Abstract
Hypoxia-inducible factor-1 (HIF-1) is a key mediator of oxygen homeostasis that was first identified as a transcription factor that is induced and activated by decreased oxygen tension. Upon activation, HIF-1 upregulates the transcription of genes that promote adaptation and survival under hypoxic conditions. HIF-1 is a heterodimer composed of an oxygen-regulated subunit known as HIF-1α and a constitutively expressed HIF-1β subunit. In general, the availability and activity of the HIF-1α subunit determines the activity of HIF-1. Subsequent studies have revealed that HIF-1 is also activated by environmental and physiological stimuli that range from iron chelators to hormones. Preclinical studies suggest that HIF-1 activation may be a valuable therapeutic approach to treat tissue ischemia and other ischemia/hypoxia-related disorders.
The focus of this review is natural product-derived small molecule HIF-1 activators. Natural products, relatively low molecular weight organic compounds produced by plants, animals, and microbes, have been and continue to be a major source of new drugs and molecular probes. The majority of known natural product-derived HIF-1 activators were discovered through pharmacological evaluation of specifically selected individual compounds. The combination of natural products chemistry with appropriate high-throughput screening bioassays could provide an alternative approach to discover novel natural product-derived HIF-1 activators. Potent natural product-derived HIF-1 activators that exhibit a low level of toxicity and side effects hold promise as new treatment options for diseases such as myocardial and peripheral ischemia, and as chemopreventative agents that could be used to reduce the level of ischemia/reperfusion injury following heart attack and stroke.
Keywords: HIF-1, Natural Product, Tissue Ischemia, Therapeutic Angiogenesis, Molecular-Target, Small Molecule Activator, Chemoprevention, Ischemia/Reperfusion Injury
Introduction
Over the course of time, multicellular organisms have evolved tightly regulated oxygen delivery systems to ensure oxygen dependent energy production. In the human body, high levels of oxygen (hyperoxia) can cause oxygen toxicity while low levels (hypoxia) are associated with hypoxia/ischemia-related diseases such as ischemic and neoplastic disorders. At the organism level, the body responds to changes in oxygen levels by altering respiration rate and blood flow. At the cellular level, changes in oxygen levels can trigger responses from a network of signaling pathways that lead to alterations in gene expression patterns.
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that is activated by hypoxic conditions [1]. It is composed of a HIF-1α subunit and a HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator or ARNT) subunit, both are members of the basic helix-loop-helix (bHLH) PER-ARNT-SIM (PAS) family of transcription factors [2]. Over seventy genes have been identified as HIF-1 target genes and the list is still growing [3]. These genes encode proteins that are involved in many aspects of cellular physiology, ranging from cellular metabolism, cell proliferation/survival/death, cytoskeletal structure, cell adhesion/motility, angiogenesis, erythropoiesis, vascular tone, to drug resistance. It is no surprise that HIF-1 plays a crucial role in development, physiological processes such as wound healing, and pathological processes such as tumor progression and tissue ischemia [3–5].
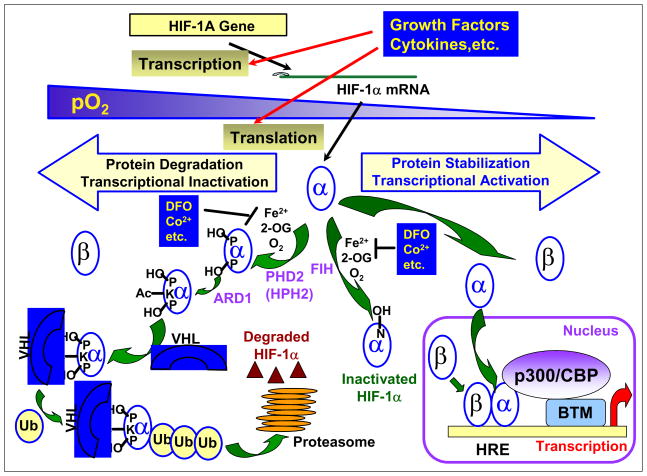
As a key regulator of oxygen homeostasis, HIF-1 is tightly regulated by the level of oxygen. Initial studies revealed that the HIF-1α subunit is degraded rapidly under normoxic conditions and stabilized under hypoxic conditions, while the HIF-1β subunit is constitutively expressed [2]. One of the breakthroughs in elucidating the pathways and mechanisms involved in HIF-1 activation is the discovery that prolyl hydroxylation of HIF-1α protein is followed by the von Hippel-Lindau disease tumor suppressor (pVHL)-mediated, oxygen-dependent degradation of HIF-1α protein, Fig. (1) [6–10]. Under normoxic conditions, the HIF-1α subunit is hydroxylated by prolyl hydroxylases in the presence of oxygen and iron. The prolyl hydroxylated HIF-1α subunit is then recognized by an E3 ubiquitin ligase complex that contains pVHL, polyubiquitinated, and degraded by the 26S proteosome. When the cellular oxygen level is reduced, prolyl hydroxylation is inhibited. Inhibition of HIF-1α protein prolyl hydroxylation and degradation leads to an increase in HIF-1α protein level. Oxygen-dependent regulation of HIF-1 activity also occurs at the transactivation level [11–17]. The HIF-1α subunit is hydroxylated by asparaginyl hydroxylase in the presence of oxygen and iron [12–15]. Hydroxylation of the asparagine-803 in HIF-1α abrogates the interaction between HIF-1α and the coactivator CBP/p300 [CREB(cAMP-response element-binding protein)-binding protein/E1A-binding protein; 300kD] [16–17]. Upon reduction in oxygen concentration, the asparaginyl hydroxylation of HIF-1α protein is blocked. The stabilized and activated HIF-1α protein translocates into the nucleus where it heterodimerizes with the HIF-1β subunit and binds to hypoxia response elements (HREs) present on the promoters of HIF-1 target genes. This complex recruits coactivators such as CBP/p300 and enhances transcription. Kinetic studies suggest that the prolyl hydroxylases that modify HIF-1α protein are more sensitive to decreases in oxygen tension than the asparaginyl hydroxylase that modifies HIF-1α protein [18]. The pathways and mechanisms that connect oxygen sensing, hydroxylation, and HIF-1 are extensively discussed in a number of recent reviews [19–26]. In addition to hydroxylation, HIF-1 activity is also regulated by other post-translational modifications that include phosphorylation, acetylation, S-nitrosation, SUMOylation, and ubiquitination [26].
Fig. (1).
Oxygen-Dependent Regulation of HIF-1α Stabilization and Activation
From the aspect of drug discovery, target validation is a critical step towards finding effective therapeutic agents. Results from extensive preclinical and clinical studies support HIF-1 as a valid molecular target for anti-cancer drug discovery [3–4, 27–31]. Overexpression of the HIF-1α subunit is associated with advanced disease stages, poor prognosis, and treatment resistance among cancer patients, while inhibition of HIF-1 retards tumor growth in animal models. A number of recent reviews have summarized the progress towards the discovery of potential cancer therapeutic agents that inhibit HIF-1 [3, 27–30]. Intense research efforts directed at the discovery of novel HIF-1 inhibitors for the treatment of cancer are currently underway in academic, industrial, and government laboratories.
Agents that activate HIF-1 may prevent ischemia/reperfusion injuries and speed recovery from tissue ischemia. The focus of this review is natural product-derived activators of HIF-1 and their therapeutic potential. In contrast to the general impression among the lay public that “natural products” and “herbal medicines” are interchangeable terms, in the field of natural products chemistry and drug discovery the term “natural product” refers to small organic compounds that are generally thought to be “secondary metabolites” that play a role in chemical defense or otherwise enhance survival. These are typically low molecular weight compounds produced by animals, plants, and microbes. Several “primary metabolites” (compounds required for the normal biochemical and/or physiological function of an organism) such as certain steroids, carbohydrates, and a prostaglandin have been shown to regulate HIF-1. In light of the significant roles these compounds play on HIF-1 mediated-signaling pathways, primary metabolites that regulate HIF-1 activation are included in this review. Natural products have been a major source of new drugs for centuries and the chemical diversity offered by natural products has not been matched by other chemical approaches. Statistics show that over 60% of the approved anticancer, antihypertensive, and antimigraine medications are of natural origin (natural products or synthetic compounds based on natural product models) [32]. A large number of the molecular probes currently used in biomedical research are natural products (i.e. rapamycin, genistein, wortmannin, actinomycin D, cycloheximide, geldanamycin, etc.) or derived from natural products (i.e. PD98059, LY294002, etc.). In this review, natural product-derived HIF-1 activators, grouped by their chemical or structural classes, will be discussed in regard to their discovery, mechanism of action, and therapeutic potential.
