Abstract
Associative processing in the cerebral hemispheres was examined using ERPs and visual half-field (VF) methods. Associative strength was manipulated using asymmetrically associated pairs: viewed in one order (forward), there was a strong prime-to-target association, but in the backward order predictability was weak. N400 priming was greater for forward than backward pairs in both VFs and not different across VF, suggesting similar semantic representations and automatic meaning activation in the two hemispheres. However, a frontal P2 enhancement for forward pairs restricted to the LH suggests that it uses context to predict likely upcoming words. Also, greater late positive complex priming for backward pairs in the LH than the RH reveals a LH advantage for strategically reshaping meaning activation for weakly related and/or non-canonically ordered pairs. The results link asymmetries in word processing with those observed at the sentence level.
Introduction
Research investigating the neural bases of language processing has revealed that, despite robust left hemisphere (LH) dominance for important aspects of language production, the right hemisphere (RH) also contributes in significant and complementary ways to language comprehension (see reviews in Beeman & Chiarello, 1998). Studies of patients with RH damage have uncovered a number of comprehension deficits, such as difficulty understanding non-literal language (e.g., Brownell, 1988; Klepousniotou & Baum, 2005), processing indirect requests (e.g., Stemmer, Giroux, & Joanette, 1994), comprehending humor (e.g., Bihrle, Brownell, Powelson, & Gardner, 1986), and drawing inferences (e.g., Beeman, 1993). Even word-level comprehension seems to be affected. For instance, Brownell and colleagues found that when presented with words (warm) that have both literal meanings (meaning related to blanket) and connotative meanings (meaning related to loving), patients with RH damage were selectively impaired in activating the connotative meanings, perhaps pointing to a deficit in processing less expected information (Brownell, Potter, Michelow, & Gardner, 1984; Brownell, Simpson, Bihrle, Potter, & Gardner, 1990; for an alternative view, see Tompkins, 1990; Gagnon, Goulet, Giroux, & Joanette, 2003).
Studies of differential hemispheric contributions to meaning apprehension have also been extended to individuals with intact brains (e.g., Beeman, 1993; Titone, 1998; Coulson, Federmeier, Van Petten, & Kutas, 2005) using the visual half-field (VF) presentation technique. In this technique, stimuli are presented in the visual periphery, such that they are apprehended and initially processed by the contralateral cerebral hemisphere; that is, stimuli presented in the right visual field (RVF) are initially apprehended by the LH and stimuli presented in the left visual field (LVF) are initially apprehended by the RH. The resulting temporal and information-quality advantage afforded to the directly stimulated hemisphere induces processing biases, which have been successfully exploited to examine hemispheric asymmetries in lexico-semantic processing at multiple levels.
A number of hemispheric differences in semantic processing have been documented in studies using the VF technique, including, for example, greater priming (facilitation) for unassociated, categorically-related word pairs (dog-goat) with LVF/RH as compared to RVF/LH presentation (Chiarello, Burgess, Richards, & Pollock, 1990). Theories that have been put forward to explain such asymmetries have broadly fallen into two categories: those that posit that the two hemispheres differ in their basic semantic representations and/or automatic semantic activation processes, and those that propose that differences arise, instead, in the controlled mechanisms that are recruited to integrate incoming semantic information with current context and/or to reshape the initially-formed semantic representation.
As an example of the former type of account, Deacon and her colleagues have proposed that there are fundamental differences in how each hemisphere represents semantic information (Deacon et al., 2004). They hypothesize that concepts are represented in a localist, associative network in the LH but in a distributed, feature-based network in the RH. Evidence for this view comes from VF studies with neural (ERP: event-related brain potential) measures of semantic priming that showed that purely lexically-associated word pairs (dog-bone) were facilitated only in the RVF/LH whereas unassociated, categorically-related word pairs with strong feature overlap (mosquito-flea) were selectively facilitated in the LVF/RH (Deacon et al., 2004; Grose-Fifer & Deacon, 2004).
Although a number of studies have found greater or selective activation of categorically-related words in the RH (Chiarello et al., 1990; Chiarello & Richards, 1992; Deacon et al., 2004; Grose-Fifer & Deacon, 2004), other studies have found significant – and even preferential – LH priming for these type of word pairs under conditions that facilitate automatic semantic processing (Abernethy & Coney, 1990; Koivisto, 1997; Collins, 1999; see also, Abernathy & Coney, 1996; Koivisto, 1998). Likewise, the relatively fewer studies that have investigated lexical associative processes in the two hemispheres have revealed that the RH, much like the LH, is capable of appreciating relationships between words based on association (Chiarello et al., 1990; Coney, 2002; Coulson et al., 2005). These findings are difficult to reconcile with accounts that posit a fundamental difference in the conceptual structure instantiated in the two hemispheres.
Other views place the locus of semantic processing asymmetries, not in the nature of the information stored in semantic memory as such, but rather in how conceptual information initially becomes active. One account of this type is the “coarse coding hypothesis” (Jung-Beeman, 2005), which proposes that the LH and the RH differ in the granularity of their semantic activation. In particular, the coarse coding hypothesis suggests that the LH activates a narrow set of concepts strongly related to a given input, whereas the RH activates a broad range of concepts including those that may only be weakly related. Data showing that although strongly associated word pairs (sofa-chair) from a category are primed in both hemispheres, unassociated pairs from the same category (lamp-chair) are primed only in the LVF/RH have been taken as support for coarse coding (Chiarello et al., 1990; Chiarello, 1991). These results suggest that the LH may have a propensity to look for close semantic relationships between words, treating weakly related information as if it was unrelated. Other evidence in line with coarse coding has come from the summation-priming paradigm, in which participants saw a lateralized target (wedding) preceded by three weakly related (white-ceremony-tuxedo) or unrelated primes (Beeman et al., 1994). The results revealed facilitation in naming accuracy for targets preceded by weakly related information only in the LVF/RH. This pattern is explained by coarse coding as resulting from more diffuse activation in the RH, allowing activation to spread to the target even from more distantly related concepts.
Although these studies and others (Faust & Lavidor, 2003; Coulson & Williams, 2005) have pointed to a RH advantage for processing weakly-related information under some circumstances, other studies have failed to find evidence consistent with the central tenet of the coarse coding hypothesis that the RH activates a broader set meanings (Richards & Chiarello, 1995; Livesay & Burgess, 2003; Meyer & Federmeier, 2007; Kandhadai & Federmeier, 2007, 2008). For instance, prior work with behavioral and ERP measures revealed no evidence for hemispheric differences in the automatic activation of multiple, divergent meanings of words (Kandhadai & Federmeier, 2007, 2008) and even evidence for LH dominance in processing the secondary sense of words with multiple meanings (Meyer & Federmeier, 2007). However, measures of more effortful semantic analysis have found some data suggesting that meaning selection may be more focal in the LH (Meyer & Federmeier, 2008).
Indeed, the second broad class of theories has placed the locus of hemispheric asymmetries at stages beyond initial semantic activation, suggesting that differences arise from how attentionally-driven control processes come online to shape that initial activation over time (Chiarello, Senehi, & Nuding, 1987; Burgess & Simpson, 1988; Nakagawa, 1991; Chiarello, Richards, & Pollock, 1992; for an alternate view, see, Koivisto, 1999; Koivisto & Laine, 2000). For example, some have suggested that a broader range of meanings are ultimately maintained in the RH because only the LH is able to use controlled processes to inhibit contextually-irrelevant information, thereby acting to narrow the range of the final set of active meanings (Burgess & Simpson, 1988; Nakagawa, 1991). Burgess and Simpson (1988), for instance, investigated priming for the dominant and subordinate meaning of ambiguous words at short (35 ms) and long (750 ms) stimulus onset asynchronies (SOAs). For RVF/LH targets, their results showed facilitation for the dominant meaning at both SOAs, with facilitation for the subordinate meaning declining at the longer SOA compared to the shorter SOA. In fact, at the long SOA, response times to subordinate meanings were actually longer than response times to unrelated words, suggesting a LH mechanism that actively inhibits the non-dominant meanings of ambiguous words. However, for LVF/RH targets, the results showed decay in facilitation for the dominant meaning and increased facilitation for the subordinate meaning at the longer SOA. These patterns of priming suggest that controlled processes may act to establish a more constrained set of meanings in the LH as compared with the RH.
