Abstract
A randomized, controlled, blinded clinical trial was performed at a research feedlot in western Canada. Auction-market-derived steers (n = 288) were randomly assigned to 1 of 3 treatments: 1) no antimicrobials on arrival; 2) oxytetracycline in the starter ration for 14 d; and 3) long-acting oxytetracycline subcutaneously on day 0. Minimal inhibitory concentrations of 7 antimicrobials were determined for 3 generic fecal E. coli isolates per animal on arrival and throughout the feeding period. There was a low prevalence of antimicrobial resistance in generic E. coli isolates from calves on arrival. There were increased proportions of cattle with resistant E. coli isolates early in the feeding period among calves in groups 2 and 3. Individual animal treatments were not associated with increased proportions of cattle with resistant E. coli isolates preslaughter. There was no difference in the proportion of animals with E. coli isolates resistant to tetracycline between the treatment groups preslaughter. However, there were significantly more animals with tetracycline resistant isolates of E. coli preslaughter than at arrival.
Résumé
Associations entre l’usage d’antimicrobiens et la prévalence de l’antibiorésistance chez Escherichia coli d’origine fécale dans le bétail d’un parc d’engraissement de l’Ouest canadien. Un essai clinique aléatoire comparatif à l’insu a été réalisé dans un parc d’engraissement de recherche dans l’Ouest canadien. On a assigné au hasard les bouvillons achetés aux enchères (n = 288) à 1 de 3 traitements : 1) aucun antimicrobien à l’arrivée; 2) oxytétracycline dans la ration initiale pendant 14 jours; et 3) oxytétracycline de longue durée administrée par voie sous-cutanée le jour 0. Les concentrations minimales inhibitrices de 7 antimicrobiens ont été déterminées pour 3 isolats génériques d’E. coli d’origine fécale pour chaque animal à l’arrivée et pendant la période d’engraissement. Il y avait une faible prévalence de résistance antimicrobienne pour les isolats génériques d’E. coli des veaux à l’arrivée. Il y avait des proportions accrues de bouvillons avec des isolats d’E. coli résistants au début de la période d’engraissement parmi les veaux des groupes 2 et 3. Les traitements individuels des animaux n’ont pas été associés à des proportions accrues de bouvillons avec des isolats d’E. coli résistants avant l’abattage. Il n’y avait aucune différence dans la proportion d’animaux avec des isolats d’E. coli résistants à la tétracycline entre les groupes de traitements avant l’abattage. Cependant, il y avait un nombre significativement supérieur d’animaux avec des isolats d’E. coli résistants à la tétracycline comparativement à l’arrivée.
(Traduit par Isabelle Vallières)
Introduction
In fed cattle production in western Canada, antimicrobials are typically used for injectable metaphylaxis, feed prophylaxis, and individual treatment of sick animals in order to prevent or limit losses associated with bovine respiratory disease (BRD) (1,2). Bovine respiratory disease is one of the most important feedlot diseases associated with concurrent stressors, including mixing, weaning, environmental stress, and long distance transportation (2,3).
Metaphylaxis is commonly used in western Canada for groups of cattle in the fall of the year when newly weaned, auction-market-derived calves are placed in feedlots and are at high risk for BRD. Feed antimicrobials are used early in the feeding period to treat and prevent BRD. Injectable metaphylaxis and feed prophylaxis in high-risk groups of animals reduce BRD morbidity and improve average daily gain and feed efficiency (4–8). Many studies have shown economic and animal health benefits from metaphylactic and prophylactic antimicrobial use (AMU) in feedlot cattle (5,8–12). In western Canada, 20% to 50% of feedlot placements are treated with injectable metaphylactic antimicrobials on arrival, depending on the risk profile of the group of calves (2) (Calvin Booker, Feedlot Health Management Services, personal communication, 2007).
Antimicrobial use, however, has been associated with antimicrobial resistance (AMR), usually on a local scale (13–20), but most notably in Europe where the use of avoparcin as a growth promotant in pigs and poultry was associated with the presence of Enterococcus faecium that was resistant to vancomycin (21).
The objectives of this study were to determine the prevalence of AMR in fecal Escherichia coli isolates from newly weaned, auction-market-derived calves on arrival at the feedlot, and to examine associations between metaphylactic (injectable) and prophylactic (feed) antimicrobial use in groups of cattle and the prevalence of antimicrobial resistant fecal E. coli during the feeding period.
Materials and methods
Trial facilities
The trial was conducted at the small-pen research feedlot at the University of Saskatchewan, which has a one-time capacity of approximately 800 cattle. The calves were housed in open-air pens with dirt floors, 20% porosity fencing, and a central alley for feeding. Each pen was 286 m2 with feed bunks 7.4 m in length. Waterers were shared between 2 pens.
Animals
In fall 2000, 288 auction-market-derived, 256–353 kg, Charolais-cross steers were purchased in Saskatchewan and brought to the feedlot facility at the University of Saskatchewan. No information was available about the source(s) of the cattle. The calves were allowed to adjust to the feed bunks for several days while the entire group was assembled. This also ensured adequate intake of the feed antimicrobial once the trial began. The calves underwent routine processing on arrival at the feedlot, including vaccination with a multivalent Clostridial vaccine with Histophilus somni [(Fermicon 7-Somnugen, Pfizer Canada, London, Ontario) or (Ultrabac7/Somnubac, Pfizer Canada)], vaccination with a modified live vaccine for Infectious Bovine Rhinotracheitis and Parainfluenza 3 (Bovishield IBR-PI3, Pfizer Canada), treatment with a topical endectocide (Ivomec Pour-On, Mérial Canada, Baie d’Urfé, Québec), eartags, and a progesterone-estradiol benzoate hormonal implant (Synovex-S, Ayerst, Veterinary Laboratories, Guelph, Ontario). At 90 days on trial (DOT), a second vaccination with a modified live vaccine for IBR and PI-3 (Bovishield IBR-PI3) and a second hormonal implant were given before switching the animals to a finishing diet. The feeding program consisted of grass hay, barley silage, and a barley-based concentrate. Monensin sodium 3% (Rumensin; Elanco Animal Health, Guelph, Ontario) was fed to all cattle during the entire feeding period in the total mixed ration at 27 to 28 ppm dry matter (DM).
