Abstract
Purpose
Stratified squamous epithelial cells assemble a specialized protective barrier structure on their periphery, termed the cornified envelope. The purpose of this study was to evaluate the presence and distribution of cornified envelope precursors in human corneal epithelium, their expression in human corneal epithelial cell cultures, and the effect of ultraviolet radiation (UVB) and transglutaminase (TG) inhibition on their expression.
Methods
Tissue distribution of small proline-rich proteins (SPRRs) and filaggrin and involucrin was studied in human cornea sections by immunofluorescence staining. Primary human corneal epithelial cells (HCECs) from limbal explants were used in cell culture experiments. A single dose of UVB at 20 mJ/cm2 was used to stimulate these cells, in the presence or absence of mono-dansyl cadaverine (MDC), a TG inhibitor. SPRR2 and involucrin protein levels were studied by immunofluorescence staining and Western blot analysis. Gene expression of 12 proteins was investigated by semiquantitative reverse transcription–polymerase chain reaction.
Results
In human cornea tissue, SPRR1, SPRR2, filaggrin, and involucrin protein expression were detected in the central and peripheral corneal and limbal epithelium. In HCECs, SPRR2 and involucrin proteins were detected in the cytosolic fraction, and involucrin levels increased after UVB. Both SPRR2 and involucrin levels accumulated in the presence of MDC. Nine genes including involucrin, SPRR (types 1A, 1B, 2A, 2B, and 3), late envelope protein (LEP) 1 and 16, and filaggrin were expressed by HCECs. SPRR 4, loricrin, and LEP 6 transcripts were not detected. UVB downregulated SPRR (2A, 2B) and LEP 1 transcripts.
Conclusions
Various envelope precursors are expressed in human corneal epithelium and in HCECs, acute UVB stress differentially alters their expression in HCECs. The expression of envelope precursors and their rapid modulation by UVB supports the role of these proteins in the regulation of ocular surface stress. TG function may be relevant in the regulation of soluble precursors in UVB-stimulated corneal epithelium.
Stratified squamous epithelial cells assemble a specialized protective barrier structure on their periphery termed the cornified cell envelope.1 Skin keratinocytes express cornified envelope precursors involucrin,2 loricrin,3 small proline-rich proteins (SPRRs),4 late envelope proteins (LEPs),5 and filaggrin.2 As part of the development of the skin epidermis, the cornified envelope precursors become covalently cross-linked to form a 5- to 10-nm mature envelope adjacent to the cell membrane.1 This transamidation process1 is mediated by transglutaminase (TG) enzymes, and it occurs sequentially, allowing the classification of precursors into early (involucrin and SPRR) or late proteins (LEP and filaggrin).
The corneal epithelium is stratified and bears similarities to the skin epidermis in its pattern of basal to superficial polarity,6 but it is different, in that the corneal epithelium does not have a water-impermeable apical layer that is equivalent to the cornified layer in the skin epithelium. Nevertheless, involucrin, a cornified envelope precursor, is expressed in normal corneal epithelium,7 and the precursors involucrin and filaggrin are elevated in the human conjunctiva in severe ocular surface diseases, such as Stevens-Johnson syndrome and alkali burns.8 Involucrin and loricrin have been detected in keratinized corneal epithelia in a rat model of dry eye.9 Because expression of such proteins can be found in nonsquamous tissues and cell lines,4 such molecules may have a function not associated with squamous differentiation. Indeed, these proteins may have a regulatory10 rather than structural role, as shown in the conjunctiva.11
The first reason for the study of cornified envelope proteins in corneal epithelium is the involvement of these precursors in barrier function. For example, the LEPs have been linked to barrier formation in nonocular tissues.5 In the cornea, the stratified epithelium is avascular and has different barrier requirements than the skin, because oxygen and nutrients need to permeate the epithelial layers from the tear film. As such, envelope proteins may have evolved away from a simple mechanical role.
Second, cornified envelope precursors may be involved in inflammatory diseases. SPRR proteins are involved in inflammatory diseases of the skin.12 Elafin, a naturally occurring precursor in skin epidermis, is a potent anti-inflammatory agent that serves as a proteinase inhibitor.10 Proinflammatory signaling may differentially regulate SPRR13 and involucrin genes.14 Third, these precursors are involved in wound healing. In murine skin, the migrating edge of epithelium after wounding expressed involucrin.15 The fourth reason for the study of such precursors is their possible role in mucosal defense against microbes. The role of SPRR in innate defense has been reported.16,17 Last, precursors may also play a role in less common diseases in the ocular surface. SPRRs are involved in neoplastic diseases of the skin,12 and in ischemic stress in cardiomyocytes.18 Despite the possible significance of these proteins in the corneal epithelium, there have been no previous studies documenting the expression of SPRR and LEP in this tissue.
Ultraviolet irradiation19,20 is a useful method of studying the regulation of cornified envelope substrates. First, such stimulation alters the expression of these proteins in nonocular tissues and is the basis of a wound healing model.2 Second, it causes corneal surface disease.21 Third, it plays a role in corneal barrier dysfunction22 in many ocular surface diseases. Last, it activates both cellular stress23 and differentiation related pathways, such as calcium signaling, which have a prominent effect on cornified envelope protein expression.24 The study of UVB-induced cornified envelope substrate regulation and the effect of TG inhibition may therefore have implications in the treatment of barrier-, inflammation- and wound-healing–related diseases in the ocular surface. Regulation of involucrin, SPRR1, SPRR3, SPRR4, loricrin, and filaggrin by UVB has been investigated in the skin,2,25 but not in the cornea. Although the anti-inflammatory effect of TG inhibition has been studied in allergic conjunctivitis,11 the effects of TG inhibition on cornified envelope proteins have not been reported in corneal epithelial cells.
The purpose of this study was to investigate the expression of cornified envelope precursors in human corneal epithelium, to investigate the expression and UVB-induced regulation of a select panel of these precursors in cultured human corneal epithelial cells, and last, the effect of inhibiting TG function on the UVB-induced regulation.
