Abstract
To clarify the molecular mechanisms behind quantal Ca2+ release, the graded Ca2+ release from intracellular stores through inositol 1,4,5-trisphosphate receptor (InsP3R) channels responding to incremental ligand stimulation, single-channel patch-clamp electrophysiology was used to continuously monitor the number and open probability of InsP3R channels in the same excised cytoplasmic-side-out nuclear membrane patches exposed alternately to optimal and suboptimal cytoplasmic ligand conditions. Progressively more channels were activated by more favorable conditions in patches from insect cells with only one InsP3R gene or from cells solely expressing one recombinant InsP3R isoform, demonstrating that channels with identical primary sequence have different ligand recruitment thresholds. Such heterogeneity was largely abrogated, in a fully reversible manner, by treatment of the channels with sulfhydryl reducing agents, suggesting that it was mostly regulated by different levels of posttranslational redox modifications of the channels. In contrast, sulfhydryl reduction had limited effects on channel open probability. Thus, sulfhydryl redox modification can regulate various aspects of intracellular Ca2+ signaling, including quantal Ca2+ release, by tuning ligand sensitivities of InsP3R channels. No intrinsic termination of channel activity with a timescale comparable to that for quantal Ca2+ release was observed under any steady ligand conditions, indicating that this process is unlikely to contribute.
Introduction
Ca2+ release through ubiquitous, endoplasmic-reticulum (ER)-localized inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) channels is the building block of complex intracellular Ca2+ signals that control numerous physiological processes, including apoptosis, secretion, immune responses, and memory (1–3). Complex modulation of InsP3R channel activities, including biphasic regulation by cytoplasmic free Ca2+ concentration ([Ca2+]i) and cooperative activation by [InsP3], gives rise to intricate Ca2+ signals including repetitive [Ca2+]i oscillations with frequencies tuned to stimulation level, propagating [Ca2+]i waves, and highly localized Ca2+ release events known as “blips” and “puffs,” as well as “quantal release”—graded release of Ca2+ in response to incremental levels of extracellular stimulation or [InsP3] (2). Although InsP3R-mediated quantal Ca2+ release has been observed in many studies in different cell types using Ca2+ flux assays or microfluorimetry, it remains unclear how such quantal release is generated in the presence of positive feedback activation of InsP3R by released Ca2+. Several molecular mechanisms have been proposed to account for the phenomenon, including InsP3 regulation of InsP3R channel activity and intrinsic termination of InsP3R activity leading to partial emptying of intracellular Ca2+ stores (4), regulation of InsP3R channel activity by [Ca2+] in the ER lumen (5), or the presence of channels with different InsP3 sensitivities in discrete Ca2+ stores (5,6). Three InsP3R genes are expressed in vertebrate cells with alternatively spliced forms, and the isoforms can form homo- as well as heterotetrameric channels (3), suggesting that graded ligand sensitivities underlying quantal Ca2+ release may be mediated by the presence of a large diversity of InsP3R channel types within cells. Here, we have discovered that heterogeneous ligand sensitivities can be present in a uniform population of InsP3R channels. Cytoplasmic-side-out (cyto-out) nuclear membrane patches were obtained from insect cells expressing only a single InsP3R isoform, or from InsP3R-deficient DT40 cells stably transfected with the cDNA of the rat type 3 InsP3R isoform (DT40-KO-r-InsP3R-3 cells). In patches exposed alternately to optimal and suboptimal ligand concentrations, no rapid intrinsic termination of InsP3R channel activity was observed, whereas the number of channels activated depended on both InsP3 and Ca2+ concentrations. These results indicate that heterogeneous sensitivity to ligand recruitment exists even within a homogeneous population of channels with identical primary sequence. Such heterogeneity is shown to be regulated largely by reversible sulfhydryl redox modifications of the channels.
Materials and Methods
Cyto-out nuclear patch-clamp experiments with rapid bath solution perfusion
Generation and maintenance of DT40-KO-r-InsP3R-3 cells and maintenance of Sf9 cells were described (7). Nuclear patch-clamp experiments were performed using nuclei isolated from Sf9 or DT40-KO-r-InsP3R-3 cells as described (7). InsP3R current traces were acquired as described (4). The solutions perfusing excised nuclear membrane patches in the cyto-out configuration were rapidly switched, as described (8) (Fig. 1, B–C). Perfusion solutions contained 140 mM KCl, 10 mM HEPES (pH 7.3), various [InsP3], 0.5 mM Ca2+ chelator (1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA) for 40–560 nM [Ca2+]i, (2-hydroxyethyl) ethylenediaminetriacetate (HEDTA) for 2–3 μM [Ca2+]i) with various [CaCl2] to give free [Ca2+] as stated (7). The pipette solution contained 140 mM KCl, 10 mM HEPES (pH 7.3), 2 μM free [Ca2+], and 10 μM InsP3. Perfusion and pipette solutions for Sf9 nuclei contained 0.5 mM Na2ATP, and those for DT40 nuclei contained 5 mM Na2ATP to ensure that all InsP3R channels were saturated with ATP4−. Free [Ca2+]s in all experimental solutions were confirmed by fluorimetry.
Figure 1.
