Abstract
Context
Acute kidney injury (AKI) following cardiac surgery with cardiopulmonary bypass (CPB) causes increased morbidity and mortality.
Objective
Evaluate the plasma profile of biomarkers potentially involved in AKI following CPB.
Methods
In a nested case-control study, plasma levels of 27 biomarkers in 11 AKI cases were compared with 25 controls.
Results
Pre CPB, plasma levels of epidermal growth factor and macrophage inflammatory protein-1β; 2 hours following CPB, soluble vascular cell adhesion molecule-1, fractalkine and macrophage inflammatory protein-1α; and at later time points, sVCAM-1 and interleukin-6 were associated with AKI.
Conclusion
Biomarkers associated with AKI following CPB may merit further study.
Keywords: Cardiac surgery, cardiopulmonary bypass, acute renal failure, pathophysiology, biomarker
INTRODUCTION
Acute kidney injury (AKI) is a serious complication of cardiac surgery with cardiopulmonary bypass (CPB). It occurs in 5-20% of patients undergoing this procedure (Lassnigg et al., 2004, Liangos O 2005), and is associated with excessive in-hospital mortality (Lassnigg et al., 2004, Chertow et al., 1998). The extent of the host inflammatory response elicited by CPB, partly driven by circulating leukocytes as a result of contact activation by the extracorporeal circuit, is an important contributor to the development of AKI (Kuijpers et al., 1992, Elgebaly et al., 1994, Cremer et al., 1996, Wan et al., 1997). In addition, further disease mechanisms have been implicated, including ischemia reperfusion, thrombosis, and exposure to endotoxin (Laffey et al., 2002, Wan et al., 1997).
The evaluation of multiple AKI-related biomarkers, if geared toward gaining insight into disease mechanisms in both early and late stages of AKI, may potentially add important information to this field of study. By contrast, research efforts on biomarkers in human AKI have so far primarily focused on the evaluation of a few biomarkers for early detection of AKI in a clinical setting, i.e. prior to a rise in serum creatinine (Parikh et al., 2006, Haase et al., 2009, Liangos et al., 2009a, Liangos et al., 2009b). The application of multiplex assays that employ low-volume biological samples for the simultaneous measurement of multiple markers is a novel technology that can be applied to the study of human disease (Skogstrand et al., 2005, Ray et al., 2005, Kofoed et al., 2006). In this pilot nested case control study, we applied the multiplex bead array assay platform to characterize the profile of 27 circulating proteins of interest that might be incriminated in the development of AKI following CPB.
PATIENTS AND METHODS
Study design and setting
This was a nested case control study to an ongoing, large, prospective cohort study of patients undergoing on-pump cardiac surgery, conducted at two tertiary care hospitals, Tufts Medical Center (Boston, MA, USA) and Caritas St. Elizabeth’s Medical Center (Boston, MA, USA). This parent study was designed to evaluate the relationship between single nucleotide polymorphisms and AKI in the setting of CPB (Liangos et al., 2007). Pregnant women, patients with preexisting AKI, end-stage renal disease on maintenance dialysis, solid organ- or bone marrow transplant recipients, and those undergoing ‘off-pump’ or ‘minimally invasive’ coronary artery bypass grafting were excluded.
All consecutive patients over 18 years of age scheduled for on-pump cardiac surgery (elective, urgent or emergent coronary artery bypass grafting, valve surgery or both) who were enrolled into the principal cohort between January 2004 and October 2007 were eligible for selection into the nested case control study.
We selected 11 patients with AKI as defined by an increase in serum creatinine by ≥ 50% within 72 hours following CPB, to constitute our case group. This definition was based on stage 1 of the original RIFLE classification of AKI (Bellomo et al., 2002, Mehta and Chertow, 2003), which has been widely used in studies of AKI following cardiac surgery (Kuitunen et al., 2006, Mishra et al., 2005, Parikh et al., 2006). Using a nearest-neighbor matching technique (MatchIt package, R software (Ho et al., 2007)), we also selected 25 AKI-free patients as defined by a peri-operative serum creatinine fluctuation of less than 0.2 mg/dl, to constitute our control group. The nearest neighbor matching technique selects the best control match for each case by using the logit distance measure. This method represents a propensity score matching using a logistic regression model for predicting the case status and includes age, sex, pre-operative left ventricular ejection fraction, surgery type, and CPB perfusion time as covariates in the model. We did not perform an exact or coerced matching on any of the above covariates. Written informed consent was obtained from all study participants, and the institutional review boards of the two participating centers approved the study protocol.
Data collection
Medical records of study participants were reviewed prospectively to retrieve pre-operative variables including baseline demographic characteristics and coexisting conditions. Intra-operative variables included surgery type, aortic cross-clamp time, and CPB perfusion time; and post-operative variables included serial serum creatinine values, the Acute Physiology and Chronic Health Evaluation (APACHE) II score (Knaus et al., 1985) calculated on post-operative day-1, total mechanical ventilation time, and length of stay in the intensive care unit.
Blood sampling
EDTA-anticoagulated blood was collected at enrolment (prior to CPB), and 2, 24 and 48 hours after the discontinuation of CPB. Samples were kept on ice and processed within 30 minutes of collection. For plasma separation, samples were centrifuged for 10 min at 3,000 RPM, followed by an additional 10 min at 13,000 RPM after transfer of the supernatant, in order to remove platelets. Plasma samples were then aliquoted and stored at −80°C until assayed.
