Abstract
Allergic conjunctivitis (AC) is elicited by immediate hypersensitivity responses to environmental agents. It is initiated by a Th2-dominated immune response that is characterized by production of IgE antibodies and eosinophilic infiltration. By using an experimental mouse model of AC induced by short ragweed (SRW) pollen, we show that sensitized Jα18−/− mice, which lack type I NKT cells, and CD1d−/− mice, which lack type I and type II NKT cells, exhibited a decrease in tearing, lid edema, conjunctival edema and vasodilatation and eosinophil infiltration into the conjunctiva when compared with wild-type (WT) mice in both Th1- and Th2-prone hosts (C57BL/6 and BALB/c mice, respectively). This demonstrates that NKT cells are needed for both the early and late phases of AC. Adoptive transfer of SRW-primed CD4+ T cells from Jα18−/− mice into naive WT BALB/c mice revealed that NKT cells were needed for the maximal induction of allergen-specific Th2 cells. Results from adoptive transfer of SRW-primed CD4+ T cells from WT BALB/c mice to naive Jα18−/− mice indicated that NKT cells were also needed for the expression of AC produced by allergen-primed CD4+ T cells. The decreased expression of AC in NKT cell-deficient mice was correlated with significant reduction in the production of Th2 cytokines in SRW pollen-sensitized mice compared with WT mice and in the capacity of SRW pollen-sensitized CD4+ T cells to mediate ocular inflammation when the hosts were confronted with SRW pollen at the ocular surface.
Keywords: allergy, atopy, ocular surface, SRW pollen, Th2
Introduction
Allergic conjunctivitis (AC) describes a variety of ocular inflammatory diseases that affect the lid, conjunctiva and, on occasion, the cornea (1). It has been estimated that AC affects 20% of the world's population (2, 3). A mild form of AC, known as seasonal AC, is induced by environmental irritants such as grass, ragweed and birch pollens (2). More severe forms, such as atopic keratoconjunctivitis or vernal keratoconjunctivitis, display more chronic symptoms and can involve the cornea (3, 4). The development of AC consists of two phases: an early phase and a late phase reaction. The early phase reaction occurs within minutes of allergen exposure and is initiated when the allergen cross-links specific IgE antibodies bound via FcεRI receptors on the surface of ocular mast cells resulting in the release of histamine, leukotrienes, proteases, prostaglandins and cytokines (3, 5). The early phase reaction is characterized by tearing, lid edema, conjunctival edema (chemosis) and vasodilatation of conjunctival blood vessels. The late phase reaction appears hours after allergen exposure and involves the infiltration of inflammatory cells, especially eosinophils, into the conjunctiva (3). IL-4 is produced by primed Th2 cells after allergen encounter and is believed to be involved in helping B cells secrete IgE, while IL-5, which is produced by Th2 cells, activates and promotes the maturation of infiltrating eosinophils (6). Another Th2 cytokine, IL-13, is implicated in increasing mucus production in allergic responses (7). Although Th2 cells are important in ocular allergies, they alone may not be sufficient to produce an allergic response. Other cells such as NKT cells are thought to contribute to Th2-mediated immune responses (8).
NKT cells are a unique subgroup of lymphocytes that differ from conventional αβ T cells in that they produce both Th1 (IFN-γ) and Th2 (IL-4) cytokines upon stimulation (9, 10). NKT cells recognize lipid antigen presented by a non-classical MHC molecule, CD1d (11). Two types of NKT cells have been described: type I NKT cells are defined by their invariant TCR Vα14Jα18 in mice and Vα24Jα18 in humans and are referred to as invariant NKT (iNKT) cells (11). Type II NKT cells express a diverse non-Vα14 TCR (12). Studies on NKT cells have produced contradictory results as to their function. While some studies implicate NKT cells in controlling immune responses against infection and tumors, others suggest that they act to protect self-tissue from damage caused by pro-inflammatory immune responses (11).
NKT cells have been implicated in allergen-induced, immune-mediated diseases such as allergic asthma and contact dermatitis (7, 8, 13). Recent studies in mice have found that NKT cells play an essential role in the development of airway hyperreactivity (AHR), a hallmark of asthma. Mice lacking type I NKT cells have a decreased ability to develop AHR and display reduced airway eosinophilia, decreased IL-4 and IL-5 in the bronchoalveolar lavage fluid (BALF) and reduced antigen-specific IgE compared with wild-type (WT) mice (8,14–16). These results are in agreement with those found by Akbari et al. (7) who found that AHR does not develop in Jα18−/− mice, which lack type I NKT cells, or CD1d−/− mice, which lack both type I and type II NKT cells. These findings demonstrate that NKT cells are required for maximal pulmonary eosinophilic infiltration, Th2 cytokine production and elevated serum IgE levels in mice with AHR. The role of NKT cells in allergic asthma in humans is surrounded by controversy. While some studies demonstrate a pronounced increase in the numbers of NKT cells in BALF of patients with allergic asthma (17–19), others have not (20–22). In this report, we determined and characterized the role of type I and type II NKT cells in the development of short ragweed (SRW) pollen-induced AC.
