Abstract
In the acute-care setting, it is widely accepted that elderly patients have increased morbidity and mortality compared with young healthy patients. The reasons for this, however, are largely unknown. Although animal modeling has helped improve treatment strategies for young patients, there are a scarce number of studies attempting to understand the mechanisms of systemic insults such as trauma, burn, and sepsis in aged individuals. This review aims to highlight the relevance of using animals to study the pathogenesis of these insults in the aged and, despite the deficiency of information, to summarize what is currently known in this field.
Keywords: Burn injury, blunt trauma, penetrating trauma, hemorrhage, traumatic brain injury, sepsis, elderly
INTRODUCTION
With advances in treatment strategies during the last few decades, the overall mortality in elderly patients in the acute-care setting has decreased considerably (1, 2). However, these patients still tend to require more aggressive treatment strategies and have an increased length of stay in the hospital (2–6). In addition, for the elderly patients who survive a systemic insult, regardless of the etiology, more end up in long-term care facilities, have greater postdischarge disabilities, and report to have a decreased quality of life than younger patients (7–10). Because these outcomes translate into a greater financial and societal burden, improvement in care for elderly patients in the acute-care setting is warranted.
Pathogenesis of a systemic insult
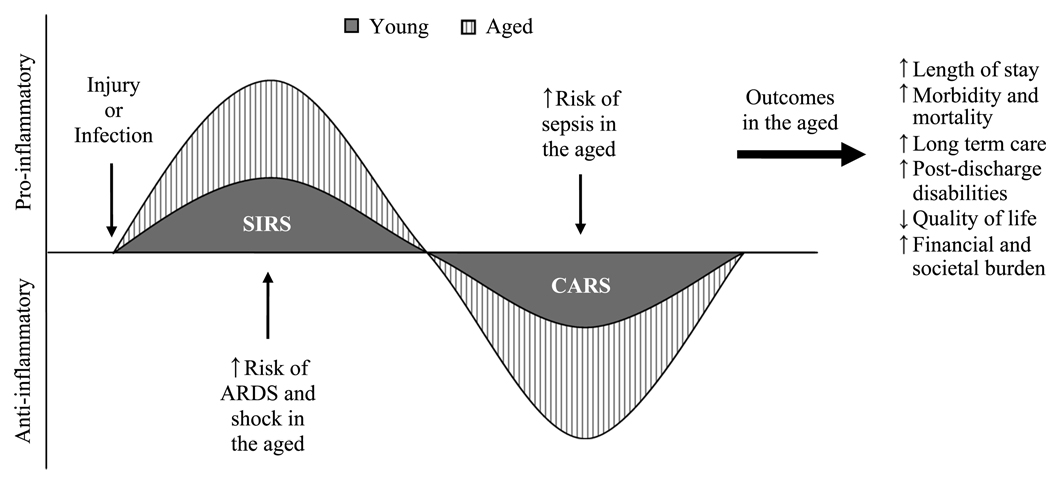
After nearly all types of systemic insults, the leading causes of death are multiple organ failure (MOF) and complications due to sepsis (11–16). For both of these outcomes, disease progression initiates from the original site of injury or infection (13, 17–19) and leads to the release of proinflammatory mediators such as IL-6, IL-8, and platelet-activating factor into circulation (17, 18, 20–23). After this phase—commonly termed the systemic inflammatory response syndrome (SIRS) (16, 24)—a global immunosuppressive phase can be observed, marked by increased interferon-γ (IFN-γ) and IL-10, as well as decreased proinflammatory cytokines (Fig. 1) (25–27). In the initial proinflammatory phase, the patient is at a significant risk for early MOF as a result of inflammatory-mediated destruction of vital organs such as the heart and lungs (13, 28) and shock, whereby hypoperfusion renders tissues incapable of sustaining aerobic metabolism (29). In the later phase, when immunosuppression dominates, the risk for MOF is more likely to result from infection and sepsis (11–13, 30). In elderly patients, compromises in both phases can be seen after a systemic insult, thus placing them at an increased risk for both early and late MOF (Fig. 1). It is therefore crucial to fully understand the aberrant responses that occur with age to improve outcomes after systemic injury in this population. Moreover, because the inflammatory component of sepsis is similar to the systemic response to severe burn or trauma, it is also important to examine the effects of aging on the reaction to injury complicated by sepsis.
FIG. 1. The effects of aging on the evolution of the systemic response after injury or infection.
In response to a serious injury or infection, there is a systemic release of proinflammatory mediators leading to SIRS, followed by CARS. In the aged patients, the degree of SIRS and CARS is exaggerated, causing an increased risk for ARDS and shock during SIRS and an increased risk for sepsis during compensatory anti-inflammatory response syndrome. Graphically, this is illustrated by a relative increase in the area under the curve. This response in the elderly patients translates into worse outcomes, as indicated. ARDS indicates acute respiratory distress syndrome; CARS, compensatory anti-inflammatory response syndrome; SIRS, systemic inflammatory response syndrome.
Age as an independent risk factor after a systemic insult
There is a large body of literature from major centers around the world that aim to identify risk factors for predicting mortality from a variety of different insults. Although the injury severity score (ISS) has been found to be one of the best predictors of outcome of trauma (2, 31, 32), its value for elderly patients is controversial. Some report that when the ISS is the same or even lower in old patients compared with younger ones, their mortality rates still remain significantly higher (2, 33–36). Others have shown that elderly patients have an overall higher mortality rate simply because their in juries are more severe (37, 38). Many speculate that the most probable explanation for increased morbidity and mortality in the elderly trauma patient is the existence of comorbidities such as cardiovascular disease and diabetes (38). However, even when controlling for the presence of one or more pre-existing conditions, elderly patients are still at a significantly increased risk of developing complications and dying than younger patients (4, 35). Richmond et al. (36) have proposed that a greater predictor of mortality in the aged trauma patient is the total number of insults initially sustained or acquired in the hospital, including age itself.
The biology of aging
Although a number of theories have emerged, most investigators who study the process of aging agree that the phenotypic manifestations that occur with age are a result of the accumulation of cellular damage over time (39–43). When cells are exposed to stressors such as UV radiation and reactive oxygen species, they tend to respond by entering into a senescent phase in an attempt to resist significant damage (39, 41, 42). If the damage is enough to render the cell incapable of repair, cells will likely undergo apoptosis (42). The ability of a cell to enter into senescence for self-preservation naturally makes it resistant to tumorigenesis because senescent cells lose the capacity to proliferate Although this seems of great benefit to an individual, the accumulation of senescent cells within a tissue can eventually lead to functional defects (44). In fact, the “aging phenotype” has been associated with the accumulation of senescent cells within various tissues of both rodents and humans, as well as within age-related pathological lesions such as atherosclerotic plaques, arthritic joints, and tumorous growths (44–48). Although seemingly counterproductive to the survival of a species, it is thought that the aging phenotype has actually been selected for in the population because many of the relevant genes are paradoxically associated with increased survival during the reproductive years (49). The concept that the same genes that are protective early in life, but cause detrimental effects that manifest later in life, is called antagonistic pleiotropy (49). What determines how an individual will age, therefore, is not only dependent on the exposure to damaging agents (endogenous or exogenous) but also on the genetic makeup that determines the response to them (40, 41, 50–52).
It is important to note that cellular senescence can occur in most, if not all, cells of the body, including those of the immune system The effects of immunosenescence result in decreased T-cell memory, decreased naive T cells with involution of the thymus, and a chronic inflammatory state, which has come to be termed “inflamm-aging” (53). Marked by an elevation in IL-6, TNF-α, and C-reactive protein, this inflammatory state of elderly patients is associated with a decreased ability to combat infections and resist tumor formation, and with an increased susceptibility to autoimmune diseases (54–57). It is thought that this proinflammatory state of elderly patients is also what significantly increases the risk for developing complications after injury or infection, as previously indicated.