Methods
Materials and Reagents
Cell culture dishes, plates, centrifuge tubes, and other plastic ware were purchased from BD Biosciences (Lincoln Park, NJ). Dulbecco's modified Eagle's medium (DMEM), Ham's F-12, amphotericin B, gentamicin, and 0.25% trypsin/0.03% EDTA solution were from Invitrogen-Gibco (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Bovine insulin, hydrocortisone, human EGF, cholera toxin A subunit, human transferrin, sodium selenite, dimethyl sulfoxide (DMSO) and Triton X-100 were from Sigma-Aldrich (St. Louis, MO). Mono-dansyl cadaverine (MDC) was from Invitrogen (Eugene, OR). OCT compound was obtained from Sakura Finetek USA. Inc. (Torrance, CA and the mounting medium (Immu-Mount) was from Thermo-Shandon (Pittsburgh, PA) Optisole-GS was purchased from Bausch and Lomb Inc. (Rochester, NY). Anti-fade medium (Gel/Mount M01) was from Biomeda Corp. (Foster City, CA).
The extraction reagent (TRIzol) was from Invitrogen-Gibco (Grand Island, NY). PCR buffer II (E12848) was obtained from Applied Biosystems (Foster City, CA). M-MuLV reverse transcriptase (Ready-To-Go You-Prime First-Strand Beads) were obtained from GE Healthcare, Inc. (Piscataway, NJ). PCR master mix (AmpErase UNG, Taqman MGB) probes for SPRR1A, SPRR1B, SPRR2A, and SPRR2B with assay IDs Hs00954595_s1, Hs00824893_m1, Hs01655189_sH, and Hs00863465_m1, respectively, were from Applied Biosystems.
The protein extraction kit (MPER) and BCA protein assay were from Pierce Biotechnology (Rockford, IL). Gel for protein electrophoresis (Ready Gel; 4%–15% Tris-HCl) was from Bio-Rad (Hercules, CA). Polyvinylidene difluoride (PVDF) membranes (Immobilon-P) were from Millipore (Billerica, MA). The electrophoretic transfer buffer was a Tris/glycine buffer from Bio-Rad. The TTBS wash buffer composed of 0.1% Tween 20 (Bio-Rad), 50 mM Tris and 0.15 M sodium chloride (pH 7.5). The chemiluminescent reagent (Luminol) was from Santa Cruz Biotechnology (Santa Cruz, CA).
Goat anti-rabbit AlexaFluor 488 antibodies (A11008) and goat anti-mouse AlexaFluor antibodies (A21151) were from Invitrogen-Molecular Probes (Carlsbad, CA). Goat anti-rabbit (no. 31460) and goat anti-mouse (no. 31430) horseradish peroxidase (HRP)–conjugated antibodies were from Pierce Biotechnology.
Human Corneal Tissue
Fresh human corneal tissues were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). Details of tissue preparation have been described.7 Briefly, the specimens were cut and embedded in a mixture of 75% OCT and 25% mounting medium (by volume; ImmuMount; Thermo-Shandon) and frozen in liquid nitrogen. Frozen sections (6–10 μm thick) were used for immunostaining. Human corneoscleral tissues, which did not meet the criteria for clinical use, from donors aged 2 to 94 years were obtained from the Lions Eye Bank of Texas (Houston, TX) for the purpose of limbal explant culture. The details of the donors' condition, tissue procurement, and length of preservation were supplied by the Eye Bank. These tissues were preserved (Optisol-GS; Chiron, Irvine, CA) at 4°C until they were processed for culture.
Cell Culture Procedure
Primary human corneal epithelial cells (HCECs) were grown from cadaveric limbal explants, as described previously.26 Briefly, corneoscleral tissues were rinsed with Hanks' balanced solution containing 50 μg/mL gentamicin and 1.25 μg/mL amphotericin B. After the central cornea, excess sclera, iris, corneal endothelium, conjunctiva and Tenon's capsule were carefully removed, the remaining limbal rim was cut into 12 equal pieces (∼2 mm2 each). Two pieces with the epithelium side up were directly placed in a well of a six-well culture plate or into a 35-mm dish, and they were covered with a drop of FBS overnight. The explants were then cultured in supplemented hormonal epithelial medium (SHEM), which was a 1:1 mixture of DMEM and Ham's F12 medium containing 5 ng/mL epidermal growth factor (EGF), 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite, 0.5 μg/mL hydrocortisone, 30 ng/mL cholera toxin A, 0.5% dimethyl sulfoxide (DMSO), 50 μg/mL gentamicin, 1.25 μg/mL amphotericin B, and 5% FBS, at 37°C in 5% CO2 and 95% humidity.
Cells grown for 7 to 12 days with 50% to 70% confluence were designated “young” cell cultures. Cells grown to 70% to 99% confluence, usually grown for 13 to 17 days, were designated “subconfluent.” whereas cells grown for 18 to 21 days and noted to be 100% confluent were labeled “confluent cells.” “Older” cells referred to cells that were overconfluent, grown for more than 21 days, and noted to have a relatively more opaque appearance. For experiments with UVB, cell cultures were observed daily under inverted light microscopy, and experiments commenced when cells became just confluent.
Corneal stroma were known to maintain a suitable microenvironment for human limbal epithelial stem cells and to control the plasticity of corneal epithelial differentiation.27 For this reason, we speculate that human corneal epithelial cells cultured from human limbal explants may be able to differentiate and stratify on porcine corneal stroma.
Porcine eyes were ordered and obtained from VisionTech, Inc. (Mesquite, TX). After the eye's corneal epithelium was completely removed with a surgical blade, a femtosecond YAG laser (IntraLase Corp., Irvine, CA) was used to create stromal discs. These stromal discs were cut to a diameter of 9 mm and thickness of 90 μm and were then placed on a culture plate containing 12 mm membrane insert supports (Corning Inc., Acton, MA). A piece of human cadaveric limbal tissue approximately 2 × 2 mm2 in size was placed epithelium side up on each stromal disc and cultured in SHEM medium, as described earlier. After 3 weeks, the entire insert was trimmed with scissors, and frozen sections obtained as described previously.7 Hematoxylin and eosin staining was performed as described previously.7
Immunofluorescent Staining
Immunofluorescent staining was performed as described previously.7 Briefly, frozen human corneas were thawed and fixed with acetone at 4°C for 5 minutes. After blocking with 5% normal goat serum in phosphate-buffered saline (PBS) for 30 minutes, a primary antibody (Table 1) was applied at the specified dilution in 5% goat serum overnight at 4°C. These and subsequent steps were performed at room temperature. After washing with PBS, the appropriate AlexaFluor 488 secondary antibody (1:300 dilution or 6.7 μg/mL) was applied in 5% goat serum for 30 minutes in a dark incubation chamber. Counterstaining of cell nuclei was performed using 1 μg/mL propidium iodide in PBS for 2 minutes. After further washing, a coverslip was applied with gel. Staining patterns were examined and photographed with an epifluorescence microscope (Eclipse 400; Nikon, Melville, NY) with a digital camera (1200; DMX, Garden City, NY). Image acquisition was performed with the camera software (ACT-1, ver. 2.12 software; Nikon, Melville, NY). Appropriate negative controls were incubated as just described, in the absence of primary antibody, but with the various secondary antibodies.
