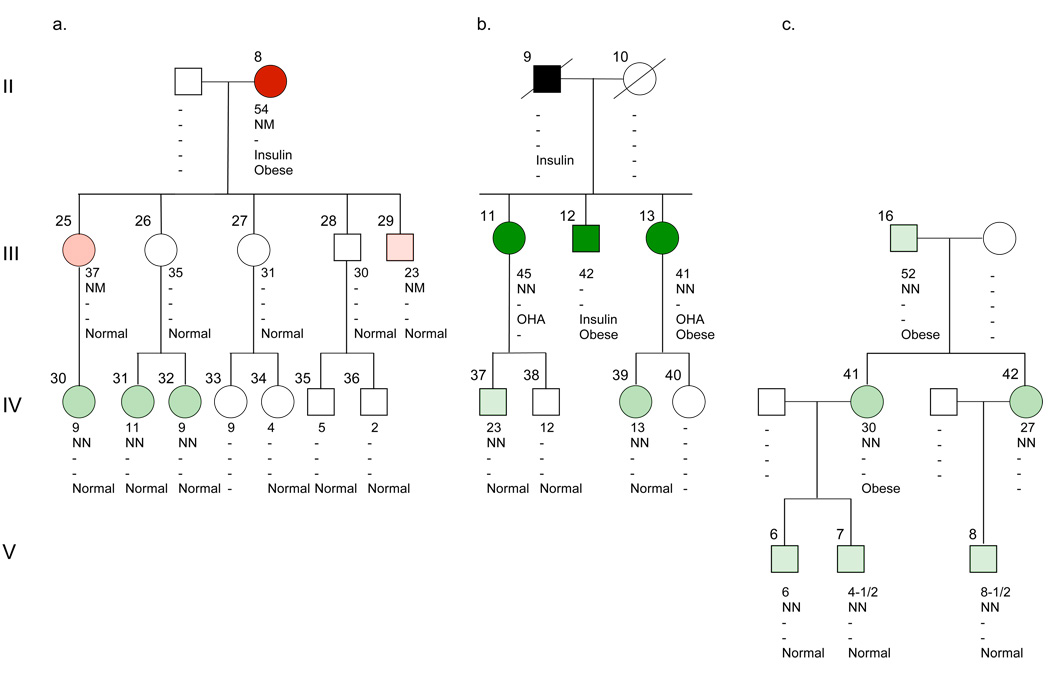
Figure 2.
Offspring of II-8 (panel A), II-9 (panel B) and III-16 (panel C). The symbols and abbreviations are described in the legend to Figure 1.
Biochemical assays
Assays were performed in the Michigan Diabetes Research and Training Center Chemistry Core Laboratory. All blood samples were collected on ice and stored at −70 °C until assayed. Plasma glucose was measured on a Cobas Mira Plus analyzer using a hexokinase method. Inter-assay variables are 3.15% at 84 mg/dL and 2.8% at 292 mg/dL (n=224). HbA1c was measured in whole blood using the assay kit Unimate 3 from Roche Diagnostics. HbA1c and total hemoglobin (Hb) are determined from hemolysate, prepared on-board the Cobas Mira Chemistry analyzer from whole blood. HbA1c is measured from the hemolysate by a latex enhanced turbidimetric immunoassay. The final test result is determined from the HbA1c/Hb ratio, including a conversion formula to match a HPLC reference method. The inter-assay variations are 3.7% at 5.7% HbA1c and 3.8% at 10.7% HbA1c (n=45). Immunoreactive insulin was measured by double-antibody radioimmunoassay. Limit of sensitivity for the assay is 2.1 µU/ml, and inter-assay and intra-assay variables are 3.4% and 2.7% respectively at 25 µU/ml.
C-peptide was measured using a solid-phase two-site chemiluminescent immunometric assay and Immulite Analyzer (Siemens Healthcare Diagnostics Inc.) (intra-assay CV 3.9%). Calibration range is 0.1–15 ng/ml.