We therefore propose that both injury and sepsis in the elderly are actually “two-hit” processes, whereby increased age primes the immune system and makes an individual vulnerable to MOF after a major insult. As in other two-hit models of systemic inflammation, the occurrence of the second challenge results in an exaggerated response, greater than that which could be achieved by either challenge alone (11, 58–60). Be cause these situations clinically pose the greatest challenge, developing effective treatment strategies to combat them is of great importance. Here, we review various animal models of systemic insult in the context of aging to elucidate some of the common modalities that can be targeted for therapy. Unfortunately, this picture is missing a great deal of substance, so insights regarding what may be happening clinically are, for the most part, unknown. Because the cost and effort to obtain aged animals is prohibitive, even for small animals, this re view will not focus on large animal models.
AGING AND ANIMAL MODELS OF SYSTEMIC INSULT
Burn injury
General models
The most common animal model used to study the response to burn is the generation of a scald injury of the desired total body surface area (61–64). This model, originally proposed by Walker and Mason (65), generates a full-thickness, third-degree burn. Other models involve using a heated stainless steel template (66) or an open flame to generate the burn (67, 68).
Burn injury and aging
As with sepsis, aged individuals who have burn injury have significantly greater mortality relative to young individuals. For example, a moderate-sized burn covering 20% of the body produces a mortality of only 5.5% in healthy, young adult patients, whereas elderly patients with the same burn size have a mortality of up to 75% (1, 69, 70). As in aged humans (71, 72), aged rodents exhibit higher mortality after burn injury (73). When we monitored survival in young and aged mice after a 15% total body surface area burn, our results showed 100% survival in the young mice–with no changes in their ability to eat, drink, groom, or ambulate—whereas only 33% of the aged mice survived (73).
The use of this moderate-sized scald injury model has also allowed us to investigate the effects of aging on systemic and cellular inflammatory and immune responses. At 24 h after burn injury, serum levels of IL-6 were almost double that of aged burn-injured mice relative to young burn-injured mice (74). Experimental evidence further demonstrates that advanced age accentuates the immunosuppression after burn injury (75). For example, aged mice had a significant decrease in delayed-type hypersensitivity (DTH). In addition, splenic lymphocytes from aged burn-injured mice produced higher levels of TH2 cytokines and had decreased proliferation in response to concanavalin A in vitro relative to young burn-injured mice (75, 76). A depressed immune response may likely predispose the elderly to a poorer outcome after burn injury in part because of an increased susceptibility to infection and sepsis (73, 75–77).
Because low (proestrus) levels of estrogen suppress the production of proinflammatory cytokines, including IL-6 (77–79), we tested the therapeutic efficacy of estrogen administration on immune responses after burn injury in aged female mice (74, 80). In our studies, estrogen supplementation markedly improved survival after burn injury relative to aged burn-injured mice given a placebo (74, 80). This intervention also reduced serum levels of IL-6 (74) and restored cellular immune responses in aged mice given a burn injury, as evidenced by a partial restoration of the DTH response (74). In addition, a recovery in IFN-γ production by cultured splenocytes, but not IL-4 levels, suggested a restoration of the TH1-TH2 shift in aged burn-injured mice (26). To further explore the involvement of IL-6 in the postburn immunosuppressive phase in aged mice, we gave anti–IL-6 antibody after burn injury and examined systemic and organ-specific immune responses. This treatment partially restored the DTH response in aged burn-injured mice and improved splenocyte proliferation and cytokine production relative to aged burn-injured mice receiving control immunoglobulin G (E.J. Kovacs, unpublished observations). In summary, our therapeutic strategies aimed at attenuating the proinflammatory state in aged mice after burn have beneficial effects on survival and the immune response.
As with other scenarios that lead to systemic inflammation, the lungs are often the first to fail after burn injury (81). Relative to young burn-injured patients, elderly patients have increased mortality when pulmonary dysfunction is present (82, 83). Recently, we examined lung pathologic findings in young and aged mice after scald injury and found that pulmonary neutrophils were higher in the lungs of aged, burn-injured mice at 24 h after injury compared with those of young mice receiving the same injury (84). Likewise, lung levels of the neutrophil chemokine KC were consistently higher in aged, burn-injured mice at both 6 and 24 h after injury compared with the young.
Although the pulmonary effects of burn injury in the elderly have been described, few have investigated the underlining mechanisms (85–88). Administration of anti-CXCR2 antibody completely abrogated the excessive pulmonary pathologic findings seen at 24 h in aged burn-injured mice (84). Whereas the consequences of this intervention on pulmonary function still need to be addressed, these results indicate that decreasing pulmonary neutrophil infiltration may prevent remote organ damage after injury, especially in elderly patients.
Blunt or penetrating trauma
General models
A number of animal models exist for both blunt and penetrating trauma; the most common are laparotomy and fracture [reviewed in Refs. (29, 89, 90)]. Penetrating injuries tend to involve larger vessels, and, therefore, progression to hemorrhagic shock is fast (89, 90). For blunt injuries, on the other hand, a greater degree of cellular damage is generated, and hemorrhage typically occurs gradually, leading to the development of metabolic acidosis (90).
Blunt or penetrating trauma and aging
In the clinical setting, the overall severity of injury and risk of death from both blunt or penetrating trauma is reported as being significantly increased or not different between young and aged patients (2, 3, 6, 34, 38) even when stratifying for ISS (4, 37, 91) or the presence of comorbidities (35). Regardless of whether mortality rates are different, aged patients consistently have an increased length of stay in the hospital compared with younger patients (3, 6, 38).
Interestingly, sex differences have also been found after blunt or penetrating trauma, suggesting a role for sex hormones in the response to injury (2). Some studies indicate that for blunt trauma, young women have a decreased mortality rate than young men, but that this survival benefit is lost in women older than 50 years (when menopause typically begins) (92–94). For penetrating trauma, on the other hand, no significant differences in mortality were seen between young men and women; however, women older than 50 years had decreased survival (95). Results from both models show that proestrus levels of estrogen are protective, possibly because of its effect on the hemodynamic response to hemorrhage and inflammation.
The most frequent cause of blunt or penetrating trauma in aged individuals is due to falls (2, 3, 5, 8). As many as 75% of emergency department consultations are related to falls in elderly patients, and, in 2002, the overall cost of falls in the aged was approximately $19 billion (5, 96, 97). With the proportion of aged individuals in our population growing, it is estimated that this figure will reach $43.8 billion by 2020 (97). These statistics indicate that elderly patients sustaining a traumatic injury still use a great deal of resources. However, despite the large volume of literature on accepted animal models of trauma, there are currently no data published on the effects of aging on blunt or penetrating trauma in animal models.
Trauma and hemorrhagic shock
General models
In the clinical setting, traumatic injury is typically accompanied by hemorrhage and can often lead to hemorrhagic shock. Therefore, models that only control for the inciting injury may not fully capture the true clinical picture. As a result, some laboratories have extended the models of blunt and penetrating trauma to include exsanguination with or without reperfusion or resuscitation [reviewed in Ref. (90)]. It has been found that, regardless of the mechanism, the initial injury initiates the inflammatory response, whereas the subsequent hemorrhage leads to tissue hypo-perfusion, ischemia, tissue reperfusion, and exacerbation of the systemic inflammatory response (90, 98).