All sick animals were treated for individual animal illness using routine feedlot protocols developed by the feedlot veterinarian and were kept in the trial. Animals that were identified as sick during the first 21 DOT, with a rectal temperature over 40.5°C and with no other identifiable cause of disease were considered to have BRD. The first line treatment was tilmicosin (Table 1). Relapses were defined as cases needing re-treatment for BRD within a 2-week period in which further clinical signs referable to the respiratory tract were seen. The 2nd line of treatment was florfenicol, and the 3rd treatment, if necessary, was trimethoprim/sulfadoxine (Table 1). The other disease condition in which individual animal antimicrobial treatments were required was bovine interdigital necrobacillosis (footrot). The case definition of footrot was a lame animal with a swollen foot and no other cause for the lameness. The suggested treatment protocol was ceftiofur sodium (Table 1). Feedlot staff also used Procaine Penicillin G for the treatment of footrot [(Depocillin, Intervet Canada, Whitby, Ontario) or (Ethacillin, Pfizer Canada)] at 500 000 IU per 50 kg BW.
Table 1.
Antimicrobials used and animal defined dose for a feedlot animal (ADDFeedlot) equivalenta
| Antimicrobials used in study (Concentration) | Dose equivalent | ADDFeedlot |
|---|---|---|
| Ceftiofur 50 mg/mL, IM (Excenel sterile powder; Pharmacia Animal Health, Orangeville, Ontario) | 1 mg/kg BW | 1 |
| Florfenicol 300 mg/mL, SQ (Nuflor; Schering-Plough Animal Health, Pointe Claire, Quebec) | 40 mg/kg BW | 4 |
| Penicillin G procaine 300 mg/mL,b IM (Depocillin; Intervet Canada, Whitby, Ontario) | 33 mg/kg BW | 1.6 |
| Penicillin G procaine 300 mg/mL, IM (Ethacilin; Rogar/STB, London, Ontario) | 33 mg/kg BW | 1.6 |
| Sulfamethazine 15 g/bolus, orally (Sulfamethazine bolus, Professional Veterinary Laboratories, Winnipeg, Manitoba) | 1 bolus/80 kg BW | 1 |
| Tilmicosin 300 mg/mL, SQ (Micotil, Provel; Guelph, Ontario) | 10 mg/kg BW | 3 |
| Trimethoprim 40 mg/mL/Sulfadoxine 200 mg/mL, IM (Trivetrin injection; Schering-Plough Animal Health) | 16 mg/kg BW | 1 |
BW — body weight.
ADDFeedlot — Animal defined dose for a feedlot animal was used to quantify the number of actual individual antimicrobial treatments given at the approved dose of the antimicrobial. This does not include feed antimicrobials or routine metaphylaxis. This measurement accounted for the dosage and duration of action of the antimicrobial.
No longer available in Canada.
Experimental design
Three treatment groups were compared in this trial: 1) control — no antimicrobials were given on arrival; 2) prophylaxis — oxytetracycline (Terramycin*-50 Premix, Pfizer Canada) at 110 g/kg active ingredient was fed in the starter ration at 2 g/animal per day for 14 d beginning at 0 DOT; and 3) metaphylaxis — 20 mg/kg BW of long-acting oxytetracycline (Liquamycin* LA-200*, Pfizer Canada) was administered subcutaneously at 0 DOT. The oxytetracycline premix was fed at a dose higher than the approved level but this is a common dose used in western Canadian feedlots. In Canada, the oxytetracycline label claim is for bloat prevention at 75 mg/animal/day.
Adjoining pens with shared waterers were randomly assigned to 1 of the 3 treatments using computer-generated random numbers. Twelve steers were randomly assigned, using computer- generated random numbers, by weight blocks into each of the 24 pens. Animals were moved through the handling facilities in a specified order every time they were handled during the trial: control, metaphylaxis, and then prophylaxis groups. Handling facilities were cleaned daily after all treatments/processing was completed. Fresh fecal samples, of approximately 25 to 40 g, were collected from the rectum of each steer. A new, plastic obstetrical glove was used for each animal. The samples were placed into clean styrofoam cups with lids, labeled and taken to the lab within 2 h. Fecal samples were collected on arrival prior to treatment (designated as 0 DOT but taken as each truckload arrived), after metaphylactic treatment was finished (7 DOT), at the end of the prophylactic treatment (15 DOT), and also 35 DOT, 70 DOT, 100 DOT, 150 DOT, and preslaughter. This preslaughter sample, collected 24 h prior to slaughter, varied between 168 and 248 DOT based on when the steers were ready for slaughter. Cattle were shipped for slaughter with a back fat measurement of 8 mm on ultrasound and a maximum finish weight of 750 kg. The feedlot workers and the laboratory staff were blinded to the treatment groups and the objectives of the study.
Laboratory analysis
Fresh feces were cultured overnight on MacConkey’s agar. Identification of E. coli was confirmed by standard biochemical tests. Three individual isolates were randomly chosen for subculture in litmus milk and stored at −70°C until a large group of antimicrobial susceptibilities could be performed together. The subcultures were thawed and immediately cultured on blood agar. From each blood agar plate, E. coli colonies were inoculated into phosphate buffered saline (PBS) to make a standard suspension of 0.5 MacFarland. This suspension was delivered onto Mueller-Hinton agar using a replicator. The MICs of 7 antimicrobials of interest in animal and human health, were determined using the Mueller-Hinton agar dilution method. The antimicrobials tested were ampicillin (AMP), enrofloxacin (ENR), gentamicin (GEN), sulfamethoxazole (SMX), tetracycline (TCY), trimethoprim (TMP), and trimethoprim/ sulfanilamide (TMP/SSS). The Mueller-Hinton plates were cultured at 37°C and antimicrobial susceptibilities were read between 18 and 24 h. A control strain of E. coli ATCC 25922 was included with each plate. Laboratory procedures, antimicrobial breakpoints, and interpretation were according to the Clinical and Laboratory Standards Institute (CLSI) standards (22,23).
