Abstract
Activation of p38γ modulates the integrity of the complex formed by the human discs large protein (hDlg) with cytoskeletal proteins, which is important for cell adaptation to changes in environmental osmolarity. Here we report that, in response to hyperosmotic stress, p38γ also regulates formation of complexes between hDlg and the nuclear protein polypyrimidine tract-binding protein-associated-splicing factor (PSF). Following osmotic shock, p38γ in the cell nucleus increases its association with nuclear hDlg, thereby causing dissociation of hDlg-PSF complexes. Moreover, hDlg and PSF bind different RNAs; in response to osmotic shock, p38γ causes hDlg-PSF and hDlg-RNA dissociation independently of its kinase activity. These findings identify a novel nuclear complex and suggest a previously unreported function of p38γ, which is independent of its catalytic activity and could affect mRNA processing and/or gene transcription to aid cell adaptation to osmolarity changes in the environment.
Keywords: PSF, hDlg, Inactive p38γ, Knock-in mice, Osmotic shock, p38γ
Introduction
Increased osmolarity in the cell environment activates signalling pathways necessary for cell adaptation to prolonged hyperosmotic exposure. The p38 mitogen-activated protein kinases (MAPKs) are crucial for both early response and long-term cell adaptation to osmotic stress (Burg et al., 2007; Cuenda and Rousseau, 2007). Although all four p38 MAPK subfamily members (p38α, p38β, p38γ and p38δ) are activated in response to hyperosmotic shock (Cuenda and Rousseau, 2007), activation of the p38γ (MAPK12) isoform is particularly rapid and strong (Sabio et al., 2005; Sabio et al., 2004). Sudden exposure of cells to high osmolar shock induces rapid shrinkage because of water efflux from the cell. To maintain cell integrity and homeostasis in these conditions, adaptive responses are needed to restore cell volume and reinforce the cytoskeletal architecture. We recently proposed a regulatory pathway for cell adaptation to a hyperosmolar environment that involves p38γ and its substrate hDlg, which modulates composition of cytoskeletal hDlg by phosphorylating one of its components (Sabio et al., 2005).
hDlg (also known as DLG1 and dlgh1) is the human orthologue of the Drosophila tumour suppressor Dlg. It belongs to the membrane-associated guanylate kinase (MAGUK) scaffold-protein family, whose members have similar structural organization; they are composed of a basic core of a variable number of PDZ domains, an SH3 domain and a catalytically inactive guanylate-kinase-like region (Funke et al., 2005). hDlg is a cytosolic protein that is recruited beneath the plasma membrane following cell contact. It acts as a scaffold, anchor and adaptor protein, allowing assembly of multiprotein complexes and their connection to downstream signalling molecules and/or to cytoskeleton-associated molecules (Funke et al., 2005). The hDlg PDZ domains bind to the C-terminal peptide motif S/T-x-V/L in a number of proteins, including ion channels, receptors, cell adhesion molecules, cytoskeletal components, and several oncoproteins (Funke et al., 2005). hDlg is expressed in most tissues; its biological functions are linked to establishing and maintaining cell polarity and adhesion integrity in epithelial cells and to the organization of neuronal and immunological synapses (Funke et al., 2005; Humbert et al., 2003; Krummel and Macara, 2006).
The regulation of hDlg in the assembly of multi-component protein complexes has yet to be elucidated. In recent years, phosphorylation has emerged as a mechanism that regulates the function of hDlg scaffold proteins (Laprise et al., 2004; Mauceri et al., 2007; Sabio et al., 2005). In response to cell stress, hDlg is hyperphosphorylated by p38γ, which is unique among the MAPK in possessing a C-terminal sequence that docks directly to PDZ domains of different proteins; moreover, p38γ phosphorylation of PDZ-domain-containing proteins is dependent on this interaction (Hasegawa et al., 1999; Sabio et al., 2005; Sabio et al., 2004). hDlg is targeted to the cytoskeleton by its association with guanylate-kinase-associated protein (GKAP), and p38γ-catalysed phosphorylation of hDlg triggers its dissociation from GKAP, releasing it from the cytoskeleton (Sabio et al., 2005).
To better understand the role of hDlg phosphorylation by p38γ when cells are exposed to hyperosmotic stress, we examined the effect of p38γ phosphorylation of hDlg on its ability to form complexes with distinct proteins. We found that hDlg binds to the polypyrimidine tract-binding (PTB) protein-associated-splicing factor (PSF) and to a related protein, the RNA- and DNA-binding protein p54nrb (NonO). PSF and p54nrb associate in vivo, and belong to the same family of nucleic-acid-binding proteins (Shav-Tal and Zipori, 2002). PSF is a nuclear protein that localizes in or proximal to nuclear ‘paraspeckle’ structures (Fox et al., 2002). It is an RNA-binding protein that was initially termed splicing factor, although it was recently shown to regulate several cellular processes including transcription, pre-mRNA processing, nuclear retention of defective RNA, as well as DNA unwinding and repair (Shav-Tal and Zipori, 2002; Zolotukhin et al., 2003). We report here that hDlg binds to various RNAs, probably through PSF, and that cell exposure to hyperosmotic shock causes dissociation of the hDlg-PSF complex and dissociation of this complex from RNA. We also show that under osmotic stress, p38γ modulates dissociation of hDlg from RNA and PSF in the nucleus, independently of its kinase activity.