Alkaloids and Other Amino Acid Derivatives
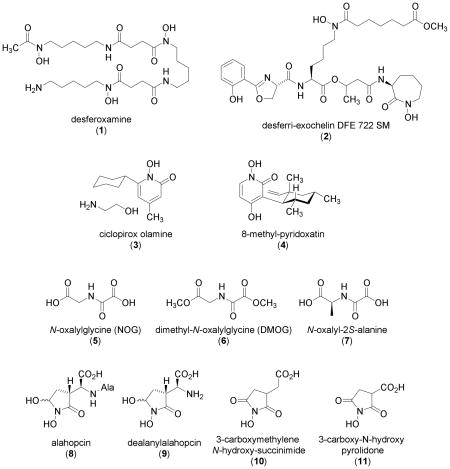
The first natural product shown to activate HIF-1 was deferoxamine (1) [33]. Deferoxamine (1) was first identified as a siderochrome from Streptomyces pilosus that chelates ferric ions [Fe3+; Fe (III)] [34]. The metal free mesylate salt form (desferoxamine mesylate, Desferal Mesylate®) is used clinically as a heavy metal antagonist for the treatment of iron and aluminum poisoning [35]. Wang and coworkers demonstrated that 1 induces the DNA-binding activity of HIF-1 and increases erythropoietin (a HIF-1 target gene) mRNA levels in cultured cells [Hep3B human hepatoma and Chinese hamster ovary (CHO)] [33]. Deferoxamine (1) has since been widely used as a hypoxia mimetic to activate HIF-1. The prolyl and asparaginyl hydroxylases that destabilize and inactivate HIF-1α protein are Fe(II)- and 2-oxoglutarate dependent oxygenases. Since 1 is water soluble and has a chelation preference for Fe(III), it is likely that 1 inhibits HIF prolyl and asparaginyl hydroxylases by preventing the uptake of cellular iron ions and thus activates HIF-1.
The tuberculosis pathogen Mycobacterium tuberculosis secretes desferri-exochelins (high-affinity iron-binding siderophores) to extract iron ions from host proteins [36]. Treatment of cells with desferri-exochelin DFE 722 SM (2) induced HIF-1α protein, activated HIF-1, and increased the expression of known HIF-1 targets such as VEGF (vascular endothelial growth factor) and NIP3 (BCL2/adenovirus E1B 19-kD protein-interacting protein 3) in human breast tumor MDA468 cells [37]. The fact that iron-bound ferri-exochelin did not activate the HIF-1 pathway suggests that iron chelation is required for the activation of HIF-1. While both deferoxamine (1) and desferri-exochelins are high affinity Fe(III) chelators, desferri-exochelin DFE 722 SM (2) is at least ten times more potent than deferoxamine at HIF-1 activation [37]. Desferri-exochelin (2) is highly lipophilic, thus allowing it to readily enter cells and chelate intracellular iron ions. In contrast, 1 is hydrophilic, limiting its ability to penetrate cell membranes. This apparent difference in polarity may contribute to the difference in potency between these two iron chelators. Desferri-exochelin DFE 722 SM (2) most likely functions by inhibiting the Fe(II)-dependent prolyl and asparaginyl hydroxylases that modify, destabilize, and inactivate HIF-1α protein.
Ciclopirox olamine [3, Loprox®, 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone 2-aminoethanol] is a commonly used antimycotic agent to treat fungal infections of the skin and nail [38]. In human heptoma HepG2 cells, 3 was shown to stabilize HIF-1α protein, induce HIF-1α protein nuclear translocation, facilitate the binding of HIF-1 to hypoxia-response elements, and promote HIF-1 activation [39]. Ciclopirox olamine (3) is at least ten times more potent than deferoxamine (1) at activating HIF-1 in vitro and ciclopirox olamine-induced HIF-1 activation can be blocked by Fe(II) and Al(III) ions [39]. It is likely that 3 also acts as an iron chelator and activates HIF-1 through the inhibition of HIF prolyl and asparaginyl hydroxylases that destabilize and inactivate HIF-1α protein under normoxic conditions. Compound 3 was shown to induce the expression of HIF-1 targets such as VEGF, GLUT-1 (glucose transporter-1), and aldolase in vitro (HepG2 cells), and stimulate angiogenesis (consequence of VEGF induction) in vivo [mouse skin wound model and chicken embryo chorioallantoic membrane (CAM) assay] [40]. However, 3 failed to induce HIF-1 target genes in an ex vivo organ model (isolated rat kidneys perfused with 3) [40].
The compound 8-methyl-pyridoxatin (4) is structurally related to ciclopirox olamine (3) and induces erythropoietin expression in human hepatoma Hep3B cells [41]. Compound (4) has been shown to activate HIF-1 in an engineered CHO cell-based reporter assay and induce HIF-1α protein in HepG2 cells [39]. Neither the effect of 4 on the expression of other HIF-1 target genes nor the mechanism of action has yet been reported.
The HIF prolyl and asparaginyl hydroxylases are members of the Fe(II)- and 2-oxoglutarate (2OG)-dependent oxygenase superfamily. Most members of this superfamily, including those that modify HIF, couple the oxidative decarboxylation of 2OG with the hydroxylation of substrate [20–24]. Analogues of 2OG such as N-oxaloylglycine (5, NOG) were originally developed as inhibitors of prolyl-4-hydroxylase, one of the Fe(II)- and 2OG-dependent oxygenases [42]. Both 5 and an ester of NOG known as DMOG (6, dimethyl-oxalylglycine) have been shown to induce HIF-1α protein through the inhibition of prolyl hydroxylation [7]. The ester form of NOG (6) is more potent in cell-based assays, presumably due to increased membrane permeability [17]. Subsequently, 6 was shown to inhibit the asparaginyl hydroxylation of HIF-1α protein and activate HIF-1 [12]. Analysis of X-ray crystallographic data of HIF asparaginyl hydroxylase (factor-inhibiting HIF, or FIH) complexed with either substrates or inhibitors indicates that 5 inhibits FIH by displacing the co-substrate 2OG from the active site and hindering the nucleophilic attack at the 2-carbonyl group [43]. In studies that employed recombinant FIH protein expressed by insect cells, 5 inhibited FIH and human type I collagen prolyl-4-hydroxylase (C-P4H-I) with the same potency, and inhibited HIF prolyl hydroxylases (HPH or PHD) with reduced efficiency (4- to 25-fold increase in Ki values, depending on each isoenzyme) [44, 45]. Among the known C-P4H-I inhibitors, compounds such as N-((3-hydroxy-6-chloroquinolin-2-yl)carbonyl)-glycine and 3-hydroxypyridine-2-carbonyl-glycine are selective inhibitors of HPHs relative to their affects on FIH [44, 45]. By contrast, 3,4-dihydroxybenzoate and pyridine-2,5-dicarboxylate are significantly more selective inhibitors of FIH, relative to their effect on HPHs, and pyridine-2,4-dicarboxylate has a comparable inhibitory effect on both FIH and HPHs [44, 45]. The alanine derivative N-oxalyl-2S-alanine (7), but not its epimer N-oxalyl-2R-alanine inhibited both HIF prolyl and asparaginyl hydroxylases [7, 43]. A structure-activity relationship study conducted using a cell-free assay system identified a number of additional 2OG analogues that inhibit HPHs [46]. The natural products alahopcin (8) and dealanylalahopcin (9) were first isolated from Streptomyces albulus subsp. ochragerus and both compounds exhibited weak inhibitory activity against the collagen prolyl hydroxylase [47–49]. Two dealanylalahopcin analogues 3-carboxymethylene N-hydroxy succinimide (10) and 3-carboxy-N-hydroxy pyrollidone (11) were subsequently shown to act as HPH inhibitors [50]. Since most of these HPH inhibitors were identified in cell-free assay systems, whether these substances can specifically activate the HIF-1 pathway in relevant biological systems remain to be demonstrated.
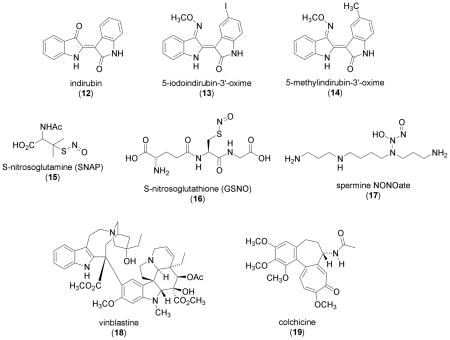
The level of HIF-1α protein has been shown to decrease over extended incubation (24 h) under either anoxic or hypoxic (0.1% O2) conditions in human colon carcinoma RKO cells [51]. This same study revealed that two derivatives of the natural product indirubin (12) [5-iodoindirubin-3'-oxime (13) and 5-methylindirubin-3'-oxime (14)] prevented the decrease in HIF-1α protein through the inhibition of GSK3β (glucogen synthase kinase 3β) and increased HIF-1α protein translation. Neither indirubin derivative affected the level of HIF-1α protein under normoxic conditions. Whether this indirubin derivative-induced stabilization of HIF-1α protein is associated with increased HIF-1 activity was not addressed. Indirubin (12) is the active ingredient of Danggui Longhui Wan, a traditional Chinese remedy used to treat various diseases including chronic myelocytic leukemia [52]. It has been shown that indirubin derivatives such as compound (13) inhibit GSK3β, cyclin-dependant kinase-1/cyclin B (CDK1/cyclin B), and cyclin-dependant kinase-5/p25 (CDK5/p25) kinases with similar potency [53]. A structurally related compound indirubin-3'-monoxime inhibited a panel of kinases with IC50 values in the nanomolar range [54]. The apparent lack of selectivity observed in these studies raises question as to the actual mechanism of action for these indirubin derivativres and whether or not the reported increase in HIF-1α protein will translate into an actual increase in HIF-1 activity.