Statistical analysis
The measurement, ADDFeedlot, was used to quantify the number of individual antimicrobial treatments given at the approved dose of the antimicrobial. This does not include feed antimicrobials or routine metaphylaxis, as these were evaluated separately. This measurement accounted for the dosage and duration of action of the antimicrobial (Table 1). The concept of ADDFeedlot has not been used previously in this type of study and was based on that of defined daily dose (DDD) used in the human literature (24,25). Each antimicrobial treatment was described as 0, 1, 2, or 3 ADDFeedlot (Table 1), based on manufacturers’ label claims.
Isolates classified as either susceptible or intermediate were considered sensitive for this analysis. Resistant animals were defined as those with 1 or more isolates resistant to 1 or more antimicrobials at a specific time period. Multidrug resistant animals were defined as those with 1 or more isolates resistant to more than 3 antimicrobials at a specific time period. The hierarchical structure of the data involved 24 pens with 12 calves per pen (n = 288). There were 3 treatment groups (control, prophylaxis, metaphylaxis) allocated at the pen level, with 8 pens per treatment. Fecal samples were collected at the animal level, as were antimicrobial treatments given to animals for disease treatment. There were 3 isolates analyzed per animal, for a total of 864 isolates per test day, and 6912 isolates total over the 8 unevenly spaced sampling days.
Descriptive statistics were calculated using commercial software. Baseline differences in resistance to 5 antimicrobials (AMP, SMX, TCY, TMP/SSS, and TMP) on arrival between the 3 treatment groups were assessed using logistic regression. Five arrival models were built, 1 for each binary outcome (SPSS for Windows 15.0.0, SPSS, Chicago, Illinois, USA), for BRD, footrot, and resistance to the 5 antimicrobials. No adjustments for clustering were done at arrival as no information was available on the purchase lots of the animals. Exact confidence intervals for animal-level prevalence estimates were calculated (PEPI v 4; Sagebrush Press, Salt Lake City, Utah, USA).
A logistic regression model using generalized estimating equations was used to examine treatment effects, with adjustment for repeated measurements within animal using an autoregressive correlation structure (Proc Genmod, SAS for Windows v. 9.1; SAS Institute, Cary, North Carolina, USA). Binary AMR outcomes evaluated were resistance to AMP, SMX, and TCY, at the animal-level, as these were the most prevalent resistance outcomes in this trial and in the literature (26). The first variables examined were treatment group (metaphylaxis, prophylaxis, or control) and sample time (time 1–8) following the design of the clinical trial. Unconditional associations were first examined between each outcome and each potential risk factor. A multivariable model was built for each outcome if risk factors were identified as potentially significant through unconditional associations (P ≤ 0.20).
The effect of individual animal antimicrobial treatments on the proportion of resistant isolates preslaughter was examined using a marginal logistic regression model. Generalized estimating equations were used, while adjusting for clustering by pen with an exchangeable correlation structure (Proc Genmod, SAS for Windows v. 9.1; SAS Institute). Individual animal antimicrobial treatments used (Treated, Treated in last 100 DOT, ADDFeedlot Overall, ADDFeedlot in last 100 DOT) were assessed as covariates in the model that included group treatment (metaphylaxis or prophylaxis) using a forward stepwise method. The individual animal antimicrobial use covariates were not independent from each other, so they were not evaluated in the same model. Individually, they represented slightly different aspects of AMU so they were all investigated. No risk factors were forced into the model. Risk factors excluded from the final model were checked for confounding first. First order interaction terms of biological significance were examined in the final model.
A marginal logistic regression model using generalized estimating equations was also used to specifically examine the treatment and time effect in this trial between arrival and pre-slaughter, with adjustment for clustering within animal using an autoregressive correlation structure (Proc Genmod, SAS for Windows v. 9.1, SAS Institute). The models were further developed as described.
Results
The mean weight and standard deviation (s) of the control, prophyaxis, and metaphylaxis groups on arrival were 289.0 (s = 18.1), 288.6 (s = 18.4), 289.0 (s = 19.0), respectively. The cumulative incidence of bovine respiratory disease (BRD) morbidity was 19% (54/288) during this trial; the incidence was 22.9%, 16.7%, and 13.5% in the control, prophyaxis, and metaphylaxis groups, respectively. These values were not significantly different at the pen level (P = 0.27). The incidence of footrot morbidity overall was 23% (67/288). The incidence was 27.1%, 20.8%, and 21.9% in the control, prophylaxis, and metaphylaxis groups, respectively. These values were also not significantly different at the pen level (P = 0.50). The average DOT that an animal was treated for individual animal disease was 148 d. Samples could not be collected from 2 animals on arrival, 4 animals at D7, 3 animals at D15, 5 animals at D35, 3 animals at D70, 4 animals at D105, 5 animals at D154, and 15 animals preslaughter. The overall proportion of mortality for this trial was 2.8% (8/288), and 1 of the fatalities occurred within the metaphylaxis group, 2 within the control group, and 5 within the prophylaxis group.
Arrival
There were no isolates with resistance to GEN or ENR. On arrival at the feedlot, there was a relatively low prevalence of AMR in fecal E. coli isolates; this was also true at the animal level (Table 2). Resistance to the 5 antimicrobials did not vary significantly among the treatments groups on arrival (P > 0.13).
Table 2.
