Abstract
It has been previously established that living cells, including mesenchymal stem cells, stiffen in response to elevation of substrate stiffness. This stiffening is largely attributed to the elevation of the tractions at the cell base that is associated with increases in cell spreading on more-rigid substrates. We show here, surprisingly, that mouse embryonic stem cells (ESCs) do not stiffen when substrate stiffness increases. As shown recently, these cells do not increase spreading on more-rigid substrates either. However, these ESCs do increase their basal tractions as substrate stiffness increases. We conclude that these ESCs exhibit mechanical behaviors distinct from those of mesenchymal stem cells and of terminally differentiated cells, and decouple its apical cell stiffness from its basal tractional stresses during the substrate rigidity response.
Cytoskeletal stiffening as a result of elevation of cytoskeletal tension (prestress) has been established as a key feature of anchorage-dependent cells such as terminally differentiated cells (1,2) and mesenchymal stem cells (3). This feature is consistent with the model that a living cell behaves as a prestress-supported, integrated network (1), which may have important implications for vital cell functions such as cell spreading (4), substrate rigidity sensing (2,5), gene expression (6), and cell proliferation and apoptosis (7). Recently we have reported that embryonic stem cells (ESCs) exhibit unique features of high intrinsic softness that dictates stress-induced cell spreading and differentiation (8). Unlike most other anchorage-dependent cells, these ESCs do not increase cell spreading on stiffer substrates (8). However, it remains unclear how these cells change their tractions and stiffness on different substrates.
Materials and Methods
Undifferentiated mouse embryonic stem cells (W4, 129/SvEv) were cultured and maintained in standard feeder free conditions on collagen-1 coated dishes, as described previously (8). Stiffness and traction measurements were performed on single individual cells ∼8 h after the ESCs were plated. Stiffness at the apical surface of an individual single cell was measured using either an RGD- or a FN-coated magnetic bead at 17.5 Pa and 0.3 Hz (8). RGD or FN was coated at 50 or 25 μg/mg bead, respectively.
Because it is known that fibronectin and collagen-1 bind to different integrin subsets, we point out that using the RGD-bead or the FN-bead may not engage the same set of cytoskeletal proteins as the collagen-1 coated substrate at the basal surface and thus may not be so simple to relate to basal tractions. These single ESCs appear viable and healthy because they exhibit normal ES cell shape, proliferate and form ES colonies, and have normal ES cell-doubling time (∼10.5 h).
Cell root-mean-square (RMS) tractions at the basal surface were quantified by measuring embedded fluorescent submicrometer particle displacement fields in the gel following published methods (8). The substrate stiffness of the gel was varied by altering bis-acrylamide crosslinker concentrations (0.04, 0.06, 0.1, and 0.3%) and polyacrylamide concentration (3%, 3%, 5%, and 5%) and the corresponding stiffnesses were 0.35, 0.6, 3.5, and 8.0 kPa using published protocols (2,4).
Results
To examine how mouse ESCs might alter their mechanical functions in response to substrate rigidity, we seeded single ESCs on collagen-1 coated polyacrylamide gels of various stiffness in the presence of ESC culture medium (leukemia inhibitory factor, i.e., +LIF) which maintains their pluripotency. The individual ESCs were all undifferentiated cells, as indicated by the high expression level of Oct3/4 (pou5f1), a primary marker for pluripotency (see Fig. S1 in the Supporting Material). As the substrate stiffness increased, cell tractions at the basal surface increased (Fig. 1 A), consistent with all previously published results in mesenchymal stem cells or terminally differentiated cells (3,5), although the projected areas of these ESCs did not change (8). Surprisingly, the stiffness of the ESCs, measured at the apical surface of the cells with magnetic twisting cytometry using either Arg-Gly-Asp (RGD)-coated beads or fibronectin (FN)-coated beads, did not exhibit corresponding increases with the substrate stiffness (Fig. 1 B). This peculiar result of the ESCs is different from those of other anchorage-dependent cells such as fibroblasts (5), although ESCs are also characterized as anchorage-dependent cells that depend on adhesion and cell shape change for survival. To investigate whether these ESCs can stiffen at all in response to mechanical stimulation, we applied periodic small stresses (17.5 Pa at 0.3 Hz) to the individual ESC seeded on collagen-1 coated soft substrate of 0.6 kPa. As shown in Fig. 2 B, the cell started to stiffen ∼5 min after the onset of stress application, accompanied by simultaneous elevations in tractions at the basal surface of the same cells (Fig. 2, A and C).