Molecular genetic testing
Family members were sent an Oragene™ DNA Self-Collection kit (DNA Genotek Inc., Ottawa, ON, Canada) to obtain a saliva sample for DNA. The IPF1 gene was amplified in two segments using PCR and the Elongase Amplification System (Invitrogen, Carlsbad, CA) with the following primers: exon 1, forward 5’-AAC GCC ACA CAG TGC CAA ATC-3’ and reverse 5’-TTA GTC CGA CCC GGG ATA ATC-3’; and exon 2, 5’-GTT GGG CTG CGT GGG TG-3’ and the reverse primer 5’-CCT GAG AGA GCG GGT TTT CC-3’. The PCR conditions were: 94 °C 1 minute, 35 cycles of 94 °C for 30 seconds, 60 °C for 30 seconds and 68 °C for 90 seconds; 68 °C for 10 minutes and 10 °C for storage. We used identical primers to sequence both strands of the two exons using a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA). The data analysis was done using the Mutation Surveyor software, version 3.0 (SoftGenetics, State College, PA).
To determine the extent of shared sequence conservation (i.e. identity-by-descent (IBD)) in the region of the IPF1 gene between the Kentucky-Michigan and Virginia probands, we genotyped DNA from the two probands on the Affymetrix Genome-Wide Human SNP Array 6.0, which includes probes for more than 906,600 SNPs. The data were pruned for minor allele frequency (>5% in HAPMAP CEU), missing data (only perfect call rate accepted) and linkage disequilibrium (intermarker distance >0.2 cM). Since the inheritance pattern of the disease is consistent with a rare recessive genetic model, standard methods of detecting extended haplotypes shared IBD are not applicable because they assume that the probability that two ostensibly unrelated individuals share both segments of a chromosome IBD is negligible. Given that these patients possess the same mutation in homozygous form, another method was developed to identify the shared region. An artificial family structure including two consanguineous loops was created to relate the unrelated probands (Figure A1), and test for regions in their genomes homozygous by descent. We conducted linkage analyses with the software program ALLEGRO [18] assuming a fully penetrant rare recessive model (f2 = 1; q = 0.0001), and using allele frequencies estimated from the HapMap CEU samples.
RESULTS
History of IPF1 mutation Pro63fsX60
The genetic studies of the Kentucky-Michigan and Virginia probands with pancreatic agenesis showed that they shared a single homozygous 2.5 Mb region (chr 13: 27,374,368 - 29,837,334 bp, NCBI build 36.1) (Figure A2). This region includes the IPF1 gene (chr 13: 27,392,168-27,398,451 bp). The size of this shared region suggests that the Pro63fsX60 mutation emerged in a recent ancestor common to both probands, and that a complex pedigree structure connects these two patients.
Michigan-Kentucky proband
Ultrasound examination at approximately one year of age revealed a very small amount of tissue in the region of the head of the pancreas that was interpreted as being pancreatic tissue (data not shown).
Parents and sister
The parents of the proband were heterozygous for the Pro63fsX60 mutation. The father (IV-23) was 23 years old with a BMI of 39 (Fig. 1B). He had a history of polyuria, polydipsia and thirst for 2 months at age 12 years and again for the last 3 months. Blood sugar levels have been 200–215 mg/dl since age 15 years. At age 17, he had postprandial blood glucose levels over 300 mg/dl, and once 461 mg/dl. He has been obese since age 12 years with maximum weight of 260 pounds. At age 13–14 years, while a member of his high school football team, he lost weight to 160 pounds while following a low caloric diet and a strenuous physical training program. The mother of the proband (IV-24), age 21 years, and BMI 41.8, has been obese since age 12 years. She was diagnosed with gestational diabetes at ages 17 and 20 years, respectively, with blood glucose levels of 235 and 200 mg/dl, respectively. She was treated with insulin during both pregnancies. The 3-year-old sister (V-4) is also a carrier. She has had postprandial blood glucose tests since the birth of her brother. Intermittently, blood sugar levels have ranged between 200 and 270 mg/dl while at other times they have been between 90 and 110 mg/dl.