Methods
Animals
C57BL/6 (H-2b) and BALB/c (H-2d) were purchased from the University of Texas (UT) Southwestern Mouse Breeding Facility. Jα18−/− mice on C57BL/6 and BALB/c backgrounds were generated as previously described and kindly provided by Masaru Taniguchi, RIKEN Research Center for Allergy and Immunology, Yokohama, Japan (23). CD1d−/− mice on C57BL/6 and BALB/c backgrounds were kindly provided by Mark A. Exley, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA. Jα18−/− and CD1d−/− mice were bred at the UT Southwestern Medical Center Animal Resource Center. All mice were used at 6–8 weeks of age. The animal studies were approved by the Institutional Review Board of the UT Southwestern Medical Center at Dallas. Animals were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology statement about the Use of Animals in Ophthalmic and Vision Research.
Induction of AC by active immunization
AC was induced as previously described (2, 24). Briefly, mice were immunized with 50 μg of SRW pollen (International Biologicals, Piedmont, OK, USA) in 5 mg of alum (Thermo Fisher Scientific Pierce, Rockford, IL, USA) by intraperitoneal (i.p.) injection on day 0. AC was induced by a multihit topical challenge in which immunized mice were given 1.5 mg of SRW pollen in 10 μl PBS in the right eye from days 10 to 16. Mice were examined clinically for signs of immediate hypersensitivity responses 20 min after each topical challenge with SRW pollen or PBS. Each parameter (lid edema, tearing, conjunctival vasodilatation and conjunctival edema) was scored on a scale ranging from 0 to 3 (18). A score of 0 indicated that there was no evidence of the respective parameter; 1+, mild response distinctly greater than the naive control; 2+, moderate change in respective parameter that could be noted by biomicroscopy, but not with naked eye; and 3+, severe response that could be perceived with naked eye.
In vivo treatment of anti-CD1d
Mice were treated with intravenous (i.v.) injections of rat anti-mouse CD1d mAb (hybridoma HB323; American Type Culture Collection, Manasas, VA, USA) and rat-IgG (Sigma-Aldrich, St Louis, MO, USA) isotype control three times a week (50 micrograms per injection) beginning 7 days prior to immunization.
Cytokine ELISA
Mice were killed 17 days after sensitization with SRW pollen and their spleens removed. Single-cell suspensions of splenocytes were prepared by gently processing between the ends of two sterile frosted slides. 1 × 107 cells per milliliter were incubated with 25 μg ml−1 of soluble SRW pollen extract (Greer Laboratories, Lenoir, NC, USA) for 48 h in 2 ml of RPMI supplemented with 10% FCS, 2 mM L-glutamine (Cambrex, Charles City, IA, USA), 1 mM sodium pyruvate (Cambrex), 1% penicillin–streptomycin–fungizone (Cambrex), 1% non-essential amino acids (Cambrex), 1% HEPES buffer (Cambrex) and 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich). Six hours before harvest, 1 μg ml−1 ionomycin (Sigma-Aldrich) and 25 ng ml−1 phorbol 12-myristate 13-acetate (Sigma-Aldrich) were added to stimulate cytokine release. ELISAs for IL-4, IL-5, IL-13 and IFN-γ were performed on culture supernatants according to the manufacturer's instructions (R&D Systems).
Induction of AC by adoptive transfer of primed CD4+ T cells
Spleens were removed from Jα18−/− or WT BALB/c (or C57BL/6) mice 17 days after sensitization with SRW pollen. CD4+ T cells were isolated using a magnetic microbead system (Miltenyi Biotec, Auburn, CA, USA). Single-cell suspensions of splenocytes were prepared by gently processing between the ends of two sterile frosted slides. Splenocytes were incubated with anti-mouse CD4-coated magnetic beads in bead buffer (0.5% BSA in PBS, pH 7.2) for 15 min at 4°C. Unattached beads were washed off with bead buffer. Cells not attached to magnetic beads passed through the column and were discarded. CD4+ cells attached to magnetic beads were retained in the column and eluted with bead buffer. One spleen equivalent of enriched CD4+ T cell suspensions (7.0 × 106 to 9.0 × 106 cells per recipient) were injected i.v. into naive BALB/c or C57BL/6 mice (<2% were double positive for NK1.1 and TCR-β). Four days later, mice were challenged topically with SRW pollen as previously described.
Reconstitution of NKT cells
Spleen cells were processed as described above. T cells were isolated using a magnetic microbead system (Miltenyi Biotec). Splenocytes were incubated with biotin antibody cocktail for 10 min followed by an anti-biotin microbead incubation for 15 min at 4°C. Unattached beads were washed off with bead buffer. Cells not attached to magnetic beads were collected as the enriched T cell population. T cell-negative cells were retained in the column and were eluted with bead buffer. Enriched T cells were stained with PE mouse anti-mouse NK1.1 (BD Bioscience Pharmingen, San Jose, CA, USA) and allophycocyanin conjugated hamster anti-mouse TCR-β (BD Bioscience Pharmingen) and incubated for 30 min. The stained cells were isolated using BD FACSAria. NK1.1+ TCR-β+ cells were used as purified NKT cells. NKT cells (2.0 × 105 to 4.5 × 105 cells per recipient) were i.v. injected into naive C57BL/6 mice prior to immunization with SRW pollen. Ten days later, mice were challenged topically with SRW pollen as previously described.