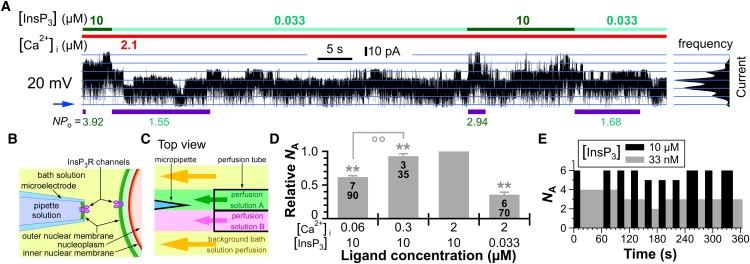
More Sf9 InsP3R channels were activated in the same nuclear membrane patch by more favorable ligand conditions. (A) InsP3R channel current trace from a cyto-out membrane patch excised from an isolated Sf9 nucleus containing many InsP3R channels with high Po. In this and subsequent current traces, cytoplasmic ligand concentrations in perfusion solutions are indicated by color bars at top, blue arrow indicates the closed-channel current level, blue lines mark evenly spaced current levels derived from the current amplitude histogram shown on the right, and the applied transmembrane potential used is indicated. NPo values during each exposure to various [InsP3]s are tabulated below the current trace. T99% durations evaluated for each exposure are indicated by the purple bars below the trace. (B) Schematic diagram showing the orientation of InsP3R channels in an isolated nucleus (right) and an excised cyto-out nuclear membrane (left). (C) Schematic diagram showing the arrangement of the rapid perfusion solution exchange system. The micropipette tip and excised cyto-out nuclear membrane were exposed alternately to perfusion solutions A or B by moving the perfusion tube so that the micropipette tip crossed the interface between the two perfusion solutions. (D) Plot of relative NA for Sf9 InsP3R channels in various ligand conditions. In this and subsequent relative NA or Po plots, ∗ and ∗∗ indicate statistically significant deviation from 1 (p < 0.05 and p < 0.005, respectively, by paired Student t-test); °° indicates statistically significant difference between values of relative NA connected by the line (p < 0.005 by unpaired t-test); numbers tabulated in the bars are of membrane patches (upper) and solution switches (lower) used to determine the relative NA values. Error bars indicate mean ± SE. (E) Plot of the number of InsP3R channels activated by various [InsP3] conditions in the same membrane patch used in A.
Counting active InsP3R channels in an isolated membrane patch
From a patch-clamp current record, NPo was evaluated as equal to either (〈i〉 − ic)/io, where 〈i〉 is the mean current, ic is the closed-channel background current, and io is the single-channel current; or (∑nTn)/(∑Tn), where Tn is the time during which the recorded current was at the nth level and n is from 0 (closed-channel current level) to NA, the maximum number of open-channel current levels observed and, therefore, the number of active channels in the patch. Tn was determined by Qub software (9). Assuming that all observed channels were identical, the single-channel open probability is Po = NPo/NA. To ensure that NA observed when the patch was exposed to optimal [InsP3] and [Ca2+]i (Nopt) was accurate (with confidence level >99%), only records >T99%(Nopt, NPo/Nopt) were analyzed. As a first-order estimate, assuming that all channels in a patch are identical and gate with simple, time-independent single-exponential kinetics,
where Tres is the empirically determined temporal resolution of the acquisition system (0.2 ms) (10), and τo is the mean open-channel duration determined from single-channel on-nucleus current records analyzed using Qub software. NA observed when the patch was exposed to suboptimal ligand concentrations (Nsub) was evaluated using only exposures with durations longer than a more conservative 99%-confidence duration: T99%(Nsub, NPo/Nopt) derived assuming that all Nopt channels activated by optimal ligand conditions contributed to the NPo measured. In Sf9 cells, expression level of the endogenous channels in the nuclear membrane was relatively low, with a mean Nopt of 3.8 ± 0.4, so that it was possible to determine NA properly in ligand conditions in which Po is as low as 0.2: T99% = 80 s for Nopt = 4 and τo ∼ 40 ms (10). In DT40-KO-r-InsP3R-3 cells, expression level of the recombinant channels was significantly higher, with a mean Nopt of 10.8 ± 1.0, so it was generally possible to determine NA for ligand conditions in which Po > 0.5: T99% = 20 s for Nopt =10 and τo ∼ 10 ms (observed in these experiments). Only patches with lower Nopt were used for determining NA for Po < 0.5. Although abrupt, irreversible channel inactivation was consistently observed for InsP3R from both Sf9 and DT40-KO-r-InsP3R-3 cells during nuclear patch-clamp experiments in on-nucleus or excised luminal-side-out configurations, the rate of channel inactivation was significantly slower in excised cyto-out membrane patches, for unknown reason(s). Thus, long continuous current records of InsP3R channels with multiple exposures (>T99%) to various ligand conditions were regularly obtained for Sf9 InsP3R (>200 s) and InsP3R in DT40-KO-r-InsP3R-3 cells (>10 min).
[Ca2+]i imaging
DT40-KO-r-InsP3R-3 cells were preexposed to culture medium with or without 10 mM dithiothreitol (DTT) for 2 h. Cells were loaded with fura-2 and imaged as described previously (11). Cells were sequentially exposed to perfusion solutions containing 0, 50 ng/ml, and 5 μg/ml anti-IgM antibody to stimulate InsP3R-mediated Ca2+ release through the B-cell receptor pathway (12). In experiments using DTT-pretreated cells, dye-loading and perfusion solutions contained 10 mM DTT. The average [Ca2+]i (with the baseline level subtracted) observed during exposure to one anti-IgM antibody concentration was used as a measure of the InsP3R-mediated [Ca2+]i signal intensity.
Results
Graded recruitment by ligands of endogenous InsP3R channels in insect Sf9 cells
The number of channels activated (NA) by different cytoplasmic ligand conditions in the same excised cyto-out nuclear membrane patch was monitored by rapid perfusion switching between optimal and suboptimal ligand concentrations (8) (Fig. 1, B and C). The relative NA observed at a solution switch is the ratio of NA in suboptimal ligand conditions to NA in optimal conditions immediately before or after the solution switch. If ligand concentrations only affect channel Po, then all activatable channels present in the patch should gate under both conditions, albeit with different Po, and relative NA should be 1. However, more InsP3R channels were consistently observed in optimal conditions (Fig. 1, A and D). Furthermore, there was a direct correlation between relative NA and the level of ligand stimulation. For example, fewer channels were activated by 60 nM Ca2+ compared with the more favorable 300 nM in the same membrane patches (Fig. 1 D), even though both concentrations are suboptimal (10). To ensure that NA was not underestimated due to reduced Po in the suboptimal conditions, the patches were exposed to each solution for long periods (>T99%) before the next solution switch, enabling NA to be determined with >99% confidence (see Materials and Methods). It is interesting that even though fewer InsP3R channels were activated by suboptimal ligand conditions, frequently the same number was activated (±1) in sequential exposures to the same suboptimal conditions (Fig. 1, A and E), suggesting that the number of channels that can be activated is not determined by a stochastic process. These observations indicate that even within a population of Sf9 InsP3R channels in close physical proximity, there are heterogeneous sensitivities to ligand recruitment.