Michigan-Kentucky pedigree
We identified 110 individuals in the 5-generation pedigree through interviews with members of generations III and IV. Of these 110 subjects, 53 were genotyped. The pedigree includes 34 subjects with a known diagnosis of diabetes, 18 of whom have been genotyped for the Pro63fsX60 mutation (Fig. 1A and 1B). One subject (the proband) is homozygous for the mutation (V-5) and 9 are heterozygous. Eight diabetic subjects do not carry the mutation and are classified as having type 2 diabetes. Four diabetic subjects can be presumed to be carriers of the mutation (I-4, II-2, II-12, and III-7). Ten subjects who are heterozygous carriers of the mutation have not been tested by blood glucose determinations (Fig. 1 and 2). The ages at diagnosis of MODY4 range from intermittent diabetes at age 2-1/2 years, to the teens and up to the early forties. Where information is available, 14 diabetic subjects have been treated with oral hypoglycemic agents and 9 with insulin [(4 are mutation carriers (II-8, III-6, III-8, V-5) and two are presumptive carriers (I-4, III-7)]. Coronary heart disease at an early age has been reported in II-7 and in 3 siblings of generation III (III-3–5) of whom 2 are known mutation carriers (Fig. 1A).
Obesity is a prominent feature of the this pedigree (Fig. 1). It has been reported in 26 subjects of generations I–IV. As all but 5 subjects were unavailable for examination, it is recorded on the basis of a relative’s statement of the presence of gross obesity, without data for height, weight, or BMI. Most likely there is an underestimation of its presence. It occurs in subjects regardless of whether they are carriers of the IPF1 mutation (N=10), presumptive carriers (N=4), non-carriers (N=8) or non-genotyped subjects (N=7). Obesity has not been observed in children under the age of 12 years.
Oral glucose tolerance tests
We were able to carry out glucose tolerance tests on five members of the Michigan-Kentucky pedigree. Other family members did not live in close proximity to the University of Michigan and testing of additional subjects was, therefore, not possible. None of these five subjects had been treated with oral hypoglycemic agents before these tests. As shown in Table 1, both the proband’s father (IV-23) and mother (IV-24) had fasting hyperglycemia and elevated post-glucose plasma glucose levels. Both had greatly elevated insulin and C-peptide levels in the fasting state (insulin 98 and 39 µU/ml, respectively), and high postprandial levels. The proband’s sister (V-4) who has intermittent diabetes had, at age 2 ½ years, a high normal fasting plasma glucose level of 104 mg/dl (similar to what was found in both parents of the Swiss patient with permanent neonatal diabetes due to IPF-1 deficiency [16]) and an elevated ½ hour glucose level. Her insulin levels were in the normal range for a 3 year old child [19] (Table 1). The proband’s maternal uncle (IV-25), who has a BMI of 31.8 and does not carry the IPF mutation, had a glucose tolerance test in the diabetic range with elevated fasting and post-glucose insulin levels. The paternal uncle (IV-22), also not a carrier, with a BMI of 31.4, had a normal glucose tolerance test, a similarly fasting insulin level but considerably higher post-glucose levels than did subject IV-25.
Table 1.
Oral Glucose Tolerance Tests in Members of the Michigan-Kentucky Pedigree with and without the IPF1 Mutation Pro63fsX60 (1.75 gm glucose per kg ideal body weight)
| ID# | Relationship to Proband |
Age (years) |
Weight (kg) |
BMI (kg/m2) |
IPF1 Mutation |
Oral Glucose Tolerance Test (hour) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | ½ | 1 | 1½ | 2 | HbA1c | |||||||
| IV-23 | Father | 23 | 116 | 39 | Yes | Glucose (mg/dl) | 155 | 283 | 305 | 282 | 265 | 6.1 |
| Insulin (µU/mL) | 98 | 229 | 246 | 261 | 284 | |||||||
| C-peptide (ng/mL) | 8.0 | 14.6 | 17.6 | 19.2 | 21.4 | |||||||
| IV-24 | Mother | 21 | 110 | 41.8 | Yes | Glucose (mg/dl) | 147 | 241 | 219 | 152 | 160 | 5.7 |
| Insulin (µU/mL) | 39 | 164 | 135 | 66 | 100 | |||||||
| C-peptide (ng/mL) | 5.0 | 14.9 | 15.8 | 13.4 | 13.7 | |||||||
| V-2 | Sister* | 2.5 | 14 | 17.7 | Yes | Glucose (mg/dl) | 104 | 204 | 141 | 135 | 116 | 5.4 |
| Insulin (µU/mL) | 6 | 63 | 26 | 34 | 19 | |||||||
