Abstract
Vitamins are essential constituents of our diet that have long been known to influence the immune system. Vitamins A and D have received particular attention in recent years as these vitamins have been shown to have an unexpected and crucial effect on the immune response. We present and discuss our current understanding of the essential roles of vitamins in modulating a broad range of immune processes, such as lymphocyte activation and proliferation, T-helper-cell differentiation, tissue-specific lymphocyte homing, the production of specific antibody isotypes and regulation of the immune response. Finally, we discuss the clinical potential of vitamin A and D metabolites for modulating tissue-specific immune responses and for preventing and/or treating inflammation and autoimmunity.
“A vitamin is a substance that makes you ill if you don’t eat it.” (Albert Szent-Gyorgyi, Nobel Prize in Physiology or Medicine, 1937).
The statement by Albert Szent-Gyorgyi epitomizes the impact of vitamins on the body’s vital organs, including the immune system. Vitamins (vital amines) are organic compounds that are required in trace amounts in the diet because they cannot be synthesized in sufficient quantities by an organism1. Vitamins and their metabolites are essential for a large number of physiological processes, fulfilling diverse functions as hormones and antioxidants, as regulators of tissue growth and differentiation, in embryonic development and in calcium metabolism, among others1.
In addition, vitamins have a role in the immune system, which extends to both innate and adaptive immune responses. Although some vitamins, such as vitamins C and E and members of the B complex, can act in a relatively nonspecific manner in the immune system (for example, as antioxidants)2–4, other vitamins, such as vitamins A and D, can influence the immune response in highly specific ways (see Supplementary information S1 (table)). Here we review the most important effects of vitamins on the immune system, with special emphasis on vitamins A and D, which have received particular attention owing to recent discoveries of their multi-faceted interactions with the immune system. Vitamins A and D are notably distinct from other vitamins in that their respective bioactive metabolites, retinoic acid and 1,25-dihydroxyvitamin D3 (1,25(OH)2VD3), have hormone-like properties. Both of these metabolites are synthesized from their vitamin precursors by different tissues and cells in the body and exert their effects on target cells remotely by binding to nuclear-hormone receptors.
Vitamin metabolism in the immune system
Vitamin D
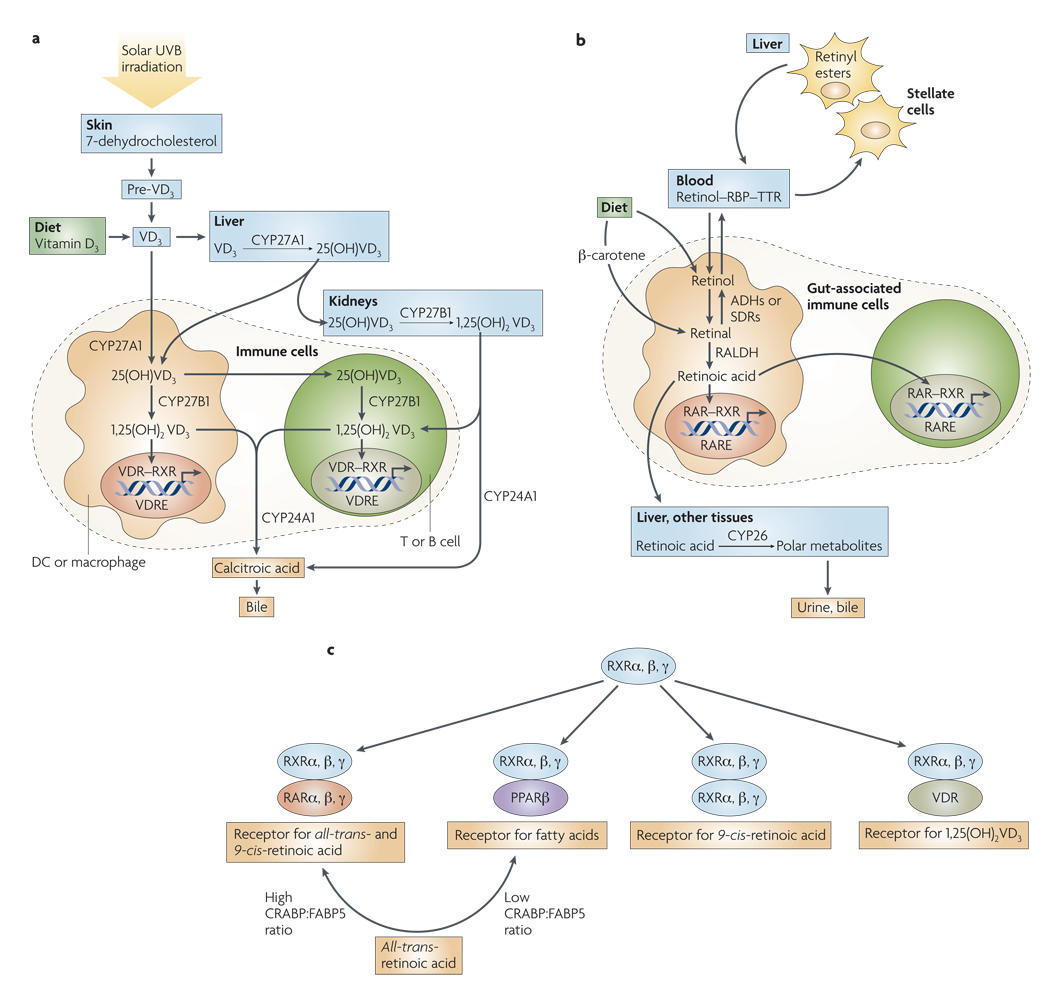
Vitamin D3 (VD3), the most physiologically relevant form of vitamin D, is synthesized in the skin from 7-dehydrocholesterol5, a process which depends on sunlight, specifically ultraviolet B radiation (wavelengths of 270–300 nm). Alternatively, it can be acquired in the diet or in vitamin supplements5 (FIG. 1a). VD3 is then converted in the liver to 25-dihydroxyvitamin D3 (25(OH)VD3), which is the main circulating form of VD3. Finally, 25(OH)VD3 is metabolized in the kidneys to 1,25(OH)2VD3, the most physiologically active VD3 metabolite5. In addition to being processed in the liver and the kidneys, VD3 can also be metabolized by cells of the immune system5,6 (FIG. 1a). In this way, 1,25(OH)2VD3 is concentrated locally in those lymphoid microenvironments that contain physiologically high concentrations of VD3, thereby increasing its specific action and also limiting potentially undesirable systemic effects, such as hypercalcaemia and increased bone resorption7.
Figure 1. Overview of vitamin A and D metabolism.
a | Vitamin D3 (VD3) is acquired in the diet or synthesized in the skin and hydroxylated in the liver to 25(OH)VD3, the main circulating form. 25(OH)VD3 is then hydroxylated in the kidneys by the cytochrome P450 protein CYP27B1 to become 1,25(OH)2VD3, the physiologically most active metabolite, which then reaches the blood where it has multiple systemic effects. Cells of the immune system, including macrophages, dendritic cells (DCs), T and B cells express the enzymes CYP27A1 and/or CYP27B1, and therefore can also hydroxylate 25(OH)VD3 to 1,25(OH)2VD3. 1,25(OH)2VD3 acts on immune cells in an autocrine or paracrine manner by binding to the vitamin D receptor (VDR). 24-hydroxylase (CYP24A1) catabolizes 1,25(OH)2VD3 to its inactive metabolite, calcitroic acid, which is excreted in the bile. b | Vitamin A (also known as retinol) is obtained from the diet and transported in the blood as a complex with retinol-binding protein (RBP) and transthyretin (TTR). In the liver, retinol is esterified to retinyl esters and stored in stellate cells. In other tissues, including gut-associated immune cells, retinol is oxidized to retinal by alcohol dehydrogenases (ADHs) or short chain dehydrogenase/reductases (SDRs). Retinal is then oxidized to all-trans-retinoic acid in an irreversible reaction that is catalysed by retinal dehydrogenases (RALDHs). Retinoic acid acts on immune cells by binding to the retinoic acid receptor (RAR). Retinoic acid is catabolized in the liver and in other tissues by the enzyme CYP26 and its metabolites are eliminated in the bile and urine. c | Retinoid X receptors (RXRs) can form RXR–RAR ( receptor for all-trans- and 9-cis-retinoic acid), RXR–PPARβ (peroxisome-proliferator-activated receptor β)(receptor for fatty acids), RXR–RXR (receptor for 9 cis-retinoic acid), or RXR–VDR (receptor for 1,25(OH)2VD3) complexes. The ratio between cellular retinoic acid-binding proteins (CRABPs) and fatty acid-binding protein 5 (FABP5) might determine whether retinoic acid signals through RAR–RXR or PPARβ–RXR, leading to different functional outcomes. RARE, retinoic acid response element; VDRE, VD response element.
Activated T cells (and probably also B cells) can only perform the final step of converting 25(OH)VD3 to 1,25(OH)2VD3 (REFS 6,8). However, macrophages and some dendritic cells (DCs), such as monocyte-derived DCs and dermal DCs, express the two sets of enzymes needed to convert VD3 into 1,25(OH)2VD3 (REFS 6,7,9).
Finally, the enzyme 24-hydroxylase, which is most abundant in the kidney and intestine10, catabolizes 1,25(OH)2VD3 to its inactive metabolite, calcitroic acid, which is then excreted in the bile.
Vitamin A
Vitamin A is obtained from the diet either as all-trans-retinol, retinyl esters or β-carotene11,12 (FIG. 1b). All-trans-retinol is esterified to retinyl esters and stored in the liver, mostly in the stellate cells11,12. In the tissues, all-trans-retinol and β-carotene are oxidized to all-trans-retinal by alcohol dehydrogenases or short chain dehydrogenase reductases, which are ubiquitously expressed enzymes11,12. All-trans-retinal is then oxidized to all-trans-retinoic acid through an irreversible reaction catalysed by retinal dehydrogenases (RALDHs), the expression of which is tightly controlled. A related metabolite of all-trans-retinal, 9-cis-retinoic acid, can be formed either by spontaneous isomerization of all-trans-retinoic acid or through oxidation of 9-cis-retinal by RALDH13. However, 9-cis-retinoic acid has not been shown to be synthesized in vivo13.
In adult mammals, RALDH can be found in some gut-associated cells, including intestinal epithelial cells (IECs)14,15 and gut-associated DCs, such as DCs from Peyer’s patches and mesenteric lymph nodes15,16. Interestingly, DCs from Peyer’s patches express RALDH-1 mRNA and protein, whereas DCs from mesenteric lymph nodes express mRNA encoding RALDH-2. Although the functional relevance of this differential RALDH isoform expression is currently unclear, it indicates that there might be more than one pathway or environmental stimulus that renders DCs capable of synthesizing retinoic acid from vitamin A. In addition, IECs also express RALDH-1 and can metabolize vitamin A to retinoic acid in vitro14, which indicates that they are another potential source of retinoic acid in the gut mucosa. The relative contributions and in vivo relevance of these different sources of retinoic acid in the gut are yet to be determined.
Nuclear receptors for vitamin metabolites
Locally produced 1,25(OH)2VD3 can act on immune cells in an autocrine or paracrine manner. On complexing with 1,25(OH)2VD3, the nuclear vitamin D receptor (VDR) heterodimerizes with nuclear receptors of the retinoic X receptor (RXR) family — which has three main isoforms: α, β and γ — and binds to VD3 response elements (VDREs) in the promoters of VD3-responsive genes (FIG. 1c).
