Abstract
Anemia is prevalent in patients with chronic kidney disease (CKD) and is associated with a lower quality of life and a higher risk of adverse outcomes including cardiovascular disease and death. Anemia management in CKD patients currently revolves around the use of erythropoiesis-stimulating agents (ESAs) and supplemental iron. However, many patients do not respond adequately and/or require high doses of these medications. Furthermore, recent clinical trials have shown that targeting higher hemoglobin levels with conventional therapies leads to increased cardiovascular morbidity and mortality, particularly when higher doses of ESAs are used, and in patients who are poorly responsive to therapy. One explanation for the poor response to conventional therapies in some patients is that these treatments do not fully address the underlying cause of the anemia. In many CKD patients, like patients with other chronic inflammatory diseases, poor absorption of dietary iron and inability to utilize the body's iron stores contributes to the anemia. Recent research suggests that these abnormalities in iron balance may be caused by elevated levels of the key iron regulatory hormone hepcidin. This article reviews the pathogenesis of anemia in CKD, the role and regulation of hepcidin in systemic iron homeostasis and the anemia of CKD, and the potential diagnostic and therapeutic implications of these findings.
Keywords: Anemia, chronic kidney disease, dialysis, inflammation, hepcidin
BACKGROUND
Iron is required for hemoglobin synthesis in the production of red blood cells. Iron is also a constituent of several proteins, which carry out essential housekeeping functions, and thus is critical for cell growth and survival. However, excess iron can generate free radicals that damage lipid membranes, proteins, and nucleic acids, leading to cell death. As a result, iron levels must be tightly regulated, both on a cellular level and systemically. Hepcidin is now recognized to be a key mediator of systemic iron homeostasis. A role for hepcidin in the pathogenesis of anemia of chronic kidney disease (CKD) is increasingly being elucidated.
CASE VIGNETTE
A 55 year-old woman with hemodialysis-dependent end-stage renal disease (ESRD) secondary to diabetic nephropathy had persistent anemia despite escalating erythropoiesis-stimulating agent (ESA) dosing. Serum hemoglobin level was 7.5 g/dl (reference range, 12-16 g/dL), and hematocrit was 23.4 % (reference range, 36-46 %). Serum iron was 22 μg/dL (reference range, 30-160 μg/dL), total iron binding capacity was 188 μg/dL (reference range, 230-404 μg/dL), and serum transferrin saturation was 11.7%, consistent with low circulating levels of iron. However, the serum ferritin was elevated at 1315 ng/mL (reference range, 10-200 ng/mL). The patient had a failed arteriovenous fistula and arteriovenous graft, and now has a tunneled catheter for hemodialysis access. She has a history of coronary artery disease, severe peripheral vascular disease, calciphylaxis, multiple episodes of skin and line infections, and a toe amputation for a non-healing ulcer. This scenario of anemia, ESA resistance, hypoferremia, and high serum ferritin is not uncommon in the CKD/ESRD population, and anemia management in these patients is currently problematic. It has recently been hypothesized that elevated hepcidin levels may contribute to functional iron deficiency, anemia, and ESA resistance in this setting.
PATHOGENESIS
Anemia of CKD
Anemia is prevalent in patients with CKD and contributes to a lower quality of life.1 Anemia in CKD patients is also associated with numerous adverse outcomes, including hospitalization, cardiovascular disease, cognitive impairment, and mortality.1 Inadequate production of erythropoietin is commonly thought to be the most important factor in the pathogenesis of anemia in these patients, and many patients are treated with ESAs. However, approximately 10-20% of patients are poorly responsive to ESAs.1 Prospective randomized controlled clinical trials, including the US Normal Hematocrit Study and the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) study raised concerns about the safety of ESAs when dosed to target higher hemoglobin levels, particularly when using higher doses and in patients who are poorly responsive to therapy.2-4 This has resulted in a FDA black box warning on the product labeling of ESAs and significant controversy over the management of anemia in CKD patients.
In fact, the etiology of anemia in CKD is multifactorial.5-6 In addition to relative erythropoietin deficiency, shortened erythrocyte survival6 and the erythropoiesis inhibitory effects of accumulating uremic toxins also contribute to the anemia of CKD.5-6 Importantly, CKD patients also have several abnormalities in systemic homeostasis of iron, an essential component in the production of red blood cells.5-6 First, hemodialysis patients in particular are typically in negative iron balance, losing approximately 1-3 grams of iron per year, due in part to blood trapping in the dialysis apparatus and repeated phlebotomy.6 Second, many patients are on ESAs to manage their anemia, which depletes iron stores by driving increased production of red blood cells.6 Third, it has been recognized that CKD patients also have impaired absorption of dietary iron. Randomized controlled trials have shown that oral iron is no better than placebo to treat iron deficiency in patients on hemodialysis.1,7-9 These abnormalities of iron metabolism in CKD patients may result in true iron deficiency, manifest by a low serum transferrin saturation and low ferritin, which can be treated with supplemental iron. However, many patients also have a functional iron deficiency or reticuloendothelial cell iron blockade, characterized by low levels of circulating iron that limit erythropoiesis, even in the face of adequate or elevated body iron stores. The management of these patients is less clear.1 This reticuloendothelial cell iron sequestration is characteristic of anemia of inflammation (also known as anemia of chronic disease) seen not only in CKD patients, but also in patients with many other chronic diseases, including autoimmune disorders, chronic infections, and malignancy.5 Indeed, many patients with CKD have a chronic inflammatory state, which may be due to an increased incidence of infections and/or induction of inflammatory cytokines by the hemodialysis procedure.5-6,10 Recent research suggests that the impaired intestinal iron absorption and impaired release of iron from body stores in CKD patients, like in other patients with anemia of inflammation, may be caused by an excess of the key iron regulatory hormone hepcidin.11-13
Systemic Iron Homeostasis and Hepcidin
Iron enters the body by absorption from dietary sources in the duodenum. Iron circulates bound to transferrin and is delivered primarily to the bone marrow for erythropoiesis. Senescent erythrocytes are phagocytosed by reticuloendothelial macrophages to recycle iron back into the circulation. Iron storage and release also occurs in hepatocytes. Sloughing of enterocytes and bleeding are the only significant means for removing iron from the body (reviewed in 14-16; Fig. 1). On average, approximately 1 to 2 mg of iron is provided on a daily basis by intestinal absorption, and this is balanced by an equal amount of iron loss by epithelial shedding in the gastrointestinal tract and blood loss in menstruating women. The majority of the iron required for erythropoiesis, approximately 20 to 25 mg per day, is provided by iron recycling from senescent erythrocytes. The circulating pool of transferrin-bound iron is much smaller, approximately 3 mg, and therefore must be turned over every few hours in order to ensure an adequate supply of iron for erythropoiesis. Iron excretion is not a regulated process, and there is no physiologic mechanism for removing larger amounts of iron, even in conditions of severe iron overload. Systemic iron balance is therefore maintained by tight regulation of iron absorption from the diet and iron release from reticuloendothelial and hepatocyte stores.14-16
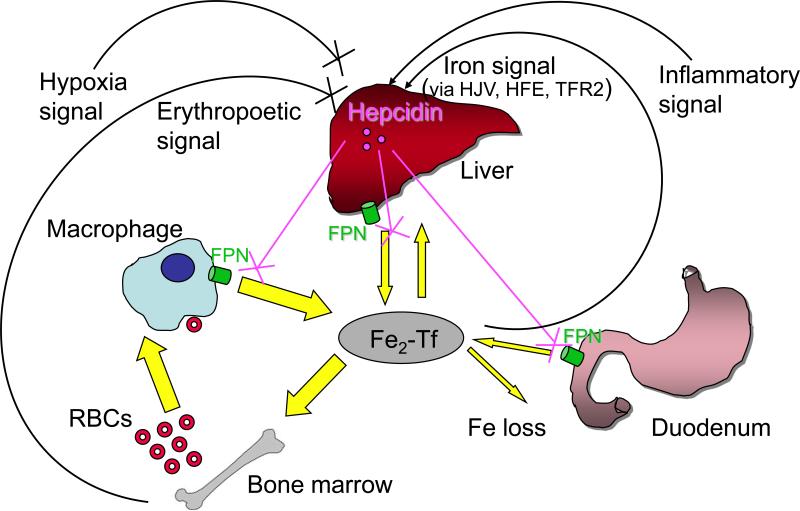
Figure 1.