Table 1.
Antibodies Used in Immunofluorescence and Western Blots
| Antigen | Category | Catalog No. | Source | Working Dilution or Concentration |
|---|---|---|---|---|
| Indirect Immunofluorescent staining | ||||
| SPRR 1A | Rabbit polyclonal | ab18580 | Abcam, Inc., Cambridge, MA | 1: 50 |
| Filaggrin | Mouse monoclonal | BT-576 | Biomedical Technologies Inc, Stoughton, MA | 1: 50 |
| Western blot and immunofluorescent staining | ||||
| SPRR 2A/2B | Rabbit polyclonal | APO-25N-001 | Alexis Biochemicals, San Diego, CA | 1: 500 (WB) 1: 100 (IF) |
| Involucrin | Mouse monoclonal | MS-126, Ab-1 clone SY5 | Lab Vision, Fremont, CA | 1: 100 or 2 μg/mL (WB and IF) |
WB, Western blot; IF, immunofluorescent staining.
In the case of immunostaining for cell cultures, the procedure was identical with two exceptions. After the cultured cells were washed with PBS, the wells were fixed with 100% methanol in −20°C for 15 minutes, followed by another wash with PBS and incubation with 0.2% Triton X-100 in PBS for 10 minutes, and then another wash with PBS before nonspecific binding was blocked with goat serum.
UVB Procedure
UVB was administered with a UVB lamp (UVM57; UVP, Upland, CA) that principally emitted medium ultraviolet wavelengths. The power output of the lamp was monitored using a radiometer (UVX; UVP), and a 302-nm sensor probe was placed under the UVB source where the irradiated cell cultures would be placed. A single dose of UVB at 20 mJ/cm2 was used to stimulate cells in a sterile culture hood, which generally corresponds to 24 seconds of continuous exposure after stabilization of power. Culture wells that did not receive any UVB exposure were used as the control. The media for all cell cultures (including nonirradiated controls) were aspirated before irradiation and replaced immediately after the irradiation. MDC was used as a cell-permeable competitive inhibitor of TG at concentrations of 40 and 60 μg/mL. This is an amine substrate28 with a high affinity for transglutaminase, serving as a substrate as well as an inhibitor by projecting an alkyl side chain toward the active center of the enzyme. Incubation with the inhibitor, where appropriate, was for 1 hour before any UVB exposure, and continued after the UVB exposure until the end of the experiment.
Polymerase Chain Reaction
Reverse transcription–polymerase chain reaction (PCR) was performed as described previously.29 Briefly, total cellular RNA was extracted 6 and 24 hours after UVB (TRIzol; Invitrogen-Gibco), after being washed with PBS. Reverse transcription was performed with magnesium chloride (5 mM), deoxynucleotides (1 mM each), RNAase inhibitor (1 U/μL), reverse transcriptase (2.5 U/μL), and random hexamers (2.5 uM) in PCR buffer II. PCR was performed with a custom-designed nucleotide primer against a unique part of the respective human mRNA or cDNA (Table 2). cDNA amplification was performed with an individually optimized number of cycles and temperatures for each primer. Controls included the housekeeping gene GADPH, as well as nonirradiated samples incubated for the same duration as the irradiated samples. The relative amount of mRNA detected was indicated by the relative intensity of the ethidium bromide stained bands (which was an indicator of the amount of amplicon produced at the end of the PCR) on electrophoresis of PCR products in 2% agarose gel. Band intensities were analyzed using (1D ver. 3.6.2 software; Eastman-Kodak, New Haven, CT).
Table 2.
Primers Used in Polymerase Chain Reaction
| Cornified Envelope Precursor | NCBI No. | Primer Sequence | Expected Product Size (bp) |
|---|---|---|---|
| SPRR1A | NM 005987 | F: tggccactggatactgaaca R: cccaaatccatcctcaaatg |
230 |
| SPRR1B | NM 003125 | F: cattctgtctcccccaaaaa R: atgggggtataagggagctg |
172 |
| SPRR2A | NM 005988 | F: tatttggctcacctcgttcc R: ccaggacttcctttgctcag |
187 |
| SPRR2B | NM 006945 | F: taattggctcacctcgttcc R: gagctgctgctctttctgct |
229 |
| SPRR3 | BC017802 | F: ttccacaacctggaaacaca R: ttcagggaccttggtgtagc |
200 |
| SPRR4 | NM 173080 | F: caagtgaagcagccttgtca R: atctggtagccaggatggtg |
222 |
| LEP1 | Marshall et al.5 | F: cctccagtctcttcctgctg R: tgatcactcatttcacccga |
270 |
| LEP6 | Marshall et al.5 | F: ccatctaacttgctgtctgacc R: aggaagatgaagttgcccct |
496 |
| LEP16 | Marshall et al.5 | F: ctttgttttgtccaggacaa R: cctgtggtggttcaggaag |
210 |
| Filaggrin | AF043380 | F: gctctaggcactcagcatcc R: gagccgtctcctgattgttc |
235 |
| Involucrin | NM_005547 | F: ggactgcctgagcaagaatgtg R: taagctgctgctctgggttt |
121 |
| Loricrin | NM000427 | F: gtgggagcgtcaagtactcc R: tagagacgcctccgtagctc |
243 |
| GADPH | M33197 | F: gccaaggtcatccatgacaac R: gtccaccaccctgttgctgta |
498 |
For the quantification of mRNA level fluctuations of SPRR1A, 1B, 2A and 2B, real-time PCR was used. The first-strand cDNA was synthesized from 1 μg of total RNA with random hexamer using M-MuLV reverse transcriptase (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare), as previously described.30,31 Real-time PCR was performed using specific probes (Taqman MGB; Applied Biosystems) a universal PCR master mix (AmpErase UNG; Applied Biosystems), and a commercial thermocycler (Smart Cycler System; Cepheid, Sunnyvale, CA), according to the manufacturer's recommendations. Assays were performed in duplicate, in two different experiments. A nontemplate control was included, to detect DNA contamination. The GAPDH gene was used as an endogenous reference for each reaction, to correct differences in the amount of total RNA added. The results were analyzed by the comparative CT method30 where target multiple of change = 2–ΔΔCt. The cycle threshold (Ct) was determined as the cycle at which the primary (fluorescent) signal crosses a user-defined threshold. The results were normalized by the Ct of GAPDH and the relative mRNA level in the nonirradiated or the youngest cell group (calibrator).