Similarly, retinoic acid exerts its multiple effects by binding to nuclear receptors of the retinoic acid receptor (RAR) family, which also has three main isoforms: α, β and γ. These form RAR–RXR heterodimers, which interact with retinoic acid response elements (RAREs) within the promoters of retinoic acid-responsive genes11,12. RAR proteins are ubiquitously expressed and are also upregulated by retinoic acid11,12. As mentioned above, RXR proteins can also pair with VDR proteins or form RXR–RXR homodimers, which are specific receptors for 9-cis-retinoic acid but not for all-trans retinoic acid (hereafter referred to as retinoic acid) (although 9-cis-retinoic acid can also signal through RAR–RXR heterodimers). In addition, RXR proteins are partners for other nuclear receptors, such as thyroid hormone receptor, peroxisome proliferator-activated receptor (PPAR) and liver X receptor, among others17. Therefore, it is possible that, given their common RXR nuclear binding partners, some ligands, such as 1,25(OH)2VD3 and retinoic acid, might antagonize each other’s effects18.
Notably, retinoic acid can also bind and signal through the PPARβ (also known as PPARδ) nuclear receptor19. Whether signalling occurs through RAR or PPARβ depends on the ratio of cellular retinoic acid-binding proteins (CRABPs) to fatty acid-binding protein 5 (FABP5), which ultimately determines the partitioning of retinoic acid between the two types of receptors19. In this model, a high CRABP:FABP5 ratio promotes RAR signalling by CRABP-mediated ‘channelling’ of retinoic acid to RAR, which results in growth inhibition and apoptosis in some cell lines, whereas a low ratio favours FABP5-mediated delivery of retinoic acid to PPARβ and survival in the same cells19. However, although this is a potentially attractive mechanism for regulating retinoic acid responses, its significance in the immune system is yet to be determined.
Immunomodulatory role of vitamin D
The influence of VD3 metabolites in the immune system, particularly of 1,25(OH)2 VD3, has been known for more than 20 years20,21. In vitro, 1,25(OH)2VD3 exerts a marked inhibitory effect on adaptive immune cells (FIG. 2). It inhibits T-cell proliferation20,21, the expression of interleukin-2 (IL-2)21–23 and interferon-γ (IFNγ) mrNA and protein in T cells24,25, and CD8 T-cell-mediated cytotoxicity26. The decrease in the production of IL-2 and IFNγ by 1,25(OH)2VD3 is partially mediated by binding of the VDR–RXR complex to the VDRE in the promoters of genes encoding IL-2 (REF. 27) and IFNγ (REF. 28). The anti-proliferative effect could be explained, at least in part, by the decrease in IL-2 production, as proliferation is partially rescued by adding exogenous IL-2 (REFS 21,22,29). These inhibitory effects of 1,25(OH)2VD3 are most pronounced in the memory T-cell compartment30, which is concomitant with the higher expression of VDR in effector and memory T cells compared with naive T cells31. Moreover, 1,25(OH)2VD3 enhances nonspecific T-cell suppressor activity, as measured by the ability of 1,25(OH)2VD3-treated T cells to suppress primary mixed-lymphocyte reactions and cytotoxic T-cell responses26.
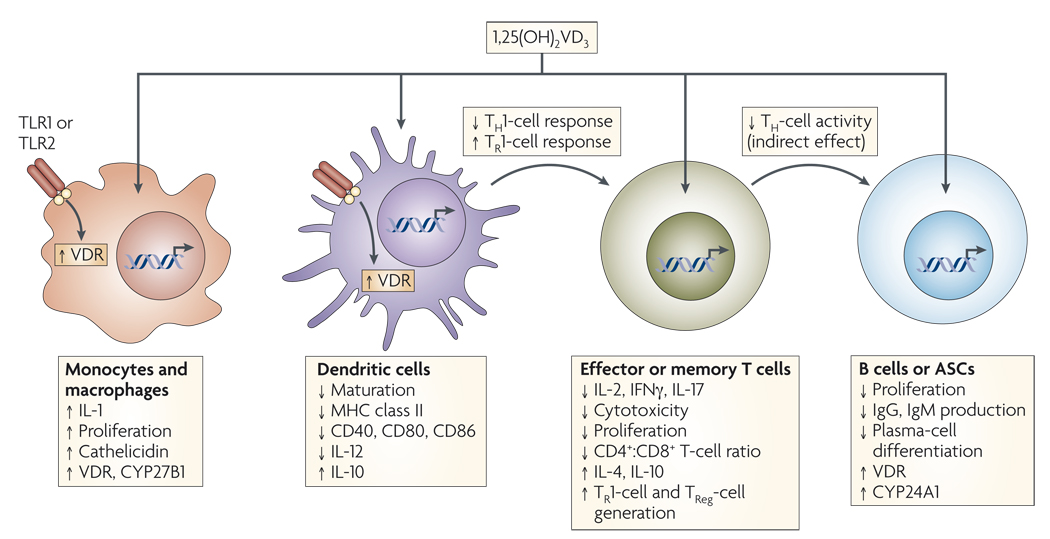
Figure 2. Mechanisms of vitamin D immunomodulation.
Systemic or locally produced 1,25(OH)2VD3 exerts its effects on several immune-cell types, including macrophages, dendritic cells (DCs), T and B cells. Macrophages and DCs constitutively express vitamin D receptor (VDR), whereas VDR expression in T cells is only upregulated following activation. In macrophages and monocytes, 1,25(OH)2VD3 positively influences its own effects by increasing the expression of VDR and the cytochrome P450 protein CYP27B1. Certain Toll-like-receptor (TLR)-mediated signals can also increase the expression of VDR. 1,25(OH)2VD3 also induces monocyte proliferation and the expression of interleukin-1 (IL-1) and cathelicidin (an antimicrobial peptide) by macrophages, thereby contributing to innate immune responses to some bacteria. 1,25(OH)2VD3 decreases DC maturation, inhibiting upregulation of the expression of MHC class II, CD40, CD80 and CD86. In addition, it decreases IL-12 production by DCs while inducing the production of IL-10. In T cells, 1,25(OH)2VD3 decreases the production of IL-2, IL-17 and interferon-γ (IFNγ) and attenuates the cytotoxic activity and proliferation of CD4+ and CD8+ T cells. 1,25(OH)2VD3 might also promote the development of forkhead box protein 3 (FOXP3)+ regulatory T (TReg) cells and IL-10-producing T regulatory type 1 (TR1) cells. Finally, 1,25(OH)2VD3 blocks B-cell proliferation, plasma-cell differentiation and immunoglobulin production. ASCs, antibody-secreting cells.
Overall, the net result of 1,25(OH)2VD3 action on T cells is to block the induction of T-helper-1 (TH1)-cell cytokines, particularly IFNγ, while promoting TH2-cell responses, an effect mediated both indirectly by decreasing IFNγ production and directly by enhancing IL-4 production7. The activity of 1,25(OH)2 VD3 on effector T-cell differentiation is further enhanced by its effect on antigen-presenting DCs, in which it suppresses the synthesis of IL-12, a cytokine that promotes TH1-cell responses32,33. Furthermore, 1,25(OH)2VD3 also inhibits TH17-cell responses, probably owing in part to its capacity to inhibit IL-6 and IL-23 production34,35, and induces the reciprocal differentiation and/or expansion of forkhead box protein 3 (FOXP3)+ regulatory T (TReg) cells35–37.
In addition to its inhibitory effects on T cells, 1,25(OH)2VD3 decreases B-cell proliferation, plasma-cell differentiation and IgG secretion8,20 (FIG. 2). It has been suggested that the effect of 1,25(OH)2VD3 on B cells might be indirectly mediated through the effect it has on antigen-presenting-cell (APC) function and/or T-cell help38. Indeed, there are conflicting reports concerning the expression of VDR by B cells8,31,39, leaving it unclear whether 1,25(OH)2VD3 can act directly on B cells.
Interestingly, 1,25(OH)2VD3 inhibits mitogen-stimulated IgG production by B cells from patients with inactive systemic lupus erythematosus (SLE), but not the spontaneous IgG production by cells from patients with active SLE40. Thus, it is possible that fully differentiated memory B cells and/or antibody-secreting cells (ASCs) are refractory to 1,25(OH)2VD3-mediated inhibition. In addition, serum levels of 1,25(OH)2VD3 are significantly decreased in patients with SLE, especially during active disease8. So, it is tempting to speculate that a decreased level of 1,25(OH)2VD3 could have an exacerbating role in SLE pathogenesis by releasing a basal physiologic or ‘tonic’ brake in humoral immunity.
Cells of the innate immune system can also be inhibited by 1,25(OH)2VD3 (FIG. 2), which is known to inhibit the differentiation, maturation and immunostimulatory capacity of DCs by decreasing the expression of MHC class II molecules and of CD40, CD80 and CD86 (REFS 7,9,33,41). Furthermore, VDR-deficient mice have increased numbers of mature DCs in skin-draining lymph nodes41. In addition, 1,25(OH)2VD3 decreases the synthesis of IL-12 (REFS 32,33) and simultaneously increases the production of IL-10 by DCs33. The net result is a decrease in TH1-cell responses and probably an induction of IL-10-producing T regulatory type 1 (TR1) cells7.
Although 1,25(OH)2VD3 primarily has inhibitory effects on the adaptive immune response, some of its effects on innate immune cells are stimulatory. For example, 1,25(OH)2VD3 can stimulate human monocyte proliferation in vitro42 and has been shown to increase the production of both IL-1 and the bactericidal peptide cathelicidin by monocytes and macrophages5,23.
Unexpectedly, in spite of the potentially important role of 1,25(OH)2VD3 in maintaining immune homeostasis, VDR-deficient mice have a normal composition of immune-cell populations and reject allogeneic and xenogeneic transplants at the same rate as wild-type mice43. However, to carefully dissect the in vivo effects of VD3 metabolites on the immune response, it will be necessary to study the effect of VD3 in animal models in which VDR deficiency is restricted to T cells, B cells and myeloid cells.
Immunomodulatory role of vitamin A
Effects on adaptive immune-cell subsets
Vitamin A metabolites can also affect some aspects of the adaptive immune response (FIG. 3). Retinoic acid enhances cytotoxicity44 and T-cell proliferation45, the latter probably mediated, at least in part, by enhancing IL-2 secretion and signalling in T cells45. Consistent with an in vivo role for vitamin A in T-cell function, vitamin A-deficient mice have defects in TH-cell activity46. A possible mechanism for this observation is that in the setting of vitamin A deficiency, retinoic acid does not compete with 1,25(OH)2VD3 for their common nuclear binding partner RXR and, therefore, the inhibitory effects of 1,25(OH)2VD3 on T-cell function (including TH-cell activity) are not offset by retinoic acid.
