Abstract
Factors intrinsic or extrinsic to individuals, such as their quality or the quality of competition in their social environment, can influence their communication signalling effort. We hypothesized that telencephalic monoamine secretion mediates the effects of a male’s own quality and quality of his social environment on his sexual signalling effort. The duration of a male European starling’s (Sturnus vulgaris) principal sexual signal, his song, positively correlates with several aspects of his quality, including his reproductive success, immunocompetence, and ability to attract mates. Therefore, the length of songs to which he is exposed reflects, in part, the quality of competition in his social environment. We manipulated the quality of the competitive environment by exposing male starlings to long or short songs for one week. We measured the length of songs produced by experimental males to gauge their quality, counted the number of songs they produced to gauge singing effort, and quantified telencephalic monoamine metabolism using HPLC. Singing effort increased with the length of the males’ own songs and with the length of songs to which we exposed them. Norepinephrine metabolism in Area X of the song control system was negatively correlated with the subjects’ mean song length and singing effort. Serotonin metabolism in the caudomedial mesopallium of the auditory telencephalon increased with the length of songs to which we exposed the subjects and with their singing effort. This raises the hypothesis that serotonin and norepinephrine secretion in the telencephalon help mediate the effects of extrinsic and intrinsic factors on signalling effort.
Keywords: bird song, monoamines, neuroplasticity, norepinephrine, serotonin
INTRODUCTION
The costs and benefits of producing communication signals can vary (Gil and Gahr, 2002), and therefore individuals should modulate their signalling effort (e.g. Sockman et al., 2005b) according to extrinsic factors, such as characteristics of the physical environment or the quality of competition among conspecifics, and to intrinsic factors, such as the signaller’s own physiological state or competitive quality. Such context-dependent, behavioral plasticity requires the neural processing of these factors, followed by production of the appropriate motor programs involved in modulating signal production.
In most songbird species, adult males attract mates and repel competing males by producing learned vocalizations known as song (Catchpole and Slater, 1995). Song learning and production are controlled by a network of interconnected forebrain nuclei, including HVC (initialism used as a proper name), the Robust Nucleus of the Arcopallium (RA), the Magnocellular Nucleus of the Anterior Nidopallium (MAN), and Area X (Nottebohm et al., 1976). HVC and RA form part of a motor pathway involved in song production, whereas MAN and Area X form part of an anterior forebrain pathway involved in song learning and plasticity (Brainard, 2004). Auditory input from an individual’s own songs or songs from another individual ascends via midbrain, thalamic, and telencephalic areas to the reciprocally connected caudomedial mesopallium (CMM) and caudomedial nidopallium (NCM) of the auditory telencephalon (Vates et al., 1996). The former provides input to the song control system via direct projections to HVC (Bauer et al., 2008).
Across vertebrate taxa, central monoamines mediate the effects of intrinsic and extrinsic factors on behavioral plasticity (Jacobs and Azmitia, 1992; Dave et al., 1998; Appeltants et al., 2002; Berridge and Waterhouse, 2003; Hurley et al., 2004; Bell et al., 2007), including plasticity in song output (Barclay and Harding, 1988; 1990; Barclay et al., 1991; 1996; Ball et al., 2003; Sasaki et al., 2006). For example, castration of zebra finches (Taeniopygia guttata) decreases norepinephrine turnover in the RA and male courtship behaviors, whereas hormone replacement increases norepinephrine turnover in the RA and restores courtship behavior to normal levels (Harding et al., 1983; Barclay and Harding, 1988; 1990). Furthermore, pharmacological depletion of norepinephrine levels in the RA results in a decrease in both the number of song bouts and the frequency of courtship displays (Barclay et al., 1991), suggesting that the stimulatory effects of elevated sex steroid hormones on these male sexual behaviors is mediated, at least in part, by norepinephrine metabolism in the RA.
In European starlings (Sturnus vulgaris), a male’s mean song length reflects some aspects of his quality, in that it positively correlates with his reproductive success, immunocompetence, age, and ability to attract mates and repel competing males (Feare, 1984; Eens et al., 1991; Mountjoy and Lemon, 1991; 1996; Eens, 1997; Gentner and Hulse, 2000; Duffy and Ball, 2002). Therefore, to manipulate an extrinsic factor — the quality of competitors in the song environment — we chronically exposed male starlings to either long or short songs for 1 week. To assess an intrinsic factor — the male’s own quality — we measured the length of songs produced during and immediately following this playback period. Although dichotomizing factors into the extrinsic and intrinsic is of dubious value, given that the two types of factors can interact to such an extent as to make them virtually inseparable, we use these terms as a simple way to distinguish between the quality of a signaller’s perceived competitors and the signaller’s own quality, respectively. To investigate the hypothesis that telencephalic monoamine secretion mediates the effects of a male’s own quality and quality of his social environment on his signalling effort, we examined how singing effort was affected by the male’s own song quality and song environment and then quantified the concentrations of serotonin, dopamine, and norepinephrine metabolites in the song control nuclei and auditory telencephalon.
MATERIALS AND METHODS
Animals and Housing
European starlings were captured on 12 December 2005 in Pennsylvania, USA (41.75°N 80.35°W) and transferred to large, outdoor flight cages at the University of North Carolina at Chapel Hill (USA), where we conducted the university-approved study (IACUC protocol 04-241.2). For the entire study, we provided the birds with food (Daily Maintenance, Roudybush; Woodland, CA, USA) and water ad libitum. We identified males based on the presence of a blue proximal region of the bill (Kessel, 1951) and later confirmed their sex (see Experiment Procedure). On 10 May 2006, we paired 20 male subjects in 10 indoor cages on a 16 h light and 8 h dark (16 L : 8 D) photoperiod to drive the birds first through a reproductive-like state and then into a non-reproductive state (Nicholls et al., 1988), thus synchronizing the birds in their reproductive cycle. On 13 July, we changed the photoperiod to 8 L : 16 D to begin the process of re-instating sensitivity to reproductive stimuli (Nicholls et al., 1988).
Experiment Procedure
On 18 October, we transferred four subjects (two of the pairs mentioned above) individually into four sound-attenuation chambers located together in one room on an 11 L : 13 D photoperiod (Session 1). We had equipped each foam-lined chamber with a cage having two perches, an air intake and fan-driven exhaust, a fluorescent light that maintained the 11 L : 13 D photoperiod within the chamber, and a speaker (Pioneer Corp. TS-G1040R). We powered speakers by a daisy chain of four mono-block amplifiers interfaced with a computer.
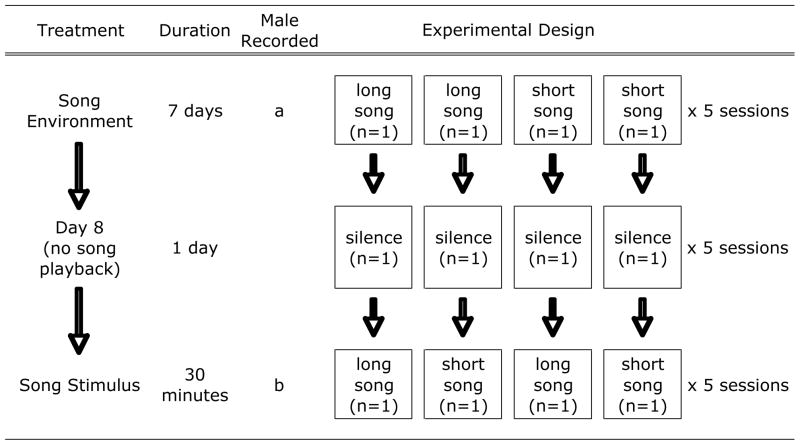
The next day, we began the simultaneous broadcast of one of two acoustic treatments, a set of long songs or a set of short songs, (hereafter termed song-environment treatment; see Song Recordings Used for Playback), through each chamber’s speaker (Table 1). We played the song at approximately 70 dB at 5 cm from the speaker to approximate the amplitude of songs that a free-living male would experience from a nearby conspecific male competitor. We balanced treatment levels between members of the original pairs described above and spatially interspersed among the chambers each replicate of the long song environment with each replicate of the short song environment. Using procedures described previously (Sockman and Salvante, 2008), we exposed the subjects to the song sets for 5.5 h per day for 7 days at partially randomized 30-min intervals during the photophase only and randomized the order of songs played within each 30-min period. Broadcasts began at the onset of the photophase each day. No more than two 30-min broadcasts occurred in a row (i.e., without at least one intervening, 30-min silent period), and no broadcast occurred during the last 30 min of the photophase each day. This approach, along with the use of an 11 L : 13 D photoperiod, was intended to mimic the natural sexual signalling environment of free-living starlings early in the breeding season. We broadcast white-noise in the room to help mask sound between chambers. On the 8th day of the session (hereafter termed day 8), the subjects received no song playback (Table 1).