Western Blot Analysis
Protein extraction was performed after incubation of cells for 24 hours after UVB irradiation. HCECs were lysed with MPER after aspiration of media and washing with PBS. Lysates were vortexed and centrifuged at 13,000g for 15 minutes. Pellets (the insoluble fraction) were solubilized by addition of 50 to 75 μL of 1% sodium dodecyl sulfate (SDS) followed by vigorous vortexing.
Western blot analysis was performed as previously described.30 Briefly, protein concentration was determined using the BCA method according to the manufacturer's instructions. The same mass of protein (40 μg) was loaded per gel lane for electrophoresis. The separated proteins were transferred to a PVDF membrane. Blots were washed in TTBS, and all subsequent steps performed at room temperature. Blocking for nonspecific proteins was performed for 1 hour in 5% skimmed milk.
Western blot analysis were performed using the primary antibodies specified in Table 1. All dilutions were made in 5% skimmed milk in TTBS. Primary antibody incubation was for 1 hour. After further washing, the appropriate secondary HRP conjugated antibody was applied for a further hour in a rocker, at a dilution of 1:5000. Chemiluminescence was detected using Luminol and the imaging system (2000R; Eastman Kodak).
Statistical Analysis
All experiments were performed at least three times. Statistical analysis was performed on computer (Microsoft Excel, Redmond, WA, and Prism 4 for Windows, ver. 4.03; GraphPad Software Inc, San Diego, CA). Analysis of variance (ANOVA) with Tukey post hoc testing was used for statistical comparisons, and statistical significance was evaluated at the level of α = 0.05.
Results
Expression of Cornified Envelope Proteins in Human Cornea Tissue and Epithelial Cultures
Expression of cornified envelope proteins was evaluated in fresh frozen human corneal tissue sections using immunofluorescent staining. SPRR1, SPRR2, filaggrin, and involucrin (Fig. 1) were present in the central, peripheral corneal epithelium, and limbal epithelium. SPRR1 and SPRR2 staining was noted in all layers but accentuated in the wing-cell layer and apical layer in the central cornea, staining was absent in some parts in the basal layer of the limbus. Involucrin was noted in the superficial layers of the central cornea and limbus. Filaggrin staining was weak in all layers with some accentuation in the superficial most layers.
Figure 1.
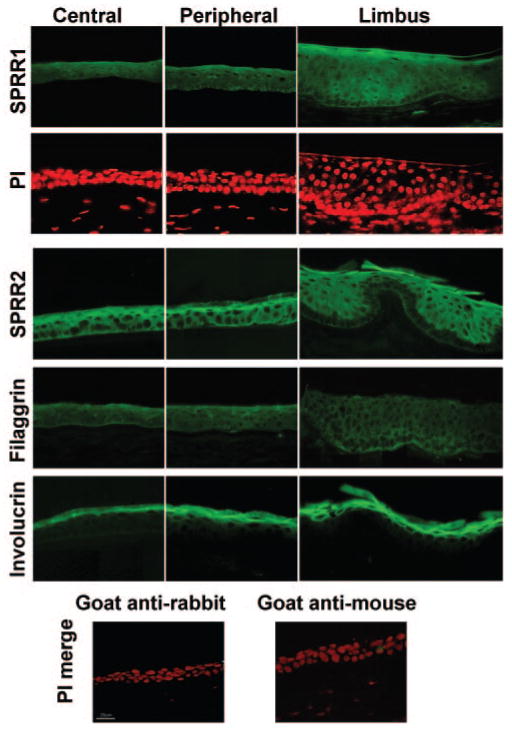
Immunofluorescence images of human cornea tissue sections showing reactivity against SPRR1, and -2, filaggrin, and involucrin. Bottom: Negative control images merged with images of propidium iodide counterstaining.
Immunofluorescent staining of these proteins was also evaluated in confluent HCECs. Staining for both SPRR2 and involucrin proteins was noted (Fig. 2A, top panel), in the cytoplasma of the cells. SPRR2 and involucrin were detected by Western blot analysis in these confluent HCECs, with greater immunoreactivity for SPRR2 and involucrin proteins noted in the soluble fraction compared to the insoluble fraction (Fig. 2B).
Figure 2.
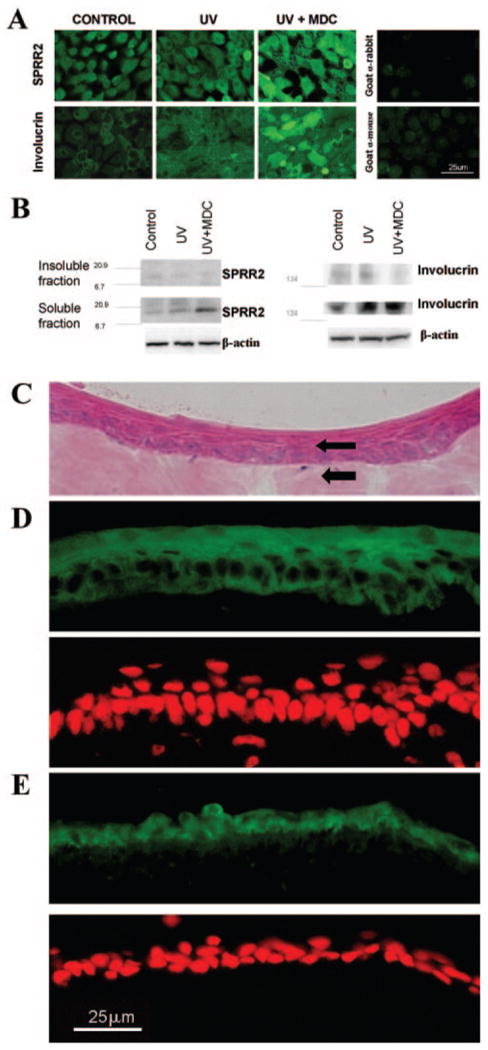
Immunofluorescence images (A) and Western blot analysis (B) performed on confluent HCECs at 24 hours after UVB. Control, control cultures; UV, UVB-treated cultures; UV+MDC, UVB treatment + MDC; PI, propidium iodide. (C) H&E staining of human corneal epithelial cells (top arrow) grown on porcine corneal stroma (bottom arrow). (D) Immunofluorescent staining image for SPRR2 (top) and propidium iodide counterstaining (bottom). (E) Immunofluorescent staining for involucrin (top) and propidium iodide counterstaining (bottom).