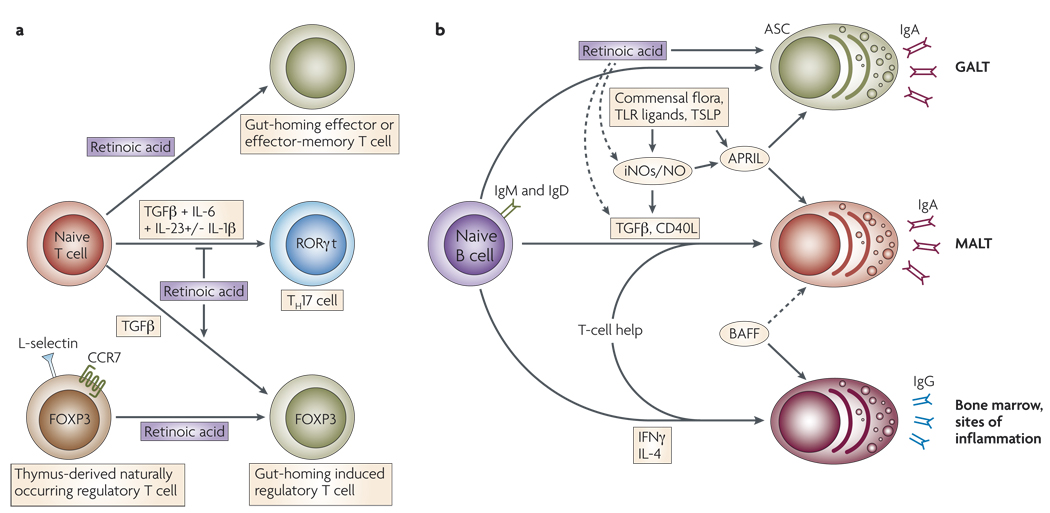
Figure 3. Effects of vitamin A metabolites on gut mucosal immunity.
a | In addition to upregulating the expression of gut-homing receptors, retinoic acid has also been reported to promote T-helper-2 (TH2)-cell differentiation. Moreover, retinoic acid blocks the differentiation of T helper 17 (TH17) cells and induces forkhead box protein 3 (FOXP3)+regulatory T (TReg) cells in the presence of transforming growth factor-β (TGFβ) by reciprocally downregulating receptor-related orphan receptor-γt (RORγt) and inducing FOXP3 expression in T cells, respectively. Retinoic acid also enhances the TGFβ-driven induction of TReg cells and induces gut-homing receptor expression in both naturally occurring and induced TReg cells. TH17-cell differentiation requires TGFβ, interleukin-6 (IL-6), IL-23 and, in humans, IL-1β. b | B cells activated in non-mucosal lymphoid tissues, such as peripheral lymph nodes and spleen, mostly become IgG+ antibody-secreting cells (ASCs) and home to the bone marrow and sites of inflammation. By contrast, B cells activated in mucosal-associated lymphoid tissues (MALT) give rise to IgA+ ASCs. In MALT (including the gut-associated lymphoid tissue; GALT), TGFβ and CD40 ligand (CD40L) are essential for the generation of T-cell-dependent IgA responses, whereas BAFF (B-cell-activating factor) and APRIL (a proliferation-inducing ligand) are important for T-cell-independent IgA responses. APRIL is induced by Toll-like receptor (TLR) signals, commensal flora and thymic stromal lymphopoietin (TSLP). Inducible nitric oxide synthase (iNOS), which is also upregulated by TLR signals and commensal flora, produces nitric oxide (NO), allows proper TGFβ signalling and induces the production of APRIL and BAFF by dendritic cells. Thus, iNOS and NO are essential for both T-cell-dependent and -independent IgA responses. In the GALT, retinoic acid might contribute directly to the differentiation of T-cell-independent (and probably also T-cell-dependent) IgA+ ASCs. In addition, retinoic acid might contribute indirectly to T-cell-dependent and -independent IgA responses by inducing iNOS expression.
Retinoic acid can inhibit B-cell proliferation47,48, although it has also been found to enhance B-cell activation under some conditions49,50. In addition, retinoic acid inhibits B-cell apoptosis. These effects are mediated through binding of vitamin A metabolites to RAR receptors51.
Notably, it has been reported that a distinct set of vitamin A metabolites classified as retro-retinoids can also affect general lymphocyte functions such as B-cell proliferation52 and T-cell activation and proliferation52. 14-hydroxy-retroretinol (14HRR) has a positive effect on proliferation, whereas anhydroretinol blocks B-cell proliferation and induces apoptosis in T cells53. Retroretinoids do not signal through RAR or RXR and, as 14HRR and anhydroretinol can antagonize each other’s effects, it has been suggested that they might compete for a common, as-yet unknown receptor53.
Retinoic acid can also modulate antigen presentation by exerting direct effects on DC function. For example, retinoic acid increases the expression of matrix metalloproteinases, thereby increasing the migration of tumour-infiltrating DCs to the draining lymph nodes, which have the potential to boost tumour-specific T-cell responses54. In addition, in the presence of inflammatory stimuli, such as tumour-necrosis factor (TNF), retinoic acid enhances DC maturation and antigen-presenting capacity, both of which are effects mediated by RXR receptors55. However, it should be noted that DCs pre-treated with retinoic acid can apparently store this metabolite50, which when released could ultimately act directly on T cells and/or other cells and contribute to the final outcome of an immune response.
Vitamin A metabolites also modulate more specific functional aspects of the immune response, such as the TH1–TH2-cell balance and the differentiation of TReg cells and TH17 cells (FIG. 3a). Vitamin A deficiency correlates with decreased TH2-cell responses56 and, conversely, vitamin A supplementation blocks the production of TH1-cell cytokines in vitro and in vivo57,58. These effects of vitamin A on TH1–TH2-cell differentiation are mediated by retinoic acid. In fact, retinoic acid promotes TH2-cell differentiation by inducing IL4 gene expression59. Moreover, retinoic acid blocks the expression of the TH1-cell master regulator T-bet and induces TH2-cell-promoting transcription factors, such as GATA3 (GATA-binding protein 3), macrophage-activating factor (MAF) and signal transducer and activator of transcription 6 (STAT6)57,60. Interestingly, vitamin A supplementation was correlated with an increase in disease severity in a mouse model of asthma, whereas vitamin A deficiency had the opposite effect, which was associated with a decrease in TH2-cell cytokines61. It has been proposed that retinoic acid exerts its TH2-cell-promoting effect indirectly through the modulation of APCs62. However, retinoic acid can also act directly on T cells to induce TH2-cell differentiation57,60 through RAR proteins57.
Several types of T cells that have dominant immunomodulatory effects have been described. The best characterized are TReg cells that express the transcription factor FOXP3. Although transforming growth factor-β (TGFβ) drives the generation of induced TReg cells in peripheral tissues63, it was recently demonstrated by several groups that this process can be significantly enhanced by retinoic acid64,65 (FIG. 3a). In addition, DCs from the gut-associated lymphoid tissue (GALT) or small intestinal lamina propria also enhance TReg-cell differentiation in a retinoic acid-dependent manner16,66. Notably, one recent study indicated that macrophages and not DCs are responsible for inducing TReg cells in the intestinal lamina propria67, and that DCs are mainly involved in the induction of TH17 cells at this site67,68. The reasons for these seemingly discrepant results are unclear. In addition to inducing FOXP3, retinoic acid also upregulates gut-homing receptors on TReg cells, targeting these cells to the gut mucosa65,66. The relative contribution of in situ-generated gut TReg cells to both oral and peripheral tolerance is yet to be determined.
The differentiation of TReg cells and TH17 cells is reciprocally regulated by cytokine signals69. Exposure of activated CD4+ T cells to TGFβ alone induces TReg cells, whereas the combination of TGFβ with IL-6, IL-1β and IL-23 or IL-21 blocks FOXP3 induction and induces TH17-cell differentiation69,70. However, exposure of CD4+ T cells to retinoic acid together with TGFβ and IL-6 negates the TH17-cell-promoting effect of IL-6 by enhancing the induction of TReg cells and blocking the induction of retinoic-acid-receptor-related orphan receptor-γt (RORγt), a key transcription factor for TH17-cell differentiation64,71. It should be noted that this effect is only observed when retinoic acid is used over a certain threshold concentration68. In fact, low concentrations of retinoic acid seem to be necessary for TH17-cell differentiation68. So, retinoic acid has a dual role in maintaining immunological tolerance: it favours the induction of TReg cells and can simultaneously either block or enhance TH17-cell differentiation, depending on its concentration.
Effects on immunoglobulin isotypes
An important feature of activated B cells is their capacity to undergo immunoglobulin class-switching and to give rise to different antibody isotypes. TH1- and TH2-cell cytokines differentially influence antibody class-switching: the TH1-cell cytokine IFNγ promotes switching to IgG2a and IgG3, whereas the TH2-cell cytokine IL-4 induces the production of IgG1 and IgE and suppresses the generation of IgG2b and IgG3 (REF. 72). DCs can also modulate B-cell activation and antibody class-switching73, which could occur indirectly by influencing TH-cell differentiation74. However, DCs can also directly promote the induction of specific immunoglobulin isotypes. GALT-resident DCs efficiently induce the generation of IgA+ ASCs when cultured with activated B cells in vitro75,76. Several cytokines and other bioactive factors are involved in the capacity of GALT-resident DCs to induce IgA+ ASCs, including TGFβ1 (REFS 16,67,74,77), IL-6 (REFS 75,76), APRIL (a proliferation-inducing ligand)78 and nitric oxide79.
GALT- and lamina propria-resident DCs also contribute to the generation of IgA+ ASCs by a mechanism depending, at least in part, on retinoic acid68,76 (FIG. 3b). The presence of retinoic acid efficiently induces IgA secretion by lipopolysaccharide (LPS)-activated splenocytes80 or by LPS-stimulated B cells cultured with spleen DCs68. However, the presence of retinoic acid during the activation of purified B cells is not sufficient to impart these effects68,76. Retinoic acid-induced IgA secretion requires either IL-5 (REFS 76,80) or IL-6 (REFS 75,76), which are known to possess a general adjuvant role in IgA production73,81,82. In fact, both retinoic acid and IL-6 are required for GALT-resident DCs to induce optimal IgA production by mouse and human B cells in vitro75,76. Vitamin A-depleted mice have decreased numbers of IgA+ ASCs in the small bowel lamina propria76,83, consistent with an in vivo role for retinoic acid in gut mucosal IgA responses. In addition, oral administration of a RAR agonist significantly increases serum IgA levels in rats84. Interestingly, exposure to retinoic acid together with IL-6 or IL-5 is not sufficient to induce IgA+ ASCs from in vitro-activated naive B cells in the absence of DCs76. Therefore, it is likely that DCs provide some necessary B-cell survival and differentiation signals that are not tissue-specific.
It was recently shown that inducible nitric oxide synthase (iNOS; also known as NOS2A) and nitric oxide are crucial for the generation of IgA+ ASCs79. Interestingly, the promoter of the iNOS gene contains a RARE that is directly activated by retinoic acid bound to its nuclear RARα–RXR heterodimeric receptor85. Moreover, intraperitoneal administration of either retinoic acid or a RAR agonist enhances LPS-induced iNOS expression in several organs and increases plasma levels of nitrate and nitrite in rats86. Therefore, retinoic acid might also indirectly contribute to IgA secretion by inducing iNOS and nitric oxide expression.
Precursors of retinoids in the diet (which include β-carotene and retinyl esters) are absorbed from the gut lumen and can be metabolized to produce retinoic acid in IECs14 and in DCs that reside near the germinal centres in Peyer’s patches15. The proximity of Peyer’s patches to the gut bacterial flora and to other potential sources of retinoic acid and cytokines, such as IECs, could help to explain the predominance of IgA class-switching that occurs in Peyer’s patches compared with mesenteric lymph nodes87. Consistent with a role for vitamin A in gut IgA production, rats or mice depleted of vitamin A have decreased levels of total IgA in intestinal lavages and decreased mucosal antigen-specific IgA responses, which correlates with decreased protection against infections and oral bacterial toxins56,88,89. Furthermore, vitamin A supplementation prevents the decline in IgA levels observed in malnourished mice58. In addition to a direct effect on ASCs, it should be considered that vitamin A deficiency could also decrease IgA secretion in the gut by reducing the expression of the polymeric immunoglobulin receptor, leading to a decrease in the secretion of dimeric IgA to the gut lumen88,89. Moreover, although vitamin A-deficient mice have decreased numbers of IgA+ ASCs in the small bowel76,83, their serum IgA levels are normal76, which indicates that retinoids are not absolutely required for generating IgA+ ASCs in other mucosal compartments.