One group of amino acid-derived natural products that activate HIF-1 is nitric oxide (NO) donors. Initial studies revealed that NO inhibits HIF-1 activation under hypoxic conditions [55–57]. Later, Kumara and coworkers discovered that the HIF-1 binding site in the human VEGF promoter actually mediates NO-induced activation of VEGF transcription under normoxic conditions [58]. The NO donor S-nitroso-N-acetyl-D,L-penicillamine (15, SNAP) induced HIF-1α protein accumulation, HIF-1 binding activities, and activated transcription from the VEGF promoter in A-172 human glioblastoma and Hep3B cells. Another NO donor, 3-(hydroxy-1-(1-methylethyl)-2-nitrosohydrazino)-1-propanamine (NOC5) also activated the VEGF promoter in a cell-based reporter assay. In bovine pulmonary artery endothelial and rat aortic smooth muscle cells, the NO donor diazen-1-ium-1,2-diolate (NOC-18), induced HIF-1α protein and HIF-1 DNA binding, augmented HIF-1β protein expression, and activated expression of the HIF-1 target gene heme oxygenase-1 (HO-1) [59]. The induction of HIF-1 by NOC-18 is both dose- and time-dependent (optimal induction: 500 μM, 3–4 h). It was observed that Angeli's salt (a NO donor that generates NO−, nitroxyl equivalents) failed to induce HIF-1 activity, while S-nitrosoglutathione (16, GSNO) (an endogenous NO donor that generates NO−, nitrosonium equivalents) exerted a similar ability to induce HIF-1 as NOC-18. This indicates that a NO− equivalent-mediated electrophilic nitrosylation reaction may take place during NO-induced HIF-1 activation. Reversal of NOC-18-induced HIF-1 activation by dithiothreitol (DTT) suggests a mechanism that involves intracellular S-nitrosylation or oxidation of protein thiols. Subsequent studies have revealed that S-nitrosylation stabilizes HIF-1α protein and S-nitrosylation of Cys-800 promotes the interaction between HIF-1α protein and the co-activator p300, thus enhancing HIF-1 activation [60, 61]. Diazenium diolate NO donors that include spermine NONOate (17, a complex of NO with the natural product spermine), diethylamine NONOate, and diethyltryamine NONOate each induced HIF-1α protein in a dose- and time-dependent manner in multiple cell lines (proximal tubular LLC-PK1, human breast carcinoma MCF-7, MB231, and MB157) [62, 63]. Using a combination of NO donors that activate HIF-1 and pharmacological inhibitors of selected pathways, studies from numerous groups have revealed that NO donor-induced HIF-1 activation requires the presence of NO, is independent of the guanylate cyclase/guanosine 3',5'-monophosphate (cGMP) pathway, activates the PI3K/AKT/mTOR (phosphoinositol 3-kinase/protein kinase B/mammalian target of rapamycin) pathway to increase the synthesis of HIF-1α protein, and is sensitive to alterations in the cellular redox environment [59, 63–66]. Biochemical studies indicate that NO can bind to the iron in the active sites of HIF hydroxylases, block O2 binding and inhibit the hydroxylation reaction [23]. Inhibition of the hydroxylases that destabilize and inactivate HIF-1α protein may also contribute to NO-mediated HIF-1 activation in cell-based studies. Both the concentration and duration of NO released by structurally different NO donors should be considered when interpreting the results of these HIF-1 activation studies. Various NO concentration thresholds have been demonstrated to activate different signaling pathways under normoxic conditions [63]. The activation of HIF-1 by NO is most likely the overall outcome of modulating distinct pathways with different mechanisms.
Microtubule-depolymerizing agents (MDA) disrupt the microtubule network and block tumor cell division. The plant alkaloids vinblastine (18) and colchicine (19), and the synthetic MDA nocodazole, each induced HIF-1α protein at the concentrations that also maximally disrupted microtubules in various cell lines (A549 human lung carcinoma, MCF-7, Jurkat human T-cell leukemia, and NIH-3T3 murine embryonic fibroblast) [67]. The induction of HIF-1α protein by MDAs requires microtubule disruption, nuclear factor-κB (NF-κB)-dependent transcription, and functional VHL protein. The MDA-induced HIF-1α protein is transcriptionally active, as evidenced by increased expression of the HIF-1 target iNOS ( inducible nitric oxide synthase). Taxol, an anti-tumor agent that promotes microtubule polymerization and stabilization, did not induce HIF-1 activation.
Phenolic Compounds
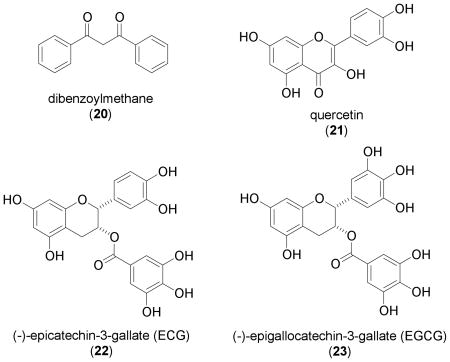
Dibenzoylmethane (20, DBM), a minor beta-diketone found in licorice (Glycyrrhiza glabra), was shown to stabilize HIF-1α protein in multiple cell lines that include, human prostate carcinoma LNCaP and PC-3, human embryonic kidney 293 (HEK 293), and rat neonatal primary cardiomyocytes [68]. The induction of HIF-1α protein correlated with increased HIF-1 activity (HEK 293 cells) and increased expression of the HIF-1 target gene VEGF at the secreted protein level (LNCaP cells and primary cardiomyocytes). Two structurally related compounds (dibenzoylpropane and curcumin) did not induce HIF-1α protein in HEK 293 cells. The fact that DBM-induced HIF-1α protein is non-ubiquitinated suggests that 20 may prevent HIF-1α protein degradation through the inhibition of prolyl hydroxylase. Dibenzoylmethane has been shown to inhibit 7,12-dimethylbenz[a]anthracene (DMBA)-induced DNA damage, mammary tumorigenesis, and lymphoma/leukemia in several animal models [69–71]. However, it is particularly important to note that the concentration of 20 that activates HIF-1 [68] is within the range of its IC50 values for cytotoxicity in LNCaP and PC-3 cells [72]. Whether dibenzoylmethane (20) has therapeutic potential for the treatment of ischemic diseases remains to be addressed in a biologically relevant system.
The flavonoid quercetin (21) is commonly found in red wine, grapes, and many other plants. Under normoxic conditions, quercetin (3,3',4',5,7-pentahydroxyflavone) was shown to activate HIF-1 in HeLa cells and murine brain endothelial cells (MBEC) that overexpress HIF-1α (maximum activation: 50 μM in HeLa and 100 μM in MBEC cells) [73]. The same study demonstrated that 21 stabilizes HIF-1α protein in HeLa cells, induces HIF-1α protein nuclear translocation, and increases the expression of the HIF-1 targets VEGF and GLUT-1 in MBEC cells. A decrease in HIF-1 activity was observed when quercetin and hypoxia were combined. Since 21 is known to be a broad-spectrum protein kinase inhibitor [54, 74], it is unlikely inhibition of Ser/Thr kinases is the only mechanism for quercetin-induced HIF-1 activation, as proposed by the authors. A recent study demonstrated that quercetin (21) also inhibits factor-inhibiting HIF (FIH), the asparaginyl hydroxylase that inactivates HIF-1α protein under normoxic conditions [75]. Such FIH inhibitory activity may also contribute to the ability of quercetin to activate HIF-1.
Green tea [dried fresh leaves of the plant Camellia sinensis L. Ktze., (Theaceae)] is one of the most popular drinks worldwide. Green tea catechins that include (–)-epicatechin (EC), (–)-epigallocatechin (EGC), (–)-epicatechin-3-gallate (22, ECG), and (–)-epigallocatechin-3-gallate (23, EGCG) are believed to be responsible for the health promoting benefits of drinking green tea. In human breast carcinoma T47D cells, ECG (22) was found to activate HIF-1 at high concentrations (100 μM) [76]. The most widely studied catechin EGCG (23) also produced a modest increase in HIF-1 activity. Despite the fact that 22 and 23 are nearly identical structurally, except for one hydroxyl group, EGCG (23) is relatively unstable in aqueous solution [76–78]. Further investigation of the ECG-induced activation of HIF-1 revealed that 22 induces the accumulation of nuclear HIF-1α protein and activates the expression of HIF-1 targets that include VEGF, GLUT-1 and CDKN1A (cyclin-dependant kinase inhibitor 1A; p21waf1/cip1) [76]. The observation that both the induction of HIF-1α protein and the activation of HIF-1 can be blocked by iron ions and ascorbate suggests that 22 may activate HIF-1 by chelating the iron ions that are required for the post-translational modifications that destabilize and inactivate HIF-1α protein [76]. Whether ECG (22), EGCG (23), and green tea extract products can activate HIF-1 in other models remains to be addressed. It is interesting to note that EGCG (23) and green tea extract have been shown to protect against ischemia/reperfusion injury, one of the intended therapeutic targets for HIF-1 activators [79–82].
Terpenes/Steroids
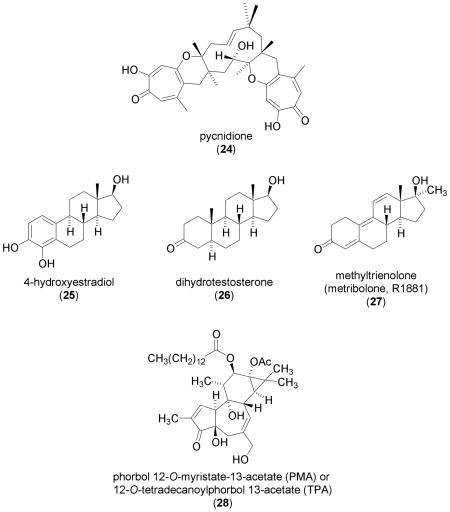
Three structurally related sesquiterpene-tropolones (pycnidione, epolone A and epolone B) were originally identified as natural products capable of inducing erythropoietin (EPO) in Hep3B cells [83]. Erythropoietin is one of the genes activated by HIF-1. Pycnidione (24) activated HIF-1 in a stable CHO cell line that expresses luciferase under the control of HREs from the transferrin gene (a target of HIF-1). The greatest levels of activation were observed with 24 at 8 μM under normoxic conditions and at 4 μM under hypoxic conditions (1% O2) [39]. At higher concentrations, 24 is cytotoxic. Induction of HIF-1α protein was observed in the presence of 24 in HepG2 cells [39]. The effect of 24 on other HIF-1 target gene expression and the mechanism of action were not reported. Judging by their structural similarities and comparable abilities to induce EPO, it is likely that epolones A and B can function like pycnidione (24) to activate HIF-1.