A number of methods to generate hemorrhagic shock have been described (89). In a fixed-pressure hemorrhage, the MAP of the animal is maintained at levels significantly less than normal for a defined period of time—typically between 30 and 90 min. In a fixed-volume hemorrhage, up to 50% of the total blood volume is removed at one time. Although these two models are the easiest to control and monitor in the laboratory, they are also the least clinically relevant, mostly because they ignore the body’s intrinsic responses to hemorrhage (27, 99–103). Uncontrolled hemorrhage models are most similar to what happens in trauma patients, but the results are highly variable when performed in the laboratory. Although the hemodynamics of these models differ greatly, they all tend to show an exacerbated systemic response compared with trauma alone, especially when followed by reperfusion or resuscitation (15, 29), which is consistent with what is seen in trauma patients (104).
Trauma, hemorrhagic shock, and aging
Whereas there are no data on traumatic injury alone, a few rodent studies have been conducted to investigate the effects of increased age on trauma in combination with hemorrhagic shock. In response to laparotomy followed by hemorrhage, Schneider et al. (105) found an increased proliferation and release of the TH1 cytokines IL-2 and IFN-γ in splenic T cells from young mice after CD3 activation but no response at all after injury in aged mice. As shown in other models (26), this study by Schneider et al. also showed that, whereas young mice generate a TH1 response after a systemic injury, aged mice tend to generate a TH2 response by releasing more IL-4 and IL-10 (105). These data suggest that aged mice have a dysfunctional T-cell response after injury, which may be responsible for the increased risk for infection seen in elderly trauma patients.
This same laboratory has also shown that, in addition to changes seen with aging, there is also an effect of sex hormones on the T-cell responses to injury. As previously described, sex differences after trauma are lost with aging presumably because of the change in estrogen with menopause. In murine models, it was shown that young female mice have a robust T-cell response after trauma in combination with hemorrhage, whereas young males showed decreased responses (105, 106). In aged mice receiving trauma and hemorrhage, the responses were reversed: females had a suppressed T-cell response, whereas males were not different from sham-injured mice (106). As with clinical observations, these studies show that estrogen levels in females during their reproductive years are protective in the setting of traumatic injury.
Schneider et al. (107) have also shown that total levels of pluripotent stem cells in the bone marrow are significantly decreased in aged mice receiving trauma and hemorrhage compared with young mice receiving the same injury. As a result, whereas young mice were able to rapidly expand hematopoietic subsets after trauma and hemorrhage, aged mice were not. Interestingly, this study also showed that the ability for hematopoietic stem cells in the bone marrow to differentiate into both lymphoid and monocyte/macrophages after trauma and hemorrhage was more robust in young females compared with young males, and that this sex difference was again lost with age (107).
Traumatic brain injury
General models
Although traumatic brain injury (TBI) models seem to be just another extension on the injury models listed previously, the outcomes are often quite different. The central nervous system has limited plasticity, and, therefore, treatment strategies after trauma to this system must include methods of neuroprotection such as hypothermia and glutamate release inhibitors (108). Moreover, along with the systemic inflammatory changes seen in other types of trauma (109–112), TBI patients frequently develop primary endocrine failure, which can significantly complicate treatment (14, 98).
Similar to models of hemorrhagic shock and sepsis, modeling TBI to what occurs clinically has been difficult; this type of injury is often complex and frequently involves some degree of both focal deficits and diffuse injury. In addition, our understanding of higher cognitive function is limited, so the ability to reproduce clinical outcomes precisely is near impossible (113) However, a number of animal models for head trauma have been developed to mimic the human response, mostly in rodents. Whereas a critical comparison of the models within each of these categories is beyond the scope of this review, it can be said that the results obtained from these studies are highly dependent on the method of injury. Overall, these models can be separated into two major categories: focal or diffuse injury (113, 114). Because each of these types of injury are quite different and can be generated a number of ways (impact of a blunt object, penetration of an object through the skull, hematomas, coup-contrecoup contusions, etc.), it is critical to understand that the results of each animal model must be applied to the particular human condition it mimics (113, 114). The most common animal model is application of direct mechanical force using either the lateral fluid percussion model or the cortical impact model [reviewed in Refs. (104, 114)].
TBI and aging
As with other types of trauma, mortality in the elderly population with TBI is approximately double that of younger trauma patients (10, 33, 115), and falls are the primary reason for this type of injury (5, 8, 116, 117). One of the most critical reasons for studying TBI in elderly patients, both clinically and in the laboratory, is that many patients older than 65 years are more likely to have an occult presentation (33, 117, 118). These patients can present with no evidence of skull fracture, altered level of consciousness, or focal neurological deficits, the main clinical tools for a physician to assess the degree of brain injury in younger patients, yet still have a significantly higher risk of death or decreased functional status after discharge (8, 10, 33, 115, 117–119).
As with the other trauma models described in this review, a limited number of animal studies exist that analyze the effects of aging on the response to TBI. Regardless of the method to generate TBI experimentally, many parallels to the clinical picture of TBI in the elderly have been found. Specifically, it has been shown that aged rats have a greater risk of death and development of functional deficits after injury than younger rats receiving TBI (120). Moreover, Maughan et al. (121) have found that, even when adjusting the severity of the injury to match young and aged rats for mortality, aged animals have significant functional losses. Magnetic resonance images of mice receiving TBI in a controlled cortical impact model show that the blood-brain barrier of aged mice is leakier and has more neurodegeneration at 3 days after injury compared with that of young animals receiving the same injury (122). Consistent with the concept of inflammaging, some laboratories observed increased basal levels of p21 (a cell cycle regulation protein), brain-derived neurotropic factor, and IL-1β mRNA, as well as decreased levels of the antioxidant ascorbate in the brains of aged rodents in the absence of injury (123–125). As in the other models, TBI caused an even greater increase in oxidative and metabolic stress, DNA damage, and inflammation (marked by increased brain-derived neurotropic factor and IL-1β) in brains of aged compared with young mice (124). Furthermore, aged mice were unable to raise levels of either urate or ascorbate, both of which have been shown to provide neuro-protection against oxidative damage after TBI, in the hippocampus and corpus callosum (125). To date, no observations on the systemic effects of TBI in aged mice have been made. Because both age and TBI significantly affect the endocrine response, animal modeling may be a valuable tool to study the effects of both factors. Overall, these few studies demonstrate that rodent models of TBI in the aged are clinically relevant and reproducible. Again, because TBI in the elderly is an increasing problem in our society, it is important to expand these studies to better understand the mechanisms that lead to worse outcomes in elderly relative to younger patients.
Sepsis
General models
Again, one of the most frequent complications of both burn injury and trauma is sepsis (11, 12). Sepsis is clinically defined as an acute systemic infection (bacterial, fungal, or parasitic) combined with tachycardia, tachypnea, hyper or hypothermia, and leukocytosis or leu-kopenia (126, 127). Progression of the response to sepsis can eventually lead to septic shock, hypotension, circulatory collapse, and death. Several animal models have been developed in an attempt to mimic the complex of symptoms seen in the human response. The experimental settings include bolus injections of bacteria (128), administration of microbial components such as LPS (129), and cecalligation and puncture (CLP) (130). The discussion regarding the advantages and disadvantages of the different models of sepsis in the ability to mimic human disease is beyond the scope of the present review However, it is important to note that, although the different models have replicated some of the features of human sepsis, they have failed to fully reproduce the condition (128). For example, in the model using LPS, pro-inflammatory mediators reach much higher peak levels and disappear earlier than in humans (126). Using CLP, on the other hand, proinflammatory mediators peak later and do not reach the levels achieved with direct LPS injection (126, 131, 132). Currently, CLP has become the more accepted model for the study of experimental sepsis, with the limitation that specific host-pathogen interactions are dependent on the poly-microbial origin (128, 133).
