Abstract
AIM: To compare the effects of four Bifidobacteria strains (Bifidobacteria L66-5, L75-4, M13-4 and FS31-12, originated from normal human intestines) on weight gain, lipid metabolism, glucose metabolism in an obese murine model induced by high-fat diet.
METHODS: Forty-eight Sprague-Dawley rats were randomly divided into six groups. Control group received standard chow, model group received high-fat diet, and intervention groups received high-fat diet added with different Bifidobacteria strains isolated from healthy volunteers’ fresh feces. All rats were executed at the 6th weekend. Body weight (BW), obese indexes, oral glucose tolerance test, serum and liver lipid and serum insulin (INS) were tested. Liver lipid deposition was classified pathologically.
RESULTS: Compared with the model group, B. M13-4 improved BW gains (264.27 ± 26.91 vs 212.55 ± 18.54, P = 0.001) while B. L66-5 induced a decrease in BW (188.47 ± 11.96 vs 212.55 ± 18.54, P = 0.043). The rest two strains had no significant change in BW. All the four strains can reduce serum and liver triglyceride and significantly alleviate the lipid deposition in liver. All strains showed a trend of lowing serum and liver total cholesterol while B. L66-5 and B. FS31-12 did so more significantly. In addition, all the four strains showed no significant differences in serum INS and glucose level.
CONCLUSION: The response of energy metabolism to administration of Bifidobacteria is strain dependent. Different strains of Bifidobacteria might drive different directions of fat distribution.
Keywords: Bifidobacterium, Obesity, Serum lipid, Body weight
INTRODUCTION
Obesity is becoming a global epidemic, and a major contributor to increased incidence of serious chronic diseases such as type 2 diabetes, cardiovascular diseases, hepatic and skeletal muscle insulin resistance, and certain forms of cancer[1]. Several strategies have been used to treat obesity, including diet control, exercise, behavior therapy, medications and surgery. However, the diet control and exercise are too hard to be strictly carried on. Also, undesirable side effects of drugs have restricted their therapeutic use. Fortunately, some recent interesting researches on obesity are helping to validate a new approach to control this medical disorder.
Recent researches have demonstrated that obesity may lead to the composition shift of gut microbiota in both mice and humans. Fewer Bacteroidetes and more Firmicutes are colonized in the gut of obese people and animals, shown by some 16S-rRNA-gene-sequence-based comparative surveys of gut bacteria[2,3]. Weight loss makes the ratio of Bacteroidetes to Firmicutes up-regulated in humans[3]. Dietary inclusion of Lactobacillus, which belongs to Bacteroidetes and/or Bifidobacterium, can improve obesity both in murine model and humans[4-6]. The intentional manipulation of community structure of gut microbiota may be a novel strategy to treat obesity.
Bifidobacterium is one of the most numerous “probiotic” in mammalian gut among commensal bacteria, which belongs to Actinomycetes and also a kind of lactic acid bacteria. It can help Bacteroides degrade polysaccharides[7] and inhibit exogenous cholesterol absorption from the small intestine[8]. Although most researches focus on the hypocholesteremia effect of Lactobacillus[4,9-11], a study showed that a strain of Bifidobacterium longum exhibited a more significant effect in lowering serum total cholesterol than a mixed culture of Streptococcus thermophilus and Lactobacillus delbrueckii subsp.bulgaricus (SL) both in rats and humans[6]. In contrast, probiotics VSL#3 (a combination of Streptococcus thermophilus and several species of Lactobacillus and Bifidobacteria) was found to increase liver fat with no significant changes in comprehensive metabolic panel or in body weight (BW) in a clinical research[12]. These results suggested that different strains may have their specificities in colonization or function. Furthermore, Bifidobacterium longum can induce an expansion of Bacteroides. Thetaiotaomicron’s substrates range under co-colonized condition, while another Bifidobacterium species, B. animalis showed no significant impact[7]. Bifidobacteria preparations are safe, widely-used, and well-tolerant. Thus, some specific strains of Bifidobacteria related to lipid metabolism and BW may be a potential therapeutic candidate for management of obesity.
In order to screen out more efficient strains in obese management among different strains of Bifidobacteria originated from normal human intestines, we established an obese model in rats induced by high-fat diet, and compare their effects on weight gain, lipid metabolism and glucose metabolism. Several strains of Bifidobacteria showed dependant effects in obese control in this study, thus would act as potential therapeutic candidates.