Human corneal epithelial cells grown on porcine stroma were able to stratify to multiple layers (Fig. 2C). SPRR2 (Fig. 2D) and involucrin (Fig. 2E) staining were observed in these cells, SPRR2 was present throughout the layers and in some cases, more intense in the suprabasal and superficial layers (Fig. 2D). Involucrin staining was patchy and relatively stronger in the thinner epithelial layers (Fig. 2E).
mRNA Expression of Cornified Envelope Precursors
Cornified envelope mRNA expression was evaluated in confluent cultured cells. Among the 12 proteins studied, mRNA expression for nine genes were detected, whereas three genes were not. Involucrin, SPRR (1A, 1B, 2A, 2B and 3), LEP (1,16) and filaggrin were observed (Fig. 3), whereas PCR of up to 60 cycles using the SPRR4, loricrin, or LEP6 primers shown in Table 2 did not produce any detectable amplicons.
Figure 3.
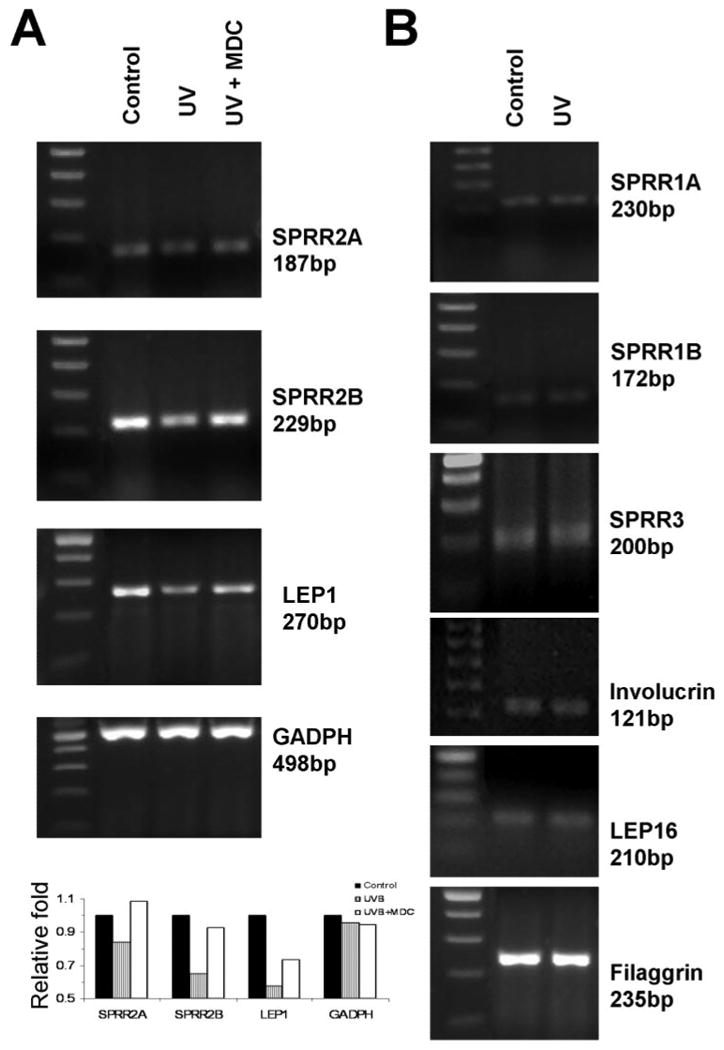
Reverse transcription PCR for cornified envelope protein precursors in cultured human corneal epithelial cells. (A) Regulation of cornified envelope mRNA by UVB at 6 hours: SPRR2A, SPRR2B, and LEP-1, as well as GADPH. The effect of MDC on the UVB-induced change is also shown. The expected size of the amplicons is shown. Leftmost lane: The molecular size ladder in 100-bp increments. Bottom: densitometric intensities of bands relative to the control band. (B) Cornified envelope mRNA not up- or downregulated by UVB.
Semiquantitative PCR showed that mRNA of SPRR1A, 1B, 2A, and 2B were downregulated when overconfluent or older cultured HCECs was compared to subconfluent HCECs (Fig. 4A). To confirm this finding, real-time PCR was used to compare the mRNA levels of these 4 precursors. A significant association was found between the mRNA levels of SPRR1A (Fig. 4B), SPRR1B (Fig. 4B), SPRR2A (not shown), and SPRR2B (not shown) and the state of confluence of the HCECs, with P = 0.023, 0.022, 0.0106, and 0.011, respectively. Figure 4B also shows the statistically significant multiple comparisons on post hoc testing.
Figure 4.
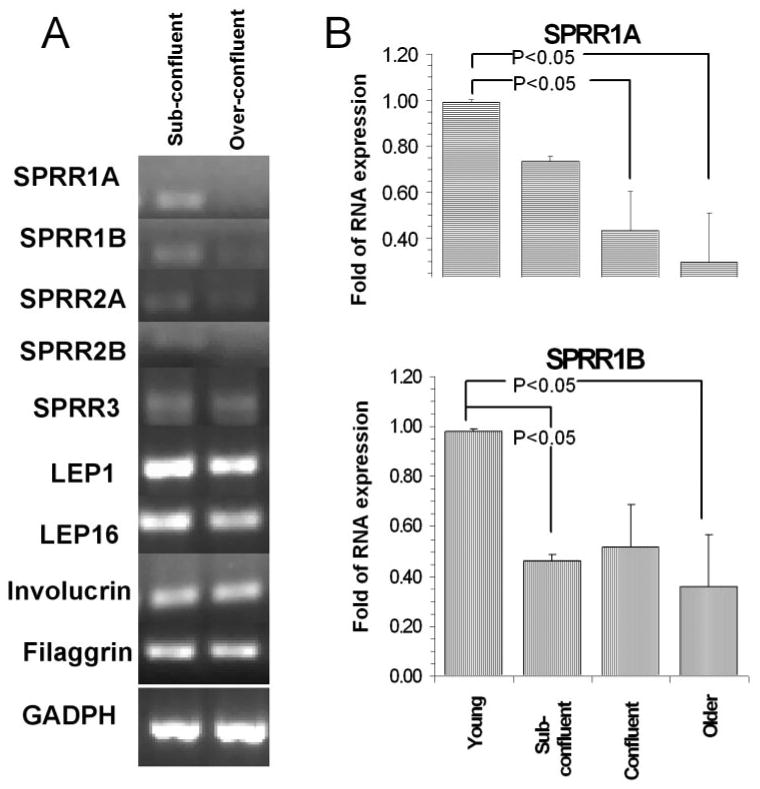
Effect of cell confluence on the mRNA expression of envelope proteins. (A) Semiquantitative PCR showing relative mRNA expression of cornified envelope precursors in the subconfluent and overconfluent cells. (B) Real-time PCR results for RNA expression of SPRR1A and -1B with different confluence and age of cells in culture. The change in expression (x-fold) was relative to the youngest cell category. Error bars, SEM. Probabilities refer to the results of ANOVA. The brackets indicate the statistically significant comparisons on post hoc testing.