| C-peptide (ng/mL) | 0.7 | 6.3 | 6.1 | 5.6 | 3.9 | |||||||
| IV-25 | Maternal uncle |
20 | 103 | 31.8 | No | Glucose (mg/dl) | 99 | 195 | 207 | 210 | 215 | 5.4 |
| Insulin (µU/mL) | 34 | 167 | 135 | 186 | 277 | |||||||
| C-peptide (ng/mL) | 3.0 | 11.1 | 12.5 | 15.4 | 17.7 | |||||||
| IV-22 | Paternal uncle | 19 | 90 | 31.4 | No | Glucose (mg/dl) | 108 | 159 | 160 | 112 | 111 | 5.7 |
| Insulin (µU/mL) | 34 | 280 | 284 | 248 | 151 | |||||||
| C-peptide (ng/mL) | 2.4 | 14.6 | 18.3 | 15.5 | 13.6 | |||||||
In last 9 months, many 2-hr post-dinner blood glucose levels ranged between 200–270 mg/dL while others were 96–100 mg/dL.
Note that HbA1c concentrations for IV-23, IV-24, and IV-25 are not diagnostic for diabetes (≤ 6.5%) while FPG or OGTT values are.
The lipid profile for the mother was as follows: cholesterol 169 mg/dl, triglycerides 74 mg/dl, high density lipoprotein cholesterol 40 mg/dl and low density lipoprotein cholesterol 114 mg/dl and those of the type 2 diabetic uncle were 199, 114, 38, and 138 mg/dl, respectively.
DISCUSSION
The clinical phenotype of the proband including severe neonatal diabetes and exocrine pancreatic insufficiency suggested a diagnosis of pancreatic agenesis [6,7,14,16]. However, an ultrasound study at 12 months of age suggested a very small amount of tissue in the region of the head of the pancreas that was interpreted as possible pancreatic tissue. Dr. William Clarke, together with a radiologist, recently reexamined the original ultrasound performed in the Virginia proband in 1991. The report states “There may be a small amount of pancreatic tissue in the region of the head but no definite pancreatic tissue in the region of the body or tail (William Clarke, personal communication). Thus, pancreatic agenesis in both probands may not be complete. Further studies of both subjects may provide insight as to the biological role of IPF1 in pancreatic development in humans. In this regard, it is interesting to note that mice lacking IPF1 have a dorsal pancreatic bud that undergoes limited proliferation. This outgrowth does not contain insulin- or amylase-positive cells but glucagon-expressing cells are found [20]. These findings support the interpretation of the ultrasound examinations above.
The Michigan-Kentucky pedigree, includes members with permanent neonatal diabetes, MODY4 and type 2 diabetes. Among the presently known diabetic subjects who have been genotyped, there are 10 diabetic subjects who carry the IPF1 mutation (and 4 who were not genotyped but in whom it can be presumed; I-4, II-2, II-12, III-7) and 8 subjects who do not and are classified as type 2 diabetic patients. In addition, 2 diabetic subjects who have not been genotyped (III-12, and III-15) are most likely type 2 diabetic subjects (Fig. 1B) as none of their siblings carried the IPF mutation. The presence of obesity in the majority of the adolescent and adult MODY4 and type 2 diabetic subjects suggests that the family harbors an obesity predisposing gene(s), the nature of which is unknown, in addition to environmental/behavioral risk factors.
The proband’s father (IV-23) and mother (IV-24) had high insulin concentrations during the oral glucose tolerance tests. These obese MODY4 subjects are characterized by fasting and post glucose hyperinsulinemia (Table 1). Their responses are very similar to what is seen in the mildly diabetic, obese, but mutation-negative brother of the mother (IV-25). The hyperinsulinemia seen in these MODY4 subjects is most likely a consequence of the obesity and associated insulin resistance as seen in type 2 diabetic subjects. The hyperinsulinemia does indicate that there is at least a partial compensatory response in these subjects and it is not materially compromised, at least initially, due to the presence of the Pro63fsX60 mutation in IPF1. In view of the small number of subjects available for testing (Table 1), further comparison between mutation carrier and mutation negative diabetic subjects is not possible. In contrast to the robust insulin response to the insulin resistance of obesity in these Pro63fsX60 diabetic carriers is the finding in IPF1 haplo-insufficient mice that show a limited compensatory insulin response to insulin resistance [21]. This suggests that hemizygous man is different from hemizygous mice.