Hepcidin is a central regulator of systemic iron homeostasis. Serum iron levels are determined by the balance of iron entry from intestinal absorption, macrophage iron recycling, and mobilization of hepatocyte stores, versus iron utilization, primarily by erythroid cells in the bone marrow. A peptide hormone secreted by the liver, hepcidin controls iron release into the plasma by downregulating cell-surface expression of the iron export protein ferroportin (FPN; encoded by the SLC40A1 gene) on absorptive enterocytes, macrophages, and hepatocytes. Hepcidin production is inhibited by erythropoetic drive and hypoxia, to ensure iron availability for erythropoiesis. Hepcidin production is stimulated by iron (via the hemochromatosis proteins HFE, hemojuvelin [HJV], and transferrin receptor 2 [TFR2]) as a negative feed back loop to maintain steady state iron levels. Hepcidin production is also stimulated by inflammation, thereby sequestering iron from invading pathogens in the setting of infection, but also causing the hypoferremia of anemia of chronic disease. Abbreviation: RBC, red blood cell.
It is now well established that hepcidin is a central mediator of systemic iron homeostasis.15-16 A small peptide hormone of 25 amino acids, hepcidin is produced and secreted predominantly by hepatocytes, circulates in the bloodstream, and is excreted by the kidneys.17-19 Hepcidin regulates systemic iron balance by binding and inducing internalization and degradation of ferroportin, an iron channel on the surface of enterocytes, macrophages, and hepatocytes, which is important in iron export into the plasma.20-21 Hepcidin thereby decreases both intestinal iron absorption and iron release from reticuloendothelial and hepatocyte stores (Fig. 1). The central importance of hepcidin in systemic iron homeostasis has been established by animal models and human patients with abnormalities in hepcidin expression. Hepcidin null mice22-23 and humans with mutations in the hepcidin gene develop severe iron overload24, while transgenic mice overexpressing hepcidin and human patients with hepcidin secreting adenomas have profound iron deficiency anemia.25-26
Regulation of Hepcidin Expression
Consistent with its role as a central regulator of systemic iron homeostasis, hepcidin expression is regulated in response to iron, erythropoietic demand, hypoxia, and inflammatory signals (Fig. 1). Iron administration increases hepcidin expression, thus providing a feedback mechanism to limit further iron absorption18, 27-28, while anemia and hypoxia inhibit hepcidin expression, thus increasing iron availability for erythropoiesis.27 Hepcidin expression is also induced by inflammation18, 27-29, which is thought to be part of the host defense mechanism to fight infection and cancer by limiting iron availability. However, in chronic inflammatory states, this leads to a deficiency of iron available for erythropoiesis, and this is thought be the mechanism underlying the reticuloendothelial iron sequestration, intestinal iron absorption impairment, and low circulating iron levels characteristic of anemia of chronic disease.5, 15-16 The signaling pathways by which iron, erythropoietic demand, hypoxia and inflammation affect hepcidin expression are increasingly being elucidated.
Hepcidin Deficiency and Iron Overload Disorders
Recent progress toward understanding the mechanisms that regulate hepcidin expression has emerged from studies of the genetic iron overload disorder hereditary hemochromatosis (reviewed in 30). This heterogeneous disorder is characterized by a failure to prevent excess iron from entering the bloodstream from enterocytes and reticuloendothelial macrophages, leading to progressive tissue iron deposition and subsequent multi-organ damage. In addition to mutations in the gene encoding hepcidin (HAMP), mutations in the genes encoding the hemochromatosis protein HFE31, transferrin receptor-2 (TFR2)32, and hemojuvelin (HJV; official gene symbol, HFE2)33 also cause hereditary hemochromatosis. Among other tissues, these proteins are all expressed in the liver31, 33-34, where hepcidin is produced. All patients and animal models with hemochromatosis due to mutations in these genes have inappropriately low levels of hepcidin expression33, 35-42, suggesting that 1) hepcidin deficiency and consequent unregulated ferroportin activity are the common pathogenic mechanisms for iron overload in these diseases, and 2) HFE, TFR2, and HJV are all involved in the regulation of hepcidin expression by iron (Fig. 1).
RECENT ADVANCES
Molecular Mechanisms of Hepcidin Regulation: Hepcidin Activators
Hepcidin Regulation by the BMP6-HJV-SMAD Signaling Pathway
Mutations in the HJV gene are the most common cause of the more severe juvenile-onset form of hereditary hemochromatosis, and result in a phenotype similar to mutations in the gene encoding hepcidin itself.24,33 A link between the bone morphogenetic protein (BMP) signaling pathway and iron metabolism was discovered when HJV was demonstrated to be a BMP co-receptor43 and BMP signals were shown to regulate hepcidin expression (Figure 2).43-44
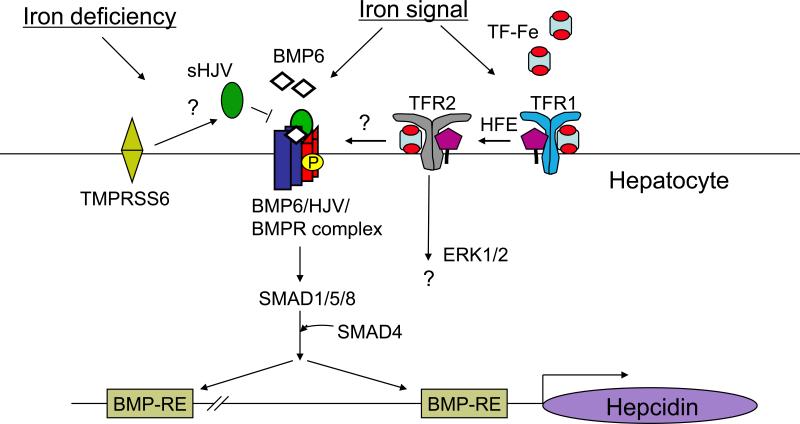
Figure 2.
Schematic diagram depicting the proposed role of bone morphogenetic protein (BMP) signaling pathway, HJV, HFE, and TFR2 in iron sensing and hepcidin regulation in the liver. In response to iron, bone morphogenetic protein 6 (BMP6) binds to the BMP co-receptor hemojuvelin (HJV) as part of the BMP6/HJV/BMPR complex on the hepatocyte membrane to activate the SMAD1/5/8 pathway. Activated SMAD complexes bind directly to BMP responsive elements (BMP-REs) on the hepcidin promoter to induce hepcidin transcription. Holotransferrin (TF-Fe) competes for HFE binding to transferrin receptor 1 (TFR1), causing HFE displacement to form a complex with TFR2 and TF-Fe to induce hepcidin expression, possibly through an interaction with the BMP6-HJV-SMAD signaling pathway and/or alternative signaling pathways such as the ERK1/2 pathway. In the setting of iron deficiency, the serine protease TMPRSS6 inhibits hepcidin expression by cleaving membrane-bound HJV to form soluble HJV, thereby inhibiting downstream SMAD signaling by loss of membrane-bound HJV and sequestration of BMP6 ligand. Abbreviations: BMPR, BMP receptor, TF, transferrin.