Hemispheric differences in processing larger language units, such as the ability to glean message level information from sentences (e.g., Faust & Chiarello, 1998) and/or to use sentence context information to prepare for the processing of upcoming words (e.g., Federmeier & Kutas, 1999; Wlotko & Federmeier, 2007) have been attributed to similar factors. For example, one such account, the “PARLO (Production Affects Reception in Left Only)” framework (Federmeier, 2007), posits that the LH engages in predictive preactivation of likely upcoming information based on the current context. The RH, instead, is suggested to process word meanings in a more bottom-up fashion, thus integrating incoming information with the current context in a posthoc fashion. Across word and sentence processing, these accounts share in common the idea that the LH has different or more efficacious mechanisms for controlled semantic processing.
In sum, hemispheric asymmetries in semantic processing have been alternately ascribed to representational differences, differences in the breadth of automatic semantic activation, and differences in the recruitment of controlled top-down semantic mechanisms to apprehend meaning. Of course, these accounts are not mutually exclusive, as hemispheric asymmetries at multiple processing stages could collectively contribute to the semantic processing differences that have been observed. Developing our understanding of how semantic processing unfolds in the two hemispheres would thus be enhanced by the use of a continuous multidimensional measure that can track semantic activation over time. Such a measure would allow the differentiation of effects arising at the level of automatic semantic activation and those arising through the influence of more strategic processing mechanisms. In the present study, we do this through the measurement of event-related brain potentials (ERPs). ERPs also have the advantage of providing a measure that can be collected in the absence of any on-line behavioral task (allowing a cleaner separation of general semantic processing differences from task-specific asymmetries) and that provides information about eye movements (allowing one to ascertain that participants maintain central fixation during lateralized presentation).
The literature using ERPs to study semantic processing, including the relatively small set of studies that has specifically looked at word level processing asymmetries (e.g., Atchley & Kwasny, 2003; Deacon et al., 2004; Coulson et al., 2005; Bouaffre & Faïta-Ainseba, 2007; Kandhadai & Federmeier, 2008), points to several aspects of the ERP signal that are likely to be informative about how the two hemispheres glean meaning from words. First, effects of language manipulations can sometimes be seen in the form of amplitude modulations of the frontal P2, a component that peaks about 225 ms after stimulus onset and that has been linked to higher order visual processing (e.g., Luck & Hillyard, 1994). The P2 is part of the normal visual evoked potential, the series of voltage deflections that is elicited by the apprehension of any visual stimulus and that reflects perceptual analysis and the allocation of visuo-spatial attention. Constraining contextual information that renders some perceptual information more likely and/or that changes participants' attentional state can affect the P2 to words, and has been shown to do so in a manner that varies across the two hemispheres (Federmeier, Mai, & Kutas, 2005; Wlotko & Federmeier, 2007; Huang, Lee, & Federmeier, submitted). In particular, frontal P2 responses to stimuli presented in the RVF (but not the LVF) have been shown to be larger in more constraining contexts and have been linked to LH mechanisms that prepare the language processing system for the apprehension of predictable words.
Another ERP component that is especially useful in the investigation of relatively automatic aspects of meaning apprehension is the N400. The N400 is a negative-going potential, distributed over centro-posterior scalp sites, that peaks approximately 400 ms after the onset of any meaningful or potentially meaningful stimulus (Kutas & Hillyard, 1980b). The amplitude of the N400 is sensitive to factors known to affect ease of semantic access, including repetition, word frequency, and the presence of supportive context information (for a review, see Kutas & Federmeier, 2000), including manipulations of semantic priming (Bentin, McCarthy, & Wood, 1985). However, it is insensitive to most syntactic (Kutas & Hillyard, 1983) or perceptual (Kutas & Hillyard, 1980a) manipulations, making it a functionally specific index of semantic processing. Effects on the N400 seem to be occurring implicitly, during relatively automatic stages of processing, because they can be observed even under masked stimulus presentation conditions (Deacon, Hewitt, Yang, & Nagata, 2000; Misra & Holcomb, 2003; for an alternate view, see Brown & Hagoort, 1993), during the attentional blink (Rolke, Heil, Streb, & Henninghausen, 2001), and during implicit recognition in amnesia (Olichney et al., 2000).
Whereas P2 and N400 effects seem to reflect initial aspects of perceptual and semantic processing, respectively, they are followed by components that have been shown to be quite sensitive to more explicit and effortful aspects of processing. One such component, which we will refer to as the Late Positive Complex (LPC), is an extended, posterior positivity that has been observed in a wide range of memory and language processing studies. In the memory literature, the LPC has been linked to explicit recollection, as, unlike the N400, it is affected by brain damage producing amnesia (e.g., Olichney et al., 2000; Düzel, Vargha-Khadem, Heinze, & Mishkin, 2001). In syntactic processing studies, where late postivity has often been referred to as the P600, it has been linked to more effortful syntactic reanalysis (e.g., Osterhout & Holcomb, 1992; Hagoort, Brown, & Groothusen, 1993) or integration (Kaan, Harris, Gibson, & Holcomb, 2000) likely to occur in ungrammatical, syntactically ambiguous, or syntactically complex sentences. Finally, in the semantic processing literature, the LPC has been associated with explicit aspects of semantic retrieval, integration, and revision (Van Petten, Kutas, Kluender, Mitchiner, & McIsaac, 1991; Swaab, Brown, & Hagoort, 1998). This positivity has also been linked to the controlled detection of thematic violations (e.g., mouse chasing the cat) and restructuring of thematic elements in sentences (Kolk, Chwilla, Van Herten, & Oor, 2003; Hoeks, Stowe, & Doedens, 2004). Thus, the LPC has been implicated in a wide-variety of attentionally-driven, controlled linguistic processes such as violation-detection, revision, and integration.
Thus, given that ERPs provide concurrent measures of perception, semantic activation, and semantic selection/revision, our goal in the present study was to further elucidate the source(s) of hemispheric asymmetries in semantic processing. In particular, we combined ERPs with VF presentation in order to investigate each hemisphere's sensitivity to lexical associative relations, which have been less well-studied than feature-based similarity. Participants saw pairs of words that were asymmetrically associated (e.g., mortgage-house), affording a manipulation of associative strength while keeping semantic content relatively constant. In the forward direction, there was a strong association from prime to target, such that the target was the primary associate of the prime. However, when these pairs were presented in the backward direction, the association from prime to target was weak. Pairs were thematically related and shared minimal or no semantic features in common. Participants saw central primes followed by lateralized targets and were told to pay attention to all the items that appeared on the screen, as their memory for the word pairs would be tested later (in an offline cued recall task).
The use of pure lexical associates (without feature overlap) allows a test of Deacon et al.'s (2004) hypothesis that localist associative networks are confined to the LH, resulting in impoverished lexical associative processes in the RH. This theory would thus predict N400 priming only for RVF/LH trials, likely greater for the strongly associated forward pairs than the weakly associated backward pairs. In contrast, the coarse coding hypothesis (Jung-Beeman, 2005) posits that the RH more strongly activates words that are only weakly related to a given cue. The use of lexical associations has the advantage of providing a quantifiable measure of the strength of the semantic relationship between the words in the pairs. In the present design, then, the coarse coding hypothesis would predict greater N400 facilitation, indicative of stronger automatic semantic activation, when the targets from the weakly associated backward pairs are presented in the LVF/RH as compared with the RVF/LH. More focal semantic activation in the LH might correspondingly predict greater RVF than LVF priming for the strongly associated forward pairs.
Finally, because lexical association is defined by the predictability of the target given the prime, this paradigm has the potential to capture, in a word priming paradigm, the kind of control processes that have been implicated as one source for semantic processing asymmetries. For example, the forward condition should afford the possibility for prediction, which, in sentence processing studies has been associated with P2 enhancements specific to RVF/LH presentation (Federmeier et al., 2005; Wlotko & Federmeier, 2007). In contrast, in the backward condition, where the prime-to-target association is weak, semantic integration processes might be recruited to allow an appreciation of the strong semantic relationship in the target-to-prime direction. Such effortful semantic processing would be expected to manifest as increases in the LPC component, which some theories (e.g., Nakagawa, 1991; Chiarello, 1998) would postulate should be more prevalent for RVF/LH than LVF/RH trials.
Methods
Participants
Thirty-two right-handed native English speakers (17 men and 15 women) between the ages of 18 and 27 (mean age: 20.5 yrs) participated and received either cash or course credit for their time. None had exposure to any other language before age five. All participants were right-handed, with 11 reporting left-handed family members. Mean handedness quotient was 0.82 (range: 0.35-1.0) as measured by the Edinburgh handedness inventory (Oldfield, 1971), where “1” is strongly right handed and “-1” is strongly left-handed. Participants were screened for normal vision and had no history of neuropsychological or psychiatric disorders.
