Abstract
The possibility that effector T cells can be converted into forkhead box P3+ regulatory T cells (Tregs) has potential therapeutic implications. To analyze the relationship between Th1 effectors and Tregs, we have used a model of systemic autoimmunity in which both effector and Tregs arise from a single population specific for a transgene-encoded systemic protein. In vitro, the presence of IFN-γ inhibits Treg generation during activation. Using IFN-γ reporter mice, we demonstrate that IFN-γ–producing cells tend not to develop into Tregs, and Th1 priming of T cells prior to cell transfer limits the number of forkhead box P3+ T cells generated in vivo. Moreover, transfer of IFN-γ−/− or STAT1−/− T cells resulted in an increase in the number of Tregs. These data support a role for Th1 effector molecules and transcription factors in the control of peripheral Treg generation and demonstrates the limited plasticity of Th1 populations.
CD25+ forkhead box P3 (Foxp3)-expressing regulatory T cells (Tregs) are generated in the thymus by self-Ag recognition and in peripheral lymphoid organs presumably in response to both self- and foreign Ags (1-3). Some of the major unresolved issues about peripherally generated Tregs are the mechanism of their generation, their ability to arise from naive or activated cell populations, and the signals that drive and control their development in vivo. It has been shown that CD4+ T cell lineages, including Th1 and Th2 cells, become progressively refractory to conversion after successive cell divisions (4, 5). More recent studies on Th17 cells and Tregs suggest that their stability is not fixed and these populations appear to be able to convert their phenotype even from a differentiated state (6, 7). However, the conversion of other effector T cells to Tregs has not been examined in detail, particularly in vivo.
The goals of this study were to examine the ability of differentiated Th 1 effector cells to convert into Tregs in vivo and to examine the cytokine control of peripheral Treg generation. We have developed an experimental model in which CD4+ T cells specific for OVA are transferred into lymphopenic mice expressing a transgene that produces OVA as a systemic protein (sOVARag−/−) (8). We have previously shown that the Ag-specific T cells rapidly develop into Th17 and Th1 effector cells, resulting in a systemic inflammatory disease. In mice that survive the acute disease, effector cells are gradually controlled by Foxp3+ Tregs (9). This well-established system is valuable in that both effector and regulatory cells arise from a single population of CD4+ T cells in response to cognate Ag. In addition, this model affords the ability to track and characterize Tregs generated in vivo.
The sequential development of effector and regulatory cells led to the question of whether cytokine producing effector cells could give rise to Foxp3+ Tregs in vivo. To examine the relationship of effector cells and Tregs in this model, we tracked the fate of Th1 effectors using OVA-specific T cells expressing a fluorescent IFN-γ reporter. In this study, we report that Treg cells tend not to develop from differentiated Th1 effector cells. Instead, we demonstrate that STAT1, the transcription factor downstream of IFN-γ, is a powerful inhibitor of Treg development. These results demonstrate the limited plasticity of Th1 effector T cell populations and the importance of cytokine control in peripheral Treg generation.
Materials and Methods
Mice
DO11.10 and IFN-γ−/− mice on a BALB/c background were obtained from The Jackson Laboratory, Bar Harbor, ME. STAT1−/− mice were purchased from Taconic Farms, Hudson, NY, and bred in accordance with Taconic’s research cross-breeding agreement. Tbet−/− mice were a gift from Dr. L. Glimcher (Harvard Medical School, Boston, MA). IFN-γ-yellow fluorescent protein (YFP) reporter mice (Yeti) were provided by Dr. R. Locksley, University of California, San Francisco, San Francisco, CA. Foxp3-GFP mice were provided by Dr. A Rudensky (Sloan-Kettering Institute, New York, NY). sOVARag−/− mice on a BALB/c background have been described previously (10). Mice were housed in microisolator cages in the University of California, San Francisco animal facility and used between 4–8 wk of age.