Multiplex bead array assay
We selected 27 gene products of interest representing 11 cytokines, 6 chemokines, 3 adhesion molecules, 4 growth factors, 2 pro-oxidant and extracellular matrix enzymes, and 1 fibrinolysis inhibitor (Appendix 1). These biomarkers were included based on a literature review, suggesting a potential role in the pathogenesis of AKI. Simultaneous quantification of these 27 proteins in the plasma was performed on the Luminex platform using two customized commercially available multiplex immunoassay panels (Linco Inc, St. Louis, MO). In brief, 25μl of standard, quality control, or plasma sample were added to each well of a 96-well plate with 25μl of the bead solution. The plate was sealed, covered with aluminum foil, and incubated overnight for 16-18 hours with agitation on a plate shaker at 4°C. The plate was then washed twice with 200μl per well of wash buffer, removing buffer by vacuum filtration between each wash. Following the addition of 25μl of a detection antibody cocktail into each well, the plate was incubated at room temperature for 1.5 hours. 25μl of a streptavidin-phycoerythrin solution was then added to each well, and the plate was incubated at room temperature for 30 min, and then analyzed on the Luminex 100 IS analyzer (Luminex, Inc., Austin, TX). The data output was saved and evaluated as median fluorescence intensity using the Luminex 100 IS curve-fitting software version 2.3. A 5-parameter weighting logistic method was used. The biomarkers were tested individually and in combination to ensure that there was no cross-reactivity between individual markers. All measurements were performed in duplicate and by a blinded investigator.
Statistical analyses
Continuous variables are presented as means (± SD) or medians (with the inter-quartile range), and categorical variables as numbers and percentages. Continuous variables were compared with the use of the Friedman test (for repeated measures over time) and the Wilcoxon rank sum test (at each time point), and categorical variables with the use of the Fisher exact test. Receiver-operating-characteristic (ROC) curves were constructed to explore the association of each biomarker with the development of AKI at the specific time points. 95% confidence interval (CI) for each area-under-the-ROC curve (AUC) was calculated. The comparison of the AUCs was performed according to the method by DeLong et al. (DeLong et al., 1988). We used different AUC cut-off values to assess the robustness of the association, suggesting possible involvement in disease mechanism (AUC 0.50-0.69, 0.70-0.89, and ≥ 0.90 for mild, moderate and strong association). All hypothesis testing was two-tailed, and a P value of less than 0.05 was used to indicate statistical significance. All statistical analyses were performed using SAS (SAS Institute, Cary, NC) version 9.1.
RESULTS
Characteristics of the AKI and control group
Table 1 displays the characteristics of the AKI and control groups. In brief, the two groups were well matched with respect to the matching variables namely age, sex, pre-operative left ventricular ejection fraction, surgery type and CPB perfusion time. The other pre- and intra-operative variables were also not significantly different between the two groups.
Table 1.
Characteristics of the nested case-control study groups
| Control group (N = 25) | AKI group (N = 11) | P-value | |
|---|---|---|---|
| Pre-operative variables | |||
| Age, years | 70 ± 12 | 74 ± 9 | 0.40 |
| Serum creatinine, mg/dl | 1.1 (0.3) | 1.1 (0.2) | 0.95 |
| Serum urea nitrogen, mg/dl | 19 (8) | 17 (5) | 0.70 |
| eGFR, ml/min*1.73 m2 | 67 (18) | 65 (8) | 0.62 |
| Women, % | 24 | 27 | 0.83 |
| Diabetes mellitus, % | 17 | 27 | 0.47 |
| Hypertension, % | 71 | 73 | 0.91 |
| History of stroke, % | 4 | 18 | 0.17 |
| Peripheral vascular disease, % | 13 | 27 | 0.28 |
| Left ventricular ejection fraction, % | 53 ± 14 | 48 ± 17 | 0.36 |
| Use of radiocontrast agents, % | 56 | 64 | 0.67 |
| Procedure status, % | |||
| Elective | 29 | 36 | 0.74 |
| Urgent | 67 | 64 | |
| Emergent | 4 | 0 | |
| Valvular surgery, % | 67% | 91% | 0.36 |
| Intra-operative variables | |||
| Aortic cross clamp time, minutes | 92 ± 33 | 99 ± 34 | 0.58 |
| CPB perfusion time, minutes | 127 ± 37 | 143 ± 53 | 0.31 |
| Post-operative variables | |||
| Day-1 APACHE II score | 9 ± 2 | 16 ± 5 | 0.002 |
| Total mechanical ventilation time, hours | 12 (9, 16) | 23 (18, 65) | 0.002 |
| Length of stay in the intensive care unit, days | 3 (2, 3) | 4 (3, 6) | 0.01 |
Data presented as mean ± SD, median (inter-quartile range) or percentage; Comparisons were performed by the Fisher exact test and Wilcoxon test. eGFR denotes estimated glomerular filtration rate according to MDRD formula (Levey et al., 1999), CPB cardiopulmonary bypass, and APACHE Acute Physiology and Chronic Health Evaluation
Figure 1 displays the peri-operative kinetics of serum creatinine, reflecting excellent separation between the AKI and control group. Post-operatively, the AKI group had a significantly higher APACHE II score, prolonged total mechanical ventilation time, and consequently prolonged stay in the intensive care unit (Table 1).
Figure 1. Perioperative profiles of serum creatinine following cardiac surgery in the acute kidney injury (AKI) (n = 11) and control groups (n = 25).
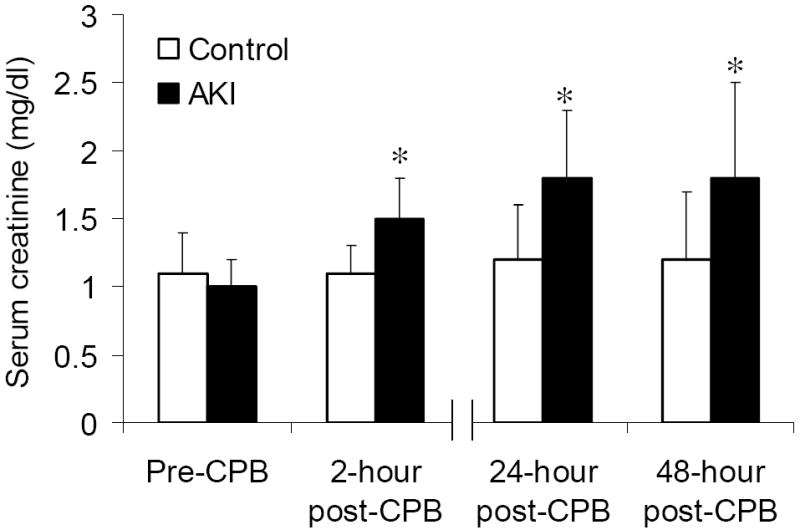
The data are presented as mean values, and the error bars represent standard deviation. CPB denotes cardiopulmonary bypass. * P < 0.02 vs. control group.