The estrogen 17β-estradiol (E2) is normally metabolized by liver cytochrome P450 enzymes into 2-hydroxy estradiol (2-OHE2) and 4-hydroxy estradiol (4-OHE2, 25). The compound 4-OHE2 (25) was demonstrated to induce HIF-1α protein in a dose- and time-dependent manner (optimal induction: 100 μM, 3 hr) in human ovarian carcinoma OVCAR-3 and A2780-CP70 cells [84]. No induction of HIF-1α protein was observed in the presence of the structurally related metabolite 2-OHE2. An increase in secreted VEGF protein (the bioactive gene product) was observed in the presence of 25 in both OVCAR-3 and A2780-CP70 cells. Mechanism of action studies that employed molecular probes suggest that 25 induces HIF-1α protein and increases secreted VEGF protein via activation of the PI3K/AKT/mTOR pathway, not the mitogen-activated protein kinase (MAPK) pathway. Given the current knowledge on HIF-1 regulation, it is possible that 4-OHE2 (25) increases HIF-1α protein synthesis and activates HIF-1 by activating the PI3K/AKT/mTOR pathway. However, further studies on 4-OHE2-induced HIF-1α protein synthesis, nuclear translocation, HIF-1 activation, and the expression of other HIF-1 target genes are necessary to support this hypothesis. Since 17β-estradiol (100 nM) was shown to inhibit the hypoxic activation of HIF-1 in Hep3B cells [85], whether the activation of HIF-1 by the 17β-estradiol metabolite 4-OHE2 (25) at 100 μM (1000 times the concentration required for inhibition by 17β-estradiol ) is of any real significance remains to be addressed in a relevant animal-based in vivo model.
The testosterone metabolite dihydrotestosterone (26, 1 nM) induces HIF-1α protein and activates HIF-1 in androgen-dependent LNCaP cells, but not in androgen-independent PC-3 cells [86]. Similar effects were observed in the presence of the metabolically stable synthetic androgen methyltrienolone (27, R1881) at a concentration of 0.1 nM. Androgens activate HIF-1 by inducing growth factors such as epidermal growth factor (EGF), that in turn enhance the synthesis of HIF-1α protein via the activation of the receptor kinase/PI3K/AKT/mTOR pathway. Since androgens are not direct stimuli for HIF-1 activation, this response requires an extended incubation time in contrast to the action of direct stimuli such as hypoxia or iron chelators.
The diterpene ester phorbol 12-O-myristate 13-acetate (28, PMA, also known as 12-O-tetradecanoylphorbol 13-acetate or TPA) which was originally isolated from the seeds of Croton tiglium L. is a potent activator of protein kinase C. Phorbol 12-O-myristate 13-acetate (28) is used as a tumor promoting agent in model systems and as a molecular probe for pathway studies [87]. In human prostate carcinoma DU145, PC-3, PPC-1, and TSU cell lines, 28 (50 - 100 nM) was shown to induce nuclear HIF-1α protein accumulation, and inhibitors of the EGF/PI3K/PTEN (phosphatase and tensin homolog)/AKT/mTOR pathway blocked the effect of PMA on HIF-1α protein [88, 89]. Induction of nuclear HIF-1α protein by 28 is followed by an increase in HIF-1 activity in DU145 cells [88]. In cultured rat neonatal cardiomyocytes, 28 has been shown to induce HIF-1α expression at the mRNA level [90]. Studies conducted in genetically engineered mouse fibroblasts suggest that PMA-induced HIF-1 activation is independent of the receptor-interacting protein (RIP)/NF-κB pathway, while the induction of HIF-1 activation by tumor necrosis factor α (TNFα) depends on the RIP/NFκB pathway [91]. A recent study revealed that 28 induces a novel HIF-1α isoform (HIF-1α785) in HEK293 cells [92]. This HIF-1α variant is devoid of exon 11 that encodes part of the oxygen-dependent degradation domain (ODDD). Phorbol 12-O-myristate 13-acetate (28) stabilizes HIF-1α785 protein without affecting HIF-1α785 mRNA level and the activity of 28 requires at least redox-sensitive and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) pathways [92]. Since 28 modulates a number of cellular signaling pathways, its HIF-1 inducing activity is most likely the combined effects of modulating a network of pathways.
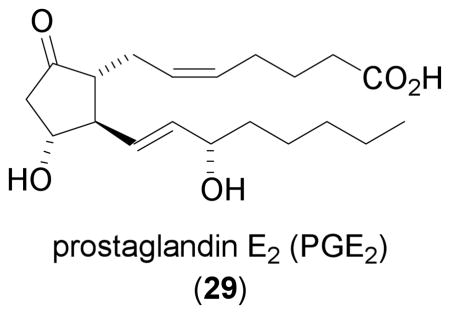
Prostaglandin E2 (PGE2)
Prostaglandin E2 (29, PGE2) was demonstrated to induce HIF-1α protein in human colon carcinoma HCT116 cells in a dose- and time-dependent manner [93]. Although modest in comparison to that triggered by hypoxia, maximal induction was observed after an 18–32 hr incubation in the presence of 29 (100 μM) . Prostaglandin E2 (29) induces HIF-1α protein by increasing HIF-1α protein synthesis. Pathway studies that employed pharmacological agents suggest that 29 activates the G-protein coupled receptor EP1, which then activates MEK/ERK and the v-src avian sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (c-src), followed by the activation of the PI3K/AKT/mTOR pathway, and leads to increased HIF-1α protein synthesis. The induction of HIF-1α protein by 29 is accompanied by an increase in the level of VEGF mRNA. In PC-3ML (a subline of PC-3) cells, 29 (1 μM) induced nuclear HIF-1α protein accumulation within 4 hr and 29 appears to be more potent than hypoxia (1% O2) at inducing HIF-1α protein [94]. The effects of PGE2 (29) on HIF-1α protein were mediated by EP2 and EP4 receptor subtypes, through activation of the MAPK and PI3K/AKT pathways.. The enzyme cyclooxygenase-2 (COX-2) has been identified as a mediator for the induction of HIF-1 and VEGF by the proinflammatory cytokine IL-1β in both A549 and colon carcinoma Caco-2 cells [95]. In A549 cells, PGE2 (one of the major products of COX-2 catalyzed metabolism of arachidonic acid) induced nuclear HIF-1α protein accumulation at concentrations as low as 0.1 μM within 1 hr. These results suggest that the effect of 29 on HIF-1 and its target genes can be mediated by more than one pathway, depending on which specific tumor type is involved. Due to the wide range of concentrations and incubation times required in different cell lines to elicit a HIF-1 response from PGE2 (29), particular caution should be taken when extrapolating these laboratory findings to the clinical setting.
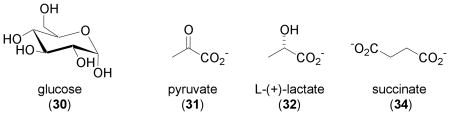
Carbohydrates and Glycolysis Products
Hypoxic conditions and a state of aerobic glycolysis are commonly found in solid tumors. One group of HIF-1 target genes is glucose transporters and the glycolytic enzymes that promote glycolysis [3]. Identification of glucose and the glycolytic end products pyruvate and lactate as HIF-1 stimuli has helped construct a two-way connection between hypoxia and aerobic glycolysis [96]. In human glioma cells (U87, U373, and U251), glucose (30), pyruvate (31), and lactate (32) were each shown to induce nuclear HIF-1α protein accumulation in a dose- and time-dependent manner. Further study using pharmacological inhibitors identified pyruvate as the key glycolytic metabolite that induces HIF-1α protein by preventing its degradation. Activation of HIF-1 targets that include VEGF, EPO, GLUT3, and aldolase A was observed in the presence of either glucose or pyruvate [96]. At the final stage of glucose oxidation, reactions in the Krebs cycle (TCA cycle or citric acid cycle) oxidize the acetyl group of acetyl CoA into CO2. One intermediate of the TCA cycle, oxaloacetate (33), induces HIF-1α protein, activates HIF-1, and induces the expression of HIF-1 target genes in U87 and U251 cells [97]. Induction of the HIF prolyl hydroxylases (HPH) HPH-1 and HPH-2 mRNAs was also observed in the presence of these endogenous 2-oxoacids (31 and 33), as well as by the other known HIF-1 stimuli that include hypoxia (1% O2), the iron chelator DFO (1), the transition metal Co2+, and the 2-oxoglutarate (2OG) analogue dimethyl-N-oxalylglycine (6) [97]. This suggests the involvement of a potential negative feedback loop that regulates HIF-1 activity. In an effort to identify the link between succinate dehydrogenase (SDH) mutations and tumor formation, Selak and coworkers revealed that succinate (34) links mitochondrial dysfunction to oncogenesis via the activation of HIF-1 [98]. The enzyme SDH is localized to the inner mitochondrial membrane and catalyzes the conversion of 34 to fumarate. Both 34 and fumarate are intermediates of the TCA cycle. Inhibition of SDH leads to an increase in 34, which is transported from mitochondria to the cytosol. Succinate (34) is the product of 2OG-dependent oxygenases and a weak inhibitor of these enzymes. Succinate (34) inhibits HIF prolyl hydroxylases in the cytosol, stabilizes HIF-1α protein, and subsequently activates HIF-1. The concentrations of 34 that were shown to inhibit HPH activity in HEK293 cell extracts are within the range of 34 found in succinate dehydrogenase deficient cells. This SDH-succinate-HIF-1 link is supported by results from clinical studies that have demonstrated that SDH mutation-related tumors such as pheochromocytoma and renal cell carcinoma are highly vascular and have activated hypoxic signaling pathways [99].