Stimuli
Asymmetrically associated word pairs (e.g., mortgage-house) were chosen based on association norms (Nelson, McEvoy, & Schreiber, 1998) with the criterion that each word pair was strongly associated (> 27%, mean: 41.2, range: 27.5-81.4) in one direction but weakly associated (<3%, mean: 0.6, range: 0-2.9) in the other direction. Of these, a final set of one hundred and twenty-eight thematically-related prime-target pairs were chosen based on screening by seven raters to remove all pairs that had synonymous, part-whole, or category-based relationships or that were deemed to share semantic features in common. Thematic relationships were defined as those shared between words that are likely to be encountered together either in a scene or in a text. Examples are given in Table 1. Half of these word-pairs (sixty-four items) appeared in the forward direction (forward pairs), such that there was a strong association from the prime to the target. The other half appeared in the backward direction (backward pairs), such that there was a weak association from the prime to the target. A second set of one hundred and twenty-eight unrelated prime-target pairs were then constructed, using the MRC Psycholinguistic Database (Coltheart, 1987) to allow systematic matching between each related prime and target and its unrelated counterpart for part of speech, length, and frequency (Kucera & Francis, 1967). Lexical characteristics of prime-target pairs for each direction (forward, backward) in related and unrelated conditions are provided in Table 2.
Table 1. Sample stimuli.
| List1 | |||
|---|---|---|---|
| Forward | Backward | ||
| Related | Unrelated | Related | Unrelated |
| Bible-God | Chest-Law | Blood-Vampire | Truth-Skipper |
| Poison-Death | Puzzle-Local | House-Mortgage | Cheese-Intruder |
| Harbor-Boat | Cotton-Site | Sleep-Pillow | Beach-Monkey |
| Sleigh-Snow | Nibble-Lie | Fish-Hook | Tool-Muse |
| Bandage-Cut | Incline-Art | Smoke-Pipe | Steel-Mess |
| List 2 | |||
| Forward | Backward | ||
| Related | Unrelated | Related | Unrelated |
| Vampire-Blood | Skipper-Truth | God-Bible | Law-Chest |
| Mortgage-House | Intruder-Cheese | Death-Poison | Local-Puzzle |
| Pillow-Sleep | Monkey-Beach | Boat-Harbor | Site-Cotton |
| Hook-Fish | Muse-Tool | Snow-Sleigh | Lie-Nibble |
| Pipe-Smoke | Mess-Steel | Cut-Bandage | Art-Incline |
Table 2. Mean values (with standard deviations in parentheses) of written log frequency and length for primes and targets in each relatedness condition.
| Forward primes / Backward targets | Forward targets / Backward primes | |||
|---|---|---|---|---|
| Relatedness type | Written log frequency | Word length | Written log frequency | Word length |
| Related | 0.84 (0.66) | 5.75 (1.30) | 1.78(0.62) | 4.53(1.15) |
| Unrelated | 0.82 (0.67) | 5.73 (1.29) | 1.78 (0.62) | 4.63 (1.16) |
Half of the targets in each condition (forward and backward for both related and unrelated) appeared in each VF. A second list was created in which the order of the primes and targets in the original list was reversed such that the forward pairs became the backward pairs and vice versa. Across participants, the same word pairs thus appeared in both strongly and weakly associated conditions. An additional set of two lists was created in which the VF of presentation from the two original lists was reversed. The resulting four lists were counterbalanced such that, across participants, each word pair appeared the same number of times in each direction (forward, backward) in each VF (RVF, LVF).
Procedure
Each participant was tested in a single session conducted in a dim, quiet testing room. Stimuli were presented one word at a time on a 21″ SVGA monitor placed at a distance of 40″ from the participant. All stimuli were in white, upper case letters presented on a black background. A red asterisk remained in the center of the screen throughout the experiment to aid participants in maintaining central fixation. A practice block preceded the experimental session, which was divided into eight equal blocks of thirty-two trials each. Participants were asked to pay attention to both the prime and the target and were told that their memory for the word pairs would be tested at the end of each block.
Each trial began with a series of pluses to indicate the beginning of a trial; these were presented centrally for 500 ms with a random SOA of 800 to 1600 ms, used to temporally jitter anticipatory potentials. The prime was then presented foveally for 200 ms. After 750 ms, the target was presented for 200 ms, at the vertical center and lateralized so that its medial edge was two degrees from horizontal center. Targets subtended an average of 2.6 degrees of horizontal visual angle (range: 1.5 to 4 degrees) and 0.5 degrees of vertical visual angle. 1300 ms after the target, a set of four exclamation points appeared in the center of the screen for 2500 ms to allow the participant to blink their eyes before the next trial resumed. The next trial sequence then began. At the end of each block, participants were given an offline cued-recall task in which they were provided with all of the primes that appeared in that block (in randomized order) and were asked to generate the target that was paired with each prime. The purpose of this task was to ensure that participants were paying attention to the primes and targets and attempting to integrate them.
Data Collection
Participants' EEG was recorded using an electrode cap containing 26 evenly-spaced Ag/AgCl electrodes (see icon in Figure 1 for arrangement). These electrode sites include Midline Prefrontal (MiPf), Left and Right Lateral Prefrontal (LLPf and RLPf), Left and Right Medial Prefrontal (LMPf and RMPf), Left and Right Mediolateral Frontal (LDFr and RDFr), Left and Right Medial Frontal (LMFr and RMFr), Left and Right Lateral Frontal (LLFr and RLFr), Midline Central (MiCe), Left and Right Mediolateral Central (LDCe and RDCe), Left and Right Medial Central (LMCe and RMCe), Midline Parietal (MiPa), Left and Right Mediolateral Parietal (LDPa and RDPa), Left and Right Lateral Temporal (LLTe and RLTe), Left and Right Medial Occipital (LMOc and RMOc), Left and Right Lateral Occipital (LLOc and RLOc), and Midline Occipital (MiOc) scalp locations. The left mastoid was used as a reference during the on-line recording. Eye movements were monitored via electrodes placed on the outer canthus of each eye (referenced to one another) and blinks were monitored via an electrode placed on the left infraorbital ridge (referenced to the left mastoid). Electrode impedances were kept below 2 k-Ohms. Brain potentials were amplified with a Sensorium 32 channel polygraph set to a band pass of 0.02 - 100 Hz, digitized at 250 Hz, and stored on a hard disk for later analysis.
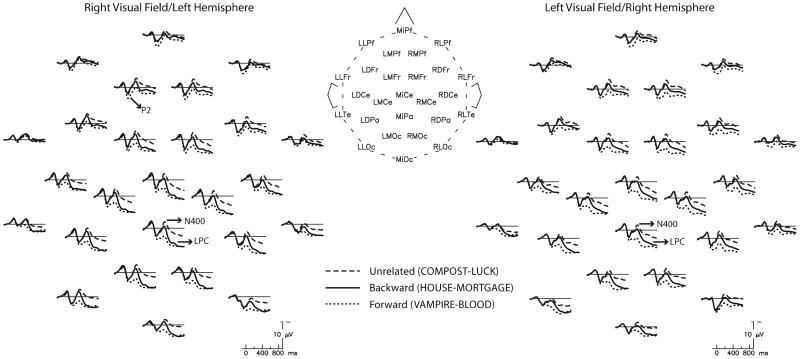
Figure 1.
Grand average (N=32) ERPs at all 26 electrode sites for target words in forward, backward and unrelated conditions. Right visual field (RVF/LH) targets are plotted on the left side of the figure and left visual field (LVF/RH) targets are plotted on the right. The head diagram shows the electrode arrangement. Negative is plotted up here and in all subsequent figures. In both VFs, N400 and LPC amplitudes were more positive for associated targets (forward and backward) compared to unrelated targets. Further, frontal P2 amplitudes to LH targets were more positive for the forward pairs than the backward pairs.
Data Analysis
Prior to analysis, the EEG was digitally filtered with a band pass of 0.2 - 20Hz (or from 0.2-8 Hz for peak measurements). ERPs were computed from 100ms before stimulus onset to 920 ms post stimulus onset. Epochs contaminated by artifacts from amplifier blocking, signal drift, or muscle activity was rejected off-line before averaging. Epochs containing eye movements were stringently rejected to ensure the validity of the VF manipulation, and those with eye blinks were corrected for those 9 participants with sufficient numbers of blinks to obtain a stable filter (Dale, 1994) and rejected otherwise. On average, 8% of trials were lost due to artifacts. Data from each participant were rereferenced offline to the algebraic mean of the left and right mastoids, and averages of artifact-free ERPs were calculated for target words in each condition after subtraction of the 100 millisecond pre-stimulus baseline.