Cell preparation, purification, and adoptive transfer
CD4+ cells were purified using positive selection according to the manufacturer’s protocol (Dynal, Invitrogen, Carlsbad, CA). A total of 2.5 × 105 to 5 × 105 naive T cells (CD4+KJ1-26+CD25−) were purified by cell sorting and adoptively transferred to recipient sOVARag−/− recipients or cultured in vitro. Activating conditions were naive DO11.10 cells cultured with with mitomycin C-treated (Sigma-Aldrich, St. Louis, MO; 1 mg/ml) BALB/c splenocytes and 1 μg/ml OVA peptide (pOVA323–339) for 5 d. For Th1-polarizing conditions, IL-12 (2.5 ng/ml) and anti–IL-4 (11B11; 10 μg/ml) were also added to the culture.
Restimulation, staining, and flow cytometry
For intracellular cytokine staining, DO11.10 T cells were recovered from peripheral lymph nodes and restimulated with mitomycin C-treated BALB/c splenocytes for 18 h in the presence of 1 mg/ml OVA peptide. Brefeldin A (Epicentre Biotechnologies, Madison, WI; 10 mg/ml) was added for the last 2 h of stimulation. For Foxp3 analysis, lymph node cells were isolated directly ex vivo and stained according to the manufacturer’s protocol (eBioscience, San Diego, CA). All samples were analyzed by flow cytometry gating on CD4+KJ1-26+ cells.
In vitro proliferation and suppressor assays
For coculture assays, responder cells were sorted from DO11.10 mice (CD4+CD25−KJ1-26+) and stimulated on BALB/c mitomycin-treated APCs with 1 μg/ml OVA peptide. Lymph nodes were harvested from sOVARag−/− recipients that had received naive DO11.10 Foxp3-GFP cells 10 d prior to transfer, and CD4+CD25+KJ1-26+GFP+ cells or CD4+CD25+KJ1-26+GFP+ from naive DO11.10 Foxp3-GFP mice were sorted and used as suppressor cells. A total of 2 × 105 responder cells and 4 × 105 APCs were used per well; Tregs were added to generate the indicated ratios. A total of 0.125 μCi/well [3H]thymidine was added during the final 12 h of culture.
Statistical analysis was done using the two-tailed t test (*p < 0.05).
Results
Relationship of IFN-γ–producing effector cells and Foxp3+ Tregs
To examine the relationship between cytokine-producing effector cells and Tregs, we used IFN-γ–YFP (Yeti) reporter mice (11) to track the fate of IFN-γ–producing cell populations in conditions known to generate Tregs in vivo. To do this, we generated DO11.10 Yeti mice and purified naive DO11.10 cells, which were devoid of Tregs (Supplemental Fig. 1), then transferred these cells into sOVARag−/− recipients as previously described (8). Lymph nodes were harvested 10 d later and analyzed for IFN-γ and Foxp3 expression by flow cytometry. At this time point, a large fraction of the transferred cells were expressing YFP (IFN-γ mRNA) and were producing IFN-γ protein, whereas a small number of cells were Foxp3+ (Fig. 1A, 1B). Furthermore, these IFN-γ– and Foxp3-expressing cells were distinct populations (Supplemental Fig. 2).
FIGURE 1.
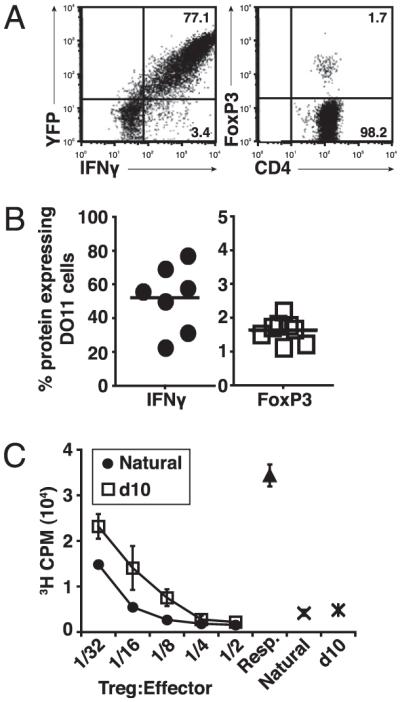
Relationship of IFN-γ–producing effector cells and Foxp3+ Tregs. A, Naive CD4+ T cells from DO11.10 Yeti mice were sorted and adoptively transferred into sOVARag−/− recipients. Lymph nodes were harvested 10 d post transfer. Representative FACS plots of IFN-γ and Foxp3 gated on DO11.10 cells. B, Cumulative data from four experiments for the percentage of IFN-γ+ or Foxp3+ cells; note the difference in scales. C, Naive T cells from DO11.10 Foxp3-GFP reporter mice were sorted and adoptively transferred into sOVARag−/− recipients. GFP+ Tregs cells were sorted 10 d post transfer (d10 Tregs) and compared with GFP+ Tregs from DO11.10 Foxp3-GFP reporter mice (natural). These cells were cultured with CD4+CD25− (responder) cells from DO11.10/Rag−/− mice and mitomycin-treated APCs for 72 h. Data are representative of two comparable experiments.