Results
hDlg interacts with PSF in a osmotic-stress-dependent manner
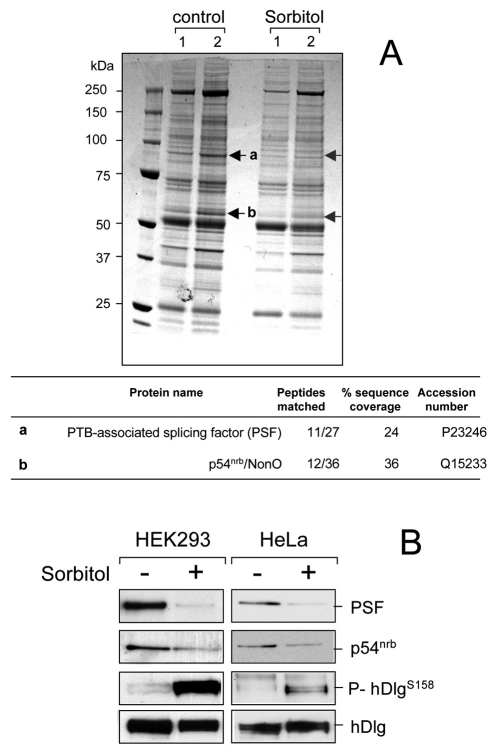
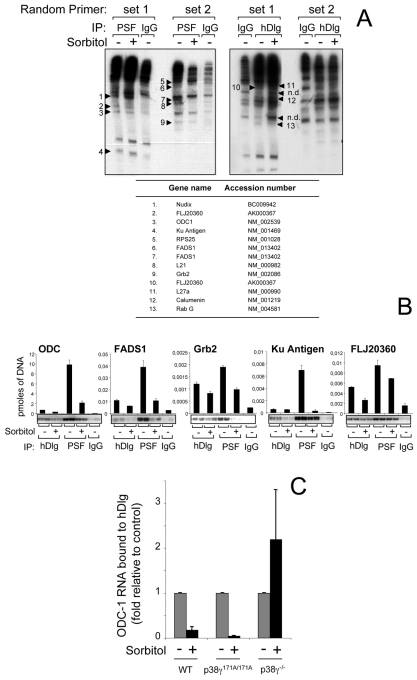
To study whether phosphorylation of hDlg by p38γ affects its binding to proteins, we used immunoprecipitation with anti-hDlg antibody to isolate endogenous hDlg protein complexes from unstimulated HEK293 cells or cells treated with sorbitol (osmotic shock) (Fig. 1A). Several bands were found in all pellets, but pellets from unstimulated cells showed a ~90 kDa band that was absent in pellets from stimulated cells. We also found a ~54 kDa band whose intensity decreased notably in pellets from stimulated compared with unstimulated cells (Fig. 1A). Tryptic peptide-mass fingerprinting identified these bands as PSF and one of its binding partners, p54nrb. PSF co-localized with hDlg in the nucleus, indicating their possible interaction (supplementary material Fig. S1). Immunoblot analysis confirmed the specific association of PSF and of p54nrb with endogenous hDlg in HEK293 and HeLa cells (Fig. 1B). PSF was associated with endogenous hDlg from unstimulated cells, but not from cells exposed to osmotic shock, the condition in which hDlg is phosphorylated at Ser158 by p38γ (Fig. 1B; supplementary material Fig. S1) (Sabio et al., 2005), suggesting that hDlg phosphorylation might affect its binding to PSF and to p54nrb. Since we observed more marked dissociation of hDlg-PSF than of the hDlg-p54nrb complex after osmotic shock, we analysed the PSF interaction in more detail.
Fig. 1.
Cell stress regulates association of hDlg with the splicing factors PSF and p54nrb. (A) Endogenous hDlg was immunoprecipitated, using 2 μg anti-hDlg antibody per sample, from 5 mg (lanes 1) or 10 mg (2) protein lysates from HEK293 cells, unstimulated or exposed to 0.5 M sorbitol (15 minutes). Protein bands a and b were excised from the gel, digested in-gel with trypsin, and their identity determined by mass fingerprinting. The number of peptides, percentage of sequence coverage and the accession number for each protein are given in the table. (B) Endogenous hDlg was immunoprecipitated, using 2 μg anti-hDlg antibody per sample, from 0.2 mg lysates from HEK293 or HeLa cells, unstimulated or stimulated as in A. Pellets were immunoblotted with anti-PSF or anti-p54nrb, with an antibody against hDlg phosphorylated at Ser158 (P-hDlgS158) and an antibody that recognises both unphosphorylated and phosphorylated hDlg.
p38γ phosphorylation of hDlg does not regulate its association with PSF
To determine whether hDlg phosphorylation modulates the interaction with PSF, we first examined the hDlg-PSF association in mouse embryonic fibroblasts (MEFs) from wild-type (WT) mice or mice lacking p38γ and p38δ (p38γ/δ−/− MEFs). We previously showed that p38γ phosphorylates hDlg in osmotically stressed WT cells; nonetheless, hDlg is also phosphorylated in p38γ−/− cells owing to the compensatory activity of other p38 MAPKs, p38δ and p38α (Sabio et al., 2005). We therefore used p38γ/δ−/− MEFs in combination with the p38α/β inhibitor SB203580, a condition in which hDlg is not phosphorylated after osmotic stress (Sabio et al., 2005). PSF interacted with hDlg in untreated WT or p38γ/δ−/− MEFs, but when cells were exposed to osmotic shock, PSF dissociated from hDlg in WT but not in p38γ/δ−/− cells (Fig. 2A). Pre-incubation with SB203580 did not affect association or dissociation of the hDlg-PSF complex (Fig. 2A).
Fig. 2.
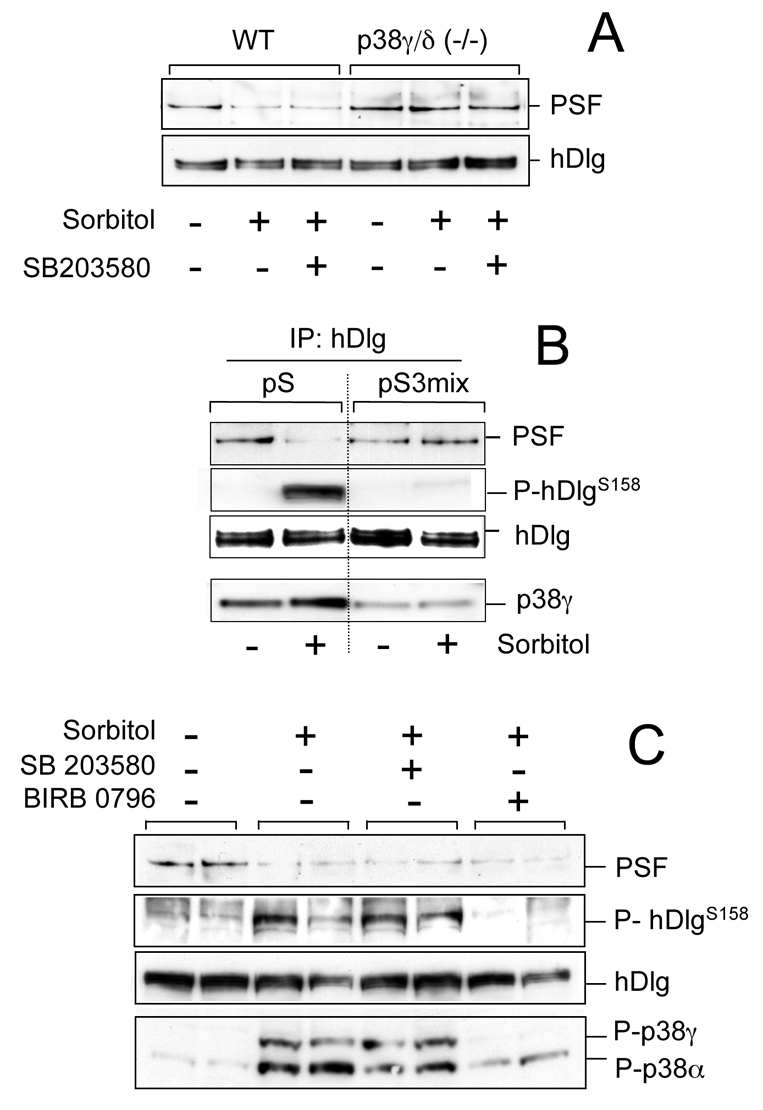
p38γ phosphorylation of hDlg does not regulate its interaction with PSF. (A) WT or p38γ/δ−/− MEFs were incubated (1 hour) alone or with 10 μM SB203580, then exposed to 0.5 M sorbitol (15 minutes). Endogenous hDlg was immunoprecipitated with anti-hDlg antibody and pellets immunoblotted with anti-PSF or anti-hDlg antibody. (B) HEK293 cells were transfected with empty pSuper vector (pS) or with a mixture of siRNA constructs to knock down p38γ (pS3mix). Cells were treated as in A, endogenous hDlg was immunoprecipitated from 0.5 mg cell lysates and pellets were immunoblotted with anti-PSF, anti-P-hDlgS158 or anti-hDlg antibody. Total lysates were immunoblotted with an antibody against total p38γ. (C) HEK293 cells were incubated alone or with 10 μM SB203580 or 1 μM BIRB 0796 (1 hour), then exposed to 0.5 M sorbitol (15 minutes). Endogenous hDlg was immunoprecipitated with anti-hDlg antibody and pellets were immunoblotted as in B. Total lysates were immunoblotted with an antibody that recognises phosphorylated p38γ and p38α.