MATERIALS AND METHODS
Preparation of bacterial cultures
Four Bifidobacteria strains were isolated from healthy volunteers’ fresh feces in our facility, and identified according to biochemical characteristics (API 20A biochemical strip, BioMerieux sa). The four strains were named B. L66-5, B. L75-4, B. M13-4 and B. FS31-12, respectively, and maintained at -80°C in our laboratory. The strains were all grown in MRS medium under anaerobic condition. When measured at 600 nm (A600), the exponential and stationary growth reached an optical density of 1-2 and 1-4.5, respectively. The correspondence between absorbance and bacterial counts was established (1 mL of culture at A600 = 1 contains about 108 colony-forming units, CFU). The number of CFU administered was routinely verified by plating. Strains were harvested by centrifugation at 2000 × g for 20 min, washed twice with neutral saline, and resuspended at 1 × 108 CFU/mL concentration in neutral saline, and 0.4 mL bacterial solution was administered to each rat by intragastric gavage.
Animals and diet
Forty-eight 3-wk-old male Sprague-Dawley (SD) rats were purchased from Slaccas Lab Animal Ltd, Shanghai, China, weighing 50-70 g. The animals were housed in individual stainless steel cages under standard conditions (20-22°C, 50%-55% humidity, 12/12 h dark/light cycle). The rats were fed with a solid standard chow [DongChuang Lab Animal Ltd., Hunan, China, including 17.53% (wt/wt) protein, 6.08% (wt/wt) fat, and 59.98% (wt/wt) carbohydrate, calories (1250 kj/100 g)] for 1 wk. After this adaptation period, 48 rats were randomly assigned to six dietary treatment groups, 8 rats in each group. Each rat was fed diet 13 g/d from the 1st wk, which was then increased by 2 g/d per week. Control group was fed on a standard chow, and the other groups were fed on a high-fat diet (HFD)(DongChuang Lab Animal Ltd, HuNan, China, 16.52% (wt/wt) protein, 25.17% (wt/wt) fat, and 56.66% (wt/wt) carbohydrate, calories (1810 kj/100 g). Each interventional group was administered with B. L66-5, B. L75-4, B. M13-4 and B. FS31-12, respectively. Model and control groups were given equivalently 0.9% saline. All rats had free access to water and were supplied with bacteria liquid or 0.9% saline by intragastric gavage at a fixed time every day. The assigned diets were given to the rats for 6 wk. BW was measured weekly and caloric intake was accounted finally. All the rats were sacrificed by ether for further studies. The care and use of animals followed our institutional and national guidelines and all experimental procedures involving animals were approved by the ethics committee of the Central South University.
Oral glucose tolerance test
At the 6th weekend after dietary treatment, the rats were deprived of diet for 12 h, then given glucose solution (5 g/kg) by intragastric gavage. Blood samples were drawn from tails to do the oral glucose tolerance test (OGTT) test. Serum glucose was measured at 0, 30, 60, 90 and 120 min by fast blood glucose meter (OneTouch-II, Johnson, America).
Assay for weight gain and fat index
After the OGTT test, the rats were fasted for 12 h and euthanized with ether. Liver, retroperitoneal (RET) and epididymal (EPI) white adipose tissues were immediately removed and weighed.
Measurement: (1) Lee’s index: (bodyweight)1/3 × 103/stem length(length from nasal tip to anus); (2) boby fat index: viscera fat/body weight ratio (viscera fat includes RET and EPI white adipose tissues); and (3) liver index: liver/body weight ratio.
Assay for serum triglycerides, total cholesterol and insulin
Blood samples were collected immediately in sterile tubes by heart puncture. Serum was collected by centrifugation at 2000 × g for 15 min at 4°C. The serum samples were analyzed for triglycerides (TG), total cholesterol (TCH) and insulin according to protocols of triglycerides fluid monoreagent (GPO-PAP) and cholesterol oxidase peroxidase-amidopyrine (CHOD-PAP) analysis (CHOD-PAP) and radioimmunoassay (Dongou Bio-Tech Ltd, Wenzhou and 3v Bio-Tech Ltd, Weifang, China).
