Abstract
Therapeutic strategies for transplantation of pancreatic islet cells are urgently needed to expand β-cell mass by stimulating islet cell proliferation and/or prolonging islet cell survival. Control of the islets by different growth factors provides a potential venue for augmenting β-cell mass. In the present study, we show the expression of the biologically active splice variant-1 (SV-1) of growth hormone-releasing hormone (GHRH) receptor in rat insulinoma (INS-1) cells as well as in rat and human pancreatic islets. In studies in vitro of INS-1 cells, the GHRH agonist JI-36 caused a significant increase in cell proliferation and a reduction of cell apoptosis. JI-36 increased islet size and glucose-stimulated insulin secretion in isolated rat islets after 48–72 h. At the ultrastructural level, INS-1 cells treated with agonist JI-36 revealed a metabolic active stimulation state with increased cytoplasm. Coincubation with the GHRH antagonist MIA-602 reversed the actions of the agonist JI-36, indicating the specificity of this agonist. In vivo, the function of pancreatic islets was assessed by transplantation of rat islets under the kidney capsule of streptozotocin-induced diabetic non-obese diabetic-severe combined immunodeficiency (NOD-SCID) mice. Islets treated with GHRH agonist JI-36 were able to achieve normoglycemia earlier and more consistently than untreated islets. Furthermore, in contrast to diabetic animals transplanted with untreated islets, insulin response to an i.p. glucose tolerance test (IPGTT) in animals receiving islets treated with agonist Jl-36 was comparable to that of normal healthy mice. In conclusion, our study provides evidence that agonists of GHRH represent a promising pharmacological therapy aimed at promoting islet graft growth and proliferation in diabetic patients.
Keywords: diabetes, islet proliferation, regenerative therapies
Transplantation of pancreatic islet cells is a valid treatment option for selected patients with brittle diabetes. Under current protocols, the main therapeutic goal that can be reliably achieved is improved glycemic control and prevention of severe hypoglycemic episodes. Insulin independence can only be achieved for a limited time after repeated transplantations (1) due to insufficient islet mass and progressive loss of islets over time. Therefore, efforts to improve islet transplantation focus on improving the exploitation of mechanisms governing β-cell proliferation and growth as well as islet quality (2–4).
Several growth factors that may have potential for enhancing β-cell mass have been identified (5). A natural growth factor-mediated adaptation of islet cell mass occurs due to increased demand during pregnancy as well as with obesity (6). In addition, promotion of islet cell growth has been linked to glucagon-like peptide 1 (GLP-1), obestatin, and ghrelin (4, 7, 8). Surprisingly, little attention has been given to the possible role of growth hormone-releasing hormone (GHRH) or its agonists. In his Nobel lecture more than 60 y ago, Bernardo Houssay described the critical role of the “hypophysis in carbohydrate metabolism and in diabetes” (9). He observed that extracts of the anterior pituitary gland can produce a stimulation and hyperplasia of islets under certain conditions. With the advent of stem cell biology and regenerative medicine, there has now been a renewed interest in elucidating the role of hypothalamic-pituitary growth factors in islet cell regulation.
GHRH stimulates the release of growth hormone (GH) from the pituitary and has been the focus of intense studies since its structure was described in 1982 (10, 11). The full biological activity of GHRH resides in the N-terminal 1–29 amino acid sequence of this peptide (12). GHRH and the pituitary type of GHRH receptor as well as its splice variants are expressed in many human tissues (i.e., ovary, testis, pancreas, colon, esophagus, breast, kidney, liver, prostate, lungs, and thymus) (13–15).
Recent study has shown that rat GHRH promoted survival of cardiomyocytes in vitro and protected rat hearts from ischemia-reperfusion injury (16). The detection of the GHRH receptor (GHRH-R) on the cardiomyocyte sarcolemma supports the view that GHRH may elicit direct signal transduction within the heart, independent of the GH/IGF1 axis per se (17). Synthetic GHRH agonists, such as JI-36 (GHRH-A), are more potent and longer-acting than native GHRH (18, 19). Recently, we showed that GHRH-agonist JI-36 has a favorable cardiac effect, attenuating infarct size as well as the progressive decrease of cardiac structure and function following myocardial infarction (MI) (16).