To verify the role of p38γ in modulating the hDlg-PSF interaction, we used a specific small-interfering RNA (siRNA) approach to knock down p38γ expression in HEK293 cells. A mixture of three pSUPER constructs (pS3mix) decreased p38γ expression by >80% without affecting levels or activation of other MAPK (supplementary material Fig. S2). We then transfected cells with control siRNA, the empty pSUPER (pS) vector, or with pS3mix and examined phosphorylation of endogenous hDlg and its association with PSF after hyperosmotic stress. siRNA reduced hDlg phosphorylation by >90% in lysate from sorbitol-treated cells (Fig. 2B; supplementary material Fig. S2) and blocked PSF dissociation from hDlg in cells exposed to osmotic shock (Fig. 2B).
To explore the possibility that p38γ phosphorylation regulates dissociation of hDlg-PSF, we examined this interaction in cells treated with SB203580, which inhibits p38α/β, and BIRB0796, which at high concentrations blocks p38γ activation and/or activity and hDlg phosphorylation (Fig. 2C) (Kuma et al., 2005). Both inhibitors failed to impede hDlg-PSF dissociation in response to hyperosmotic stress, although BIRB0796 blocked hDlg phosphorylation and p38γ activation (Fig. 2C). In contrast to results from experiments with cells lacking p38γ, the results using kinase inhibitors indicate that p38γ phosphorylation of hDlg does not modulate hDlg-PSF interaction. Our data suggest that p38γ regulates PSF association with hDlg independently of its kinase activity.
p38γ regulates association of PSF with hDlg
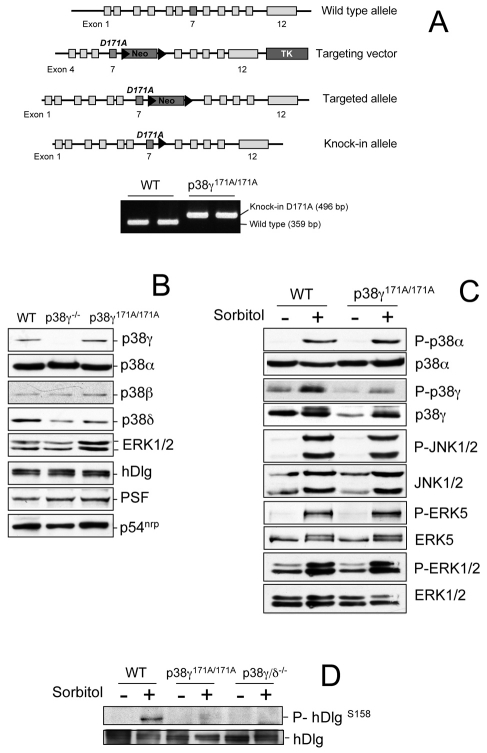
In light of these results, and to show that the hDlg-PSF complex is regulated by p38γ independently of its kinase activity, we used cells derived from knock-in mice expressing catalytically inactive p38γ. Since the p38MAPKs are a clear example of functional redundancy owing to the large number of related family members, and this redundancy could account for the failure to detect an apparent phenotype in p38γ−/− mice, we generated a catalytically inactive p38γ (D171A) knock-in mouse (p38γ171A/171A). The targeting construct was designed to replace WT exon 7 of the p38γ gene, which encodes Asp171, with a mutant exon 7 form that encodes alanine at this position (Fig. 3A). PCR analysis (Fig. 3A) confirmed replacement of the WT with the mutant exon. Western blot using p38γ antibodies indicated similar p38γ levels in p38γ171A/171A and WT MEFs (Fig. 3B). We confirmed that expression of all p38 isoforms, ERK1/2, JNK1/2, ERK5, hDlg, PSF and p54nrp levels were similar in p38γ171A/171A and in WT MEFs (Fig. 3B,C). Sorbitol treatment activated p38α, ERK1/2, JNK1/2 and ERK5 pathways in these cells (Fig. 3C). Osmotic shock caused hDlg phosphorylation in WT, but not in p38γ171A/171A MEFs or in negative-control p38γ/δ−/− cells (Fig. 3D), indicating that p38γ alone is responsible for hDlg phosphorylation in these conditions, and that the p38γ expressed in p38γ171A/171A cells is inactive.
Fig. 3.
Generation and characterization of the inactive p38γ knock-in mouse. (A) Diagram showing the endogenous p38γ gene, the targeting construct vector generated, the targeted allele with the neomycin selection cassette still present (Neo), and the targeted allele with the neomycin cassette removed by Cre recombinase. The grey boxes represent exons and the black triangles, the LoxP sites. The knock-in allele with the D171A mutation in exon 7 is marked (dark grey). Genomic DNA purified from tail biopsy sample was used as a template for PCR, resolved on a 1% agarose gel and examined by ethidium bromide staining. (B) Lysates from WT, p38γ−/− or p38γ171A/171A MEFs (30-50 μg of protein) were immunoblotted with specific antibodies for each protein indicated. (C) WT or p38γ171A/171A MEFs were exposed to 0.5 M sorbitol (15 minutes) and activation of the indicated MAPK was examined using phospho-specific antibodies. To analyse activation of p38γ, this isoform was immunoprecipitated with anti-p38γ antibody from 2 mg cell lysates and immunoblotted with the p38 phospho-specific antibody. (D) WT, p38γ171A/171A or p38γ/δ−/− MEFs were treated as in C and endogenous hDlg immunoprecipitated with anti-hDlg antibody from 0.5 mg protein lysate. Pellets were immunoblotted with anti-P-hDlgS158 or anti-hDlg.