Assay for liver lipid
Liver tissues (100 mg) were pulverized in liquid nitrogen to prepare 10% tissue homogenate. These homogenates were extracted under 4°C with chloroform: methanol (2:1) for 48 h, then centrifuged at 12 000 × g/min for 15 min at 4°C. The concentrations of TG and TCH in supernatant were determined according to the protocols.
Liver histopathology
Liver tissues were routinely fixed in paraffin-embedded sections, and stained with hematoxylin and eosin (HE). To detect lipid droplets, sections were stained with Sudan IV and counterstained with hematoxylin. Each histologic section was observed for 5 fields of high power field. The classification and degree of fatty deposition are as follows: mild fatty degeneration (+): fatty hepatocytes occupying 30%-50% of the hepatic parenchyma, moderate fatty degeneration (++): 50%-75%, and severe fatty degeneration (+++): > 75%.
Statistical analysis
All data were presented as the mean ± SD. Univariate analysis of variance test was applied to determine the statistical significance of the difference among the groups, using the General Linear Models procedure of SPSS15.0 (SPSS Inc., Chicago, IL, USA). Some rank/frequency data were analyzed by nonparametric test, with the significance level set at P < 0.05.
RESULTS
Caloric intake, BW increment and obesity indexes in high-fat diet treated rats administered with different strains of Bifidobacteria
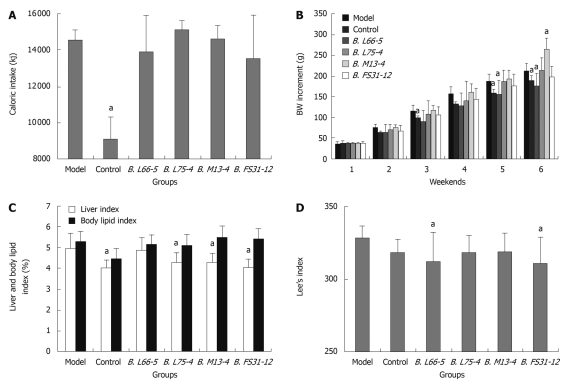
No rat died throughout the study. Caloric intake in control group (standard chow) was significantly lower than that of model group (HFD) (9055.68 ± 1246.62 kj vs 14562.32 ± 541.55 kj, P < 0.05). However, among model group and the four Bifidobacteria groups which were fed on high-fat diets, caloric intake showed no significant difference (P > 0.05). These indicate that the different groups of HFD rats had a similar caloric consumption (Figure 1A). The weight of all groups was increased every week, especially the model group and group B. M13-4. At the end of the 3rd wk, the BW increment in group B. L66-5 was significantly less than in model group (90.26 ± 27.06 g vs 115.75 ± 15.13 g, P < 0.05), and further increased (156.05 ± 33.19 g vs 186.98 ± 16.25 g, P < 0.05) at the end of 5th wk. At the 6th weekend, weight increment in group B. L66-5 was much less than the model group (175.19 ± 31.24 g vs 212.55 ± 18.54 g, P < 0.05). However, the BW increment was significantly higher in group B. M13-4 than in the model group (264.27 ± 26.91 g vs 212.55 ± 18.54 g, P < 0.05). No significant change of BW was found in group B. L75-4 and group B. FS31-12 compared with the model group (Figure 1B).
Figure 1.
Caloric intake, body weight increment and obesity indexes in high-fat diet treated rats with different strains of Bifidobacteria. A: Sum of caloric intake in all groups at the 6th weekend; B: Body weight (BW) increment in all groups for 6 wk; C: Liver index and body lipid index in all groups; D: Lee’s index in all groups. Results are shown as mean ± SD (n = 8). aP < 0.05 vs model group.
Both the Lee’s and liver indexes were decreased in all interventional groups. The liver index was obviously lower in control, B. L75-4, B. M13-4 and B. FS31-12 groups than in model group (4.04% ± 0.36%, 4.27% ± 0.50%, 4.27% ± 0.47%, 4.04% ± 0.36% vs 4.98% ± 0.72%, P < 0.05). The body lipid index showed no significant differences among the groups (P > 0.05) (Figure 1C). The Lee’s index was significantly lower in groups B. L66-5 and B. FS31-12 than in model group (312.65 ± 20.18, 311.22 ± 17.52 vs 327.98 ± 8.90, P < 0.05, Figure 1D).