Sepsis and aging
Unlike other systemic insults, sepsis is the most frequent systemic insult in the elderly population; the mean age of intensive care unit patients meeting the criteria for severe sepsis is 63.8 years (7). Moreover, elderly patients account for 65% of sepsis cases, yielding a relative risk that is 13.1 times higher than younger patients (7). In relation to clinical outcomes after sepsis, age alone was found to be an independent predictor of mortality (7). Currently, there are no studies analyzing the effects of aging on the development of sepsis after burn or trauma. Examining the response of aged animals to sepsis alone, however, may provide insight into the mechanisms behind the triple hit of injury complicated by sepsis in elderly patients.
More than all other models of systemic insult, LPS administration is the most widely studied in aged animals mostly because of its simplicity and reproducibility. However, results from these studies need to be analyzed carefully because the injection of LPS does not necessarily generate the same response. We and others have found that aged mice given intraperitoneal LPS or CLP show higher mortality when compared with the young (131, 134, 135). Regardless of age, the mortality rate is higher in the CLP model when compared with the LPS model (131). In one study, Turnbull et al. (136) monitored survival during a 10-day period using a moderate injury consisting of a double puncture of the cecum with a 25-gauge needle. Here, 75% of young mice compared with only 25% of aged mice survived (136).
We have previously indicated that one of the main elements involved in the pathogenesis of the systemic inflammatory response in the elderly is the presence of an exaggerated proinflammatory state. As a result, both LPS administration and CLP generate higher serum levels of IL-6 and TNF-α in aged mice relative to young mice (131, 134–137). Interestingly, higher plasma levels of IL-6 were also observed in aged mice that died compared with young nonsurvivors (136), suggesting an association between IL-6 levels and mortality, as previously described (17). No age-associated differences were found in serum IL-1β levels, but IL-10 levels were significantly higher in aged mice after either CLP or LPS relative to those of young mice receiving the same insult (131, 137).
The dysregulated inflammatory response in elderly patients has also been shown to alter other systemic defense systems. After trauma or infection, the acute-phase response is initiated by the host in an effort to prevent additional tissue damage, to destroy infectious organisms, and to trigger the repair processes that are necessary to restore homeostasis (138–140). When compared with young mice, aged mice receiving LPS exhibited decreased mRNA expression of acute-phase proteins such as α1-acid glycoprotein and albumin (141). However, aged mice receiving intraperitoneal LPS had higher serum levels of serum amyloid A (SAA) and normal levels of LPS-binding protein relative to those of young mice (135). Interestingly, because IL-6 is one of the main stimulators of the acute-phase response, aged IL-6 knockout mice receiving LPS had improved survival when compared with aged wild-type mice (135). In addition, aged LPS-treated IL-6 knockout mice had serum levels of SAA and corticosterone, normally found to be elevated in the aged (142, 143), which were markedly reduced compared with aged wild-type mice (135).
Regardless of age, acute lung injury is a major cause of morbidity and mortality in patients with sepsis (12, 144). At 24 h after intraperitoneal injection of LPS, we found that aged mice similarly had a highly exacerbated pulmonary inflammatory response compared with that of young mice receiving the same insult (145). Relative to young mice, IL-6 mRNA expression was greater in the lungs and hearts from aged mice subjected to CLP (146). Because proinflammatory cytokines may contribute to the development of organ dysfunction during septic shock (147, 148), their augmented expression in the organs of aged mice may enhance tissue injury, thus contributing to the age-associated morbidity and mortality.
The presence of an early hypermetabolic phase followed by a hypometabolic phase is characteristic of the progression of systemic insults, including sepsis (137, 149). The timing of the transition from hypermetabolism to hypometabolism, as evaluated by changes in serum glucose levels, did not seem to be affected by advanced age in mice after CLP (137). However, during the hypometabolic phase, aged mice developed a more profound hypoglycemia than young mice after CLP (137). Temperature instability is another hallmark of sepsis, ranging from hyperthermia to hypothermia (131, 150). The degree of hypothermia after CLP was more pronounced in aged mice than in young mice (131). In addition, a difference in the severity of hypothermia was found between aged mice that survived and those that died, suggesting that the mortality in the aged mice can be predicted by the degree of hypothermia (131).
Whereas mortality in elderly patients with sepsis seems to be dependent, in part, on the reduced tolerance of organ systems to stress, there is a lack of information regarding the mechanisms involved in age-related tissue vulnerability to sepsis. Regardless of age, apoptotic cell death has been found in many cell types in septic patients especially in lymphoid organs and in the gastrointestinal tract (151–153). Apoptotic cell death after CLP in aged mice was also found to be significantly higher in the spleens and gut epithelium, relative to young mice after CLP (154). One possibility for the mechanism causing increased apoptosis in aged mice after CLP includes the effect of activation of TNF receptor 1 and the death domain–associated activation of caspase 3 (155–157). Because TNF-α is augmented in aged relative to young mice during CLP (136, 137), it is possible that TNF-α may play an important role in sepsis-induced apoptosis in aged individuals.
CONCLUSIONS
Although elderly patients do not comprise most patients in trauma or burn units, their mortality rates are often significantly higher even when adjusting for injury severity. In addition, this patient population tends to be undertriaged, use more resources, have an increased length of stay in the hospital, and incur greater hospital charges than younger patients. Moreover, elderly patients requiring acute care often have worse postdischarge outcomes compared with younger patients. In particular, they have an increased risk of developing septic complications. Although there are proven clinical differences in this patient population, there seems to be a lack of effort to understand why they fare worse after a systemic challenge (Table 1). Perhaps the cost of obtaining aged animals, their decreased tolerance to inflammation, and the ethics involved in generating injury discourage investigators from pursuing this cause. The purpose of this review was to elucidate the benefits of animal modeling in the setting of systemic inflammation in aged individuals and the clinically relevant situation of multiple hits to implore others to expand our knowledge in this field. Not discussed, however, is the potential benefit of using in silico models to simulate the response to injury and infection so as to avoid the issues involved with using live animals.
TABLE 1.
Comparing the known effects of aging in models of burn, trauma, and sepsis
| Survival | Circulating proinflammatory mediators |
Circulating anti-inflammatory mediators |
Immune cell responses |
Remote organ damage |
Metabolic response |
Sex differences |
References | |
|---|---|---|---|---|---|---|---|---|
| Burn injury | ↓ | ↑ IL-6 | ? | ↑TH2 cytokines in vitro; ↓ DTH response; ↓ splenocyte proliferation in vitro |
↑ Lung | ? | ? | 22, 72, 74, 75, 83 |
| Blunt or penetrating trauma |
? | ? | ? | ? | ? | ? | ? | — |
| Trauma hemorrhage | ? | ? | ? | ↑ TH2, no TH1 cytokines in vitro; ↓ splenocyte proliferation in vitro |
? | ? | More robust immune cell response in young females; no differences in aged |
104, 106 |
| TBI | ↓ | ? | ? | ? | ? | ? | ? | 119, 120 |
| Sepsis | ↓ | ↑ IL-6, IL-1β, TNF-α, SAA |
↑ IL-10 and corticosterone |
? | ↑ Lung and liver | More profound hypoglycemia |
? | 132, 135, 136, 137, 138, 146, 147 |
DTH indicates delayed-type hypersensitivity; SAA, serum amyloid A
It has been speculated that many clinicians maintain the stigma that elderly patients in the acute-care setting will most likely die, and, therefore, they withhold aggressive treatment despite the potential for complete recovery (91). After reviewing the current body of literature on the effects of age on injury and sepsis, it is evident that these patients are more complicated than younger patients mainly because of the existence of comorbid conditions, occult presentations, and an overall dysregulation of the inflammatory response. Instead of trying to fit these patients into the standards of care set for younger individuals, there is a call for generating a new or distinct systematic approach to the elderly patient with severe injury or infection (37); suggestions have even included creating a geriatric consult service (36). This may involve either simply treating these patients more aggressively or using novel methods of treatment.