Finally, GHRH has been shown to promote angiogenesis by increasing vascular endothelial growth factors (VEGF) (20). VEGF and vascularization play a crucial role in β- cell function and islet regeneration (21, 22). In the present study, we show expression of GHRH receptor splice variant-1 (SV-1) (23, 24) in rat insulinoma INS-1 cells as well as in rat and human pancreatic islets. We also analyzed the effect of a synthetic GHRH agonist on β- cell survival and cell proliferation in vitro and in vivo. In addition, we tested the effect of this agonist, JI-36, on β-cells before transplantation in a diabetic animal model.
Results
Expression of Receptor for GHRH in Insulinoma Cells and in Rat Islet Cells.
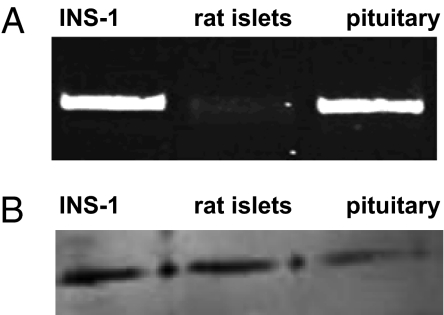
RT-PCR analysis showed expression of GHRH receptor (564 bp) in INS-1 cells and in rat islets. Rat pituitary was used as a positive control (Fig. 1A). In addition, the protein of the biologically more-active splice variant SV-1 of GHRH receptor was detected in INS-1 and rat islets by Western blotting (39.5 kDa). Rat pituitary was used as a positive control (Fig. 1B).
Fig. 1.
The expression of GHRH-R based on mRNA levels (A) and receptor detection by Western blots (B) was shown in INS-1 cells and primary rat islets. Rat pituitary was used as a positive control.
Immunohistochemical Confirmation of the Expression of GHRH Receptor Protein in Insulinoma Cells and Rat and Human Islets.
Immunohistochemical analysis showed pronounced GHRH-R immunostaining of INS-1 cells (Fig. 2 A and D), rat (Fig. 2 B and E), and human (Fig. 2 C and F) islets. To confirm the localization of GHRH-R on β-cells, costaining for insulin was performed (Fig. 2F).
Fig. 2.
Immunohistochemical staining of GHRH-R in INS-1 cells (A and D), rat islets (B and E), and human pancreatic islets (C and F). To show the presence of GHRH-R protein on islet β-cells, costaining with insulin was performed (F).
Ultrastructural Analysis of Insulinoma Cells Before and After Incubation with the GHRH Agonist.
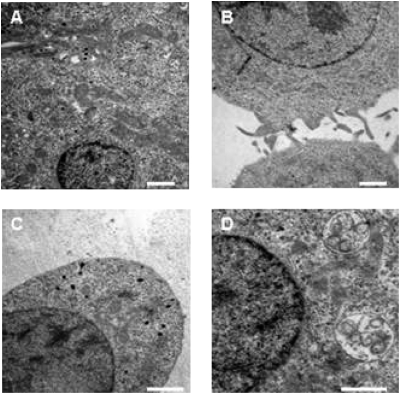
INS-1 cells under normal culture conditions were characterized by secretory granules close to the cell membrane (Fig. 3A). The cell surface itself extended long filopodia and other membrane protrusions (Fig. 3B). Treatment of islet cells with 10−6 M JI-36 produced an enlargement of the cell membrane and the volume of the cytoplasm. This was accompanied by the disappearance of membrane protrusions (Fig. 3C). Furthermore, mitochondria and lysosomes were also enlarged; the latter contained numerous vesicles, indicating intracytoplasmatic digestion of the contents (peptides, proteins) of secretory vacuoles, after the vacuoles fuse with lysosomes. Additional changes became obvious in the cell nucleus, demonstrating an increased amount of heterochromatin as well as nucleoli (Fig. 3D). These morphological changes suggest an increased active metabolic state of the islet cells.
Fig. 3.
Ultrastructural analysis of INS-1 cells. These cells under normal culture conditions show secretory granules frequently lining up at the cell membrane as well as a substantial number of cell membrane protrusions and filopodia (A and B). In contrast, islet cells treated with GHRH agonist JI-36 (10−6 M) reveal an enlargement of the cell membrane and disappearance of filopodia. Furthermore, hyperplasia and enlargement of mitochondria as well as a conspicuous inclusion of vesicles into lysosomes could be found. An increasing amount of heterochromatin and nucleoli in the cell nucleus was also documented. These signs suggest a more-active metabolic state of the islet cells (C and D). (Scale bar: 1 μm.)
Cell Proliferation Studies on Insulinoma Cells.