Vitamin metabolites and lymphocyte homing
Although naive lymphocytes migrate mainly through secondary lymphoid organs, effector and memory lymphocytes acquire ‘traffic’ molecules that endow them with the capacity to migrate to select extralymphoid tissues and to sites of inflammation. Of the extralymphoid compartments, the gastrointestinal mucosa and the skin are the two main body surfaces exposed to environmental antigens and are also the two paradigmatic tissues for which tissue-specific adhesion and chemoattractant receptors (also known as homing receptors) have been characterized in detail. For example, effector and memory lymphocytes migrating to the small bowel require expression of the α4β7-integrin and CC-chemokine receptor 9 (CCR9), whereas those migrating to the skin rely on the expression of ligands for E- and P-selectin and CCR4 or CCR10 (REF. 73) (FIG. 4a). It has been demonstrated that the lymphoid microenvironment in which lymphocytes are activated determines the set of homing receptors that they acquire — for example, T cells activated in skin-draining lymph nodes acquire skin-homing receptors whereas those activated in the GALT acquire gut-homing receptors90. In the lymphoid microenvironment, DCs are essential for efficient T-cell activation91. Several groups have shown that DCs from Peyer’s patches and mesenteric lymph nodes are sufficient to induce the expression of α4β7-integrin and CCR9 and therefore imprint gut-homing capacity on activated mouse T cells92–96 and mouse and human B cells68,76, whereas lymphocytes activated by mouse DCs from peripheral lymph nodes preferentially acquire skin-homing receptors95,96.
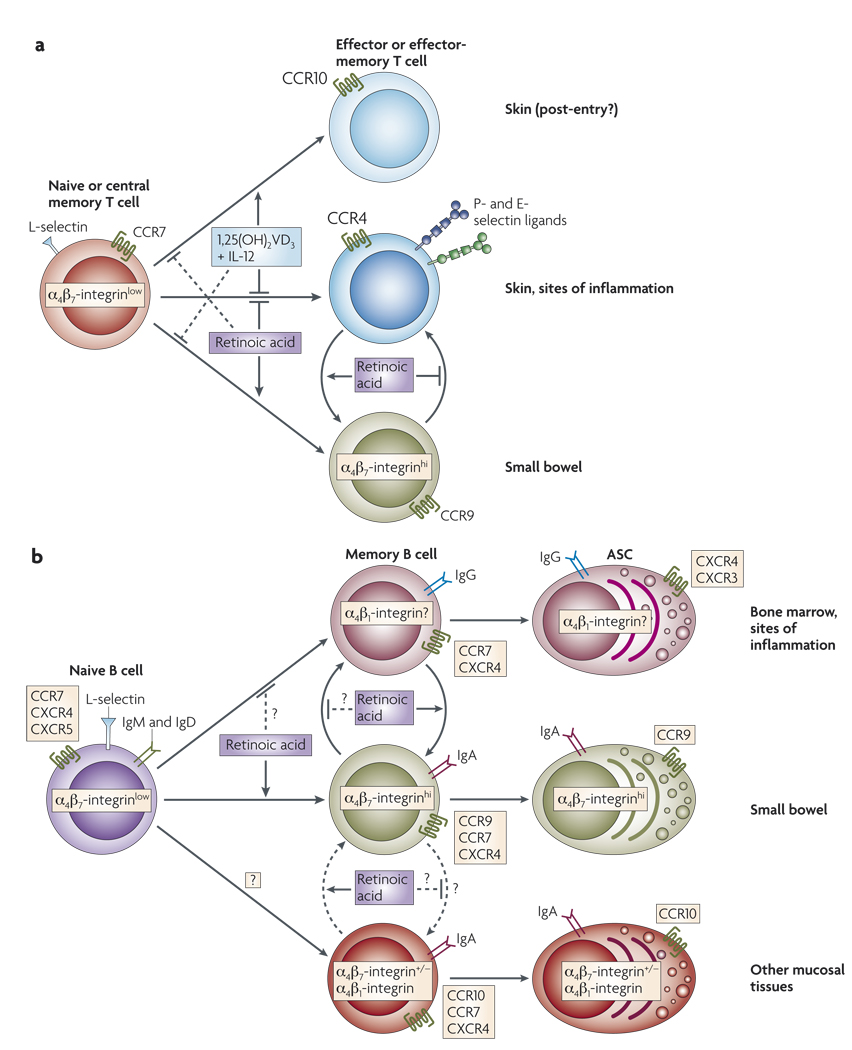
Figure 4. Roles of retinoic acid and 1,25(OH)2VD3 in tissue-specific lymphocyte homing.
a | Retinoic acid produced by gut-associated lymphoid tissue (GALT)-resident dendritic cells and probably by other cells, such as intestinal epithelial cells, potently induces the expression of the gut-homing receptors α4β7-integrin and CC-chemokine receptor 9 (CCR9) by activated CD4+ and CD8+ T cells. Retinoic acid also blocks the induction of skin-homing receptors by T cells, including CCR4 and the ligands for E- and P-selectin. Effector and memory T cells exhibit plasticity in their homing commitment: skin-homing T cells can become gut-homing T cells and vice versa if they are restimulated either with or without retinoic acid, respectively. In the presence of interleukin-12 (IL-12), 1,25(OH)2VD3 (the main circulating vitamin D3 (VD3) metabolite) induces the expression of skin-associated CCR10 by human (but not mouse) T cells. However, 1,25(OH)2VD3 blocks the induction of E-selectin ligands and therefore inhibits skin-homing. So, it is possible that 1,25(OH)2VD3 induces CCR10 expression after T cells have homed to the skin to retain them in the epidermis (in which the CCR10 ligand CCL27 is expressed). 1,25(OH)2VD3 also antagonizes the upregulation of gut-homing receptors, whereas retinoic acid reciprocally blocks the induction of CCR10 expression by 1,25(OH)2VD3. b | Like T cells, B cells also exhibit plasticity in their homing commitment and can either acquire or lose gut-homing potential when reactivated with or without retinoic acid, respectively. Retinoic acid induces the expression of α4β7-integrin and CCR9 on activated B cells and antibody-secreting cells (ASCs). It is unknown whether retinoic acid alters the homing of B cells and ASCs to other tissues, such as the bone marrow or sites of inflammation. ASCs in mucosal tissues (mostly IgA+ ASCs) also express CCR10, although it is unclear where and how this receptor is upregulated by these cells.
How do GALT-resident DCs imprint lymphocytes with a gut-homing phenotype? More than 25 years ago it was shown that rats suffering from both protein-caloric and vitamin A deficiencies exhibited impaired migration of recently activated mesenteric lymphocytes to the small intestinal mucosa97. Protein-caloric malnutrition without vitamin A deficiency did not affect lymphocyte migration97. Adoptive transfer experiments showed that impaired lymphocyte migration was observed only when donor lymphocytes were from protein-caloric-deficient and vitamin A-deficient rats and not when wild-type cells were transferred to protein-caloric-deficient and vitamin A-deficient recipients97. This suggests that vitamin A deficiency mainly affected lymphocyte migratory capacity, but not the target tissues97. More recently, it was described that vitamin A-depleted rats had a marked decrease in the number of IgA+ ASCs and CD4+ T cells in the ileum83. The molecular basis for these observations was recently determined in a study that showed that mice depleted of vitamin A had decreased numbers of effector and memory T cells in the gut mucosa, but not elsewhere15. The vitamin A metabolite retinoic acid was sufficient to induce the expression of α4β7-integrin and CCR9 by activated T cells, even in the absence of DCs15. Blocking retinoic acid receptors of the RAR family significantly decreased the induction of α4β7-integrin expression by T cells by GALT-resident DCs, which shows that retinoic acid is essential for the gut-imprinting capacity of the DCs15. Consistently, GALT-resident DCs, unlike DCs from other tissues, express RALDH enzymes, which are essential for retinoic acid biosynthesis15. Together, these results indicate that retinoic acid is pivotal for the imprinting of gut-homing T cells.
Although vitamin A deficiency decreases the number of T and B cells in the small bowel lamina propria15,76,83,97, it does not affect lymphocyte migration to the colon97. Analogously, GALT-resident DCs imprint T and B cells with homing capacity for the small bowel, but they do not induce colon-homing T cells93. Therefore, retinoic acid is neither necessary nor sufficient to imprint colon-homing lymphocytes. The molecular signals that are responsible for lymphocyte homing to the colon and the reasons why T-cell migration to this compartment is controlled differently from homing to the small bowel are still to be determined.
Regarding the migration of ASCs, it has been proposed that CCR10 might have a role in the homing of IgA+ ASCs to the colon, mammary glands and probably to other mucosal compartments73 (FIG. 4b). However, it is currently unclear how CCR10 expression is induced by ASCs. Recent reports indicate that IgA+ ASCs might acquire CCR10 expression in colonic patches or in iliac lymph nodes following rectal immunization98 and that the expression of this receptor can also be induced by 1,25(OH)2VD3 in human ASCs39. However, 1,25(OH)2VD3 does not induce CCR10 expression in murine ASCs in vitro and VDR-deficient mice have normal numbers of CCR10+ IgA+ ASCs39, which indicates that 1,25(OH)2VD3 might not be necessary for the induction of CCR10 expression by B cells in vivo, at least in mice.
In vitro-generated tissue-tropic lymphocytes retain marked plasticity; skin-homing T cells can be converted to gut-homing T cells and vice versa if they are re-stimulated with or without GALT-resident DCs, respectively95,96. Similarly, previously activated B cells can be re-educated and acquire or lose gut-homing potential when they are restimulated with or without retinoic acid, respectively76. The decrease in α4β7-integrin and CCR9 expression observed in T and B cells that are activated in the absence of retinoic acid might be a default differentiation mechanism. However, it is also possible that other factors can actively contribute to the downregulation of gut-homing-receptor expression by lymphocytes. As both RAR and VDR must form heterodimers with RXR to signal, it is possible that 1,25(OH)2VD3 could actively antagonize the effects of retinoic acid by competing for the same nuclear partner18. Consistent with this possibility, 1,25(OH)2VD3 blocks the retinoic acid-induced upregulation of gut-homing receptors on human T cells6,99. However, whether this retinoic acid antagonism by 1,25(OH)2VD3 has a regulatory role in the imprinting of gut-homing lymphocytes in vivo remains unknown.
In addition to imprinting a gut-homing phenotype on lymphocytes, retinoic acid and GALT-resident DCs also block the upregulation of the skin-homing receptors CCR4 and ligands for P- and E-selectin by T cells15,95. Therefore, acquisition of a skin-homing phenotype might be the default pathway for T-cell activation in the absence of retinoic acid or when RAR signalling is blocked100. Nonetheless, as VD3 as well as 1,25(OH)2VD3 can be synthesized in the skin101, it is conceivable that, like retinoic acid in the gut, 1,25(OH)2VD3 might have a reciprocal role in imprinting lymphocyte homing to the skin. In agreement with this possibility, it was recently shown that 1,25(OH)2VD3 synergizes with IL-12 to induce the expression of skin-associated CCR10 by human T cells6. However, it was also shown that 1,25(OH)2VD3 actually blocks the upregulation of ligands for E-selectin6,99 and the expression of fucosyltransferase-VII (REF. 99), an enzyme essential for the synthesis of selectin ligands102. This was correlated with decreased homing of T cells to the inflamed skin in a model of contact hypersensitivity induced by oxazolone99. Although these data indicate that 1,25(OH)2VD3 might block skin-homing, it should be noted that these experiments were carried out without IL-12 supplementation, which could potentially counteract the negative effect of 1,25(OH)2VD3 on the expression of E-selectin ligands and skin-homing. It is also possible that 1,25(OH)2VD3 induces CCR10 expression by T cells after they have homed to the skin to increase their retention in this tissue. In fact, because keratinocytes express the CCR10 ligand CC-chemokine ligand 27 (CCL27), it has been proposed that CCR10 upregulation might promote T-cell trafficking to and/or retention in the epidermis6.