To examine the function of the peripherally generated Foxp3+ cells, we generated DO11.10 Foxp3-GFP reporter mice (1). Naive T cells were transferred into sOVARag−/− recipients. At 10 d posttransfer, GFP+ cells were purified and assayed for suppressive activity. During a standard coculture assay, the Tregs generated in vivo were as suppressive as “natural” Tregs purified from Foxp3-GFP DO11.10 mice, indicating that the Foxp3+ T cells generated in vivo are functional Tregs (Fig. 1C).
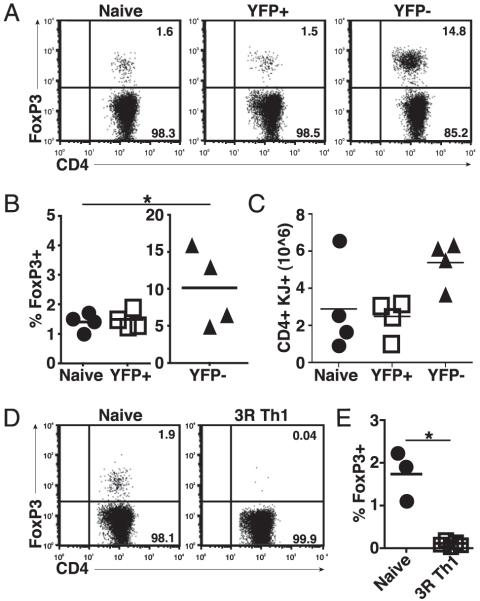
We then focused on determining whether Th1 effector cells could develop into Tregs. Naive DO11.10 Yeti cells were primed in vitro under Th1 conditions. After 5 d of culture, cells were sorted into YFP+ (IFN-γ+) and YFP− (IFN-γ−) populations and transferred into sOVARag−/− recipients. At 10 d posttransfer, YFP+ donor cells had similar percentages of Foxp3+ cells as seen with naive DO11.10 cells, suggesting that at least some IFN-γ–producing cells are able to convert to Tregs (Fig. 2A, 2B). In contrast, the percentage of Foxp3+ cells recovered upon transfer of sorted YFP− donor cells was much greater (Fig. 2A, 2B). Furthermore, there was an increase in the total number of CD4+ T cells recovered upon transfer of YFP− cells, although not statistically significant, suggesting that IFN-γ may inhibit proliferation and/or promote death of activated T cells (Fig. 2C). These results indicate that these T cells, which fail to produce IFN-γ during Th1 priming, convert into Tregs more efficiently than their IFN-γ–producing counterparts.
FIGURE 2.
Reduced Treg development from IFN-γ–producing cells. Naive CD4+ T cells from DO11.10 Yeti mice were sorted and cultured under Th1 conditions. After 5 d, cells were sorted into YFP− and YFP+ populations and transferred into sOVARag−/− recipients; for comparison, freshly sorted naive cells were also transferred. Lymph nodes were harvested 10 d post transfer. A, Representative FACS plots show Foxp3 expression gated on DO11.10 cells. B, The percentage of Foxp3+ T cells for three experiments (note the difference in scales). C, The number of Foxp3+ T cells for three experiments. D, Naive CD4+ T cells from WT DO11.10 mice were sorted and cultured under Th1 conditions for three rounds of stimulation (3R Th1) and transferred into sOVARag−/− recipients. Lymph nodes were harvested 10 d post transfer. Representative FACS plots are shown gated on DO11.10 cells. E, The number of Foxp3+ T cells for three experiments.