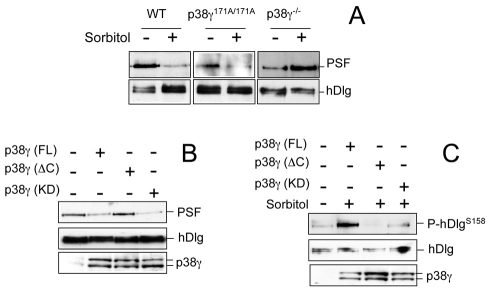
Examination of the hDlg-PSF interaction in cells with catalytically inactive or no p38γ, showed that PSF interacted with hDlg in untreated WT, p38γ171A/171A or p38γ−/− MEFs; following osmotic shock, PSF dissociated from hDlg in WT and p38γ171A/171A but not in p38γ−/− cells (Fig. 4A), indicating that p38 binding to hDlg, and not its phosphorylation, regulates hDlg-PSF dissociation.
Fig. 4.
p38γ regulates hDlg-PSF association independently of its catalytic activity. (A) WT, p38γ171A/171A or p38γ/δ−/− MEFs were treated as in Fig. 3C and endogenous hDlg immunoprecipitated using anti-hDlg antibody from 0.2 mg of protein lysate. Pellets were immunoblotted with anti-PSF or anti-hDlg antibody. Similar results were obtained in two independent experiments. (B) HEK293 cells were transfected with empty GFP-vector, GFP-p38γ(FL), GFP-p38γ(ΔC) or GFP-p38γ(KD). Endogenous hDlg was immunoprecipitated as in A, and pellets were immunoblotted with anti-PSF or anti-hDlg. (C) Cells were transfected as in B and exposed to 0.5 M sorbitol (15 minutes). Endogenous hDlg was immunoprecipitated, and pellets were immunoblotted with anti-P-hDlgS158 or anti-hDlg. Similar results were obtained in at least two independent experiments. Levels of p38γ expression in B and C were checked by immunoblotting 20 μg cell extract with anti-p38γ antibody.
We then further examined whether hDlg-PSF dissociation after osmotic stress was affected by p38γ binding to hDlg. p38γ binds to hDlg-PDZ domains 1 and 3 through its C-terminal (ETXL) motif (Sabio et al., 2005). We analysed PSF binding to hDlg in HEK293 cells expressing p38γ(FL) (full length, which binds and phosphorylates hDlg), p38γ(ΔC) (which lacks the last four amino acids and neither binds nor phosphorylates hDlg), or p38γ(KD) [kinase dead, residue D171 replaced by Ala, which binds but does not phosphorylate hDlg (Sabio et al., 2005)]. PSF co-immunoprecipitated with hDlg in untransfected control cells or in cells expressing p38γ(ΔC), but not in p38γ(FL)- or p38γ(KD)-expressing cells (Fig. 4B). Osmotic shock induced hDlg phosphorylation only in p38γ(FL)-expressing cells (Fig. 4C). All these results confirm that PSF dissociation from hDlg is regulated by p38γ, is independent of hDlg phosphorylation and might depend on hDlg association with p38γ.
Osmotic stress induces p38γ accumulation in the nucleus
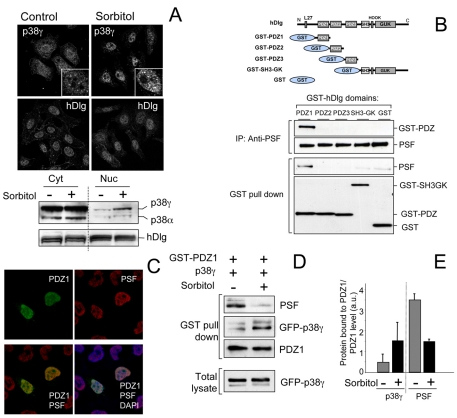
Immunolocalization studies showed that p38γ and hDlg had the same diffuse cytoplasmic and nuclear localization in resting cells (Fig. 5A). Following hyperosmotic shock, hDlg distribution between cytoplasm and nucleus was unaffected, although the localization of hDlg at the cell membrane was partially lost (Fig. 5A), whereas p38γ accumulated in the nucleus showing some co-localization with PSF in paraspeckles (Fig. 5A; supplementary material Fig. S3). In addition, we have found that inactive p38γ171A mutant also accumulated in the nucleus in response to sorbitol treatment (supplementary material Fig. S4). We thus considered that p38γ translocation and nuclear accumulation after sorbitol stimulation might cause hDlg-PSF complex dissociation and hDlg-p38γ complex formation. To test this idea, we used cells expressing hDlg PDZ domain 1 (PDZ1), because (1) p38γ binds to this domain (Sabio et al., 2005), (2) experiments to define the hDlg region necessary for binding to PSF showed that PSF complex interacted only with hDlg PDZ1 in situ (Fig. 5B), and (3) in cells expressing PDZ1, it was found only in the nucleus where it co-localizes with PSF (Fig. 5C; supplementary material Fig. S5). As predicted, PSF dissociated from PDZ1 after sorbitol stimulation, whereas p38γ binding to PDZ1 increased significantly (Fig. 5D,E). These results suggest that nuclear accumulation of p38γ after sorbitol treatment regulates PSF complex association with hDlg in the nucleus by competing for the same binding domain.
Fig. 5.
p38γ accumulates in the nucleus after osmotic stress and regulates hDlg-PSF interaction. (A) HeLa cells, unstimulated or treated with 0.5 M sorbitol (15 minutes), were stained with anti-p38γ or anti-hDlg antibodies and analysed by fluorescence microscopy. Inserts in the top panels show a higher magnification of p38γ localization in cell nuclei. Cells were fractionated and 30 μg protein from cytoplasm (Cyt) and nuclear (Nuc) fractions were immunoblotted using anti-p38γ, anti-p38α and anti-hDlg antibodies. (B) Different hDlg domains were expressed in HEK293 cells (top). After transfection, cells were lysed and GST-proteins purified by pull-down. Endogenous PSF was immunoprecipitated using anti-PSF antibody. Pellets were immunoblotted using anti-PSF or anti-GST antibody. (C) HEK293 cells were transfected with GST-PDZ1, stained with anti-GST and/or anti-PSF antibody, and analysed by fluorescence microscopy. Nuclei are stained with DAPI. Results were similar in four independent experiments in HEK293 cells and in two independent experiments in HeLa cells. (D) HEK293 cells expressing GST-PDZ1 and GFP-p38γ were treated with sorbitol as in A. GST-PDZ1 was purified by pull-down immunoprecipitation and pellets were immunoblotted using anti-PSF, anti-GST or anti-GFP antibodies. Total lysates were immunoblotted with anti-GFP antibody to examine p38γ expression. (E) Band intensity of blots in D (anti-PSF, anti-GST for PDZ1 and anti-GFP for p38γ) were quantified and the relative amount of p38γ or PSF in pellets was calculated. Histogram values are means ± s.e.m. of four independent experiments.