Finally, 1,25(OH)2VD3 might also affect leukocyte migration by blocking chemokine synthesis at effector sites. For instance, 1,25(OH)2VD3 decreased the expression of CCL2, CCL3, CXCl10 and subsequent monocyte infiltration in experimental autoimmune encephalomyelitis (EAE)103. Similarly, a 1,25(OH)2VD3 analogue decreased the production of the chemokines CCL2, CCL5, CXCl10 and consequent TH1-cell infiltration in non-obese diabetic (NOD) mice, a model of type 1 diabetes104.
Effects of antioxidant vitamins on immunity
It has been known for more than 30 years that some vitamins with antioxidant properties, including vitamin A, vitamin B6 (pyridoxine), vitamin C (ascorbic acid) and particularly vitamin E, have protective effects on animal models of atherosclerosis and ischaemia-reperfusion injury (IRI)2–4. Vitamin E collectively refers to eight related compounds (tocopherols and tocotrienols), of which α-tocopherol has the greatest bioavailability and is the best characterized105. Vitamin E decreases the release of reactive oxygen species by monocytes106 and the expression of CD11b and very late antigen 4 (VLA4), thereby decreasing monocyte adhesion to the endothelium106. Vitamin E also blocks the release of pro-inflammatory cytokines, including IL-1, IL-6, TNF and the chemokine IL-8, by monocytes and macrophages107,108. Moreover, vitamin E prevents the upregulation of the adhesion molecules vascular cell-adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1) on the endothelium induced by oxidized low-density lipo protein (LDL)109 and IL-1β110, as well as the upregulation of E-selectin and some chemokines108. Reactive oxygen species activate the nuclear factor-κB (NF-κB) pathway106, which initiates many pro-inflammatory events. Therefore, the therapeutic antioxidant effect of these vitamins could be explained, at least in part, by their capacity to decrease NF-κB activation.
Vitamin E can also act directly on T cells by decreasing IFNγ production111 and CD95L (also known as FASL)112 expression, thereby helping to decrease inflammation and immune-mediated tissue damage. These effects on macrophages and T cells are believed to be important for the protective effect for vitamin E in animal models of atherosclerosis108,113 and IRI114,115. Consistent with a potential physiological role for vitamin E in preventing atherosclerosis, hyperlipidemic mice that are deficient in α-tocopherol transfer protein, which is important for transporting α-tocopherol and for preventing its degradation, have more severe atherosclerosis116.
It was recently shown that vitamin C prevents oxidative damage during ischaemia reperfusion in rats117 and humans4. Notably, it was shown that vitamin C but not vitamin E prevented leukocyte adhesion to the microvascular endothelium in hamster models of oxidative endothelial stress induced by cigarette smoke or oxidized LDL118,119. Differences in lipophilicity might potentially have an impact in the distribution and/or location of vitamins C and E and partially account for the differential effect of these vitamins. Therefore, it is possible that combined supplementation of vitamins C and E could offer synergistic benefits. The antioxidant and/or anti-atherogenic role for other vitamins, such as vitamin B6 and vitamin K, is less well documented and somewhat controversial, with evidence both in favour120,121 and against122 a protective role for these vitamins in atherosclerosis.
Finally, it should be noted that many of the encouraging results in animal models have not been consistently translated into a significant therapeutic benefit in controlled clinical trials of vitamin supplementation for the prevention of cardiovascular diseases and IRI121. Therefore, it remains to be determined whether antioxidant vitamins will prove to be useful for the treatment and/or prevention of these ailments in humans.
Vitamin metabolites in immunotherapy
Vitamin D
Given that 1,25(OH)2VD3 has a physiological protective role in dampening or limiting potentially pathogenic immune responses at the cellular level, one might predict that interfering with its effects could predispose to hypersensitivity or autoimmunity7. Consistent with this idea, levels of serum 1,25(OH)2VD3 are often decreased in patients with type I diabetes123 and SLE8,124, and 1,25(OH)2VD3 levels are inversely correlated with disease activity in patients with rheumatoid arthritis125. Moreover, VD3 deficiency accelerates intestinal inflammation in IL-10-deficient mice, which develop inflammatory bowel disease126. VD3 deficiency might also predispose to type I diabetes7,127. However, although children with rickets (typically caused by insufficient dietary supply of vitamin D) have a higher incidence of diabetes than VD3-sufficient children7, they are also more susceptible to infection7,128, which suggests that VD3 might also be important for protective immune responses. This effect could be partially mediated by the capacity of 1,25(OH)2VD3 to increase the bactericidal capacity of macrophages5,23.
Given its immunomodulatory properties, 1,25(OH)2VD3 or its analogues might be clinically useful for the treatment of inflammatory and auto immune diseases. Administration of 1,25(OH)2VD3 or an analogue prevented proteinuria and prolonged life span in a mouse model of experimental SLE129,130. In addition, 1,25(OH)2VD3 prevented EAE in mice103,131,132, an effect that was dependent on IL-10 and IL-10 receptor signalling132. As 1,25(OH)2VD3 in combination with glucocorticoids induces IL-10-producing TR1 cells133, it is possible that 1,25(OH)2VD3 might exert its therapeutic effect, at least in part, through the generation of TR1 cells. However, induction of FOXP3+ TReg cells might also have an important role35–37. Other models of experimental autoimmunity in which 1,25(OH)2VD3 has shown a therapeutic benefit include insulitis in NOD mice127, prostatitis34 and rheumatoid arthritis7,134. 1,25(OH)2VD3 can also block cutaneous contact hypersensitivity99, an effect that could be mediated in part by blocking the induction of skin-homing-receptor expression by lymphocytes99. Consistent with its anti-inflammatory role, a 1,25(OH)2VD3 analogue has been successfully used as a therapy for psoriasis135,136. However, it should be pointed out that topical skin application of 1,25(OH)2VD3 also has the potential to trigger allergic dermatitis by increasing TH2 cell-mediated responses137.
It has been proposed that 1,25(OH)2VD3 could also be used as an adjuvant in immunomodulatory therapy in transplantation. 1,25(OH)2VD3 and a 1,25(OH)2VD3 analogue prolonged the survival of mouse cardiac allografts138,139, and decreased the rates of allograft rejection and increased survival in a rat model of liver transplantation140 and in fully mismatched mouse pancreatic islet transplants141. In addition, a 1,25(OH)2VD3 analogue provided significant protection from graft-versus-host disease (GVHD) in rats142. Moreover, 1,25(OH)2VD3 and a 1,25(OH)2VD3 analogue significantly prevented chronic allograft rejection in a rat model of renal transplantation143 and delayed chronic allograft rejection in a mouse model of aortic transplantation139.
Interestingly, polymorphisms in VDR are associated with a higher incidence of GVHD in patients who undergo bone-marrow transplantation144, which indicates that 1,25(OH)2VD3 might also have a role in suppressing alloreactive immune responses in humans. In agreement with this possibility, 1,25(OH)2VD3 supplementation has been shown to have a beneficial effect by improving allograft function of human renal transplants145, which is especially relevant considering that renal insufficiency is associated with decreased 1,25(OH)2VD3 synthesis146,147. Importantly, although 1,25(OH)2VD3 helps to prevent transplant rejection, it does not seem to interfere significantly with protective immune responses against pathogens148. Therefore, it is possible that once 1,25(OH)2VD3 induces a ‘homeostatic’ immunomodulatory threshold, it does not exert further immunosuppression.
Although 1,25(OH)2VD3 could be a potentially useful immunomodulatory agent for clinical use, it can cause some serious adverse effects, in particular the induction of hypercalcaemia and bone resorption7. Therefore, multiple drug development efforts are aimed at finding 1,25(OH)2VD3 analogues that exert immunomodulation without causing significant hypercalcaemia7. Indeed, long-term administration of a 1,25(OH)2VD3 analogue efficiently decreased serum levels of IL-2 and IgG in mice without significant adverse side effects149, and some 1,25(OH)2VD3 analogues have been successfully used in autoimmune disease models, such as EAE, to decrease the doses of conventional immunosuppressive drugs7. These results indicate that 1,25(OH)2VD3 analogues may be an effective and safer alternative to 1,25(OH)2VD3 for immune modulation.
Vitamin A
Given the crucial role of retinoic acid in imprinting a gut-homing capacity on T and B cells15,76, as well as its potential to promote the differentiation of IgA+ ASCs76,80, it is not surprising that vitamin A deficiency is associated with impaired intestinal immune responses56,88,89 and increased mortality associated with gastrointestinal and respiratory infections150. Conversely, vitamin A supplementation correlates with a significant decrease in diarrhoea and mortality in HIV-infected or malnourished children76,151,152. Therefore, retinoic acid or RAR agonists could be used for targeting T- and B-cell responses to the gut mucosa for vaccination purposes.
In addition, given that retinoic acid can potentiate the TGFβ-mediated induction of TReg cells while antagonizing the differentiation of pro-inflammatory TH17 cells64, treatment with retinoic acid together with TGFβ could be a useful strategy to generate TReg cells for treating inflammatory pathologies affecting not only the intestine, but also peripheral tissues. In fact, retinoic acid and RAR-agonists have been successfully used in some models of autoimmune inflammation, such as EAE153,154, adjuvant arthritis155,156 and experimental nephritis157. Retinoids have also been successfully used to treat psoriasis158 and they are effective in treating and/or preventing contact dermatitis in mice and humans159,160. In these models, therapeutic effects partially correlated with the induction of TH2-cell responses153 and decreased expression of α4β7-integrin on effector T cells157, but the role of retinoic acid in the induction of TReg cells and the inhibition of TH17 cells in these settings has yet to be assessed.
Concluding remarks
Although 1,25(OH)2VD3 clearly exerts immunomodulatory activity in vitro and in vivo, its relative physiological role in maintaining immune tolerance and in shaping immune responses is still unclear. Moreover, as retinoic acid and 1,25(OH)2VD3 can potentially antagonize each other’s effects6,18,99, it will be important to dissect the interplay between 1,25(OH)2VD3, retinoic acid and other mechanisms of immunomodulation in vivo.
1,25(OH)2VD3 can upregulate CCR10 on human T cells and ASCs6,39 while blocking the expression of skin- and gut-homing receptors6,99. However, the in vivo relevance of the effects of 1,25(OH)2VD3 on CCR10 expression by T cells that are infiltrating the skin and by IgA+ ASCs that are migrating to the gut lamina propria remains to be determined. In addition, although GALT-resident DCs enhance IgA secretion and induce gut-homing effector lymphocytes ex vivo, it will be important to determine the relative in vivo contribution of DCs versus other potential sources of retinoic acid in the gut (such as IECs), and to determine whether there are retinoic acid-independent mechanisms of imprinting a gut-homing phenotype. Moreover, as GALT-resident DCs can also enhance the differentiation of TGFβ-induced TReg cells, it will be necessary to determine the in vivo scenarios in which GALT-resident DCs and retinoic acid promote either effector or suppressive T-cell responses. Along these same lines, it will be important to study the contribution of these in situ-generated gut TReg cells compared with their systemic counterparts in maintaining immune tolerance at intestinal and extra-intestinal sites.
