Summary
Signals orchestrating productive CD4+ T-cell responses are well documented; however, the regulation of contraction of CD4+ T-cell effector populations following the resolution of primary immune responses is not well understood. While distinct mechanisms of T-cell death have been defined, the relative importance of discrete death pathways during the termination of immune responses in vivo remains unclear. Here we review the current understanding of cell-intrinsic and cell-extrinsic variables that regulate contraction of CD4+ T-cell effector populations through multiple pathways that operate both initially during T-cell priming and later during the effector phase. We discuss the relative importance of antigen-dependent and antigen-independent mechanisms of CD4+ T-cell contraction during in vivo responses, with a special emphasis on influenza virus infection. In this model, we highlight the roles of greater differentiation and presence in the lung of CD4+ effector T cells, as well as their polarization to particular T-helper subsets, in maximizing contraction. We also discuss the role of autocrine interleukin-2 in limiting the extent of contraction, and we point out that these same factors regulate contraction during secondary CD4+ T-cell responses.
Keywords: CD4+ T cells, immunologic memory, T-cell contraction, influenza
Introduction
During a primary immune response, naive CD4+ T cells recognizing antigen in the context of activated antigen-presenting cells (APCs) expand many fold and differentiate into large highly activated populations of effector cells that make their way to the site of infection and throughout tissues (1, 2). Depending on the inflammatory environment and several other variables during priming, CD4+ T cells can become polarized into one or more distinct effector subsets with specialized functions and attributes. The best characterized of such subsets are T-helper 1 (Th1) and Th2, long associated with promoting effective cell-mediated and humoral responses, respectively (3). More recently, additional subsets have been described including Th17, T-follicular helper (Tfh), and various regulatory populations (4–6) (Table 1). However, substantial heterogeneity and plasticity, as assessed by cytokine production patterns, has been observed within subsets, especially when generated in vivo (7, 8), and during an infection, it seems most likely that multiple ‘polarized’ CD4+ T-cell subsets are generated (9). These effector cells secrete large quantities of potent cytokines, chemokines, and immunoregulatory proteins and also exert cell-based effector mechanisms such as cytotoxicity in response to low doses of antigen without the need for costimulation (10). These functions make them both extremely effective when combating a pathogen but also dangerous to the host, because the inflammatory responses that they induce can damage host tissue if unchecked as well as potentially trigger autoimmunity. Indeed, CD4+ T effectors play a central role in the development of several experimental models of autoimmunity including experimental autoimmune encephalitis (EAE), rheumatoid arthritis, and colitis (11–13). Thus, the clearance of the activated effector cells after the resolution of infection and the selection of an appropriate cohort of CD4+ T cells to transition to resting memory are of great importance.
Table 1.
CD4 T cell effector subsets
| Th Subset | Polarizing Factors |
Transcription Factors |
Signature Cytokines |
Susceptibility to AICD |
|---|---|---|---|---|
| Th1 | IL-12, IFN-γ | T-bet | IFN- γ, TNF, IL-2 | ++++ |
| Th2 | IL-4 | GATA-3 | IL-4, IL-5, IL-13 | + |
| Th17 | TGF-β, IL-6 | ROR-γt | IL-17, IL-21, IL-22 | ++ |
| TFh | IL-6, IL-21 | Bcl-6 | IL-4, IL-21 | ? |
| Treg | TGF-β, RA | Foxp3 | IL-10 | ? |
Following the resolution of a primary immune response, usually correlating with the decline of inflammation and antigen clearance, a large majority of activated CD4+ T-cell effectors die via apoptosis to leave a small but relatively stable population of memory cells (14). While many of the signals and steps leading to the generation of effector CD4+ T-cell subsets are well defined (2, 15–17), the processes and triggers involved in the contraction of activated effectors are not well understood, especially in vivo. Generally, apoptosis in T cells is triggered through two distinct pathways both leading to the activation of caspase cascades, DNA breaks, and ultimately cell death (18). The ‘extrinsic pathway’ is initiated through the triggering of death receptors within the tumor necrosis factor (TNF) receptor family by their specific ligands and functions through initiator caspases 8 and 10 (19–22). The ‘intrinsic’ pathway is triggered through damage to mitochondrial membranes and initiator caspase 9 (22, 23). Non-apoptotic death pathways can also influence the contraction of CD4+ T-cell effector populations (24).