For each of the systemic inflammatory conditions reviewed here, a number of similar aspects can be found (Table 1). However, because the pathogenesis of each carries its own uniqueness, it is not enough to fully accept the conclusions regarding one situation to treat another. For example, the condition of sepsis is driven by the presence of a microorganism; burn injury produces a great degree of tissue damage, causing the loss of thermal and barrier regulation generated by the skin; cardiovascular and hemodynamic responses are a major component to trauma combined with hemorrhage; TBI requires central nervous system protection and prevention against primary endocrine dysfunction. Underlying each of these situations is the immune/inflammatory parameters that drive their progression from bad to worse. Because it is known that aged individuals have significant compromises in their immune system in the absence of injury, it is important to consider age as the first “hit” in the face of injury. In particular, IL-6 seems to be a common factor between inflamm-aging and each of these models of systemic inflammation (17, 20, 23, 77, 135, 142, 158, 159). Methods to modulate the exaggerated inflammatory response in the aged may therefore be promising. Animal studies from our laboratory and others have shown the benefit in inhibiting one or more components of inflammation in aged mice after injury, such as administration of anti—IL-6 antibody (E.J. Kovacs, unpublished observations), chemokine blockers (84, 160), and estrogen (74). Although these therapies have shown benefit in rodents, the controversy over whether they can translate to the clinic still remains. Because the studies on the effectiveness of new treatment strategies are not being performed in animal models, it can be decades before any novel therapies for elderly patients sustaining a systemic insult are developed. Until then, it is of great importance that clinicians derive standards of care for elderly patients in the acute-care setting that are not based on the typical response of young, healthy individuals.
ACKNOWLEDGMENTS
The authors thank Pamela Witte, M.D., as Director of the Immunology and Aging Program at Loyola University and Joslyn Albright, M.D., for thoughtful discussion.
This study was supported by the National Institutes of Health (grant nos. R01 AG18859 to E.J.K., R01 GM042577 to R.L.G., R01 AA12034 to E.J.K., F30 AG029724 to V.N.), Institutional Training Grant T32 AA13527 (to E.J.K), Illinois Excellence in Academic Medicine Grant, and Ralph and Marian C Falk Research Trust.
REFERENCES
- 1.Miller SF, Bessey PQ, Schurr MJ, Browning SM, Jeng JC, Caruso DM, Gomez M, Latenser BA, Lentz CW, Saffle JR, Kagan RJ, Purdue GF, Krichbaum JA. National Burn Repository 2005: a ten-year review. J Burn Care Res. 2006;27:411–436. doi: 10.1097/01.BCR.0000226260.17523.22. [DOI] [PubMed] [Google Scholar]
- 2.National Trauma Data Bank Annual Report. [Accessed March 28, 2008];2007 Available at: http://www.facs.org/trauma/ntdb/ntdbannualreport2007.pdf.
- 3.Gomberg BF, Gruen GS, Smith WR, Spott M. Outcomes in acute orthopaedic trauma: a review of 130,506 patients by age. Injury. 1999;30:431–437. doi: 10.1016/s0020-1383(99)00138-2. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MD, Tracy JK, Meyer W, Pasquale M, Napolitano LM. Trauma in the elderly: intensive care unit resource use and outcome. J Trauma. 2002;53:407–414. doi: 10.1097/00005373-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron E, Clement J, Lavoie A, Ratte S, Bamvita JM, Aumont F, Clas D. A simple fall in the elderly: not so simple. J Trauma. 2006;60:268–273. doi: 10.1097/01.ta.0000197651.00482.c5. [DOI] [PubMed] [Google Scholar]
- 6.Roth BJ, Velmahos GC, Oder DB, Vassiliu P, Tatevossian R, Demetriades D, Belzberg H, Alo K. Penetrating trauma in patients older than 55 years: a case-control study. Injury. 2001;32:551–554. doi: 10.1016/s0020-1383(01)00079-1. [DOI] [PubMed] [Google Scholar]
- 7.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 8.Mosenthal AC, Livingston DH, Lavery RF, Knudson MM, Lee S, Morabito D, Manley GT, Nathens A, Jurkovich G, Hoyt DB, et al. The effect of age on functional outcome in mild traumatic brain injury. 6-month report of a prospective multicenter trial. J Trauma. 2004;56:1042–1048. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Goecke M, Sharkey P, Brenneman F. Long-term outcomes after injury in the elderly. J Trauma. 2003;54:486–491. doi: 10.1097/01.TA.0000051588.05542.D6. [DOI] [PubMed] [Google Scholar]
- 10.LeBlanc J, de Guise E, Gosselin N, Feyz M. Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Inj. 2006;20:779–790. doi: 10.1080/02699050600831835. [DOI] [PubMed] [Google Scholar]
- 11.Davis KA, Santaniello JM, He LK, Muthu K, Sen S, Jones SB, Gamelli RL, Shankar R. Burn injury and pulmonary sepsis: development of a clinically relevant model. J Trauma. 2004;56:272–278. doi: 10.1097/01.TA.0000108995.64133.90. [DOI] [PubMed] [Google Scholar]
- 12.Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma. 2003;54:959–966. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- 13.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Post-injury multiple organ failure a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. discussion 510–512. [DOI] [PubMed] [Google Scholar]
- 14.Deitch EA, Dayal SD. Intensive care unit management of the trauma patient. Crit Care Med. 2006;34:2294–2301. doi: 10.1097/01.CCM.0000233857.94604.73. [DOI] [PubMed] [Google Scholar]
- 15.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Baue AE. MOF, MODS, and SIRS: what is in a name or an acronym? Shock. 2006;26:438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 17.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccolo MT, Wang Y, Verbrugge S, Warner RL, Sannomiya P, Piccolo NS, Piccolo MS, Hugli TE, Ward PA, Till GO. Role of chemotactic factors in neutrophil activation after thermal injury in rats. Inflammation. 1999;23:371–385. doi: 10.1023/a:1020213717336. [DOI] [PubMed] [Google Scholar]
- 19.Stubbe HD, Greiner C, Westphal M, Rickert CH, Aken HV, Eichel V, Wassmann H, Daudel F, Hinder F. Cerebral response to norepinephrine compared with fluid resuscitation in ovine traumatic brain injury and systemic inflammation. Crit Care Med. 2006;34:2651–2657. doi: 10.1097/01.CCM.0000239196.17999.B7. [DOI] [PubMed] [Google Scholar]
- 20.Mimasaka S, Hashiyada M, Nata M, Funayama M. Correlation between serum IL-6 levels and death: usefulness in diagnosis of “traumatic shock”? Tohoku J Exp Med. 2001;193:319–324. doi: 10.1620/tjem.193.319. [DOI] [PubMed] [Google Scholar]
- 21.Botha AJ, Moore FA, Moore EE, Peterson VM, Silliman CC, Goode AW. Sequential systemic platelet-activating factor and interleukin 8 primes neutrophils in patients with trauma at risk of multiple organ failure. Br J Surg. 1996;83:1407–1412. doi: 10.1002/bjs.1800831027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs EJ, Duffner LA, Gregory MS, Grabowski KA. Anti IL-6 antibody restores cell-mediated immunity in aged mice after scald injury; Paper presented at: Immunology 2000, AAI/CIS Joint Meeting; Seattle, WA. 2000. May, [Google Scholar]
- 23.Woiciechowsky C, Schoning B, Cobanov J, Lanksch WR, Volk HD, Docke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002;52:339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA. 1994;271:226–233. [PubMed] [Google Scholar]