Prevalence (and count) of fecal E. coli isolates resistant to specific antimicrobials and prevalence (and count) of animals with 1 or more fecal E. coli isolates resistant to specific antimicrobials on arrival at the feedlot
| Antimicrobial resistancea | Isolate-level prevalence as percent (out of 858) | Exact 95% confidence interval for isolate-level prevalence (as percent) | Animal-level prevalence as percent (out of 286) | 95% confidence interval animal-level prevalence (as percent) |
|---|---|---|---|---|
| AMP | 1.9 (16) | 1.1–3.0 | 3.5 (10) | 2.0–6.0 |
| ENR | 0.0 (0) | naa | 0.0 (0) | na |
| GEN | 0.0 (0) | na | 0.0 (0) | na |
| SMX | 4.7 (40) | 3.4–6.3 | 8.0 (23) | 5.1–12.4 |
| TCY | 9.8 (84) | 7.9–12.0 | 16.4 (47) | 12.0–22.0 |
| TMP/SSS | 1.2 (10) | 0.6–2.1 | 1.7 (5) | 0.8–3.8 |
| TMP | 1.3 (11) | 0.6–2.3 | 2.1 (6) | 1.0–4.2 |
| AMPp | 0.1 (1) | 0.0–0.6 | na | na |
| SMXp | 1.0 (9) | 0.5–2.0 | na | na |
| TCYp | 5.9 (51) | 4.5–7.7 | na | na |
| AMP TCY | 0.1 (1) | 0.0–0.6 | na | na |
| AMP TMP | 0.1 (1) | 0.0–0.6 | na | na |
| TCY SMX | 1.6 (14) | 0.9–2.7 | na | na |
| AMP TCY SMX | 0.9 (8) | 0.4–1.8 | Na | na |
| AMP TCY TMP/SSS TMP | 0.1 (1) | 0.0–0.6 | Na | na |
| TCY SMX TMP/SSS TMP | 0.6 (5) | 0.2–1.4 | Na | na |
| AMP TCY SMX TMP/SSS TMP | 0.5 (4) | 0.1–1.2 | Na | na |
| No resistance | 88.9 (763) | 86.6–90.9 | 81.1 (232) | 74.9–86.1 |
| Resistance to 1 or more antimicrobials | 11.1 (95) | 9.1–13.4 | 18.9 (54) | 74.9–86.1 |
| Resistance to 3 or more antimicrobials | 2.1 (18) | 1.2–3.3 | 3.1 (9) | 1.9–5.2 |
AMP — ampicillin, ENR — enrofloxacin, GEN — gentamicin, SMX — sulfamethoxazole, TCY — tetracycline, TMPSSS — trimethoprim/sulfanilamide, TMP — trimethoprim, AMPp — ampicillin phenotype, SMXp — sulfamethoxazole phenotype, TCYp — tetracycline phenotype.
AMU associations with AMR
This clinical trial looked at antimicrobial interventions in a research feedlot under commercial feedlot management conditions. Some of the animals developed disease conditions that had to be treated with antimicrobials on an individual animal basis. There were 100/288 animals (35%) treated on an individual animal basis during the feeding period. The median ADDFeedlot for individual animal treatments was 0 (range: 0 to 13.2) (Table 3). There were 57/276 individual animals (21%) treated in the last 100 d before slaughter, mostly for footrot (Table 3). Individual animal treatments were also broken down by group (Table 4).
Table 3.
Animal defined dose for a feedlot animal (ADDFeedlot) over the entire feeding period and over the last 100 days of the feeding perioda
| ADDFeedlot Overall | Animal Count | Overall ADDFeedlot | ADDFeedlot Last 100 days | Animal Count | Last 100 days ADDFeedlot |
|---|---|---|---|---|---|
| 0 | 188 | 0 | 0 | 219 | 0 |
| 1 | 38 | 38 | 1 | 5 | 5 |
| 1.6 | 29 | 46.4 | 1.6 | 39 | 62.4 |
| 2 | 3 | 6 | 2 | 1 | 2 |
| 3 | 2 | 6 | 3.2 | 7 | 22.4 |
| 3.2 | 5 | 16 | 4.2 | 1 | 4.2 |
| 4 | 1 | 4 | 4.8 | 1 | 4.8 |
| 4.2 | 1 | 4.2 | 6.2 | 1 | 6.2 |
| 4.6 | 13 | 59.8 | 6.4 | 1 | 6.4 |
| 4.8 | 1 | 4.8 | 8.8 | 1 | 8.8 |
| 6.2 | 3 | 18.6 | |||
| 6.4 | 1 | 6.4 | |||
| 8 | 1 | 8 | |||
| 11.8 | 1 | 11.8 | |||
| 13.2 | 1 | 13.2 | |||
| Total | 288 | 243.2 | Total | 276 | 122.2 |
ADDFeedlot — Animal defined dose for a feedlot animal was used to quantify the number of individual antimicrobial treatments given at the approved dose of the antimicrobial. This does not include feed antimicrobials or routine metaphylaxis. This measurement accounted for the dosage and duration of action of the antimicrobial.
Table 4.
Number of treatments broken down by study group
| Study group | Number of pens treated | Number of animals treated overall | Number of animals treated in the last 100 days of the feeding period | Overall ADDFeedlot | ADDFeedlot of animals treated in the last 100 d of the feeding period |
|---|---|---|---|---|---|
| Prophylaxis | 8 | 27 | 18 | 77.6 | 37 |
| Metaphylaxis | 8 | 32 | 18 | 70.6 | 38.8 |
| Control | 8 | 41 | 26 | 95 | 46.4 |
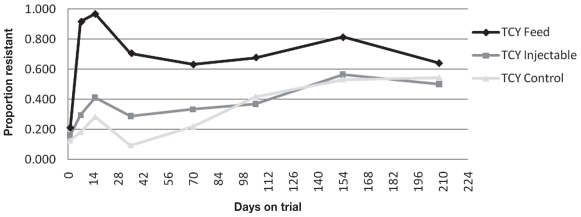
The proportion of animals with at least 1 of the 3 isolates resistant to AMP, SMX, or TCY changed over time (Table 5). The associations between treatment and AMR for TCY and SMX also varied with time on feed (Figures 1 to 3). At Day 15, an animal was 115 times (P < 0.0001; 95% CI: 34.5 to 386.5) more likely to have 1 or more fecal E. coli isolates resistant to TCY than at Day 0, when oxytetracycline was used in the feed. At Day 15, an animal was 3.7 times (P < 0.0001; 95% CI: 2.0 to 6.9) more likely to have 1 or more fecal E. coli isolates resistant to TCY than at Day 0, when injectable oxytetracycline was administered on arrival. At Day 15, an animal was 2.7 times (P = 0.009; 95% CI: 1.3 to 5.9) more likely to have 1 or more fecal E. coli isolates resistant to TCY than at Day 0, in the control group. The same trend was present at Day 150 when animals were 16.7, 6.9, and 7.9 times more likely to have 1 or more fecal E. coli isolates resistant to TCY than at Day 0 in the prophylaxis, metaphylaxis, and control groups, respectively [(P < 0.0001; 95% CI: 8.5 to 33.0), (P < 0.0001; 95% CI: 3.3 to 14.3), (P < 0.0001; 95% CI: 3.8 to 12.6)].