Results
Behavior
Target responses in the off-line cued recall task were coded as correct only if participants provided the exact word that was paired with the prime. On average, participants were able to recall the exact target for 101 (39.5%) of the 256 prime words, indicating that they paid attention to the stimuli. Percent recall was subjected to an analysis of variance (ANOVA) with 2 levels of VF (RVF, LVF), 2 levels of relatedness (related, unrelated), and 2 levels of directionality (forward, backward) as factors. There was no main effect of VF (F1,31 = 2.07, p = 0.16, partial η2 = 0.063) or interactions of VF with other factors. The main effect of relatedness was significant, with more accurate recall to related targets compared to unrelated targets (64.2% vs. 14.9%, F1,31 = 652.76, p < 0.05, partial η2 = 0.95). There was also a main effect of directionality, with more accurate recall in the forward than in the backward (50.4% vs. 28.5%, F1,31 = 367.82, p < 0.05, partial η2 = 0.92) condition. Relatedness and directionality interacted (F1,31 = 99.10, p < 0.05, partial η2 = 0.76) because, as expected, the effect of directionality was primarily carried by the related condition (advantage for forward pairs was 34% in the related and 10% in the unrelated condition).
Electrophysiological Recordings
Grand average ERP waveforms for targets in each word-pair type (forward, backward, unrelated) at all 26 electrode sites are shown in Figure 1. Target words in all conditions elicited the pattern characteristic of ERPs to visual stimuli. These components included, over posterior sites, an initial positivity (P1) peaking around 50 ms, a negativity (N1) at around 125 ms, and a positivity (P2) around 250 ms, and, over frontal sites, a negativity (N1) peaking around 150 ms and a positivity (P2) peaking around 250 ms. Confirming the success of the VF manipulation, sensory components were larger and earlier over sites contralateral to presentation VF and were followed (from ∼300 ms to the end of the epoch) by a lateralized, posterior negative-going effect (selection negativity) seen in all prior VF-ERP studies (e.g., Federmeier and Kutas, 1999). Visual evoked potentials were followed by a centro-posterior negativity peaking around 400 ms (N400) and a posterior positivity (LPC) between about 500 and 900 ms. Effects on the frontal P2, the N400, and the LPC were characterized using repeated measures analyses of variance (ANOVAs). Electrode main effects and interactions are not reported, as these were not of theoretical significance.
Frontal P2
Frontal P2s were measured as the largest local peak (defined as a maximum that is larger than both the previous and the subsequent point to avoid confounds from the edges of the measurement time window) between 220 and 270 ms. Measurements were taken at five medial frontal channels (midline prefrontal and left and right medial prefrontal and frontal sites) based on the typical scalp distribution of the P2 and with the goal of avoiding overlap with immediately following N400 effects over more posterior sites. These local peak amplitudes were subjected to an ANOVA with 2 levels of VF (RVF, LVF), 2 levels of relatedness (related, unrelated), 2 levels of directionality (forward, backward) and 5 levels of electrodes as factors. There were main effects of visual field (F1,31 = 5.72, p < 0.05, partial η2 = 0.16), relatedness (F1,31 = 6.69, p < 0.05, partial η2 = 0.18), and directionality (F1,31 = 26.70, p < 0.05, partial η2 = 0.46). Overall, P2 amplitudes were larger (more positive) with RVF as compared to LVF presentation (4.37 vs. 3.76 μV), for related than unrelated targets (4.33 μV vs. 3.80 μV), and for forward as compared with backward pairs (4.75 μV vs. 3.39 μV). None of the interactions was significant.
Planned pairwise comparisons between the related and corresponding (lexically matched) unrelated conditions for each VF and directionality condition revealed a significant priming effect (increased positivity to related than unrelated targets) limited to forward pairs presented in the RVF (related: 5.54 μV, unrelated: 4.50 μV; F1,31 = 4.97, p < 0.05, partial η2 = 0.14); all other pairwise comparisons were non-significant. Comparisons on the difference wave (a point by point subtraction of the related from the lexically matched unrelated ERPs for each condition) revealed that, for RVF presentation, forward pairs elicited greater P2 priming than did backward pairs (1.03 μV vs. -0.19 μV, F1,31 = 4.18, p < 0.05, partial η2 = 0.12); this comparison was not significant for LVF presentation (F1,31 < 1, partial η2 = 0.0003). Figure 2 shows the effect pattern.
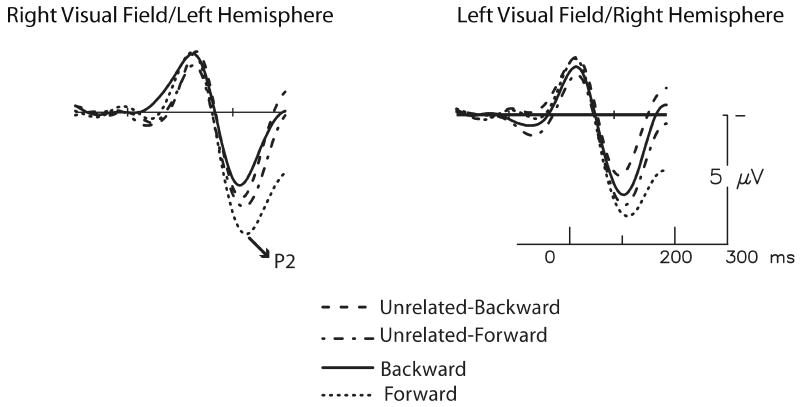
Figure 2.
A shortened time window (-100 to 300 ms) is used to show P2 responses for all conditions (forward, backward, unrelated-forward, unrelated-backward) at a representative medial prefrontal channel (LMPf). RVF/LH targets are plotted on the left and LVF/RH targets on the right. A selective P2 enhancement is evident for the predictable forward targets when initially presented to the LH.
Thus, exactly replicating the pattern seen for sentential constraint (Federmeier et al., 2005; Wlotko & Federmeier, 2007), the results suggest a selective P2 enhancement for related targets in the forward pairs, where the target words were predictable from the primes, limited to targets presented to the RVF/LH.
N400
N400 mean amplitudes were measured between 350-500 ms over the 11 centro-posterior channels (marked on Figure 3) where N400 effects tend to be most prominent. These were subjected to a repeated measures ANOVA with 2 levels of VF (RVF, LVF), 2 levels of relatedness (related, unrelated), 2 levels of directionality (forward, backward), and 11 levels of electrode as factors. There was no main effect of visual field (F1,31 < 1, partial η2 = 0.009). However, there was a main effect of relatedness with reduced (more positive) N400 amplitudes to related targets compared to unrelated targets (1.52 μV vs. -1.32 μV, F1,31 = 46.69, p < 0.05, partial η2 = 0.60). There was also a main effect of directionality with reduced N400 amplitudes in the forward condition compared to those in the backward condition (0.91 μV vs. -0.71 μV, F1,31 = 59.38, p < 0.05, partial η2 = 0.66). The only significant interaction was between relatedness and directionality (F1,31 = 35.19, p < 0.05, partial η2 = 0.53). Follow-up comparisons revealed that, overall, for both forward and backward conditions, the related targets elicited significantly more positive N400s than their unrelated counterparts (Fwd: 2.99 μV vs. -1.18 μV, F1,31 = 59.3, p < 0.05, partial η2 = 0.66; Bwd: 0.06 μV vs. -1.46 μV, F1,31 = 15.15, p < 0.05, partial η2 = 0.33). However, whereas responses to the unrelated targets were comparable across forward and backward conditions (F1,31 = 1.19, partial η2 = 0.04), responses to related targets were more facilitated (smaller N400s) in the forward than in the backward condition (F1,31 = 71.23, p < 0.05, partial η2 = 0.70).