Graded ligand recruitment of homogeneous recombinant InsP3R channels
Although only one InsP3R gene has been identified in invertebrates, Drosophila and Caenorhabditis elegans InsP3R can exist as splice variants (3). Thus, variations in the primary sequence of the endogenous Sf9 InsP3R as a cause of the observed heterogeneous ligand sensitivities cannot be ruled out. To determine whether heterogeneous ligand sensitivities exist in a population of channels with identical primary sequences, we used a stably transfected cell line (DT40-KO-r-InsP3R-3) derived from DT40-InsP3R-KO cells (12) that have all three endogenous InsP3R genes knocked out, so that only recombinant rat type 3 InsP3R (InsP3R-3) is expressed (7). All InsP3R channels in these cells must be homotetrameric, with subunits having the same primary sequence. Remarkably, graded activation by ligands of these homogeneous InsP3R channels was also observed, with relative NA increasing gradually as ligand conditions became progressively more favorable (Fig. 2, A and C and Fig. S1 A in the Supporting Material). Also reminiscent of Sf9 channels, repeated exposures of patches to the same suboptimal conditions consistently activated the same number of channels (±1). These observations demonstrate that a population of InsP3R channels with homogeneous primary sequence can exhibit intrinsic heterogeneous sensitivities to recruitment by Ca2+i and InsP3.
Figure 2.
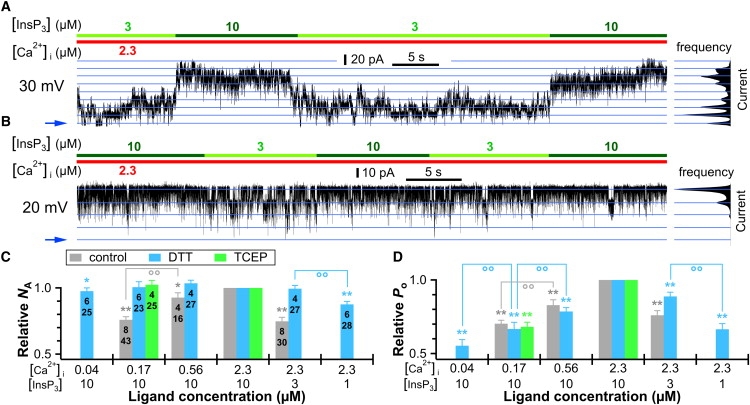
Effects of sulfhydryl reduction on graded ligand recruitment and activity level of homotetrameric recombinant rat type 3 InsP3R channels consisting of subunits with identical primary sequence expressed in DT40-KO-r-InsP3R-3 cells. (A and B) Current trace of control (A) and DTT-treated (B) InsP3R-3 channels alternately exposed to optimal and suboptimal [InsP3] conditions. (C and D) Plots of relative NA (C) and relative Po (D) of control, DTT-treated (3 mM for >60 min) and TCEP-treated (6 mM for >90 min) InsP3R-3 channels in various ligand conditions. Note that the ranges of the relative NA and Po axes are from 0.5 to 1 to emphasize the change in Po and NA in various ligand conditions. Convention for symbols ∗, ∗∗ and °° is the same as in Fig. 1.
Heterogeneous ligand sensitivity is largely regulated by reversible redox modulation
The heterogeneous ligand sensitivities observed in a homogeneous population of InsP3R channels cannot be easily modeled by kinetic schemes involving various activation states. Thus, it seems more reasonable to assume that the homogeneous population of channels becomes heterogeneous due to posttranslational modifications. Regulation of InsP3R channel activity by phosphorylation, redox status, and protein interaction has been documented (3), although the possible roles of such regulation in “increment detection” (6,13,14), or graded recruitment of InsP3R by ligands, have not been studied. In preliminary studies, isolated DT40-KO-r-InsP3R-3 nuclei treated with 0.7 unit/μl bovine intestinal alkaline phosphatase for 30 min at room temperature exhibited a ligand-dependent change in NA similar to that seen with untreated nuclei. In contrast, the heterogeneous sensitivity was largely abrogated by exposure of isolated nuclei to DTT (3 mM for at least 60 min) (Fig. 2, B and C, and Fig. S1, B and C). Of note, the normal dependence of channel Po on [InsP3] and [Ca2+]i was unchanged after the channels were exposed to DTT, with Po decreasing when [Ca2+]i was reduced from optimal 2.3 μM to suboptimal 170 and 40 nM, and when [InsP3] was reduced from saturating 10 μM to subsaturating 3 and 1 μM (Fig. 2 D). This indicates that the effects of ligand concentrations on the number of channels activated and on Po are independent. Similar results were obtained with another sulfhydryl reducing reagent, tris(2-carboxyethyl)phosphine (TCEP; 6 mM for at least 90 min) (Fig. 2, C and D, and Fig. S1 D), indicating that sulfhydryl reduction of InsP3R regulates these effects.
We attempted to observe acute effects of DTT on InsP3R channel NA in continuous cyto-out nuclear patch-clamp experiments with pipette and perfusion solutions containing DTT using nuclei that had not been preexposed to DTT. However, the decrease in NA normally observed at [Ca2+]i reduction from 2 μM to 170 nM was not altered by 30 min exposure to DTT, even when [DTT] was raised to 20 mM for ∼7 min. Because cyto-out nuclear patches rarely remained stable under constant perfusion for >20 min, we probed the duration of DTT exposure required to abrogate the heterogeneity in InsP3R [Ca2+]i sensitivity by using nuclei first exposed to 3 mM DTT for ∼20 min. Cyto-out membrane patches from these nuclei were then used in experiments with pipette and perfusion solutions containing DTT (3 mM). In two experiments, a change in NA was observed at the beginning but was abrogated during the experiments. Total DTT exposure times that eliminated heterogeneity of channel [Ca2+]i sensitivity were 37 and 55 min. Thus, in our in vitro experimental conditions, a long exposure to a reducing environment is required to substantially reduce the heterogeneity in InsP3R channel ligand sensitivity.