Table 1.
Song Treatments and Experimental Design
 |
We equipped each chamber with an omni-directional microphone (Sennheiser ME 62) that was interfaced with a computer to record and store the songs produced by the subjects. During the photophase of each of the first 8 days, we collected audio recordings of the songs produced by the subjects. We programmed the computer to store the recordings only if 27 consecutive peaks in the recording’s oscillogram exceeded an amplitude threshold for at least 3 s (Sound Analysis Pro Software, version 1.02). Stored recordings began 20 ms prior to the peak train and ended when sound dropped below the amplitude threshold for 10 s. We selected the amplitude, peak-count, and time thresholds in order to minimize the recording of playback song and other sounds and to maximize the recording of the subjects’ songs. Nonetheless, we generated a large number of audio files, many of which contained cage noises produced when the subjects moved in addition to the subjects’ songs.
As part of another study, we then exposed one of the four subjects to a set of novel long songs and another subject to a set of novel short songs for 30 min (hereafter termed song-stimulus treatment) beginning 4 h after the onset of the photophase on the 9th day of the session (see Song Recordings Used for Playback) (Table 1). We exposed the remaining two subjects to the song-stimulus treatment 30 min later. By balancing the song-stimulus treatment levels between members of the same song-environment treatment group, we generated four treatment groups (but see Song Recordings Used for Playback): 1) long song environment, long song stimulus; 2) long song environment, short song stimulus; 3) short song environment, long song stimulus; 4) short song environment, short song stimulus.
At 90 min following onset of the song-stimulus treatment, we weighed the subjects, rapidly decapitated them, and removed their brains. Using previously described protocols (Sockman and Salvante, 2008), we froze one hemisphere (alternating left and right with each treatment group), fixed the other hemisphere and stored both at −80°C. We removed testes from each individual and dried them at 60°C until they reached a constant dry mass. We repeated these procedures for a total of five sessions of four subjects each, resulting in a total of 20 subjects divided among five replicates for each of the four treatment groups, counterbalanced among the chambers and sessions.
Song Recordings Used for Playbacks
Details of the song recordings used for playback have been described previously (Gentner and Hulse, 2000). Briefly, for the song-environment treatment, a library of complete song bouts was recorded from a single, laboratory-housed male directing song at a female. From these, 12 songs were selected, which, based on length, were divided into two sets of six: a long-song set and a short-song set with mean song lengths of 55.2 and 26.0 s, respectively. The song sets used for the song-stimulus treatment were constructed in the same manner, except that the songs were recorded from a different male and the mean song lengths of the long-song and short-song sets were 55.6 and 25.4 s, respectively. Importantly, neither total song nor total silence duration differed between the long-song and short-song sets for either treatment, and therefore we exposed all males to the exact same amount of song, regardless of group. By necessity, the two levels of the treatment also differed in the repetition rate of individual songs. However, a previous study reported that song length (or a correlate thereof), but not repetition rate, affected expression of immediate, early genes in the auditory forebrain of female European starlings (Gentner et al., 2001). We assume that this sensitivity specifically to a correlate of song length over song repetition rate also applies to our dependent measures on males, but we do not know this with certainty.
We included the song-stimulus treatment as part of another study examining the effects of the song-environment treatment on neural sensitivity to the length of a novel song stimulus. Because our measurements of singing effort and mean song length preceded the song-stimulus treatment (see explanation below), they were not affected by it. Additionally, the two levels of the song-stimulus treatment were balanced across levels of the song-environment treatment. Consequently, we do not discuss the effects of the song-stimulus treatment further. However, because the brains were collected following exposure to the song-stimulus treatment, we measured monoamine metabolite levels after the subjects were exposed to the song-stimulus treatment. Consequently, we include song-stimulus treatment as a variable in all analyses of monoamine metabolite concentrations to control for its potential to affect monoamine metabolism.
Because both levels of our song-environment treatment were constructed from the recordings of a single male, we cannot extend our conclusions to the effects of song length in general (Kroodsma et al., 2001; Wiley, 2003). However, a previous study showed that females in a mate-choice context prefer this specific long-song set to this specific short-song set (Gentner and Hulse, 2000), and consequently our results are based on responses to stimuli that are known to differ in their attractiveness to females and, presumably, to differ in the content of information about the competitive quality of the signaller.
Quantification of Male Singing Behavior
We used a male’s song count as an indicator of his singing effort. For this study we used songs from day 8 only, because song count was most variable on this day. We defined a song as a series of at least two different motifs containing a combined total of at least 5 notes, wherein we defined a note as a continuous trace on a spectogram and a motif as a group of notes arranged in a fixed order (Adret-Hausberger and Jenkins, 1988; Eens et al., 1989). Although eleven birds sang on day 9, there are important reasons for not using day 9 in the analyses. First, it is difficult to interpret day 9 song because birds were sacrificed at different times during the day, and therefore, some males had longer than others to sing. Second, and most importantly, in this study we are interested in the effects of the song-environment treatment, and not the song-stimulus treatment, on singing effort. Therefore, the best way to assess the effect of the song-environment treatment on singing effort without any confounding effects of the song-stimulus treatment was to focus on singing effort on day 8. Given these constraints, day 8 was also the time point nearest to that of our tissue collection and therefore the time point most relevant to our measurements of monoamine metabolites.
There were too many song files to analyze them all, so we employed the following sampling technique. Using a random number generator, we selected approximately 45% of the song files recorded on day 8. We distinguished song files from songs because individual files sometimes contained multiple songs, and individual songs sometimes spanned multiple files. The proportion of files selected from each individual reflected the proportion of all files that the individual produced. For example, if all 20 subjects produced a combined total of 1000 song files on day 8, we analyzed 450 of these files (45% of 1000 files = 450 files). If 25% of the total number of song files on day 8 came from individual X, then we randomly selected and analyzed 113 of individual X’s day-8 song files (113 files = 25% of 450 song files analyzed from day 8). Within this random subset of song files, we then counted the number of songs and measured the lengths of their computer-drawn spectograms (using the software Raven, version 1.2.1, Cornell Lab of Ornithology).
Quantification of Monoamine Metabolites and Protein
When monoamines are secreted into the synapse, enzymes metabolize them. Thus, as indices of monamine secretion, we quantified levels of their principal metabolites (Moore, 1986). Using previously described protocols (Sockman and Salvante, 2008), we measured tissue concentrations of the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC; hereafter termed dopamine metabolite), the norepinephrine metabolite 3-methoxy-4-hydroxy-phenylglycol (MHPG; hereafter termed norepinephrine metabolite), and the serotonin metabolite 5-hydroxyindolacetic acid (5-HIAA; hereafter termed serotonin metabolite) using reversed-phase high-pressure liquid chromatography (HPLC) with electrochemical detection (Kilts et al., 1981). Briefly, we separated compounds using a Monochrom C18 3 μm column (100 × 4.6 mm, MetaChem) with a mobile phase (1 L, pH 3.9) consisting of sodium phosphate (7.1 g), citric acid (5.76 g), disodium EDTA (50 mg), sodium octyl sulfonate (350 mg) and methanol (13%). We maintained electrode potential at 650 mV with respect to an Ag/AgCl reference electrode. We prepared standard solutions containing a fixed amount (30 ng) of the internal standard (isoproterenol, Sigma) and variable amounts of each monoamine metabolite. We included a five-point standard curve in each assay (5 × 50 μL injections), and used linear regression to fit a line through the standard curve points (R2 > 0.99 for each of the components in each assay).
We cut 300-μm sagittal sections from the frozen hemispheres on a cryostat maintained at −10°C, thaw mounted them onto glass microscope slides, and rapidly refroze them on pulverized dry ice. From each of three consecutive sections starting at the midline and using a chilled 1-mm (i.d.) custom-made, thin-walled stainless steel spring-loaded punch, we micropunched a region of the CMM, a caudo-dorsal region of the NCM (NCMd), and a ventral region of the NCM (NCMv), each for which the anatomical boundaries have been described (Sockman et al., 2002) (Fig. 1a). We also sampled from the four principal brain nuclei within the song control system (see introduction). We chose the areas from which to sample based on comparison with the canary and zebra finch brain atlases, and on anatomical boundaries defined by NISSL-staining of the song control nuclei in sections from the alternate, fixed hemisphere from the same birds (Sockman et al., 2009). We micropunched MAN from one section that was approximately 2100 μm from the midline and a region of Area X from three consecutive sections starting approximately 1500 μm from the midline (Fig. 1b). We also took micropunches from a region of HVC from four consecutive sections starting approximately 2400 μm from the midline, and from a region of the RA from two consecutive sections starting approximately 2400 μm from the midline (Fig. 1c).