- 25.Durbin EA, Gregory MS, Messingham KA, Fontanilla CV, Duffner LA, Kovacs EJ. The role of interleukin 6 in interferon-gamma production in thermally injured mice. Cytokine. 2000;12:1669–1675. doi: 10.1006/cyto.2000.0768. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs EJ, Duffner LA, Plackett TP. Immunosuppression after injury in aged mice is associated with a TH1-TH2 shift, which can be restored by estrogen treatment. Mech Ageing Dev. 2004;125:121–123. doi: 10.1016/j.mad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CP, Schwacha MG, Chaudry IH. The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim Biophys Acta. 2004;1689:22–32. doi: 10.1016/j.bbadis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Demling RH, LaLonde C, Liu YP, Zhu DG. The lung inflammatory response to thermal injury: relationship between physiologic and histologic changes. Surgery. 1989;106:52–59. [PubMed] [Google Scholar]
- 29.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8:373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarlowe MH, Duffy A, Kannan KB, Itagaki K, Lavery RF, Livingston DH, Bankey P, Hauser CJ. Prospective study of neutrophil chemokine responses in trauma patients at risk for pneumonia. Am J Respir Crit Care Med. 2005;171:753–759. doi: 10.1164/rccm.200307-917OC. [DOI] [PubMed] [Google Scholar]
- 31.Santaniello JM, Luchette FA, Esposito TJ, Gunawan H, Reed RL, Davis KA, Gamelli RL. Ten year experience of burn, trauma, and combined burn/trauma injuries comparing outcomes. J Trauma. 2004;57:696–700. doi: 10.1097/01.ta.0000140480.50079.a8. dicussion 700–701. [DOI] [PubMed] [Google Scholar]
- 32.Davis JW, Kaups KL. Base deficit in the elderly: a marker of severe injury and death. J Trauma. 1998;45:873–877. doi: 10.1097/00005373-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. discussion 223–224. [DOI] [PubMed] [Google Scholar]
- 34.Schulman AM, Claridge JA, Young JS. Young versus old: factors affecting mortality after blunt traumatic injury. Am Surg. 2002;68:942–947. discussion 947–948. [PubMed] [Google Scholar]
- 35.Bergeron E, Lavoie A, Clas D, Moore L, Ratte S, Tetreault S, Lemaire J, Martin M. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma. 2003;54:478–485. doi: 10.1097/01.TA.0000037095.83469.4C. [DOI] [PubMed] [Google Scholar]
- 36.Richmond TS, Kauder D, Strumpf N, Meredith T. Characteristics and outcomes of serious traumatic injury in older adults. J Am Geriatr Soc. 2002;50:215–222. doi: 10.1046/j.1532-5415.2002.50051.x. [DOI] [PubMed] [Google Scholar]
- 37.Tornetta P, 3rd, Mostafavi H, Riina J, Turen C, Reimer B, Levine R, Behrens F, Geller J, Ritter C, Homel P. Morbidity and mortality in elderly trauma patients. J Trauma. 1999;46:702–706. doi: 10.1097/00005373-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Nagy KK, Smith RF, Roberts RR, Joseph KT, An GC, Bokhari F, Barrett J. Prognosis of penetrating trauma in elderly patients: a comparison with younger patients. J Trauma. 2000;49:190–193. doi: 10.1097/00005373-200008000-00003. discussion 193–194. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Sierra F. Is (your cellular response to) stress killing you? J Gerontol A Biol Sci Med Sci. 2006;61:557–561. doi: 10.1093/gerona/61.6.557. [DOI] [PubMed] [Google Scholar]
- 42.Campisi J. Aging, tumor suppression and cancer: high wire-act! Mech Ageing Dev. 2005;126:51–58. doi: 10.1016/j.mad.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 43.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Paradis V, Youssef N, Dargere D, Ba N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 46.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faragher RG, Kipling D. How might replicative senescence contribute to human ageing? Bioessays. 1998;20:985–991. doi: 10.1002/(SICI)1521-1878(199812)20:12<985::AID-BIES4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 48.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 49.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 50.Cournil A, Kirkwood TB. If you would live long, choose your parents well. Trends Genet. 2001;17:233–235. doi: 10.1016/s0168-9525(01)02306-x. [DOI] [PubMed] [Google Scholar]
- 51.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Guarente L, Picard F. Calorie restriction–the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflammaging An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 54.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 55.Doria G, Frasca D. Ageing and genetic control of immune responsiveness. Immunol Lett. 1994;40:231–233. doi: 10.1016/0165-2478(94)00061-1. [DOI] [PubMed] [Google Scholar]
- 56.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 58.Samonte VA, Goto M, Ravindranath TM, Fazal N, Holloway VM, Goyal A, Gamelli RL, Sayeed MM. Exacerbation of intestinal permeability in rats after a two-hit injury: burn and Enterococcus faecalis infection. Crit Care Med. 2004;32:2267–2273. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- 59.Moore EE, Moore FA, Harken AH, Johnson JL, Ciesla D, Banerjee A. The two-event construct of postinjury multiple organ failure. Shock. 2005;24 suppl 1:71–74. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 60.Maegele M, Sauerland S, Bouillon B, Schafer U, Trubel H, Riess P, Neugebauer EA. Differential immunoresponses following experimental traumatic brain injury, bone fracture and “two-hit”–combined neurotrauma. Inflamm Res. 2007;56:318–323. doi: 10.1007/s00011-007-6141-3. [DOI] [PubMed] [Google Scholar]
- 61.Tinsley JH, Teasdale NR, Yuan SY. Myosin light chain phosphorylation and pulmonary endothelial cell hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol. 2004;286:L841–L847. doi: 10.1152/ajplung.00341.2003. [DOI] [PubMed] [Google Scholar]
- 62.Sayeed MM. Inflammatory/cardiovascular-metabolic responses in a rat model of burn injury with superimposed infection. Shock. 2005;24 suppl 1:40–44. doi: 10.1097/01.shk.0000191412.56343.1e. [DOI] [PubMed] [Google Scholar]
- 63.Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ. Neutrophil chemokine production in the skin following scald injury. Burns. 1999;25:403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- 64.Mulligan MS, Till GO, Smith CW, Anderson DC, Miyasaka M, Tamatani T, Todd RF, 3rd, Issekutz TB, Ward PA. Role of leukocyte adhesion molecules in lung and dermal vascular injury after thermal trauma of skin. Am J Pathol. 1994;144:1008–1015. [PMC free article] [PubMed] [Google Scholar]
- 65.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Hansbrough JF, Wikstrom T, Braide M, Tenenhaus M, Rennekampff OH, Kiessig V, Bjursten LM. Neutrophil activation and tissue neutrophil sequestration in a rat model of thermal injury. J Surg Res. 1996;61:17–22. doi: 10.1006/jsre.1996.0074. [DOI] [PubMed] [Google Scholar]
- 67.Katahira J, Murakami K, Schmalstieg FC, Cox R, Hawkins H, Traber LD, Traber DL. Role of anti–l-selectin antibody in burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1043–L1050. doi: 10.1152/ajplung.00305.2001. [DOI] [PubMed] [Google Scholar]
- 68.Gore DC, Rinehart A, Asimakis G. Temporal changes in cellular energy following burn injury. Burns. 2005;31:998–1002. doi: 10.1016/j.burns.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Wibbenmeyer LA, Amelon MJ, Morgan LJ, Robinson BK, Chang PX, Lewis R, 2nd, Kealey GP. Predicting survival in an elderly burn patient population. Burns. 2001;27:583–590. doi: 10.1016/s0305-4179(01)00009-2. [DOI] [PubMed] [Google Scholar]
- 70.Jeng JC. Patrimonie de Docteur Baux–Baux scores >> 100 gleaned from 170,791 admissions: a glimmer from the National Burn Repository. J Burn Care Res. 2007;28:380–381. doi: 10.1097/BCR.0B013E318053D3F4. [DOI] [PubMed] [Google Scholar]