Effect of CPB on plasma biomarker profiles
As shown in Table 2, in response to CPB, there was a significant increase in plasma level of several cytokines (e.g. IL-1α, IL-4, IL-6, and IL-10), chemokines (e.g. IL-8, MCP-1, MIP-1α, and MIP-1β), adhesion molecules (e.g. sE-selectin, sICAM-1, and sVCAM-1), and G-CSF, MPO, MMP-9, and PAI-1 (P < 0.05 by the Friedman test), while other markers, such as GMCSF, TGF α and IL-2, showed no apparent changes in plasma level in response to CPB (data not shown). Three distinct patterns of response to CPB could be identified with a subset of biomarkers peaking at 2 hours (IL-1Ra, IL-10, MPO, MIP-1β, IP-10, and MMP-9), at 24 hours (IL-6, G-CSF, MCP-1, and PAI-1), and at 48 hours (IL-1α, IL-4, IL-8, MIP-1α, fractalkine, sE-selectin, sICAM-1, and sVCAM-1).
Table 2.
Selected, time-dependent, AKI-independent plasma biomarker response.
| Pre-CPB | 2-hour post-CPB | 24-hour post-CPB | 48-hour post-CPB | P-value | |
|---|---|---|---|---|---|
| Cytokines | |||||
| IL-1α, pg/ml | 3.2 (3.2, 10.9) | 3.2 (3.2, 4.5) | 14.3 (3.2, 97.2) | 11.6 (3.2, 1032.7) | 0.006 |
| IL-1Ra, pg/ml | 112.4 (38.5, 322.0) | 824.3 (67.1, 5149.1) | 202.9 (45.2, 703.4) | 204.8 (72.5, 560.2) | <0.0001 |
| IL-4, pg/ml | 3.2 (3.2, 3.2) | 3.2 (3.2, 5.3) | 53.8 (3.2, 322.1) | 36.7 (3.2, 931.5) | 0.02 |
| IL-6, pg/ml | 4.2 (3.2, 20.3) | 68.9 (34.1, 208.6) | 216.9 (95.1, 353.0) | 159.6 (115.1, 318.2) | <0.0001 |
| IL-10, pg/ml | 6.6 (3.2, 27.5) | 193.3 (44.2, 412.6) | 70.7 (25.0, 177.3) | 45.4 (11.3, 164.1) | <0.0001 |
| Chemokines | |||||
| IL-8, pg/ml | 3.2 (3.2, 12.8) | 14.5 (3.2, 49.0) | 21.0 (5.7, 57.7) | 21.7 (4.7, 49.7) | <0.0001 |
| MCP-1, pg/ml | 52.7 (11.3, 117.6) | 163.1 (78.7, 329.6) | 133.8 (78.5, 178.4) | 197.1 (112.3, 255.1) | <0.0001 |
| MIP-1α, pg/ml | 3.2 (3.2, 4.7) | 3.8 (3.2, 7.2) | 3.3 (3.2, 10.3) | 5.5 (3.2, 13.3) | 0.002 |
| MIP-1β, pg/ml | 3.2 (3.2, 32.2) | 50.6 (11.4, 142.9) | 3.2 (3.2, 20.9) | 4.7 (3.2, 59.8) | 0.001 |
| IP-10, pg/ml | 235.4 (57.0, 776.2) | 421.1 (235.6, 937.2) | 243.3 (126.2, 425.8) | 244.4 (165.7, 596.7) | 0.01 |
| Fractalkine, pg/ml | 9.5 (3.2, 30.1) | 3.2 (3.2, 13.5) | 17.3, (3.2, 43.8) | 14.3 (3.2, 70.8) | 0.001 |
| Adhesion molecules | |||||
| sE-selectin, ng/ml | 5.5 (4.2, 6.9) | 3.7 (2.4, 5.8) | 5.9 (4.7, 9.8) | 10.1 (6.9, 14.3) | <0.0001 |
| sICAM-1, ng/ml | 68.5 (26.1, 143.6) | 41.8 (31.0, 96.9) | 94.8 (32.9, 196.9) | 189.1, (76.1, 254.5) | <0.0001 |
| sVCAM-1, ng/ml | 134.7 (77.8, 177.4) | 133.2 (103.7, 198.3) | 209.8 (169.5, 251.0) | 248.5 (198.8, 272.3) | <0.0001 |
| Growth factors | |||||
| G-CSF, pg/ml | 3.2 (3.2, 3.2) | 31.4 (3.2, 224.8) | 178.3 (30.4, 551.6) | 57.6 (14.8, 194.0) | <0.0001 |
| Other markers | |||||
| MPO, ng/ml | 1.7 (0.5, 2.9) | 3.89 (2.9, 7.3) | 2.15 (1.5, 5.5) | 3.40 (2.1, 7.7) | 0.005 |
| MMP-9, ng/ml | 1.0 (0.4, 1.9) | 4.1 (1.6, 7.9) | 2.0 (0.8, 3.2) | 1.4 (0.9, 2.3) | <0.0001 |
| PAI-1, ng/ml | 1.9 (0.9, 4.0) | 3.8 (2.1, 9.3) | 5.44 (2.9, 16.1) | 4.60 (2.6, 7.6) | 0.0004 |
Data presented as median (25th, 75th percentile); P value by the Friedman Test.