Figure 1.
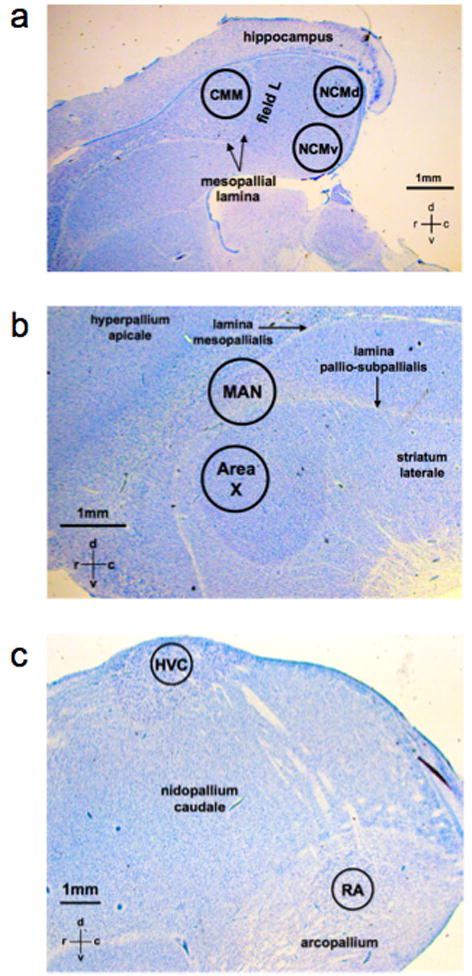
Photomicrographs of regions within the forebrain of European starlings sampled for HPLC analysis of central monoamine metabolism. Sample punches (1 mm i.d.) taken from a) the caudomedial mesopallium (CMM), dorsal caudomedial nidopallium (NCMd), and ventral caudomedial nidopallium (NCMv) of the auditory telencephalon, and b) the magnocellular nucleus of the anterior nidopallium (MAN), Area X, c) the robust nucleus of the arcopallium (RA) and HVC (initialism used as a proper name) of the song control system.
We expelled the tissue punches into 1.9-ml polypropylene microcentrifuge tubes, froze them on dry ice and stored them at −80 °C until assay. Immediately before assay we added mobile phase (225 μl) containing 30 ng isoproterenol to each tube. We sonicated the samples and then centrifuged them at 16,000 g for 15 minutes at 4°C. We aspirated the supernatant and injected 50 μl from each sample into the HPLC system. We calculated the metabolite contents by first correcting their peak heights for percent recovery of the internal standard (i.e., the height of the isoproterenol peak) for each sample, and then comparing the values to those obtained for the corresponding metabolites in the standard curve.
We measured the protein content of each sample by dissolving the remaining sample pellet in 100 μl 0.2 N NaOH and performing the Bradford protein-dye binding assay (Quick Start Bradford Protein Assay, Bio-Rad) with bovine serum albumin (Bio-Rad) as a standard on a μQuant microplate spectrophotometer (BioTek) (Bradford, 1976).
Analyses
Our data consisted of a combination of fixed (e.g., song environment, mean song length) and hierarchically-structured random (e.g., individual nested within session) effects, each of which may differ from the others in its correlation structure. In addition, a few samples were lost during HPLC, rendering our dataset unbalanced. Therefore, we analyzed these data in a mixed, multilevel modeling framework using the software Stata IC 10.0 for the Macintosh (Stata Corporation, College Station, TX, USA), which readily accommodates unbalanced, hierarchically-structured combinations of fixed and random effects (Burton et al., 1998; Goldstein et al., 2002; Rabe-Hesketh and Skrondal, 2005). We primarily used Stata’s command for multilevel mixed-effects linear regression but, for the song count data, we instead used the command for multilevel mixed-effects Poisson regression. These models estimated parameters with restricted maximum likelihood and used z-tests to test the null hypothesis that a coefficient equalled 0. For more information on the rationale for and approach to mixed, multilevel modeling frameworks, see Sockman et al. (2008).
For the majority of analyses, we nested observation (the individual bird’s measurement) within session as a random intercept. However, for some metabolite responses in some brain areas, these initial models failed to converge on a solution. In these instances, we reanalyzed the data without the random intercept. With three monoamines of interest measured in each of seven brain areas, there is a large potential for spurious results. Therefore, to maximize the rigor of our analytical strategy, we established a three-branch decision tree to investigate how the effects of song environment and mean song length on singing effort may be mediated by the secretion of monoamines in the auditory telencephalon and the song control nuclei (Fig. 2). Relationships resulting in p values greater than or equal to 0.05 were deemed unreliable and thus excluded from further consideration. For the first branch of this decision tree, we determined whether song environment, mean song length, or song stimulus predicted (p < 0.05) each monoamine metabolite within each brain area of interest. Also, if an area-specific monoamine metabolite was related to both song environment and mean song length, it had to have had a consistent relationship with both parameters (i.e., both positive or both negative) to be considered a potential candidate for mediating the effects of these parameters on singing effort. This is because singing effort increased with an increase in each parameter (see Results). This first branch of our decision tree resulted in a subset of area-specific metabolite concentrations that were associated with either song environment or mean song length (see Results).
Figure 2.
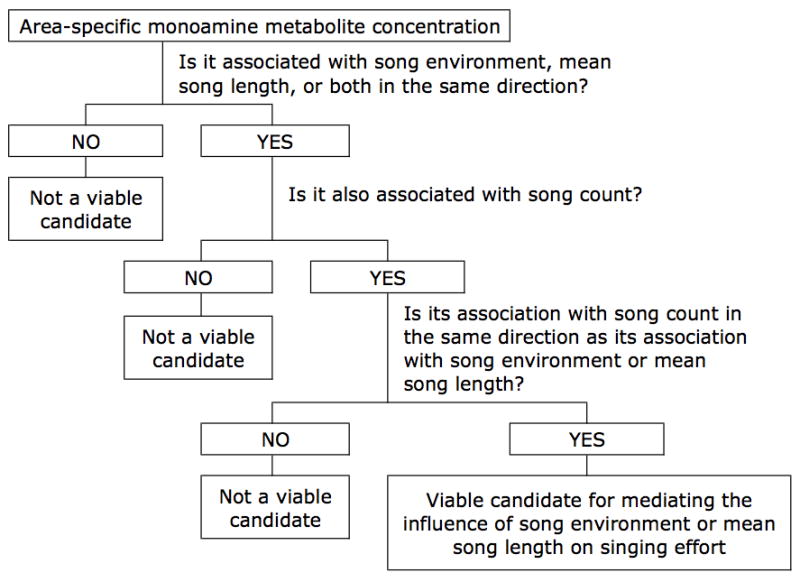
Three-branch decision tree for determining viable area-specific monoamine metabolite candidates for mediating the influence of song environment and mean song length on singing effort.
The association of these area-specific metabolite concentrations with song environment or mean song length does not necessarily indicate that they are related in any way to the mediation of singing effort. At a minimum, the metabolite concentration should also be correlated with song count. Hence, in the second branch of our decision tree, we determined whether or not any of the above subset of area-specific monoamine metabolite concentrations predicted song count (Fig. 2). From the initial set of area-specific metabolite concentrations related to song environment or mean song length, this second approach generated a sub-subset of area-specific metabolite concentrations that were also related to song count.
Although a single area-specific metabolite concentration may correlate with both song count and with song environment or mean song length, the monoamine for that metabolite does not necessarily mediate the effects of these factors on singing effort. Additionally, the correlations should be in the same direction (both positive or both negative) because both song environment and mean song length were positively correlated with song count (see Results section below). Therefore, in the third branch of our decision tree, we identified only those area-specific metabolite concentrations that were correlated in the same direction with both singing effort and with at least one of either song environment or mean song length (Fig. 2).
We then assessed which of song environment, mean song length and the viable monoamine metabolite candidates from the third branch of our decision tree best predicted song count on day 8. For this final model, we also applied an information theoretic approach by calculating Akaike’s Information Criterion (AIC, Burnham and Anderson, 2003) for a null model containing only an intercept and for each model in which we added the individual factors above in all possible combinations. A model’s AIC value reflects its goodness of fit relative to its number of parameters, using the log likelihood of the model while penalizing for each parameter. The model with the most efficient combination of parameters is that with the lowest AIC value. Figures depict means ± SEM and p-values from models described above.