Other Activators of HIF-1
Two groups of chemicals commonly used to induce chemical hypoxia are iron chelators and transition metals. Natural product-derived iron chelators that activate HIF-1 were discussed earlier in this review. Synthetic iron chelators such as 2,2'-pyridyl and 1,10-phenanthroline can also activate HIF-1 and the expression of HIF-1 target genes [40, 100]. A compound that is structurally related to 1,10-phenanthroline, known as FG-0041 inhibits the activity of HPH-2 (PHD-2; EGLN-1), induces HIF-1α protein, and activates the expression of the HIF-1 target VEGF [101]. Cobalt was the first transition metal shown to activate HIF-1 [102, 103]. Other metals such as nickel, chromium (VI), and copper have also been shown to activate HIF-1 [104–107]. The organomercurial compound mersalyl activates HIF-1 and its target genes via the insulin-like growth factor-1 receptor and MAPK pathway [108]. Recently, it was demonstrated that cobalt and nickel activate HIF-1 by depleting intracellular ascorbate, a co-factor for the HIF hydroxylases that destabilize and inactivate HIF-1α protein [109]. A separate study revealed that the Ca2+ chelator BAPTA (1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid) activates HIF-1 in SH-SY5Y human neuroblastoma cells [110].
There is increasing evidence that supports HIF-1 as a general regulator of cellular responses to environmental, extracellular, and intracellular signals. Non-hypoxic physiological stimuli such as growth factors, cytokines, and hormones can also induce HIF-1α protein and activate HIF-1. Activation of HIF-1 by insulin, insulin-like growth factor (IGF)-1 and IGF-2 leads to an increased expression of HIF-1 target genes such as IGF-2, IGF-binding protein (IGFBP)-2 and IGFBP-3 and forms an autocrine loop that promotes proliferation [111, 112]. Other growth factors that include EGF, basic fibroblast growth factor (bFGF), and heregulin have all been shown to activate HIF-1 in various cell lines [88, 112–114]. Both PI3K and MAPK signaling pathways are involved in mediating the induction and activation of HIF-1 by growth factors [112–117]. Activation of the PI3K/AKT/mTOR pathway relieves translational inhibition and enhances translation of HIF-1α mRNA [113]. These growth factors activate HIF-1 by increasing HIF-1α protein synthesis, in contrast to the mechanism of stimuli such as hypoxia and iron chelators that inhibit the degradation of HIF-1α protein. Cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL-1β) induce HIF-1α protein under normoxic conditions and the induction can be augmented by hypoxic treatments [118, 119]. TNFα-induced HIF-1α protein stabilization and HIF-1 activation involve reactive oxygen species (ROS)-sensitive pathways, activation of the PI3K/AKT and MAPK pathways, and receptor-interacting protein (RIP)-dependent activation of NFκB [65, 91, 120, 121]. A recent study demonstrated that TNFα increases HIF-1α protein synthesis [122]. Activation of HIF-1 by IL-1β requires pathways similar to those used by TNFα [95, 115, 123, 124]. Most of the studies indicate that IL-1β induces HIF-1α protein accumulation. One study demonstrated that IL-1 also rapidly increases HIF-1α mRNA levels [125]. The vascular hormones angiotensin II and thrombin activate HIF-1 via stimulation of both HIF-1α transcription and translation [126–128]. The mechanisms used by these hormones to activate HIF-1 were summarized in a recent review [129]. Recently, thyroid hormone and follicle-stimulating hormone were added to the list of hormones that activate HIF-1 [130, 131]. Other physiological factors such as the redox protein thioredoxin-1 (Trx-1) and oxidized low-density lipoprotein (oxLDL) can also induce HIF-1α protein and activate HIF-1, suggesting that HIF-1 may play a role in tumors that over-express Trx-1 and diseases related to oxLDL such as atherosclerosis [132, 133].
Studies conducted using genetically engineered mice deficient in HIF-1α established that HIF-1 plays a critical role in inflammatory responses [134–136]. These observations provide an explanation for the activation of HIF-1 by inflammatory stimuli that range from viral proteins to bacteria lipopolysaccharide (LPS) [137, 138].
Potential Therapeutic Applications of HIF-1 Activators
The intended therapeutic targets for HIF-1 activators are tissue ischemia and related diseases. These conditions may include peripheral, cerebral and myocardial ischemia, reperfusion injury following heart attack or stroke, wound healing, organ transplant, and retinopathy of prematurity in neonates. Current approaches to activate HIF-1 include HIF-1α gene therapy [139–141], small molecules that inhibit the HIF hydroxylases [40, 45, 101, 142], and peptides that block the degradation of HIF-1α protein [143, 144]. Results from studies in transgenic mice indicate that HIF-1 activation may have a therapeutic advantage over direct VEGF activation, in regard to the promotion of angiogenesis with lower incidence of side effects (i.e. vessel leakage associated with VEGF over-expression) [145].
As an example, the therapeutic potential of HIF-1 activation for the treatment of cardiovascular diseases is discussed. Atherosclerotic coronary artery disease progresses from vascular stenosis (narrowing of the vessel) and ischemia to vessel occlusion followed by myocardial infarction (death of the cells that depend on perfusion from the occluded vessel). One adaptive physiological response to chronic myocardial ischemia is to form new collateral blood vessels that function as replacement for the occluded vessels. In patients, the extent of coronary collateralization correlates with good prognosis after myocardial infarction [146]. Results from animal studies suggest that the ability to respond to ischemia and form new blood vessels is most likely influence by both genetic and environmental factors. For example, age plays a significant role in ischemia-induced neovascularization as older mice and rabbits exhibit impaired collateralization following femoral artery ligation [147–149]. Intervention with HIF-1 activators represents a potential therapeutic approach to compensate for the impaired ischemia-induced collateral vessel formation. In a rat acute myocardial infarction model, HIF-1α gene therapy with a HIF-1α/VP16 hybrid enhanced angiogenesis and reduced infarct size [140]. Reintroduction of blood flow to an ischemic organ causes reperfusion injury, which represents a second phase of injury in diseases such as heart attack and stroke. Activation of HIF-1 by intermittent hypoxia protected isolated rodent hearts against ischemia-reperfusion injury [150]. Constitutive expression of HIF-1α hybrids from recombinant adenoviral vectors protected cultured rat neonatal cardiomyocytes against ischemia-reperfusion injury [151]. These results suggest that HIF-1 activators may also prevent ischemia-reperfusion injuries through a preconditioning mechanism. Ironically, desferri-exochelin which was recently shown to activate HIF [37], has also been shown to prevent cardiac reperfusion injury in cultured rat adult cardiac myocytes and in isolated rabbit hearts [152]. Desferri-exochelin also inhibited human vascular smooth muscle cell proliferation in vitro and prevented restenosis in a porcine coronary artery restenosis model [153, 154]. The high incidence of restenosis has limited the success of coronary angioplasty for the treatment of obstructive coronary artery lesions. Whether HIF-1 activation can prevent the development of restenosis after vascular injury has not been conclusively demonstrated.
Perspectives
Natural products and natural product-derived compounds have contributed significantly as important molecular probes to elucidate the process of HIF-1 activation and may ultimately serve as therapeutically useful HIF-1 activators. It is important to emphasize that HIF-1 responds not only to hypoxia, but also to numerous physiological and non-physiological stimuli such as exogenous low molecular weight natural products. Hypoxia appears to be the universal stimulus for HIF-1 activation and other activators are relatively cell type-specific. Most of the reported HIF-1 activators have only been examined in cell culture-based systems. While some of these studies were performed at physiologically relevant concentrations, other studies were performed at concentrations far beyond those obtained physiologically. Several studies were even conducted at or above established toxic concentrations. One critical point that must be kept in mind is that the induction of HIF-1α protein does not necessarily translate into HIF-1 activation. Several investigators have defined HIF-1 activators as substances/conditions that induce HIF-1α protein accumulation. This may not be true, since proteasome inhibitors such as MG-132 and lactacystin induce HIF-1α protein by preventing proteasomal degradation but these compounds actually inhibit HIF-1 activation [155]. The known HIF-1 activators can be simply classified into two general classes based on their effect(s) on HIF-1α protein: 1) inhibitors of HIF-1α protein degradation and inactivation; and 2) activators of HIF-1α transcription and translation. The majority of the agents shown to activate HIF-1 have been identified from specific experiments to examine the response of individually selected test compounds, rather than from using bioassays to screen for novel activators. Therefore, enormous potential remains for the discovery of new classes of HIF-1 activators through the use of high-throughput screening-based drug discovery approaches that would examine either natural product-based or synthetic-based chemical libraries. The complexity of pathways that regulate the production and function of HIF-1 poses a challenge for the identification of new HIF-1 activators that are suitable for drug development. Both environmental and genetic factors related to each specific disease target need to be considered when establishing proper in vitro models and developing bioassays for the purpose of HIF-1 activator-based drug discovery. Since ischemic tissues have intrinsically impaired vascularization, drug delivery is critical to achieve the desired therapeutic efficacy of HIF-1 activators. The therapeutic activation of HIF-1 should be treated as a finely tuned process since excessive HIF-1 activation may produce undesired pathological consequences. The ideal therapeutic HIF-1 activator will be one that both prevents and treats tissue ischemia without producing significant side effects. Only time will tell if specific HIF activators will meet the expectations of ideal molecular-targeted drugs.