To determine the reversibility of the abrogation of heterogeneous InsP3R ligand sensitivities by sulfhydryl reducing reagents, DT40-KO-r-InsP3R-3 nuclei were first incubated with 6 mM TCEP for 120 min, to substantially reduce the heterogeneity, and then exposed to H2O2 (5 mM for at least 60 min) before cyto-out nuclear patch-clamp experiments were performed. H2O2 treatment completely restored heterogeneous ligand sensitivities to the same levels observed in untreated channels (Fig. 3 A and Fig. S1 E). Relative Po of the H2O2-treated channels was also very similar to those of untreated channels in all suboptimal ligand concentrations (Fig. 3 D). Similar effects on NA were obtained with thimerosal (TMS) (Fig. 3 A), another oxidizing reagent that has been shown to modify InsP3R-mediated Ca2+ release (15–19). However, TMS-treated InsP3R channels exhibited substantially lower Po (0.38 ± 0.06) under optimal ligand conditions than either untreated (0.72 ± 0.02) or H2O2-treated channels (0.73 ± 0.01) (Fig. 3, C and E). Furthermore, whereas a negligible amount of channel run-down (decrease in NA with time) was observed in cyto-out patch-clamp experiments using untreated or DTT-, TCEP-, or TCEP⇒H2O2-treated InsP3R channels, TMS-treated InsP3R channels inactivated rapidly, with a time constant of ∼80 ± 40 s (N = 5), even though saturating InsP3 was continuously present (Fig. S1 F). Thus, TMS has complex effects on the gating behaviors of InsP3R channels not observed with H2O2.
Figure 3.
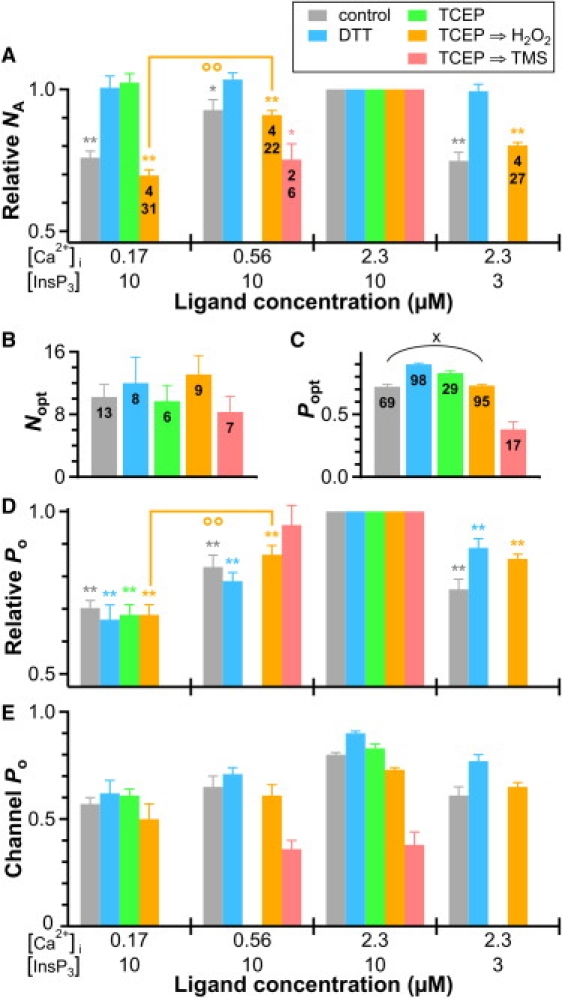
The effects of sulfhydryl reduction on relative NA and Po of InsP3R-3 channels are reversible. (A) Relative NA plot shows effects of exposure to H2O2 (5 mM for >60 min) and TMS (100 μM for >90 min) after TCEP treatment, in comparison with those of other redox treatments. Convention for symbols ∗, ∗∗ and °° is the same as in Fig. 1. (B) Plot of number of channels observed in optimal ligand conditions Nopt after various redox treatments. There is no significant difference between any of the Nopt values (p > 0.05 by one-way ANOVA test). Total numbers of cyto-out membrane patches used are tabulated. (C) Plot of optimal Po (Popt) observed after the redox treatments. All Popt values are significantly different from one another (p < 0.05 by unequal variance t-test) except that for control channels and that for channels treated with TCEP and then H2O2, connected by the round bracket marked with ×. Total numbers of Popt measurements performed are tabulated. (E) Plot of InsP3R channel Po in different ligand conditions after various redox treatments. Note that the range of the channel Po axis is 0 to 1.
We performed a series of cyto-out patch-clamp experiments after various durations of H2O2 exposure to gauge the rate of restoration by H2O2 of ligand sensitivity heterogeneity for TCEP-treated channels. Whereas 45 min was the longest H2O2 exposure after which there was still no change in NA when [Ca2+]i was dropped from 2.3 μM to 170 nM, the change in NA with [Ca2+]i decrease was restored in all six experiments with H2O2 exposures >58 min. Thus, a long exposure to H2O2 was required to modify InsP3R channel ligand sensitivities under our in vitro experimental conditions.
None of the redox treatments significantly altered the number of InsP3R channels activated by optimal ligand conditions, Nopt (Fig. 3 D). Thus, regardless of their redox status, all functional InsP3R channels can be similarly activated by optimal ligand stimulation. In contrast, the number of channels activated by suboptimal ligand conditions is significantly increased by a reducing environment and decreased by an oxidizing one (Fig. 3 A). On the other hand, channel Po was only modestly changed (∼10%) by all redox reagents except TMS over a wide range of ligand concentrations (Fig. 3, C–E). Thus, the main effect of the redox status of InsP3R channel is to determine the number of channels that can be activated in suboptimal ligand conditions by modulating the sensitivities of the channel to activation by InsP3 and Ca2+i.