These results also suggested that progressive Th1 differentiation may inhibit conversion of effector cells to Foxp3+ Tregs. To examine the impact of the extent of Th1 commitment on the ability of IFN-γ–producing cells to convert into Tregs, wild-type (WT) DO11.10 cells were primed for three cycles of Th1-polarizing conditions in vitro and subsequently transferred into sOVARag−/− recipients. In these studies, repeated Th1 priming led to a near-complete inhibition of Treg generation (Fig. 2D, 2E), suggesting that although IFNγ-producing cells are able to convert to Foxp3+ Tregs at low levels, terminally differentiated Th1 effector cells do not exhibit this flexibility.
Inhibition of Treg development by IFN-γ
The ability of YFP− cells to preferentially generate Tregs in vivo suggested that IFN-γ production from the YFP+ donor cells might inhibit the generation of peripheral Tregs in vivo. To formally test the influence of IFN-γ on peripheral Treg generation, we transferred naive or in vitro-activated IFN-γ−/− DO11.10 T cells that were devoid of Tregs prior to transfer (Supplemental Fig. 3) into sOVARag−/− mice. The percentage of Foxp3+ T cells after 10 d in vivo was significantly greater from IFN-γ−/− than from WT T cells, independent of the activation status of the transferred cells (Fig. 3A, 3B). Similar to earlier experiments, we recovered slightly more total T cells from recipient mice that received IFN-γ−/− donor cells, although the differences were not statistically significant (Fig. 3C). To confirm the impact of IFN-γ on the generation of Tregs, we cultured WT or IFN- γ−/− DO11.10 T cells under activating conditions in vitro and assayed for the presence of Foxp3+ cells. IFN-γ−/− DO11.10 cells generated a higher percentage of Foxp3+ T cells over time than WT DO11.10 cells (Fig. 3D). The influence of IFN-γ was further confirmed by the addition of exogenous IFN-γ, which markedly decreased the frequency of Foxp3+ T cells generated in vitro (Fig. 3D). These results support the conclusion that the Th1 response, and specifically IFN-γ, is a natural inhibitor of peripheral Treg generation.
FIGURE 3.
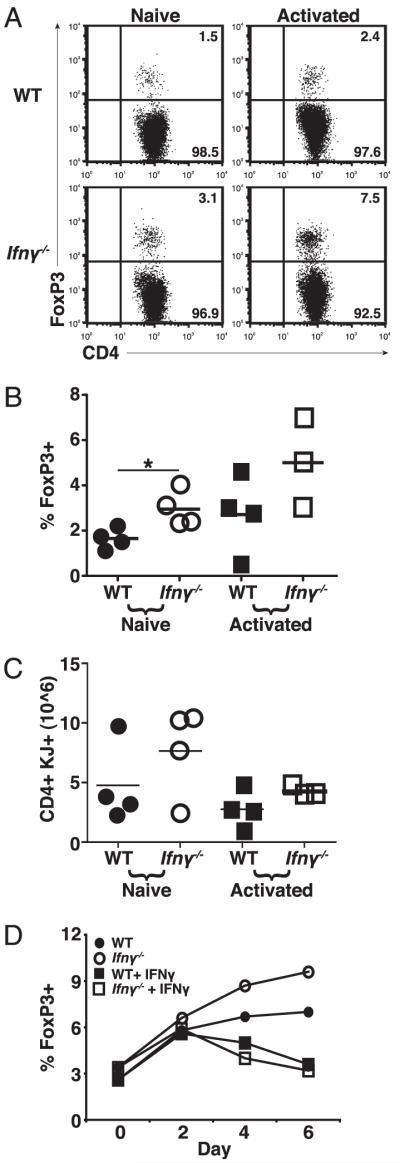
IFN-γ inhibits development of Foxp3+ cells. A, Naive T cells from WT and IFN-γ−/− DO11.10 mice were cultured in activating conditions for 5 d and then transferred to sOVARag−/− recipients; for comparison, freshly sorted naive cells were also transferred. Lymph nodes were harvested 10 d post transfer. A, Representative plots of Foxp3+ T cells gated on DO11.10 cells. B, The percentage of Foxp3+ T cells for three experiments. C, The number of Foxp3+ T cells for three experiments. D, Naive CD4+ T cells from WT and IFN-γ−/− DO11.10 mice were cultured in activating conditions ± exogenous IFN-γ for Foxp3 expression on days 2, 4, and 6. Data are representative of two experiments.