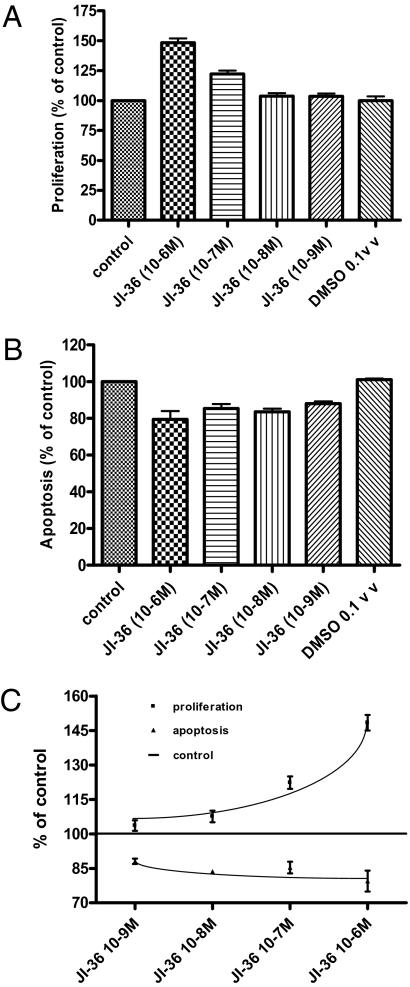
Incubation of INS-1 cells with JI-36 (10−6 to 10−9 M) for 24–96 h caused a significant and dose-dependent increase in cell proliferation rates. The most effective concentration of the agonist was 10−6 M, with a 50% increase after 72 h (Fig. 4 A and C). Coincubation of INS-1 cells with the GHRH agonist JI-36 (10−6 M) and the GHRH antagonist MIA-602 (10−6 M) for 72 h reversed the proliferation-stimulating effect of the agonist.
Fig. 4.
In vitro effects of GHRH agonist JI-36 on INS-1 cells. (A) JI-36 (10−6 M) stimulated cell proliferation significantly (50% increase compared with control) after 72 h in culture (n = 3). (B) Apoptosis as indicated by activity of caspases 3 and 7 was significantly reduced by 20% after treatment with JI-36 (10−6 M) for 72 h (n = 3). (C) JI-36 treatment dose-dependently increased cell proliferation and conversely decreased the rate of apoptosis in INS-1 cells with the maximum effect at 10−6 M. ***P < 0.001; **P < 0.01; *P < 0.05.
Cell Apoptosis Studies on Insulinoma Cells.
Incubation of INS-1 cells with JI-36 (10−6-10−9 M) for 24–96 h resulted in a significant decrease in degree of cell apoptosis as measured by the reduction of activity of caspases 3 and 7. The maximal antiapoptotic effect was seen after 72 h; the most effective concentration of the agonist causing this effect was 10−6 M (Fig. 4 B and C).
Determination of Islet Number and Islet Volume.
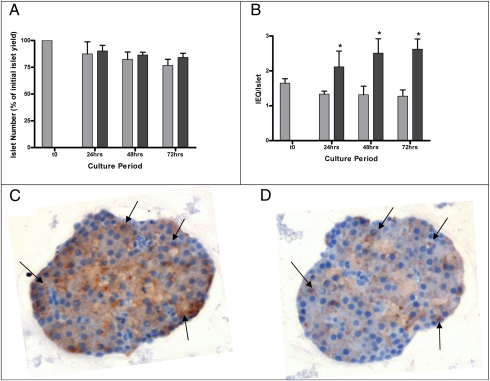
Cultures of isolated rat islets in the presence of JI-36 showed no relevant change in number of islets over time compared with control islets (Fig. 5A). Calculation of islet equivalents (IEQ) by relative conversion into islets of 150 μm diameter showed a significant increase in IEQ/islet ratio, indicating a relative “islet growth” after 48 h, and up to 72 h, following exposure to JI-36 (Fig. 5B).
Fig. 5.
Effect of JI-36 on islet number and islet size in vitro (n = 4). (A) The number of islets decreased slightly over time in culture, with no difference between treatment group and control. The bars represent the percentage of islet number compared with islet yield right after isolation (t0). (B) When converted to islet equivalents (IEQ), a significant difference between JI-36-treated islets and controls was seen after 24 h and continued to increase over time. Gray bars represent control group (n = 4), black bars represent JI-36-treated islets (n = 4). *P < 0.05. (C and D) Immunostaining of islet serial sections for insulin (C, brown staining) and Ki-67 (D, brown staining) showed colocalization (arrowheads) of the proliferation marker within β-cells.