Histology
Eyes from mice were removed 17 days after sensitization and fixed in 10% formalin for histology. Paraffin-embedded tissue sections were stained with Congo Red. Differential cell counts were performed by counting all inflammatory cells in the forniceal conjunctiva in the histological section of each mouse. Inflammatory cells were counted in masked fashion by two investigators and were recorded as eosinophils, neutrophils or mononuclear cells.
Statistics
Clinical scores and ELISA data are represented as the mean ± SD. Inflammatory cell counts are represented as mean ± SE. Comparison between the WT-immunized and knockout immunized mice was made using Student's t-test (24). Significance of the histological data was tested by Student's t-test. P values <0.05 were considered significant.
Results
Th1- and Th2-prone hosts develop AC
Experiments were performed to determine if the early and late phase of AC could be induced in both Th1- and Th2-prone hosts (C57BL/6 and BALB/c mice, respectively). AC was induced by i.p. sensitization with SRW pollen followed by topical challenges with SRW pollen administered to the right eye for seven consecutive days. The early phase of AC was assessed by evaluating the clinical phenotype of the disease within 20 min of each daily topical challenge with SRW pollen (i.e. days 10–16) and by scoring tear production, lid edema, chemosis and conjunctival vasodilatation (2). Since the clinical scores were taken within minutes of topical challenge, they represent ‘early phase’ responses. Both WT BALB/c and C57BL/6 mice developed the clinical phenotype of AC. However, the severity of AC was significantly less in C57BL/6 mice compared with BALB/c mice (data not shown).
The late phase of AC is characterized by the infiltration of inflammatory cells consisting primarily of eosinophils and occurs 6–12 h after allergen challenge. Compared with the PBS controls, WT BALB/c and C57BL/6 mice had an increase in the number of eosinophilic infiltration into the conjunctiva (data not shown).
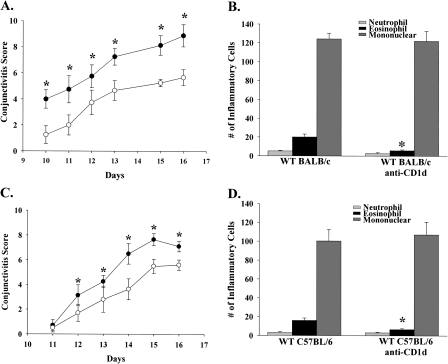
The possibility that NKT cells were needed for the maximal expression of AC was also examined. Accordingly, WT BALB/c mice were treated with either anti-CD1d mAb or an isotype control antibody. WT BALB/c mice treated with anti-CD1d antibody had a decreased expression of the early phase of AC compared with the isotype control antibody-treated group (Fig. 1A). There was also a decrease in the number of infiltrating eosinophils into the conjunctive (Fig. 1B). WT C57BL mice treated with anti-CD1d antibody also demonstrated the same trend, a reduction in the clinical manifestation of AC as well as diminished eosinophil infiltration into the conjunctiva (Fig. 1C and D).
Fig. 1.
Blocking NKT cell activation results in decreased expression of AC. Clinical AC scores in (A) BALB/c or (C) C57BL/6 mice treated with either anti-CD1d (open circle) antibody or an isotype control antibody (closed circle) prior to sensitization and challenge with SRW pollen. (B and D) Eosinophilic infiltrations into the conjunctive of SRW pollen-challenged mice. (n = 10) This graph is representative of two independent experiments; *P < 0.05.
Type I and type II NKT cells are needed for the early and late phases of AC
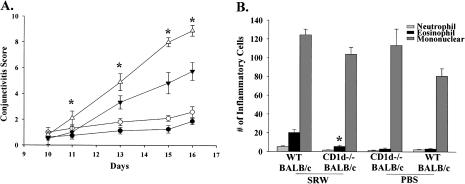
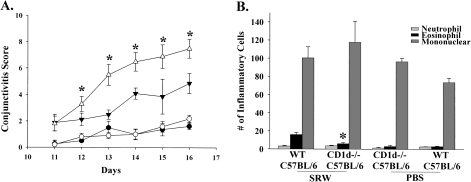
Initial experiments in WT mice treated with anti-CD1d mAb suggested that NKT cells (type I and type II) might be required to elicit maximal expression of AC. To further explore the role of NKT cells in AC, we assessed the clinical expression of AC in CD1d−/− mice, which lack type I and type II NKT cells. CD1d−/− BALB/c (Fig. 2A) and CD1d−/− C57BL/6 (Fig. 3A) mice had decreased tear production, lid edema, chemosis and conjunctival vasodilatation compared with WT mice. Eosinophilic infiltration into the conjunctivae of CD1d−/− BALB/c (Fig. 2B) and CD1d−/− C57BL/6 (Fig. 3B) mice was significantly decreased compared with the WT counterparts. Thus, type I and type II NKT cells are needed to elicit the early and late phase of AC in both Th1- and Th2-prone hosts.