Effects of UVB on Cornified Envelope Protein and mRNA Expression
The effects of UVB irradiation on expression of cornified envelope proteins (at 24 hours) and mRNA (at 6 and 24 hours) in confluent HCECs were evaluated. As noted by immunofluorescent staining (Fig. 2A, middle panel) and Western blot (Fig. 2B), UVB induced the expression of involucrin, but not SPRR2 protein. By semiquantitative RT-PCR, UVB was observed to downregulate SPRR (2A, 2B) and LEP 1 mRNA at 6 hours relative to controls incubated for the same duration (Fig. 3A), whereas no obvious UVB induced change was detected for the other transcripts (Fig. 3B). Changes detected at 6 hours (Fig. 3A) were less evident at 24 hours (not shown).
Effect of MDC on Cornified Envelope Expression
UVB-induced mRNA expression of cornified envelope precursors was evaluated in the presence of the TG inhibitor MDC in confluent HCECs at 6 and 24 hours. This inhibitor suppressed the UVB-induced change in mRNA expression in SPRR2A, SPRR2B, and LEP1 (Fig. 3A).
Immunofluorescent staining of confluent HCECs, 24 hours after UVB, showed that incubation with MDC increased the level of fluorescence when staining was performed for SPRR2 and involucrin (Fig. 2A, bottom panel). This finding was confirmed by Western blot on confluent HCECs 24 hours after UVB, where accumulation of SPRR2 and involucrin in the cytoplasmic fraction was observed in the presence of MDC (Fig. 2B). This suggests that there was reduced incorporation of SPRR2 and involucrin into the insoluble envelope.
Discussion
Our results of a survey of various cornified envelope precursor proteins show their presence and distribution in human corneal epithelial tissue and the selective expression of some members in HCECs. For the first time, the gene expression of members of late envelope proteins (LEP) in an ocular cell type is reported. Culture confluence and UVB are observed to regulate the mRNA expression of some of these precursors. Furthermore, inhibition of transglutaminase (TG) appears to affect expression of certain members.
Expression of cornified envelope protein subtypes varies in different tissues: SPRR1 is restricted to appendageal areas, SPRR2 in sublingual and tongue epithelium and SPRR3 in esophageal epithelium.12 LEP-1 to -5 are found in the skin, whereas LEP-14 and -15 are found in the esophagus and LEP-7 and -16, in the heart.5 Most (9/12) of the cornified envelope precursor mRNAs in corneal epithelial cells are detected in the control (non-irradiated) HCECs, especially in younger cells. In skin epidermis envelope assembly involves formation of a scaffold by ubiquitous proteins such as involucrin, and the “reinforcement” proteins which differ between epithelial cell types.32 In the corneal epithelium, SPRR may be the favored reinforcement protein, whereas in the skin, loricrin is preferred.32 SPRR members have a variable number of proline-rich peptide repeats in the central domains and span different lengths in their cross-bridging roles.32
In our study, the human corneal epithelial cells grown on porcine stroma were able to stratify and differentiate. The pattern of SPRR2 and involucrin staining was similar to those we observed in central and peripheral human corneal sections. At the same time, we found that SPRR transcripts decrease with increasing confluence. Conditions unique to cell culture, such as crowding and competition for nutrients may affect the SPRR transcript level even in nonconfluent monolayer cells. This may be a function of proliferative rate or cellular turnover and may be unrelated to differential expression of the SPRR protein in stratified cultures. Expression of cornified envelope proteins has been noted to differ between cultured cells and tissue (e.g., cultured keratinocytes express loricrin poorly whereas skin keratinocytes express it abundantly).3
We have shown that mRNA levels of cornified envelope substrates are regulated by UVB. In previous studies involucrin, loricrin, and filaggrin were altered within 48 hours in ultraviolet irradiated human skin grafted to nude mice.33 In the skin, UVB downregulates involucrin and SPRR3, but upregulates SPRR4 level and its incorporation into the envelope.25 Loricrin and filaggrin are downregulated, whereas involucrin and SPRR1 are unaffected after UVB irradiation of in vitro reconstructed skin.2 In corneal epithelial cells, the involucrin promoter may bind stress-induced transcription factors.34,35 Differences in dosage, wavelength, and regimen of ultraviolet irradiation make it difficult to compare results between studies. We observed discrepancies between protein and mRNA levels of involucrin and SPRR2A and -2B after UV treatment. The possible reasons for these “discrepancies” may relate to regulatory factors such as RNA stability, degradation, translational initiation and protein degradation (ubiquitylation, sumoylation).36–39 In addition, the SPRR2 antibody may react against some members of SPRR2 family other than SPRR2A and -2B.
The effects of MDC were: first, to suppress the UVB-induced reduction in mRNA levels of some precursors (SPRR2A and -2B and LEP1) and, second, to increase the soluble protein levels (SPRR2 and involucrin) after UVB exposure. How does the inhibitor oppose the UVB-induced decrease of mRNA levels? Proinflammatory cytokines and stress pathways interact at the SPRR1A promoter in nonocular cell types.18 Because TG inhibition reduces the TG-mediated increase in phospholipase11 and NFκB activation,40 addition of inhibitor may alter the composition of the transcriptional complex in favor of transcription. Why does MDC increase the level of envelope protein after UVB? This is most likely due to the inhibition of cross-linking by TG. Covalent cross-linkage is expected to shift the soluble precursors to insoluble cornified envelope. Therefore, if UVB stimulates the cellular utilization of soluble precursors in this fashion, TG inhibitor is expected to block the shift and increase the detectable soluble precursor. The ability of TG to enzymatically cross-link substrates has been previously shown in the skin.41,42 Because soluble involucrin is located in the cytoplasm and not at the perimembranous cell envelope,1 an additional regulatory step may involve TG-involucrin interaction43 or targeting of involucrin to the cell periphery.