RESULTS
Singing Behavior
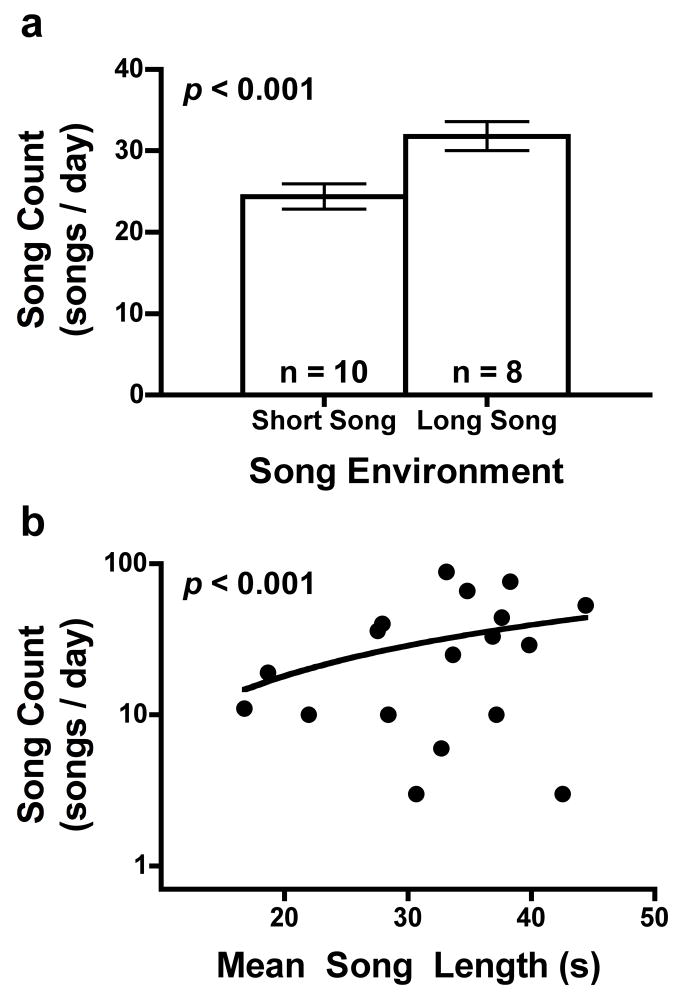
Males exhibited considerable variation in song count (singing effort) on day 8 (range: 0 to 431 songs). Moreover, day-8 song count increased with the quality (length) of both the song environment (p < 0.001; Fig. 3a) and the male’s own song (p < 0.001; Fig. 3b) (Table 2). Males that sang on day 8 (n = 18) exhibited over 2.5-fold inter-individual variation in mean song length (range: 16.5 to 43.1 s). However, we found no effect of song environment on mean song length (z = −0.04, p > 0.2), indicating that our extrinsic factor did not significantly affect our intrinsic factor.
Figure 3.
Change in song count with respect to a) song environment (mean ± SE) and b) the length of evoked songs (Y axis has a log-10 scale because count data tend to follow a Poisson distribution)..
Table 2.
Statistical effects of song environment* and mean song length (s) on song count (n = 18 males).
| Independent variables | Estimate | Standard Error | z Score | p-value |
|---|---|---|---|---|
| Song Environment | 0.4657 | 0.0874 | 5.33 | < 0.001 |
| Mean Song Length | 0.1029 | 0.0121 | 8.48 | < 0.001 |
| Intercept | −0.2731 | 0.4784 | −0.57 | > 0.2 |
Coded 0 for short song, 1 for long song
It is possible that the effect of song environment on song count may have been due to an effect on reproductive development. However, males in the long song environment (14.37 ± 3.57 mg) had similar dry testes masses to those of males exposed to the short song environment (11.06 ± 2.08 mg) (p > 0.2) and therefore, were not likely advanced reproductively, though, it should be noted that testis mass is at best a very gross measure of reproductive development.
What Factors Best Explain Variation in Singing Effort?
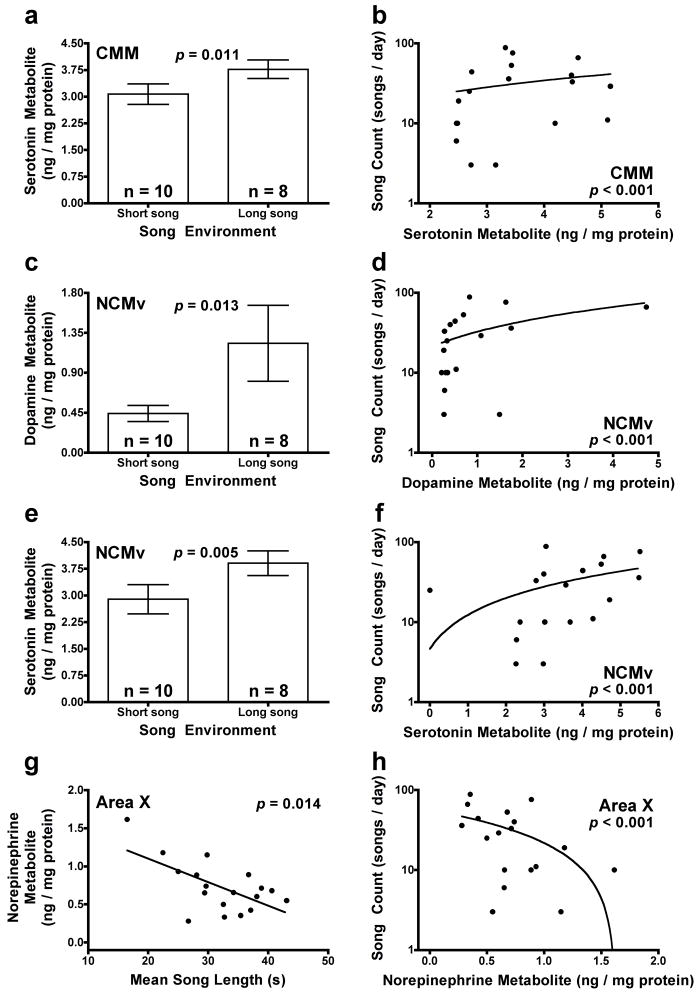
Using our three-branch decision tree, we found four viable monoamine candidates whose area-specific metabolism was correlated with both singing effort and either song environment or mean song length and in a consistent direction (both positive or both negative) (Fig. 4). Concentrations of serotonin metabolite in the CMM and the NCMv and of dopamine metabolite in the NCMv were consistently positively related to both song environment (p = 0.011, Fig. 4a; p = 0.005, Fig. 4e; and p = 0.013, Fig. 4c, respectively) and song count on day 8 (p < 0.001, Fig. 4b; p < 0.001, Fig. 4f; and p < 0.001, Fig. 4d, respectively) (Table 3, 5). Similarly, concentrations of norepinephrine metabolite in Area X were negatively correlated with both mean song length (p = 0.014; Fig. 4g) and song count on day 8 (p < 0.001; Fig. 4h) (Table 4, 5). The relationship between dopamine metabolite in the NCMv and song count appears as though it might be driven by a single point with a high value for the concentration of dopamine metabolite (Fig. 4d). However, when that value is removed from the analysis, the relationship remains reliable (coefficient = 0.442 ± 0.104, z = 4.23, p < 0.001). In addition to being related to song environment and song count, the concentration of serotonin metabolite in the NCMv was also negatively related to the song-stimulus treatment (p = 0.033; Table 3).
Figure 4.
Region-specific monoamine candidates for mediating the effects of song environment and mean song length on singing effort. Serotonin metabolism in the CMM (a, b), dopamine metabolism in the NCMv (c, d) and serotonin metabolism in the NCMv (e, f) were positively associated with both song environment and song count. Norepinephrine metabolism in Area X was negatively associated with both g) mean song length and h) song count. Y axes showing song counts have a log-10 scale because count data tend to follow a Poisson distribution.
Table 3.