Mechanisms of suppression of peripheral Tregs
The experiments described above show that IFN-γ can inhibit the generation of Foxp3+ cells. STAT1 and Tbet, two transcription factors known to promote Th1 differentiation, are both downstream of the IFN-γ receptor (12, 13). To examine their role in the control of peripheral Treg generation, we transferred Tbet−/− or STAT1−/− DO11.10 cells primed under Th1 conditions to sOVARag−/− recipients. In the absence of Tbet, the frequency of Foxp3+ cells recovered was the same as with WT T cells. However, in the absence of STAT1, there was a significant increase in Foxp3+ T cell (Treg) recovery (Fig. 4A, 4B) with no increase and actually a modest decrease in total T cell recovery (Fig. 4C). These data indicate that the inhibitory effect of IFN-γ on Treg generation is a Tbet-independent and STAT1-dependent phenomenon.
FIGURE 4.
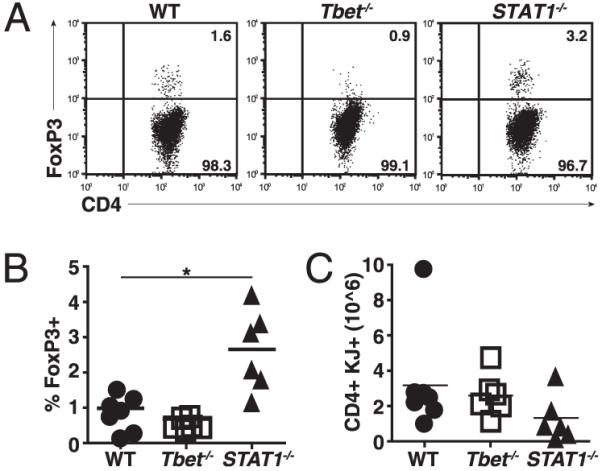
STAT1 but not Tbet inhibits peripheral Treg generation. Naive CD4+ T cells from WT, Tbet−/−, or STAT1−/− DO11.10 mice were sorted, cultured under Th1 conditions, and transferred to sOVARag−/− recipients. Lymph nodes were harvested 10 d post transfer. A, Representative plots of Foxp3 stains gated on DO11.10. B, The percentage of Foxp3+ T cells for three experiments. C, The number of Foxp3+ T cells from three experiments.
Discussion
The studies described here used a single-Ag model for the generation of effector cells and Tregs in vivo to explore the relationships between these cell populations. Our results indicate that differentiated Th1 effectors do not efficiently develop into Foxp3+ Tregs in vivo (Fig. 2). This contrasts with the ability of Th17 cells to readily develop into Tregs, at least in vitro, suggesting that Th17 cells are more plastic than differentiated Th1 or Th2 effectors (6, 14). Recent data have also shown that Foxp3+ Tregs can convert into Th1 (15) and Th17 (6, 7) effector cells in vivo. Taken together, the conclusion of these studies is that populations of differentiated T cells vary considerably in their plasticity and interconvertibility.
The failure of differentiated Th1 cells to convert into Tregs is likely because IFN-γ inhibits the peripheral generation of Tregs. This conclusion is supported by data showing enhanced Treg generation from IFN-γ−/− T cells in vitro and in vivo and the ability of exogenous IFN-γ to decrease Treg recovery in vitro (Figs. 2, 3). Much of the previous work on the effects of IFN-γ on Treg development have relied on in vitro systems and have given contradictory results. Some groups have shown that IFN-γ can inhibit Treg generation or function (16, 17), but other studies show that IFN-γ can actually enhance generation and function (18, 19). Our studies are the first to show that IFN-γ made by one T cell population can inhibit the generation of Tregs from the same population in response to prolonged Ag encounter in vivo. It has been suggested that the suppressive effect of IFN-γ is related to cell death induced by reactive oxygen species (16), our studies show that IFN-γ has only a modest effect on total cell recoveries. It has been previously demonstrated that IFN-γ produced by Th1 effectors serves to eliminate terminally differentiated Th1 cells in vivo (20, 21). It is unclear if the inhibition of Treg generation is related to the ability of IFN-γ to promote death of some effector cells or inhibit their proliferation.