Acknowledgments
Support for this effort was provided by the NIH/NCI CA-98787-01, NOAA NURP/NIUST NA16RU1496, and USDA/Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
List of Abbreviations
- 2-OHE2
2-hydroxy estradiol
- 2OG
2-oxoglutarate
- 4-OHE2
4-hydroxy estradiol
- AKT
Protein kinase B
- ARNT
Aryl hydrocarbon receptor nuclear translocator
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid
- bFGF
basic fibroblast growth factor
- bHLH
basic helix-loop-helix
- CAM
Chicken embryo chorioallantoic membrane
- CBP
CREB (cAMP-response element-binding protein)-binding protein
- CDK1
Cyclin-dependant kinase-1
- CDK5
Cyclin-dependant kinase-5
- CDKN1A
Cyclin-dependant kinase inhibitor 1A
- cGMP
Guanosine 3',5'-monophosphate
- CHO
Chinese hamster ovary
- COX-2
Cyclooxygenase-2
- C-P4H-I
Human type I collagen prolyl-4-hydroxylase
- c-src
v-src avian sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog
- DBM
Dibenzoylmethane
- DMBA
7,12-dimethylbenz[a]anthracene
- DMOG
Dimethyl-oxalylglycine
- DTT
Dithiothreitol
- E2
17β-estradiol
- EC
Epicatechin
- ECG
Epicatechin-3-gallate
- EGC
Epigallocatechin
- EGCG
Epigallocatechin-3-gallate
- EGF
Epidermal growth factor
- EPO
Erythropoietin
- ERK
Extracellular signal-regulated kinase (same as MAPK)
- FIH
Factor-inhibiting HIF; HIF asparaginyl hydroxylase
- GLUT-1
Glucose transporter-1
- GSK3β
Glucogen synthase kinase 3β
- GSNO
S-nitrosoglutathione
- HIF-1
Hypoxia-inducible factor-1
- HO-1
Heme oxygenase-1
- HPH
HIF prolyl hydroxylase (same as PHD)
- HRE
Hypoxia response element
- IGF
Insulin-like growth factor
- IGFBP
Insulin-like growth factor binding protein
- IL-1β
Interleukin-1β
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase (same as ERK)
- MBEC
Murine brain endothelial cells
- MDA
Microtubule-depolymerizing agent
- MEK
Mitogen-activated protein kinase kinase; MAPKK; MAPK/ERK kinase
- mTOR
Mammalian target of rapamycin
- NFκB
Nuclear factor-κB
- NIP3
BCL2/adenovirus E1B 19-kD protein-interacting protein 3
- NO
Nitric oxide
- NOC5
3-(hydroxy-1-(1-methylethyl)-2-nitrosohydrazino)-1-propanamine
- NOC-18
Diazen-1-ium-1,2-diolate
- NOG
N-oxaloylglycine
- ODDD
Oxygen-dependent degradation domain
- oxLDL
Oxidized low-density lipoprotein
- PAS
PER-ARNT-SIM
- p300
E1A-binding protein, 300 kD
- PGE2
Prostaglandin E2
- PHD
Prolyl hydroxylase domain-containing protein (same as HPH)
- PI3K
Phosphoinositol 3-kinase
- PMA
Phorbol 12-O-myristate 13-acetate (same as TPA)
- PTEN
Phosphatase and tensin homolog
- VHL
von Hippel-Lindau disease tumor suppressor
- RIP
Receptor-interacting protein
- SDH
Succinate dehydrogenase
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
- TNF-α
Tumor necrosis factor α
- TPA
12-O-tetradecanoylphorbol 13-acetate (same as PMA)
- Trx-1
Thioredoxin-1
- VEGF
Vascular endothelial growth factor
References
- 1.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–54. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop- helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 4.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–8. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- 5.Poellinger L, Johnson RS. HIF-1 and hypoxic response: the plot thickens. Curr Opin Genet Dev. 2004;14:81–5. doi: 10.1016/j.gde.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 7.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 9.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 11.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 13.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277:26351–5. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 15.McNeill LA, Hewitson KS, Claridge TD, Seibel JF, Horsfall LE, Schofield CJ. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. Biochem J. 2002;367:571–5. doi: 10.1042/BJ20021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Structural basis for HIF-1α/CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA. 2002;99:5271–6. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1α. Proc Natl Acad Sci USA. 2002;99:5367–72. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- 19.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–83. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781–90. doi: 10.1046/j.1432-1033.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- 21.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J Cell Sci. 2003;116:3041–9. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 22.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–23. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 23.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 24.Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov Today. 2004;9:704–11. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–82. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 26.Brahimi-Horn C, Mazure N, Pouyssegur J. Signalling via the hypoxia-inducible factor-1α requires multiple posttranslational modifications. Cell Signal. 2005;17:1–9. doi: 10.1016/j.cellsig.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–11. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 28.Welsh SJ, Powis G. Hypoxia inducible factor as a cancer drug target. Curr Cancer Drug Targets. 2003;3:391–405. doi: 10.2174/1568009033481732. [DOI] [PubMed] [Google Scholar]
- 29.Powis G, Kirkpatrick L. Hypoxia inducible factor-1α as a cancer drug target. Mol Cancer Ther. 2004;3:647–54. [PubMed] [Google Scholar]
- 30.Paul SA, Simons JW, Mabjeesh NJ. HIF at the crossroads between ischemia and carcinogenesis. J Cell Physiol. 2004;200:20–30. doi: 10.1002/jcp.10479. [DOI] [PubMed] [Google Scholar]
- 31.Hopfl G, Ogunshola O, Gassmann M. HIFs and tumors--causes and consequences. Am J Physiol Regul Integr Comp Physiol. 2004;286:R608–23. doi: 10.1152/ajpregu.00538.2003. [DOI] [PubMed] [Google Scholar]
- 32.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 33.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–5. [PubMed] [Google Scholar]
- 34.Bickel H, Hall GE, Keller-Schierlein W, Prelog V, Vischer E, Wettstein A. Metabolic products of Actinomycetaceae. XXVII. Constitutional formulas of ferrioxamine B. Helvetica Chimica Acta. 1960;43:2129–38. [Google Scholar]
- 35.Anonymous. In: AHFS Drug Information 2004. McEvoy GK, editor. Bethesda: American Society of Health-System Pharmacists, Inc./American Hospital Formulary Service; 2004. pp. 2870–3. [Google Scholar]
- 36.Gobin J, Moore CH, Reeve JR, Jr, Wong DK, Gibson BW, Horwitz MA. Iron acquisition by Mycobacterium tuberculosis: isolation and characterization of a family of iron-binding exochelins. Proc Natl Acad Sci USA. 1995;92:5189–93. doi: 10.1073/pnas.92.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong TW, Horwitz LD, Moore JW, Sowter HM, Harris AL. A mycobacterial iron chelator, desferri-exochelin, induces hypoxia-inducible factors 1 and 2, NIP3, and vascular endothelial growth factor in cancer cell lines. Cancer Res. 2002;62:6924–7. [PubMed] [Google Scholar]
- 38.Anonymous. In: AHFS Drug Information 2004. McEvoy GK, editor. Bethesda: American Society of Health-System Pharmacists, Inc./American Hospital Formulary Service; 2004. pp. 3358–61. [Google Scholar]
- 39.Wanner RM, Spielmann P, Stroka DM, Camenisch G, Camenisch I, Scheid A, Houck DR, Bauer C, Gassmann M, Wenger RH. Epolones induce erythropoietin expression via hypoxia-inducible factor-1α activation. Blood. 2000;96:1558–65. [PubMed] [Google Scholar]
- 40.Linden T, Katschinski DM, Eckhardt K, Scheid A, Pagel H, Wenger RH. The antimycotic ciclopirox olamine induces HIF-1α stability, VEGF expression, and angiogenesis. FASEB J. 2003;17:761–3. doi: 10.1096/fj.02-0586fje. [DOI] [PubMed] [Google Scholar]
- 41.Cai P, Smith D, Cunningham B, Brown-Shimer S, Katz B, Pearce C, Venables D, Houck D. 8-methyl-pyridoxatin: A novel N-hydroxy pyridone from fungus OS-F61800 that induces erythropoietin in human cells. J Nat Prod. 1999;62:397–9. doi: 10.1021/np980450t. [DOI] [PubMed] [Google Scholar]
- 42.Cunliffe CJ, Franklin TJ, Hales NJ, Hill GB. Novel inhibitors of prolyl 4-hydroxylase. 3. Inhibition by the substrate analogue N-oxaloglycine and its derivatives. J Med Chem. 1992;35:2652–8. doi: 10.1021/jm00092a016. [DOI] [PubMed] [Google Scholar]
- 43.Elkins JM, Hewitson KS, McNeill LA, Seibel JF, Schlemminger I, Pugh CW, Ratcliffe PJ, Schofield CJ. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1α. J Biol Chem. 2003;278:1802–6. doi: 10.1074/jbc.C200644200. [DOI] [PubMed] [Google Scholar]
- 44.Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–80. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 45.Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- 46.Mole DR, Schlemminger I, McNeill LA, Hewitson KS, Pugh CW, Ratcliffe PJ, Schofield CJ. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–80. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- 47.Higashide E, Horii S, Ono H, Mizokami N, Yamazaki T, Shibata M, Yoneda M. Alahopcin, a new dipeptide antibiotic produced by Streptomyces albulus subsp. ochragerus subsp. nov. J Antibiot (Tokyo) 1985;38:285–95. doi: 10.7164/antibiotics.38.285. [DOI] [PubMed] [Google Scholar]
- 48.Higashide E, Kanamaru T, Fukase H, Horii S. Isolation of dealanylalahopcin, a new amino acid, and its biological activity. J Antibiot (Tokyo) 1985;38:296–301. doi: 10.7164/antibiotics.38.296. [DOI] [PubMed] [Google Scholar]
- 49.Horii S, Fukase H, Higashide E, Yoneda M, Nishida H, Sakai H, Hirota A, Isogai A. Structure of alahopcin (nourseimycin), a new dipeptide antibiotic. J Antibiot (Tokyo) 1985;38:302–11. doi: 10.7164/antibiotics.38.302. [DOI] [PubMed] [Google Scholar]