Figure 1.
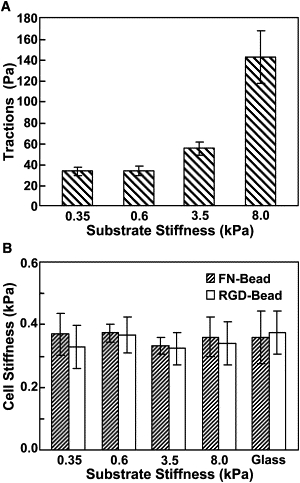
Traction and stiffness of mouse ESCs decouple on different substrate stiffness. (A) Cell root-mean-square (RMS) traction at the basal surface increases with the increase of substrate stiffness. There is no significant difference in ESC tractions between substrate stiffness of 0.35 and 0.6 kPa (p > 0.91), but significant differences are observed when plated on higher substrate stiffnesses: 0.6 and 3.5 kPa (p < 0.02) and 3.5 and 8.0 kPa (p < 0.006) (n = 11, 23, 13, or 13 cells on 0.35, 0.6, 3.5, or 8.0 kPa). (B) Stiffness of ESCs remained relatively constant regardless of changes in substrate stiffness (no statistical differences between different substrates). On substrate of 0.35, 0.6, 3.5, and 8.0 kPa or glass, n = 10, 10, 16, 14, or 12 cells for fibronectin-coated beads (FN-Bead); n = 15, 47, 28, 13, or 13 cells for RGD-coated beads (RGD-Bead). Mean ± SE.
Figure 2.
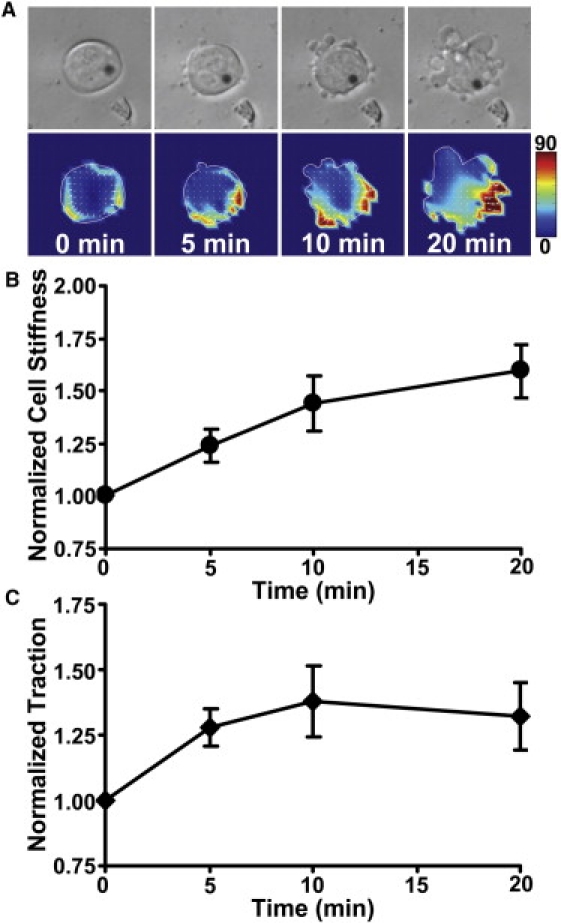
Apical cell stiffening and basal traction elevation in response to mechanical stress in ESCs. (A) Time-lapse images of a representative cell on a 0.6-kPa substrate show an increase in basal traction after onset of mechanical stress. (B) Normalized cell stiffness as a function of stress application duration shows apical cell stiffening in response to mechanical stimulation (p < 0.03, 0.02, and 0.006 comparing 5, 10, and 20 min with time 0; n = 6 cells; mean ± SE). (C) Normalized RMS traction in the same ESCs as in panel B in response to mechanical stress shows an elevation in basal traction. (p < 0.007, 0.001, and 0.024 comparing 5, 10, and 20 min with time 0; n = 6 cells; mean ± SE).