Immunohistochemical staining of the islets, after 72 h in culture with JI-36, for insulin and the proliferation marker Ki-67, showed colocalization of the two markers, indicating an induced proliferation, specifically although not exclusively in β-cells (Fig. 5 C and D).
Measurement of Islet Membrane Integrity.
Rat islets were evaluated by fluorescent microscopy using FDA/PI staining. We observed no difference in islet viability between the groups after 24, 48, and 72 h in culture (72-h time point: 93 ± 2.2% for control islets, 96 ± 3.3% for islets exposed to JI-36; n = 4). Morphological appearance following dithizone staining also did not differ between treatment groups.
Effects of JI-36 on Glucose-Stimulated Insulin Secretion.
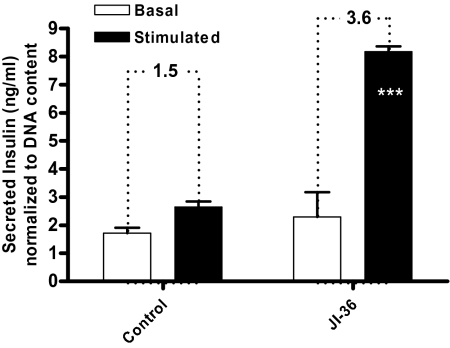
In a static model of glucose-stimulated insulin secretion, exposure to JI-36 for 48 h resulted in a slight increase of insulin release into the culture media after 1 h at basal (3.3 mM) glucose concentration as compared with control (2.3 ± 0.5 ng/mL vs. 1.7 ± 0.1 ng/mL; n = 5). Upon stimulation with high levels of glucose (16.7 mM), insulin release from treated islets was significantly increased (3.6-fold) relative to insulin release at basal glucose concentration, whereas untreated islets augmented insulin release only 1.5-fold (8.2 ± 0.2 ng/mL compared with control 2.6 ± 0.2 ng/mL; n = 5; P < 0.001; Fig. 6). Thus, treatment of rat islets in vitro more than doubled total insulin release upon stimulation.
Fig. 6.
Effect of GHRH agonist JI-36 on glucose-stimulated insulin secretion. After equilibration at 3.3 mM glucose, islets were stimulated with high glucose concentration of 16.7 mM for 1 h. Exposure to JI-36 did not cause a relevant difference in insulin secretion at basal conditions. Glucose challenge resulted in a significantly increased insulin release 3.6-fold relative to insulin release at basal glucose concentration when compared with untreated islets that increased insulin release 1.5-fold relative to insulin release at basal glucose concentration (n = 5). Overall, pretreatment with JI-36 resulted in a more than double insulin release upon glucose stimulation compared with control (***P < 0.001).
Performance of Islets Exposed to JI-36 in Vivo.
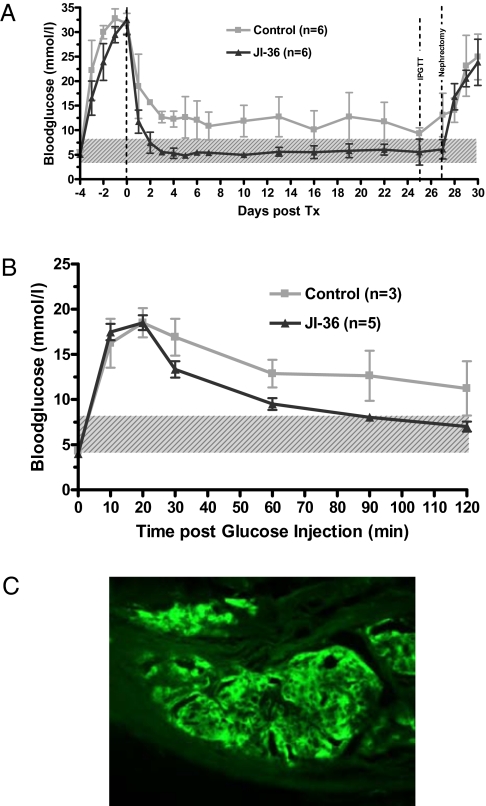
For all islet preparations tested, animals transplanted with islets previously exposed to agonist Jl-36 consistently performed better, with blood glucose levels reaching the range of normal healthy mice. When evaluated at day 25, five of six animals from the JI-36 group were “cured,” and one animal showed partial graft function. In comparison, in the control group, only three animals were normoglycemic, two had impaired graft function, and one showed graft failure (Fig. 7A). In the i.p. glucose tolerance test (IPGTT), the JI-36 group showed an insulin response comparable to that of normal healthy mice, whereas control islets in animals, classified as cured on the basis of attaining normoglycemia before challenge, exhibited delayed and inadequate responses to glucose challenge (Fig. 7B).