Effects of Bifidobacteria strains on glucose and lipid metabolism in high-fat diet treated rats
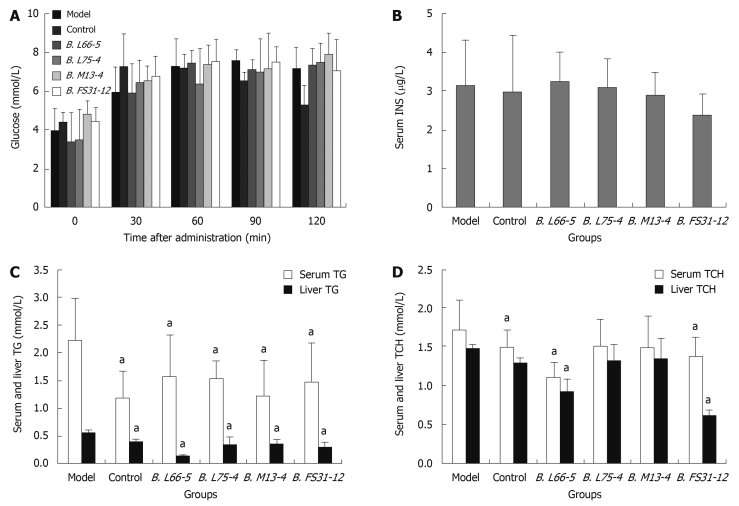
Effect of four Bifidobacteria strains on serum glucose: In control group, the peak of serum glucose appeared at 30 min and returned to normal at 120 min when glucose was significantly lower than in model group (5.32 ± 0.98 vs 7.17 ± 1.08, P < 0.05). In high-fat diet fed groups, the peak was postponed to 60-120 min and lasted longer than that in control group. In these groups, the serum glucose did not return to normal until 120 min. The serum glucose at 5 time points showed no significant differences among model group and interventional groups (P > 0.05) (Figure 2A).
Figure 2.
Effects of Bifidobacteria strains on glucose and lipid metabolism in high-fat diet treated rats. A: Effect of four Bifidobacteria strains on serum glucose; B: Serum insulin (INS) concentration in all groups; C: Serum and liver triglycerides (TG) in all groups; D: Serum and liver total cholesterol (TCH) in all groups. Results are shown as mean ± SD (n = 8), aP < 0.05 vs model group.
Changes of serum insulin, TG and TCH and liver TG and TCH: The serum insulin (INS) showed no significant differences among the groups (P > 0.05) (Figure 2B). The TG levels in serum decreased significantly in all intervention groups compared with the model group (1.59 ± 0.73, 1.54 ± 0.30, 1.23 ± 0.65, 1.47 ± 0.70 vs 2.23 ± 0.76, P < 0.05), so did the TG levels in supernatant of liver homogenate in those groups (0.13 ± 0.02, 0.31 ± 0.16, 0.34 ± 0.08, 0.29 ± 0.10 vs 0.56 ± 0.04, P < 0.05) (Figure 2C). In groups B. L66-5 and B. FS31-12, the TCH level in serum and liver were obviously lower than in model group (1.11 ± 0.18, 1.37 ± 0.26 vs 1.72 ± 0.38, P < 0.05 and 1.27 ± 0.08, 0.62 ± 0.6 vs 1.47 ± 0.05, P < 0.05, Figure 2D), while the other strains also showed a downward trend (P > 0.05).