BMPs are members of the TGF-β (transforming growth factor β) superfamily of signaling molecules. BMP ligands bind to cell-surface type I and type II serine threonine kinase receptors. Upon formation of the complex, type I receptors phosphorylate type II receptors, which then phosphorylate intracellular SMAD1, SMAD5, and SMAD8 proteins. These SMAD proteins form a complex with common mediator SMAD4, and the SMAD complex translocates to the nucleus to modulate gene transcription (Reviewed in 45).
As a co-receptor, HJV binds to BMP ligands and BMP receptors and enhances intracellular SMAD signals in response to low levels of BMP ligands.43 HJV is selective for its interaction with BMP ligands and receptors. HJV binds to BMP2, BMP4, BMP5, and BMP6, but not BMP7 or BMP9.43, 46-47 HJV signals through all three BMP type I receptors (ALK2, ALK3, and ALK6), but only two of the three BMP type II receptors (ActRIIA and BMPRII).48 Notably, HJV can alter type II receptor utilization by BMP ligands, allowing BMP ligands that normally signal through BMPRII to signaling through ActRIIA.48 This may be one mechanism by which HJV enhances SMAD signaling in the liver where ActRIIA is the predominant BMP type II receptor expressed.48
In liver-derived cell cultures, BMP-SMAD signals increase hepcidin expression at the transcriptional level.43-44,46,49 HJV enhances hepcidin induction in response to BMPs,43 while HJV mutants associated with hemochromatosis have impaired ability to generate BMP signals and induce hepcidin expression, and livers from Hjv null mice have evidence of reduced BMP signaling in the liver.43 These data suggest that mutations in HJV trigger iron overload by causing impaired hepatic BMP-SMAD signaling, decreased hepcidin expression, and consequent unregulated ferroportin activity.43
Several additional lines of evidence support the importance of the BMP-HJV-SMAD signaling pathway in regulating hepcidin expression and iron homeostasis in vivo. Targeted disruption of the common BMP/TGF-β signaling mediator Smad4 in the mouse liver results in low hepcidin levels and iron overload, similar to the phenotype seen in hemochromatosis.44 Promoter mutational analysis has identified two specific motifs on the hepcidin promoter, which appear to mediate hepcidin induction to BMP-SMAD signals.50-53 A mutation in one of these BMP responsive elements on the hepcidin promoter has been associated with a significantly more severe iron overload phenotype in a patient with hemochromatosis due to the most common HFE mutation, C282Y (a cysteine to tyrosine change at amino acid 282).54 In mice, BMP administration increases hepatic hepcidin expression and reduces serum iron, while administration of BMP inhibitors reduces hepcidin expression, mobilizes reticuloendothelial cell iron stores, and increases serum iron levels.46-47, 55
More recent data has identified BMP6 as a ligand for HJV and a key endogenous regulator of hepcidin expression and iron metabolism in vivo.47,56 A genome-wide liver transcription profile from mice fed on diets of variable iron content identified Bmp6 as one of the mRNAs, and the only BMP ligand, that was decreased by a low iron diet and increased by a high iron diet, concordantly with hepcidin.57 BMP6 binds to HJV, and data from a bioinhibition assay suggests that HJV may have higher binding affinity for BMP6 compared with other BMP ligands, although this remains to be shown directly.46-47 Administration of specific BMP6 inhibitors in vivo reduces hepcidin expression and increases serum iron.47 Importantly, Bmp6 null mice have an iron overload phenotype that resembles juvenile hemochromatosis due to HJV mutations with reduced hepcidin expression, increased ferroportin expression, increased serum iron, and tissue iron overload.47, 56 Taken together, these data support the central role of the BMP6-HJV-SMAD signaling pathway in regulating hepcidin expression and systemic iron balance in vivo (Figure 2).
Hepcidin Regulation by Iron
Iron stimulates hepcidin expression, providing an important feedback mechanism to maintain steady-state iron levels. A principal mechanism by which iron stimulates hepcidin expression is through activation of the BMP6-HJV-SMAD signaling pathway (Figure 2). As discussed above, chronic increases in dietary iron increase liver Bmp6 mRNA expression concordantly with hepcidin expression, and hepatic Bmp6 mRNA levels are significantly positively correlated with liver iron concentration in mice.57-58 Additionally, acute iron administration to mice increases phosphorylation of SMAD1/5/8 in the liver within 1 hour.55 The ability of iron-saturated transferrin (holotransferrin) to increase hepcidin expression in primary hepatocyte cultures is dependent on BMP-SMAD signaling because it is blocked by BMP signaling pathway inhibitors.59 Similarly, the ability of iron to induce hepcidin expression in zebrafish is blocked by a BMP inhibitor.55 Thus, the BMP6-SMAD signaling pathway in the liver is activated by iron, and the ability of iron to induce hepcidin expression is dependent on BMP-SMAD signaling. The mechanisms by which iron levels are sensed to increase in BMP6-HJV-SMAD signaling are still not fully understood. It has been postulated that HFE, TFR2, and transferrin receptor 1 (TFR1) may be involved in this process.60-61
Mutations in HFE, encoding an atypical class I major histocompatibility complex (MHC) molecule, are the most common cause of hereditary hemochromatosis, and result in a less severe adult-onset phenotype compared with HJV or HAMP mutations.30-31 HFE has been shown to bind to both transferrin receptors TFR1 and TFR2.62-63 TFR1 is a ubiquitously expressed cell surface receptor which binds transferrin and mediates transferrin-dependent iron uptake into cells (reviewed in 14). The binding site for HFE on TFR1 overlaps with the binding site for transferrin and holotransferrin competes for HFE binding to TFR1.62, 64-67 TFR2 is a TFR1 homologue that has more restricted expression, predominantly in the liver,34 and mutations in TFR2 are a much more rare cause of adult-onset hereditary hemochromatosis.31-32 The central importance of the liver expression of HFE and TFR2 for the regulation of iron metabolism is supported by the fact that hepatocyte-specific conditional knockout of either Hfe or Tfr2 in mice recapitulates the iron overload phenotype seen in global Hfe or Tfr2 knockout mice.68-69 One model proposes that when circulating iron levels increase, holotransferrin binds to TFR1 in the liver, displacing HFE, which is then able to upregulate a hepcidin via an interaction with TFR2 and transferrin (Figure 2).60-61 It is notable, however, that human patients with mutations in both alleles of HFE and TFR2 present with a more severe juvenile-onset form of hemochromatosis compared with the adult-onset hemochromatosis typical of either mutation alone70, and that combined Hfe null/Tfr2 null mice have a more severe hepcidin deficiency, iron overload phenotype compared with either single Hfe null or Tfr2 null mice.71 These data suggest that the mechanisms by which HFE and TFR2 regulate hepcidin expression and iron homeostasis are not entirely overlapping.
Whether HFE and/or TFR2 induce hepcidin expression via an interaction of the BMP6-HJV-SMAD signaling pathway and/or a separate as yet uncharacterized signaling pathway is still not fully understood (Figure 2). Notably, Hfe null mice have appropriately increased hepatic Bmp6 mRNA levels relative to their increased body iron burden, but inappropriately low levels of phosphorylated SMAD1/5/8 protein and Id1 mRNA, a target gene upregulated by BMP6-SMAD signaling.58,72 Furthermore, BMP6 induction of hepcidin is decreased in primary hepatocyte cultures from Hfe null mice versus wildtype mice.58 This suggests that HFE regulates hepcidin expression through an interaction with the BMP6-SMAD signaling pathway.58
Some studies have suggested that TfR2 can activate the mitogen activated protein kinase (MAPK) pathway, including ERK1/ERK2 and p38 MAP kinases.73-74 Although one study suggested that ERK1/ERK2 pathway activation may be involved in iron-saturated transferrin induction of hepcidin expression in conjunction with the BMP-SMAD pathway in primary hepatocyte cultures74, a direct demonstration of the relevance of the ERK/MAPK signaling pathway in hepcidin regulation and iron homeostasis in vivo is lacking.