Peri-operative plasma biomarker profiles in the AKI and control group
Figure 2 displays the peri-operative profiles of the plasma biomarkers that achieved significant or near significant fluctuations over the 4 time points, between the AKI and control group. In brief, prior to surgery, EGF and MIP-1β levels were significantly higher (P < 0.05) in the AKI group compared to the control group, with a non-significant trend toward lower G-CSF levels (P = 0.07). Early after exposure to CPB, at the 2-hour time point, sVCAM-1, fractalkine and MIP-1α levels were significantly higher (P < 0.05) in the AKI group compared to the control group, with a non-significant trend toward lower G-CSF (P = 0.09) and higher MPO (P = 0.09) levels. At the later, 24-hour and 48-hour post-CPB time points, sVCAM-1 and IL-12 levels were significantly lower (P < 0.05) in the AKI group compared to the control group, with a non-significant trend toward higher MIP-1β (P = 0.08) and lower IL-6 (P = 0.08) levels.
Figure 2. Perioperative profiles of selected plasma biomarkers following cardiac surgery in the acute kidney injury (AKI) (n = 11) and control groups (n = 25).
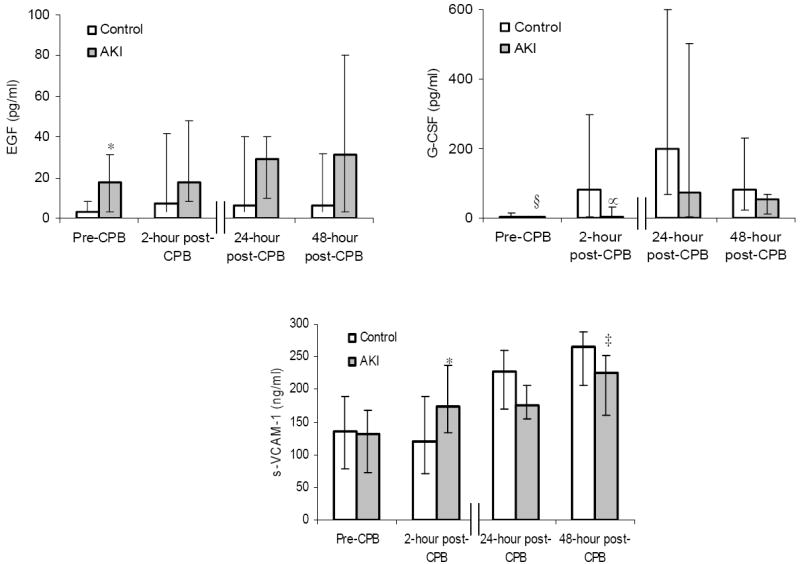
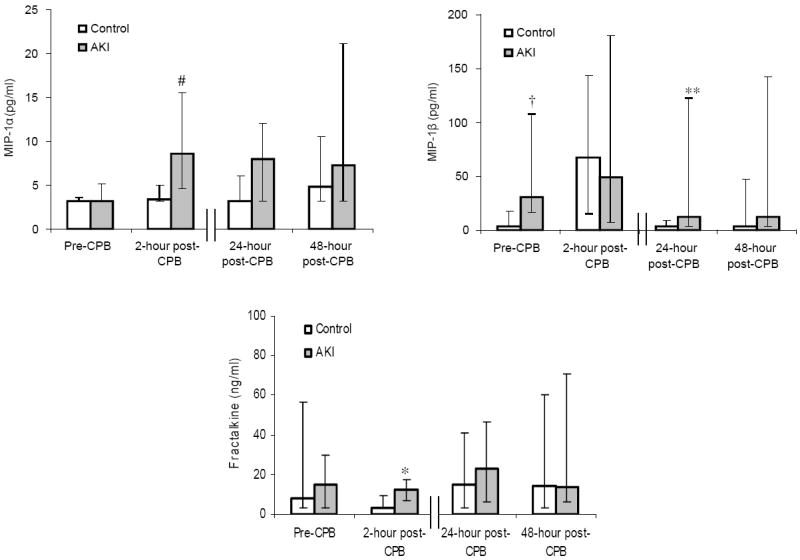
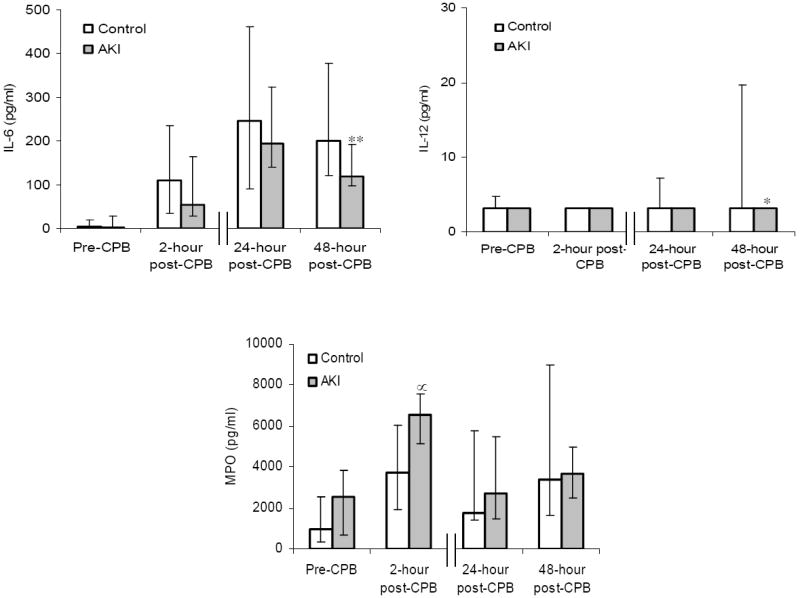
The data are presented as median values, and the error bars represent 25th and 75th percentile values. † P = 0.02, ‡ P = 0.03, * P = 0.04, § P = 0.07, ** P = 0.08, ∝ P = 0.09 vs. control group.