Fig. 2.
Type I and II NKT cells are needed for the early and late phase of AC in Th2-prone hosts. (A) Clinical scores for AC in CD1d−/− BALB/c mice (closed inverted triangle) and WT BALB/c mice (open triangle) that were sensitized and challenged topically with SRW pollen. CD1d−/− BALB/c mice (closed circle) and WT BALB/c mice (open circle) were challenged topically with PBS and served as negative controls. (B) Eosinophilic infiltration into the conjunctivae of CD1d−/− BALB/c mice and WT BALB/c mice. (n = 12) This graph is representative of two independent experiments; *P < 0.05.
Fig. 3.
Type I and II NKT cells are needed for the early and late phase of AC in Th1-prone hosts. (A) Clinical scores for AC in CD1d−/− C57BL/6 mice (closed inverted triangle) and WT C57BL/6 mice (open triangle) that were challenged with SRW pollen. CD1d−/− C57BL/6 mice (closed circle) and WT C57BL/6 mice (open circle) were challenged with PBS and served as negative controls. (B) Eosinophilic infiltration into the conjunctivae of CD1d−/− C57BL6 mice and WT C57BL/6 mice. (n = 10) This graph is representative of two independent experiments; *P < 0.05.
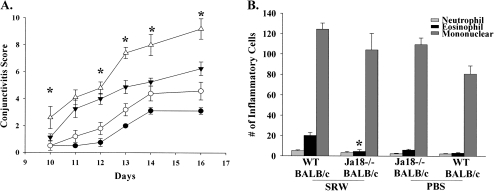
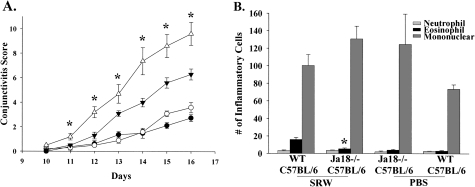
To determine if type I NKT cells are needed for AC, we employed Jα18−/− mice, which lack only type I NKT cells. Jα18−/− BALB/c (Fig. 4A) and Jα18−/− C57BL/6 (Fig. 5A) mice displayed a decrease in the clinical expression (i.e. early phase) of AC when compared with WT mice, which confirmed that type I NKT cells are needed for the early phase of AC. Compared with CD1d−/− mice, Jα18−/− mice expressed more severe AC, suggesting that there is an additive effect in the reduction of AC when both type I and type II NKT cells are missing. Histological analysis demonstrated that the number of eosinophilic infiltration into the conjunctivae of Jα18−/− BALB/c (Fig. 4B) and Jα18−/− C57BL/6 (Fig. 5B) mice was significantly decreased in NKT cell-deficient mice in both strains compared with their WT counterparts. These results indicate that NKT-deficient mice in both Th1- and Th2-prone strains have diminished early phase reactions, as defined by the clinical scores as well as the late phase reactions as demonstrated by a decreased eosinophilic infiltration into the conjunctiva.
Fig. 4.
Type I NKT cells are needed for the early and late phase of AC in Th2-prone hosts. (A) Clinical scores of Jα18−/− BALB/c (closed inverted triangle) and WT BALB/c mice (open triangle) that were sensitized and challenged with SRW pollen compared with Jα18−/− mice. Jα18−/− BALB/c mice (closed circle) and WT BALB/c mice (open circle) were challenged with PBS and served as negative controls. (B) Eosinophilic infiltration into the conjunctivae of Jα18−/− BALB/c and WT BALB/c mice. (n = 15) This graph is representative of three independent experiments; *P < 0.05.
Fig. 5.
Type I NKT cells are needed for the early and late phase of AC in Th1-prone hosts. (A) Clinical sores of Jα18−/− C57BL/6 mice (closed inverted triangle) and WT C57BL/6 mice (open triangle). Jα18−/− C57BL/6 mice (closed circle) and WT C57BL/6 mice (open circle) were challenged with PBS and served as negative controls. (B) Eosinophilic infiltration into the conjunctivae of Jα18−/− C57BL/6 mice and WT C57BL/6 mice. (n = 10); This graph is representative of two independent experiments. *P < 0.05.
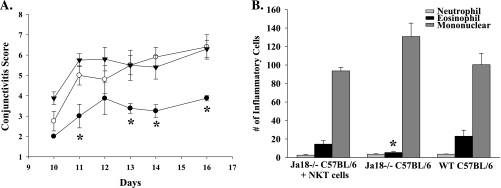
To show that the absence of NKT cells was responsible for the reduction of AC in Jα18−/− mice, we reconstituted Jα18−/− mice with NKT cells before immunization with SRW pollen. Adoptive transfer of cells double positive for NK1.1 and TCR-β fully reconstituted the ability for Jα18−/− mice to develop both early and late phase responses of AC when compared with WT C57BL/6 mice treated with SRW pollen (Fig. 6A and B). As before, Jα18−/− mice displayed reduced early and late phase responses of AC (Fig. 6A and B). The restoration of early and late phase responses show that Jα18−/− mice were otherwise identical to WT C57BL/6 mice; these results verify that NKT cells are required for the development of AC.