Hepcidin Regulation by Inflammation
Hepcidin excess is thought to be the mechanism underlying the low circulating iron levels and reticuloendothelial cell iron sequestration that is a hallmark of anemia of inflammation.5,16 Indeed, inflammation is a potent inducer of hepcidin expression.18,27-29 The most well-characterized mechanism is direct transcriptional activation of hepatic hepcidin expression by interleukin 6 (IL-6) binding to its receptor complex containing gp130 to activate janus kinase (JAK) and activator of transcription 3 (STAT3), which binds to a conserved DNA element in the proximal hepcidin promoter (Figure 3).75-77 Other proinflammatory cytokines, such as IL-1, may also play a role in hepcidin induction.78 Notably, hepcidin induction by IL-6 appears to require an intact BMP-SMAD signaling pathway because hepatocyte-specific loss of common mediator Smad444, administration of soluble HJV46, or administration of a small molecule inhibitor of BMP type I receptor kinase activity55 all impair IL-6 induction of hepcidin expression. The crosstalk between these pathways appears to occur in part at the level of the hepcidin promoter, where the proximal BMP responsive element and the STAT3 binding element are in close proximity, because mutations in the proximal BMP responsive element severely impair hepcidin induction by IL-6 (Figure 3).52
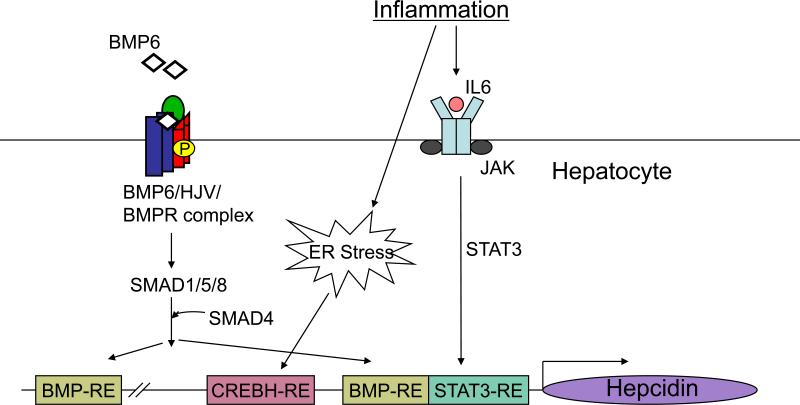
Figure 3.
Schematic diagram depicting the molecular mechanisms by which inflammation activates hepcidin transcription in the liver. Inflammation mediated by cytokines (e.g., IL-6) leads to activation of the JAK/STAT3 pathway. Activated STAT3 binds directly to a STAT3-responsive element (STAT3-RE) on the hepcidin promoter to induce hepcidin transcription. The JAK/STAT3 pathway depends on an intact BMP responsive element (BMP-RE) that is adjacent to the STAT3-RE for full activity. Inflammation may also activate the endoplasmic reticulum (ER) stress pathway using a CREBH-responsive element (CREBH-RE) on the hepcidin promoter to induce hepcidin. Abbreviations: BMP, bone morphogenetic protein; CREBH, cyclic AMP response element-binding protein H; JAK, janus kinase; STAT3, activator of transcription 3.
More recently, a second mechanism has been characterized by which these proinflammatory cytokines and bacterial lipopolysaccharide (LPS) may induce hepatic hepcidin expression. Proinflammatory cytokines and LPS activate endoplasmic reticulum (ER) stress and the unfolded protein response, and increase expression and cleavage of CREBH (cyclic AMP response element-binding protein H)79, which activates transcription of acute phase response genes in the liver.79 ER stress also increases hepcidin expression through CREBH binding and transactivation of the hepcidin promoter (Figure 3).80 ER stress has also been suggested to transcriptionally regulate hepcidin expression through CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) and C/EBPα, although this has not been linked directly to inflammation.81
While the role of liver-derived hepcidin in systemic iron homeostasis has been best characterized, hepcidin is also produced in monocytes/macrophages, and is induced in these cells by LPS and certain bacterial pathogens through Toll-like receptors and possibly also the IL-6/STAT3 pathway.82-86. In contrast to the liver, hepcidin expression in macrophages is not induced by iron.82 Hepcidin production stimulated by bacterial pathogens or LPS in monocytes/macrophages decreases ferroportin mRNA expression and ferroportin protein cell surface expression in an autocrine fashion.82-83,86 Although the amount of hepcidin production in macrophages is much less than in the liver83, hepcidin induction in macrophages in the setting of infection may contribute to the host defense by acting locally to limit iron availability to invading pathogens.82-84,86
Molecular Mechanisms of Hepcidin Regulation: Hepcidin Inhibitors
Soluble Hemojuvelin
HJV is attached to the plasma membrane by a GPI (glycosylphosphatidylinositol) anchor. In addition to its cell associated form, soluble forms of HJV have been detected in the conditioned media of transfected cells and in the blood of humans and other animals.87-93 Mechanisms for release soluble HJV from the cell appears to involve cleavage by the proprotein convertase furin or the transmembrane serine protease 6 TMPRSS6 (see hepcidin regulation by iron deficiency below).91-94 Notably, administration of exogenous soluble HJV has been shown to act as an inhibitor of BMP signaling and hepcidin expression, presumably by binding to BMP ligands and preventing their interaction with cell surface signaling receptors.46,59,87 The quantity and physiologic relevance of endogenous forms of soluble HJV in vivo is still poorly understood, although some data suggests that soluble HJV may be reduced by iron loading and increased by iron deficiency and hypoxia.87,89-90,92 It has been hypothesized that while GPI-anchored, membrane HJV acts as a co-receptor to enhance the BMP-SMAD signaling and hepcidin expression, generation of soluble HJV inhibits the BMP-SMAD signaling and hepcidin expression, either exclusively by removing the enhancing effects of the membrane form of HJV, or with the additive effect of sequestering BMP ligands (Figure 2).43,46,59 Interestingly, HJV is expressed not only in the liver, but also in skeletal and cardiac muscle,33 where its function is currently unknown. It has been proposed that HJV expression in these other tissues may serve as a source of soluble HJV.87,95
Hepcidin Regulation by Anemia
Anemia and ESA administration are potent inhibitors of hepcidin expression, allowing greater iron availability for erythropoiesis.27,96 The dominance of the inhibitory effect of anemia and erythropoeitic drive over the stimulatory effects of iron on hepcidin regulation is evidenced in β-thalassemias. In this disease, hepcidin levels remain low due to very high erythropoietic activity even in the face of increasing serum iron and tissue iron deposition that ultimately lead to fatal iron overload.97-98 Although ESAs have been shown to inhibit hepcidin transcription in isolated liver cells in vitro through erythropoietin receptor signaling and inhibition of (C/EBPα) binding to the hepcidin promoter99, two studies suggested that in vivo, the inhibitory effects of anemia or ESAs on hepcidin expression require erythropoietic activity.100-101 These studies showed that inhibition of erythropoiesis by chemotherapy, an ESA-blocking antibody, or irradiation prevented hepcidin suppression by either ESAs or anemia.100-101 It has been suggested that proliferating erythrocyte precursors may secrete a substance that circulates to the liver to inhibit hepcidin expression. Recently, two modulators of the TGF-β/BMP superfamily signaling pathway, growth and differentiation factor 15 (GDF15) and twisted gastrulation (TWSG1), were reported to be secreted by erythroblast precursors and play a role in hepcidin suppression in thalassemia.102-103 GDF15 is a TGF-β superfamily ligand that activates SMAD2 and SMAD3, and TWSG1 can function as a BMP agonist or antagonist.104-107 Notably, an iron phenotype was not described in mice with GDF15 dysregulation, and sera from patients with sickle cell anemia or myelodysplastic syndrome did not have elevated GDF15 levels102,108-109, suggesting that the role of these proteins in hepcidin suppression may be limited to anemias like β-thalassemia with ineffective erythropoiesis.