Identification of mRNA bound to hDlg and/or PSF
As PSF is an RNA-binding protein, we sought to identify which physiological RNA targets of PSF were also hDlg (or hDlg-PSF) complex targets, and to examine whether RNAs binding to hDlg-PSF complex is regulated by PSF association-dissociation with hDlg. We immunoprecipitated each protein separately from HEK293 extracts. The RNA species in the immunoprecipitates were purified and amplified by a differential display-based method and resolved on a denaturing polyacrylamide gel (Fig. 6A). We excised bands observed only when anti-hDlg or anti-PSF was used, but not IgG alone, and re-amplified and cloned the DNA. We detected eight distinct RNA species in the PSF immunoprecipitate and four different RNA species in the hDlg immunoprecipitate (Fig. 6A).
Fig. 6.
Osmotic stress decreases RNA binding to hDlg and PSF. (A) Endogenous PSF or hDlg were immunoprecipitated, with anti-PSF or anti-hDlg antibody, from HEK293 cells, alone or treated with 0.5 M sorbitol (15 minutes); IgG immunoprecipitation was used as negative control. RNA in the pellets was purified by a differential display-based method using set 1 or set 2 of random primers and resolved on a 6% denaturing polyacrylamide gel. Bands of interest were excised, re-amplified and cloned for identification (bands are indicated as 1 to 13; unidentified bands are labelled n.d.); band identity and GenBank accession number are shown (bottom). (B) Extracts from were prepared as in A, hDlg or PSF immunoprecipitated and the levels of bound RNA identified in A were determined using specific primers for each RNA by real-time PCR. The RNAs identified were also amplified by RT-PCR using specific primers and resolved in an agarose gel. (C) WT, p38γ171A/171A or p38γ−/− MEF extracts were prepared as in A, hDlg was immunoprecipitated and the levels of bound ODC1 RNA determined using specific primers by real-time PCR.
To confirm these results, we used a more sensitive quantitative method. Specific primers were generated and real-time PCR performed. Only the RNA encoding ornithine decarboxylase-1 (ODC-1), FADS1, Ku Antigen, Grb2 and FLJ20360 decreased after cell treatment (Fig. 6B). In all cases, PSF bound more RNA molecules than hDlg. This suggested that binding of these RNAs to the hDlg-PSF complex is regulated by PSF association-dissociation with hDlg.
Our results show that the amount of PSF-associated ODC1 RNA exceeded that of the other RNAs by 250- to 5000-fold (Fig. 6B). ODC-1 is the first enzyme in polyamine synthesis and is essential for normal development and tissue repair in mammals. In addition, both ODC1 mRNA expression and its activity might be affected by changes in osmolarity (Hayashi et al., 2002). We therefore studied whether p38γ affected ODC1 RNA binding to hDlg under osmotic stress. Examination of the hDlg-ODC1 RNA interaction in WT, p38γ171A/171A or p38γ−/− MEFs showed that following osmotic shock, ODC1 RNA dissociated from hDlg in WT and p38γ171A/171A, but not in p38γ−/− cells (Fig. 6C). Moreover, the amount of ODC1 RNA that interacted with hDlg increased in p38γ−/− MEFs in response to sorbitol, coinciding with the increase in hDlg-associated PSF in the same conditions (Fig. 4A). These findings indicate that the association of hDlg with RNA, probably through PSF, is regulated by p38γ.
Discussion
To better understand how p38γ cascade signalling regulates the scaffold activity of hDlg, the prototype of the MAGUK family, we used a proteomic approach to characterize hDlg complexes. Mass spectrometry analysis of bound protein showed that in resting cells, hDlg binds to nuclear proteins, to the splicing factor PSF and to the related protein p54nrb, but that the complex dissociates following osmotic stress. Despite the fact that hDlg localises to the nucleus as well as to cytoplasm and the plasma membrane (Inesta-Vaquera et al., 2009; Sabio et al., 2005), to date only the nuclear protein Net1 is reported to interact with and promote hDlg translocation to the nucleus (Garcia-Mata et al., 2007). Although nuclear hDlg is thought to be important for undifferentiated epithelial cell growth (Roberts et al., 2007), its precise role has not been described. Based on its function at the plasma membrane, nuclear hDlg might also have a structural role and associate different protein complexes at the nuclear matrix. Other MAGUK proteins such as the calmodulin-associated serine/threonine kinase (CASK) (Hsueh et al., 2000) and ZO-1 (Balda and Matter, 2000), which act as scaffold proteins at cell junctions and synapses, translocate to the nucleus and contribute to the transcriptional regulation of specific genes.
Osmotic shock triggered dissociation of hDlg-PSF complexes in cells expressing inactive p38γ, but not in cells lacking p38γ, indicating that regulation of the hDlg-PSF complex is independent of p38γ kinase activity. The idea that kinases act by direct phosphorylation of their substrates is changing; for example, following osmotic stress, the yeast p38-related MAPK Hog-1 is recruited to chromatin as an integral component of transcription complexes to regulate gene expression (Alepuz et al., 2001; Proft and Struhl, 2002). Experiments in mammalian cells also suggest a phosphorylation-independent role for p38γ in K-Ras transformation, although the precise mechanism for this regulation remains unknown (Tang et al., 2005). We generated a knock-in mouse expressing inactive p38γ, which will be a useful tool to validate the hypothesis that this kinase might act through its recruitment to cell complexes. Knock-in mice expressing inactive p38MAPK are a powerful approach in the study of the role of both kinase-substrate and kinase-binding protein interaction. Moreover, cells from this mouse will help to overcome the problems inherent in assessing protein interactions using overexpression approaches. Analysis of knockout cells for different p38 isoforms showed clear functional redundancy among p38 family members (Sabio et al., 2005). Although hDlg is phosphorylated by p38δ in p38γ knockout cells, in vitro experiments showed that in the presence of inactive p38γ, hDlg phosphorylation by p38δ is abolished (supplementary material Fig. S6) (Sabio et al., 2005); this indicates that p38γ binding to hDlg impairs its phosphorylation by other kinases.