Fig. 7.
Islet transplantation (300 islet equivalents) beneath the kidney capsule of streptozotocin- induced diabetic NOD-SCID mice. (A) After transplantation, the control group showed a delayed decrease in blood glucose levels, and only three of six animals showed stable normoglycemia during the follow-up period. Animals receiving a graft of islets pretreated with JI-36 showed rapid and persistent recovery from diabetes (five of six animals). (B) On day 25 following islet transplantation, animals with normal glucose control were subjected to an IPGTT. Though control animals responded in a delayed and insufficient manner to the glucose challenge, the group treated with JI-36 was able to revert initial hyperglycemia to normal ranges within 2 h. Shaded area highlights normal range of blood glucose. (C) Pancreatic islets were treated with JI-36 before transplantation beneath the kidney capsule. Representative immunostained section for insulin (green) shows stable graft integration after 27 d.
Islet grafts retrieved on day 27 after transplantation were immunostained for insulin and showed stable graft integration (Fig. 7C).
Discussion
The main finding of the present study is that GHRH agonist JI-36 improves β-cell survival and growth as well as metabolic function. We have shown the expression of mRNA and protein for GHRH in both rodent and human islets. The GHRH agonist reduced programmed cell death of β-cells. This was reversed by an antagonist of GHRH. Finally, pretreatment with GHRH agonist improved β-cell engraftment and metabolic function of islets following transplantation under the kidney capsule in the streptozotocin-induced diabetic mice. Furthermore, islets treated with the GHRH agonist before transplantation into diabetic NOD-SCID mice were able to produce normoglycemia in these mice earlier and more consistently than islets sham-treated without JI-36. In addition, JI-36 exposed islets showed a stronger response upon glucose challenge compared with untreated islets in vitro and in vivo
GH itself and IGF1, as well as GH-releasing peptides such as ghrelin and other GH secretagogues, have been shown to increase β-cell proliferation in transplanted human and fetal rat islets (25, 26). This study, however, shows the potential role of a GHRH agonist in islet cell proliferation and survival. The detection of the GHRH receptor on β-cells in rat and human islets supports the view that GHRH may exert a direct signal transduction within the pancreas independent and/or in addition to the effects mediated by the GH/IGF1 pathways.
Though ghrelin and other GH secretagogues may have pleiotropic actions with potentially unexpected side effects, the administration of GHRH may offer a more physiological approach due to its direct actions. Our ultrastructural analysis shows an increase of β-cell cytoplasm with a reduction of cell extensions and filopodia. Interestingly, a recent study has shown a beneficial effect of another hypothalamic-releasing hormone, corticotrophin-releasing hormone, on β-cell proliferation (27), further emphasizing an important connection between the hypothalamic-pituitary axis and the integrity of insulin-producing cells in the pancreas. Synthetic agonists of GHRH such as JI-36 are more potent and longer acting than native GHRH or other growth factors. This may open new therapeutic options. Because there are millions of patients with type 1 diabetes, and the availability of pancreatic islet donors is extremely limited, reaching less than a few hundred per year, there is a desperate need for the development of methods for increasing the efficiency of β-cell function and islet cell mass. In vitro expansion of islet cell function and mass by the use of growth factors is therefore of great interest. If future studies can show that this strategy can be safely applied in vivo, treatment with GHRH analog may have a tremendous impact also on the prevention and treatment of type 2 diabetes patients. A major feature of diabetes mellitus type 2 is the progressive loss of β-cell mass over time, very similar to the situation with transplanted human islets.