Cellular redox potential modulates InsP3R-mediated Ca2+ signaling
The physiological significance of the observed redox modulation of heterogeneous ligand sensitivities of InsP3R was evaluated by studying the effects of DTT treatment on InsP3R-mediated Ca2+ signaling in intact DT40-KO-r-InsP3R-3 cells. Cells were sequentially exposed to increasing concentrations of anti-IgM antibody (Ab) to stimulate InsP3R-mediated Ca2+ release through the endogenous B-cell antigen receptor pathway (12). The average [Ca2+]i above the basal level was used as a measure of Ca2+ signal intensity (Fig. 4). Although increasing concentrations of Ab elicited incrementally higher [Ca2+]i signals in both control and DTT-treated cells, [Ca2+]i signals in DTT-treated cells were consistently higher. For example, [Ca2+]i signals in DTT-treated cells exposed to 50 ng/ml Ab were comparable to those in control cells exposed to saturating (5 μg/ml) [Ab]. In contrast, saturating [Ab] elicited similar [Ca2+]i signals in DTT-treated and control cells. Of note, [Ca2+]i signals were significantly elevated in DTT-treated cells even in the absence of Ab stimulation, and comparable to those in control cells exposed to subsaturating (50 ng/ml) [Ab]. Although InsP3R-mediated Ca2+ release is only one of a number of mechanisms controlling [Ca2+]i signaling, these effects of DTT are remarkably consistent with those expected based on the effects of redox modification on single InsP3R channel activity since sulfhydryl reduction of InsP3R channels increases the number of activated channels in response to suboptimal ligand stimulation, but does not affect the number of channels activated in response to strong stimulation. These results demonstrate that InsP3R-mediated Ca2+ signaling can be modulated by the cellular redox potential, with possible isoform specificity, and they suggest that this occurs largely through regulation of the number of channels involved in Ca2+ release. Furthermore, these results also suggest that graded recruitment of InsP3R channels by incremental ligand stimulation plays a significant role in the regulation of Ca2+ signaling at the cellular level.
Figure 4.
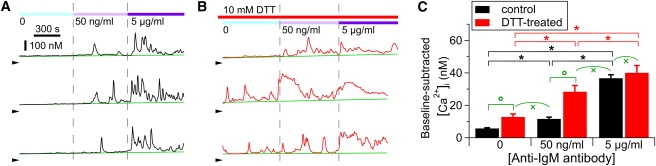
DTT treatment increases sensitivity of anti-IgM-induced InsP3R-mediated [Ca2+]i signals in DT40-KO-r-InsP3R-3 cells. (A and B) [Ca2+]i in control (A) and DTT-treated (B) DT40-KO-r-InsP3R-3 cells exposed to various [anti-IgM antibody] levels. Color bars at top indicate [anti-IgM antibody] and [DTT] in perfusion solutions. Green lines mark baseline [Ca2+]i levels. Arrowheads indicate [Ca2+]i = 0. (C) Plot of averaged Ca2+ signal levels observed in 310 control and 152 DTT-treated cells. ∗ and ° indicate statistically significant differences between signal levels connected by the square bracket, with p < 0.05 by paired and unpaired t-test, respectively. × indicates no significant difference between mean signal levels connected by round bracket (p > 0.05 by unpaired t-test).
Discussion
Graded recruitment of single InsP3R channels by incremental ligand activation
Graded recruitment of individual InsP3R channels by increasing levels of ligand stimulation was observed previously in a study of the endogenous channels in insect Sf9 cells using on-nucleus patch-clamp electrophysiology (10). Not only did the channel Po increase in more favorable ligand conditions, as expected, but the mean number of active channels also increased, which was unexpected. However, technical issues limited confidence in the conclusion that only a subset of channels could respond to intermediate levels of ligand stimulation. The cytoplasmic solution inside the micropipette could not be changed (8), so the number of active channels could be monitored under just one set of ligand conditions in each experiment. Although factors that randomly affect the number of active channels recorded, like the size of the micropipette tip and the expression level of the InsP3R, might cancel out over large numbers of experiments, systematic factors, like possible ligand-induced InsP3R channel clustering (20), could not be properly controlled for. In this study, graded recruitment of InsP3R channels was directly evaluated by recording the number of active channels in the same excised cyto-out membrane patches under optimal and suboptimal ligand conditions. For both endogenous InsP3R channels in insect Sf9 cells and recombinant rat InsP3R-3 channels expressed in chicken DT40 B cells with no endogenous InsP3R background, the number of activated channels was observed to be a graded function of the strength of ligand stimulation.
The inability to activate all the channels in subsaturating [InsP3] cannot be accounted for by kinetic schemes involving channels in some ligand-bound sequestered state(s) being unable to open (10), because channels in such states cannot be activated without prior dissociation of bound ligand. In contrast, more InsP3R channels were activated when [InsP3] or [Ca2+] were rapidly stepped from subsaturating to saturating levels. In a similar way, kinetic schemes involving identical channels, as in the case of the recombinant InsP3R-3-expressing cells, cannot account for the observation that the same number of channels remained unactivated in repeated steps to suboptimal ligand concentrations, suggesting that a nonstochastic process was involved. These observations demonstrate that multiple levels of sensitivity to ligand (both InsP3 and Ca2+i) stimulation exist, even among a population of homotetrameric recombinant InsP3R channels consisting of subunits with identical primary sequences.
It is important to recognize that ligand recruitment of InsP3R channels is a different kind of ligand regulation of the channels that is unrelated to and independent of the ligand regulation of InsP3R channel Po. This is most clearly demonstrated by the observation that as the heterogeneity in ligand sensitivity of the channels was abrogated by sulfhydryl reducing agents (Fig. 3 A), the channel Po in suboptimal ligand conditions was nevertheless significantly lower than Popt (Fig. 3 E). If the reducing treatment increases NA at suboptimal ligand conditions by enhancing the capacity of the ligand to increase channel Po, then the channel Po observed at suboptimal ligand conditions should increase also.