Hepatic lipid deposition in high-fat diet induced rats treated with different strains of Bifidobacteria
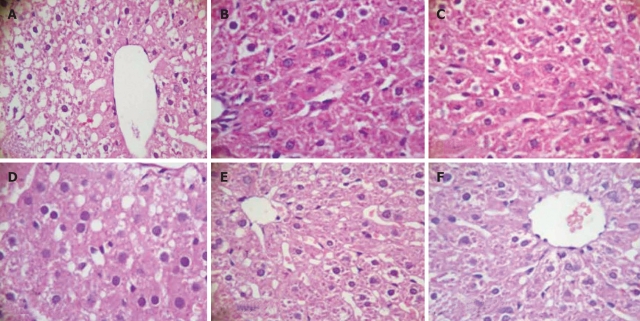
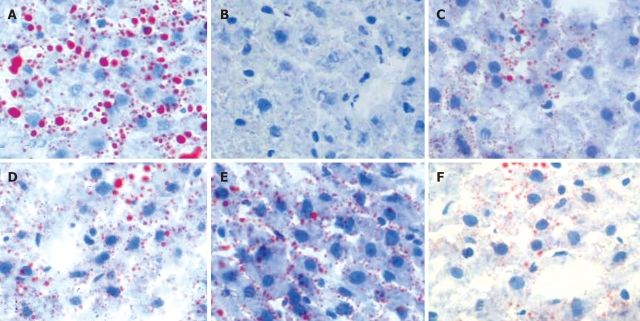
Moderate degree of microvesicular steatosis was observed in model group. No fatty vacuolization was found in groups B. L66-5 and B. FS31-12. Hepatocyte steatosis was obviously alleviated in groups B. L75-4 and B. M13-4 compared with model group (χ2 = 30.754, P = 0.000) (Table 1 and Figure 3). Sudan IV staining showed that a plenty of scarlet lipid droplets deposited in the livers of model group, which confirmed the results shown in HE staining. Expectedly, lipid droplets were obviously decreased in all intervention groups compared with the model group, especially in groups B. L66-5 and B. FS31-12 (Figure 4).
Table 1.
Degrees of liver lipid deposition in all groups
| Groups | n |
Degree of fatty deposition1 |
|||
| - | + | ++ | +++ | ||
| Model | 8 | 0 | 7 | 1 | 0 |
| Control | 8 | 8 | 0 | 0 | 0 |
| B. L66-5 | 8 | 8 | 0 | 0 | 0 |
| B. L75-4 | 8 | 6 | 2 | 0 | 0 |
| B. M13-4 | 8 | 5 | 3 | 0 | 0 |
| B. FS31-12 | 8 | 8 | 0 | 0 | 0 |
The standard of classification and score for the degree of lipid deposition in liver was referred to the liver histopathology mentioned in MATERIALS AND METHODS.
Figure 3.
Hepatic tissue sections of each group in HE staining (HE, light microscope, × 400). A: Model; B: Control; C: B. L66-5; D: B. L75-4; E: B. M13-4; F: B. FS31-12.
Figure 4.
Hepatic tissue sections of each group in Sudan IV-staining (Sudan IV, light microscope, × 400), and lipid droplets are scarlet. A: Model; B: Control; C: B. L66-5; D: B. L75-4; E: B. M13-4; F: B. FS31-12.
DISCUSSION
To demonstrate the relationship between the administration of different strains of Bifidobacteria and the status of glucose and lipid metabolism, we established a murine obese model based on a 6-wk administration of high-fat diet (HFD), characterized by a significant increase of BW gain, fat mass, TG and TCH in serum and liver, and obesity indexes. Our results demonstrated that administration of the four Bifidobacteria (Bifidobacteria L66-5, L75-4, M13-4 and FS3-1-1-2) played a role in reducing serum and liver TG and TCH, as well as liver lipid deposition. Furthermore, to our surprise, among the four strains of Bifidobacteria, two contrary results were yielded in BW changes: B. M13-4 showed a significant increase in BW while B. L66-5 showed a decrease in BW based on a similar caloric consumption. Bifidobacteria L75-4 and FS3-1-1-2 strains had no significant effect in BW change. However, all the four strains showed no significant influence on serum INS and glucose level. Based on our results, different Bifidobacteria strains lead to different responses of energy and fat metabolism in rat models.