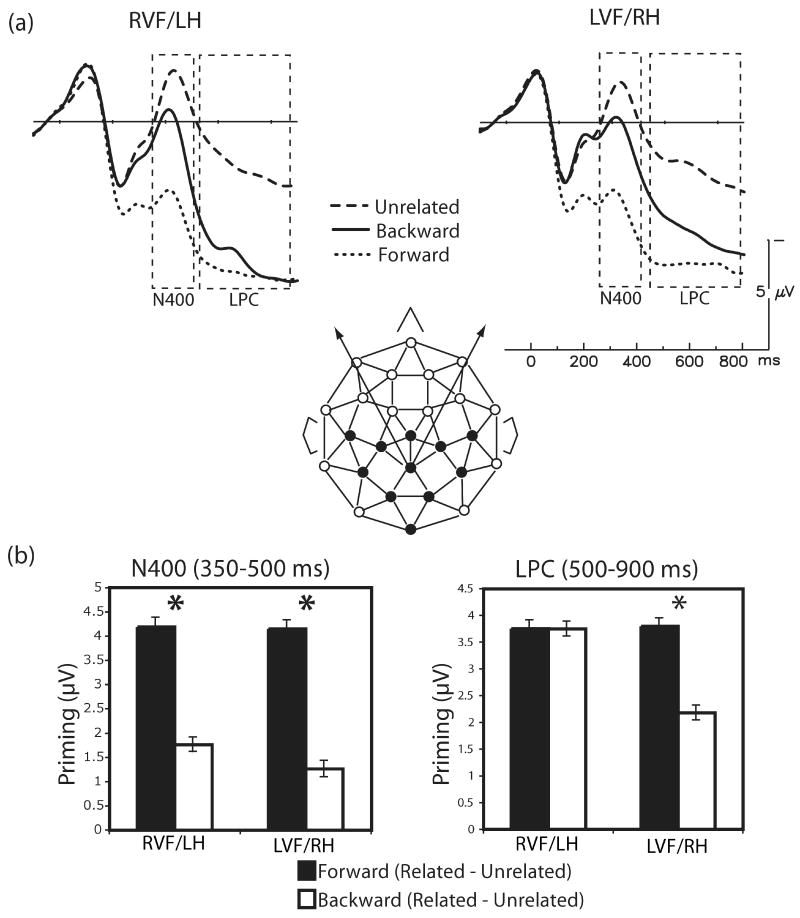
Figure 3.
(a) Grand average ERPs to targets in the forward, backward and unrelated pairs at a representative channel (MiPa), with RVF/LH targets plotted on the left and LVF/RH targets plotted on the right. In both VFs, N400 amplitudes (350 to 500 ms) were sensitive to associative strength, with the smallest N400s to the strongly associated forward pairs and weaker (but significant) priming for the weakly associated backward pairs, as compared with unrelated targets. LPC amplitudes (500 to 900 ms) were modulated by associative strength for LVF/RH targets, but not for RVF/LH targets, which showed equal priming for forward and backward pairs. (b) In both VFs, there was greater N400 priming for forward pairs than backward pairs, and no difference in N400 effect sizes across VF. LPC priming tracked patterns of N400 priming for LVF/RH targets, but LPC priming for both forward and backward targets in the RVF/LH was indistinguishable and comparable to that seen for LVF/RH forward targets.
Planned comparisons were conducted to focus in on priming effects within and across VF. As for the P2, analyses were conducted on the difference waves (subtraction of the related ERPs from lexically matched unrelated ERPs). As can be seen in Figure 3, N400 priming was greater in the forward than in the backward condition in both VFs (RVF: 4.19 μV vs. 1.76 μV, F1,31 = 25.83, p < 0.05, partial η2 = 0.45; LVF: 4.15 μV vs. 1.26 μV, F1,31 = 18.09, p < 0.05, partial η2 = 0.37). Comparisons across VF revealed no significant differences in priming for either the forward or backward conditions (Fwd: F1,31 < 1, partial η2 = 0.0003; Bwd: F1,31 < 1, partial η2 = 0.02).
In summary, N400 priming effects, taken to index relatively automatic aspects of initial semantic activation to the target words, were seen for both the forward and backward conditions in both VFs, and effects of associative strength were also evident in both VFs, with greater priming for the more strongly associated forward pairs. Comparisons across VF revealed no evidence to support the prediction that associative priming would be more robust for the LH compared to the RH (Deacon et al., 2004) or that priming for weak (backward) associates would be greater in the RH (Jung-Beeman, 2005).
LPC
The 11 electrode sites used for the N400 analyses were also analyzed in the later time window (500-900 ms) to examine effects on LPC mean amplitudes. A repeated measures ANOVA with 2 levels of VF (RVF, LVF), 2 levels of relatedness (related, unrelated), 2 levels of directionality (forward, backward), and 11 levels of electrode as factors revealed a marginal main effect of visual field, with more positive LPC amplitudes to RVF compared to LVF (3.35 μV vs. 2.94 μV, F1,31 = 3.24, p < 0.09, partial η2 = 0.09) targets. There were also main effects of relatedness with more positive LPCs to related compared to unrelated targets (4.84 μV vs. 1.46 μV, F1,31 = 120.60, p < 0.05, partial η2 = 0.80), and of directionality, with more positive LPCs in the forward than in the backward condition (3.44 μV vs. 2.86 μV, F1,31 = 7.71, p < 0.05, partial η2 = 0.20). Similar to the pattern seen on the N400, there was a relatedness by directionality interaction (F1,31 = 4.98, p < 0.05, partial η2 = 0.14). LPCs were enhanced for related relative to unrelated targets in both the forwards and the backwards conditions (Fwd: 5.32 μV vs. 1.54 μV, F1,31 = 93.10, p < 0.05, partial η2 = 0.75; Bwd: 4.34 μV vs. 1.38 μV, F1,31 = 89.83, p < 0.05, partial η2 = 0.74). However, while the unrelated targets were comparable across directionality, the related targets elicited significantly more positive LPC activations in the forward condition compared to the backward condition (F1,31 = 8.95, p < 0.05, partial η2 = 0.22).
Different from the pattern seen on the N400, the overall ANOVA also revealed a marginal interaction between VF and relatedness (F1,31 = 3.90, p < 0.07, partial η2 = 0.11) and a significant three-way interaction among VF, relatedness and directionality (F1,31 = 4.77, p < 0.05, partial η2 = 0.13). Planned comparisons on the difference waves (subtraction of related from matched unrelated ERPs) revealed that, in the LVF/RH, there was significantly more priming in the forward compared to the backward condition (3.80 μV vs. 2.18 μV, F1,31 = 6.52, p < 0.05, partial η2 = 0.17). However, in the RVF/LH, there were no significant differences in the amount of LPC priming across directionality (forward: 3.76 μV; backward: 3.75 μV; F1,31< 1, partial η2 ≈ 0). Comparisons across VF revealed that the amount of LPC priming was statistically equivalent for the forward condition, whereas there was significantly greater LPC priming in the backward condition for RVF/LH compared with LVF/RH targets (F1,31 = 7.17, p < 0.05, partial η2 = 0.19). Figure 3 shows the effect pattern.
Thus, LPC effects, taken to index more strategic/explicit semantic integration processes elicited in response to the target, were different for RVF/LH and LVF/RH processing. In both VFs, responses to related targets were enhanced relative to those to unrelated targets. However, in the LVF/RH, effects of associative strength, also observed on the N400, were maintained into the LPC time window, with more positivity to related targets in the forward than in the backward condition. However, in the RVF/LH, LPC priming was statistically equivalent across directionality, reflecting an enhanced LPC for the related targets in the backward condition. These results suggest a LH benefit for strategically appreciating the semantic association in the backward condition.
Discussion
This study used ERPs in conjunction with VF presentation methods to examine multiple aspects of lexical associative processing in the two cerebral hemispheres; to our knowledge, this is the first study to investigate hemispheric asymmetries in sensitivity to associative strength using ERPs. Strength of lexical association was manipulated using asymmetrically associated word pairs. When the words were presented in one order (the forward condition), there was high predictability of the target from the prime (strong association); indeed, the target was the prime's top associate. However, presented in the opposite order (the backward condition), there was low predictability of the target given the prime (weak association). We were thus able to manipulate associative strength – and, correspondingly, predictability – within the same pairs of words. Participants read the stimuli without making a response, and were later tested on their memory for the word pairs in an off-line cued recall task.
This paradigm allowed us to test the predictions of several prominent views of how the two hemispheres store, access, maintain, and reshape word meaning information. Some views have attributed semantic processing asymmetries to fundamental differences in the conceptual networks instantiated in each hemisphere, with concepts postulated to be represented in a localist, associative network in the LH but in a distributed, feature-based network in the RH (Deacon et al., 2004). Consistent with this hypothesized representational difference, Deacon et al. found N400 facilitation for categorically-related word pairs only with LVF/RH presentation but facilitation for lexically-associated pairs only with RVF/LH presentation (Deacon et al., 2004; Grose-Fifer & Deacon, 2004). In contrast, other ERP studies (Coulson et al., 2005) have also observed association-based priming effects on the N400 with LVF/RH presentation. However, Coulson et al. (2005) did not specifically eliminate associated pairs that might also have had semantic feature overlap. In the present study, therefore, we used only word pairs that were associated thematically; they were not synonyms, did not share categorical or whole-part relations, and did not have obvious feature overlap.