Aside from the antioxidant effects of vitamins C and E that have been demonstrated in animal models of cardiovascular disease and IRI, there is a lack of published information on the impact of these and other vitamins, such as vitamin B6 and K120–122, on the adaptive immune system and in other inflammatory settings, such as autoimmune diseases. Whether these vitamins will offer a therapeutic benefit in the settings of human cardiovascular diseases, IRI and other pathologies remains unclear.
Although many open questions remain, there is promise that vitamin A and D metabolites or their analogues have the potential to be used in clinical settings for therapeutic benefit. In particular, it will be important to assess the impact of using 1,25(OH)2VD3 analogues as an adjuvant immunomodulatory therapy in the setting of autoimmune diseases and in transplant recipients. It will also be important to determine the net effects of retinoic acid or synthetic RAR-agonists, especially in the intestine, where these agents appear to have a role in enhancing immune responses. The capacity of vitamin A metabolites to foster gut-homing T cells might improve strategies of mucosal vaccination or aid in decreasing pathogenic immunity by potentiating the induction of TReg cells.
Supplementary Material
Acknowledgements
We thank S. Davis for editorial assistance and E. Villablanca for critical reading of this manuscript. J.R.M. is grateful to I. Ramos for constant support. J.R.M. is supported by grants from the Crohn’s & Colitis Foundation of America, the Cancer Research Institute, the Howard M. Goodman Fellowship and the Center for the Study of IBD (DK 43351). M.I. is supported by the Grants-in-Aid from Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology, the Naito Foundation and the Uehara Memorial Foundation. U.H.v.A. is supported by National Institutes of Health grants AI061663, AI069259, AI072252, HL56949 and AR42689.
Glossary
- Stellate cells
(Also known as Ito cells). Types of pericytes found in the hepatic perisinusoidal space that are the main reservoirs of retinol in the liver.
- Intestinal epithelial cells (IECs)
A tight monolayer of cells covering the luminal surface of the intestine. They are specialized in the absorption of nutrients and also serve as a mechanical and immunological barrier with the external environment (the intestinal lumen).
- Peyer’s patches
Groups of lymphoid nodules present in the small intestine (usually the ileum). They are found massed together on the intestinal wall, opposite the line of attachment of the mesentery. Peyer’s patches consist of a dome area, B-cell follicles and interfollicular T-cell areas.
- Mesenteric lymph nodes (MLNs)
Lymph nodes located at the base of the mesentery. They collect lymph (including cells and antigens) draining from the intestinal mucosa.
- TH17 cells (T helper 17 cells)
A subset of CD4+ T helper cells that produce interleukin-17 (IL-17) and are thought to be important in inflammatory and autoimmune diseases. Their generation involves TGFβ, IL-6, IL-23 or IL-21, IL-1β and the transcription factor RORγt.
- TReg cells (Regulatory T cells)
Specialized types of CD4+ T cells that can suppress the effector responses of other immune cells. These cells provide a crucial mechanism for the maintenance of peripheral self-tolerance and are characterized by the expression of the transcription factor forkhead box P3.
- Systemic lupus erythematosus (SLE)
An autoimmune disease in which autoantibodies specific for DNA, RNA or proteins associated with nucleic acids form immune complexes. These complexes damage small blood vessels, especially in the kidneys. Patients with SLE generally have abnormal B- and T-cell function as well as rashes, arthritis, kidney disease and central-nervous-system involvement.
- Antibody-secreting cells (ASCs)
Cells specialized in secreting immunoglobulins. Although they originate from activated B cells, ASCs lose the expression of surface immunoglobulins and other B-cell markers and upregulate plasma cell markers, such as CD138 in mice or CD27 in humans.
- TR1 cells (T regulatory type 1 cells)
A population of regulatory T cells that arises in the periphery after an encounter with antigen in the presence of interleukin-10 (IL-10) and that regulates immune responses through the secretion of IL-10 and transforming growth factor-β. They suppress T-cell responses, downregulate the expression of co-stimulatory molecules and pro-inflammatory cytokines by antigen-presenting cells and favour the production of IgD, IgA and IgG by B cells.
- Gut-associated lymphoid tissue (GALT)
Lymphoid structure associated with the intestinal mucosa, including cryptopatches, isolated lymphoid follicles, Peyer’s patches and caecal and colonic patches.
- Small intestinal lamina propria
Connective tissue between the intestinal epithelium and the intestinal muscularis mucosae layer, which contains various myeloid and lymphoid cells, including macrophages, dendritic cells, T cells and B cells.
- Colonic patches
Structures resembling Peyer’s patches that are scattered throughout the colon. They have been implicated in the generation of colonic immune responses.
- Experimental allergic encephalomyelitis (EAE)
An experimental model of the human disease multiple sclerosis. Autoimmune disease is induced in experimental animals by immunization with myelin or peptides derived from myelin. The animals develop a paralytic disease with inflammation and demyelination in the brain and spinal cord.
- Type 1 diabetes
A chronic autoimmune disease that is characterized by the T-cell-mediated destruction of β cells (which secrete insulin) in the pancreas. Patients with type 1 diabetes develop hyperglycaemia and can develop diabetes-associated complications in multiple organ systems, owing to a lack of insulin. Diabetes in non-obese diabetic mice is a model of type I diabetes.
- Atherosclerosis
A chronic disorder of the arterial wall characterized by endothelial damage that gradually induces deposits of cholesterol, cellular debris, calcium and other substances. These deposits eventually lead to plaque formation and arterial stiffness.
- Ischaemia-reperfusion injury (IRI)
Cellular damage caused by the return of blood supply to a tissue after a period of inadequate blood supply. The absence of oxygen and nutrients causes cellular damage, such that restoration of the blood flow results in inflammation.
- Rheumatoid arthritis
An immunological disorder that is characterized by symmetrical polyarthritis, often progressing to crippling deformation after years of synovitis. It is associated with systemic immune activation, with the presence of acute-phase reactants in the peripheral blood and with rheumatoid factor (immunoglobulins specific for IgG), which form immune complexes that are deposited in many tissues.
- Inflammatory bowel disease (IBD)
A chronic condition of the intestine that is characterized by severe inflammation and mucosal destruction. The most common forms in humans are ulcerative colitis and Crohn’s disease, which are believed to be T helper 2 (TH2)- and TH1-type diseases, respectively. However, interleukin-23 and TH17 cells have also recently been shown to be involved in the pathology of IBD.
- Graft-versus-host disease (GVHD)
An immune response mounted against the recipient of an allograft by immunocompetent donor T cells that are derived from the graft. Typically, it is seen in the context of allogeneic bone-marrow transplantation.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
α4β7| CCR4 | CCR9 | CCR10 | FOXP3| IFNγ | IL-2| iNOS | PPARβ | RAR | RXR | TNF | VDR
FURTHER INFORMATION
Ulrich H. von Adrian’s homepage: http://www.cbr.med.harvard.edu/labs/vonandrian
Rodrigo Mora’s homepage: http://www.massgeneral.org/gastroenterology/research/research_mora.html
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
References
- 1.Rosenberg IH. Challenges and opportunities in the translation of the science of vitamins. Am. J. Clin. Nutr. 2007;85:325S–327S. doi: 10.1093/ajcn/85.1.325S. [DOI] [PubMed] [Google Scholar]
- 2.Beetens JR, Coene MC, Verheyen A, Zonnekeyn L, Herman AG. Influence of vitamin C on the metabolism of arachidonic acid and the development of aortic lesions during experimental atherosclerosis in rabbits. Biomed. Biochim. Acta. 1984;43:S273–S276. [PubMed] [Google Scholar]
- 3.Llesuy S, et al. Effect of vitamins A and E on ischemia-reperfusion damage in rabbit heart. Mol. Cell. Biochem. 1995;145:45–51. doi: 10.1007/BF00925712. [DOI] [PubMed] [Google Scholar]
- 4.Pleiner J, et al. Intra-arterial vitamin C prevents endothelial dysfunction caused by ischemia-reperfusion. Atherosclerosis. 2008;197:383–391. doi: 10.1016/j.atherosclerosis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6. Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nature Immunol. 2007;8:285–293. doi: 10.1038/ni1433. This paper reports that 1,25(OH)2VD3 is synthesized by dermal DCs and induces CCR10 expression by human T cells, presumably increasing their epidermotropism.
- 7.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J. Steroid. Biochem. Mol. Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 9.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3–1α-hydroxylase and production of 1α, 25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 10.Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1α, 25-dihydroxyvitamin D3. Endocrinology. 1997;138:2233–2240. doi: 10.1210/endo.138.6.5170. [DOI] [PubMed] [Google Scholar]
- 11.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J. Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 12.Moise AR, Noy N, Palczewski K, Blaner WS. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46:4449–4458. doi: 10.1021/bi7003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lera AR, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nature Rev. Drug Discov. 2007;6:811–820. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- 14.Lampen A, Meyer S, Arnhold T, Nau H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J. Pharmacol. Exp. Ther. 2000;295:979–985. [PubMed] [Google Scholar]
- 15. Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. This paper reports for the first time that retinoic acid is necessary and sufficient to induce gut-homing receptors on T cells. It is also shown that gut-associated DCs can metabolize dietary vitamin A into retinoic acid, which explains their capacity to imprint gut-homing lymphocytes.
- 16. Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. This paper shows that CD103+ DCs from mesenteric lymph nodes induce TReg cells by a mechanism involving TGFβ and retinoic acid.
- 17.Germain P, et al. International union of pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 18.Bastie JN, et al. 1α 25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol. Endocrinol. 2004;18:2685–2699. doi: 10.1210/me.2003-0412. [DOI] [PubMed] [Google Scholar]
- 19. Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. This paper demonstrates that the intracellular ratio between cellular retinoic acid binding protein (CRABP) and fatty acid binding protein (FABP) determines whether retinoic acid acts through RAR or PPARβ nuclear receptors, which translates as distinct functional outcomes.
- 20.Lemire JM, Adams JS, Sakai R, Jordan SC. 1α, 25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Invest. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J. Clin. Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. References 20 and 21 are among the first reports to clearly demonstrate that 1,25(OH)2VD3 can exert a powerful immunomodulatory effect on T and B cells ex vivo.