Statistical effects of song environment*, song stimulus*, and mean song length (s) on monoamine metabolite levels (ng/mg protein) in the caudomedial mesopallium (CMM), dorsal caudomedial nidopallium (NCMd), and ventral caudomedial nidopallium (NCMv) of the auditory telencephalon (n = 18 males).
| Area-specific monoamine metabolite | ||||
|---|---|---|---|---|
| Independent variables | Estimate | Standard Error | z Score | p-value |
| Dopamine metabolite in CMM | ||||
| Song Environment | 0.4193 | 0.3936 | 1.07 | > 0.2 |
| Song-Stimulus Treatment | −0.6609 | 0.4050 | −1.63 | 0.103 |
| Mean Song Length | 0.0024 | 0.0348 | 0.07 | > 0.2 |
| Intercept | 0.7962 | 1.2062 | 0.66 | > 0.2 |
| Norepinephrine metabolite in CMM | ||||
| Song Environment | 0.2476 | 0.1242 | 1.99 | 0.046 |
| Song-Stimulus Treatment | 0.1035 | 0.1275 | 0.81 | > 0.2 |
| Mean Song Length | −0.0135 | 0.0095 | −1.42 | 0.155 |
| Intercept | 1.1084 | 0.3334 | 3.32 | < 0.001 |
| Serotonin metabolite in CMM | ||||
| Song Environment | 1.0236 | 0.4031 | 2.54 | 0.011 |
| Song-Stimulus Treatment | −0.5574 | 0.4137 | −1.35 | 0.178 |
| Mean Song Length | −0.0218 | 0.0309 | −0.71 | > 0.2 |
| Intercept | 4.0433 | 1.0817 | 3.74 | < 0.001 |
| Dopamine metabolite in NCMd | ||||
| Song Environment | 0.1325 | 0.1238 | 1.07 | > 0.2 |
| Song-Stimulus Treatment | −0.0996 | 0.1273 | −0.78 | > 0.2 |
| Mean Song Length | −0.0160 | 0.0105 | −1.52 | 0.128 |
| Intercept | 0.8565 | 0.3651 | 2.35 | 0.019 |
| Norepinephrine metabolite in NCMd | ||||
| Song Environment | 0.0989 | 0.1834 | 0.54 | > 0.2 |
| Song-Stimulus Treatment | 0.0170 | 0.1885 | 0.09 | > 0.2 |
| Mean Song Length | −0.0127 | 0.0153 | −0.83 | > 0.2 |
| Intercept | 1.1905 | 0.5319 | 2.24 | 0.025 |
| Serotonin metabolite in NCMd | ||||
| Song Environment | 0.6173 | 0.6872 | 0.90 | > 0.2 |
| Song-Stimulus Treatment | −1.3394 | 0.7053 | −1.90 | 0.058 |
| Mean Song Length | −0.1266 | 0.0526 | −2.40 | 0.016 |
| Intercept | 8.0397 | 1.8441 | 4.36 | < 0.001 |
| Dopamine metabolite in NCMv | ||||
| Song Environment | 1.1129 | 0.4504 | 2.47 | 0.013 |
| Song-Stimulus Treatment | −0.8647 | 0.4623 | −1.87 | 0.061 |
| Mean Song Length | −0.0099 | 0.0349 | −0.28 | > 0.2 |
| Intercept | 1.1888 | 1.2230 | 0.97 | > 0.2 |
| Norepinephrine metabolite in NCMv | ||||
| Song Environment | 0.4327 | 0.1642 | 2.63 | 0.008 |
| Song-Stimulus Treatment | −0.2832 | 0.1688 | −1.68 | 0.093 |
| Mean Song Length | −0.0326 | 0.0138 | −2.37 | 0.018 |
| Intercept | 1.7773 | 0.4786 | 3.71 | < 0.001 |
| Serotonin metabolite in NCMv | ||||
| Song Environment | 1.4067 | 0.5038 | 2.79 | 0.005 |
| Song-Stimulus Treatment | −1.1028 | 0.5181 | −2.13 | 0.033 |
| Mean Song Length | −0.0460 | 0.0437 | −1.05 | > 0.2 |
| Intercept | 4.9051 | 1.5161 | 3.24 | < 0.001 |
Coded 0 for short song, 1 for long song
Table 5.
Statistical effects of monoamine metabolite levels (ng/mg protein) in select regions of the auditory telencephalon (see Table 3 for abbreviations) and song control system (see Table 4 for abbreviations) on song count (n = 16 males for dopamine metabolite in HVC; n = 18 males for all other monoamine metabolites). Comparisons were made between the directions of the significant relationships (i.e. positive or negative correlations) and the direction of the previously reported significant relationships between the monoamine metabolite of interest and either song environment or mean song length.
| Independent Variables | Estimate | Standard Error | z Score | p-value |
|---|---|---|---|---|
| Norepinephrine metabolite in CMM | −0.1485 | 0.1752 | −0.85 | > 0.2 |
| Intercept | 3.2895 | 0.3057 | 10.76 | < 0.001 |
| Serotonin metabolite in CMM | 0.4769 | 0.0552 | 8.64 | < 0.001 |
| Intercept | 1.4405 | 0.3809 | 3.78 | < 0.001 |
| CONSISTENT with positive relationship with song environment | ||||
| Serotonin metabolite in NCMd | 0.1784 | 0.0278 | 6.43 | < 0.001 |
| Intercept | 2.5322 | 0.3046 | 8.31 | < 0.001 |
| INCONSISTENT with negative relationship with mean song length | ||||
| Dopamine metabolite in NCMv | 0.2145 | 0.0348 | 6.16 | < 0.001 |
| Intercept | 2.9645 | 0.2451 | 12.10 | < 0.001 |
| CONSISTENT with positive relationship with song environment | ||||
| Serotonin metabolite in NCMv | 0.0200 | 0.0100 | 5.78 | < 0.001 |
| Intercept | 2.3342 | 0.2451 | 7.99 | < 0.001 |
| CONSISTENT with positive relationship with song environment | ||||
| Dopamine metabolite in MAN | −0.0204 | 0.0366 | −0.56 | > 0.2 |
| Intercept | 3.1957 | 0.2833 | 11.28 | < 0.001 |
| Norepinephrine metabolite in Area X | −0.5311 | 0.1469 | −3.61 | < 0.001 |
| Intercept | 3.5351 | 0.2633 | 13.43 | < 0.001 |
| CONSISTENT with negative correlation with mean song length | ||||
| Dopamine metabolite in Area X | 0.0020 | 0.0010 | 2.15 | 0.032 |
| Intercept | 2.8397 | 0.3128 | 9.08 | < 0.001 |
| INCONSISTENT with negative correlation with mean song length | ||||
| Dopamine metabolite in HVC | −0.0217 | 0.1763 | −0.12 | > 0.2 |
| Intercept | 3.1811 | 0.2879 | 11.05 | < 0.001 |
Table 4.
Statistical effects of song environment*, song-stimulus*, and mean song length (s) on monoamine metabolite levels (ng/mg protein) in the magnocellular nucleus of the anterior nidopallium (MAN; n = 18 males), Area X (n = 18 males), HVC (initialism used as a proper name; n = 16 males), and the robust nucleus of the arcopallium (RA; n = 18 males) of the song control system.