We observed significant, and indistinguishable, N400 priming effects for these purely associated pairs with both RVF/LH and LVF/RH presentation. Perhaps most importantly, both hemispheres also proved to be equally sensitive to associative strength, manifesting larger priming effects for the strongly associated forward pairs than for the weakly associated backward pairs, with no VF difference in the size of the priming effect for either forward or backward pairs. Since these were the same words, simply presented in a different order, any feature overlap between them should have been essentially identical in the two cases, yet the RH was as sensitive as the LH to the difference in associative strength between the forward and backward orders. Behavioral studies have found a similar pattern. For example, Coney (2002), using prime-target pairs of varying associative strength, showed that lexical decision times in both VF were linearly related to associative strength. Together, this set of data argues against the idea that the organization of semantic information in the RH renders it insensitive to lexical relationships based on association rather than semantic feature overlap.
The fact that Deacon et al. (2004) failed to find evidence for association-based priming in the LVF/RH is puzzling, but may be related to methodological differences between that study and those that have found LVF/RH associative priming effects (e.g., Coney, 2002; Coulson et al., 2005). For instance, Deacon et al. (2004) employed a strongly right-handed participant pool with a mean handedness quotient of 0.95 (range: 0.85-1.0) as measured by the Edinburgh handedness inventory (Oldfield, 1971). We believe that the participant pool employed in the present study, with a mean handedness quotient of 0.82 (range: 0.35-1.0), may be more representative of the typical distribution of right-handed native English speakers. Importantly, however, the pattern of results remains the same when we restrict analyses to only the most lateralized subset of participants (n = 23, mean: 0.91, range: 0.77-1.0). Therefore, the differences in the distribution of handedness quotients across the studies do not seem to be responsible for the differences in the effect patterns observed.
Another methodological difference between Deacon et al. (2004) and the present study is with regard to prime lateralization. Whereas Deacon et al. (2004) lateralized both the targets and the primes (to the same VF), the current study and that of Coulson et al. (2005) used centrally-presented primes/context words and lateralized targets. Interestingly, a similar inconsistency as a function of prime location has been observed in the literature using behavioral measures, in which priming for lexically associated words was observed in both VFs when primes were presented centrally but was not observed in either VF when primes were lateralized (see, Chiarello et al., 1990). Although the methodological and theoretical implications of lateralizing the primes are still unclear, the results from the current study and many that have preceded it demonstrate that lateralization of the target alone is sufficient to produce significant and reproducible hemispheric asymmetries. Additional lateralization of the prime has a downside, in that it will tend to decrease prime perceptibility, perhaps especially in the LVF/RH given the RH's overall poorer word apprehension abilities (Jordan et al., 2003), which could reduce priming effects. Furthermore, central presentation of the prime has the added advantage of allowing greater cross-talk between the word priming literature and that looking at asymmetries in sentence processing, in which (by necessity) only the target is lateralized (Faust, Babkoff, & Kravetz, 1995; Titone, 1998; Federmeier & Kutas, 1999; Coney & Evans, 2000; Coulson et al., 2005; Wlotko & Federmeier, 2007).
A second prominent theory, the coarse coding hypothesis (Jung-Beeman, 2005) posits that the initial spread of semantic activation is different in the two hemispheres, with the LH focally activating typical or contextually-relevant meaning features but the RH weakly activating a more diffuse set of concepts. In the context of the present study, coarse coding predicts greater LVF/RH than RVF/LH priming for the weakly associated backwards pairs. Because priming is assessed on the N400 component, known to be a functionally specific index of early, implicit aspects of semantic processing (see, e.g., review by Federmeier and Laszlo, in press), the use of ERPs provides an especially strong test of the hypothesis that processing asymmetries arise via differences in the breath of automatic spreading activation.
We found that both hemispheres showed N400 facilitation (relative to unrelated targets) for the weakly associated targets in the backward condition, and there was no evidence that this priming was greater in the LVF/RH. These results are consistent with failures to find evidence for coarse coding based on the number, rather than the strength, of lexical associative links in mediated priming studies (Richards & Chiarello, 1995; Livesay & Burgess, 2003), failures to find evidence for a RH benefit in N400 priming for the secondary sense of words with multiple meanings (Meyer & Federmeier, 2007), and failures to find RH advantages for integrating across weakly related words and/or maintaining multiple senses of words (Kandhadai & Federmeier, 2007, 2008). Taken as a whole, then, the ERP data, supported by convergent behavioral findings, do not support the hypothesis that there are notable differences in how the two cerebral hemispheres represent or initially access meaning information from words.
Other views suggest that meaning processing asymmetries arise from differences in the hemispheres' use of top-down, control mechanisms, rather than in basic semantic representation and/or activation processes. Such control mechanisms would likely play an especially important role during the processing of higher-order language structures, and, in fact, consistent hemispheric differences have been found in impact of sentence context information on word processing (see, e.g., review by Federmeier, Wlotko, & Meyer, 2008). The role of such processes in word priming paradigms would likely be more variable. However, because associative strength is defined by predictability, manipulations of associative strength offer the possibility of capturing, in a word priming paradigm, the kind of expectancy-based processes that have been linked to hemispheric asymmetries in sentence processing (e.g., Faust & Chiarello, 1998; Federmeier, 2007) and that have also been suggested as a source for hemispheric differences in word processing (e.g., Chiarello, 1985; Chiarello et al., 1987; Burgess & Simpson, 1988; Chiarello et al., 1992).
For example, one such account, the “PARLO” framework (Federmeier, 2007), suggests that asymmetries in language processing arise because of differences in the hemispheres' ability to use top-down mechanisms to prepare for the processing of expected stimuli. This view postulates that language production mechanisms, which are more highly developed and efficacious in the LH, are also used during comprehension to predict likely upcoming words based on current context and to preactivate features of those words at multiple levels. The RH, instead, is believed to process words in a more bottom-up fashion, integrating them into the larger language context as they arise. On this account, then, priming asymmetries would not be due to the nature of the semantic relationship or the semantic distance between two words as such, but rather depend on the predictive validity of different types of cues in different processing environments.
Sentence processing studies have consistently observed similar levels of N400 facilitation in the two hemispheres when context information is either very strong or weak, with differences most apparent at intermediate levels of contextual strength where prediction can provide a basis for facilitation not afforded by integration alone (see, e.g., Wlotko and Federmeier, 2007). Based on these sentence-level effects, we would not expect to see N400 asymmetries for the strong forward and weak backward associates employed in the present study. However, enhanced P2 responses – limited to RVF/LH presentation – have often been observed to words in constraining sentence contexts, perhaps reflecting a preparatory attentional response elicited by language contexts that afford a strong expectation for particular upcoming stimuli (Federmeier & Kutas, 2002; Federmeier et al., 2005; Wlotko & Federmeier, 2007). Consistent with that data and with the predictions of the PARLO framework, in the current study we observed a significant P2 enhancement that was restricted to related targets in the forward condition and seen only with RVF/LH presentation. These results thus support the idea that LH-based prediction mechanisms, thus far primarily characterized in sentence processing studies, also operate during the processing of minimal word-level contexts that promote strong expectations for particular words (see also Koivisto, 1999).
In addition to predicting, the LH has also been suggested to be better at using controlled semantic processing mechanisms to reshape initial semantic activation (Burgess & Simpson, 1988; Chiarello et al., 1987; Chiarello et al., 1992; Nakagawa, 1991; Collins, 1999). This type of explanation has been put forward, for example, to explain RVF/LH advantages in selecting contextually appropriate word meanings (e.g., Nakagawa, 1991). Such controlled, strategic semantic processing mechanisms would be expected to manifest on the LPC (e.g., Swaab et al., 1998; Kolk et al., 2003; Hoeks et al., 2004) and might play a particularly important role in processing for the weakly associated backward pairs, in allowing an appreciation of the much stronger reverse – i.e., target-to-prime – semantic relationship in these pairs. Indeed, we observed equivalent LPC enhancements for forward targets in both hemispheres, but significantly greater LPC priming for the backward targets in the RVF/LH compared to the LVF/RH. Strikingly, in the RVF/LH, LPC facilitation for the backward targets was equivalent to that for the forward targets. This pattern suggests that the LH may strategically amplify weak but relevant meaning activation and/or reorder language elements – in the current task, allowing it to then better appreciate the strong reverse association from the target to the prime in the backward condition. Indeed, these results converge with behavioral studies that have found a LH benefit for controlled semantic processing (e.g., Nakagawa, 1991) and with findings in the functional neuroimaging literature that have emphasized a LH role in semantic processing that requires working memory and/or executive-control (e.g., Kapur et al., 1994; Gabrieli et al., 1996; Wagner, Desmond, Demb, Glover, & Gabrieli, 1997).