The phenotype of Pro63fsX60 carriers in the Michigan-Kentucky pedigree differs from that observed in the Virginia pedigree where a decreased fasting insulin level and a decreased insulin response to glucose were found in 7 non-obese members [15]. The results suggests that the beta cell defect in non-obese Pro63fsX60 diabetic carriers is due to a functional defect at least early in the natural history of the disease.
Previous studies suggest that the mutant protein may act in a dominant-negative fashion [22]. This particular mutation may be unique and the net effect of IPF1 expression and function may be greater than that expected due to simple hemizygosity. Perhaps this accounts for the phenotypic differences between carriers in our family compared to those of another family with different mutations (16).
In general, obesity is uncommon in MODY diabetic subjects [1]. However, obesity and hyperinsulinemia occur sporadically in some MODY families. In the RW HNF-4α/MODY1 pedigree, obesity is present in two diabetic members (mother IV-25, with insulin requiring diabetes and daughter, age 7, fasting plasma glucose 203 mg/dl, HbA1c 9.7%, fasting immunoreactive insulin 27 µU/ml) [23]. A 13.5 year old obese Israeli diabetic boy (BMI 29.8, blood glucose 417 mg/dl, HbA1c 11.8%) with a mutation in the HNF-1α/MODY3 gene had an elevated fasting C-peptide level of 1,489 pmol/l [24]. In a Czech study, diabetes was associated with a mutation in the NeuroD1/MODY6 gene in 2 families, of whom all members were obese and probands had high fasting C-peptide levels [25]. Also, in the original description of NeuroD1/MODY6 form of diabetes [9], diabetic members of one of two families were obese and had “relatively high insulin levels”. Thus, obesity and obesity-induced insulin resistance and hyperinsulinemia have been observed in subjects with MODY1, MODY3, MODY4, and MODY6 and thus may be a general phenomenon. Moreover, it suggests that beta-cell compensation is not compromised in these individuals, at least at the time they were studied. . Obese MODY and type 2 diabetic patients are phenotypically indistinguishable and can only be differentiated by genetic studies [24].
In the Virginia MODY4 pedigree, the average age at diagnosis of diabetes at 35 years (range 17–67 years) suggests that expression of diabetes occurs at a later age in MODY4 than observed for other subtypes of MODY [7]. As MODY may be a largely asymptomatic disease in younger decades, particularly in non-obese subjects, age of diagnosis has limited meaning as hyperglycemia may exist for years to decades before a diagnosis is made. Age at diagnosis of MODY4 in the Michigan-Kentucky family ranges from intermittent diabetes, at age 2-1/2 years, to the teens and up to the early forties. The occurrence of obesity with an IPF1 mutation may be a contributing factor for the earlier diagnosis of diabetes in these members as compared to those in the Virginia pedigree.
Supplementary Material
ACKNOWLEDGMENTS
Funded by the Biochemistry Core of the Michigan Diabetes Research and Training Center (DK020572) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD) , U.S. Public Health Service Grant M-01-RR-00042 to the General Clinical Research Center and CTSA Grant UL1RR024986, all at The University of Michigan. Work at the University of Chicago was funded by the U.S. Public Health Service Grant DK020595 (University of Chicago Diabetes Research and Training Center) and by a gift from the Kovler Family Foundation. We thank Dr. William L. Clarke for providing the sputum specimen from the Virginia proband, Dr. Doris Stoffers for her helpful advice and Ms. Kinga Skowron for her help in preparing the figures.