A model that can account for our observations is illustrated in Fig. 5. In this model, a channel remains closed, with Po = 0, when ligand (Ca2+ or InsP3) concentrations are below a threshold ([ligand]thres). When [ligand] increases above [ligand]thres, the channel starts to gate, with Po jumping from 0 to a finite value. Further increases in [ligand] raise channel Po, in accordance with the ligand dependence of channel Po observed in previous single-channel studies (3). Thus, [ligand]thres is a measure of the sensitivity of the channel to ligand recruitment, which is different from the ligand dependence of channel Po. The graded recruitment of channels by increasing levels of ligand activation observed in this study implies that many degrees of ligand sensitivity (i.e., [ligand]thres) exist even among homotetrameric channels, as shown in Fig. 5. Steady-state patch-clamp experiments measuring Po of these channels at various [ligand]s will always observe active channels with identical channel Po, detecting no apparent heterogeneity in the ligand regulation of channel Po. However, in experiments in which the ligand concentration is changed, the number of active channels observed will change with [ligand] due to the heterogeneity in [ligand]thres among the channels.
Figure 5.
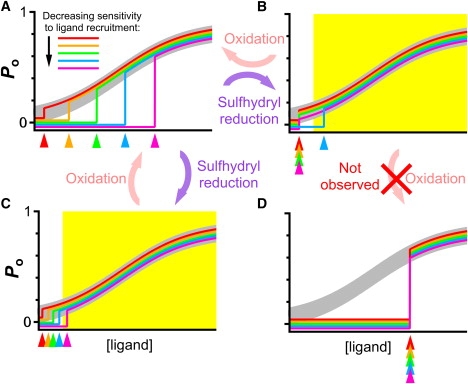
Model to account for graded ligand recruitment of InsP3R channels due to heterogeneous ligand sensitivities. (A) Plot of channel Po versus [ligand] curves for InsP3R channels. Each color curve represents the ligand dependence of Po for InsP3R channels with a different sensitivity to ligand recruitment. [ligand]thres values are indicated by colored arrowheads. The thick gray curve represents the homogeneous ligand dependence of channel Po observed for all the channels. (B and C) Possible effects of sulfhydryl reduction treatment on sensitivities of InsP3R channels, homogenizing channel sensitivities (B) or sensitizing the channels (C). Range of physiologically relevant [ligand] is indicated by yellow region. (D) A possible effect of H2O2 treatment on InsP3R channel ligand sensitivities that was not observed in this study.
Sulfhydryl redox modification largely accounts for heterogeneous InsP3R ligand sensitivities
All three InsP3R isoforms contain tens of cysteine residues (3), which can be in different redox states (21). We found that the heterogeneity in sensitivity to InsP3 and Ca2+i activation of homotetrameric InsP3R-3 channels consisting of subunits with identical primary sequence was mostly abrogated by treatment with sulfhydryl reducing agents (DTT or TCEP). That such effects were observed in excised nuclear membrane patches suggests that they were caused by modifications of the intrinsic sulfhydryl redox status of the channels. Although sulfhydryl reduction enabled more channels to become activated under suboptimal ligand conditions, it did not increase the total number of activatable channels (Fig. 3 B). Furthermore, sulfhydryl reduction only modulated channel Po to a modest extent (∼10%) in all ligand conditions (Fig. 3 E). Thus, the major effect of sulfhydryl reduction on single InsP3R channel gating behaviors is to enable them to be recruited into activity in less favorable conditions of [InsP3] and [Ca2+]i. In our model, this can be achieved either by homogenizing the sensitivity of the channels so that most channels have the same low [ligand]thres (Fig. 5 B) or by sensitizing the channels so that their [ligand]thres, although still different, are mostly lower than the relevant physiological [ligand] range (Fig. 5 C). In both cases, heterogeneity in sensitivity of the channels to ligand recruitment observed within the physiological [ligand] range is reduced.
The effects of DTT or TCEP were completely reversed by H2O2. This is remarkable, because if sulfhydryl reduction changes the [ligand]thres of most channels to the same low value (Fig. 5 B), then prolonged treatment of the channels with H2O2 (as used in our protocol) might be expected to change the [ligand]thres of most/all channels to the same high value (Fig. 5 D). The observed restoration of channel heterogeneity by H2O2 suggests either that there is some intrinsic difference among the InsP3R channels that prevents H2O2 from changing the [ligand]thres of all of them to the same high value, or that sulfhydryl reduction sensitizes all the channels to ligand recruitment while retaining their differences in [ligand]thres (Fig. 5 C) and H2O2 restores them to their original, different ligand sensitivities (Fig. 5 A).
Besides H2O2, TMS (100 μM) also reversed the effects of sulfhydryl reduction, but it had additional effects of reducing Po under optimal conditions and inducing rapid irreversible channel inactivation. This agrees with reports that high concentrations (>2 μM) of TMS, but not other biological oxidizing agents, irreversibly suppress InsP3R-mediated Ca2+ release in various cell types (17,22,23). These TMS-specific effects may be due to reactions involving the mercury present in TMS (24).
Our observations that treatment with sulfhydryl reducing agents enables InsP3R channels to be recruited by less favorable [Ca2+]i and [InsP3] conditions and enhances submaximally stimulated InsP3-mediated Ca2+ release in DT40-KO-r-InsP3R-3 cells disagree with observations from previous studies that InsP3R-mediated Ca2+ signaling is potentiated by treatment with sulfhydryl oxidizing regents (e.g., oxidized glutathione, tert-butyl hydroperoxide, and low concentrations of TMS (15,16,18,23,25,26)) and inhibited by DTT (17,27). However, different sensitivity to TMS treatment has been observed for different InsP3R isoforms (18,23,28). Furthermore, enhancement of Ca2+ release by the sulfhydryl reducing reagent NADH (29) and by high concentrations (1 mM) of DTT, reduced glutathione, or β-mercaptoethanol in the presence of inhibitory levels (1 mM) of TMS (17) have been reported. Thus, the variability in the observed effects of redox modifications of InsP3R-mediated Ca2+ release, especially in intact or permeabilized cells, may be due to the complexity of the systems used. It may also suggest that InsP3R channels contain multiple functional redox-sensing thiol groups that mediate different effects on channel activity with different susceptibilities to modification by different redox reagents.