In conclusion, we were able to examine multiple aspects of lexical associative processing in the cerebral hemispheres using ERPs, which provide a continuous, multidimensional measure that can track the processing of word meaning over time and distinguish effects of automatic semantic processing from those elicited by more strategic processing mechanisms. Our findings augment a growing body of work that fails to find evidence for hemispheric asymmetries in semantic representation and/or automatic semantic activation processes. Instead, our data suggest that there are differences in how the two hemispheres use more controlled aspects of processing to shape that initial semantic activation over time. In particular, the LH seems more likely and/or better able to predict likely upcoming words based on current context and to use strategic mechanisms to reshape and amplify weak but relevant meaning relations. This work thus provides an important link between asymmetries in word level processing and the kinds of hemispheric differences that have been uncovered at the sentence level.
Acknowledgments
Thanks to the undergraduate members of the Cognition and Brain Lab at the University of Illinois for assistance with data collection. Support from NIA grant AG026308 to Kara D. Federmeier is gratefully acknowledged.
References
- Abernathy M, Coney J. Semantic category priming in the left hemisphere. Neuropsychologia. 1996;34:339–350. doi: 10.1016/0028-3932(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Abernethy M, Coney J. Semantic and phonemic priming in the cerebral hemispheres. Neuropsychologia. 1990;28:933–945. doi: 10.1016/0028-3932(90)90109-2. [DOI] [PubMed] [Google Scholar]
- Atchley RA, Kwasny K. Using event related potentials to examine hemispheric differences in semantic processing. Brain and Cognition. 2003;53:133–138. doi: 10.1016/s0278-2626(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Beeman M. Semantic processing in the right hemisphere may contribute to drawing inferences from discourse. Brain and Language. 1993;1:80–120. doi: 10.1006/brln.1993.1006. [DOI] [PubMed] [Google Scholar]
- Beeman M, Chiarello C. Right hemisphere language comprehension: Perspectives from cognitive neuroscience. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Beeman M, Friedman R, Grafman J, Perez E, Diamond S, Lindsay M. Summation priming and coarse semantic coding in the right hemisphere. Jounal of Cognitive Neuroscience. 1994;6:26–45. doi: 10.1162/jocn.1994.6.1.26. [DOI] [PubMed] [Google Scholar]
- Bentin S, McCarthy G, Wood CC. Event-related potentials associated with semantic priming. Electroencephalography and Clinical Neurophysiology. 1985;60:343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Bihrle AM, Brownell HH, Powelson JA, Gardner H. Comprehension of humorous and nonhumorous materials by left and right brain-damaged patients. Brain and Cognition. 1986;5:399–411. doi: 10.1016/0278-2626(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Bouaffre S, Faïta-Ainseba F. Hemispheric differences in the time-course of semantic priming processes: Evidence from event-related potentials (ERPs) Brain and Cognition. 2007;63:123–135. doi: 10.1016/j.bandc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Brown C, Hagoort P. The processing nature of the N400: Evidence from masked priming. Journal of Cognitive Neuroscience. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Brownell HH. Appreciation of metaphoric and connotative word meaning by brain-damaged patients. In: Chiarello C, editor. Right hemisphere contributions to lexical semantics. New York: Springer; 1988. pp. 19–31. [Google Scholar]
- Brownell HH, Potter HH, Michelow D, Gardner H. Sensitivity to lexical denotation and connotation in brain-damaged patients: A double dissociation? Brain and Language. 1984;22:253–265. doi: 10.1016/0093-934x(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Brownell HH, Simpson TL, Bihrle AM, Potter HH, Gardner H. Appreciation of metaphoric alternative word meanings by left and right brain-damaged patients. Neuropsychologia. 1990;28:375–383. doi: 10.1016/0028-3932(90)90063-t. [DOI] [PubMed] [Google Scholar]
- Burgess C, Simpson GB. Cerebral hemispheric mechanisms in the retrieval of ambiguous word meanings. Brain and Language. 1988;33:86–103. doi: 10.1016/0093-934x(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Chiarello C. Hemisphere dynamics in lexical access: Automatic and controlled priming. Brain and Language. 1985;26:146–172. doi: 10.1016/0093-934x(85)90034-3. [DOI] [PubMed] [Google Scholar]
- Chiarello C. Interpretation of word meanings by the cerebral hemispheres: one is not enough. In: Schwanenflugel P, editor. The Psychology of Word Meanings. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 251–278. [Google Scholar]
- Chiarello C. On codes of meaning and the meaning of codes: Semantic access and retrieval within and between hemispheres. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: Perspectives from cognitive neuroscience. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. pp. 141–160. [Google Scholar]
- Chiarello C, Burgess C, Richards L, Pollock A. Semantic and Associative priming in the cerebral hemispheres: Some words do, some words don't … sometimes, some place. Brain and Language. 1990;38:75–104. doi: 10.1016/0093-934x(90)90103-n. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Richards L. Another look at categorical priming in cerebral hemispheres. Neuropsychologia. 1992;30:381–392. doi: 10.1016/0028-3932(92)90111-x. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Richards L, Pollock A. Semantic additivity and semantic inhibition: Dissociable processes in the cerebral hemispheres? Brain and Language. 1992;42:52–76. doi: 10.1016/0093-934x(92)90056-k. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Senehi J, Nuding S. Semantic Priming with abstract and concrete words: Differential asymmetry may be post-lexical. Brain and Language. 1987;18:153–163. doi: 10.1016/0093-934x(87)90060-5. [DOI] [PubMed] [Google Scholar]
- Collins M. Differences in semantic category priming in the left and right cerebral hemispheres under automatic and controlled processing conditions. Neuropsychologia. 1999;37:1071–1085. doi: 10.1016/s0028-3932(98)00156-0. [DOI] [PubMed] [Google Scholar]
- Coltheart M. MRC Psycholinguistic Database: Machine Usable Dictionary. The Quarterly Journal of Experimental Psychology. 1987;33A:497–505. [Google Scholar]
- Coney J. The effect of associative strength on priming in the cerebral hemispheres. Brain and Cognition. 2002;50:234–241. doi: 10.1016/s0278-2626(02)00507-9. [DOI] [PubMed] [Google Scholar]
- Coney J, Evans KD. Hemispheric asymmetries in the resolution of lexical ambiguity. Neuropsychologia. 2000;38:272–282. doi: 10.1016/s0028-3932(99)00076-7. [DOI] [PubMed] [Google Scholar]
- Coulson S, Federmeier KD, Van Petten C, Kutas M. Right hemisphere sensitivity to word and sentence-level context. Journal of Experimental Psychology: Learning, Memory and Cognition. 2005;31:129–147. doi: 10.1037/0278-7393.31.1.129. [DOI] [PubMed] [Google Scholar]
- Coulson S, Williams RF. Hemispheric asymmetries and joke comprehension. Neuropsychologia. 2005;43:128–141. doi: 10.1016/j.neuropsychologia.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Dale AM. Source localization and spatial discriminant analysis of event-related potentials: Linear approaches. University of California San Diego; LaJolla, CA: 1994. [Google Scholar]
- Deacon D, Grose-Fifer J, Yang CM, Stanick V, Hewitt S, Dynowska A. Evidence for a new conceptualization of semantic representation in the left and right cerebral hemispheres. Cortex. 2004;40:467–478. doi: 10.1016/s0010-9452(08)70140-0. [DOI] [PubMed] [Google Scholar]
- Deacon D, Hewitt S, Yang C, Nagata M. Event-related potentials indices of semantic priming using masked and unmasked words: evidence that the N400 does not reflect a post-lexical process. Cognitive Brain Research. 2000;9:137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Düzel E, Vargha-Khadem F, Heinze HJ, Mishkin M. Brain activity evidence for recognition without recollection after early hippocampal damage. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8101–8106. doi: 10.1073/pnas.131205798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Babkoff H, Kravetz S. Linguistic processes in the two cerebral hemispheres: Implications for modularity vs. interactionism. Journal of Clinical and Experimental Neuropsychology. 1995;17:171–192. doi: 10.1080/01688639508405117. [DOI] [PubMed] [Google Scholar]
- Faust M, Chiarello C. Constraints on Sentence Priming in the Cerebral Hemispheres: Effects of Intervening Words in Sentences and Lists. Brain and Language. 1998;63:219–236. doi: 10.1006/brln.1997.1941. [DOI] [PubMed] [Google Scholar]