Recent researches illustrated that gut microbiome should be considered as a set of genetic factors that, together with host genotype and life style (energy intake and expenditure), contribute to the pathophysiology of obesity. Turnbaugh et al[13] observed microbiota samples of obese mice, after transferring the microbiota to germ-free lean mice, significant fat gain was obtained and calorie extraction improved. BW increase has also been observed after administration of Bifidobacterium. In a preterm infant study in 1997, the authors added Bifidobacterium breve (about 0.5 × 109 live bacteria) to very low-birth weight infant formula, and their BW gain became significantly greater than in control group after administration for 4 wk[14]. In other cultures, Lactobacillus rhamnosus GG (1 × 107 cfu/g) also led to a weight growth in term infants after being supplemented to formulas for 4 mo[15]. However, Bifidobacterium longum[16], Bifidobacterium lactis[17,18], and Bifidobacteria combined with several species of lactobacilli plus fructooligosaccharides[19] have not shown any effects. Their similar concentrations and intervention periods indicated different effects in vivo. In this study, B. M13-4 strain can decrease serum and liver TG, TCH and liver index, while no apparent changes were found in body lipid index and Lee’s index with an obvious BW gain. It suggests that B. M13-4 alleviated lipid deposition in liver although more fat was accumulated in the body. The mechanisms may be complex: (1) Intestinal microbiota can help the host to digest polysaccharides and absorb monosaccharides and short-chain fatty acids, which finally converse to lipids[20]; (2) Gut microbiota can modulate some signal pathways associated with energy balance in the gut epithelium: Gpr41, a short-chain fatty-acid binding G protein-coupled receptor, and peptide tyrosine tyrosine[21]; (3) Bifidobacteria can also help the host to eradicate Campylobacter[22] or Eandida and Enterococcus[23] to stabilize their intestinal flora; and (4) The amount of visceral fat is positively correlated with the insulin sensitivity[24,25]; the possible effect in improving insulin sensitivity to alleviating visceral adiposity of probiotics is limited in our study and worth further studies. Host colonized by B. M13-4 absorbed more fat and transmitted them into body fat. This may contribute to the patients with fat/energy malabsorption. We should also reappraise the probiotics use in healthy and obese people for their potential effects such as fat/energy over-absorptions and weight over-growth.
To our surprise, rats colonized by B. L66-5 showed a weight loss. The opposite outcomes in B. M13-4 and B. L66-5, and strains showing no effect in BW, including B. L75-4 and B. FS3-1-1-2, and other strains, including Lactobacillus acidophilus ATCC 43121[4], Lactobacillus gasseri SBT2055[5] and Bifidobacterium longum[6], VSL#3[12], Lactobacillus reuteri[26], may result from different interactions between strains and intestinal microbia, inappropriate dosage, variability in end-points, and subjects. Anyhow, B. L66-5 was specific in regulating fat harvest and utilization, and may be a new therapeutic candidate for weight control.
All the four strains showed their effects in reducing serum TG and TCH, especially the strain B. L66-5. Administration of B. L66-5 decreased the serum and liver TG and TCH and BW, and alleviated liver lipid deposition. Recent studies showed that probiotics had hypocholesteremia effects in both rat and human, including Bifidobacterium longum[6], Lactobacillus acidophilus ATCC 43121[4], Lactobacillus plantarum MA2[9], Lactobacillus gasseri[27], Lactobacillus reuteri[26,28], Bacillus polyfermenticus SCD[29], etc. Some specific probiotics strains could reduce serum TCH and TG, and increase the ratio of high-density lipoprotein/low-density lipoprotein (HDL/LDL). The mechanisms involved may be as follows: (1) assimilation of cholesterol by bacterial cells; (2) deconjugation of bile acids by bacterial acid hydrolyses (reduces cholesterol reabsorption, increases cholesterol excretion of deconjugated bile salts, and increases cholesterol uptake by low-density lipoprotein receptor pathway in the liver as a compensatory response); (3) cholesterol binding to bacterial cell walls; and (4) inhibition of hepatic cholesterol synthesis and/or redistribution of cholesterol from plasma to the liver through the action of short-chain fatty acids, the end products of carbohydrate fermentation in the gut[30]. Furthermore, our results showed an individual amelioration effect in hepatic steatosis. The degree of fatty deposition in liver was obviously alleviated in all intervention groups as shown by liver histopathology. Probiotics VSL#3 also showed an effect in improving high-fat-diet induced hepatic steatosis in rats through lowering liver inflammatory signaling, increasing the expression of peroxisome proliferators-activated receptor α and hepatic natural killer T cell numbers[31,32]. However, the signaling capabilities of the four Bifidobacteria need further studies.
The four strains showed no significant differences in serum INS and glucose level. Similar negative results were also documented by Esposito et al[32] and Sato et al[5]. In our study, we fed the rats with high-fat diet for 6 wk, and none had a significant change of INS level, while in other HFD induced SD rats, insulin resistance did not occur until 8 mo or 36 wk[33,34]. So the next experimental period may prolong to 8 mo or longer.