Effects of sulfhydryl redox modification of InsP3R channels on intracellular Ca2+ signaling
Our results suggest that reversible posttranslational redox modification of InsP3R channels can be a physiological mechanism that regulates intracellular Ca2+ signaling by fine-tuning the ligand sensitivities of the channels. This is supported by the strong correlations between the effects of DTT treatment on InsP3R ligand sensitivity and the intensity of [Ca2+]i signals generated by extracellular stimuli in DT40-KO-r-InsP3R-3 cells. DTT treatment only increased the number of channels activated by suboptimal ligand concentrations, and it substantially enhanced the intensity of Ca2+ signals only in cells stimulated by subsaturating agonist concentrations. In our in vitro experiments with isolated nuclei, prolonged (tens of minutes) exposures to relatively high concentrations of sulfhydryl redox reagents were required to significantly alter the ligand sensitivities of InsP3R channels. In contrast, much shorter (1- to 5-min) exposures to sulfhydryl redox reagents could alter InsP3R-mediated Ca2+ release in permeabilized cells (16,26,27). The difference may be the result of loss in the isolated nuclei used in our experiments of soluble cytosolic oxidases and reductases that catalyze redox modifications of InsP3R in vivo. Our results suggest that the sulfhydryl redox state of the InsP3R may be quite stable naturally despite changes in the local redox potential until it comes into contact with an appropriate oxidoreductase to catalyze its redox modification. With specific localization of enzymes that regulate local cytoplasmic and ER luminal redox potentials (30) and of oxidoreductases that catalyze redox modifications of various thiol groups in the InsP3R, the distribution of InsP3R channels with different ligand sensitivities could be highly compartmentalized. Such compartmentalization can play a significant role in determining the spatial characteristics of intracellular Ca2+ signals, like generating InsP3R channels with high InsP3 sensitivity at highly localized discrete “hot spots” to release Ca2+ locally in “blips” under resting or low-stimulation conditions (31), and forming regions with InsP3R channels with lower InsP3 and Ca2+i sensitivities to constrain Ca2+ release to “puffs” at suboptimal stimulation conditions so that propagating Ca2+ waves will only be triggered when stimulation exceeds some critical level (32). Sulfhydryl redox modification of InsP3R channel ligand sensitivities provides an intricate means to allow the intracellular Ca2+ signaling pathway to be regulated by the cellular redox status. Future studies of the effects of redox modifications of InsP3R channels properties can shed light on how physiological processes regulating the oxidative state of cells can affect Ca2+ signaling and how pathologic conditions resulting in abnormal cellular redox status can lead to Ca2+ signaling dysregulation.
Graded InsP3R channel recruitment and quantal Ca2+ release
Our observations validate heterogeneous ligand sensitivities of InsP3R channels as a mechanism that can contribute to the phenomenon of InsP3R-mediated quantal Ca2+ release (6). With InsP3R channels that have different InsP3 sensitivities in different discrete intracellular Ca2+ stores, incremental [InsP3] can progressively empty stores with less sensitive InsP3R channels (5,6). Existence of ligand sensitivity heterogeneity among InsP3R channels containing subunits with identical primary sequence explains how quantal Ca2+ release can be generated by purified homotetrameric cerebellar InsP3R reconstituted into lipid vesicles (33,34). Our study suggests that discrete Ca2+ stores containing InsP3R channels with different InsP3 sensitivities can be generated by different redox environments around the stores.
Graded recruitment of InsP3R by ligands can also increase the dynamic range of Ca2+ release in response to stimulation. The amount of Ca2+ released by a population of InsP3R channels is proportional to the activity (Po) of individual channels and the number of channels activated (NA). As shown previously by Ionescu et al. (10) and more clearly here, ligand regulation of InsP3R Po and NA are independent. When [InsP3] increases as a result of extracellular stimulation, more Ca2+ will be released from the ER Ca2+ store than one would expect from the increase in channel Po alone because of the recruitment of more activated channels. Heterogeneous [Ca2+]i sensitivities of the channels will in turn generate stronger positive feedback in Ca2+-induced Ca2+ release because of the increase in both Po and NA generated by more favorable [Ca2+]i. Thus, graded recruitment of InsP3R channels by ligands can enhance the increase in Ca2+ release generated by higher [InsP3] and contribute to the transformation of localized Ca2+ release events (puffs) into propagating Ca2+ waves in response to more intense intracellular or extracellular stimulation (31,32,35).
Finally, it has been suggested that time-dependent intrinsic reduction or termination of InsP3R channel activity leading to partial emptying of Ca2+ stores can be a mechanism contributing to quantal Ca2+ release (36–39). However, within the timescale for termination of the fast phase of quantal Ca2+ release (∼10 s) (6,13,14), no intrinsic time-dependent reduction in Po or NA has been observed for Sf9 channels after [InsP3] jumps from 0 to saturating (8) or subsaturating levels (33 nM; n = 92), or here in either Sf9 channels or recombinant channels in DT40-KO-r-InsP3R-3 cells in various combinations of constant suboptimal ligand conditions. Abrupt channel inactivation in constant [InsP3] has been observed in nuclear patch-clamp studies of endogenous channels from Sf9 cells (10) and type 1 InsP3R channels from Xenopus oocytes (38), but with long mean channel activity duration (20–30 s) even under suboptimal [InsP3] and [Ca2+]i conditions. The mean activity duration before abrupt channel inactivation was even longer for recombinant type 3 InsP3R channels from DT40-KO-r-InsP3R-3 cells (120 s). Thus, intrinsic reduction or termination of InsP3R channel activity is unlikely to contribute significantly to InsP3R-mediated quantal Ca2+ release.
Supporting Material
One figure is available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(10)00532-1.
Supporting Material
Acknowledgments
This work was supported by National Institutes of Health grants R01 GM074999 to D.-O.D.M., R01 GM065830-06A1 to D.-O.D.M. and J.K.F., and R01 MH059937 to J.K.F.