Abbreviations
- MODY
maturity-onset diabetes of the young
- IBD
identity-by-descent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971–980. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 2.Hattersley AT, Turner RC, Permutt MA, et al. Linkage of type 2 diabetes to the glucokinase gene. Lancet. 1992;339:1307–1310. doi: 10.1016/0140-6736(92)91958-b. [DOI] [PubMed] [Google Scholar]
- 3.Froguel P, Zouali H, Vionnet N, et al. Familial hyperglycemia due to mutations in glucokinase: definition of a subtype of diabetes mellitus. N Engl J Med. 1993;328:697–702. doi: 10.1056/NEJM199303113281005. [DOI] [PubMed] [Google Scholar]
- 4.Yagamata K, Furata H, Oda N, et al. Mutations in the hepatocyte nuclear factor-4α gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 5.Yamagata K, Oda N, Kaisaki PJ, et al. Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3) Nature. 1996;384:455–458. doi: 10.1038/384455a0. [DOI] [PubMed] [Google Scholar]
- 6.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 7.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 8.Horikawa Y, Iwasaki N, Hara M, et al. Mutation in hepatocyte nuclear factor-1beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 9.Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23:323–328. doi: 10.1038/15500. [DOI] [PubMed] [Google Scholar]
- 10.Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38:54–62. doi: 10.1038/ng1708. [DOI] [PubMed] [Google Scholar]
- 11.Molven A, Ringdal M, Nordbo AM, et al. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131–1136. doi: 10.2337/db07-1467. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse IPF1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright NM, Metzger DL, Borowitz SM, Clarke WL. Permanent neonatal diabetes mellitus and pancreatic exocrine insufficiency resulting from congenital pancreatic agenesis. Am J Dis Child. 1993;147:607–609. doi: 10.1001/archpedi.1993.02160300013005. [DOI] [PubMed] [Google Scholar]
- 15.Clocquet AR, Egan JM, Stoffers DA, et al. Impaired insulin secretion and increased insulin sensitivity in familial maturity-onset diabetes of the young 4 (insulin promoter factor 1 gene) Diabetes. 2000;49:1856–1864. doi: 10.2337/diabetes.49.11.1856. [DOI] [PubMed] [Google Scholar]
- 16.Schwitzgebel VM, Mamin A, Brun T, et al. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88:4398–4406. doi: 10.1210/jc.2003-030046. [DOI] [PubMed] [Google Scholar]
- 17.Thomas IH, Saini NK, Adhikari A, et al. Neonatal diabetes mellitus with pancreatic agenesis in an infant with homozygous IPF1 Pro63fsX60 mutation. Pediatric Diabetes. 2009;10:492–496. doi: 10.1111/j.1399-5448.2009.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudbjartsson DF, Jonasson K, Frigge ML, Kong A. Allegro, a new computer program for multipoint linkage analysis. Nat Genet. 2000;25:12–13. doi: 10.1038/75514. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbloom AL, Wheeler L, Bianchi R, Chin FT, Tiwary CM, Grgic A. Age-adjusted analysis of insulin responses and abnormal glucose tolerance tests in children and adolescents. Diabetes. 1975;24:820–828. doi: 10.2337/diab.24.9.820. [DOI] [PubMed] [Google Scholar]
- 20.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoffers DA, Stanojevic V, Habener JF. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J Clin Invest. 1998;102:232–241. doi: 10.1172/JCI2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fajans SS, Bell GI. Maturity-onset diabetes of the young: A model for genetic studies of diabetes mellitus. In: LeRoith D, Taylor SF, Olefsky JM, editors. Diabetes Mellitus: A Fundamental and Clinical Text. Second Edition. Chapter 70. Philadelphia, PA: J.P. Lippincott Company; 2000. pp. 691–705. [Google Scholar]
- 24.Weintrob N, Stern E, Klipper-Aurbach, Philip M, Gat-Yablonski G. Childhood obesity complicating the differential diagnosis of maturity-onset diabetes of the young and type 2 diabetes. Pediatric Diabetes. 2008;9:60–64. doi: 10.1111/j.1399-5448.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonsorcikova L, Pruhova S, Cinek O, et al. Autosomal inheritance of diabetes in two families characterized by obesity and a novel H241Q mutation in NEUROD1. Pediatric Diabetes. 2008;9:367–372. doi: 10.1111/j.1399-5448.2008.00379.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.