Table 5.
Antimicrobial resistance at animal levela at each time period (n = 2257)
| Antimicrobial resistanceb | Treatment group | Arrival | Day |
Pre-slaughter | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 15 | 35 | 70 | 105 | 154 | |||||
| AMP | Prophylaxis | 2/96 | 14/95 | 15/94 | 4/92 | 7/92 | 7/92 | 19/92 | 14/84 | 82/737 |
| Metaphylaxis | 6/94 | 7/95 | 23/95 | 5/94 | 8/96 | 3/95 | 12/94 | 9/94 | 73/757 | |
| Control | 2/96 | 4/94 | 5/96 | 1/96 | 4/96 | 8/96 | 9/96 | 10/94 | 43/764 | |
| SMX | Prophylaxis | 11/96 | 70/95 | 65/94 | 42/92 | 34/92 | 45/92 | 45/92 | 24/84 | 336/737 |
| Metaphylaxis | 6/94 | 13/95 | 14/94 | 14/96 | 23/95 | 30/94 | 23/94 | 6/94 | 138/757 | |
| Control | 6/96 | 6/94 | 10/96 | 7/96 | 7/96 | 16/96 | 27/96 | 26/94 | 105/764 | |
| TCY | Prophylaxis | 20/96 | 87/95 | 91/94 | 65/92 | 58/92 | 62/92 | 75/92 | 54/84 | 512/737 |
| Metaphylaxis | 15/94 | 28/95 | 39/95 | 27/94 | 32/96 | 35/95 | 53/94 | 47/94 | 276/757 | |
| Control | 12/96 | 17/94 | 27/96 | 9/96 | 21/96 | 40/96 | 51/96 | 51/94 | 228/764 | |
At least 1 of the 3 isolates had this phenotype.
AMP — ampicillin, ENR — enrofloxacin, GEN — gentamicin, SMX — sulfamethoxazole, TCY — tetracycline, TMP/SSS — trimethoprim/sulfanilamide, TMP — trimethoprim.
Figure 1.
Proportion of animals with one or more isolates resistant to tetracycline (TCY) described over time.
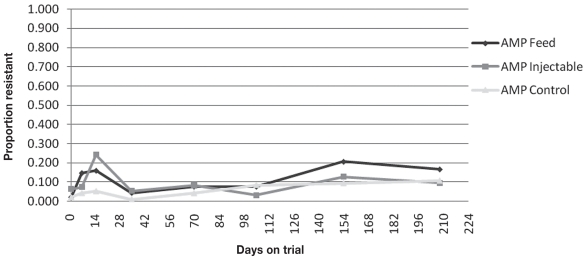
Figure 3.
Proportion of animals with one or more isolates resistant to ampicillin (AMP) described over time.
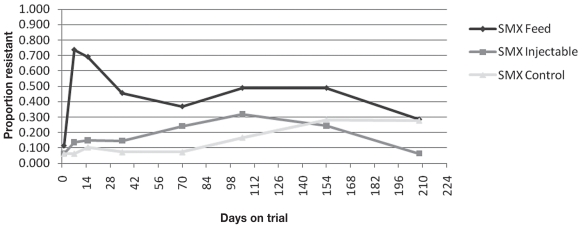
At Day 15, an animal was 17.4 times (P < 0.0001; 95% CI: 8.6 to 35.0) more likely to have 1 or more fecal E. coli isolates resistant to SMX than at Day 0, when oxytetracycline was used in the feed. At Day 15, an animal was 2.8 times (P = 0.04; 95% CI: 1.1 to 7.5) more likely to have 1 or more fecal E. coli isolates resistant to SMX than at Day 0, when injectable oxytetracycline was administered on arrival. There was no significant increase in the likelihood of an animal in the control group to have 1 or more fecal E. coli isolates resistant to SMX at Day 15 compared to Day 0. The same trend was present at Day 150, as with TCY resistance, where animals were 7.4, 7.0, and 5.9 times more likely to have 1 or more fecal E. coli isolates resistant to SMX than at Day 0 in the prophylaxis, metaphylaxis and control groups, respectively [(P < 0.0001; 95% CI: 3.6 to 15.1), (P < 0.0001; 95% CI: 2.7 to 18.2), (P = 0.0003; 95% CI: 2.2 to 15.4)].
The final model for AMP contained treatment group and sample time, but no interaction term. Feed oxytetracycline was associated with a significantly higher proportion of animals with 1 or more fecal E. coli isolates resistant to AMP compared to the control group, while adjusting for sampling time. With adjustment for sampling time, an animal in the prophylaxis group was 2.2 times more likely to have 1 or more AMP resistant fecal E. coli isolates than an animal in the control group (P = 0.0001; 95% CI: 1.5 to 3.2). An animal in the metaphylaxis group was 1.9 times more likely to have one or more fecal E. coli isolates resistant to AMP than an animal in the control group (P = 0.0290; 95% CI: 1.1 to 3.4), with adjustment for sampling time. Ampicillin resistance on days 7, 15, 150 and preslaughter was significantly higher from Day 0 after adjusting for treatment group (P < 0.0171). No measures of individual animal antimicrobial treatment (Treated, Treated in last 100 DOT, ADDFeedlot Overall, ADDFeedlot in last 100 DOT) were associated with a higher proportion of E. coli isolates resistant to TCY, SMX, or AMP preslaughter.
The only significant variable in models built to compare only arrival and preslaughter results for TCY, SMX, and AMP was time. The proportion of animals with 1 or more E. coli isolates resistant to tetracycline was not different between the treatment groups preslaughter (P > 0.14). The odds of an animal having at least 1 isolate resistant to TCY preslaughter were 6.4 times higher than at arrival (P < 0.0001; 95% CI: 4.3 to 9.6). The odds of an animal having at least 1 isolate resistant to SMX preslaughter were 4.2 times higher than at arrival (P < 0.0001; 95% CI: 2.6 to 7.0). The odds of an animal having at least 1 isolate resistant to AMP preslaughter were 3.8 times higher than at arrival (P = 0.0003; 95% CI: 1.8 to 8.0).