- 22.Lemire JM, et al. 1,25-dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J. Immunol. 1985;134:3032–3035. [PubMed] [Google Scholar]
- 23.Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell. Immunol. 1986;98:311–322. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 24.Reichel H, Koeffler HP, Tobler A, Norman AW. 1α,25-dihydroxyvitamin D3 inhibits γ-interferon synthesis by normal human peripheral blood lymphocytes. Proc. Natl Acad. Sci. USA. 1987;84:3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigby WF, Yirinec B, Oldershaw RL, Fanger MW. Comparison of the effects of 1,25-dihydroxyvitamin D3 on T lymphocyte subpopulations. Eur. J. Immunol. 1987;17:563–566. doi: 10.1002/eji.1830170420. [DOI] [PubMed] [Google Scholar]
- 26.Meehan MA, Kerman RH, Lemire JM. 1,25-dihydroxyvitamin D3 enhances the generation of nonspecific suppressor cells while inhibiting the induction of cytotoxic cells in a human MLR. Cell. Immunol. 1992;140:400–409. doi: 10.1016/0008-8749(92)90206-5. [DOI] [PubMed] [Google Scholar]
- 27.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell. Biol. 1995;15:5789–5799. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-γ gene. Eur. J. Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Matsui T, Nakao Y, Koizumi T, Nakagawa T. & Fujita, T 1,25-dihydroxyvitamin D3 regulates proliferation of activated T-lymphocyte subsets. Life Sci. 1985;37:95–101. doi: 10.1016/0024-3205(85)90630-7. [DOI] [PubMed] [Google Scholar]
- 30.Muller K, Bendtzen K. Inhibition of human T lymphocyte proliferation and cytokine production by 1,25-dihydroxyvitamin D3. Differential effects on CD45RA+ and CD45R0+ cells. Autoimmunity. 1992;14:37–43. doi: 10.3109/08916939309077355. [DOI] [PubMed] [Google Scholar]
- 31.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 32.D’Ambrosio D, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-κB downregulation in transcriptional repression of the p40 gene. J. Clin. Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penna G, Adorini L. 1α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 34.Penna G, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J. Immunol. 2006;177:8504–8511. doi: 10.4049/jimmunol.177.12.8504. [DOI] [PubMed] [Google Scholar]
- 35.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1 /Th 17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 36.Gorman S, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4 + CD25+ cells in the draining lymph nodes. J. Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 37.Penna G, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+ Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 38.Muller K, Heilmann C, Poulsen LK, Barington T, Bendtzen K. The role of monocytes and T cells in 1,25-dihydroxyvitamin D3 mediated inhibition of B cell function in vitro. Immunopharmacology. 1991;21:121–128. doi: 10.1016/0162-3109(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 39.Shirakawa AK, et al. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J. Immunol. 2008;180:2786–2795. doi: 10.4049/jimmunol.180.5.2786. [DOI] [PubMed] [Google Scholar]
- 40.Chong PJ, et al. 1,25 dihydroxyvitamin-D3 regulation of immunoglobulin production in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. J. Autoimmun. 1989;2:861–867. doi: 10.1016/0896-8411(89)90012-7. [DOI] [PubMed] [Google Scholar]
- 41.Griffin MD, et al. Dendritic cell modulation by 1α, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl Acad. Sci. USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohta M, Okabe T, Ozawa K, Urabe A, Takaku F. 1α, 25-dihydroxyvitamin D3 (calcitriol) stimulates proliferation of human circulating monocytes in vitro. FEBS Lett. 1985;185:9–13. doi: 10.1016/0014-5793(85)80730-4. [DOI] [PubMed] [Google Scholar]
- 43.Mathieu C, et al. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J. Bone Miner. Res. 2001;16:2057–2065. doi: 10.1359/jbmr.2001.16.11.2057. [DOI] [PubMed] [Google Scholar]
- 44.Dennert G, Lotan R. Effects of retinoic acid on the immune system: stimulation of T killer cell induction. Eur. J. Immunol. 1978;8:23–29. doi: 10.1002/eji.1830080106. [DOI] [PubMed] [Google Scholar]
- 45.Ertesvag A, Engedal N, Naderi S, Blomhoff HK. Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion. J. Immunol. 2002;169:5555–5563. doi: 10.4049/jimmunol.169.10.5555. [DOI] [PubMed] [Google Scholar]
- 46.Carman JA, Smith SM, Hayes CE. Characterization of a helper T lymphocyte defect in vitamin A-deficient mice. J. Immunol. 1989;142:388–393. [PubMed] [Google Scholar]
- 47.Blomhoff HK, et al. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J. Biol. Chem. 1992;267:23988–23992. [PubMed] [Google Scholar]
- 48.Ballow M, Xiang S, Wang W, Brodsky L. The effects of retinoic acid on immunoglobulin synthesis: role of interleukin 6. J. Clin. Immunol. 1996;16:171–179. doi: 10.1007/BF01540916. [DOI] [PubMed] [Google Scholar]
- 49.Ertesvag A, Aasheim HC, Naderi S, Blomhoff HK. Vitamin A potentiates CpG-mediated memory B-cell proliferation and differentiation: involvement of early activation of p38MAPK. Blood. 2007;109:3865–3872. doi: 10.1182/blood-2006-09-046748. [DOI] [PubMed] [Google Scholar]
- 50. Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 2007;179:3504–3514. doi: 10.4049/jimmunol.179.6.3504. This paper shows that DCs can efficiently store retinoic acid, which can be ‘released’ during lymphocyte activation.
- 51.Lomo J, et al. RAR-, not RXR, ligands inhibit cell activation and prevent apoptosis in B-lymphocytes. J. Cell. Physiol. 1998;175:68–77. doi: 10.1002/(SICI)1097-4652(199804)175:1<68::AID-JCP8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 52.Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by 14-hydroxy-4, 14-retro-retinol. Science. 1991;254:1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- 53.O’Connell MJ, et al. Retro-retinoids in regulated cell growth and death. J. Exp. Med. 1996;184:549–555. doi: 10.1084/jem.184.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darmanin S, et al. All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J. Immunol. 2007;179:4616–4625. doi: 10.4049/jimmunol.179.7.4616. [DOI] [PubMed] [Google Scholar]
- 55. Geissmann F, et al. Retinoids regulate survival and antigen presentation by immature dendritic cells. J. Exp. Med. 2003;198:623–634. doi: 10.1084/jem.20030390. This paper shows that retinoic acid can also act on DCs, modulating their antigen-presenting capacity.
- 56.Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80:581–586. [PMC free article] [PubMed] [Google Scholar]
- 57.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 58.Nikawa T, et al. Vitamin A prevents the decline in immunoglobulin A and Th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J. Nutr. 1999;129:934–941. doi: 10.1093/jn/129.5.934. [DOI] [PubMed] [Google Scholar]
- 59.Lovett-Racke AE, Racke MK. Retinoic acid promotes the development of Th2-like human myelin basic protein-reactive T cells. Cell. Immunol. 2002;215:54–60. doi: 10.1016/s0008-8749(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 60.Dawson HD, et al. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J. Immunol. 2008;180:1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- 62.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J. Nutr. 2002;132:3736–3739. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- 63.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nature Rev. Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 64. Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. This paper shows that retinoic acid enhances TReg-cell differentiation while blocking differentiation towards TH17 cells.
- 65. Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070719. This paper shows that retinoic acid enhances TReg -cell differentiation and imprints TReg cells with gut tropism.
- 66. Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. This paper demonstrates that lamina propria DCs induce de novo TReg -cell differentiation in a retinoic acid-dependent manner.
- 67.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 68.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 69.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 70. Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-b and induction of the nuclear receptor RORγt. Nature Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. This paper re-assesses the role of TGFβ in human TH17-cell differentiation, showing that, similar to mouse TH17 cells, human TH17 cells also require this cytokine for their differentiation.
- 71. Ivanov II, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. This paper demonstrates that the transcription factor RORγt is necessary and sufficient for TH17-cell differentiation.
- 72.Snapper CM, Marcu KB, Zelazowski P. The immunoglobulin class switch: beyond “accessibility”. Immunity. 1997;6:217–223. doi: 10.1016/s1074-7613(00)80324-6. [DOI] [PubMed] [Google Scholar]
- 73.Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm. Bowel Dis. 2008;14:275–289. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- 74.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato A, et al. CD11b+ Peyer’s patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J. Immunol. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 76. Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. This paper shows that retinoic acid is a mechanistic link between gut-homing imprinting of B cells and differentiation of IgA-secreting cells.
- 77.Travis MA, et al. Loss of integrin αv β8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 79. Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. This paper shows that iNOS is required for IgA production in all mucosal compartments. It is also reported that gut-associated DCs express iNOS in a TLR-dependent manner.
- 80.Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell. Immunol. 1999;192:41–47. doi: 10.1006/cimm.1998.1438. [DOI] [PubMed] [Google Scholar]
- 81.Nikawa T, et al. Impaired vitamin A-mediated mucosal IgA response in IL-5 receptor-knockout mice. Biochem. Biophys. Res. Commun. 2001;285:546–549. doi: 10.1006/bbrc.2001.5138. [DOI] [PubMed] [Google Scholar]
- 82.Ramsay AJ, et al. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 83. Bjersing JL, Telemo E, Dahlgren U, Hanson LA. Loss of ileal IgA+ plasma cells and of CD4+ lymphocytes in ileal Peyer’s patches of vitamin A deficient rats. Clin. Exp. Immunol. 2002;130:404–408. doi: 10.1046/j.1365-2249.2002.02009.x. This paper shows that vitamin A depletion markedly decreases the number of IgA-antibody-secreting cells in the rat ileum.
- 84.Kuwabara K, Shudo K, Hori Y. Novel synthetic retinoic acid inhibits rat collagen arthritis and differentially affects serum immunoglobulin subclass levels. FEBS Lett. 1996;378:153–156. doi: 10.1016/0014-5793(95)01440-3. [DOI] [PubMed] [Google Scholar]
- 85.Zou F, et al. Retinoic acid activates human inducible nitric oxide synthase gene through binding of RARα/RXRα heterodimer to a novel retinoic acid response element in the promoter. Biochem. Biophys. Res. Commun. 2007;355:494–500. doi: 10.1016/j.bbrc.2007.01.178. [DOI] [PubMed] [Google Scholar]
- 86.Seguin-Devaux C, et al. Enhancement of the inducible NO synthase activation by retinoic acid is mimicked by RARα agonist in vivo. Am. J. Physiol. Endocrinol. Metab. 2002;283:E525–E535. doi: 10.1152/ajpendo.00008.2002. [DOI] [PubMed] [Google Scholar]
- 87.Bergqvist P, Gardby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 2006;177:7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 88.Sirisinha S, Darip MD, Moongkarndi P, Ongsakul M, Lamb AJ. Impaired local immune response in vitamin A-deficient rats. Clin. Exp. Immunol. 1980;40:127–135. [PMC free article] [PubMed] [Google Scholar]
- 89.Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunology. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 90.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 93.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 94.Johansson-Lindbom B, et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mora JR, et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dudda JC, et al. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J. Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 97. McDermott MR, et al. Impaired intestinal localization of mesenteric lymphoblasts associated with vitamin A deficiency and protein-calorie malnutrition. Immunology. 1982;45:1–5. This paper shows that vitamin A depletion decreases the migration of mesenteric lymph node lymphoblasts to the small bowel (but not the colon).
- 98.Chang SY, et al. Colonic patches direct the cross-talk between systemic compartments and large intestine independently of innate immunity. J. Immunol. 2008;180:1609–1618. doi: 10.4049/jimmunol.180.3.1609. [DOI] [PubMed] [Google Scholar]
- 99. Yamanaka KI, et al. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J. Allergy Clin. Immunol. 2007;121:148–157. doi: 10.1016/j.jaci.2007.08.014. This paper shows that 1,25(OH)2VD3 decreases the expression of ligands for E- and P-selectin, inhibits T-cell homing to the inflamed skin and decreases the expression of gut-homing receptors.