| Area-specific monoamine metabolite | ||||
|---|---|---|---|---|
| Independent variables | Estimate | Standard Error | z Score | p-value |
| Dopamine metabolite in MAN | ||||
| Song Environment | −1.0367 | 0.4692 | −2.21 | 0.027 |
| Song-Stimulus Treatment | −0.2369 | 0.4847 | −0.49 | > 0.2 |
| Mean Song Length | 0.0500 | 0.0496 | 1.01 | > 0.2 |
| Intercept | 0.4566 | 1.7160 | 0.27 | > 0.2 |
| Norepinephrine metabolite in MAN | ||||
| Song Environment | −0.1294 | 0.3855 | −0.34 | > 0.2 |
| Song-Stimulus Treatment | 0.2359 | 0.3956 | 0.60 | > 0.2 |
| Mean Song Length | −0.0094 | 0.0295 | −0.32 | > 0.2 |
| Intercept | 1.4070 | 1.0344 | 1.36 | 0.174 |
| Serotonin metabolite in MAN | ||||
| Song Environment | −1.6444 | 1.3901 | −1.18 | > 0.2 |
| Song-Stimulus Treatment | 1.7541 | 1.4298 | 1.23 | > 0.2 |
| Mean Song Length | −0.0390 | 0.1214 | −0.32 | > 0.2 |
| Intercept | 4.6247 | 4.2144 | 1.10 | > 0.2 |
| Dopamine metabolite in Area X | ||||
| Song Environment | −0.8380 | 2.2441 | −0.37 | > 0.2 |
| Song-Stimulus Treatment | −4.7413 | 2.3031 | −2.06 | 0.040 |
| Mean Song Length | −0.4259 | 0.1719 | −2.48 | 0.013 |
| Intercept | 34.4193 | 6.0221 | 5.72 | 0.001 |
| Norepinephrine metabolite in Area X | ||||
| Song Environment | −0.0110 | 0.1277 | −0.09 | > 0.2 |
| Song-Stimulus Treatment | 0.1298 | 0.1314 | 0.99 | > 0.2 |
| Mean Song Length | −0.0278 | 0.0113 | −2.45 | 0.014 |
| Intercept | 1.5569 | 0.3929 | 3.96 | < 0.001 |
| Serotonin metabolite in Area X | ||||
| Song Environment | 0.0985 | 0.4842 | 0.20 | > 0.2 |
| Song-Stimulus Treatment | −0.6392 | 0.4970 | −1.29 | 0.198 |
| Mean Song Length | −0.0553 | 0.0371 | −1.49 | 0.136 |
| Intercept | 4.8048 | 1.2994 | 3.70 | < 0.001 |
| Dopamine metabolite in HVC | ||||
| Song Environment | −0.2669 | 0.1189 | −2.24 | 0.025 |
| Song-Stimulus Treatment | 0.0106 | 0.1207 | 0.09 | > 0.2 |
| Mean Song Length | −0.0133 | 0.0086 | −1.54 | 0.123 |
| Intercept | 1.0362 | 0.2998 | 3.46 | < 0.001 |
| Norepinephrine metabolite in HVC | ||||
| Song Environment | −0.0207 | 0.1139 | −0.18 | > 0.2 |
| Song-Stimulus Treatment | −0.1355 | 0.1157 | −1.17 | > 0.2 |
| Mean Song Length | −0.0047 | 0.0085 | −0.55 | > 0.2 |
| Intercept | 0.7531 | 0.2951 | 2.55 | 0.011 |
| Serotonin metabolite in HVC | ||||
| Song Environment | 0.3211 | 0.5966 | 0.54 | > 0.2 |
| Song-Stimulus Treatment | −0.6354 | 0.6057 | −1.05 | > 0.2 |
| Mean Song Length | 0.0395 | 0.0431 | 0.92 | > 0.2 |
| Intercept | 2.6759 | 1.5042 | 1.78 | 0.075 |
| Dopamine metabolite in RA | ||||
| Song Environment | 0.2704 | 0.1618 | 1.67 | 0.095 |
| Song-Stimulus Treatment | −0.1721 | 0.1660 | −1.04 | > 0.2 |
| Mean Song Length | −0.0236 | 0.0124 | −1.90 | 0.057 |
| Intercept | 1.3618 | 0.4342 | 3.14 | 0.002 |
| Norepinephrine metabolite in RA | ||||
| Song Environment | 0.1155 | 0.1055 | 1.09 | > 0.2 |
| Song-Stimulus Treatment | −0.0653 | 0.1089 | −0.60 | > 0.2 |
| Mean Song Length | −0.0019 | 0.0107 | −0.17 | > 0.2 |
| Intercept | 0.3066 | 0.3709 | 0.83 | > 0.2 |
| Serotonin metabolite in RA | ||||
| Song Environment | 0.8813 | 1.1273 | 0.78 | > 0.2 |
| Song-Stimulus Treatment | −0.8709 | 1.1570 | −0.75 | > 0.2 |
| Mean Song Length | −0.0110 | 0.0863 | −0.13 | > 0.2 |
| Intercept | 5.8994 | 3.0252 | 1.95 | 0.051 |
Coded 0 for short song, 1 for long song
If song environment affects singing effort via metabolism of serotonin in the CMM and the NCMv and dopamine in the NCMv, and if mean song length influences singing effort directly through norepinephrine metabolism in Area X, then we predict that when all of these factors are included in one model variation in singing effort should best be explained by monoamine metabolite levels as opposed to being explained by mean song length and song environment. In the full model, we found no reliable relationship between song count and dopamine metabolite levels in the NCMv or between song count and serotonin metabolite levels in the NCMv (both p > 0.2). Moreover, AIC values (not shown) indicated that the most efficient model did not include the concentration of dopamine metabolite in the NCMv or the concentration of serotonin metabolite in the NCMv as factors. However, AIC did support the inclusion of song environment (p = 0.002), mean song length (p < 0.001), the concentration of serotonin metabolite in the CMM (p = 0.019), and the concentration of norepinephrine metabolite in Area X (p < 0.001) in the model (Table 6). Therefore, serotonin metabolism in the CMM of male starlings may be involved in mediating the effects on singing effort of variation in the quality of competition in a male’s social environment. Similarly, the association between a male’s quality and his singing effort may be mediated, in part, by modulation of norepinephrine metabolism in Area X.
Table 6.
The best-fit model based on our data of the modulatory factors and central monoaminergic mechanisms influencing song count (n = 18 males; See Table 3 for abbreviation).
| Independent variables | Estimate | Standard Error | z Score | p-value |
|---|---|---|---|---|
| Serotonin metabolite in CMM (ng/mg protein) | 0.1737 | 0.0744 | 2.34 | 0.019 |
| Norepinephrine metabolite in Area X (ng/mg protein) | −0.7725 | 0.2098 | −3.68 | < 0.001 |
| Song environment* | 0.3807 | 0.1242 | 3.07 | 0.002 |
| Mean song length (s) | 0.0949 | 0.0131 | 7.26 | < 0.001 |
| Intercept | −0.0767 | 0.5355 | −0.14 | > 0.2 |
Coded 0 for short song, 1 for long song
Additional Monoamine Metabolite Relationships
Although only two viable candidate monoamines emerged from our analytical approach described above, we discovered additional relationships between song environment, mean song length, song count, and monoamine metabolism in the auditory telencephalon and song control nuclei that did not fulfill all of the criteria in our decision tree, but may nonetheless provide insight into the relationships between region-specific monoamine metabolism and plasticity in singing effort and other behaviors. We found that although males exposed to the long song environment had higher concentrations of norepinephrine metabolite in the CMM (p = 0.046; Table 3) and lower concentrations of dopamine metabolite in the MAN (p = 0.027; Table 4) and HVC (p = 0.025; Table 4) than males exposed to the short song environment, song count on day 8 was not related to any of these monoamine metabolites (p > 0.2 in all cases; Table 5). Furthermore, the positive correlations between the concentrations of both serotonin metabolite in the NCMd and dopamine metabolite in Area X and song count on day 8 (p < 0.001 and p = 0.032; Table 5) were not consistent with their negative relationships with mean song length (p = 0.016; Table 3 and p = 0.013; Table 4). Consequently, we did not consider the metabolism of norepinephrine in the CMM, dopamine in the MAN, HVC and Area X, and serotonin in the NCMd as being directly involved in the neural pathways mediating the effects of song environment and mean song length on song count. However, the secretion of these monoamines in these brain areas may be influenced by the context-dependent changes in singing effort or be involved in the neural pathways mediating the effects of song environment and mean song length on other behaviors.
DISCUSSION
Context-dependent modulation of communication behavior is influenced by both extrinsic factors that can be attributed to aspects of the signalling environment and factors that are intrinsic to the signaller, such as his condition or quality. Birdsong is an excellent example of how the extrinsic physical and social environment and intrinsic quality, motivation, and reproductive state of the signaller influence communication behavior. However, how the songbird brain integrates these intrinsic and extrinsic factors and modulates signalling effort accordingly is poorly understood. The present study is consistent with the hypothesis that norepinephrine and serotonin metabolism in the telencephalon may, in part, mediate the effects of some intrinsic and extrinsic factors on male singing effort.
Male Song Quality, Norepinephrine in the Song Control System, and Singing Effort
Our results are consistent with the hypothesis that the association between a male’s intrinsic song quality and his singing effort may be mediated by modulation of norepinephrine secretion in Area X. In male zebra finches electrophysiological and immediate early gene activity in Area X are lower when song is “directed” towards a conspecific, which often occurs in the context of courtship, than when the singer is not oriented towards a conspecific, and is thus singing “undirected” song (Jarvis et al., 1998; Hessler and Doupe, 1999). A proposed purpose of undirected singing is song practice (Jarvis et al., 1998). The increase in Area X activity during practice bouts may be due to Area X’s perceptual role in processing information about a male’s own song (Mello and Ribeiro, 1998; Doupe and Konishi, 1991), making for the intriguing possibility that this area is involved in mediating the influence of a male’s own song quality on his singing effort.
Sex steroids, which are elevated during courtship, are likely involved in the mechanism linking intrinsic male song quality, norepinephrine secretion in Area X, and singing effort. Castration and hormone replacement studies on zebra finches suggest that the combination of low sex steroid levels and elevated norepinephrine secretion in Area X has a suppressive effect on singing effort (Barclay and Harding, 1988; 1990). Furthermore, pharmacological treatment that reduces noradrenergic input to the forebrain results in an increase in immediate early gene expression in Area X during directed singing (Castelino and Ball, 2005), further supporting the idea that norepinephrine suppresses neural activity in Area X.