We and others have previously shown that by improving the quality of islets and by a careful quality control of the islets before transplantation, the results can be substantially improved (2). Furthermore, multiple studies performed recently have clearly shown that β- cells are able to replicate under basal conditions and that β-cell mass can be augmented in response to a variety of physiological and/or pathophysiological stimuli (28). Indeed, it has become obvious that the major source of new β-cells during adult life is more likely due to the proliferation of preexisting β-cells than the differentiation of progenitor or stem cells in the pancreas (29). Therefore, improving β-cell function and replication in vivo may be an important therapeutic strategy for both the prevention and the cure of diabetes mellitus. Although our study was mainly performed in rodents, we have also shown expression of the receptor in human islet cells. On the basis of previous studies with other growth factors, it is appropriate to extrapolate that human islets will have the same potential to expand and improve islet cell mass in a fashion similar to the results observed in our animal models. In addition to refining quality of islet cells and islet cell function before transplantation, it may be possible to improve islet engraftment and reduce the number of islets needed for a successful outcome by using a short-term in vivo exposure to the agonist. Previous work has shown that temporary systemic administration of growth factors such as hepatocyte growth factor (HGF) may improve graft survival and blood glucose control in vivo (30). This, however, requires further study in vivo to adequately address safety issues and the risk of uncontrolled proliferation and tumorigenesis.
In summary, the current long-term efficacy of clinical islet transplantation is rather low. One of the major underlying factors for this outcome is the loss of islet mass over time. Therefore, the exploration of mechanisms promoting islet proliferation and growth is critically important for further progress in the field. The application of synthetic GHRH agonist for islet proliferation in vitro as well as graft function and survival in vivo in therapies of diabetes, and our study showing the importance of local autocrine and paracrine GHRH in β-cell regulation and growth, suggest a promising regenerative therapeutic potential for patients with diabetes.
Materials and Methods
Peptide Analogs Preparation.
GHRH agonist Jl-36 and GHRH antagonist MIA-602 were synthesized in the laboratory of author A.V.S. (17, 19, 20).
Rat Insulinoma Cell Line.
Rat insulinoma cells (INS-1) were cultured in RPMI medium 1640 (PAA) supplemented with 2 mM L-glutamine, 10% FBS, 1 mM Na-pyruvate, 50 μM 2-mercaptoethanol, and 100 U/mL penicillin-streptomycin (Gibco) in a humidified 5% CO2/95% O2 atmosphere at 37 °C. The culture medium was changed every other day. Cells were grown for 72 h before experimentation. GHRH agonist JI-36 (10−6 to 10−9 M) and GHRH antagonist MIA-602 (10−6 to 10−7 M) were used for 24–96 h, respectively.
Isolation of Rat Pancreatic Islets.
Pancreatic islets were isolated from male Wistar rats according to guidelines established by the University of Dresden Institutional Animal Care and Use Committee. Animals were anesthetized by 3% isoflurane; digestion solution (Collagenase V; Sigma-Aldrich) was injected in situ via the pancreatic common bile duct. Islets were purified by centrifugation on a discontinuous Ficoll gradient (Mediatech). Purified islets were maintained in culture media (CMRL 1066; Mediatech) supplemented with 10% FBS at 37 °C in a 5% CO2 incubator. Volume and purity were determined by microscopic sizing after staining with dithizone (Sigma-Aldrich).
Isolation of Human Pancreatic Islets.
Human pancreata from cadaver donors were obtained through Eurotransplant following consent for research use obtained from the next of kin and authorization by the German Foundation for Organ Transplantation. Islets were isolated using a modification of the automated Ricordi method (31). Briefly, collagenase NB1, neutral protease (Serva Electrophoresis), and DNase (Roche) were infused into the main pancreatic duct. Islets were separated from exocrine tissue by centrifugation on a continuous-density Biocoll gradient (Biochrom) in a COBE 2991 cell processor. For determination of purity and islet yield, islet samples were stained with dithizone (Sigma-Aldrich) and sized using an eyepiece reticle and inverted microscope. Islets were cultured in CMRL 1066 (Mediatech) containing 2.5% human serum albumin at 37 °C in a 5% CO2 incubator before experimentation.
Islet Equivalent Determination.
Triplicate samples of 100–300 islets were stained with dithizone (Sigma-Aldrich), which binds zinc ions present specifically in islet β-cells, and sized using an eyepiece reticle and inverted microscope (32). All islets with a diameter >50 μm were divided into classes of 50-μm increments (i.e., 50–100, 100–150, 150–200, etc.) for calculation of islet equivalents (IEQ). Each diameter class was converted into the mean volume of 150-μm diameter islets by a relative conversion factor. These factors allow converting the total islet number from any preparation into IEQ.
Exposure of INS-1 Cells and Rat and Human Islets to GHRH Analogs.
INS-1 cells were grown for 72 h before experimentation; islets were collected immediately after the isolation procedure and divided into three treatment groups: (i) culture media with vehicle (DMSO) as a control group, (ii) culture media containing GHRH agonist JI-36 (10−6 M), and (iii) culture media with JI-36 plus GHRH antagonist MIA-602 (10−6 M). Media change and addition of the analogs was performed after 24 h and 48 h in islet cultures and every other day in INS-1 cell cultures.