Full details of the mechanism by which p38γ regulates the hDlg-PSF complex after osmotic shock remain to be elucidated. We found that p38γ accumulates in the nucleus of stimulated cells and that p38γ binding to nuclear hDlg PDZ1 increases, whereas binding of PSF to nuclear hDlg PDZ1 decreases. This leads us to suggest that both proteins compete for the same interaction domain in hDlg and that the increase in nuclear p38γ concentration after sorbitol stimulation displaces PSF from hDlg. We have shown in cells that both p38γ and PSF bind to PDZ1 of hDlg; however, whereas p38γ possesses a sequence in its C-terminal half that can associate with PDZ domains (Sabio et al., 2005) PSF does not. Therefore, we cannot exclude the possibility that hDlg and PSF associate in vivo (in cells) through another unknown protein. Additionally, the intracellular localization of p38MAPK after activation remains unclear; p38α activation by DNA double-strand breaks causes nuclear translocation of this isoform (Wood et al., 2009). Here, we report that p38γ accumulates in the nucleus after osmotic stimulation of cells, but not following other p38γ-activating stimuli such as UV irradiation, which also caused a significantly less dissociation of the hDlg-PSF complex (supplementary material Fig. S7). This indicates that the nature of the stimulus might determine p38γ distribution, and that sorbitol signals could release p38γ from docking molecules that retain it in the cytosol. This nuclear accumulation might be a response mechanism to sorbitol that facilitates phosphorylation of p38γ targets. We found that hDlg is phosphorylated in the nuclear fraction of sorbitol-stimulated cells (supplementary material Fig. S1). A nuclear role for p38γ, including functional interaction with hDlg, would not exclude its distinct cytoplasmic role in modulating the hDlg-cytoskeleton complex. Indeed, through its ability to shuttle between cytoplasm and nucleus, p38γ/hDlg might provide an important connection between two processes that are crucial for adaptation to osmotic stress: gene expression and cytoskeletal reorganization. This study thus indicates that, through interactions with PSF-hDlg and potentially to other transcription factors, p38γ might be involved in the transcriptional regulation of adaptation programs.
PSF is an RNA-binding protein with two consensus RNA-binding domains; however, only a few RNAs are reported to bind PSF (Buxade et al., 2008; Cobbold et al., 2008; Figueroa et al., 2009; Zolotukhin et al., 2003). In addition, we also show that hDlg binds different RNAs, because hDlg does not possess a consensus RNA-binding domain, it is likely that hDlg-RNA binding occurs through its association with PSF and/or other RNA-binding proteins such as p54nrb. Binding of RNA encoding ODC-1, FADS1, Ku antigen, Grb2 and FLJ20360 to hDlg and PSF decreased after osmotic stress in cells. Moreover, our data show that the hDlg-ODC1 RNA complex is regulated by p38γ, and suggest that in response to osmotic stress p38γ might control RNA binding to hDlg by affecting its association with PSF. It has been suggested that ODC1 mRNA expression might be affected by changes in the osmolarity (Hayashi et al., 2002). We nonetheless have not observed, under any of our experimental conditions, changes in total ODC1 RNA (supplementary material Table S2) or any effect on ODC1 splicing (supplementary material Fig. S8). Although further experiments are needed to address this question, we cannot rule out the possibility that the binding of ODC1 RNA to the hDlg-PSF complex might affect other processes, such as RNA stability or localization, or even the transcription of certain genes.
In summary, we identify a nuclear complex formed by hDlg-PSF-RNA that is regulated by the p38γ kinase (Fig. 7). By associating with hDlg, p38γ controls this complex independently of its catalytic activity. Our findings suggest that p38γ has a role in regulating signalling events involved in mRNA processing and/or gene transcription that would aid cell adaptation to osmolarity changes in the environment.
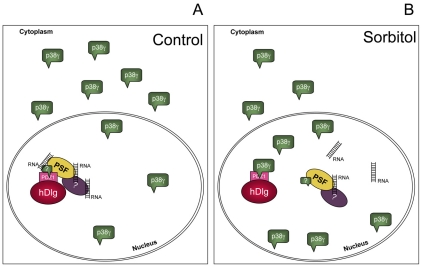
Fig. 7.
Model of regulation of the hDlg-PSF-RNA complex by the p38γ kinase. (A) Under normal physiological conditions, in resting cells, p38γ is localized mainly in the cytoplasm of the cell, whereas the hDlg-PSF-RNA complex is associated in the nucleus of the cell. (B) Changes in the osmolarity of the environment causes the accumulation of p38γ in the nucleus, which increases its binding to hDlg, The nuclear interaction of p38γ with hDlg through its PDZ domain 1 leads to hDlg dissociation from PSF and RNAs in the nucleus.
Materials and Methods
Materials
Protein-G-Sepharose and [γ-32P]ATP were purchased from Amersham Pharmacia Biotech. Protease-inhibitor cocktail tablets and Taq Polymerase were purchased from Roche. Precast polyacrylamide gels, running buffer and transfer buffer were from Invitrogen (Paisley, UK). SB203580 was obtained from Calbiochem (Nottingham, UK) and BIRB0796 was made by custom synthesis (Kuma et al., 2005). Colloidal Coomassie Blue (Invitrogen, Groningen, The Netherlands). The generation of p38γ−/− and p38γ/δ−/− MEFs used in this study has been described previously (Sabio et al., 2005). Other chemicals were of the highest purity available and purchased from Merck (Poole, UK) or Sigma (Poole, UK).
Antibodies
Anti-p38γ and anti-p38δ antibodies for immunoblotting were raised and purified as described elsewhere (Cuenda et al., 1997; Goedert et al., 1997). An antibody that recognises p38γ in immunoflurescence staining was generated by injecting sheep with p38γ N- and C-terminal peptides. The antibody was affinity-purified on a GST-p38γ-antigen-Sepharose column (Sabio et al., 2004). Antibody that recognises the heatshock protein 27 (Hsp27) (phosphorylated at Ser15) was described previously (Knebel et al., 2001). Antibodies that recognise p38α phosphorylated at Thr180 and Tyr182 (these antibodies also recognise phosphorylated p38γ), ERK1/ERK2, phosphorylated ERK1/ERK2 (Thr202/Tyr204), JNK1/JNK2, phospho-JNK1/JNK2 (Thr183/Tyr185) and c-jun were purchased from New England Biolabs (Hitchin, UK) whereas p38α antibody was obtained from Upstate (Dundee, UK). Anti-ERK5 and anti-GST antibodies were obtained from the Division of Signal Transduction Therapy (Dundee, UK) and mouse anti-p38β antibody was purchased from Zymed (San Francisco, CA). Anti-PSF antibody was from Sigma and anti-p54 antibody from BD Biosciences. Phospho-specific antibodies that recognise hDlg phosphorylated at Ser158, Thr209, Ser431 or Ser442 were raised against the peptides VSHSHIpSPIK (residues 152-161), NTDSLEpTPTYVNG (residues 203-215), DNHVpSPSSYLGQTPASPARYSPISK and DNHVSPSSYLGQTPApSPARYSPISK (residues 427-451) of rat hDlg as described elsewhere (Sabio et al., 2005). Anti-hDlg was generated by injecting sheep at Diagnostics Scotland (Pennicuik, UK) with glutathione-S-transferase (GST)-tagged hDlg (Sabio et al., 2005), and used to immunoprecipitate endogenous hDlg and also to detect it in western blots. An antibody that recognises green fluorescence protein (GFP), and secondary antibodies Alexa Fluor 488-conjugated donkey anti mouse and Alexa Fluor 594-conjugated donkey anti-sheep were from Molecular Probes (Leiden, The Netherlands). Rabbit anti-sheep IgG, goat anti-rabbit and rabbit anti-mouse peroxidase-conjugated IgG antibodies and peroxidase-conjugated Protein-G were obtained from Perbio Science UK (Tattenhall, UK).