Hepcidin Regulation by Hypoxia
Hypoxia is also a potent inhibitor of hepcidin expression even in the absence of anemia.27 The mechanism for this is incompletely understood, but seems to be related to the hypoxia inducing factor (HIF) pathway, which also mediates expression levels of erythropoietin and many other hypoxia-induced genes.110 HIFs are heterodimeric transcription factors consisting of an alpha regulatory subunit (HIF-1α, HIF-2α, or HIF-3α) and a ubiquitously expressed beta subunit (HIF-1β, also known as ARNT). Under normoxic, iron-sufficient conditions, the HIF-α subunit is hydroxylated by oxygen and iron-dependent 2-oxoglutarate-dependent oxygenases, ubiquitinated by von Hippel–Lindau (VHL) protein, and degraded. Under hypoxic or iron-depleted conditions, hydroxylase activity is inhibited and the HIF-α subunit accumulates, translocates to the nucleus, heterodimerizes with the ARNT, and binds to specific hypoxia-responsive promoter elements (HREs) of target genes to modulate gene transcription (reviewed in 110). Consistent with a role for the HIF pathway in regulating hepcidin expression in vivo, mice with a liver specific deletion of Vhl, whose absence increases HIF activity, have reduced hepcidin levels, while a double deletion of Arnt in combination with Vhl in the liver restored normal hepcidin levels, showing the specificity of the effect of the Vhl deletion on HIF activity.111 However, the mice with the liver specific deletion of Vhl also had increased erythropoietin levels and polycythemia111, suggesting that another factor related to erythropoietic drive could have contributed to hepcidin suppression. Although one report suggests that HIF-1α binds directly to HREs in the hepcidin promoter, and HIF binding suppresses hepcidin gene transcription111, conflicting data have been reported in other studies.112-113 Alternative pathways suggested by other studies include other HIF-1 independent, 2-oxoglutarate-dependent pathways113 or pathways involving reactive oxygen species.112 Interestingly, furin, which cleaves HJV, and TFR1 are also encoded by HIF target genes.92, 114-115 Thus, HIF could suppress hepcidin expression indirectly by reducing BMP-SMAD–mediated hepcidin induction and/or reducing HFE/TFR2-mediated hepcidin induction.92, 114-116
Hepcidin Regulation by Iron Deficiency
Iron deficiency also inhibits hepcidin expression. The HIF pathway described above may be one mechanism by which this occurs, since the 2-oxoglutarate-dependent dioxygenases that hydroxylate HIF-α subunits are dependent on iron as well as oxygen.110 In mouse studies, Hif-1α is increased in the liver by an iron deficient diet.111 However, deletion of Hif1a in the liver in one study had no effect117, and in a second study rescued only a small percentage of the hepcidin decrease induced by an iron deficient diet.111 Similarly, a liver-specific deletion of Arnt, which inactivates all Hif-α isoforms (Hif-1α, Hif-2α, and Hif-3α) only slightly rescued the hepcidin suppression induced by an iron deficient diet and had no effect on serum iron or red blood cell parameters.117 Notably, Hif-2α does appear to play an important role in iron homeostasis in the intestine by increasing expression of proteins important for iron uptake by duodenal enterocytes.117-118
TMPRSS6 also appears to play an important role in hepcidin suppression by iron deficiency. 119-121 Mutations in TMPRSS6 in humans and mice leads to iron-refractory iron deficiency anemia (IRIDA), a congenital form of iron deficiency anemia that is unresponsive to oral iron and only partially responsive to parenteral iron treatment.119-121 Patients and mice with mutations in TMRPSS6 have inappropriately high hepcidin expression relative to their iron deficiency and anemia, suggesting that hepcidin excess is the mechanism causing the IRIDA, and that TMPRSS6 normally functions as a hepcidin suppressor in response to iron deficiency.119-121 The mechanism by which TMPRSS6 inhibits hepcidin expression is incompletely understood. TMPRSS6 is predominantly expressed in the liver.122 In vitro studies where TMPRSS6 is overexpressed suggest that TMPRSS6 can cleave HJV and inhibit hepcidin induction by HJV, BMPs, and IL-6 (presumably due to loss of cell surface HJV and/or the presence of soluble HJV as described previously) (Figure 2).119,94 Whether HJV cleavage occurs at physiologic levels of TMPRSS6 expression in vivo remains to be determined. Notably, combined Tmprss6/Hjv mutant mice have an iron overload phenotype that resembles Hjv null mice, consistent with a genetic interaction between TMPRSS6 and the BMP-HJV-SMAD pathway.123
A recent study also suggests that GDF15 levels may be increased by cellular iron deficiency in vitro and GDF15 levels are modestly increased in human sera in the setting of iron deficiency or iron chelator administration.124 However, whether this plays a physiologic role in hepcidin suppression by iron deficiency in vivo is still unknown.
Hepcidin Assays
Hepcidin excess has been postulated to play a role in the anemia of CKD/ESRD patients due to reduced renal clearance and induction by inflammatory stimuli, particularly in patients who are ESA resistant. However, it is only recently that hepcidin assays have been developed to start to investigate these hypotheses.
Hepcidin is synthesized in the liver as an 84 amino acid prepropeptide and processed by peptidase cleavage to a 60 amino acid propeptide (prohepcidin), followed by furin and related proprotein convertase cleavage to yield the mature carboxy terminal 25 amino acid hepcidin (hepcidin-25).125 Hepcidin-25 is a cationic peptide that forms a hairpin loop stabilized by 4 disulfide bonds.126-127 In addition to prohepcidin and hepcidin-25, 22 and 20 carboxy terminal amino acid forms of hepcidin are also found in the circulation and/or urine, most likely due to N-terminal truncation of hepcidin-25, although the mechanism by which hepcidin-22 and hepcidin-20 are generated are still poorly understood.19, 128-129 Hepcidin-25 is the bioactive form of hepcidin, while the other forms of hepcidin have little or no biological activity.130-132 The fractional excretion of hepcidin is reported to be approximately 3-5%, either because it is not freely filtered or because it is reabsorbed, similar to other small peptides that are reabsorbed and degraded in proximal tubules.11, 133 Indeed, hepcidin was recently reported to bind specifically to α2-macroglobulin, and it has been estimated that approximately 89% of circulating hepcidin is protein-bound.134
Development of immunochemical methods to detect mature hepcidin have been complicated by hepcidin's small size and its conservation among animal species.133 The first described hepcidin assay was an immunodot assay to measure urinary hepcidin.28-29 However, this assay is semi-quantitative, laborious, and not suitable for serum hepcidin measurements.11,133 A commercially available immunoassay was developed to detect serum prohepcidin, but prohepcidin levels do not correlate with biological activity, iron status, or inflammation.135-136 Others have developed mass spectroscopic techniques to measure mature hepcidin in serum and urine. Although this technique has the potential advantage of being able to distinguish among hepcidin-25, hepcidin-22, and hepcidin-20, these assays depend on expensive equipment that is not widely available, and most are semi-quantitative, although more recent refinements are improving the quantitative ability.128,133,137-141 In the last year, immunoassays to quantitate mature serum hepcidin have now been developed, as well as an assay based on competition against 125I-hepcidin-25 binding to a peptide identical to the ferroportin hepcidin binding site.11-12,142-144 A recently published round robin study comparing these various mass spectrometry and immunochemical based methods to quantify urinary and plasma mature hepcidin has shown that the absolute hepcidin concentration differs widely between methods, but the Spearman correlations between individual sample mean hepcidin values obtained by most methods were generally high for seven of eight methods tested.145 The analytical variance is generally low and similar for all methods, indicating the potential suitability of all methods to distinguish hepcidin levels of different samples.145 It was hypothesized that the differences in absolute hepcidin levels between methods may be due to the use of different calibrators, hepcidin aggregation, hepcidin protein-binding, and/or the existence of hepcidin-25, hepcidin-22, and hepcidin-20 isoforms that may be detected to some extent by immunochemical methods, depending on the antibody used.145 This report called for efforts to further harmonize the various hepcidin assays.145
Hepcidin Excess and the Anemia of CKD
Using the above-mentioned immunoassays to measure mature hepcidin, several groups have now confirmed that hepcidin levels are elevated in CKD and ESRD patients,11-13 and inversely correlate with GFR12-13, suggesting that reduced renal clearance contributes to hepcidin elevation in this patient population. Hepcidin levels are reduced by dialysis, but return to pre-dialysis levels before the next dialysis session.146 Interestingly, one study using a mass spectrometry based technique to distinguish among hepcidin-25, hepcidin-20, and hepcidin-22 suggested that although hepcidin-25 was increased in ESRD patients, and total hepcidin (encompassing hepcidin-25, hepcidin-20, and hepcidin-22) was inversely correlated with estimated GFR in CKD patients not requiring dialysis, no significant correlation was found between hepcidin-25 and estimated GFR in CKD patients not requiring dialysis.147 These results will need to be confirmed in larger studies.