Fluorescein Diacetate-Propidium Iodide Viability Staining.
Small aliquots of islets were transferred in PBS-containing Petri dishes. Fluorescein diacetate (FDA) and propidium iodide (PI) were added to the samples at a final concentration of 0.5 and 75 μM, respectively. Using a fluorescence microscope, 100 islets were assessed for cell viability by estimating the percentage of viable cells (green) vs. nonviable cells (red) within each islet. The percentage of viable cells was then calculated (33).
Measurement of Insulin Secretion by Static Challenge with Glucose.
For static insulin secretion in response to glucose challenge, islets were transferred into Petri dishes containing oxygenated Krebs–Ringer bicarbonate buffer (137 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4-7 H2O, 2.5 mM CaCl2-2 H2O, 25 mM NaHCO3, 0.25% BSA) and preincubated in 3.3 mM glucose at 37 °C (5% CO2) for 30 min. Groups of 8–10 islets from the equilibration cultures were transferred to fresh oxygen-saturated media containing either 3.3 or 16.7 mM glucose and then incubated an additional 60 min in a 37 °C water bath with gentle shaking. Secreted insulin in the media was measured by ELISA (Millipore) and values normalized to extracted islet DNA (Quant-iT PicoGreen; Invitrogen).
In Vivo Islet Functional Assessment.
NOD-SCID mice (MTZ breed) with induced diabetes were used as islet recipients following guidelines established by the University of Dresden Institutional Animal Care and Use Committee. Diabetes was induced by a single i.p. injection of 180 mg/kg streptozotocin (Sigma-Aldrich). Serum glucose was then measured daily using an Ascensia Elite glucometer (Bayer). Mice were considered diabetic if nonfasting blood glucose was >350 mg/dL for 2 or more consecutive d. Rat islet preparations were used for transplantation. Islets from each preparation were divided into two groups, and JI-36 (10−6 M) or vehicle (DMSO) was added to the culture media. Islets were cultured for 48 h before transplantation. After culture, samples of 300 IEQ were washed in transplant media (Ringer acetate with 5% glucose and 10% FBS) and transplanted to beneath the left kidney capsule. The animal experiments and housing were in accordance with institutional guidelines and German animal regulations.
Posttransplant Follow-Up.
The mice were observed for 30 d after transplantation. The nonfasting blood glucose levels were measured daily during the first week and twice a week thereafter. On day 25, mice were subjected to an IPGTT. Two days later, grafts were removed. This led to a recurrence of the diabetic state. This suggests that restoration and maintenance of normoglycemia was to the result of islet graft function.
Definition of Metabolic Control.
On follow-up, sustained nonfasting blood glucose levels of ≤10 mM (≤180 mg/dL) were defined as “cure,” 10–18 mM (180–320 mg/dL) as “partial function” of transplanted islets, and levels above 18 mM (>320 mg/dL) as “graft failure.”
Glucose Tolerance Test.
Mice were fasted overnight (at least 6 h) before examination. A glucose solution was given at 3 g/kg body weight i.p., and blood glucose was recorded before injection and 15, 30, 45, 60, 90, and 120 min following glucose injection. Nontransplanted mice were used as controls and tested concurrently.
For details of RT-PCR, Western blot analysis, immunohistochemical analysis, fluorescent immunohistochemistry of transplanted pancreatic islets, electron microscopy, measurement of cell proliferation, caspase activity, and statistical analysi, see SI Text.
Supplementary Material
Acknowledgments
We thank Linda Gebauer for technical help, Silke Langer for her assistance with immunohistochemistry, and Doreen Streichert for help with electron microscopy. We thank Kathleen Eisenhofer and Martina Haberland for help in the preparation of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft Grant SFB 655 “From Cells to Tissues” (to M.E.B. and S.R.B.), the Dresden Tumor Centre of Excellence, the Centre for Regenerative Therapies Dresden, and the Paul Langerhans Institute Dresden. Studies in Miami were supported by the Medical Research Service of the Veterans Affairs Department and University of Miami Miller School of Medicine Departments of Pathology and Medicine, Division of Hematology/Oncology (A.V.S.) and the Austin Weeks Family Endowment for Urologic Research (N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005098107/-/DCSupplemental.