Plasma biomarker diagnostic performance for AKI
Results of the ROC analysis over the 4 time points are summarized in Figure 3 for 8 selected biomarkers. In brief, prior to surgery, amongst all 27 measured biomarkers, only plasma EGF (AUC 0.708; 95% CI 0.508, 0.907; P = 0.04), MIP-1β (AUC 0.739; 95% CI 0.557, 0.921; P = 0.01), and G-CSF (AUC 0.652; 95 CI 0.556, 0.748; P = 0.002) predicted the development of AKI. At the 2-hour post-CPB time point, 4 of the 27 biomarkers were associated with the development of AKI, namely sVCAM-1 (AUC 0.731; 95% CI 0.557, 0.906; P = 0.01), fractalkine (AUC 0.727; 95% CI 0.546, 0.908; P = 0.01), MPO (AUC 0.699; 95% CI 0.497, 0.901; P = 0.05), and G-CSF (AUC 0.652; 95% CI 0.556, 0.748; P = 0.002). At the 24-hour post-CPB time point, only IL-12 (AUC 0.636; 95% CI 0.541, 0.732; P = 0.01) was associated with development of AKI. At the late 48-hour post-CPB time point, 3 markers maintained an association with development of AKI: sVCAM-1 (AUC 0.742; 95% CI 0.558, 0.926; P = 0.01), IL-6 (AUC 0.700; 95% CI 0.507, 0.893; P = 0.04) and IL-12 (AUC 0.693; 95% CI 0.556, 0.829; P = 0.01).
Figure 3. Diagnostic accuracy of selected plasma biomarkers according to the selected perioperative time points following cardiac surgery.
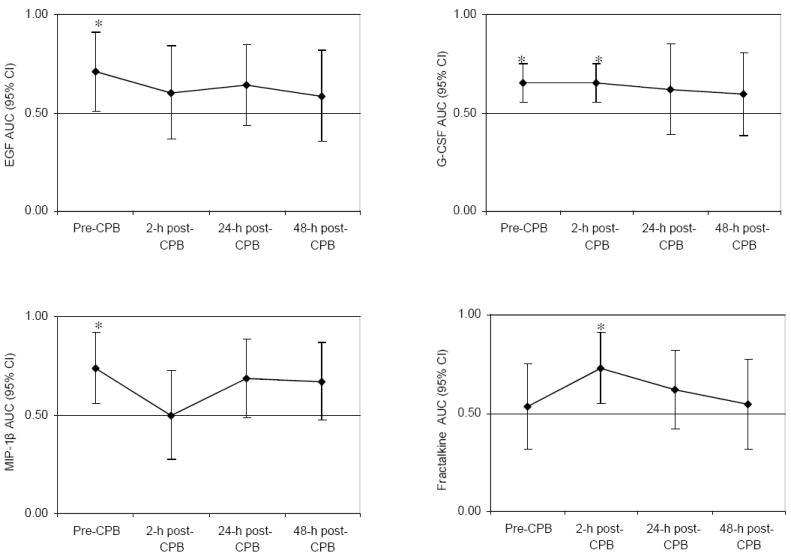

The area under the receiver-operating-characteristic curve (AUC) is shown (with 95% confidence interval) for the 8 biomarkers measured on plasma samples obtained prior to surgery, 2, 24, and 48 hours after discontinuation of cardiopulmonary bypass (CPB), for the detection of acute kidney injury. * P = 0.04; † P = 0.01; ‡ P = 0.002; ** P = 0.05.
DISCUSSION
The measurement of blood and urine biomarkers is becoming increasingly important in the study of disease mechanisms and for the early detection of kidney disease. As a result, there is a growing demand for rapid, precise, and cost-effective measurement of such analytes in both clinical and research laboratory settings (Elshal and McCoy, 2006). Although the enzyme linked immunosorbent assay has traditionally been considered the standard for quantitative analysis of biomarkers, this technique is not well suited for high throughput measurement of multiple analytes. The multiplex bead array assay allows for a quantitative measurement of multiple analytes simultaneously in small volumes of biological fluids, using an automated platform.
In the present pilot nested case control study, we explore the feasibility of measuring multiple proteins in serial plasma samples obtained from patients undergoing cardiac surgery with CPB, using the Luminex platform. Our goal was to identify biomarkers of interest that are potentially linked to the development of AKI, in an effort to elucidate disease mechanisms and provide directions for future research.
The hypotheses to be tested were that several pathophysiologic pathways might be involved in mediating kidney injury following CPB, of which most can be classified as (1) mediators of inflammation, including cytokines (IL-1α, IL-1β, IL-1Ra, IL-2, IL-4, IL-6, TNF-α, IFN-γ, IL-12, IL-10), chemokines (IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, fractalkine) and adhesion molecules (sE-selectin, sICAM-1, sVCAM-1); (2) oxidative stress mediators (MPO); and (3) growth factors (G-CSF, GM-CSF, EGF, VEGF). We also included the protease MMP-9 and the thrombogenic factor PAI-1 in our exploratory analyses. We found that 16 of 27 gene products were up-regulated in response to CPB, with several-fold increases in circulating levels of MIP-1α, and, as early as 2 hours following CPB. Some biomarkers displayed a sustained increase following CPB (IL-6, IL-8, MCP-1, MPO, PAI-1, and G-CSF), whereas others displayed only a transient increase (IL-10, MIP-1β, MMP-9, and IP-10). Other markers displayed a significant but delayed response including IL-1α, IL-4, sE-selectin, and sICAM-1. Our findings confirm some of the markers previously shown to be up-regulated in response to CPB (Wan et al., 1997, Tomic et al., 2005, Laffey et al., 2002, Meldrum and Donnahoo, 1999). One may speculate that an early and sustained response pattern of a marker indicates a stronger association with CPB-induced tissue injury and therefore a more important pathophysiological role in this setting. On the other hand, other CPB-independent injurious stimuli in the postoperative setting, such as reduced cardiac output (Gillies et al., 2005, Giannessi et al., 2007) and surgical wound repair processes (Henry and Garner, 2003), may also play a causative role in the delayed/sustained as compared to the early and transient patterns of response.