- 50.Schlemminger I, Mole DR, McNeill LA, Dhanda A, Hewitson KS, Tian YM, Ratcliffe PJ, Pugh CW, Schofield CJ. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg Med Chem Lett. 2003;13:1451–4. doi: 10.1016/s0960-894x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 51.Schnitzer SE, Schmid T, Zhou J, Eisenbrand G, Brune B. Inhibition of GSK3beta by indirubins restores HIF-1α accumulation under prolonged periods of hypoxia/anoxia. FEBS Lett. 2005;579:529–33. doi: 10.1016/j.febslet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Xiao Z, Hao Y, Liu B, Qian L. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk Lymphoma. 2002;43:1763–8. doi: 10.1080/1042819021000006295. [DOI] [PubMed] [Google Scholar]
- 53.Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, Eisenbrand G, Meijer L. Indirubins inhibit glycogen synthase kinase-3β and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–60. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 54.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Christou H, Morita T, Laughner E, Semenza GL, Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5' enhancer. J Biol Chem. 1998;273:15257–62. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- 56.Sogawa K, Numayama-Tsuruta K, Ema M, Abe M, Abe H, Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc Natl Acad Sci USA. 1998;95:7368–73. doi: 10.1073/pnas.95.13.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–44. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 58.Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D'Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–97. [PubMed] [Google Scholar]
- 59.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58:1197–203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 60.Sumbayev VV, Budde A, Zhou J, Brune B. HIF-1α protein as a target for S-nitrosation. FEBS Lett. 2003;535:106–12. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 61.Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1α protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003;549:105–9. doi: 10.1016/s0014-5793(03)00807-x. [DOI] [PubMed] [Google Scholar]
- 62.Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1α under the influence of nitric oxide. Blood. 2001;97:1009–15. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 63.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1α, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA. 2004;101:8894–9. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandau KB, Faus HG, Brune B. Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI 3K pathway. Biochem Biophys Res Commun. 2000;278:263–7. doi: 10.1006/bbrc.2000.3789. [DOI] [PubMed] [Google Scholar]
- 65.Sandau KB, Zhou J, Kietzmann T, Brune B. Regulation of the hypoxia-inducible factor 1α by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001;276:39805–11. doi: 10.1074/jbc.M107689200. [DOI] [PubMed] [Google Scholar]
- 66.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–8. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 67.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. Microtubule disruption utilizes an NFκB-dependent pathway to stabilize HIF-1α protein. J Biol Chem. 2003;278:7445–52. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- 68.Mabjeesh NJ, Willard MT, Harris WB, Sun HY, Wang R, Zhong H, Umbreit JN, Simons JW. Dibenzoylmethane, a natural dietary compound, induces HIF-1α and increases expression of VEGF. Biochem Biophys Res Commun. 2003;303:279–86. doi: 10.1016/s0006-291x(03)00336-x. [DOI] [PubMed] [Google Scholar]
- 69.Lin CC, Lu YP, Lou YR, Ho CT, Newmark HH, MacDonald C, Singletary KW, Huang MT. Inhibition by dietary dibenzoylmethane of mammary gland proliferation, formation of DMBA-DNA adducts in mammary glands, and mammary tumorigenesis in Sencar mice. Cancer Lett. 2001;168:125–32. doi: 10.1016/s0304-3835(01)00506-7. [DOI] [PubMed] [Google Scholar]
- 70.Huang MT, Lou YR, Xie JG, Ma W, Lu YP, Yen P, Zhu BT, Newmark H, Ho CT. Effect of dietary curcumin and dibenzoylmethane on formation of 7,12-dimethylbenz[a]anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis. 1998;19:1697–700. doi: 10.1093/carcin/19.9.1697. [DOI] [PubMed] [Google Scholar]
- 71.Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1998;19:1039–43. doi: 10.1093/carcin/19.6.1039. [DOI] [PubMed] [Google Scholar]
- 72.Jackson KM, DeLeon M, Verret CR, Harris WB. Dibenzoylmethane induces cell cycle deregulation in human prostate cancer cells. Cancer Lett. 2002;178:161–5. doi: 10.1016/s0304-3835(01)00844-8. [DOI] [PubMed] [Google Scholar]
- 73.Wilson WJ, Poellinger L. The dietary flavonoid quercetin modulates HIF-1α activity in endothelial cells. Biochem Biophys Res Commun. 2002;293:446–50. doi: 10.1016/S0006-291X(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 74.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welford RW, Schlemminger I, McNeill LA, Hewitson KS, Schofield CJ. The selectivity and inhibition of AlkB. J Biol Chem. 2003;278:10157–61. doi: 10.1074/jbc.M211058200. [DOI] [PubMed] [Google Scholar]
- 76.Zhou YD, Kim YP, Li XC, Baerson SR, Agarwal AK, Hodges TW, Ferreira D, Nagle DG. Hypoxia-inducible factor-1 activation by (-)-epicatechin gallate: potential adverse effects of cancer chemoprevention with high-dose green tea extracts. J Nat Prod. 2004;67:2063–9. doi: 10.1021/np040140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demeule M, Michaud-Levesque J, Annabi B, Gingras D, Boivin D, Jodoin J, Lamy S, Bertrand Y, Beliveau R. Green tea catechins as novel antitumor and antiangiogenic compounds. Curr Med Chem Anti-Canc Agents. 2002;2:441–63. doi: 10.2174/1568011023353930. [DOI] [PubMed] [Google Scholar]
- 78.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–6. [PubMed] [Google Scholar]
- 79.Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88:1555–69. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 80.Hirano R, Momiyama Y, Takahashi R, Taniguchi H, Kondo K, Nakamura H, Ohsuzu F. Comparison of green tea intake in Japanese patients with and without angiographic coronary artery disease. Am J Cardiol. 2002;90:1150–3. doi: 10.1016/s0002-9149(02)02787-x. [DOI] [PubMed] [Google Scholar]
- 81.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–3. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- 83.Cai P, Smith D, Cunningham B, Brown-Shimer S, Katz B, Pearce C, Venables D, Houck D. Epolones: novel sesquiterpene-tropolones from fungus OS- F69284 that induce erythropoietin in human cells. J Nat Prod. 1998;61:791–5. doi: 10.1021/np9800506. [DOI] [PubMed] [Google Scholar]
- 84.Gao N, Nester RA, Sarkar MA. 4-Hydroxy estradiol but not 2-hydroxy estradiol induces expression of hypoxia-inducible factor 1α and vascular endothelial growth factor A through phosphatidylinositol 3-kinase/Akt/FRAP pathway in OVCAR-3 and A2780-CP70 human ovarian carcinoma cells. Toxicol Appl Pharmacol. 2004;196:124–35. doi: 10.1016/j.taap.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Mukundan H, Kanagy NL, Resta TC. 17-β estradiol attenuates hypoxic induction of HIF-1α and erythropoietin in Hep3B cells. J Cardiovasc Pharmacol. 2004;44:93–100. doi: 10.1097/00005344-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 86.Mabjeesh NJ, Willard MT, Frederickson CE, Zhong H, Simons JW. Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3'-kinase/protein kinase B in prostate cancer cells. Clin Cancer Res. 2003;9:2416–25. [PubMed] [Google Scholar]
- 87.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–8. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 88.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. [PubMed] [Google Scholar]
- 89.Zhong H, Hanrahan C, van der Poel H, Simons JW. Hypoxia-inducible factor 1α and 1beta proteins share common signaling pathways in human prostate cancer cells. Biochem Biophys Res Commun. 2001;284:352–6. doi: 10.1006/bbrc.2001.4981. [DOI] [PubMed] [Google Scholar]
- 90.Ladoux A, Frelin C. Cardiac expressions of HIF-1 alpha and HLF/EPAS, two basic loop helix/PAS domain transcription factors involved in adaptative responses to hypoxic stresses. Biochem Biophys Res Commun. 1997;240:552–6. doi: 10.1006/bbrc.1997.7708. [DOI] [PubMed] [Google Scholar]
- 91.Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem J. 2003;370:1011–7. doi: 10.1042/BJ20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chun YS, Lee KH, Choi E, Bae SY, Yeo EJ, Huang LE, Kim MS, Park JW. Phorbol ester stimulates the nonhypoxic induction of a novel hypoxia-inducible factor 1α isoform: implications for tumor promotion. Cancer Res. 2003;63:8700–7. [PubMed] [Google Scholar]
- 93.Fukuda R, Kelly B, Semenza GL. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330–4. [PubMed] [Google Scholar]
- 94.Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277:50081–6. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 95.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–7. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 96.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 97.Dalgard CL, Lu H, Mohyeldin A, Verma A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem J. 2004;380:419–24. doi: 10.1042/BJ20031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 99.Pollard P, Wortham N, Barclay E, Alam A, Elia G, Manek S, Poulsom R, Tomlinson I. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol. 2005;205:41–9. doi: 10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- 100.Hodges TW, Hossain CF, Kim YP, Zhou YD, Nagle DG. Molecular-targeted antitumor agents: the Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. J Nat Prod. 2004;67:767–71. doi: 10.1021/np030514m. [DOI] [PubMed] [Google Scholar]
- 101.Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–64. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–8. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 104.Salnikow K, An WG, Melillo G, Blagosklonny MV, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999;20:1819–23. doi: 10.1093/carcin/20.9.1819. [DOI] [PubMed] [Google Scholar]