Discussion
Escherichia coli was chosen as the indicator commensal organism in this study because it is easy to isolate from all animals and is an important carcass contaminant at slaughter (27). Escherichia coli is a potential reservoir of resistance genes that could transfer resistance to zoonotic or commensal organisms that might cause disease in cattle or people (28–30). The last fecal sample was collected during the 24 h prior to slaughter shipment, to be representative of bacteria at the stage of production that might ultimately affect the consumer. Three isolates were randomly chosen from each animal to better represent the isolate variety in individual animals. Porosity fencing and waterer allocation helped prevent fecal contamination between pens assigned different treatments but all routine feedlot management procedures were carried out similar to commercial feedlot protocols with no other special procedures to avoid AMR gene dissemination. During laboratory analysis, no further passage of isolates occurred that might have contributed to loss of plasmids coding for AMR.
One of the objectives of this trial was to characterize AMR in fecal E. coli, from newly weaned, auction-market-derived calves on arrival at the feedlot, as an indication of ‘baseline’ resistance. This has not been well-characterized in feedlot cattle in western Canada and could represent resistance patterns and AMU from the herd of origin or auction market. On arrival, calves had relatively low proportions of resistant fecal E. coli isolates. In a western Canadian study with similar methodologies, the proportion of animals, on arrival, with one or more isolates resistant to TCY was similar (17.6%), but the proportion of animals with 1 or more isolates resistant to SMX (44.4%) and AMP (20.3%) was higher than in this study, perhaps due to differences in the farm of origin, AMU, and management practices (31). A recent study in western Canada analyzing E. coli isolates from calves pre-weaning had a similar adjusted isolate-level prevalence of AMP at 1.6%, TCY at 5.0%, and SMX at 4.0% (32). The proportions of isolates resistant to TCY and SMX (9.8% and 4.7%, respectively) in this study were lower than those found in a study of individual and pooled fecal E. coli samples from feedlot pens and individual feedlot animals (28.1% to 31.8% and 16.9% to 25.7%) in the USA, possibly because those samples were taken later in the feeding period (33). In this study, the proportion of animals with 1 or more TCY resistant E. coli isolates (16.4%) was also similar to that from an American study of pasture fecal samples collected from newly weaned calves where 13% to 17% of fecal samples contained 1 or more E. coli isolate resistant to TCY (34).
At the time of this trial, GEN was only licensed for intra-uterine use, ENR was not licensed for use in cattle in Canada, and no resistance was found to them at any time period during this study, or in another western Canadian study (33). These antimicrobial classes were important in human medicine so they were evaluated even though they were not commonly used, as cross-resistance and co-resistance with related and unrelated antimicrobials can occur (35).
Few studies have fully explored associations between AMU and AMR in feedlot cattle. Direct comparisons of results between studies of different species and different sectors of cattle production were not made as AMU and management factors are quite different and this may affect the results (2). The current study showed that the use of oxytetracycline in the feed was associated with an increased proportion of animals with 1 or more fecal E. coli isolates resistant to TCY and SMX. This was not surprising as the use of feed or water antimicrobials is associated with the development of AMR in other species (15,16). In pigs, Dunlop et al (15) suggested that AMR associated with feed medication overshadowed any association with individual animal treatment. Other studies in cattle have also found associations between AMR and feed antimicrobials (14,17,20).
In this study, the mass use of injectable oxytetracycline on arrival at the feedlot was associated with an increased proportion of animals with 1 or more isolates resistant to TCY and SMX, but not as strong as the association with the use of feed antimicrobials. This could be related to longer term administration of the feed antimicrobial or perhaps the route of administration. In other studies, there has been no or a much less pronounced association between injectable antimicrobials and AMR than with feed antimicrobials and AMR (13,14,18,20). The trial by Berge et al (13) also had complex interactions in the statistical analysis including that between treatment and time, as seen in this study. Findings from this trial can also be loosely compared with those from another Canadian study in which associations between AMR in fecal E. coli isolates from bulls at a test station and individual AMU were found when feed antimicrobials were also used (19). In the study by O’Connor et al (19), the use of injectable oxytetracycline in individual cattle receiving chlortetracycline in the feed was associated only with increased prevalence of resistance to chloramphenicol and sulfisoxazole in fecal E. coli isolates.
An interesting increase in the proportion of animals with 1 or more resistant isolates was noticed in all treatment groups later in the feeding period (Table 5). This later increase in the proportion of animals with 1 or more resistant isolates was not limited to the animals that originally received mass treatment with feed antimicrobials. Several explanatory hypotheses were considered. Of the 100 antimicrobial treatments given during the feeding period, 57 were given during the last 100 days on feed. The proportion of animals with 1 or more resistant isolates was not significantly associated with individual animal antimicrobial treatments late in the feeding period; however, although not statistically significant, perhaps these treatments did play a role in the increased proportion of resistant organisms either in combination with other selective forces suggested below. Or, the study might also have been lacking in power to see this association as it was not designed for this purpose. A second potential explanation is the change onto a finishing (high-grain) ration which preceded it. Another feedlot study isolated a higher proportion of TCY resistant Campylobacter spp. from animals on a finishing (high-grain) diet than from animals on a backgrounding (high-forage) diet (17,36). The change in diet may create a selective advantage for some resistant bacteria perhaps through a decrease in the pH of the rumen or perhaps feed could have been a vector of AMR genes (37). A third mechanism might involve the sharing of mobile genetic elements or bacteria between the different treatment groups over time (38). Early increases in proportions of animals with 1 or more resistant isolates were seen in animals following mass treatment with feed antimicrobials. This could be due to clonal proliferation of resistant E. coli strains related to the selective pressure from AMU; AMU result in the killing of susceptible bacterial strains (39) and allow resistant strains to flourish (18). The normal flora (based on AMR phenotype) was then re-established to some extent, after the AMU pressure decreased, similar to the Lowrance study (18). Some of the resistant isolates may have had a bacterial fitness advantage which was spread horizontally across the feedyard through mobile genetic elements. Horizontal spread has been suggested in the literature; antimicrobial resistant isolates have rapidly colonized calves (40,41). However, other studies have not described this type of widespread horizontal spread of resistance across all pens and all treatment groups (17,42). This is another area of suggested future research.