Our nested case control study designed to match the AKI and control groups to important characteristics including CPB perfusion time (an important predictor of AKI), allowed us to better decipher the differential regulation of the selected biomarkers in AKI. We observed differential expression of MIP-1β, EGF, and G-CSF prior to surgery, sVCAM-1, fractalkine, MIP-1α, G-CSF, and MPO 2-hours post-CPB, and IL-6, IL-12, MIP-1β, and sVCAM-1 at the later time points.
MIP-1α and MIP-1β are chemokines that are secreted by monocytes and lymphocytes including T-cells (Irving et al., 1990). MIP-1β is a key trigger of macrophage migration, acting through the chemokine (C-C motif) receptor 5 (CCR5) (Cheung et al., 2009) and inducing downstream signaling pathways, resulting in increased generation of reactive oxygen species (Tatara et al., 2009). MIP-1β has been implicated in the pathogenesis of acute renal allograft rejection (Fischereder, 2007) and progression of chronic kidney disease (Galkina and Ley, 2006). It is conceivable that the preexisting elevation of MIP-1β found in our study might be indicative of macrophage activation and enhanced migration prior to cardiac surgery, which in turn, could predispose to a pro-inflammatory surge in target organs in response to the CPB stimulus. In this context, our results might indicate that pre-operative immune-activation, perhaps induced by cardiac ischemia and as evidenced by elevated plasma MIP-1β levels may place patients at risk for developing AKI if subjected to cardiac surgery with CPB and may therefore serve as a preoperative renal risk marker.
Endothelial injury is an important pathophysiologic feature of ischemic AKI, particularly in the extension phase (Molitoris and Sutton, 2004). Fractalkine expression on injured endothelium functions both as a potent chemoattractant and as an adhesion molecule for the recruitment and migration of fractalkine receptor-expressing circulating inflammatory cells into sites of inflammation (Beck et al., 2003, Imai et al., 1997). A soluble form of this chemokine is a potent chemoattractant of T cells and monocytes. Increased fractalkine expression and macrophage infiltration has been observed in cisplatin-induced AKI (Lu et al., 2008). In ischemic AKI, there is upregulation of fractalkine specifically in kidney blood vessels, and fractalkine receptor inhibition is protective and is associated with reduced macrophage infiltration in the kidney (Oh et al., 2008). Fractalkine has been implicated in the pathogenesis of thrombotic microangiopathies (Ramos et al., 2007, Zanchi et al., 2008). We observed a significant increase in soluble fractalkine levels 2 hours after CPB in the AKI group as compared to the control group, and in the ROC analysis, this biomarker achieved an AUC greater than 0.700, for the prediction of AKI, suggesting potential involvement of this chemokine in disease mechanism. By contrast, other circulating markers of endothelial damage we tested were not differentially expressed between the AKI and control subjects.
Prior studies of patients undergoing on-pump cardiac surgery have shown an association of circulating sICAM-1 levels with pulmonary dysfunction (Gorlach et al., 2003), and of sE-selectin, P-selectin and sICAM-1 levels with prolonged postoperative requirement for vasopressor use (Wei et al., 2003), and post-operative development of sepsis (Paret et al., 2000). Although we found a uniform increase in plasma sICAM-1 and sE-selectin in response to CPB, these markers did not discriminate between the AKI and control groups. Similarly to E-selectin, VCAM-1 is also expressed on the surface of endothelial cells in response to inflammatory cytokines and facilitates leukocyte recruitment to sites of inflammation (Kluger, 2004). Consistent with prior observations (Andresen et al., 2002), in our study, sVCAM-1 levels also rose following CPB, but were differentially expressed displaying a biphasic response between the AKI and control group. Indeed, sVCAM-1 was significantly higher at 2 hours but significantly lower at 48 hours post-CPB in the AKI vs. control group. Such a biphasic endothelial response to inflammatory stimuli has previously been described (Kim et al., 2004, Ricard et al., 1997, Ho et al., 2009).
Finally, we also found that plasma G-CSF and EGF levels were differentially expressed between the AKI and control groups. Indeed, G-CSF levels were lower in patients with AKI prior to and 2-hours after CPB. This finding is in keeping with the hypothesis that hematopoietic stem cells may play a role in repair processes following AKI and may hasten recovery or abrogate the extension phase (Lin et al., 2003). However, this stands in contrast to a recent animal study, which found that granulocytosis induced by the exogenous administration of G-CSF, as opposed to spontaneous, physiological G-CSF fluctuations, worsened ischemia reperfusion injury (Togel et al., 2004). Surprisingly in our study, EGF, a growth factor involved in reparative processes and known to accelerate restoration of kidney function in experimental models of AKI (Hammerman, 1998), was elevated prior to surgery in the AKI group.
Our study has several strengths. We explored disease mechanism in a human model of AKI where the timed insult to the kidney was clearly defined, i.e., the CPB stimulus. Furthermore, in our nested case-control study design, using a nearest-neighbor matching technique, we were able to match for important variables that are known to predict AKI including age, pre-operative left ventricular ejection fraction, surgery type, and CPB perfusion time. This allowed us to minimize the potentially confounding effect of these variables on the study of disease mechanisms using blood as a sentinel organ. In addition, we used a high throughput customized platform that allowed us to measure simultaneously 27 biomarkers of interest over several time points, using low-volume plasma samples. These biomarkers were carefully selected based on their potential involvement in mechanisms of disease relevant to AKI. There are however, some important limitations to consider. We studied a relatively small sample of patients and performed multiple testing, and our observed associations between select biomarkers and AKI cannot prove causality. Further, our ROC analysis indicated rather mild to moderate as opposed to strong associations between these biomarkers and AKI. In addition, our AKI definition was based on incremental changes in serum creatinine. Although this creatininebased definition has been well linked to adverse clinical outcomes (Uchino et al., 2006), it may not fully capture more subtle structural kidney damage that might have occurred in both the AKI and control groups. Our findings however, support a potential role for chemokines in the development of AKI following cardiac surgery, particularly fractalkine and MIP-1β. Further studies are needed to confirm and extend our preliminary findings.