- 105.Salnikow K, Blagosklonny MV, Ryan H, Johnson R, Costa M. Carcinogenic nickel induces genes involved with hypoxic stress. Cancer Res. 2000;60:38–41. [PubMed] [Google Scholar]
- 106.Gao N, Jiang BH, Leonard SS, Corum L, Zhang Z, Roberts JR, Antonini J, Zheng JZ, Flynn DC, Castranova V, Shi X. p38 Signaling-mediated hypoxia-inducible factor 1α and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J Biol Chem. 2002;277:45041–8. doi: 10.1074/jbc.M202775200. [DOI] [PubMed] [Google Scholar]
- 107.Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, Eckhardt K, Troger J, Barth S, Camenisch G, Wenger RH. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005 doi: 10.1182/blood-2004-10-3980. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 108.Agani F, Semenza GL. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol. 1998;54:749–54. doi: 10.1124/mol.54.5.749. [DOI] [PubMed] [Google Scholar]
- 109.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem. 2004;279:40337–44. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- 110.Berchner-Pfannschmidt U, Petrat F, Doege K, Trinidad B, Freitag P, Metzen E, de Groot H, Fandrey J. Chelation of cellular calcium modulates hypoxia-inducible gene expression through activation of hypoxia-inducible factor-1α. J Biol Chem. 2004;279:44976–86. doi: 10.1074/jbc.M313995200. [DOI] [PubMed] [Google Scholar]
- 111.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998;17:5085–94. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 1999;59:3915–8. [PubMed] [Google Scholar]
- 113.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, Fang WG. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J Pathol. 2005;205:530–6. doi: 10.1002/path.1734. [DOI] [PubMed] [Google Scholar]
- 115.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Burgel T. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512:157–62. doi: 10.1016/s0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 116.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–81. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 117.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–11. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 118.Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1β and tumor necrosis factor-α stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–7. [PubMed] [Google Scholar]
- 119.El Awad B, Kreft B, Wolber EM, Hellwig-Burgel T, Metzen E, Fandrey J, Jelkmann W. Hypoxia and interleukin-1beta stimulate vascular endothelial growth factor production in human proximal tubular cells. Kidney Int. 2000;58:43–50. doi: 10.1046/j.1523-1755.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 120.Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-α-dependent regulation of HIF-1α. FEBS Lett. 2001;505:269–74. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- 121.Zhou J, Fandrey J, Schumann J, Tiegs G, Brune B. NO and TNF-α released from activated macrophages stabilize HIF-1α in resting tubular LLC-PK1 cells. Am J Physiol Cell Physiol. 2003;284:C439–46. doi: 10.1152/ajpcell.00294.2002. [DOI] [PubMed] [Google Scholar]
- 122.Zhou J, Callapina M, Goodall GJ, Brune B. Functional integrity of nuclear factor κB, phosphatidylinositol 3'-kinase, and mitogen-activated protein kinase signaling allows tumor necrosis factor α-evoked Bcl-2 expression to provoke internal ribosome entry site-dependent translation of hypoxia-inducible factor 1α. Cancer Res. 2004;64:9041–8. doi: 10.1158/0008-5472.CAN-04-1437. [DOI] [PubMed] [Google Scholar]
- 123.Qian D, Lin HY, Wang HM, Zhang X, Liu DL, Li QL, Zhu C. Normoxic induction of the hypoxic-inducible factor-1αby interleukin-1β involves the extracellular signal-regulated kinase 1/2 pathway in normal human cytotrophoblast cells. Biol Reprod. 2004;70:1822–7. doi: 10.1095/biolreprod.103.025031. [DOI] [PubMed] [Google Scholar]
- 124.Haddad JJ. Recombinant human interleukin (IL)-1β-mediated regulation of hypoxia-inducible factor-1α (HIF-1α) stabilization, nuclear translocation and activation requires an antioxidant/reactive oxygen species (ROS)-sensitive mechanism. Eur Cytokine Netw. 2002;13:250–60. [PubMed] [Google Scholar]
- 125.Thornton RD, Lane P, Borghaei RC, Pease EA, Caro J, Mochan E. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J. 2000;350:307–12. [PMC free article] [PubMed] [Google Scholar]
- 126.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–71. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 127.Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–9. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 128.Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- 129.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–40. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 130.Ma Y, Freitag P, Zhou J, Brune B, Frede S, Fandrey J. Thyroid hormone induces erythropoietin gene expression through augmented accumulation of hypoxia-inducible factor-1. Am J Physiol Regul Integr Comp Physiol. 2004;287:R600–7. doi: 10.1152/ajpregu.00115.2004. [DOI] [PubMed] [Google Scholar]
- 131.Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem. 2004;279:19431–40. doi: 10.1074/jbc.M401235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1α protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–95. [PubMed] [Google Scholar]
- 133.Shatrov VA, Sumbayev VV, Zhou J, Brune B. Oxidized low-density lipoprotein (oxLDL) triggers hypoxia-inducible factor-1α (HIF-1α) accumulation via redox-dependent mechanisms. Blood. 2003;101:4847–9. doi: 10.1182/blood-2002-09-2711. [DOI] [PubMed] [Google Scholar]
- 134.Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α-deficient chimeric mice. Proc Natl Acad Sci USA. 2002;99:2170–4. doi: 10.1073/pnas.052706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Makino Y, Nakamura H, Ikeda E, Ohnuma K, Yamauchi K, Yabe Y, Poellinger L, Okada Y, Morimoto C, Tanaka H. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J Immunol. 2003;171:6534–40. doi: 10.4049/jimmunol.171.12.6534. [DOI] [PubMed] [Google Scholar]
- 136.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1α. Mol Cell Biol. 2004;24:5223–34. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koury J, Deitch EA, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, Feinman R. Persistent HIF-1α activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock. 2004;22:270–7. doi: 10.1097/01.shk.0000135256.67441.3f. [DOI] [PubMed] [Google Scholar]
- 139.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–81. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 140.Shyu KG, Wang MT, Wang BW, Chang CC, Leu JG, Kuan P, Chang H. Intramyocardial injection of naked DNA encoding HIF-1α/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res. 2002;54:576–83. doi: 10.1016/s0008-6363(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 141.Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation. 2000;102:2255–61. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- 142.Milkiewicz M, Pugh CW, Egginton S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J Physiol. 2004;560:21–6. doi: 10.1113/jphysiol.2004.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Willam C, Masson N, Tian YM, Mahmood SA, Wilson MI, Bicknell R, Eckardt KU, Maxwell PH, Ratcliffe PJ, Pugh CW. Peptide blockade of HIFα degradation modulates cellular metabolism and angiogenesis. Proc Natl Acad Sci USA. 2002;99:10423–8. doi: 10.1073/pnas.162119399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M. PR39, a peptide regulator of angiogenesis. Nat Med. 2000;6:49–55. doi: 10.1038/71527. [DOI] [PubMed] [Google Scholar]
- 145.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1α. Genes Dev. 2001;15:2520–32. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heeschen C, Dimmeler S, Hamm CW, Boersma E, Zeiher AM, Simoons ML. Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation. 2003;107:524–30. doi: 10.1161/01.cir.0000048183.37648.1a. [DOI] [PubMed] [Google Scholar]
- 147.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 148.Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643–7. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 149.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–4. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- 150.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 151.Date T, Mochizuki S, Belanger AJ, Yamakawa M, Luo Z, Vincent KA, Cheng SH, Gregory RJ, Jiang C. Expression of constitutively stable hybrid hypoxia-inducible factor-1α protects cultured rat cardiomyocytes against simulated ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;288:C314–20. doi: 10.1152/ajpcell.00374.2004. [DOI] [PubMed] [Google Scholar]
- 152.Horwitz LD, Sherman NA, Kong Y, Pike AW, Gobin J, Fennessey PV, Horwitz MA. Lipophilic siderophores of Mycobacterium tuberculosis prevent cardiac reperfusion injury. Proc Natl Acad Sci USA. 1998;95:5263–8. doi: 10.1073/pnas.95.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pahl PM, Yan XD, Hodges YK, Rosenthal EA, Horwitz MA, Horwitz LD. An exochelin of Mycobacterium tuberculosis reversibly arrests growth of human vascular smooth muscle cells in vitro. J Biol Chem. 2000;275:17821–6. doi: 10.1074/jbc.M909918199. [DOI] [PubMed] [Google Scholar]
- 154.Rosenthal EA, Bohlmeyer TJ, Monnet E, MacPhail C, Robertson AD, Horwitz MA, Burchenal JE, Horwitz LD. An iron-binding exochelin prevents restenosis due to coronary artery balloon injury in a porcine model. Circulation. 2001;104:2222–7. doi: 10.1161/hc4301.097194. [DOI] [PubMed] [Google Scholar]
- 155.Mabjeesh NJ, Post DE, Willard MT, Kaur B, Van Meir EG, Simons JW, Zhong H. Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–82. [PubMed] [Google Scholar]