Competitive Environment, Serotonin in the Auditory Telencephalon, and Singing Effort
Our findings are also consistent with the hypothesis that serotonin secretion in the CMM of male starlings may be involved in mediating the effects of extrinsic social factors on singing effort. The responsiveness of the songbird CMM to conspecific male song has been well documented (Mello et al., 1992; MacDougall-Shackleton et al., 1998; Ball et al., 2006; Sockman, 2007), and the sensitivity of the female’s auditory telencephalon to variation in conspecific male song length is known to be modulated by the quality of songs in the acoustic environment (Sockman et al., 2002; 2005a). The CMM’s direct projections to HVC (Bauer et al., 2008) may allow variation in serotonergically-modulated CMM activity to translate into plasticity in singing effort.
In the inferior colliculus, the principal midbrain nucleus of the mammalian auditory system, serotonin alters the magnitude of neuronal responses to relevant conspecific acoustic signals by modulating the range of frequencies to which the neurons are sensitive, via gain control and frequency tuning of specific neurons (Hurley and Pollak, 1999; 2001; 2005; Hurley et al., 2002; 2004). It has been hypothesized that serotonin’s selective influence on auditory neurons could lead to differential pattern encoding for different auditory signals depending on the arousal state of the listener and the incoming auditory stimuli, which could ultimately lead to reconfiguration of the neural circuitry and responsiveness of the auditory system (Hurley et al., 2002; 2004). Thus, one hypothesis is that socially modulated serotonin metabolism in the CMM reshapes the HVC-projecting neurons in the CMM, changing the responsiveness of the auditory telencephalon and thus the song control system to conspecific song, resulting in an increase in singing effort.
Modulation of a Complex Communication Signal
We expected that if the effects of song environment and mean song length on singing effort were solely mediated by secretion of serotonin in the CMM and norepinephrine in Area X, respectively, then serotonin and norepinephrine secretion in these brain areas should explain variation in singing effort that would otherwise be attributed to the extrinsic and intrinsic factors. Contrary to our prediction, the best-fit model included all four parameters, and each explained a portion of the variation in singing effort. Indeed, the context-dependent modulation of singing effort is, not surprisingly, more complex than our simple dual-pathway model (forward solid arrows, Fig. 5). Additional mediating neural mechanisms must also be involved in the neural pathways through which male quality and social environment influence singing effort (dashed arrows, Fig. 5) because our intrinsic and extrinsic factors explained a portion of the variation in singing effort that was not attributable to secretion of norepinephrine in Area X or serotonin in the CMM. For example, our intrinsic and extrinsic factors may influence monoamine secretion in brain regions from which we did not sample.
Figure 5.
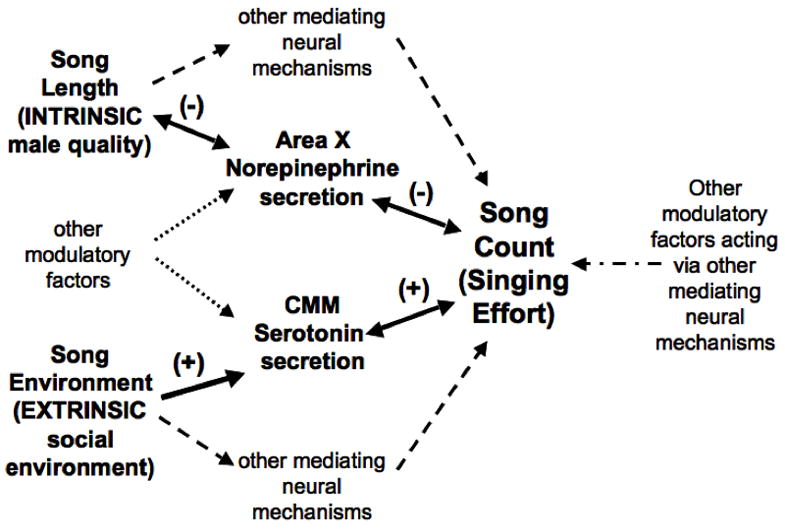
Hypothetical model by which an individual’s mean song length, its song environment, and other potential intrinsic and extrinsic factors may influence singing effort (see Fig. 1 for abbreviations). Norepinephrine secretion in Area X and serotonin secretion in the CMM may, in part, mediate the influence of male quality and the quality of the perceived social environment, respectively, on singing effort (forward solid arrows). Alternatively, the variation in singing effort may elicit the observed changes in norepinephrine secretion in Area X and serotonin secretion in the CMM (reverse solid arrows). Male quality and the quality of the perceived social environment also likely influence singing effort through alternate neural pathways (dashed arrows). Other intrinsic and extrinsic factors may also affect secretion of norepinephrine in Area X and serotonin in the CMM (dotted arrows), through which they may ultimately influence singing effort. The unexplained variation in singing effort can be attributed to alternative intrinsic and extrinsic modulatory factors acting on additional neural pathways to regulate plasticity in singing effort (dotted-dashed arrow).
Norepinephrine secretion in Area X and serotonin secretion in the CMM are also likely influenced by intrinsic and extrinsic factors other than the male’s own quality and the quality of perceived competitors (dotted arrows, Fig. 5). Noradrenergic fibers innervating the songbird auditory telencephalon and song control system project from cell bodies located within the locus coeruleus and subcoeruleus regions (Mello et al., 1998; Appeltants et al., 2000; Ball et al., 2004). Telencephalic serotonergic fibers likely originate from the raphe nuclei, as they do in mammals (Bobillier et al., 1976; Halaris et al., 1976; Moore et al., 1978; Azmitia and Segal, 1978; Klepper and Herbert, 1991; Jacobs and Azmitia, 1992). These monoaminergic cell bodies and fibers receive input from a variety of brain regions, including the hypothalamus and regions of the limbic system (Jacobs and Azmitia, 1992; Ball et al., 2004). Consequently, the extent of monoaminergic secretion in Area X and the CMM may be indirectly influenced by a multitude of intrinsic and extrinsic factors that modulate the signalling properties of these afferent connections.
Factors in addition to the quality of the male and his competitive environment also must influence his singing effort, and these other factors’ effects may be mediated by neural mechanisms other than, or in addition to, monoaminergic activity in the auditory telencephalon and song control system (dotted-dashed arrow, Fig. 5). Central and peripheral levels of sex steroids are elevated during the period of courtship and mate acquisition, often reflect individual condition and quality, and respond rapidly to social interactions (Balthazart, 1983; Wingfield et al., 1993; Ball and Balthazart, 2004; Remage-Healey et al., 2008). Variation in levels of sex steroids and their biologically active metabolites could lead to differential up-regulation and activation of androgen and estrogen receptors within the song control system and consequently modulate monoaminergic activity in these areas and singing activity in response to the male’s own condition or social interactions (Schlinger, 1997; Ball et al., 2003; Gahr, 2004; Harding, 2004; Meitzen et al., 2007; Remage-Healey et al., 2008). The signaller’s sexual motivation and arousal may also play a role in regulating signalling behavior in the context of reproduction. Numerous studies have reported relationships between the expression of sexually motivated behaviors, including male birdsong, and variation in gene expression, morphology, and neurotransmitter and neuroendocrine activity in brain regions that are associated with motivation and arousal, such as the nucleus taeniae, area ventralis of Tsai, medial bed nucleus of the stria terminalis, ventromedial nucleus of the hypothalamus, and the medial preoptic area, which includes the medial preoptic nucleus (Hull et al., 1999; Riters and Ball, 1999; Riters et al., 2000, 2004; Maney and Ball, 2003; Sewards and Sewards, 2003; Heimovics and Riters, 2005, 2006, 2007; Balthazart and Ball, 2007; Heimovics et al., 2009). Future studies examining the neural mechanisms through which other extrinsic and intrinsic factors influence singing effort are crucial for broadening our understanding of how variability in the signaller’s environment or in the signaller itself can shape adaptive behavioral plasticity.
Finally, variation in singing effort may itself elicit the observed differences in norepinephrine secretion in Area X, serotonin secretion in the CMM, and other area-specific monoamine metabolite levels that were individually related to singing effort, but either did not explain variation in singing effort in the final model or exhibited contradictory relationships with song count and mean song length or song environment, and therefore were not subjected to further analyses (see Materials and Methods and Results) (reverse solid arrows, Fig. 5). Numerous studies have shown that context-dependent variation in singing (e.g., singing directed or undirected song) or hearing this variation in one’s own songs can cause changes in the song-control and auditory-processing brain regions, including differences in neural activity, immediate early gene expression, and monoamine metabolism (Jarvis and Nottebohm, 1997; Jarvis et al., 1998; Hessler and Doupe, 1999; reviewed in Doupe et al., 2005; Sasaki et al., 2006). This may explain the significant relationships between singing effort and monoamine metabolism in brain regions that have previously been associated with either context-dependent singing, such as dopamine secretion in Area X (Hessler and Doupe, 1999; Sasaki et al., 2006), or processing context-dependent variation in song, such as serotonin secretion in the NCMv and NCMd and dopamine secretion in the NCMd (Jarvis and Nottebohm, 1997; Sockman and Salvante, 2008).