Cell culture, transfection and lysis
Human embryonic kidney (HEK) 293 cells, HeLa cells and mouse embryonic fibroblasts (MEFs), were cultured as described previously (Sabio et al., 2005; Sabio et al., 2004). Transfection of HEK293 cells was carried out using FuGENE 6 (Roche) following the protocol recommended by the manufacturer (Sabio et al., 2004). Cells were incubated in DMEM for 12 hours in the absence of serum before stimulation with 0.5 M sorbitol, then lysed in buffer A [50 mM Tris-HCl, pH 7.5, 1 mM EGTA, 1 mM EDTA, 0.15 M NaCl, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 50 mM sodium β-glycerophosphate, 5 mM pyrophosphate, 0.27 M sucrose, 0.1 mM phenylmethylsulphonyl fluoride, 1% (v/v) Triton X-100] plus 0.1% (v/v) 2-mercaptoethanol and complete proteinase-inhibitor cocktail (Roche). Lysates were centrifuged at 13,000 g for 15 minutes at 4°C, the supernatants removed, quick frozen in liquid nitrogen and stored at −80°C until used.
Immunoprecipitation from cell lysate
Extracts from MEF, HEK293 or HeLa cells, were incubated with 2 μg anti-hDlg, 1.5 μg anti-PSF or 2 μg anti-p38γ antibody coupled to protein-G-Sepharose. After incubation for 2 hours at 4°C, the captured proteins were centrifuged at 13,000 g, the supernatants discarded and the beads washed twice in buffer A containing 0.5 M NaCl, then twice with buffer A alone.
DNA constructs and proteins
All DNA constructs and proteins used in this study are described elsewhere (Sabio et al., 2005; Sabio et al., 2004).
Isolation of hDlg complexes
Extracts from HEK293 cells (5 or 10 mg protein) were incubated (2 hours, 4°C) with 4 μg hDlg antibody coupled to protein-G-Sepharose. Captured proteins were centrifuged (13,000 g) and the supernatant discarded and pellets were washed four times in buffer A. Samples were denatured and resolved by electrophoresis. To visualise hDlg-interacting proteins, 4-12% polyacrylamide gels were stained with colloidal Coomassie Blue (Invitrogen). IgG from a pre-immune serum coupled to protein-G-Sepharose were used to preclear cell lysate before performing the co-immunoprecipitation. Proteins immunoprecipitated by hDlg were excised from the gel for identification by tryptic-mass fingerprinting. Tryptic peptides were analysed on an Elite STR matrix-assisted laser desorption time of flight (MALDI-TOF) mass spectrophotometer (Perseptive Biosystems Framingham, MA) with saturated α-cyanocinnamic acid as the matrix. The mass spectrum was acquired in the reflector mode and mass calibrated internally. Tryptic peptide ions were scanned against the Swiss-Prot and NCBI databases using the MS-FIT program of Protein Prospector siRNA construction. To generate siRNA to knock down p38γ MAPK, we used the pSUPER (pS) technology (Brummelkamp et al., 2002). pSUPER vectors were generated by cloning in the oligonucleotides: (5′-GATCCCCGTTCCTCGTGTACCAGATGTTCAAGAGACATCTGGTACACGAGGAACTTTTTGGAAA-3′); (5′-GATCCCCGAGCGATGAGGCCAAGAACTTCAAGAGAGTTCTTGGCCTCATCGCTCTTTTTGGAAA-3′) and (5′-GATCCCCCCGCACACTGGATGAATGGTTCAAGAGACCATTCATCCAGTGTGCGGTTTTTGGAAA-3′). They were directed against the human p38γ MAPK sequence and selected using the siDESIGN Centre program at http://www.oligoengine.com.
Generation of inactive p38γ knock-in mice
The targeting vector, made to replace Asp171 with Ala in WT exon 7 of the p38γ gene and the heterozygous mouse (p38γ171A/+) was generated in collaboration with Artemis Pharmaceuticals (Koln, Germany).
Identification of RNA species in hDlg and PSF immunoprecipitates
RNA in immunoprecipitates was isolated and identified as described (Rousseau et al., 2002), with modifications. Unknown RNA species in the immunoprecipitate were amplified with the Genhunter RNAimage kit 4. The 3′ primer used for reverse transcription and PCR was a mix of three primers, H-T11G, H-T11C and H-T11A. The 5′ primer set for PCR amplification consisted of a mixture of four primers (H-AP25, H-AP26, H-AP27 and H-AP-28; termed random primer set 1) or the four primers H-AP29, H-AP30, H-AP31 and H-AP32 (termed random primer set 2).
Subcellular fractionation
Subcellular fractionation was performed using the ProteoExtract™, Subcellular Proteome Extraction Kit from Calbiochem (Nottingham). Immunoblotting with the following antibodies against the indicated marker proteins were carried out as control: anti-calpain for cytosolic fraction, anti-insulin receptor (InsR) for the membrane fraction and anti-CREB for the nuclear fraction. Protein quantification was performed by densitometry using ChemiDoc XRS system and the program quantity one from Bio-Rad (Hercules, CA)
Immunofluorescence staining
Cells were grown on poly-L-lysine-coated 22×22 mm glass coverslips for 24 hours before fixation. Cell were fixed with 4% paraformaldehyde for 10 minutes and permeabilised for 1 minute with 1% Triton X-100 as described (Sabio et al., 2004). Immunostaining of hDlg and p38γ were performed as previously described (Inesta-Vaquera et al., 2009; Sabio et al., 2005; Sabio et al., 2004). PSF was immunostained by using 4 μg/ml of the specific antibody and GST-PDZ1 using 5 μg/ml of anti-GST antibody.