We suggest that the process of contraction is regulated by the integration of a series of complex signals that are present both during initial priming of CD4+ T cells and later during the effector stage. In this review, we discuss what we know about some of the factors that regulate the contraction phase of CD4+ T-cell subsets generated during influenza virus infection, pointing out roles from our own studies for the extent of initial antigen exposure, the stage of differentiation and anatomical location of effectors, for CD4 subset polarization, for restimulation with antigen, and for autocrine interleukin-2 (IL-2) produced during restimulation. The complexity of regulation suggests that contraction is a critical checkpoint that preferentially selects particular CD4 subsets to persist as memory and thus helps to shape the potential of secondary responses.
T-cell responses following influenza virus infection
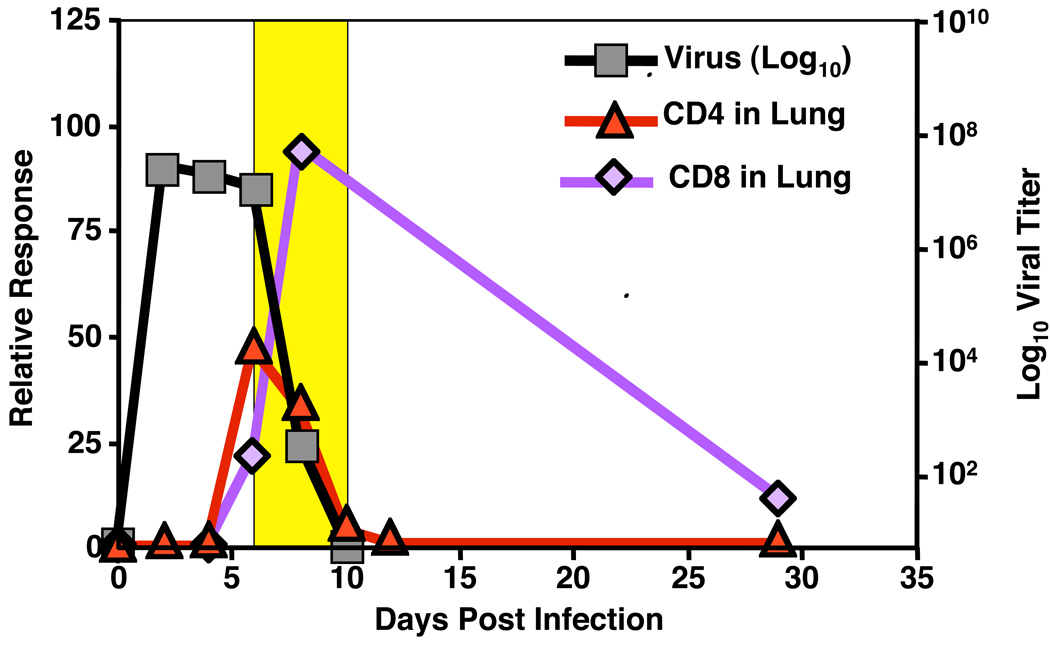
When small numbers of naive T-cell receptor (TCR) transgenic (Tg) CD4+ or CD8+ T cells recognizing influenza, such as HNT CD4+ T cells recognizing an epitope of the viral hemagglutinin (25), are introduced to naive hosts shortly before infection, in our studies and those of others using adoptive transfer models (26, 27), approximately 10% of the transferred cells ‘take’, enabling the dynamics of an epitope specific T-cell response to be characterized. Following intranasal infection, naive CD4+ and CD8+ T-cell responses initiate in the draining mediastinal lymph nodes (MLN) and later in the spleen (28, 29). Cells divide rapidly in these sites and differentiate into a spectrum of highly activated effectors over 5–6 days, as judged by phenotypic and functional criteria. A cohort of the most differentiated effectors then migrates to the lung starting at days 5–6, leading to a peak in effector numbers of both CD4+ and CD8+ T cells in the lung at days 7–8 (Fig. 1).
Fig. 1. T-cell contraction following influenza infection.
Following maximal effector responses in the lung, an acute contraction phase (yellow highlighted) occurs in the lung that is coincident with viral clearance.