Fig. 6.
Adoptive transfer of NKT cells into Jα18−/− mice restores AC. (A) Clinical score of Jα18−/− mice reconstituted with NKT-positive cells (open circle), Jα18−/− mice (closed circle) and WT C57BL/6 mice (closed inverted triangle). (B) Eosinophilic infiltration into the conjunctivae of Jα18−/− C57BL/6 mice reconstituted with NKT cells and challenged with SRW pollen. (n = 5); *P < 0.05.
NKT cell-deficient mice have diminished production of Th2 cytokines
The production of Th2 cytokines (IL-4, IL-5 and IL-13) plays a critical role in the induction of AC (25, 26). We hypothesized that the reduction in the early phase and late phase reactions of AC was due to a decrease in cytokine production by SRW-specific Th2 cells. To assess this, splenocytes from SRW pollen-sensitized Jα18−/− and WT mice were stimulated in vitro with SRW extract for 48 h and the presence of Th2 cytokines was quantified by ELISA.
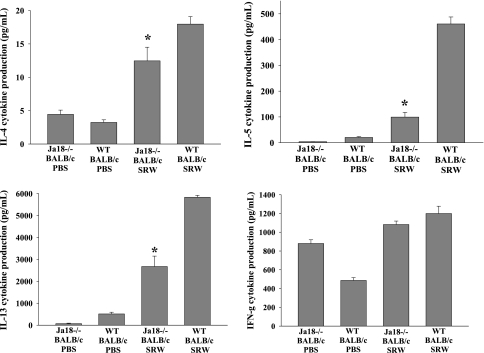
Spleen cells from Jα18−/− BALB/c mice displayed a decreased production of Th2 cytokines IL-4, IL-5 and IL-13 compared with WT mice, while the production of the Th1 cytokine, IFN-γ, was comparable to WT levels (Fig. 7). This suggests that the presence of type I NKT cells are necessary for the generation of allergen-specific Th2 cells and the optimal expression of AC.
Fig. 7.
Decreased severity of AC in NKT cell-deficient BALB/c mice is associated with diminished production of IL-4, IL-5 and IL-13. Bulk splenocytes were incubated with allophycocyanin's pulsed with SRW extract. Supernatants were collected 48 h after in vitro culture and analyzed by ELISA. (n = 10) This graph is representative of two independent experiments; *P < 0.05.
NKT cells are necessary for the efferent arm of AC (‘licensing’ stage)
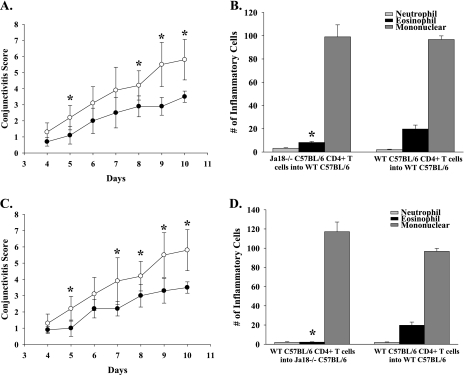
The present results indicate that NKT cells are needed for activating the afferent arm of the Th2 immune response as shown by the diminished production of Th2 cytokines that occurred in the Jα18−/− mice. However, studies on AHR in mice indicate that iNKT cells play an important role in the efferent arm of the Th2 immune response (7). That is, iNKT cells in the lung produce significant quantities of IL-4 and IL-13, which create a Th2-biased milieu in the lung. Circulating Th2 cells that enter the lung are exposed to the Th2 cytokines and are boosted or ‘licensed’ to produce Th2-based inflammation. In this case, iNKT cells act on the efferent arm of the Th2 immune response by affecting the function of previously activated Th2 cells. With this in mind, we performed adoptive transfer experiments to determine if iNKT cells might also influence the efferent arm of the immune response in AC. Accordingly, we adoptively transferred CD4+ T cells from SRW pollen-sensitized Jα18−/− BALB/c or WT BALB/c donors into naive WT BALB/c recipients. Four days after the adoptive transfer, both groups of mice were challenged topically with SRW pollen daily for seven consecutive days. Both the clinical manifestation (early phase reaction of AC) and the number of inflammatory cells present in the conjunctiva (late phase reaction of AC) were assessed clinically and histopathologically, respectively.