This study implicates norepinephrine and serotonin secretion within the male starling song control system and auditory telencephalon, respectively, as potential mediators of the effects of intrinsic and extrinsic factors on the modulation of birdsong production. Although some of the relationships we have shown are correlative, identifying these relationships are a critical first step in the discovery of neural mechanisms underlying context-dependent plasticity in singing effort. Future studies involving experimental manipulation of monoaminergic activity in specific brain regions should provide insight into the potential of these neurotransmitters as direct regulators of plasticity in communication signalling.
Acknowledgments
Contract grant sponsor: NINDS (National Institute of Neurological Disorders and Stroke); contract grant number: R01 NS055125
We thank B.A. Whitman for help in experiment set-up and data collection, R.B. Mailman and S.B. Southerland for providing and helping with the HPLC system, T.Q. Gentner for the song recordings used in the playbacks, A. Troyer and family for capturing the birds used in this study, and K. Simmons, K. Suppler, B. Stout, S. Vora, S. Cavadel, and A. Byerly for their help with bird care.
References
- Adret-Hausberger M, Jenkins PF. Complex organization of the warbling song in the European starling Sturnus vulgaris. Behaviour. 1988;107:138–156. [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002;133:221–235. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: Multiple sites of steroid hormone action and the importance of variation in song behavior. Ann NY Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: Multiple sites of action and role of ascending catecholamine projections. Ann NY Acad Sci. 2003;1007:211–231. doi: 10.1196/annals.1286.021. [DOI] [PubMed] [Google Scholar]
- Ball GF, Sockman KW, Duffy DL, Gentner TQ. A neuroethological approach to song behavior and perception in European starlings: interrelationships among testosterone, neuroanatomy, immediate early gene expression, and immune function. Adv Stud Anim Behav. 2006;36:59–121. [Google Scholar]
- Balthazart J. Hormonal correlates of behavior. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Vol. 7. New York: Academic Press; 1983. pp. 221–365. [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrin. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: Steroid effects on brain monoamines. Brain Res. 1988;459:333–343. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF, Waterman SA. Correlations between catecholamine levels and sexual behavior in male zebra finches. Pharmacol Biochem Behav. 1991;41:195–201. doi: 10.1016/0091-3057(92)90082-q. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem Behav. 1996;53:213–220. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM, Backstrom T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol Behav. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Berridge C, Waterhouse B. The locus coeruleus- noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M. The raphe nuclei of the cat brain stem: A topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976;113:449–486. doi: 10.1016/0006-8993(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brainard MS. Contributions of the anterior forebrain pathway to vocal plasticity. Ann NY Acad Sci. 2004;1016:377–394. doi: 10.1196/annals.1298.042. [DOI] [PubMed] [Google Scholar]
- Burnham HP, Anderson DR. Model Selection and Multi-model Inference. Berlin: Springer; 2003. p. 496. [Google Scholar]
- Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: An introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17:1261–1291. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context- dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur J Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song. Cambridge: Cambridge University Press; 1995. p. 256. [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Konishi M. Song-selectig auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci USA. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28:353–363. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Ball GF. Song predicts immunocompetence in male European starlings (Sturnus vulgaris) Proc R Soc Lond B. 2002;269:847–852. doi: 10.1098/rspb.2002.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: An integrated ethological approach. Adv Stud Behav. 1997;26:355–434. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Temporal and sequential organization of song bouts in the starling. Ardea. 1989;77:75–85. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Male song as a cue for mate choice in the European starling. Behaviour. 1991;116:210–238. [Google Scholar]
- Feare CJ. The Starling. Oxford: Oxford University Press; 1984. p. 328. [Google Scholar]
- Gahr M. Hormone-dependent neural plasticity in the juvenile and adult song system. Ann NY Acad Sci. 2004;1016:684–703. doi: 10.1196/annals.1298.025. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Anim Behav. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in aditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gil D, Gahr M. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol Evol. 2002;17:133–141. [Google Scholar]
- Goldstein H, Brown W, Rasbash J. Multilevel modeling of medical data. Stat Med. 2002;21:3291–3315. doi: 10.1002/sim.1264. [DOI] [PubMed] [Google Scholar]
- Halaris AE, Jones BE, Moore RY. Axonal transport in serotonin neurons of the midbrain raphe. Brain Res. 1976;107:555–574. doi: 10.1016/0006-8993(76)90144-x. [DOI] [PubMed] [Google Scholar]
- Harding CF. Hormonal modulation of singing: Hormonal modulation of the songbird brain and singing behavior. Ann NY Acad Sci. 2004;1016:524–539. doi: 10.1196/annals.1298.030. [DOI] [PubMed] [Google Scholar]
- Harding CF, Sheridan K, Walters MJ. Hormonal specificity and activation of sexual behavior in male zebra finches. Horm Behav. 1983;17:111–133. doi: 10.1016/0018-506x(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Breeding-context dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav Brain Res. 2007;176:333–343. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nature Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-meurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: Monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak G. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19:8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak G. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol. 2001;85:828–842. doi: 10.1152/jn.2001.85.2.828. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak G. Serotonin modulates responses to species-specific vocalizations in the interior colliculus. J Comp Physiol A. 2005;191:535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hear Res. 2002;168:1–11. doi: 10.1016/s0378-5955(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Jacobs BJ, Azmitia EC. Structure and function of the brain-serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Moter-driven gene expression. Proc Natl Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the birds sings: Context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculous of the rat. Brain Res. 1991;557:180–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Kessel B. Criteria for sexing and aging European starlings (Sturnus vulgaris) Bird Banding. 1951;22:16–23. [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hydroxytryptamine and four metabolically related compounds by means of reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr-Biomed. 1981;225:347–357. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroodsma DE, Byers BE, Goodale E, Johnson S, Liu W-C. Pseudoreplication in playback experiments, revisited a decade later. Anim Behav. 2001;61:1029–1033. [Google Scholar]
- MacDougall-Shackleton SA, Hulse SH, Ball GF. Neural bases of song preferences in female zebra finches (Taeniopygia guttata) NeuroReport. 1998;9:3047–3052. doi: 10.1097/00001756-199809140-00024. [DOI] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: Immunocytochemical study of dopamine-β-hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acan Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KE. Drug-induced changes in the efflux of dopamine and serotonin metabolites from the brains of freely moving rats. Ann NY Acad Sci. 1986;473:303–320. doi: 10.1111/j.1749-6632.1986.tb23625.x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: Ascending projections. J Comp Neurol. 1978;180:416–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Song as an attractant for male and female European starlings, and the influence of song complexity on their response. Behav Ecol Sociobiol. 1991;28:97–100. [Google Scholar]
- Mountjoy DJ, Lemon RE. Female choice for complex song in the European starling: A field experiment. Behav Ecol Sociobiol. 1996;38:65–71. [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canaria. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2005. p. 320. [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male Europoean starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA. Sex steroids and their actions on the birdsong system. J Neurobiol. 1997;33:619–631. [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull. 2003;61:25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Sockman KW. Neural orchestration of mate-choice plasticity in songbirds. J Ornith. 2007;148:S225–S230. [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc Roy Soc Lond B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Complimentary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J Neurobiol. 2005a;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Develop Neurobiol. 2008;68:656–668. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG, Racke DM, Campbell CR, Whitman BA. Song compeition changes the brain and behavior of a male songbird. J Exp Biol. 2009;212:2411–2418. doi: 10.1242/jeb.028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Sewall KB, Ball GF, Hahn TP. Economy of mate attraction in the Cassin’s finch. Biol Lett-UK. 2005b;1:34–37. doi: 10.1098/rsbl.2004.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Weiss J, Webster MS, Talbott V, Schwabl H. Sex-specific effects of yolk-androgens on growth of American kestrels. Behav Ecol Sociobiol. 2008;62:617–625. [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata) J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wiley RH. Is there an ideal behavioural experiment? Anim Behav. 2003;66:585–588. [Google Scholar]
- Wingfield JC, Farner DS. Encodrinology of reproduction in wold species. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Vol. 9. New York: Academic Press; 1993. pp. 163–327. [Google Scholar]