Our studies to assess the mechanism by which IFN-γ inhibits Treg development have shown that STAT1 but not Tbet is involved in this inhibition (Fig. 4). Published data suggest that STAT1-deficient mice have impaired Treg function, but the influence on the generation of Treg populations from either naive cells or effector cells was not addressed (22). It is possible that STAT1 competes with STAT5, which is necessary for Treg maintenance, or STAT1 may function indirectly by activating a pathway that inhibits Treg development. It is also a possibility that the effect of IFN-γ and STAT1 acts directly on Foxp3 expression or on other aspects of Treg development and maintenance.
The inability of Th1 cells to develop into Tregs and the inhibitory effect of IFN-γ on Treg generation suggests that once a Th1-dominant inflammatory reaction is initiated, it will likely not be controlled by peripherally generated Tregs. This implies that the transition from inflammatory disease to Treg-mediated recovery that we see after transfer of DO11.10 cells into sOVARag−/− recipients may not be a direct conversion of effector cells to Tregs. It is more likely that recovery is related to the death of differentiated Th1 cells (20) followed by the generation of Tregs from uncommitted cells. It remains to be established if the same events are associated with recovery in other inflammatory reactions and in nonlymphopenic situations. Answers to these questions may reveal novel approaches for limiting the life span and activity of pathogenic effector cells and promoting the development of protective regulatory cells.
Supplementary Material
Acknowledgments
We thank Shuwei Jiang for assistance with cell sorting and Carlos Benitez for maintenance of mice colonies.
Supported by National Institutes of Health Grants P01 AI35297, R01 AI64677, and U19 AI56388 and fellowships from the Howard Hughes Medical Institute (to D.C.) and the National Institutes of Health (to S.D.K.).
Abbreviations used in this paper
- Foxp3
forkhead box P3
- Treg
regulatory T cell
- WT
wild-type
- YFP
yellow fluorescent protein
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 4.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 5.Mullen AC, Hutchins AS, Villarino AV, Lee HW, High FA, Cereb N, Yang SY, Hua X, Reiner SL. Cell cycle controlling the silencing and functioning of mammalian activators. Curr. Biol. 2001;11:1695–1699. doi: 10.1016/s0960-9822(01)00533-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr. Opin. Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J. Immunol. 2004;173:5028–5035. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- 11.Mayer KD, Mohrs K, Crowe SR, Johnson LL, Rhyne P, Woodland DL, Mohrs M. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-γ production. J. Immunol. 2005;174:7732–7739. doi: 10.4049/jimmunol.174.12.7732. [DOI] [PubMed] [Google Scholar]
- 12.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J. Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 13.Berenson LS, Ota N, Murphy KM. Issues in T-helper 1 development—resolved and unresolved. Immunol. Rev. 2004;202:157–174. doi: 10.1111/j.0105-2896.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa H, Kato T, Tawara I, Ikeda H, Kuribayashi K, Allen PM, Schreiber RD, Old LJ, Shiku H. IFN-γ controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response. J. Immunol. 2005;175:4433–4440. doi: 10.4049/jimmunol.175.7.4433. [DOI] [PubMed] [Google Scholar]
- 17.Chang JH, Kim YJ, Han SH, Kang CY. IFN-γ-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur. J. Immunol. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 18.Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ, Bushell A. Exogenous IFN-γ ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur. J. Immunol. 2008;38:2512–2527. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J. Exp. Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulds KE, Rotte MJ, Paley MA, Singh B, Douek DC, Hill BJ, O’Shea JJ, Watford WT, Seder RA, Wu CY. IFN-γ mediates the death of Th1 cells in a paracrine manner. J. Immunol. 2008;180:842–849. doi: 10.4049/jimmunol.180.2.842. [DOI] [PubMed] [Google Scholar]
- 21.Wall DA, Hamberg SD, Reynolds DS, Burakoff SJ, Abbas AK, Ferrara JL. Immunodeficiency in graft-versus-host disease. I. Mechanism of immune suppression. J. Immunol. 1988;140:2970–2976. [PubMed] [Google Scholar]
- 22.Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J. Exp. Med. 2003;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.