Hepcidin levels correlate with markers of iron burden in the CKD population, as in other patient populations, particularly serum ferritin.11-13,146-148 In fact, in multivariate analyses, ferritin is the strongest predictor of serum hepcidin levels.12-13,147 Hepcidin levels are also increased by iron administration in the CKD population as in normal controls.11-12 Interestingly, although hepcidin is stimulated by inflammation and has been shown to be elevated in patients with inflammation as defined by high C-reactive protein (CRP) levels11, initial studies in CKD patients has not shown a consistent or robust correlation between hepcidin levels inflammatory markers such as CRP, erythrocyte sedimentation rate (ESR), or IL-6 levels.12-13,146,148 One explanation for these findings may be related to the patient populations selected for these studies, some of which excluded patients with active illness or infection.
Hepcidin levels are associated with anemia in dialysis patients, consistent with a role for hepcidin excess in the anemia of CKD; however, there is an inverse correlation between ESA dose and hepcidin levels, arguing against a diagnostic role for hepcidin as a predictor of ESA resistance.12, 148 The likely explanation for this finding is the fact that ESA administration is an inhibitor of hepcidin expression in the CKD population, as in the general population.12,146 It has been proposed that the initial decrease in hepcidin levels after starting ESAs might be a better indicator of the long-term responsiveness to ESAs.149
Given the limited number and size of studies to date, the unresolved issues surrounding hepcidin assays themselves, and the numerous factors that can modulate hepcidin levels in the CKD/ESRD population including iron administration, ESA administration, body iron burden, inflammation, renal clearance, and dialysis (Figure 4), more studies will be needed to determine whether hepcidin will have diagnostic utility as a measure of iron status, inflammatory status, and/or ESA responsiveness or resistance.
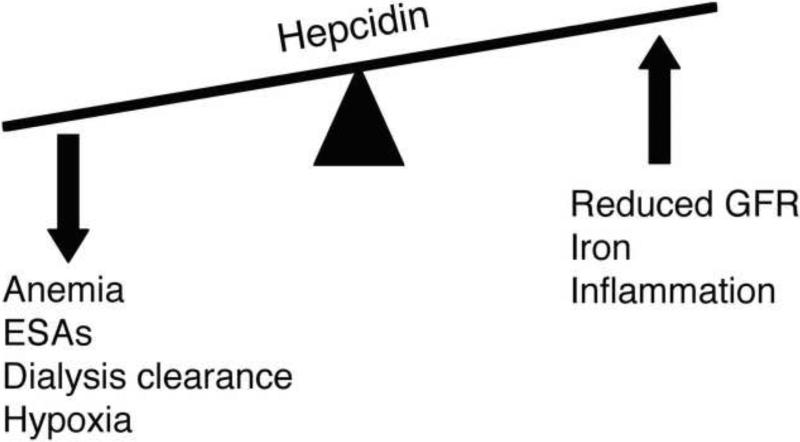
Figure 4. Hepcidin levels in chronic kidney diease and end-stage renal diease patients.
Hepcidin levels are elevated in chronic kidney disease and end-stage renal diease patients, and reflect the balance of stimulatory factors: reduced renal clearance (GFR, glomerular filtration rate), inflammation, and iron administration; and inhibitory factors: anemia, erythropoiesis-stimulating agent (ESA) administration, clearance by dialysis, and hypoxia.
Hepcidin-lowering Agents for the Treatment of Anemia of Inflammation
The notion that hepcidin excess may contribute to the dysregulation of iron homeostasis and anemia in CKD patients raises the possibility that hepcidin-lowering agents may be an effective strategy for ameliorating anemia in this patient population. While ESAs target the relative erythropoietin deficiency and supplemental iron addresses the true iron deficiency found in this patient population, hepcidin-lowering agents might be able to complement these strategies by improving iron availability from the diet and from existing body stores. Some initial small animal studies have shown that BMP inhibitors, including soluble HJV and a small molecule BMP inhibitor, can function as hepcidin lowering agents to mobilize splenic iron stores and increase serum iron levels in vivo.46, 55 It remains to be proven whether BMP inhibitors and/or other hepcidin-lowering strategies will be effective for treating of anemia of CKD. Furthermore, the potential side effects of these strategies remain unknown.
SUMMARY
Hepcidin excess is increasingly being identified as a contributing factor to anemia in CKD/ESRD patients by impairing iron absorption from the diet and iron mobilization from body stores. A multitude of factors can modulate hepcidin levels in the CKD/ESRD population: iron administration, ESA administration, body iron burden, inflammation, renal clearance, and dialysis. More studies are needed to better understand the diagnostic utility of hepcidin in CKD/ESRD patients as a measure of iron status, inflammatory status, and/or ESA resistance. More studies are needed to investigate whether hepcidin-lowering agents may have a role in treating anemia in CKD/ESRD patients.
ACKNOWLEDGEMENTS
Support: Dr Babitt is supported in part by National Institutes of Health grant K08 DK-075846, the Satellite Dialysis Young Investigator Grant of the National Kidney Foundation, and a Claflin Distinguished Scholar Award from the Massachusetts General Hospital. Dr Lin is supported in part by National Institutes of Health grants RO1 DK-069533 and RO1 DK-071837.