During the first few days following infection, virus titers in the lung increase exponentially and then level off. Once virus-specific T cells reach the lung, influenza titers of fall precipitously. When sublethal doses of virus are used to infect the animals, live virus is cleared from the lung by day 9–10. As virus is cleared, the flu-specific TCR Tg cohort of CD4+ T cells contracts with very similar kinetics (28). TCR Tg CD8+ T cells also contract, but at a slower pace, and significantly more survive long-term than corresponding CD4+ T cells (29). It is important to stress that although it is useful to follow the TCR Tg cohort to visualize these events, the host polyclonal CD8+ and CD4+ T-cell dynamics are very similar (30, 31). Similar differences in the survival of CD4+ versus CD8+ T cells following the resolution of infection have been described in a variety of other infectious models (32–34). Several factors might account for the differential rates of contraction between effector CD4+ and CD8+ T cells. A primary mechanism could involve access to T-cell survival factors. For example, CD8+ T cells may out-compete memory CD4+ T cells for IL-15 via expression of a higher affinity receptor (35, 36), thereby causing the latter population to be less fit for long-term survival. A larger population of antigen-experienced CD4+ as compared to CD8+ T cells may also require cognate interactions with antigen (37–39), though we find memory from in vitro-generated CD4+ T-cell effectors is maintained optimally in the absence of major histocompatibility complex (MHC) class II (40), arguing this is not the case for all subsets of memory. Residual antigen depots have been detected after the resolution of primary responses in diverse models (41–44), and their gradual waning over time might constrict survival niches. In support of this hypothesis, residual antigen depots present following influenza infection seem particularly capable of generating and perhaps maintaining CD4+ and CD8+ T-cell memory populations (41, 45).
T-cell contraction during influenza infection
Contraction of influenza-specific CD4+ T cells in the lung is generally the most marked, and attrition of virus-specific T cells in the lung continues for longer periods of time than that observed in the MLN and spleen (Fig. 2). These data are compatible with several different hypotheses that suggest a number of non-mutually exclusive mechanisms that may be responsible for contraction. First, the fact that contraction is more dramatic in the lung is compatible with the concept that re-exposure to antigen, which in the case of influenza, replicates and is detected almost exclusively in the lung (46, 47), could drive an antigen-induced cell death that is either absent or more limited in other organs (48). Indeed, there is convincing evidence that CD4+ T cells in the lung are being restimulated by specific antigen during the contraction phase because a significant proportion secrete interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) when they are examined ex vivo without the need for further restimulation (28). This is not observed within T-cell populations in the MLN or spleen. It is also possible that inflammatory mediators present at high relative concentrations in the lung drive some component of contraction in synergy with TCR triggering or independently (49, 50).
Fig. 2. CD4+ T-cell contraction following influenza infection.
Naive TCR Tg CD4+ T cells recognizing influenza (HNT) were transferred to BALB/c hosts then infected with a sublethal dose of virus. Donor CD4+ T-cell numbers were determined between 7 and 30 days post infection. Data presented on a Log2 scale to emphasize the early acute contraction in all sites, but more prolonged contraction seen in the lung.
Another possible explanation for the increased contraction observed in the lung is that some of the effectors, presumably those that have received the highest dose of antigen stimulation for the longest (51, 52), are programmed to die either autonomously following restimulation or by withdrawal of stimulation (27, 53, 54). The effectors that migrate to the lung could be enriched in that subset. A related hypothesis is that effectors secreting certain cytokines and other factors commit cytokine-induced suicide following induction of cytokine production (49, 55). A contrasting hypothesis is that effectors, which make or respond best to survival cytokines like IL-2, are concentrated in the periphery and that when they recognize antigen they survive at higher rates (56–59). Since contraction of effector T cells is an essential component of a successful immune response, we suggest it will involve multiple mechanisms that are closely regulated to both clear effectors that are no longer necessary nor desirable and yet leave behind a small population of resting memory cells that have been ‘educated’ to respond optimally in a secondary encounter. Below, we describe these potential mechanisms in more detail and analyze whether what we know about CD4+ T-cell contraction fits with some or all of them in the remainder of this discussion.
Activation-induced cell death
Death of effectors may be triggered through TCR interaction with specific antigen leading to enhanced contraction relative to that due to passive or spontaneous death to be discussed further on. As is the case in passive apoptotic death due to antigen and growth factor withdrawal, the susceptibility of responding CD4+ T cells to activation-induced cell death (AICD) is influenced by both cell-intrinsic and cell-extrinsic variables (19–21). The relative importance of AICD versus antigen and growth factor withdrawal in the contraction of CD4+ T-cell populations is not yet clear and most likely differs dramatically depending on a variety of factors including the tissue environment (48), surface molecule expression of responding cells (60–63), and costimulatory ligands encountered (64–66). The major pathway of AICD involves interaction of Fas (CD95) on the target cell with Fas ligand (FasL) either on the same or different cells (19). This pathway is well established in vitro in murine and human systems (67–70) but seems to be less prominent in in vivo studies (71–74).