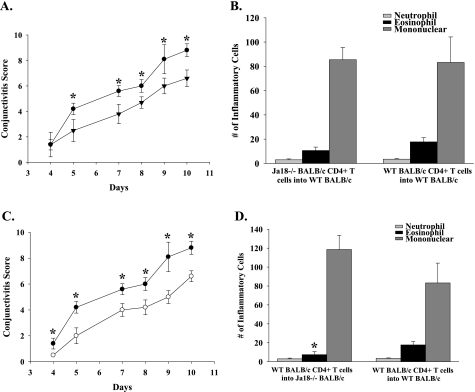
WT BALB/c naive recipients of CD4+ T cells from Jα18−/− SRW pollen-sensitized donors displayed decreased early phase signs of AC compared with WT naive recipients of CD4+ T cells from SRW pollen-sensitized WT donors (Fig. 8A). The same trend was seen in C57BL/6 mice (Fig. 9A). The reduced severity of AC was consistent with the previously observed diminished Th2 cytokine production by CD4+ T cells from the Jα18−/− mice (Fig. 7). By contrast, the WT BALB/c recipients of CD4+ T cells from Jα18−/− donors had eosinophilic infiltrations into the conjunctiva that were similar to WT recipients of CD4+ T cells from WT donors (Fig. 8B). These results suggest that NKT cells are needed for the maximal generation of Th2 cells and for the expression of the early phase reaction of AC. However, Th2 cells generated in the absence of NKT cells were capable of mediating late phase inflammation if an NKT cell repertoire was available to license this Th2 cell population in the conjunctiva. C57BL/6 mice that received CD4+ T cells from Jα18−/− mice had a reduction in eosinophilic infiltrations into the conjunctiva compared with WT recipients of CD4+ T cells from WT donors (Fig. 9B). These results indicate that in a Th1-prone host CD4+ T cells generated in the absence of NKT cells are not competent to license Th2 cells in the conjunctiva.
Fig. 8.
NKT cells participate in the afferent and efferent arms of AC. CD4+ T cells isolated from SRW-sensitized BALB/c donors were adoptively transferred into naive recipients. (A) Clinical scores of AC in naive WT recipients that received CD4+ T cells from either Jα18−/− donors (closed inverted triangle) or WT donors (closed circle) that were sensitized with SRW pollen. (B) Eosinophilic infiltration into the conjunctivae of WT recipients of CD4+ T cells from SRW-sensitized Jα18−/− or WT donors. (C) Clinical scores of AC in naive WT (closed circle) or Jα18−/− (open circle) recipients of CD4+ T cells from WT donors that were sensitized with SRW pollen. (D) Eosinophilic infiltration into the conjunctivae of Jα18−/− and WT BALB/c mice that received CD4+ T cells from WT BALB/c mice that were sensitized with SRW pollen. (n = 10) This graph is representative of two independent experiments; *P < 0.05.
Fig. 9.
NKT cells participate in the afferent and efferent arms of AC. CD4+ T cells isolated from SRW-sensitized C57BL/6 donors were adoptively transferred into naive recipients. (A) Clinical scores of AC in naive WT C57BL/6 recipients that received CD4+ T cells from either C57BL/6 Jα18−/− donors (closed inverted triangle) or WT donors (closed circle) that were sensitized with SRW pollen. (B) Eosinophilic infiltration into the conjunctivae of WT recipients of CD4+ T cells from SRW-sensitized Jα18−/− or WT C57BL/6 donors. (C) Clinical scores of AC in naive WT (closed circle) or Jα18−/− (open circle) recipients of CD4+ T cells from WT donors that were sensitized with SRW pollen. (D) Eosinophilic infiltration into the conjunctivae of Jα18−/− and WT C57BL/6 mice that received CD4+ T cells from WT C57BL/6 mice that were sensitized with SRW pollen. (n = 5); *P < 0.05.
The capacity of Th2 cells from Jα18−/− donor mice to produce late phase inflammation suggested that the previously observed diminution of AC in Jα18−/− mice sensitized with SRW pollen was due to impaired expression of Th2-based inflammation (i.e. efferent arm). This was tested by adoptively transferring CD4+ T cells from SRW pollen-sensitized WT donors to Jα18−/− recipients and assessing both the early phase and late phase reactions. The results of this adoptive cell transfer experiment demonstrated that the absence of an intact NKT cell repertoire prevented full expression of both the early phase and late phase reactions of AC by the SRW pollen-sensitized CD4+ T cells from WT mice (Figs 8C and 9(C and D)). These results suggest that NKT cells function at the end stage organ (i.e. conjunctiva) and, as in AHR, may act to license Th2 cells in the conjunctiva for the maximal expression of AC.
Discussion
AC is an immediate hypersensitivity reaction mediated by a Th2 immune response and the preferential generation of IgE antibodies. In the early phase, the allergen cross-links IgE antibodies bound via FcεRI on the surface of mast cells (3). This event occurs within minutes of allergen exposure and triggers the degranulation of mast cells releasing mediators such as histamine. The late phase reaction is evident 6–12 h after allergen challenge and is characterized by an inflammatory infiltrate consisting of neutrophils and a preponderance of eosinophils (3). Th2 cells and their cytokines are associated with many allergic disorders such as atopic dermatitis and asthma (25); however, other immune cells play important roles in the induction and expression of allergic diseases.