Financial Disclosure: Drs Babitt and Lin have ownership interest in a startup company, Ferrumax Pharmaceuticals, which has licensed technology from the Massachusetts General Hospital based on work cited here and described in prior publications. Patent applications entitled “Methods and Composition to Regulate Iron Metabolism” and “Methods and Compositions for Regulating Iron Homeostasis by Modulation of BMP6” have been submitted to the USPTO by the Massachusetts General Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47(5)(suppl 3):S1–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 4.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 6.Malyszko J, Mysliwiec M. Hepcidin in anemia and inflammation in chronic kidney disease. Kidney Blood Press Res. 2007;30(1):15–30. doi: 10.1159/000098522. [DOI] [PubMed] [Google Scholar]
- 7.Macdougall IC, Tucker B, Thompson J, Tomson CR, Baker LR, Raine AE. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996;50(5):1694–1699. doi: 10.1038/ki.1996.487. [DOI] [PubMed] [Google Scholar]
- 8.Fudin R, Jaichenko J, Shostak A, Bennett M, Gotloib L. Correction of uremic iron deficiency anemia in hemodialyzed patients: a prospective study. Nephron. 1998;79(3):299–305. doi: 10.1159/000045053. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz GS, Kahn GA, Feingold RE, Coco M, Lynn RI. An evaluation of the effectiveness of oral iron therapy in hemodialysis patients receiving recombinant human erythropoietin. Clin Nephrol. 1997;48(1):34–40. [PubMed] [Google Scholar]
- 10.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20(5):440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 12.Ashby DR, Gale DP, Busbridge M, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75(9):976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 13.Zaritsky J, Young B, Wang HJ, et al. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–1056. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 15.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18(2):394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 16.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112(2):219–30. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause A, Neitz S, Mägert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2-3):147–50. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 18.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 20.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 21.Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2009 doi: 10.3324/haematol.2009.014399. doi:10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98(15):8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesbordes-Brion JC, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108(4):1402–5. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 24.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33(1):21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100(10):3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 30.Pietrangelo A. Hereditary hemochromatosis. Biochim Biophys Acta. 2006;1763(7):700–710. doi: 10.1016/j.bbamcr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 32.Camaschella C, Roetto A, Calì A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25(1):14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 33.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 34.Kawabata H, Yang R, Hirama T, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274(30):20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 35.Bridle KR, Frazer DM, Wilkins SJ, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 36.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 37.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115(8):2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115(8):2180–6. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawabata H, Fleming RE, Gui D, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105(1):376–381. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 40.Muckenthaler M, Roy CN, Custodio AO, et al. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet. 2003;34(1):102–107. doi: 10.1038/ng1152. [DOI] [PubMed] [Google Scholar]
- 41.Nicolas G, Viatte L, Lou DQ, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34(1):97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 42.Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54(7):980–986. doi: 10.1136/gut.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 44.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 46.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111(10):5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103(27):10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truksa J, Peng H, Lee P, Beutler E. Different regulatory elements are required for response of hepcidin to interleukin-6 and bone morphogenetic proteins 4 and 9. Br J Haematol. 2007;139(1):138–147. doi: 10.1111/j.1365-2141.2007.06728.x. [DOI] [PubMed] [Google Scholar]
- 51.Truksa J, Lee P, Beutler E. Two BMP responsive elements, STAT, and bZIP/HNF4/COUP motifs of the hepcidin promoter are critical for BMP, SMAD1, and HJV responsiveness. Blood. 2009;113(3):688–695. doi: 10.1182/blood-2008-05-160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verga Falzacappa MV, Casanovas G, Hentze MW, Muckenthaler MU. A bone morphogenetic protein (BMP)-responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J Mol Med. 2008;86(5):531–540. doi: 10.1007/s00109-008-0313-7. [DOI] [PubMed] [Google Scholar]
- 53.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med. 2009;87(5):471–480. doi: 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 54.Island ML, Jouanolle AM, Mosser A, et al. A new mutation in the hepcidin promoter impairs its BMP response and contributes to a severe phenotype in HFE related hemochromatosis. Haematologica. 2009;94(5):720–724. doi: 10.3324/haematol.2008.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 57.Kautz L, Meynard D, Monnier A, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 58.Corradini E, Garuti C, Montosi G, et al. Bone Morphogenetic Protein Signaling Is Impaired in a Hfe Knockout Mouse Model of Hemochromatosis. Gastroenterology. 2009;137(4):1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110(6):2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7(3):205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett MJ, Lebrón JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403(6765):46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 63.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281(39):28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 64.Lebrón JA, West AP, Jr, Bjorkman PJ. The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. J Mol Biol. 1999;294(1):239–245. doi: 10.1006/jmbi.1999.3252. [DOI] [PubMed] [Google Scholar]
- 65.West AP, Jr, Giannetti AM, Herr AB, et al. Mutational analysis of the transferrin receptor reveals overlapping HFE and transferrin binding sites. J Mol Biol. 2001;313(2):385–397. doi: 10.1006/jmbi.2001.5048. [DOI] [PubMed] [Google Scholar]
- 66.Giannetti AM, Björkman PJ. HFE and transferrin directly compete for transferrin receptor in solution and at the cell surface. J Biol Chem. 2004;279(24):25866–25875. doi: 10.1074/jbc.M401467200. [DOI] [PubMed] [Google Scholar]
- 67.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998;95(4):1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vujić Spasić M, Kiss J, Herrmann T, et al. Hfe acts in hepatocytes to prevent hemochromatosis. Cell Metab. 2008;7(2):173–178. doi: 10.1016/j.cmet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 69.Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132(1):301–310. doi: 10.1053/j.gastro.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 70.Pietrangelo A, Caleffi A, Henrion J, et al. Juvenile hemochromatosis associated with pathogenic mutations of adult hemochromatosis genes. Gastroenterology. 2005;128(2):470–479. doi: 10.1053/j.gastro.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 71.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50(6):1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 72.Kautz L, Meynard D, Besson-Fournier C, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114(12):2515–20. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 73.Calzolari A, Raggi C, Deaglio S, et al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. 2006;119(Pt 21):4486–4498. doi: 10.1242/jcs.03228. [DOI] [PubMed] [Google Scholar]
- 74.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94(6):765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 77.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 78.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102(6):1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang K, Shen X, Wu J, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 80.Vecchi C, Montosi G, Zhang K, et al. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325(5942):877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oliveira SJ, Pinto JP, Picarote G, et al. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLoS One. 2009;4(8):e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu XB, Nguyen NB, Marquess KD, Yang F, Haile DJ. Regulation of hepcidin and ferroportin expression by lipopolysaccharide in splenic macrophages. Blood Cells Mol Dis. 2005;35(1):47–56. doi: 10.1016/j.bcmd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Peyssonnaux C, Zinkernagel AS, Datta V, Lauth X, Johnson RS, Nizet V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107(9):3727–3732. doi: 10.1182/blood-2005-06-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sow FB, Florence WC, Satoskar AR, et al. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J Leukoc Biol. 2007 Oct;82(4):934–945. doi: 10.1189/jlb.0407216. [DOI] [PubMed] [Google Scholar]
- 85.Koening CL, Miller JC, Nelson JM, et al. Toll-like receptors mediate induction of hepcidin in mice infected with Borrelia burgdorferi. Blood. 2009;114(9):1913–1918. doi: 10.1182/blood-2009-03-209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Theurl I, Theurl M, Seifert M, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111(4):2392–2399. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 87.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106(8):2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 88.Kuninger D, Kuns-Hashimoto R, Kuzmickas R, Rotwein P. Complex biosynthesis of the muscle-enriched iron regulator RGMc. J Cell Sci. 2006;119(Pt 16):3273–3283. doi: 10.1242/jcs.03074. [DOI] [PubMed] [Google Scholar]