References
- 1.Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Foskett J.K., White C., Mak D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak D.-O.D., McBride S., Foskett J.K. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parys J.B., Missiaen L., Casteels R. Mechanisms responsible for quantal Ca2+ release from inositol trisphosphate-sensitive calcium stores. Pflugers Arch. 1996;432:359–367. doi: 10.1007/s004240050145. [DOI] [PubMed] [Google Scholar]
- 6.Muallem S., Pandol S.J., Beeker T.G. Hormone-evoked calcium release from intracellular stores is a quantal process. J. Biol. Chem. 1989;264:205–212. [PubMed] [Google Scholar]
- 7.Mak D.-O.D., White C., Foskett J.K. Nuclear patch clamp electrophysiology of inositol trisphosphate receptor Ca2+ release channels. In: Putney J.W., editor. Methods in Calcium Signaling Research. 2nd ed. CRC Press; Boca Raton, FL: 2005. pp. 203–229. [Google Scholar]
- 8.Mak D.-O.D., Pearson J.E., Foskett J.K. Rapid ligand-regulated gating kinetics of single inositol 1,4,5-trisphosphate receptor Ca2+ release channels. EMBO Rep. 2007;8:1044–1051. doi: 10.1038/sj.embor.7401087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin F., Auerbach A., Sachs F. A direct optimization approach to hidden Markov modeling for single channel kinetics. Biophys. J. 2000;79:1915–1927. doi: 10.1016/S0006-3495(00)76441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ionescu L., Cheung K.H., Foskett J.K. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal [corrected] Ca2+ release. J. Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White C., Li C., Foskett J.K. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat. Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugawara H., Kurosaki M., Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. USA. 1990;87:3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor C.W., Potter B.V. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem. J. 1990;266:189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Missiaen L., Taylor C.W., Berridge M.J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352:241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- 16.Bootman M.D., Taylor C.W., Berridge M.J. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1992;267:25113–25119. [PubMed] [Google Scholar]
- 17.Kaplin A.I., Ferris C.D., Snyder S.H. Purified reconstituted inositol 1,4,5-trisphosphate receptors. Thiol reagents act directly on receptor protein. J. Biol. Chem. 1994;269:28972–28978. [PubMed] [Google Scholar]
- 18.Bultynck G., Szlufcik K., De Smedt H. Thimerosal stimulates Ca2+ flux through inositol 1,4,5-trisphosphate receptor type 1, but not type 3, via modulation of an isoform-specific Ca2+-dependent intramolecular interaction. Biochem. J. 2004;381:87–96. doi: 10.1042/BJ20040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thrower E.C., Duclohier H., Dawson A.P. The inositol 1,4,5-trisphosphate-gated Ca2+ channel: effect of the protein thiol reagent thimerosal on channel activity. Biochem. J. 1996;318:61–66. doi: 10.1042/bj3180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taufiq-Ur-Rahman A., Skupin A., Taylor C.W. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph S.K., Nakao S.K., Sukumvanich S. Reactivity of free thiol groups in type-I inositol trisphosphate receptors. Biochem. J. 2006;393:575–582. doi: 10.1042/BJ20050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirier S.N., Poitras M., Guillemette G. Thiol-reactive agents biphasically regulate inositol 1,4,5-trisphosphate binding and Ca2+ release activities in bovine adrenal cortex microsomes. Endocrinology. 2001;142:2614–2621. doi: 10.1210/endo.142.6.8195. [DOI] [PubMed] [Google Scholar]
- 23.Vanlingen S., Sipma H., Parys J.B. Modulation of type 1, 2 and 3 inositol 1,4,5-trisphosphate receptors by cyclic ADP-ribose and thimerosal. Cell Calcium. 1999;25:107–114. doi: 10.1054/ceca.1998.0010. [DOI] [PubMed] [Google Scholar]
- 24.Elferink J.G. Thimerosal: a versatile sulfhydryl reagent, calcium mobilizer, and cell function-modulating agent. Gen. Pharmacol. 1999;33:1–6. doi: 10.1016/s0306-3623(98)00258-4. [DOI] [PubMed] [Google Scholar]
- 25.Hilly M., Piétri-Rouxel F., Mauger J.P. Thiol reagents increase the affinity of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1993;268:16488–16494. [PubMed] [Google Scholar]
- 26.Bird G.S., Burgess G.M., Putney J.W. Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. J. Biol. Chem. 1993;268:17917–17923. [PubMed] [Google Scholar]
- 27.Montero M., Barrero M.J., Alvarez J. Stimulation by thimerosal of histamine-induced Ca2+ release in intact HeLa cells seen with aequorin targeted to the endoplasmic reticulum. Cell Calcium. 2001;30:181–190. doi: 10.1054/ceca.2001.0224. [DOI] [PubMed] [Google Scholar]
- 28.Vanlingen S., Sipma H., Parys J.B. Modulation of inositol 1,4,5-trisphosphate binding to the various inositol 1,4,5-trisphosphate receptor isoforms by thimerosal and cyclic ADP-ribose. Biochem. Pharmacol. 2001;61:803–809. doi: 10.1016/s0006-2952(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 29.Kaplin A.I., Snyder S.H., Linden D.J. Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-trisphosphate receptors mediates hypoxic mobilization of calcium. J. Neurosci. 1996;16:2002–2011. doi: 10.1523/JNEUROSCI.16-06-02002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zima A.V., Blatter L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Parker I., Choi J., Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 32.Marchant J., Callamaras N., Parker I. Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris C.D., Cameron A.M., Snyder S.H. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 1992;356:350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]
- 34.Hirota J., Michikawa T., Mikoshiba K. Kinetics of calcium release by immunoaffinity-purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles. J. Biol. Chem. 1995;270:19046–19051. doi: 10.1074/jbc.270.32.19046. [DOI] [PubMed] [Google Scholar]
- 35.Petersen O.H., Petersen C.C., Kasai H. Calcium and hormone action. Annu. Rev. Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- 36.Hajnóczky G., Thomas A.P. The inositol trisphosphate calcium channel is inactivated by inositol trisphosphate. Nature. 1994;370:474–477. doi: 10.1038/370474a0. [DOI] [PubMed] [Google Scholar]
- 37.Mak D.-O.D., Foskett J.K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- 38.Mak D.-O.D., Foskett J.K. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchant J.S., Taylor C.W. Rapid activation and partial inactivation of inositol trisphosphate receptors by inositol trisphosphate. Biochemistry. 1998;37:11524–11533. doi: 10.1021/bi980808k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