NKT cells play a major role in regulating the immune response by bridging the innate and adaptive immune system. They are a unique subgroup of lymphocytes that differ from conventional αβ T cells in that they recognize lipid and not peptide antigen and upon stimulation produce both Th1 and Th2 cytokines (9, 10). Two different subsets of NKT cells have been described: type I NKT cells, which express invariant Vα14Jα18 TCR in the mouse or Vα24Jα18 in the human, and type II NKT cells, which express more diverse TCRs (11). Type I NKT cells recognize lipid antigens, such as α-galactosylceramide, presented by a non-classical MHC molecule, CD1d (11). In tumor immunity, type I NKT cells have been shown to primarily mediate a protective role, while type II NKT cells suppress anti-tumor surveillance (12).
There have been conflicting reports regarding the role of NKT cells in asthma. Some studies have implicated NKT cells as being important in the development of AHR (7, 8). Type I NKT cells that produce IL-4 and IL-13 were required for the clinical expression of AHR (7) and for the maximal production of eosinophilia, Th2 cytokines and IgE (8). Jα18−/− and CD1d−/− BALB/c mice were unable to develop AHR, even though they could generate conventional Th2 cells capable of making IL-4 and IL-13 (7). Moreover, adoptive transfer of type I NKT cells, which produced IL-4 and IL-13, into Jα18−/− mice restored AHR, while administration of IL-13 restored AHR in CD1d−/− mice (7).
By contrast, others have reported that an allergic response can develop in the absence of NKT cells (27, 28). Ovalbumin (OVA)-sensitized CD1d−/− mice developed eosinophil-rich inflammation and similar levels of OVA-specific IgE comparable to WT C57BL/6 mice. These findings were supported by Morishima et al. (29) who found that airway eosinophilia, AHR and OVA-specific IgE levels were no different in Jα18−/− and WT BALB/c mice. Brown et al. (27) assessed the role of NKT cells in AHR using beta 2-microglobulin (β2m)−/− mice, which, due to the disruption of the β2m gene, impairs surface expression of CD1. They found that β2m−/− mice had no significant difference in lung resistance, airway pressure, IL-4-producing cells or serum IgE compared with WT mice. A more recent study found that a CD1d-restricted, NK1.1+ non-iNKT cell was present in β2m−/− mice and was responsible for the development of AHR (30). Possible explanations for their results include differences in genetic background, experimental protocol for OVA immunization and the time points used in the evaluations.
To our knowledge, only one other study has examined the role of NKT cells in AC. Fukushima et al. (31) found that administration of α-GalCer, which is known to stimulate type I NKT cells, significantly increased Th2 immune responses and infiltration of eosinophils into the conjunctiva when administered at the time of immunization with SRW. However, treatment with α-GalCer just prior to the first challenge resulted in decreased infiltrations of eosinophils into the conjunctiva of SRW-primed mice. This decrease in the late phase reaction of AC was associated with an increase in the frequency of CD4+CD25+ T cells (31).
The present study demonstrates that both Th1- and Th2-prone hosts develop the early and late phases of AC. C57BL/6 mice, which are Th1-prone hosts, did not develop the clinical phenotype that characterizes the early phase of AC to the same magnitude as BALB/c mice, which are Th2-prone hosts. In order to assess if NKT cells were needed for the full expression of AC, we employed two NKT cell-deficient mice: Jα18−/− and CD1d−/− mice. Jα18−/− BALB/c and Jα18−/− C57BL/6 mice had decreased clinical signs of AC and reduced eosinophil infiltration into the conjunctiva compared with WT mice. Furthermore, adoptively transferring NKT cells into Jα18−/− C57BL/6 mice fully reconstituted the early and late phase of AC. These results confirmed that NKT cells were needed for the full expression of the early and late phases of AC. Since it is not feasible to assess the role of type II NKT cells alone, we used CD1d−/− mice that lack both type I and type II NKT cells. CD1d−/− BALB/c and CD1d−/− C57BL/6 mice also had decreased clinical signs of AC and reduced eosinophil infiltration into the conjunctiva compared with WT mice. Moreover, it was evident that the clinical severity of AC in Jα18−/− mice was greater than observed in CD1d−/− mice in both strains, indicating that there might be an additive effect when both type I and type II NKT cells are absent.
We show that the decrease in the early and late phase of AC correlates with a decrease in the production of Th2 (IL-4, IL-5 and IL-13) but not Th1 (IFN-γ) cytokines by SRW-specific T cells. The dampened Th2 response was correlated with high IFN-γ levels that occurred in the NKT-deficient mice. This suggests that additional factors contribute to the decreased Th2 immune responses in these animals. As in AHR, the role of NKT cells in AC may be to license Th2 cells in the conjunctiva to induce AC, as adoptively transferred SRW-primed CD4+ T cells were unable to induce AC in Jα18−/− mice. However, unlike the conditions in AHR, NKT cells were needed at both the afferent and efferent stages of AC.
In conclusion, this study demonstrates that NKT cells are needed for development of AC. We suggest that NKT cells mediate or amplify the Th2 inflammatory responses that are needed for the maximal expression of AC. It will be important to identify the underlying molecular mechanisms that NKT cells employ for the development of AC and determine if disabling NKT cells at the ocular surface is a viable therapeutic option for the treatment of AC.
Funding
National Institutes of Health (EY0007641, EY016664); Research to Prevent Blindness.