This study demonstrated that calves arrived at the feedlot with a relatively low prevalence of AMR in commensal E. coli in the feces. Use of feed antimicrobials for disease prophylaxis in groups of calves for the first 14 DOT was associated with pronounced increases in proportions of cattle with 1 or more TCY or SMX resistant E. coli isolates early in the feeding period. The metaphylactic use of long-acting injectable oxytetracycline on arrival was also significantly associated with increased proportions of cattle with 1 or more resistant E. coli isolates during the feeding period. Individual animal AMU was not a significant risk factor in these associations. The proportion of animals with 1 or more E. coli isolates resistant to tetracycline was not different between the treatment groups preslaughter; however, there were significantly more animals with tetracycline resistance in 1 or more isolates of E. coli preslaughter than at arrival.
Figure 2.
Proportion of animals with one or more isolates resistant to sulphamethoxazole (SMX) described over time.
Acknowledgments
We thank the staff of the University of Saskatchewan Beef Cattle Research Unit for their care of the cattle and assistance with the project, Fei Huang and Jaime Gerun for technical support in the laboratory, and Kate Berezowski for data entry. CVJ
Footnotes
This paper was part of the PhD thesis of Dr. Sylvia Checkley, Department of Large Animal Clinical Sciences, University of Saskatchewan, Saskatoon, Saskatchewan.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This trial was funded in part by a research grant from the Saskatchewan Agriculture Development Fund, Agriculture Research Branch, 3085 Albert Street, Regina, Saskatchewan S4S 0B1.
References
- 1.Booker CW, Guichon PT, Schunicht OC, Wildman BK, Jim GK. Economic impact of antimicrobial use in feedlots. Bovine Proceedings. 1999;32:111–112. [Google Scholar]
- 2.Radostits OM. Herd Health: Food Animal Production Medicine. 3 ed. Philadelphia: WB Saunders; 2001. [Google Scholar]
- 3.Ribble CS, Meek AH, Shoukri MM, Guichon PT, Jim GK. Risk factors associated with fatal fibrinous pneumonia (shipping fever) in feedlot calves. Proc Am Assoc Bov Pract. 1998;31:104–109. [Google Scholar]
- 4.Schumann FJ, Janzen ED, McKinnon JJ. Prophylactic tilmicosin medication of feedlot calves at arrival. Can Vet J. 1990;31:285–288. [PMC free article] [PubMed] [Google Scholar]
- 5.Harland RJ, Jim GK, Guichon PT, Townsend HGG, Janzen ED. Efficacy of parenteral antibiotics for disease prophylaxis in feedlot calves. Can Vet J. 1991;32:163–168. [PMC free article] [PubMed] [Google Scholar]
- 6.Van Donkersgoed J. Meta-analysis of field trials of antimicrobial mass medication for prophylaxis of bovine respiratory disease in feedlot cattle. Can Vet J. 1992;33:786–795. [PMC free article] [PubMed] [Google Scholar]
- 7.Morck DW, Merrill JK, Thorlakson BE, Olson ME, Tonkinson LV, Costerton JW. Prophylactic efficacy of tilmicosin for bovine respiratory tract disease. J Am Vet Med Assoc. 1993;202:273–277. [PubMed] [Google Scholar]
- 8.Gallo GF, Berg JL. Efficacy of a feed-additive antibacterial combination for improving feedlot cattle performance and health. Can Vet J. 1995;36:223–229. [PMC free article] [PubMed] [Google Scholar]
- 9.Wittum TE, Woollen NE, Perino LJ, Littledike ET. Relationships among treatment for respiratory tract disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. J Am Vet Med Assoc. 1996;209:814–818. [PubMed] [Google Scholar]
- 10.Booker CW, Jim GK, Guichon PT, Schunicht OC, Thorlakson BE, Lockwood PW. Evaluation of florfenicol for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1997;38:555–560. [PMC free article] [PubMed] [Google Scholar]
- 11.Jim GK, Booker CW, Guichon PT, et al. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1999;40:179–184. [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb DJ, Schwartzkopf-Genswein KS, McAllister TA, Genswein BMA, Streeter M. Effect of subtherapeutic antibiotics and auction exposure on health, performance, and feeding behavior of weaned calves. Can J Anim Sci. 2006;86:457–460. [Google Scholar]
- 13.Berge ACB, Epperson WB, Pritchard RH. Assessing the effect of a single dose florfenicol treatment in feedlot cattle on the antimicrobial resistance patterns in faecal Escherichia coli. Vet Res. 2005;36:723–734. doi: 10.1051/vetres:2005027. [DOI] [PubMed] [Google Scholar]
- 14.Berge ACB, Moore DA, Sischo WM. Field trial evaluating the influence of prophylactic and therapeutic antimicrobial administration on antimicrobial resistance in fecal Escherichia coli in dairy calves. Appl Environ Microbiol. 2006;72:3872–3878. doi: 10.1128/AEM.02239-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlop RH, McEwen SA, Meek AH, Clarke RC, Black WD, Friendship RM. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow-to-finish farms in Ontario, Canada. Prev Vet Med. 1998;34:283–305. doi: 10.1016/s0167-5877(97)00095-0. [DOI] [PubMed] [Google Scholar]
- 16.Funk JA, LeJeune JT, Wittum TE, Rajala-Schultz PJ. The effect of subtherapeutic chlortetracycline on antimicrobial resistance in the fecal flora of swine. Microbial Drug Resistance. 2006;12:210–218. doi: 10.1089/mdr.2006.12.210. [DOI] [PubMed] [Google Scholar]
- 17.Inglis GD, McAllister TA, Busz HW, et al. Effects of subtherapeutic administration of antimicrobial agents to beef cattle on the prevalence of antimicrobial resistance in Campylobacter jejuni and Campylobacter hyointestinalis. Appl Environ Microbiol. 2005;71:3872–3881. doi: 10.1128/AEM.71.7.3872-3881.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowrance TC, Loneragan GH, Kunze DJ, et al. Changes in antimicrobial susceptibility in a population of Escherichia coli isolated from feedlot cattle administered ceftiofur crystalline-free acid. Am J Vet Res. 2007;68:501–507. doi: 10.2460/ajvr.68.5.501. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor AM, Poppe C, McEwen SA. Changes in the prevalence of resistant Escherichia coli in cattle receiving subcutaneously injectable oxytetracycline in addition to in-feed chlortetracycline compared with cattle receiving only in-feed chlortetracycline. Can J Vet Res. 2002;66:145–150. [PMC free article] [PubMed] [Google Scholar]