References
- 1.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7:38–47. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann R, Spinas GA, Moritz W, Weber M. Has time come for new goals in human islet transplantation? Am J Transplant. 2008;8:1096–1100. doi: 10.1111/j.1600-6143.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 4.Reimann M, et al. An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol Ther. 2009;121:317–331. doi: 10.1016/j.pharmthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen JH, Svensson C, Galsgaard ED, Møldrup A, Billestrup N. Beta cell proliferation and growth factors. J Mol Med. 1999;77:62–66. doi: 10.1007/s001090050302. [DOI] [PubMed] [Google Scholar]
- 6.Lingohr MK, Buettner R, Rhodes CJ. Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes? Trends Mol Med. 2002;8:375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- 7.Granata R, et al. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes. 2008;57:967–979. doi: 10.2337/db07-1104. [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ. Glucagon and glucagon-like peptides 1 and 2. Results Probl Cell Differ. 2010;50:121–135. doi: 10.1007/400_2009_35. [DOI] [PubMed] [Google Scholar]
- 9.Houssay BA. [Role of the hypophysis in carbohydrate metabolism and diabetes.] Folia Endocrinol Mens Incretologia Incretoterapia. 1950;3:127–136. [PubMed] [Google Scholar]
- 10.Ling N, et al. Isolation, primary structure, and synthesis of human hypothalamic somatocrinin: Growth hormone-releasing factor. Proc Natl Acad Sci USA. 1984;81:4302–4306. doi: 10.1073/pnas.81.14.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300:276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- 12.Vance ML. Growth-hormone-releasing hormone. Clin Chem. 1990;36:415–420. [PubMed] [Google Scholar]
- 13.Guarcello V, Weigent DA, Blalock JE. Growth hormone releasing hormone receptors on thymocytes and splenocytes from rats. Cell Immunol. 1991;136:291–302. doi: 10.1016/0008-8749(91)90353-d. [DOI] [PubMed] [Google Scholar]
- 14.Havt A, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khorram O, Yeung M, Vu L, Yen SS. Effects of [norleucine27]growth hormone-releasing hormone (GHRH) (1-29)-NH2 administration on the immune system of aging men and women. J Clin Endocrinol Metab. 1997;82:3590–3596. doi: 10.1210/jcem.82.11.4363. [DOI] [PubMed] [Google Scholar]
- 16.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83:303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 18.Izdebski J, et al. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1995;92:4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schally AV, Comaru-Schally AM. In: Growth Hormone Secretagogues in Clinical Practice. Bercu BB, Walker RF, editors. New York: Dekker; 1998. pp. 131–142. [Google Scholar]
- 20.Letsch M, Schally AV, Busto R, Bajo AM, Varga JL. Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and -independent prostate cancers. Proc Natl Acad Sci USA. 2003;100:1250–1255. doi: 10.1073/pnas.0337496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabs N, et al. Reduced insulin secretion and content in VEGF-a deficient mouse pancreatic islets. Exp Clin Endocrinol Diabetes. 2008;116(Suppl 1):S46–S49. doi: 10.1055/s-2008-1081486. [DOI] [PubMed] [Google Scholar]
- 22.Nikolova G, et al. The vascular basement membrane: A niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler CG, et al. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogues. Proc Natl Acad Sci USA. 2009;106:15879–15884. doi: 10.1073/pnas.0907843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höglund E, Mattsson G, Tyrberg B, Andersson A, Carlsson C. Growth hormone increases beta-cell proliferation in transplanted human and fetal rat islets. JOP. 2009;10:242–248. [PubMed] [Google Scholar]
- 26.Vasavada RC, et al. Growth factors and beta cell replication. Int J Biochem Cell Biol. 2006;38:931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Huising MO, et al. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci USA. 2010;107:912–917. doi: 10.1073/pnas.0913610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner–Weir S, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 30.Fiaschi–Taesch NM, et al. Hepatocyte growth factor enhances engraftment and function of nonhuman primate islets. Diabetes. 2008;57:2745–2754. doi: 10.2337/db08-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 32.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827–830. [PubMed] [Google Scholar]
- 33.London NJ, et al. A microfluorometric viability assay for isolated human and rat islets of Langerhans. Diabetes Res. 1989;12:141–149. [PubMed] [Google Scholar]
- 34.Ziegler CG, et al. Dehydroepiandrosterone induces a neuroendocrine phenotype in nerve growth factor-stimulated chromaffin pheochromocytoma PC12 cells. Endocrinology. 2008;149:320–328. doi: 10.1210/en.2007-0645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.