Availability of antigen is obviously one critical factor in determining the extent of AICD. When T-cell effectors generated in the periphery reach the lung of influenza-infected mice, they are exposed to high levels of viral antigen on both classical APCs (75–77) and on infected cells, mainly epithelial cells, which become MHC class II positive (78, Hernandez, Brown and Swain, unpublished data). This antigen-specific restimulation of effectors in the lung can be seen to trigger cytokine production, which is clearly observed (28), and to elicit other effector functions that contribute to viral clearance (79). This same encounter is also likely to trigger the AICD of those effectors that are susceptible and hence contribute to the contraction of the responding population. In unpublished studies, we find little or no role for Fas in the contraction of CD4+ T cells that have become effectors in vivo and are in the lung, after influenza infection. This could be related to the high levels of CD43 expressed on these cells (80), as high CD43 expression has been shown to confer resistance of CD4+ T cells to Fas-mediated death signals (63). Similarly, recent observations in an influenza model point towards engagement of CD44 as a critical signal in protecting highly activated Th1-polarized CD4+ T cells from Fas-dependent apoptosis and facilitating development of memory populations (81). Several other studies have also found significant AICD in the absence of Fas signaling, and it is possible that a wide variety of death pathways including perforin-dependent (82), TCR proximal kinase-mediated (20, 83), and exposure to reactive oxygen species (ROS) can play a role (84).
Regulation of AICD by Th polarization
Levels of susceptibility to AICD differ dramatically between differentially polarized Th subsets. It is well established that Th1-polarized effectors are more susceptible to AICD than Th2 cells (53, 85, 86). More recently, it has been suggested that Th17-polarized populations generated in vitro are also more resistant than Th1 populations to AICD (87). Most studies evaluate AICD in vitro, and few in vivo studies of AICD, especially in infectious models, have been reported. The enhanced susceptibility to AICD observed in Th1 populations is likely due to differential expression of regulation of several molecules including cytotoxic T-lymphocyte antigen-4 (CTLA-4), CD43, FasL, and granzyme B (53, 82, 85–88). Interestingly, in our and other studies (28, 80), each of these factors is upregulated significantly on lung effectors relative to those in the peripheral lymphoid organs, correlating with the greater contraction seen in the lung. Similarly, enhanced susceptibility of Th1-populations compared to Th17 populations to AICD has been correlated with reduced levels of cellular FADD-like interleukin-1β-converting enzyme inhibitory protein (c-FLIP) (89), a well-established anti-apoptotic molecule that can inhibit death receptor signaling (90, 91). Our studies indicate that the CD4 effectors in the lung of influenza-infected mice are strongly Th1-polarized, while effectors in the periphery are less strongly polarized, as indicated by the fact they make less IFNγ and more IL-2 (28). If AICD is an important contributor to contraction, the polarization of effectors in the lung could help to explain why the rate of contraction is greater there than in the peripheral lymphoid organs. Antigen and cytokine withdrawal-mediated programmed cell death of effectors, to be discussed below, is also influenced by Th subset polarization.