This study has identified the expression of LEP genes in HCECs. A limitation of the study is that we omitted protein studies for these members. We did not study such precursors as envoplakin, the S10044 proteins, and the NICE proteins.45 Because knocking out of major cornified envelope proteins has not produced marked phenotypes in animals,46 it may be important to examine these less-well-studied members. We did not investigate in situ tissue distribution of cornified envelope mRNA, or the effect of UVA radiation, which may be more relevant than UVB in clinical scenarios.47
According to the precursor-availability hypothesis for skin epidermis, the envelope structure incorporates whatever precursor is available under environmentally stressed conditions.44,48 The composition of the envelope may determine its biomechanical properties and barrier function.42 Application of the “precursor availability” hypothesis to the corneal epithelium underlies the importance of understanding the stress-induced regulation of envelope precursors in this specialized cell. Some newly discovered roles of the precursors in nonocular tissue have been found: SPRR2A may play a role in bacterial infection,16,17 and SPRR1A may protect cardiomyocytes against ischemic stress.18 It will not be surprising if these precursors have a protective role in the ocular surface.
In conclusion, SPRR1, SPRR2, filaggrin, and involucrin are expressed in human corneal epithelium. Many subtypes of cornified envelope protein precursors, including involucrin; SPRR1A, -1B, -2A, and -2B; LEP-1, -6, and -16; and filaggrin are expressed in primary cultured corneal epithelial cells. Some of these proteins are regulated by UVB exposure. SPRR in the corneal epithelial cells may be markers of an early stage of differentiation and may have additional roles in modulation of stress. Modulation of envelope precursors by inhibiting the activity of transglutaminase may have therapeutic implications in ocular surface disorders.
Acknowledgments
Supported by National Eye Institute Grants EY11915 (SCP) and EY014553 (D-QL), a fellowship from the Agency for Science Technology and Research, Singapore, an unrestricted Grant from Research to Prevent Blindness, and by the Oshman Foundation, and The William Stamps Farish Fund.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2005.
Disclosure: L. Tong, None; R.M. Corrales, None; Z. Chen, None; A.L. Villarreal, None; C.S. De Paiva, None; R. Beuerman, None; D.-Q. Li, None; S.C. Pflugfelder, None
References
- 1.Segre J. Complex redundancy to build a simple epidermal permeability barrier. Curr Opin Cell Biol. 2003;15:776–782. doi: 10.1016/j.ceb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev Biol. 1997;183:123–138. doi: 10.1006/dbio.1996.8465. [DOI] [PubMed] [Google Scholar]
- 3.Hohl D, Mehrel T, Lichti U, Turner ML, Roop DR, Steinert PM. Characterization of human loricrin: structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–6636. [PubMed] [Google Scholar]
- 4.Tesfaigzi J, Carlson DM. Expression, regulation, and function of the SPR family of proteins: a review. Cell Biochem Biophys. 1999;30:243–265. doi: 10.1007/BF02738069. [DOI] [PubMed] [Google Scholar]
- 5.Marshall D, Hardman MJ, Nield KM, Byrne C. Differentially expressed late constituents of the epidermal cornified envelope. Proc Natl Acad Sci USA. 2001;98:13031–13036. doi: 10.1073/pnas.231489198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Munozledo F. Development of a spontaneous permanent cell line of rabbit corneal epithelial cells that undergoes sequential stages of differentiation in cell culture. J Cell Sci. 1994;107:2343–2351. doi: 10.1242/jcs.107.8.2343. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura T, Nishida K, Dota A, Matsuki M, Yamanishi K, Kinoshita S. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2001;42:549–556. [PubMed] [Google Scholar]
- 9.Toshino A, Shiraishi A, Zhang W, Suzuki A, Kodama T, Ohashi Y. Expression of keratinocyte transglutaminase in cornea of vitamin a-deficient rats. Curr Eye Res. 2005;30:731–739. doi: 10.1080/02713680591005940. [DOI] [PubMed] [Google Scholar]
- 10.Molhuizen HO, Alkemade HA, Zeeuwen PL, de Jongh GJ, Wieringa B, Schalkwijk J. SKALP/elafin: an elastase inhibitor from cultured human keratinocytes. Purification, cDNA sequence, and evidence for transglutaminase cross-linking. J Biol Chem. 1993;268:12028–12032. [PubMed] [Google Scholar]
- 11.Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest. 2003;111:121–128. doi: 10.1172/JCI15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Heller-Milev M, Huber M, Panizzon R, Hohl D. Expression of small proline rich proteins in neoplastic and inflammatory skin diseases. Br J Dermatol. 2000;143:733–740. doi: 10.1046/j.1365-2133.2000.03768.x. [DOI] [PubMed] [Google Scholar]
- 13.Cabral A, Voskamp P, Cleton-Jansen AM, South A, Nizetic D, Backendorf C. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J Biol Chem. 2001;276:19231–19237. doi: 10.1074/jbc.M100336200. [DOI] [PubMed] [Google Scholar]
- 14.Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- 15.Inada R, Matsuki M, Yamada K, et al. Facilitated wound healing by activation of the transglutaminase 1 gene. Am J Pathol. 2000;157:1875–1882. doi: 10.1016/S0002-9440(10)64826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller A, O'Rourke J, Grimm J, et al. Distinct gene expression profiles characterize the histopathological stages of disease in Helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Proc Natl Acad Sci USA. 2003;100:1292–1297. doi: 10.1073/pnas.242741699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 18.Pradervand S, Yasukawa H, Muller OG, et al. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004;23:4517–4525. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest Ophthalmol Vis Sci. 2003;44:5102–5109. doi: 10.1167/iovs.03-0591. [DOI] [PubMed] [Google Scholar]
- 20.Shimmura S, Tadano K, Tsubota K. UV dose-dependent caspase activation in a corneal epithelial cell line. Curr Eye Res. 2004;28:85–92. doi: 10.1076/ceyr.28.2.85.26237. [DOI] [PubMed] [Google Scholar]