In conclusion, we established a murine obese model based on a 6-wk administration of high-fat diet, which partially resembles the disorder of energy metabolism in human. The response of glucose and lipid metabolism to several strains of Bifidobacteria was evaluated. It was indicated that administration of the four strains of Bifidobacteria resulted in decreased serum/liver TG, serum/liver TCH, and hepatic steatosis, with no significant response to glucose and INS level. To our surprise, the data we presented demonstrated that administration of strain B. L66-5 led to BW loss, decreased serum TG/TCH and decreased hepatic adiposity, while administration of strain B. M13-4 resulted in significant increase of BW gain with alleviated hepatic adipose and serum/liver TG in rats. Thus, it is concluded that the response of energy metabolism to administration of Bifidobacteria is strain dependent. Different strains of Bifidobacteria might drive different directions of fat distribution. B. M13-4 action may generate a new conception: certain probiotics may promote BW gain by more effective fat absorption, and a cautious assessment is needed before probiotics therapy is given, especially in obese people. B. L66-5 might act as a new therapeutic probiotic candidate in controlling BW gain. Further studies should focus on evaluating how the administration of these Bifidobacteria modifies gut microbiota of obese rats.
COMMENTS
Background
Obesity is becoming a global epidemic. Recent researches have demonstrated that obesity may lead to the composition shift of gut microbiota in both mice and humans. The intentional manipulation of community structure of gut microbiota may be a novel strategy to treat obesity.
Research frontiers
Bifidobacterium is one of the most numerous “probiotic” in mammalian gut among commensal bacteria, and exhibited a significant effect in lowering serum total cholesterol. Specific strains of Bifidobacteria for energy metabolism may be helpful in management of obesity.
Innovations and breakthroughs
This study evaluates the effects of the administration of four strains of Bifibofacteria in obese rats. It demonstrated an interesting action of these strains on harvest energy from nutrients and regulation of lipid storage.
Applications
The manuscript gives new and interesting information about the key role of gut microbiota in the harvest energy from nutrients and regulation of lipid storage and metabolism.
Terminology
Probiotic bacteria are defined as living microorganisms that have beneficial effects in human health.
Peer review
This study evaluates the effects of the administration of four strains of Bifibofacteria on obese rats. It demonstrated an interesting action of these strains on harvest energy from nutrients and regulation of lipid storage. It is an interesting work that gives new information about the role of gut microbiota in host metabolism.
Footnotes
Peer reviewers: Laura E Matarese, MS, RD, LDN, FADA, CNSD, Thomas E. Starzl Transplantation Institute, UPMC Montefiore, 7 South, 3459 Fifth Avenue, Pittsburgh, PA 15213, United States; Antonio Gasbarrini, MD, Professor, Department of Internal Medicine, Gemelli Hospital, Catholic University of Rome, Largo A. Gemelli 8, 00168 Rome, Italy
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 4.Park YH, Kim JG, Shin YW, Kim SH, Whang KY. Effect of dietary inclusion of Lactobacillus acidophilus ATCC 43121 on cholesterol metabolism in rats. J Microbiol Biotechnol. 2007;17:655–662. [PubMed] [Google Scholar]
- 5.Sato M, Uzu K, Yoshida T, Hamad EM, Kawakami H, Matsuyama H, Abd El-Gawad IA, Imaizumi K. Effects of milk fermented by Lactobacillus gasseri SBT2055 on adipocyte size in rats. Br J Nutr. 2008;99:1013–1017. doi: 10.1017/S0007114507839006. [DOI] [PubMed] [Google Scholar]
- 6.Xiao JZ, Kondo S, Takahashi N, Miyaji K, Oshida K, Hiramatsu A, Iwatsuki K, Kokubo S, Hosono A. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci. 2003;86:2452–2461. doi: 10.3168/jds.S0022-0302(03)73839-9. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira DI, Gibson GR. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit Rev Biochem Mol Biol. 2002;37:259–281. doi: 10.1080/10409230290771519. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Xu N, Xi A, Ahmed Z, Zhang B, Bai X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol. 2009;84:341–347. doi: 10.1007/s00253-009-2012-x. [DOI] [PubMed] [Google Scholar]