RNA quantification with specific probes by real-time PCR
Cells were treated as indicated, then lysed and immunoprecipitated with either hDlg or PSF. The RNA present in the immunoprecipitates was isolated using the NulcleoSpin RNA purification method (Macherey-Nabel). RNA was reverse transcribed (iScript, Bio-Rad) and real-time PCR carried out using Sybr green. The primer sequences used are listed in supplementary material Table S1.
Supplementary Material
Acknowledgments
We thank J. M. Carr, in the MRC Unit, for help generating knock-in p38γ mice; the protein production and antibody purification teams at DSTT (University of Dundee) for antibody expression and purification; L. Almonacid for help with the real-time PCR and C. Mark for editorial assistance. This work was supported by: MICINN (BFU2007-67577), UK Medical Research Council, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck and Co, Merck KGaA and Pfizer. Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/15/2596/DC1
References
- Alepuz P. M., Jovanovic A., Reiser V., Ammerer G. (2001). Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell 7, 767-777 [DOI] [PubMed] [Google Scholar]
- Balda M. S., Matter K. (2000). The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 19, 2024-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp T. R., Bernards R., Agami R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550-553 [DOI] [PubMed] [Google Scholar]
- Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007). Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441-1474 [DOI] [PubMed] [Google Scholar]
- Buxade M., Morrice N., Krebs D. L., Proud C. G. (2008). The PSF.p54nrb complex is a novel Mnk substrate that binds the mRNA for tumor necrosis factor alpha. J. Biol. Chem. 283, 57-65 [DOI] [PubMed] [Google Scholar]
- Cobbold L. C., Spriggs K. A., Haines S. J., Dobbyn H. C., Hayes C., de Moor C. H., Lilley K. S., Bushell M., Willis A. E. (2008). Identification of internal ribosome entry segment (IRES)-trans-acting factors for the Myc family of IRESs. Mol. Cell. Biol. 28, 40-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Rousseau S. (2007). p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 1773, 1358-1375 [DOI] [PubMed] [Google Scholar]
- Cuenda A., Cohen P., Buee-Scherrer V., Goedert M. (1997). Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38). EMBO J. 16, 295-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa A., Kotani H., Toda Y., Mazan-Mamczarz K., Mueller E. C., Otto A., Disch L., Norman M., Ramdasi R. M., Keshtgar M., et al. (2009). Novel roles of hakai in cell proliferation and oncogenesis. Mol. Biol. Cell 20, 3533-3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. H., Lam Y. W., Leung A. K., Lyon C. E., Andersen J., Mann M., Lamond A. I. (2002). Paraspeckles: a novel nuclear domain. Curr. Biol. 12, 13-25 [DOI] [PubMed] [Google Scholar]
- Funke L., Dakoji S., Bredt D. S. (2005). Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu. Rev. Biochem. 74, 219-245 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R., Dubash A. D., Sharek L., Carr H. S., Frost J. A., Burridge K. (2007). The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity. Mol. Cell. Biol. 27, 8683-8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Cuenda A., Craxton M., Jakes R., Cohen P. (1997). Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 16, 3563-3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Cuenda A., Spillantini M. G., Thomas G. M., Buee-Scherrer V., Cohen P., Goedert M. (1999). Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J. Biol. Chem. 274, 12626-12631 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Tsujino T., Iwata S., Nonaka H., Emoto N., Yano Y., Otani S., Hayashi Y., Itoh H., Yokoyama M. (2002). Decreased ornithine decarboxylase activity in the kidneys of Dahl salt-sensitive rats. Hypertens. Res. 25, 787-795 [DOI] [PubMed] [Google Scholar]
- Hsueh Y. P., Wang T. F., Yang F. C., Sheng M. (2000). Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 404, 298-302 [DOI] [PubMed] [Google Scholar]
- Humbert P., Russell S., Richardson H. (2003). Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays 25, 542-553 [DOI] [PubMed] [Google Scholar]
- Inesta-Vaquera F. A., Centeno F., del Reino P., Sabio G., Peggie M., Cuenda A. (2009). Proteolysis of the tumour suppressor hDlg in response to osmotic stress is mediated by caspases and independent of phosphorylation. FEBS J. 276, 387-400 [DOI] [PubMed] [Google Scholar]
- Knebel A., Morrice N., Cohen P. (2001). A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 20, 4360-4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M. F., Macara I. (2006). Maintenance and modulation of T cell polarity. Nat. Immunol. 7, 1143-1149 [DOI] [PubMed] [Google Scholar]
- Kuma Y., Sabio G., Bain J., Shpiro N., Marquez R., Cuenda A. (2005). BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J. Biol. Chem. 280, 19472-19479 [DOI] [PubMed] [Google Scholar]
- Laprise P., Viel A., Rivard N. (2004). Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J. Biol. Chem. 279, 10157-10166 [DOI] [PubMed] [Google Scholar]
- Mauceri D., Gardoni F., Marcello E., Di Luca M. (2007). Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. J. Neurochem. 100, 1032-1046 [DOI] [PubMed] [Google Scholar]
- Proft M., Struhl K. (2002). Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell 9, 1307-1317 [DOI] [PubMed] [Google Scholar]
- Roberts S., Calautti E., Vanderweil S., Nguyen H. O., Foley A., Baden H. P., Viel A. (2007). Changes in localization of human discs large (hDlg) during keratinocyte differentiation are [corrected] associated with expression of alternatively spliced hDlg variants. Exp. Cell Res. 313, 2521-2530 [DOI] [PubMed] [Google Scholar]
- Rousseau S., Morrice N., Peggie M., Campbell D. G., Gaestel M., Cohen P. (2002). Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs. EMBO J. 21, 6505-6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G., Reuver S., Feijoo C., Hasegawa M., Thomas G. M., Centeno F., Kuhlendahl S., Leal-Ortiz S., Goedert M., Garner C., et al. (2004). Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem. J. 380, 19-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G., Arthur J. S., Kuma Y., Peggie M., Carr J., Murray-Tait V., Centeno F., Goedert M., Morrice N. A., Cuenda A. (2005). p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shav-Tal Y., Zipori D. (2002). PSF and p54(nrb)/NonO-multi-functional nuclear proteins. FEBS Lett. 531, 109-114 [DOI] [PubMed] [Google Scholar]
- Tang J., Qi X., Mercola D., Han J., Chen G. (2005). Essential role of p38gamma in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 280, 23910-23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. D., Thornton T. M., Sabio G., Davis R. A., Rincon M. (2009). Nuclear localization of p38 MAPK in response to DNA damage. Int. J. Biol. Sci. 5, 428-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin A. S., Michalowski D., Bear J., Smulevitch S. V., Traish A. M., Peng R., Patton J., Shatsky I. N., Felber B. K. (2003). PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol. Cell. Biol. 23, 6618-6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.