Discussion and Conclusion
Our data show that mouse ESCs clearly respond to collagen-1 coated substrate rigidity by increasing cell basal tractions. However, their apical stiffness does not change with substrate rigidity. This decoupling between basal tractions and apical stiffness as substrate rigidity varies appears to be a unique feature of these ESCs, dramatically different from that of the mesenchymal stem cells or of terminally differentiated cells (3,5).
At the time of this publication, we do not know the exact mechanism(s) underlying this stiffness-traction decoupling phenomenon. However, there are some possible explanations. It is known that the ESCs have much lower amounts of F-actin and actin bundles than their differentiated counterpart cells (8). It has been established that in terminally differentiated cells, these tensed actin bundles are essential for propagating a locally applied stress through the cytoskeleton at a distance for an integrated, concerted mechanical response (9). It is possible that the few actin bundles under the apical surface of the ESCs (10) are not sufficiently tensed so as to enable the observed elevations in tractions (i.e., myosin-II dependent tension) at the basal surface (in response to substrate rigidity) to be propagated to the apical surface.
Unlike terminally differentiated cells whose nucleus is ∼5–10 times stiffer than its cytoplasm (11,12), the huge nucleus of the ESC (8) is as soft as its cytoplasm and does not express nuclear intermediate filaments Lamin A/C (12), a stiff nuclear matrix inside the nuclear envelope. Lack of Lamin A/C in living cells contributes to a softer cytoplasm (13), possibly by softening the anchoring sites of the LINC (linker of nuclear-cytoskeleton) (14). As a result, the apical actin bundles might not be prestressed/tensed and/or mechanically integrated with the basal actin-myosin bundles. All these might have contributed to the lack of stiffening of the apical cytoskeleton in response to the traction elevation at the basal surface as substrate rigidity increases. Interestingly, it is reported that cell spreading and stiffening can be independently controlled when filamin-deficient, terminally differentiated cells are plated on collagen-1 or fibronectin (15).
In contrast, we show here that cell basal tractions and apical stiffness are independently controlled on different rigidities of substrates, although coated with the same collagen-1 concentration. Importantly, this decoupling between apical stiffness and basal tractions (and thus myosin-II dependent basal prestress) in ESCs can be abolished by applying a small oscillatory loading at the apical surface via integrins. The external stress-induced simultaneous responses in both stiffening and traction elevation shown in this report are preceded by increases in cell spreading and followed by stress-induced ESC differentiation, as evident by the observation that the pluripotent market Oct3/4 was downregulated (8).
Future studies are needed to elucidate the mechanisms of the cytoskeletal structural changes that are necessary for the observed external stress-induced coupling between stiffness and traction in ESCs.
Supporting Material
One figure is available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(10)00555-2.
Supporting Material
Acknowledgments
This work was supported by National Institutes of Health grant No. NIH R01 GM072744.
References and Footnotes
- 1.Wang N., Tolić-Nørrelykke I.M., Stamenović D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 2.Engler A.J., Griffin M.A., Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Yeung T., Georges P.C., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 5.Solon J., Levental I., Janmey P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Fabry B., Wang N. Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. Am. J. Physiol. Cell Physiol. 2002;280:C1475–C1484. doi: 10.1152/ajpcell.2001.280.6.C1475. [DOI] [PubMed] [Google Scholar]
- 7.Numaguchi Y., Huang S., Ingber D.E. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis. 2003;6:55–64. doi: 10.1023/a:1025821517679. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury F., Na S., Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu S., Chen J., Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Physiol. Cell Physiol. 2003;285:C1082–C1090. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- 10.Khatau S.B., Hale C.M., Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniotis A.J., Chen C.S., Ingber D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pajerowski J.D., Dahl K.N., Discher D.E. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.S., Hale C.M., Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale C.M., Shrestha A.L., Wirtz D. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 2008;95:5462–5475. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byfield F.J., Wen Q., Janmey P.A. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys. J. 2009;96:5095–5102. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