- 21.Kwok LS, Coroneo MT. A model for pterygium formation. Cornea. 1994;13:219–224. doi: 10.1097/00003226-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Margaritis LH, Politof TK, Koliopoulos JX. Quantitative changes and ultrastructural alterations of the cornea in response to ultraviolet light. II. Effects of amphibia; elucidation of desmosomal structure and basement membrane synthesis. Tissue Cell. 1976;8:603–614. doi: 10.1016/0040-8166(76)90034-3. [DOI] [PubMed] [Google Scholar]
- 23.Pfundt R, van Vlijmen-Willems I, Bergers M, Wingens M, Cloin W, Schalkwijk J. In situ demonstration of phosphorylated c-jun and p38 MAP kinase in epidermal keratinocytes following ultraviolet B irradiation of human skin. J Pathol. 2001;193:248–255. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH780>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- 25.Cabral A, Sayin A, de Winter S, Fischer DF, Pavel S, Backendorf C. SPRR4, a novel cornified envelope precursor: UV-dependent epidermal expression and selective incorporation into fragile envelopes. J Cell Sci. 2001;114:3837–3843. doi: 10.1242/jcs.114.21.3837. [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Jun Song X, de Paiva CS, Chen Z, Pflugfelder SC, Li DQ. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espana EM, Kawakita T, Romano A, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- 28.Parameswaran KN, Velasco PT, Wilson J, Lorand L. Labeling of epsilon-lysine crosslinking sites in proteins with peptide substrates of factor XIIIa and transglutaminase. Proc Natl Acad Sci USA. 1990;87:8472–8475. doi: 10.1073/pnas.87.21.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 31.Corrales RM, Calonge M, Herreras JM, Saez V, Mayo A, Chaves FJ. Levels of mucin gene expression in normal human conjunctival epithelium in vivo. Curr Eye Res. 2003;27:323–328. doi: 10.1076/ceyr.27.5.323.17221. [DOI] [PubMed] [Google Scholar]
- 32.Steinert PM, Candi E, Kartasova T, Marekov L. Small proline-rich proteins are cross-bridging proteins in the cornified cell envelopes of stratified squamous epithelia. J Struct Biol. 1998;122:76–85. doi: 10.1006/jsbi.1998.3957. [DOI] [PubMed] [Google Scholar]
- 33.Del Bino S, Vioux C, Rossio-Pasquier P, et al. Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br J Dermatol. 2004;150:658–667. doi: 10.1111/j.0007-0963.2004.05886.x. [DOI] [PubMed] [Google Scholar]
- 34.Adhikary G, Crish JF, Bone F, Gopalakrishnan R, Lass J, Eckert RL. An involucrin promoter AP1 transcription factor binding site is required for expression of involucrin in the corneal epithelium in vivo. Invest Ophthalmol Vis Sci. 2005;46:1219–1227. doi: 10.1167/iovs.04-1285. [DOI] [PubMed] [Google Scholar]
- 35.Adhikary G, Crish J, Lass J, Eckert RL. Regulation of involucrin expression in normal human corneal epithelial cells: a role for activator protein one. Invest Ophthalmol Vis Sci. 2004;45:1080–1087. doi: 10.1167/iovs.03-1180. [DOI] [PubMed] [Google Scholar]
- 36.Neve BP, Hoogerbrugge N, Verhoeven AJ, Birkenhager JC, Jansen H. Growth hormone restores hepatic lipase mRNA levels but the translation is impaired in hepatocytes of hypothyroid rats. Biochim Biophys Acta. 1997;1345:172–179. doi: 10.1016/s0005-2760(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 37.Schedel J, Distler O, Woenckhaus M, et al. Discrepancy between mRNA and protein expression of tumour suppressor maspin in synovial tissue may contribute to synovial hyperplasia in rheumatoid arthritis. Ann Rheum Dis. 2004;63:1205–1211. doi: 10.1136/ard.2003.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sola B, Salaun V, Ballet JJ, Troussard X. Transcriptional and post-transcriptional mechanisms induce cyclin-D1 over-expression in B-chronic lymphoproliferative disorders. Int J Cancer. 1999;83:230–234. doi: 10.1002/(sici)1097-0215(19991008)83:2<230::aid-ijc14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Tuomisto TT, Riekkinen MS, Viita H, Levonen AL, Yla-Herttuala S. Analysis of gene and protein expression during monocyte-macrophage differentiation and cholesterol loading–cDNA and protein array study. Atherosclerosis. 2005;180:283–291. doi: 10.1016/j.atherosclerosis.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Kim YS, Choi DH, et al. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004;279:53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- 41.Tarcsa E, Candi E, Kartasova T, Idler WW, Marekov LN, Steinert PM. Structural and transglutaminase substrate properties of the small proline-rich 2 family of cornified cell envelope proteins. J Biol Chem. 1998;273:23297–23303. doi: 10.1074/jbc.273.36.23297. [DOI] [PubMed] [Google Scholar]
- 42.Steinert PM, Kartasova T, Marekov LN. Biochemical evidence that small proline-rich proteins and trichohyalin function in epithelia by modulation of the biomechanical properties of their cornified cell envelopes. J Biol Chem. 1998;273:11758–11769. doi: 10.1074/jbc.273.19.11758. [DOI] [PubMed] [Google Scholar]
- 43.Phillips MA, Qin Q, Mehrpouyan M, Rice RH. Keratinocyte transglutaminase membrane anchorage: analysis of site-directed mutants. Biochemistry. 1993;32:11057–11063. doi: 10.1021/bi00092a015. [DOI] [PubMed] [Google Scholar]
- 44.Robinson NA, Lapic S, Welter JF, Eckert RL. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J Biol Chem. 1997;272:12035–12046. doi: 10.1074/jbc.272.18.12035. [DOI] [PubMed] [Google Scholar]
- 45.Marenholz I, Zirra M, Fischer DF, Backendorf C, Ziegler A, Mischke D. Identification of human epidermal differentiation complex (EDC)-encoded genes by subtractive hybridization of entire YACs to a gridded keratinocyte cDNA library. Genome Res. 2001;11:341–355. doi: 10.1101/gr.114801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Djian P, Easley K, Green H. Targeted ablation of the murine involucrin gene. J Cell Biol. 2000;151:381–388. doi: 10.1083/jcb.151.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grether-Beck S, Buettner R, Krutmann J. Ultraviolet A radiation-induced expression of human genes: molecular and photobiological mechanisms. Biol Chem. 1997;378:1231–1236. [PubMed] [Google Scholar]
- 48.Michel S, Schmidt R, Robinson SM, Shroot B, Reichert U. Identification and subcellular distribution of cornified envelope precursor proteins in the transformed human keratinocyte line SV-K14. J Invest Dermatol. 1987;88:301–305. doi: 10.1111/1523-1747.ep12466177. [DOI] [PubMed] [Google Scholar]