- 71.Kerby JD, McGwin G, Jr, George RL, Cross JA, Chaudry IH, Rue LW., 3rd Sex differences in mortality after burn injury: results of analysis of the National Burn Repository of the American. Burn Associatio? J Burn Care Res. 2006;27:452–456. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- 72.Le HQ, Zamboni W, Eriksson E, Baldwin J. Burns in patients under 2 and over 70 years of age. Ann Plast Surg. 1986;17:39–44. doi: 10.1097/00000637-198607000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Kovacs EJ, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. J Am Aging Assoc. 2002;25:3–10. doi: 10.1007/s11357-002-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76:36–41. doi: 10.1189/jlb.1103538. [DOI] [PubMed] [Google Scholar]
- 75.Plackett TP, Schilling EM, Faunce DE, Choudhry MA, Witte PL, Kovacs EJ. Aging enhances lymphocyte cytokine defects after injury. FASEB J. 2003;17:688–689. doi: 10.1096/fj.02-0452fje. [DOI] [PubMed] [Google Scholar]
- 76.Choudhry MA, Plackett TP, Schilling EM, Faunce DE, Gamelli RL, Kovacs EJ. Advanced age negatively influences mesenteric lymph node T cell responses after burn injury. Immunol Lett. 2003;86:177–182. doi: 10.1016/s0165-2478(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 77.Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Exp Gerontol. 2005;40:549–555. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 79.Saucedo R, Rico G, Basurto L, Ochoa R, Zarate A. Transdermal estradiol in menopausal women depresses interleukin-6 without affecting other markers of immune response. Gynecol Obstet Invest. 2002;53:114–117. doi: 10.1159/000053005. [DOI] [PubMed] [Google Scholar]
- 80.Gomez CR, Plackett TP, Kovacs EJ. Aging and estrogen: modulation of inflammatory responses after injury. Exp Gerontol. 2007;42:451–456. doi: 10.1016/j.exger.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hollingsed TC, Saffle JR, Barton RG, Craft WB, Morris SE. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166:592–596. doi: 10.1016/s0002-9610(05)80662-2. discussion 596–597. [DOI] [PubMed] [Google Scholar]
- 82.Clayton MC, Solem LD, Ahrenholz DH. Pulmonary failure in geriatric patients with burns: the need for a diagnosis-related group modifier. J Burn Care Rehabil. 1995;16:451–454. doi: 10.1097/00004630-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 83.Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136:25–36. [PubMed] [Google Scholar]
- 84.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83:1493–1501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duchateau J. Immunosenescence and the lung. Rev Mal Respir. 2003;20:735–741. [PubMed] [Google Scholar]
- 86.Linn BS. Age differences in the severity and outcome of burns. J Am Geriatr Soc. 1980;28:118–123. doi: 10.1111/j.1532-5415.1980.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 87.Slater H, Gaisford JC. Burns in older patients. J Am Geriatr Soc. 1981;29:74–76. doi: 10.1111/j.1532-5415.1981.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 88.Lionelli GT, Pickus EJ, Beckum OK, Decoursey RL, Korentager RA. A three decade analysis of factors affecting burn mortality in the elderly. Burns. 2005;31:958–963. doi: 10.1016/j.burns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Hirshberg A, Hoyt DB, Mattox KL. From “leaky buckets” to vascular injuries: understanding models of uncontrolled hemorrhage. J Am Coll Surg. 2007;204:665–672. doi: 10.1016/j.jamcollsurg.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Hauser CJ. Preclinical models of traumatic, hemorrhagic shock. Shock. 2005;24 suppl 1:24–32. doi: 10.1097/01.shk.0000191387.18818.43. [DOI] [PubMed] [Google Scholar]
- 91.Sharma OP, Oswanski MF, Sharma V, Stringfellow K, Raj SS. An appraisal of trauma in the elderly. Am Surg. 2007;73:354–358. [PubMed] [Google Scholar]
- 92.George RL, McGwin G, Jr, Metzger J, Chaudry IH, Rue LW., 3rd The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 93.Harbrecht BG, Peitzman AB, Rivera L, Heil B, Croce M, Morris JA, Jr, Enderson BL, Kurek S, Pasquale M, Frykberg ER, et al. Contribution of age and gender to outcome of blunt splenic injury in adults: multicenter study of the eastern association for the surgery of traum? J Trauma. 2001;51:887–895. doi: 10.1097/00005373-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, Spain DA. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 95.George RL, McGwin G, Jr, Windham ST, Melton SM, Metzger J, Chaudry IH, Rue LW., 3rd Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Colon-Emeric CS, Pieper CF, Artz MB. Can historical and functional risk factors be used to predict fractures in community-dwelling older adults? development and validation of a clinical tool. Osteoporos Int. 2002;13:955–961. doi: 10.1007/s001980200133. [DOI] [PubMed] [Google Scholar]
- 97.National Safety Council: Help seniors live better, longer: action urged to prevent brain injury. [Accessed March 10, 2008]; Available at: www.nsc.org.
- 98.Lomas-Neira JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock. 2005;24 suppl 1:33–39. doi: 10.1097/01.shk.0000191411.48719.ab. [DOI] [PubMed] [Google Scholar]
- 99.Toda Y, Takahashi T, Maeshima K, Shimizu H, Inoue K, Morimatsu H, Omori E, Takeuchi M, Akagi R, Morita K. A neutrophil elastase inhibitor, sivelestat, ameliorates lung injury after hemorrhagic shock in rats. Int J Mol Med. 2007;19:237–243. [PubMed] [Google Scholar]
- 100.Yamashita M, Taniyama M, Tamai M. Cellular localization of tumor necrosis factor–alpha mRNA and interleukin-6 mRNA in the rat liver after hemorrhagic shock. Surg Today. 2002;32:701–706. doi: 10.1007/s005950200130. [DOI] [PubMed] [Google Scholar]
- 101.Hierholzer C, Kelly E, Tsukada K, Loeffert E, Watkins S, Billiar TR, Tweardy DJ. Hemorrhagic shock induces G-CSF expression in bronchial epithelium. Am J Physiol. 1997;273:L1058–L1064. doi: 10.1152/ajplung.1997.273.5.L1058. [DOI] [PubMed] [Google Scholar]
- 102.Hierholzer C, Kelly E, Billiar TR, Tweardy DJ. Granulocyte colony-stimulating factor (G-CSF) production in hemorrhagic shock requires both the ischemic and resuscitation phase. Arch Orthop Trauma Surg. 1997;116:173–176. doi: 10.1007/BF00426067. [DOI] [PubMed] [Google Scholar]
- 103.Vallejo JG, Nemoto S, Ishiyama M, Yu B, Knuefermann P, Diwan A, Baker JS, Defreitas G, Tweardy DJ, Mann DL. Functional significance of inflammatory mediators in a murine model of resuscitated hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2005;288:H1272–H1277. doi: 10.1152/ajpheart.01003.2003. [DOI] [PubMed] [Google Scholar]
- 104.Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 105.Schneider CP, Schwacha MG, Chaudry IH. Influence of gender and age on T-cell responses in a murine model of trauma-hemorrhage: differences between circulating and tissue-fixed cells. J Appl Physiol. 2006;100:826–833. doi: 10.1152/japplphysiol.00898.2005. [DOI] [PubMed] [Google Scholar]
- 106.Kahlke V, Angele MK, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Reversal of sexual dimorphism in splenic T lymphocyte responses after trauma-hemorrhage with aging. Am J Physiol Cell Physiol. 2000;278:C509–C516. doi: 10.1152/ajpcell.2000.278.3.C509. [DOI] [PubMed] [Google Scholar]
- 107.Schneider CP, Schwacha MG, Chaudry IH. Impact of sex and age on bone marrow immune responses in a murine model of trauma-hemorrhage. J Appl Physiol. 2007;102:113–121. doi: 10.1152/japplphysiol.00848.2006. [DOI] [PubMed] [Google Scholar]
- 108.Lim HB, Smith M. Systemic complications after head injury: a clinical review. Anaesthesia. 2007;62:474–482. doi: 10.1111/j.1365-2044.2007.04998.x. [DOI] [PubMed] [Google Scholar]