- 100.Mora JR, von Andrian UH. Retinoic acid: an educational “vitamin elixir” for gut-seeking T cells. Immunity. 2004;21:458–460. doi: 10.1016/j.immuni.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp. Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 102.Maly P, et al. The α1,3 fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 103.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J. Neurosci. Res. 2007;85:2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 104.Giarratana N, et al. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J. Immunol. 2004;173:2280–2287. doi: 10.4049/jimmunol.173.4.2280. [DOI] [PubMed] [Google Scholar]
- 105.Singh U, Devaraj S. Vitamin E: inflammation and atherosclerosis. Vitam. Horm. 2007;76:519–549. doi: 10.1016/S0083-6729(07)76020-X. [DOI] [PubMed] [Google Scholar]
- 106.Jialal I, Devaraj S, Kaul N. The effect of α-tocopherol on monocyte proatherogenic activity. J. Nutr. 2001;131:389S–394S. doi: 10.1093/jn/131.2.389S. [DOI] [PubMed] [Google Scholar]
- 107.Devaraj S, Jialal I. α-tocopherol decreases interleukin-1 β release from activated human monocytes by inhibition of 5-lipoxygenase. Arterioscler. Thromb. Vasc. Biol. 1999;19:1125–1133. doi: 10.1161/01.atv.19.4.1125. [DOI] [PubMed] [Google Scholar]
- 108.Munteanu A, Zingg JM. Cellular, molecular and clinical aspects of vitamin E on atherosclerosis prevention. Mol. Aspects Med. 2007;28:538–590. doi: 10.1016/j.mam.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Cominacini L, et al. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic. Biol. Med. 1997;22:117–127. doi: 10.1016/s0891-5849(96)00271-7. [DOI] [PubMed] [Google Scholar]
- 110.Yoshikawa T, et al. α-tocopherol protects against expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 1998;7:15–19. doi: 10.1002/biof.5520070103. [DOI] [PubMed] [Google Scholar]
- 111.Winkler C, Schroecksnadel K, Schennach H, Fuchs D. Vitamin C and E suppress mitogen-stimulated peripheral blood mononuclear cells in vitro. Int. Arch. Allergy Immunol. 2007;142:127–132. doi: 10.1159/000096438. [DOI] [PubMed] [Google Scholar]
- 112.Li-Weber M, et al. Vitamin E inhibits CD95 ligand expression and protects T cells from activation-induced cell death. J. Clin. Invest. 2002;110:681–690. doi: 10.1172/JCI15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nature Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 114.Ichikawa H, et al. A novel vitamin E derivative (TMG) protects against gastric mucosal damage induced by ischemia and reperfusion in rats. Dig. Dis. Sci. 2003;48:54–58. doi: 10.1023/a:1021778229997. [DOI] [PubMed] [Google Scholar]
- 115.Onem G, et al. Neuroprotective effects of L-carnitine and vitamin E alone or in combination against ischemia-reperfusion injury in rats. J. Surg. Res. 2006;131:124–130. doi: 10.1016/j.jss.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 116.Terasawa Y, et al. Increased atherosclerosis in hyperlipidemic mice deficient in α -tocopherol transfer protein and vitamin E. Proc. Natl Acad. Sci. USA. 2000;97:13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ozcan AV, et al. The effects of iloprost and vitamin C on kidney as a remote organ after ischemia/reperfusion of lower extremities. J. Surg. Res. 2007;140:20–26. doi: 10.1016/j.jss.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 118.Lehr HA, Frei B, Arfors KE. Vitamin C prevents cigarette smoke-induced leukocyte aggregation and adhesion to endothelium in vivo. Proc. Natl Acad. Sci. USA. 1994;91:7688–7692. doi: 10.1073/pnas.91.16.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lehr HA, Frei B, Olofsson AM, Carew TE, Arfors KE. Protection from oxidized LDL-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by vitamin C but not by vitamin E. Circulation. 1995;91:1525–1532. doi: 10.1161/01.cir.91.5.1525. [DOI] [PubMed] [Google Scholar]
- 120.Endo N, et al. Antioxidant activity of vitamin B6 delays homocysteine-induced atherosclerosis in rats. Br. J. Nutr. 2006;95:1088–1093. doi: 10.1079/bjn20061764. [DOI] [PubMed] [Google Scholar]
- 121.Thomson MJ, Puntmann V, Kaski JC. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc. Drugs Ther. 2007;21:195–210. doi: 10.1007/s10557-007-6027-1. [DOI] [PubMed] [Google Scholar]
- 122.Lentz SR, Piegors DJ, Malinow MR, Heistad DD. Supplementation of atherogenic diet with B vitamins does not prevent atherosclerosis or vascular dysfunction in monkeys. Circulation. 2001;103:1006–1011. doi: 10.1161/01.cir.103.7.1006. [DOI] [PubMed] [Google Scholar]
- 123.Littorin B, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 124.Muller K, et al. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. Clin. Rheumatol. 1995;14:397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 125.Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun. Rev. 2007;7:59–64. doi: 10.1016/j.autrev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 126.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J. Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 127.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 128.Yener E, Coker C, Cura A, Keskinoglu A, Mir S. Lymphocyte subpopulations in children with vitamin D deficient rickets. Acta Paediatr. Jpn. 1995;37:500–502. doi: 10.1111/j.1442-200x.1995.tb03362.x. [DOI] [PubMed] [Google Scholar]
- 129.Abe J, et al. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3, 22-oxa-1α, 25-dihydroxyvitamin D3. J. Nutr. Sci. Vitaminol. (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 130.Lemire JM, Ince A, Takashima M. 1,25-dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12:143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 131.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J. Clin. Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 133.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cantorna MT, Hayes CE, DeLuca HF. 1,25-dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J. Nutr. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 135.Barna M, Bos JD, Kapsenberg ML, Snijdewint FG. Effect of calcitriol on the production of T-cell-derived cytokines in psoriasis. Br. J. Dermatol. 1997;136:536–541. [PubMed] [Google Scholar]
- 136.Durakovic C, Ray S, Holick MF. Topical paricalcitol (19-nor-1α, 25-dihydroxyvitamin D2) is a novel, safe and effective treatment for plaque psoriasis: a pilot study. Br. J. Dermatol. 2004;151:190–195. doi: 10.1111/j.1365-2133.2004.06002.x. [DOI] [PubMed] [Google Scholar]
- 137.Li M, et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl Acad. Sci. USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lemire JM, et al. Prolongation of the survival of murine cardiac allografts by the vitamin D3 analogue 1,25-dihydroxy-δ16-cholecalciferol. Transplantation. 1992;54:762–763. doi: 10.1097/00007890-199210000-00046. [DOI] [PubMed] [Google Scholar]
- 139.Amuchastegui S, Daniel KC, Adorini L. Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation. 2005;80:81–87. doi: 10.1097/01.tp.0000164619.49828.7a. [DOI] [PubMed] [Google Scholar]
- 140.Zhang AB, Zheng SS, Jia CK, Wang Y. Effect of 1,25-dihydroxyvitamin D3 on preventing allograft from acute rejection following rat orthotopic liver transplantation. World J. Gastroenterol. 2003;9:1067–1071. doi: 10.3748/wjg.v9.i5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gregori S, et al. Regulatory T cells induced by 1α, 25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 142.Pakkala I, Taskinen E, Pakkala S, Raisanen-Sokolowski A. MC1288, a vitamin D analog, prevents acute graft-versus-host disease in rat bone marrow transplantation. Bone Marrow Transplant. 2001;27:863–867. doi: 10.1038/sj.bmt.1702873. [DOI] [PubMed] [Google Scholar]
- 143.Hullett DA, et al. Prevention of chronic allograft nephropathy with vitamin D. Transpl. Int. 2005;18:1175–1186. doi: 10.1111/j.1432-2277.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 144.Middleton PG, et al. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30:223–228. doi: 10.1038/sj.bmt.1703629. [DOI] [PubMed] [Google Scholar]
- 145.Sezer S, Uyar M, Arat Z, Ozdemir FN, Haberal M. Potential effects of 1,25-dihydroxyvitamin D3 in renal transplant recipients. Transplant. Proc. 2005;37:3109–3111. doi: 10.1016/j.transproceed.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 146.Caravaca F, et al. Are plasma 1,25-dihydroxyvitamin D3 concentrations appropriate after successful kidney transplantation? Nephrol. Dial. Transplant. 1998;13 Suppl. 3:91–93. doi: 10.1093/ndt/13.suppl_3.91. [DOI] [PubMed] [Google Scholar]
- 147.Fleseriu M, Licata AA. Failure of successful renal transplant to produce appropriate levels of 1,25-dihydroxyvitamin D. Osteoporos. Int. 2007;18:363–368. doi: 10.1007/s00198-006-0238-3. [DOI] [PubMed] [Google Scholar]
- 148.Cantorna MT, et al. 1,25-dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation. 1998;66:828–831. doi: 10.1097/00007890-199810150-00003. [DOI] [PubMed] [Google Scholar]
- 149.Smith EA, et al. Effects of long-term administration of vitamin D3 analogs to mice. J. Endocrinol. 2000;165:163–172. doi: 10.1677/joe.0.1650163. [DOI] [PubMed] [Google Scholar]
- 150. Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. 1983;2:585–588. doi: 10.1016/s0140-6736(83)90677-3. References 149 and 150 provide epidemiological evidence that indicate a role for vitamin A in preventing child mortality in developing countries.
- 151.Sommer A, et al. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169–1173. doi: 10.1016/s0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- 152.Semba RD, et al. Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus-infected children in Uganda: a controlled clinical trial. Nutrition. 2005;21:25–31. doi: 10.1016/j.nut.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 153.Racke MK, et al. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J. Immunol. 1995;154:450–458. [PubMed] [Google Scholar]
- 154.Wang T, et al. The effect of Am-80, one of retinoids derivatives on experimental allergic encephalomyelitis in rats. Life Sci. 2000;67:1869–1879. doi: 10.1016/s0024-3205(00)00776-1. [DOI] [PubMed] [Google Scholar]
- 155.Brinckerhoff CE, Coffey JW, Sullivan AC. Inflammation and collagenase production in rats with adjuvant arthritis reduced with 13-cis-retinoic acid. Science. 1983;221:756–758. doi: 10.1126/science.6308759. [DOI] [PubMed] [Google Scholar]
- 156.Nagai H, et al. Effect of Am-80, a synthetic derivative of retinoid, on experimental arthritis in mice. Pharmacology. 1999;58:101–112. doi: 10.1159/000028272. [DOI] [PubMed] [Google Scholar]
- 157.Escribese MM, et al. Therapeutic effect of all-trans-retinoic acid (at-RA) on an autoimmune nephritis experimental model: role of the VLA-4 integrin. BMC Nephrol. 2007;8:3. doi: 10.1186/1471-2369-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van de Kerkhof PC. Update on retinoid therapy of psoriasis in: an update on the use of retinoids in dermatology. Dermatol. Ther. 2006;19:252–263. doi: 10.1111/j.1529-8019.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- 159.Ruzicka T, et al. Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: results of a randomized, double-blind, placebo-controlled, multicenter trial. Arch. Dermatol. 2004;140:1453–1459. doi: 10.1001/archderm.140.12.1453. [DOI] [PubMed] [Google Scholar]
- 160.Niwa S, et al. Effect of Am-80, a retinoid derivative, on 2, 4-dinitrofluorobenzene-induced contact dermatitis in mice. Pharmacology. 2000;60:208–214. doi: 10.1159/000028371. [DOI] [PubMed] [Google Scholar]
- 161.Stephensen CB, Moldoveanu Z, Gangopadhyay NN. Vitamin A deficiency diminishes the salivary immunoglobulin A response and enhances the serum immunoglobulin G. response to influenza A virus infection in BALB/c mice. J. Nutr. 1996;126:94–102. doi: 10.1093/jn/126.1.94. [DOI] [PubMed] [Google Scholar]
- 162.Siewert C, et al. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 163.Buck J, et al. Anhydroretinol: a naturally occurring inhibitor of lymphocyte physiology. J. Exp. Med. 1993;178:675–680. doi: 10.1084/jem.178.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.