- Faust M, Lavidor M. Semantically convergent and semantically divergent priming in the cerebral hemispheres: Lexical decision and semantic judgment. Brain Research: Cognitive Brain Research. 2003;17:585–597. doi: 10.1016/s0926-6410(03)00172-1. [DOI] [PubMed] [Google Scholar]
- Federmeier KD. Thinking ahead: the role and roots of prediction in language comprehension. Psychophysiology. 2007;44:491–505. doi: 10.1111/j.1469-8986.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M. Right words and left words: Electrophysiological evidence for hemispheric differences in meaning processing. Brain Research: Cognitive Brain Research. 1999;8:373–392. doi: 10.1016/s0926-6410(99)00036-1. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M. Picture the difference: Electrophysiological investigations of picture processing in the cerebral hemispheres. Neuropsychologia. 2002;40:730–747. doi: 10.1016/s0028-3932(01)00193-2. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Laszlo S. Time for Meaning: Electrophysiology provides insights into the dynamics of representation and processing in semantic memory. In: Ross B, editor. Psychology of Learning and Memory. Vol. 51. New York: Elsevier; in press. [Google Scholar]
- Federmeier KD, Mai H, Kutas M. Both Sides Get the Point: Hemispheric Sensitivities to Sentential Constraint. Memory and Cognition. 2005;33:871–886. doi: 10.3758/bf03193082. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Wlotko E, Meyer AM. What's “right” in language comprehension: ERPs reveal right hemisphere language capabilities. Language and Linguistics Compass. 2008;2:1–17. doi: 10.1111/j.1749-818X.2007.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, et al. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Gagnon L, Goulet P, Giroux F, Joanette Y. Processing of metaphoric and non-metaphoric alternative meanings of words after right- and left-hemispheric lesion. Brain and Language. 2003;87:217–226. doi: 10.1016/s0093-934x(03)00057-9. [DOI] [PubMed] [Google Scholar]
- Grose-Fifer J, Deacon D. Priming by natural category membership in the left and right cerebral hemispheres. Neuropsychologia. 2004;42:1948–1960. doi: 10.1016/j.neuropsychologia.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown CM, Groothusen J. The Syntactic Positive Shift (SPS) as an ERP measure of syntactic processing. Language and Cognitive Processes. 1993;8:439–483. [Google Scholar]
- Hoeks JCJ, Stowe LA, Doedens G. Seeing words in context: the interaction of lexical and sentence level information during reading. Cognitive Brain Research. 2004;19:59–73. doi: 10.1016/j.cogbrainres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Huang HW, Lee CL, Federmeier KD. Imagine that! ERPs provide evidence for distinct hemispheric contributions to the processing of concrete and abstract concepts. Neuroimage. doi: 10.1016/j.neuroimage.2009.07.031. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaan E, Harris A, Gibson E, Holcomb P. The P600 as an index of syntactic integration difficulty. Language and Cognitive Processes. 2000;15:159–201. [Google Scholar]
- Kandhadai P, Federmeier KD. Multiple priming of lexically ambiguous and unambiguous targets in the cerebral hemispheres: the coarse coding hypothesis revisited. Brain Research. 2007;1153:144–157. doi: 10.1016/j.brainres.2007.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandhadai P, Federmeier KD. Summing it up: Semantic activation processes in the two hemispheres as revealed by event-related potentials. Brain Research. 2008;1233:146–159. doi: 10.1016/j.brainres.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Rose R, Liddle PF, Zipursky RB, Brown GM, Stuss D, et al. The role of the left prefrontal cortex in verbal processing: semantic processing or willed action? Neuroreport. 1994;5:2193–2196. doi: 10.1097/00001756-199410270-00051. [DOI] [PubMed] [Google Scholar]
- Klepousniotou E, Baum SR. Processing homonymy and polysemy: Effects of sentential context and time-course following unilateral brain damage. Brain and Language. 2005;95:365–382. doi: 10.1016/j.bandl.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Koivisto M. The time course of semantic activation in the cerebral hemispheres. Neuropsychologia. 1997;35:497–504. doi: 10.1016/s0028-3932(96)00100-5. [DOI] [PubMed] [Google Scholar]
- Koivisto M. Categorical priming in the cerebral hemispheres: automatic in the left hemisphere, postlexical in the right hemisphere? Neuropsychologia. 1998;36:661–668. doi: 10.1016/s0028-3932(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Koivisto M. Hemispheric dissociations in controlled lexical-semantic processing. Neuropsychology. 1999;13:488–497. doi: 10.1037//0894-4105.13.4.488. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Laine M. Hemispheric asymmetries in activation and integration of categorical information. Laterality. 2000;5:1–21. doi: 10.1080/713754358. [DOI] [PubMed] [Google Scholar]
- Kolk HHJ, Chwilla DJ, Van Herten M, Oor PJW. Structure and limited capacity in verbal working memory: a study with event-related potentials. Brain and Language. 2003;85:1–36. doi: 10.1016/s0093-934x(02)00548-5. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present day American English, Providence. Providence, RI: Brown Univ. Press; 1967. [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Event-related brain potentials to semantically inappropriate and surprisingly large words. Biological Psychology. 1980a;11:99–116. doi: 10.1016/0301-0511(80)90046-0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980b;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Event-related brain potentials to grammatical errors and semantic anomalies. Memory and Cognition. 1983;11:539–550. doi: 10.3758/bf03196991. [DOI] [PubMed] [Google Scholar]
- Livesay K, Burgess C. Mediated priming in the cerebral hemispheres. Brain and Cognition. 2003;53:283–286. doi: 10.1016/s0278-2626(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Federmeier KD. The effects of context, meaning frequency, and associative strength on semantic selection: distinct contributions from each cerebral hemisphere. Brain Research. 2007;1183:91–108. doi: 10.1016/j.brainres.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Federmeier KD. The divided visual world paradigm: eye-tracking reveals hemispheric asymmetries in lexical ambiguity resolution. Brain Research. 2008;1222:166–183. doi: 10.1016/j.brainres.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Holcomb PJ. Event-related potential indices of masked repetition priming. Psychophysiology. 2003;40:115–130. doi: 10.1111/1469-8986.00012. [DOI] [PubMed] [Google Scholar]
- Nakagawa A. Role of anterior and posterior attention networks in hemispheric asymmetries during lexical decision. Journal of Cognitive Neuroscience. 1991;3:313–321. doi: 10.1162/jocn.1991.3.4.313. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related brain potentials elicited by syntactic anomaly. Journal of memory and language. 1992;31:785–806. [Google Scholar]
- Richards L, Chiarello C. Depth of associated activation in the cerebral hemispheres: mediated versus direct priming. Neuropsychologia. 1995;33:171–179. doi: 10.1016/0028-3932(94)00102-u. [DOI] [PubMed] [Google Scholar]
- Rolke B, Heil M, Streb J, Henninghausen E. Missed prime words within the attentional blink evoke an N400 semantic priming effect. Psychophysiology. 2001;38:165–174. [PubMed] [Google Scholar]
- Stemmer B, Giroux F, Joanette Y. Production and evaluation of requests by right hemisphere brain damaged individuals. Brain and Language. 1994;47:1–31. doi: 10.1006/brln.1994.1040. [DOI] [PubMed] [Google Scholar]
- Swaab TY, Brown C, Hagoort P. Understanding ambiguous words in sentence contexts: electrophysiological evidence for delayed contextual selection in Broca's aphasia. Neuropsychologia. 1998;36:737–761. doi: 10.1016/s0028-3932(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Titone D. Hemispheric differences in context sensitivity during lexical ambiguity resolution. Brain and Language. 1998;65:361–394. doi: 10.1006/brln.1998.1998. [DOI] [PubMed] [Google Scholar]
- Tompkins CA. Knowledge and strategies for processing lexical metaphor after right or left hemisphere brain damage. Journal of Speech, Language, and Hearing Research. 1990;33:307–316. doi: 10.1044/jshr.3302.307. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Kutas M, Kluender R, Mitchiner M, McIsaac H. Fractionating the word repetition effect with event-related potentials. Journal of Cognitive Neuroscience. 1991;3:131–150. doi: 10.1162/jocn.1991.3.2.131. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic Repetition Priming for Verbal and Pictorial Knowledge: A Functional MRI Study of Left Inferior Prefrontal Cortex. Journal of Cognitive Neuroscience. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wlotko E, Federmeier KD. Finding the right word: Hemispheric asymmetries in the use of sentence context information. Neuropsychologia. 2007;45:3001–3014. doi: 10.1016/j.neuropsychologia.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]