- 10.Segawa S, Wakita Y, Hirata H, Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiol. 2008;128:371–377. doi: 10.1016/j.ijfoodmicro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Kekkonen RA, Sysi-Aho M, Seppanen-Laakso T, Julkunen I, Vapaatalo H, Oresic M, Korpela R. Effect of probiotic Lactobacillus rhamnosus GG intervention on global serum lipidomic profiles in healthy adults. World J Gastroenterol. 2008;14:3188–3194. doi: 10.3748/wjg.14.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol. 2008;42:1117–1119. doi: 10.1097/MCG.0b013e31816d920c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 14.Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997;76:F101–F107. doi: 10.1136/fn.76.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vendt N, Grünberg H, Tuure T, Malminiemi O, Wuolijoki E, Tillmann V, Sepp E, Korpela R. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J Hum Nutr Diet. 2006;19:51–58. doi: 10.1111/j.1365-277X.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 16.Puccio G, Cajozzo C, Meli F, Rochat F, Grathwohl D, Steenhout P. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition. 2007;23:1–8. doi: 10.1016/j.nut.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Weizman Z, Alsheikh A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. J Am Coll Nutr. 2006;25:415–419. doi: 10.1080/07315724.2006.10719554. [DOI] [PubMed] [Google Scholar]
- 18.Gibson RA, Barclay D, Marshall H, Moulin J, Maire JC, Makrides M. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: a randomised controlled trial. Br J Nutr. 2009;101:1706–1713. doi: 10.1017/S0007114508084080. [DOI] [PubMed] [Google Scholar]
- 19.Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, Tancredi DJ, Bevins CL, Sherman MP. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J Pediatr Gastroenterol Nutr. 2009;48:216–225. doi: 10.1097/MPG.0b013e31818de195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tennyson CA, Friedman G. Microecology, obesity, and probiotics. Curr Opin Endocrinol Diabetes Obes. 2008;15:422–427. doi: 10.1097/MED.0b013e328308dbfb. [DOI] [PubMed] [Google Scholar]
- 21.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tojo M, Oikawa T, Morikawa Y, Yamashita N, Iwata S, Satoh Y, Hanada J, Tanaka R. The effects of Bifidobacterium breve administration on campylobacter enteritis. Acta Paediatr Jpn. 1987;29:160–167. doi: 10.1111/j.1442-200x.1987.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Hotta M, Sato Y, Iwata S, Yamashita N, Sunakawa K, Oikawa T, Tanaka R, Watanabe K, Takayama H, Yajima M. Clinical effects of Bifidobacterium preparations on pediatric intractable diarrhea. Keio J Med. 1987;36:298–314. doi: 10.2302/kjm.36.298. [DOI] [PubMed] [Google Scholar]
- 24.Axen KV, Dikeakos A, Sclafani A. High dietary fat promotes syndrome X in nonobese rats. J Nutr. 2003;133:2244–2249. doi: 10.1093/jn/133.7.2244. [DOI] [PubMed] [Google Scholar]
- 25.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 26.Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci. 1998;81:2336–2340. doi: 10.3168/jds.S0022-0302(98)70123-7. [DOI] [PubMed] [Google Scholar]
- 27.Usman , Hosono A. Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. J Dairy Sci. 2000;83:1705–1711. doi: 10.3168/jds.S0022-0302(00)75039-9. [DOI] [PubMed] [Google Scholar]
- 28.Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in mice. J Dairy Sci. 2000;83:401–403. doi: 10.3168/jds.S0022-0302(00)74895-8. [DOI] [PubMed] [Google Scholar]
- 29.Paik HD, Park JS, Park E. Effects of Bacillus polyfermenticus SCD on lipid and antioxidant metabolisms in rats fed a high-fat and high-cholesterol diet. Biol Pharm Bull. 2005;28:1270–1274. doi: 10.1248/bpb.28.1270. [DOI] [PubMed] [Google Scholar]
- 30.Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004;80:516–526. doi: 10.1136/pgmj.2003.008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905–911. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 33.Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Zhou L, Gu Y, Zhang Y, Tang J, Li F, Shang W, Jiang B, Yue X, Chen M. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br J Nutr. 2007;98:720–726. doi: 10.1017/S0007114507750870. [DOI] [PubMed] [Google Scholar]