In conclusion, in the present nested case-control study, we explored mechanisms of disease in AKI following on-pump cardiac surgery, using a multiplex bead array assay with blood as a sentinel organ. Although several inflammatory, leukocyte and oxidative stress markers, as well as growth factors were affected by CPB, only a few were associated with AKI following CPB, and might be of higher importance in the pathophysiology or for the prediction of this complex disorder, and merit further study.
Acknowledgments
The authors thank Mary C. Perianayagam, PhD for her technical assistance.
DECLARATION OF INTEREST This work was supported in part by the American Heart Association (AHA #0535367N, to Orfeas Liangos) and institutional research funds (to Orfeas Liangos). Bertrand Jaber is supported in part by a grant from the National Institutes of Health (DK077751). Dr. Goligorsky is supported by grants from the National Institutes of Health (DK54602, DK052783, DK45462 (MSG)) and the Westchester Artificial Kidney Foundation.
Appendix 1
Selected 27 plasma biomarkers
| Cytokines |
| Tumor necrosis factor-α (TNF-α) |
| Interleukin-1α (IL-1α) |
| Interleukin-1β (IL-1β) |
| Interleukin-1Ra (IL-1Ra) |
| Interleukin-2 (IL-2) |
| Interleukin-4 (IL-4) |
| Interleukin-6 (IL-6) |
| Interleukin-10 (IL-10) |
| Interleukin-12 (IL-12) |
| Interferon-γ (IFN-γ) |
| Transforming growth factor-α (TGF-α) |
| Chemokines |
| Interleukin-8 (IL-8) |
| Monocyte chemoattractant protein-1 (MCP-1) |
| Macrophage inflammatory protein-1α (MIP-1α) |
| Macrophage inflammatory protein-1β (MIP-1β) |
| Induced protein-10 (IP-10) |
| Fractalkine |
| Adhesion molecules |
| Soluble E-selectin (sE-selectin) |
| Soluble inter-cellular adhesion molecule-1 (sICAM-1) |
| Soluble vascular cell adhesion molecule-1 (sVCAM-1) |
| Growth factors |
| Granulocyte colony-stimulating factor (G-CSF) |
| Granulocyte macrophage colony-stimulating factor (GM-CSF) |
| Epidermal growth factor (EGF) |
| Vascular endothelial growth factor (VEGF) |
| Pro-oxidant enzymes |
| Myeloperoxidase (MPO) |
| Extracellular matrix enzymes |
| Matrix metalloproteinase-9 (MMP-9) |
| Fibrinolysis inhibitor |
| Plasminogen activator inhibitor-1 (PAI-1) |
Footnotes
This work was presented in part at the 40th Annual Meeting of the American Society of Nephrology, San Francisco, CA, October 31-November 5, 2007.
References
- Andresen TK, Svennevig JL, Videm V. Soluble VCAM-1 is a very early marker of endothelial cell activation in cardiopulmonary bypass. Perfusion. 2002;17:15–21. doi: 10.1191/0267659102pf531oa. [DOI] [PubMed] [Google Scholar]
- Beck G, Ludwig F, Schulte J, van Ackern K, van der Woude FJ, Yard BA. Fractalkine is not a major chemoattractant for the migration of neutrophils across microvascular endothelium. Scand J Immunol. 2003;58:180–7. doi: 10.1046/j.1365-3083.2003.01298.x. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Mehta R, Palevsky PM, Ronco C. The Acute Dialysis Quality Initiative II: the Vicenza conference. Adv Ren Replace Ther. 2002;9:290–3. doi: 10.1053/jarr.2002.35574. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Cheung R, Malik M, Ravyn V, Tomkowicz B, Ptasznik A, Collman RG. An arrestindependent multi-kinase signaling complex mediates MIP-1beta/CCL4 signaling and chemotaxis of primary human macrophages. J Leukoc Biol. 2009;86:833–45. doi: 10.1189/jlb.0908551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer J, Martin M, Redl H, Bahrami S, Abraham C, Graeter T, Haverich A, Schlag G, Borst HG. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. 1996;61:1714–20. doi: 10.1016/0003-4975(96)00055-0. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- Elgebaly SA, Houser SL, el Kerm AF, Doyle K, Gillies C, Dalecki K. Evidence of cardiac inflammation after open heart operations. Ann Thorac Surg. 1994;57:391–6. doi: 10.1016/0003-4975(94)91003-0. [DOI] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischereder M. Chemokines and chemokine receptors in renal transplantation--from bench to bedside. Acta Physiol Hung. 2007;94:67–81. doi: 10.1556/APhysiol.94.2007.1-2.7. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–77. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- Giannessi D, Colotti C, Maltinti M, Del Ry S, Prontera C, Turchi S, Labbate A, Neglia D. Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones. 2007;12:265–74. doi: 10.1379/CSC-272.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M, Bellomo R, Doolan L, Buxton B. Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Crit Care. 2005;9:266–79. doi: 10.1186/cc3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach G, Sroka J, Heidt M, Knez I, Sablotzki A, Schonburg M, Akinturk H, Roth P, Wozniak G, Vogt PR. Intracellular adhesion molecule-1 in patients developing pulmonary insufficiency after cardiopulmonary bypass. Thorac Cardiovasc Surg. 2003;51:138–41. doi: 10.1055/s-2003-40314. [DOI] [PubMed] [Google Scholar]
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Hammerman MR. Renal programmed cell death and the treatment of renal disease. Curr Opin Nephrol Hypertens. 1998;7:1–3. doi: 10.1097/00041552-199801000-00001. [DOI] [PubMed] [Google Scholar]
- Henry G, Garner WL. Inflammatory mediators in wound healing. Surg Clin North Am. 2003;83:483–507. doi: 10.1016/S0039-6109(02)00200-1. [DOI] [PubMed] [Google Scholar]
- Ho D, Imai K, King G, Stuart E. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis. 2007;15:199–236. [Google Scholar]
- Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis. 2009;53:584–95. doi: 10.1053/j.ajkd.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Irving SG, Zipfel PF, Balke J, McBride OW, Morton CC, Burd PR, Siebenlist U, Kelly K. Two inflammatory mediator cytokine genes are closely linked and variably amplified on chromosome 17q. Nucleic Acids Res. 1990;18:3261–70. doi: 10.1093/nar/18.11.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lessner SM, Sakurai Y, Galis ZS. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am J Pathol. 2004;164:1567–74. doi: 10.1016/S0002-9440(10)63715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MS. Vascular endothelial cell adhesion and signaling during leukocyte recruitment. Adv Dermatol. 2004;20:163–201. [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52:1284–93. doi: 10.1373/clinchem.2006.067595. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Hakkert BC, Hart MH, Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992;117:565–72. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettila V. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–6. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–52. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Liangos O, H WK, Wald R, Perianayagam MC, Balakrishnan VS, MacKinnon RW, Warner K, Symes JF, Li L, Kouznetsov A, Pereira BJG, Bonventre JV, Jaber BL. Urinary Kidney Injury Molecule-1 (KIM-1) and N-acetyl(beta)-D-glucosaminidase (NAG) levels in patients undergoing Cardiac Surgery with Cardiopulmonary Bypass (CPB) J Am Soc Nephrol. 2005;16:318A. [Google Scholar]
- Liangos O, Kolyada A, Perianayagam MC, Tighiouart H, Wald R, MacKinnon R, Warner K, Dolan N, Jaber BL. Increased plasma IL-8 level is associated with IL-8 -251 AA genotype and with acute kidney injury following cardiopulmonary bypass. J Am Soc Nephrol. 2007:798A. [Google Scholar]
- Liangos O, Kolyada A, Tighiouart H, Perianayagam MC, Wald R, Jaber BL. Interleukin-8 and acute kidney injury following cardiopulmonary bypass: a prospective cohort study. Nephron Clin Pract. 2009a;113:c148–54. doi: 10.1159/000232595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, Bonventre JV, Jaber BL. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009b;14:423–31. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–99. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther. 2008;324:111–7. doi: 10.1124/jpet.107.130161. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. 2003;14:2178–87. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Donnahoo KK. Role of TNF in mediating renal insufficiency following cardiac surgery: evidence of a postbypass cardiorenal syndrome. J Surg Res. 1999;85:185–99. doi: 10.1006/jsre.1999.5660. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–9. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol. 2008;294:F264–71. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- Paret G, Prince T, Keller N, Dagan O, Sasson Y, Barzilai A, Guthmann D, Barzilay Z. Plasma-soluble E-selectin after cardiopulmonary bypass in children: is it a marker of the postoperative course? J Cardiothorac Vasc Anesth. 2000;14:433–7. doi: 10.1053/jcan.2000.7942. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- Ramos MV, Fernandez GC, Patey N, Schierloh P, Exeni R, Grimoldi I, Vallejo G, Elias-Costa C, Del Carmen Sasiain M, Trachtman H, Combadiere C, Proulx F, Palermo MS. Involvement of the fractalkine pathway in the pathogenesis of childhood hemolytic uremic syndrome. Blood. 2007;109:2438–45. doi: 10.1182/blood-2006-06-026997. [DOI] [PubMed] [Google Scholar]
- Ray CA, Bowsher RR, Smith WC, Devanarayan V, Willey MB, Brandt JT, Dean RA. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. J Pharm Biomed Anal. 2005;36:1037–44. doi: 10.1016/j.jpba.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Ricard I, Payet MD, Dupuis G. Clustering the adhesion molecules VLA-4 (CD49d/CD29) in Jurkat T cells or VCAM-1 (CD106) in endothelial (ECV 304) cells activates the phosphoinositide pathway and triggers Ca2+ mobilization. Eur J Immunol. 1997;27:1530–8. doi: 10.1002/eji.1830270632. [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- Tatara Y, Ohishi M, Yamamoto K, Shiota A, Hayashi N, Iwamoto Y, Takeda M, Takagi T, Katsuya T, Ogihara T, Rakugi H. Macrophage inflammatory protein-1beta induced cell adhesion with increased intracellular reactive oxygen species. J Mol Cell Cardiol. 2009;47:104–11. doi: 10.1016/j.yjmcc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Togel F, Isaac J, Westenfelder C. Hematopoietic stem cell mobilization-associated granulocytosis severely worsens acute renal failure. J Am Soc Nephrol. 2004;15:1261–7. doi: 10.1097/01.asn.0000123692.01237.0a. [DOI] [PubMed] [Google Scholar]
- Tomic V, Russwurm S, Moller E, Claus RA, Blaess M, Brunkhorst F, Bruegel M, Bode K, Bloos F, Wippermann J, Wahlers T, Deigner HP, Thiery J, Reinhart K, Bauer M. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation. 2005;112:2912–20. doi: 10.1161/CIRCULATIONAHA.104.531152. [DOI] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–7. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–92. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- Wei M, Laurikka J, Kuukasjarvi P, Pehkonen E, Tarkka M. Soluble adhesion molecules in coronary artery bypass surgery. Asian Cardiovasc Thorac Ann. 2003;11:198–202. doi: 10.1177/021849230301100303. [DOI] [PubMed] [Google Scholar]
- Zanchi C, Zoja C, Morigi M, Valsecchi F, Liu XY, Rottoli D, Locatelli M, Buelli S, Pezzotta A, Mapelli P, Geelen J, Remuzzi G, Hawiger J. Fractalkine and CX3CR1 mediate leukocyte capture by endothelium in response to Shiga toxin. J Immunol. 2008;181:1460–9. doi: 10.4049/jimmunol.181.2.1460. [DOI] [PubMed] [Google Scholar]