- 109.Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC, Wang JY. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81:288–298. doi: 10.1016/j.lfs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 110.Nimmo AJ, Cernak I, Heath DL, Hu X, Bennett CJ, Vink R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004;38:40–47. doi: 10.1016/j.npep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 111.Hutchinson PJ, O’Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 112.Kossmann T, Hans VH, Imhof HG, Stocker R, Grob P, Trentz O, Morganti-Kossmann C. Intrathecal and serum interleukin-6 and the acute-phase response in patients with severe traumatic brain injuries. Shock. 1995;4:311–317. doi: 10.1097/00024382-199511000-00001. [DOI] [PubMed] [Google Scholar]
- 113.Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 114.Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mosenthal AC, Lavery RF, Addis M, Kaul S, Ross S, Marburger R, Deitch EA, Livingston DH. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma. 2002;52:907–911. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 116.Fletcher AE, Khalid S, Mallonee S. The epidemiology of severe traumatic brain injury among persons 65 years of age and older in Oklahoma, 1992–2003. Brain Inj. 2007;21:691–699. doi: 10.1080/02699050701426873. [DOI] [PubMed] [Google Scholar]
- 117.Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54:1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rathlev NK, Medzon R, Lowery D, Pollack C, Bracken M, Barest G, Wolfson AB, Hoffman JR, Mower WR. Intracranial pathology in elders with blunt head trauma. Acad Emerg Med. 2006;13:302–307. doi: 10.1197/j.aem.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 119.Testa JA, Malec JF, Moessner AM, Brown AW. Outcome after traumatic brain injury: effects of aging on recovery. Arch Phys Med Rehabil. 2005;86:1815–1823. doi: 10.1016/j.apmr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Hamm RJ, Jenkins LW, Lyeth BG, White-Gbadebo DM, Hayes RL. The effect of age on outcome following traumatic brain injury in rats. J Neurosurg. 1991;75:916–921. doi: 10.3171/jns.1991.75.6.0916. [DOI] [PubMed] [Google Scholar]
- 121.Maughan PH, Scholten KJ, Schmidt RH. Recovery of water maze performance in aged versus young rats after brain injury with the impact acceleration model. J Neurotrauma. 2000;17:1141–1153. doi: 10.1089/neu.2000.17.1141. [DOI] [PubMed] [Google Scholar]
- 122.Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 123.Shimamura M, Garcia JM, Prough DS, Hellmich HL. Laser capture microdissection and analysis of amplified antisense RNA from distinct cell populations of the young and aged rat brain: effect of traumatic brain injury on hippocampal gene expression. Brain Res Mol Brain Res. 2004;122:47–61. doi: 10.1016/j.molbrainres.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 124.Shah SA, Prough DS, Garcia JM, DeWitt DS, Hellmich HL. Molecular correlates of age-specific responses to traumatic brain injury in mice. Exp Gerontol. 2006;41:1201–1205. doi: 10.1016/j.exger.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Moor E, Shohami E, Kanevsky E, Grigoriadis N, Symeonidou C, Kohen R. Impairment of the ability of the injured aged brain in elevating urate and ascorbate. Exp Gerontol. 2006;41:303–311. doi: 10.1016/j.exger.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 126.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 127.Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, van der Poll T. Pre-clinical models of shock and sepsis: what can they tell us? Shock. 2005;24 suppl 1:1–6. doi: 10.1097/01.shk.0000191383.34066.4b. [DOI] [PubMed] [Google Scholar]
- 128.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24 suppl 1:7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 129.Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, Heidecke CD, Pfeffer K. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66:2300–2309. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24 suppl 1:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 131.Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273–287. doi: 10.1385/IR:24:3:273. [DOI] [PubMed] [Google Scholar]
- 133.Hyde SR, Stith RD, McCallum RE. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immun. 1990;58:619–624. doi: 10.1128/iai.58.3.619-624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25:581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 136.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 137.Hyde SR, McCallum RE. Lipopolysaccharide–tumor necrosis factor–glucocorticoid interactions during cecal ligation and puncture-induced sepsis in mature versus senescent mice. Infect Immun. 1992;60:976–982. doi: 10.1128/iai.60.3.976-982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 139.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP. New insights into the biology of the acute phase response. J Clin Immunol. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- 140.Samols D, Agrawal A, Kushner I. Acute phase proteins. In: Oppenheim JJ, Feldman M, editors. Cytokine Reference On-Line. London, UK: Academic Press; 2002. [Google Scholar]
- 141.Post DJ, Carter KC, Papaconstantinou J. The effect of aging on constitutive mRNA levels and lipopolysaccharide inducibility of acute phase genes. Ann N Y Acad Sci. 1991;621:66–77. [PubMed] [Google Scholar]
- 142.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 143.Kizaki T, Ookawara T, Iwabuchi K, Onoe K, Day NK, Good RA, Maruyama N, Haga S, Matsuura N, Ohira Y, et al. Age-associated increase of basal corticosterone levels decreases ED2high, NF-kappaBhigh activated macrophages. J Leukoc Biol. 2000;68:21–30. [PubMed] [Google Scholar]
- 144.Turnage RH, Nwariaku F, Murphy J, Schulman C, Wright K, Yin H. Mechanisms of pulmonary microvascular dysfunction during severe burn injury. World J Surg. 2002;26:848–853. doi: 10.1007/s00268-002-4063-3. [DOI] [PubMed] [Google Scholar]
- 145.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 146.Saito H, Papaconstantinou J. Age-associated differences in cardiovascular inflammatory gene induction during endotoxic stress. J Biol Chem. 2001;276:29307–29312. doi: 10.1074/jbc.M103740200. [DOI] [PubMed] [Google Scholar]
- 147.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- 149.Hill AG, Hill GL. Metabolic response to severe injury. Br J Surg. 1998;85:884–890. doi: 10.1046/j.1365-2168.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 150.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 151.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell–deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 152.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 153.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 154.Turnbull IR, Buchman TG, Javadi P, Woolsey CA, Hotchkiss RS, Karl IE, Coopersmith CM. Age disproportionately increases sepsis-induced apoptosis in the spleen and gut epithelium. Shock. 2004;22:364–368. doi: 10.1097/01.shk.0000142552.77473.7d. [DOI] [PubMed] [Google Scholar]
- 155.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)–like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- 156.Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- 157.Tsuji H, Harada A, Mukaida N, Nakanuma Y, Bluethmann H, Kaneko S, Yamakawa K, Nakamura SI, Kobayashi KI, Matsushima K. Tumor necrosis factor receptor p55 is essential for intrahepatic granuloma formation and hepatocellular apoptosis in a murine model of bacterium-induced fulminant hepatitis. Infect Immun. 1997;65:1892–1898. doi: 10.1128/iai.65.5.1892-1898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 159.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 160.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]