- 89.Zhang AS, Anderson SA, Meyers KR, Hernandez C, Eisenstein RS, Enns CA. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J Biol Chem. 2007;282(17):12547–12556. doi: 10.1074/jbc.M608788200. [DOI] [PubMed] [Google Scholar]
- 90.Silvestri L, Pagani A, Fazi C, et al. Defective targeting of hemojuvelin to plasma membrane is a common pathogenetic mechanism in juvenile hemochromatosis. Blood. 2007;109(10):4503–4510. doi: 10.1182/blood-2006-08-041004. [DOI] [PubMed] [Google Scholar]
- 91.Kuninger D, Kuns-Hashimoto R, Nili M, Rotwein P. Pro-protein convertases control the maturation and processing of the iron-regulatory protein, RGMc/hemojuvelin. BMC Biochem. 2008;9:9. doi: 10.1186/1471-2091-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111(2):924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 93.Lin L, Nemeth E, Goodnough JB, Thapa DR, Gabayan V, Ganz T. Soluble hemojuvelin is released by proprotein convertase-mediated cleavage at a conserved polybasic RNRR site. Blood Cells Mol Dis. 2008;40(1):122–131. doi: 10.1016/j.bcmd.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang AS, West AP, Jr, Wyman AE, Bjorkman PJ, Enns CA. Interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. J Biol Chem. 2005;280(40):33885–33894. doi: 10.1074/jbc.M506207200. [DOI] [PubMed] [Google Scholar]
- 96.Nicolas G, Viatte L, Bennoun M, Beaumont C, Kahn A, Vaulont S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol Dis. 2002;29(3):327–335. doi: 10.1006/bcmd.2002.0573. [DOI] [PubMed] [Google Scholar]
- 97.Kearney SL, Nemeth E, Neufeld EJ, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48(1):57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 98.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 99.Pinto JP, Ribeiro S, Pontes H, et al. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPalpha. Blood. 2008;111(12):5727–5733. doi: 10.1182/blood-2007-08-106195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vokurka M, Krijt J, Sulc K, Necas E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol Res. 2006;55(6):667–674. doi: 10.33549/physiolres.930841. [DOI] [PubMed] [Google Scholar]
- 102.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 103.Tanno T, Porayette P, Sripichai O, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114(1):181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oelgeschläger M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405(6788):757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ross JJ, Shimmi O, Vilmos P, et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410(6827):479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 106.Chang C, Holtzman DA, Chau S, et al. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410(6827):483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- 107.Xu J, Kimball TR, Lorenz JN, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98(3):342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 108.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20(10):3742–3751. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. J Biochem Mol Biol. 2006;39(6):649–655. doi: 10.5483/bmbrep.2006.39.6.649. [DOI] [PubMed] [Google Scholar]
- 110.Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7(1):28–32. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- 111.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117(7):1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi SO, Cho YS, Kim HL, Park JW. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPalpha and STAT-3. Biochem Biophys Res Commun. 2007;356(1):312–317. doi: 10.1016/j.bbrc.2007.02.137. [DOI] [PubMed] [Google Scholar]
- 113.Braliou GG, Verga Falzacappa MV, Chachami G, Casanovas G, Muckenthaler MU, Simos G. 2-Oxoglutarate-dependent oxygenases control hepcidin gene expression. J Hepatol. 2008;48(5):801–810. doi: 10.1016/j.jhep.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 114.Lok CN, Ponka P. Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem. 1999;274(34):24147–24152. doi: 10.1074/jbc.274.34.24147. [DOI] [PubMed] [Google Scholar]
- 115.Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999;274(34):24142–24146. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- 116.Mole DR. Iron homeostasis and its interaction with prolyl hydroxylases. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2790. doi:10.1089/ars.2009.2790. [DOI] [PubMed] [Google Scholar]
- 117.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9(2):152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Folgueras AR, de Lara FM, Pendás AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 122.Velasco G, Cal S, Quesada V, Sánchez LM, López-Otín C. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J Biol Chem. 2002;277(40):37637–37646. doi: 10.1074/jbc.M203007200. [DOI] [PubMed] [Google Scholar]
- 123.Truksa J, Gelbart T, Peng H, Beutler E, Beutler B, Lee P. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. Br J Haematol. 2009;147(4):571–581. doi: 10.1111/j.1365-2141.2009.07873.x. [DOI] [PubMed] [Google Scholar]
- 124.Lakhal S, Talbot NP, Crosby A, et al. Regulation of growth differentiation factor 15 expression by intracellular iron. Blood. 2009;113(7):1555–1563. doi: 10.1182/blood-2008-07-170431. [DOI] [PubMed] [Google Scholar]
- 125.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40(1):132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hunter HN, Fulton DB, Ganz T, Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277(40):37597–37603. doi: 10.1074/jbc.M205305200. [DOI] [PubMed] [Google Scholar]
- 127.Jordan JB, Poppe L, Haniu M, et al. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J Biol Chem. 2009;284(36):24155–24167. doi: 10.1074/jbc.M109.017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53(4):620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 129.Schranz M, Bakry R, Creus M, Bonn G, Vogel W, Zoller H. Activation and inactivation of the iron hormone hepcidin: Biochemical characterization of prohepcidin cleavage and sequential degradation to N-terminally truncated hepcidin isoforms. Blood Cells Mol Dis. 2009;43(2):169–179. doi: 10.1016/j.bcmd.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 130.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107(1):328–333. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106(6):2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gagliardo B, Kubat N, Faye A, et al. Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process. J Hepatol. 2009;50(2):394–401. doi: 10.1016/j.jhep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 133.Swinkels DW, Girelli D, Laarakkers C, et al. Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS One. 2008;3(7):e2706. doi: 10.1371/journal.pone.0002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peslova G, Petrak J, Kuzelova K, et al. Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood. 2009;113(24):6225–6236. doi: 10.1182/blood-2009-01-201590. [DOI] [PubMed] [Google Scholar]
- 135.Roe MA, Spinks C, Heath AL, et al. Serum prohepcidin concentration: no association with iron absorption in healthy men; and no relationship with iron status in men carrying HFE mutations, hereditary haemochromatosis patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr. 2007;97(3):544–549. doi: 10.1017/S0007114507336829. [DOI] [PubMed] [Google Scholar]
- 136.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 137.Tomosugi N, Kawabata H, Wakatabe R, et al. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108(4):1381–1387. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 138.Kemna E, Tjalsma H, Laarakkers C, Nemeth E, Willems H, Swinkels D. Novel urine hepcidin assay by mass spectrometry. Blood. 2005;106(9):3268–3270. doi: 10.1182/blood-2005-05-1873. [DOI] [PubMed] [Google Scholar]
- 139.Murphy AT, Witcher DR, Luan P, Wroblewski VJ. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood. 2007;110(3):1048–1054. doi: 10.1182/blood-2006-11-057471. [DOI] [PubMed] [Google Scholar]
- 140.Murao N, Ishigai M, Yasuno H, Shimonaka Y, Aso Y. Simple and sensitive quantification of bioactive peptides in biological matrices using liquid chromatography/selected reaction monitoring mass spectrometry coupled with trichloroacetic acid clean-up. Rapid Commun Mass Spectrom. 2007;21(24):4033–4038. doi: 10.1002/rcm.3319. [DOI] [PubMed] [Google Scholar]
- 141.Kobold U, Dülffer T, Dangl M, et al. Quantification of hepcidin-25 in human serum by isotope dilution micro-HPLC-tandem mass spectrometry. Clin Chem. 2008;54(9):1584–1586. doi: 10.1373/clinchem.2008.107029. [DOI] [PubMed] [Google Scholar]
- 142.De Domenico I, Nemeth E, Nelson JM, et al. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8(2):146–156. doi: 10.1016/j.cmet.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Grebenchtchikov N, Geurts-Moespot AJ, Kroot JJ, et al. High-sensitive radioimmunoassay for human serum hepcidin. Br J Haematol. 2009;146(3):317–325. doi: 10.1111/j.1365-2141.2009.07758.x. [DOI] [PubMed] [Google Scholar]
- 144.Koliaraki V, Marinou M, Vassilakopoulos TP, et al. A novel immunological assay for hepcidin quantification in human serum. PLoS One. 2009;4(2):e4581. doi: 10.1371/journal.pone.0004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kroot JJ, Kemna EH, Bansal SS, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94(12):1748–1752. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weiss G, Theurl I, Eder S, et al. Serum hepcidin concentration in chronic haemodialysis patients: associations and effects of dialysis, iron and erythropoietin therapy. Eur J Clin Invest. 2009;39(10):883–890. doi: 10.1111/j.1365-2362.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 147.Peters HP, Laarakkers CM, Swinkels DW, Wetzels JF. Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rate. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp546. doi:10.1093/ndt/gfp546. [DOI] [PubMed] [Google Scholar]
- 148.Kato A, Tsuji T, Luo J, Sakao Y, Yasuda H, Hishida A. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol. 2008;28(1):115–121. doi: 10.1159/000109968. [DOI] [PubMed] [Google Scholar]
- 149.Swinkels DW, Wetzels JF. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2008;23(8):2450–2453. doi: 10.1093/ndt/gfn267. [DOI] [PubMed] [Google Scholar]