- 20.Stabler SL, Fagerberg DJ, Quarles CL. Effects of oral and injectable tetracyclines on bacterial drug resistance in feedlot cattle. Am J Vet Res. 1982;43:1763–1766. [PubMed] [Google Scholar]
- 21.Bager F, Madsen M, Christensen J, Aarestrup FM. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish Poultry and pig farms. Prev Vet Med. 1997;31:95–112. doi: 10.1016/s0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute (CLSI) CLSI document M100-S16. 2006. Performance standards for Antimicrobial Susceptibility Testing; 16th Informational Supplement. (Use for Humans) [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards (NCCLS) Performance standards for Antimicrobial Disk and Dilution Susceptibility Tests for bacteria Isolates from Animals. Information Supplement M32-S1. 2004 [Google Scholar]
- 24.Austin DJ, Kakehashi M, Anderson RM. The transmission dynamics of antibiotic-resistant bacteria: The relationship between resistance in commensal organisms and antibiotic consumption. Proc Roy Soc London Ser B: Biol Sci. 1997;264:1629–1638. doi: 10.1098/rspb.1997.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci. 1999;95:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Government of Canada (GOC) Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2005. Guelph, Ontario: Public Health Agency of Canada; [Last accessed June 8, 2010]. 2007. Available from http://www.phac-aspc.gc.ca/cipars-picra/2005_e.html. [Google Scholar]
- 27.Stopforth JD, Lopes M, Shultz JE, Miksch RR, Samadpour M. Microbiological status of fresh beef cuts. J Food Prot. 2006;69:1456–1459. doi: 10.4315/0362-028x-69.6.1456. [DOI] [PubMed] [Google Scholar]
- 28.Blake DP, Hillman K, Fenlon DR, Low JC. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ideal conditions. J Appl Microbiol. 2003;95:428–436. doi: 10.1046/j.1365-2672.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 29.Linton AH, Howe K, Bennett PM, Richmond MH, Whiteside EJ. The colonization of the human gut by antibiotic resistant Escherichia coli from chickens. J Appl Microbiol. 1977;43:465–469. doi: 10.1111/j.1365-2672.1977.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 30.Winokur PL, Vonstein DL, Hoffman LJ, Uhlenhopp EK, Doern GV. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates form food animals and humans. Antimicrob Agents Chemother. 2001;45:2716–2722. doi: 10.1128/AAC.45.10.2716-2722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Checkley SL, Campbell JR, Chirino-Trejo M, Janzen ED, McKinnon JJ. Antimicrobial resistance in generic fecal Escherichia coli obtained from beef cattle on arrival at the feedlot and prior to slaughter, and associations with volume of total individual cattle antimicrobial treatments in one western Canadian feedlot. Can J Vet Res. 2008;72:101–108. [PMC free article] [PubMed] [Google Scholar]
- 32.Gow SP, Waldner CL, Rajić A, McFall ME, Reid-Smith R. Prevalence of antimicrobial resistance in fecal generic Escherichia coli isolated in western Canadian cow-calf herds. Part 1-Beef calves. Can J Vet Res. 2008;72:82–90. [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner BA, Dargatz DA, Salman MD, Morley PS, Wittum TE, Keefe TJ. Comparisons of sampling techniques for measuring the antimicrobial susceptibility of enteric Escherichia coli recovered from feedlot cattle. Am J Vet Res. 2002;63:1662–1670. doi: 10.2460/ajvr.2002.63.1662. [DOI] [PubMed] [Google Scholar]
- 34.Huston CL, Bailey RH, Best TF, Huston JE, Evans RR. Antimicrobial resistance of enteric E. coli in beef cattle treated with antibiotics. The AABP Proceedings. 2003;36:156–157. [Google Scholar]
- 35.Guardabassi L, Courvalin P. Antimicrobial resistance in bacteria of animal origin. In: Aarestrup FM, editor. Antimicrobial Resistance in Bacteria of Animal Origin. Washington: ASM Pr; 2006. [Google Scholar]
- 36.Alexander TW, Yanke LG, Topp E, et al. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl Environ Microbiol. 2008;74:4405–4416. doi: 10.1128/AEM.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dargatz DA, Fedorka-Cray PJ, Ladely SR, Ferris KE, Green AL, Headrick ML. Antimicrobial susceptibility patterns of Salmonella isolates from cattle in feedlots. J Am Vet Med Assoc. 2002;221:268–272. doi: 10.2460/javma.2002.221.268. [DOI] [PubMed] [Google Scholar]
- 38.Aarestrup FM. The origin, evolution and local and global dissemination of antimicrobial resistance. In: Aarestrup FM, editor. Antimicrobial Resistance in Bacteria of Animal Origin. Washington: ASM Pr; 2006. [Google Scholar]
- 39.Aly R, Maibach HI, Strauss WG, Shinefield HR. Effects of a systemic antibiotic on nasal bacterial ecology in man. Appl Microbiol. 1970;20:240–244. doi: 10.1128/am.20.2.240-244.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donaldson SC, Straley BA, Hegde NV, Sawant AA, DebRoy C, Jayarao BM. Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves. Appl Environ Microbiol. 2006;72:3940–3948. doi: 10.1128/AEM.02770-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyle DV, Yates CM, Chase-Topping ME, et al. Molecular epidemiology of antimicrobial-resistant commensal Escherichia coli strains in a cohort of newborn calves. Appl Environ Microbiol. 2005;71:6680–6688. doi: 10.1128/AEM.71.11.6680-6688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson SML, McAllister TA, Selinger LB, et al. Transfer of a rifampicin-resistant Escherichia coli strain among feedlot cattle. J Appl Microbiol. 2003;95:398–410. doi: 10.1046/j.1365-2672.2003.01987.x. [DOI] [PubMed] [Google Scholar]