References
- 1.Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin. Exp. Immunol. 2008;153(Suppl 1):17. doi: 10.1111/j.1365-2249.2008.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh H, Neelam S, Hurt M, Niederkorn JY. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect. Immun. 2005;73:1061. doi: 10.1128/IAI.73.2.1061-1068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dace DS, Chen PW, Niederkorn JY. CD4+ T-cell-dependent tumour rejection in an immune-privileged environment requires macrophages. Immunology. 2008;123:367. doi: 10.1111/j.1365-2567.2007.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J. Immunol. 1999;162:957. [PubMed] [Google Scholar]
- 5.Galatowicz G, Ajayi Y, Stern ME, Calder VL. Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin. Exp. Allergy. 2007;37:1648. doi: 10.1111/j.1365-2222.2007.02782.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamelmann E, Gelfand EW. IL-5-induced airway eosinophilia—the key to asthma? Immunol. Rev. 2001;179:182. doi: 10.1034/j.1600-065x.2001.790118.x. [DOI] [PubMed] [Google Scholar]
- 7.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr. Opin. Immunol. 2003;15:627. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Lisbonne M, Diem S, de Castro Keller A, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 2003;171:1637. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 9.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 10.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage. J. Exp. Med. 2002;195:835. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrosino E, Berzofsky JA, Terabe M. Regulation of tumor immunity: the role of NKT cells. Expert Opin. Biol. Ther. 2008;8:725. doi: 10.1517/14712598.8.6.725. [DOI] [PubMed] [Google Scholar]
- 12.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J. Exp. Med. 2005;202:1623. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gober MD, Fishelevich R, Zhao Y, Unutmaz D, Gaspari AA. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J. Invest. Dermatol. 2008;128:1460. doi: 10.1038/sj.jid.5701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 15.Bilenki L, Yang J, Fan Y, Wang S, Yang X. Natural killer T cells contribute to airway eosinophilic inflammation induced by ragweed through enhanced IL-4 and eotaxin production. Eur. J. Immunol. 2004;34:345. doi: 10.1002/eji.200324303. [DOI] [PubMed] [Google Scholar]
- 16.Meyer EH, Goya S, Akbari O, et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl Acad. Sci. USA. 2006;103:2782. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbari O, Faul JL, Hoyte EG, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 18.Matangkasombut P, Marigowda G, Ervine A, et al. Natural killer T cells in the lungs of patients with asthma. J. Allergy Clin. Immunol. 2009;123:1181. doi: 10.1016/j.jaci.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen Y, Yongyi B, Yuling H, et al. V alpha 24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J. Immunol. 2005;175:4914. doi: 10.4049/jimmunol.175.8.4914. [DOI] [PubMed] [Google Scholar]
- 20.Mutalithas K, Croudace J, Guillen C, et al. Bronchoalveolar lavage invariant natural killer T cells are not increased in asthma. J. Allergy Clin. Immunol. 2007;119:1274. doi: 10.1016/j.jaci.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Rock M, Yoder S, Hoskins A, Ajayi WU, Sheller JR, Dworski R. Effect of allergen challenge on the percentage of natural killer T cells in patients with atopic asthma. Ann. Allergy Asthma Immunol. 2009;102:432. doi: 10.1016/s1081-1206(10)60517-0. [DOI] [PubMed] [Google Scholar]
- 22.Vijayanand P, Seumois G, Pickard C, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356:1410. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 23.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 24.Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin. Immunol. Immunopathol. 1998;87:75. doi: 10.1006/clin.1997.4507. [DOI] [PubMed] [Google Scholar]
- 25.Fukushima A, Ozaki A, Jian Z, et al. Dissection of antigen-specific humoral and cellular immune responses for the development of experimental immune-mediated blepharoconjunctivitis in C57BL/6 mice. Curr. Eye Res. 2005;30:241. doi: 10.1080/02713680590927560. [DOI] [PubMed] [Google Scholar]
- 26.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 27.Brown DR, Fowell DJ, Corry DB, et al. Beta 2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J. Exp. Med. 1996;184:1295. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korsgren M, Persson CG, Sundler F, et al. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J. Exp. Med. 1999;189:553. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morishima Y, Ishii Y, Kimura T, et al. Suppression of eosinophilic airway inflammation by treatment with alpha-galactosylceramide. Eur. J. Immunol. 2005;35:2803. doi: 10.1002/eji.200525994. [DOI] [PubMed] [Google Scholar]
- 30.Below S, Konkel A, Zeeck C, et al. Virulence factors of Staphylococcus aureus induce Erk-MAP kinase activation and c-Fos expression in S9 and 16HBE14o- human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L470. doi: 10.1152/ajplung.90498.2008. [DOI] [PubMed] [Google Scholar]
- 31.Fukushima A, Sumi T, Fukuda K, et al. Modulation of murine experimental allergic conjunctivitis by treatment with alpha-galactosylceramide. Immunol. Lett. 2006;107:32. doi: 10.1016/j.imlet.2006.07.001. [DOI] [PubMed] [Google Scholar]