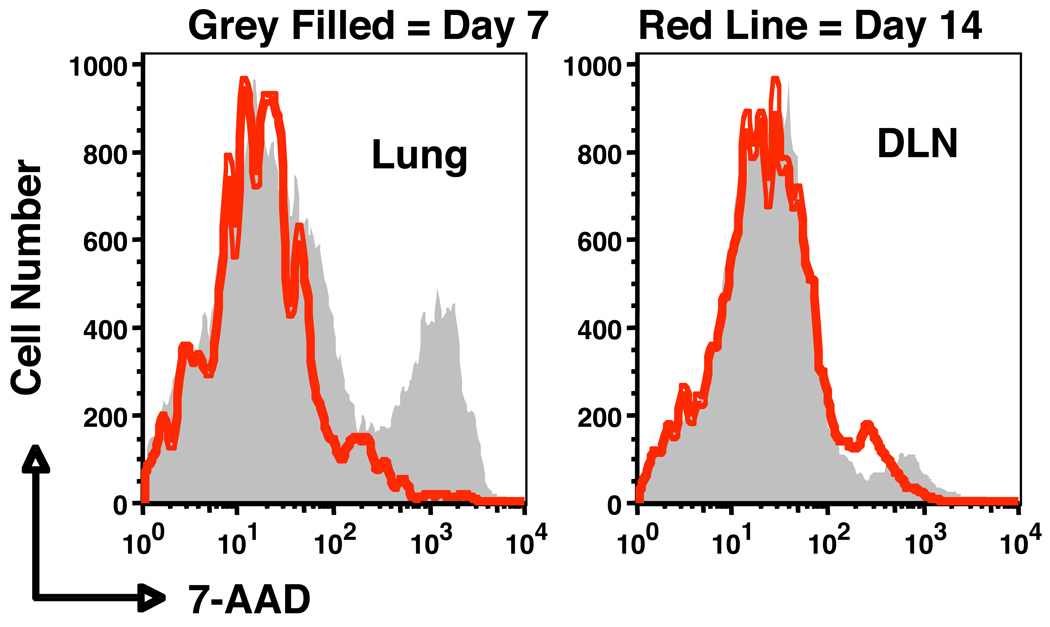
To what extent AICD actually influences contraction of CD4+ T cells following influenza infection has not been determined. To evaluate if effectors from the lung are indeed more susceptible to AICD than those in the periphery and to confirm that peak susceptibility is seen at day 7 just as contraction begins and is reduced at day 14 when contraction has largely finished, we transferred naive HNT cells to Thy-disparate hosts that were subsequently infected with influenza and harvested donor-enriched T cells from lung and MLN at 7 and 14 days after infection. We restimulated the isolated populations with anti-CD3 and then added 7-AAD and gated on the donor CD4+ T cells to evaluate whether the restimulation induced AICD (Fig. 3). Indeed, ex vivo restimulation induced substantial death in the 7 day donor CD4+ effector population from the lung but less in those cells isolated from the MLN, and susceptibility to AICD was much less in both donor cell populations recovered after 14 days. This result supports the concept that effectors in the infected tissue do have a higher susceptibility to AICD at the peak of the anti-influenza response, when they are most activated. AICD is likely to play a less prominent role in contraction following resolution of those infections in which antigen levels are low at the time effectors are most numerous or in the site where they reside (19). Instead, it would seem that AICD may play the most prominent role in regulating contraction during situations in which high amounts of antigen are present for prolonged periods, such as chronic infection or situations of autoimmunity where T-cell exhaustion must also be taken into consideration (92, 93). We are currently evaluating the extent to which specific antigen is necessary for contraction of CD4+ effectors in influenza.
Fig. 3. Lung-resident effector CD4+ T cells are most susceptible to AICD during influenza infection.
Naive HNT CD4+ T cells were transferred to naive hosts, which were then inflected with influenza. At 7 (shaded) and 14 (red line) days post infection, donor CD4+ T cells were recovered from the lung or draining lymph nodes (DLN). Cells were then restimulated with anti-CD3 for 20 hours and stained with 7-AAD to detect apoptotic cells.
Regulation of AICD by cytokines
Another critical factor regulating AICD in T-cell populations is activation status. In vitro, only highly activated and cycling effectors are susceptible to AICD, and even short periods of rest dramatically reduce the amount of AICD observed upon restimulation (20, 53, 94, 95) (Fig. 4). Since effectors in vivo quickly become resting in the absence of antigen (54, 96), we predict AICD susceptibility is also transient in vivo and only applies to activated effectors, not resting memory cells. Several cytokines can influence the relative susceptibility of effectors to AICD; however, the roles of each individual cytokine may depend on the cytokine milieu present during priming in addition to conditions present during antigen restimulation. Somewhat paradoxically, IL-2, which is a potent growth factor of effectors, has been shown to play a major role during priming in sensitizing cells to AICD (97–99). Triggering of the IL-2R upregulates FasL via a pathway involving the IL-2 inducible T-cell kinase (Itk) (99–101). Again, somewhat paradoxically given the reduced susceptibility of Th2 cells to AICD (53, 85, 86), the Th2-associated cytokine IL-4 has been shown to enhance AICD (102), sensitizing cells in an IL-2-dependent manner also involving upregulation and triggering of the IL-2R (CD25). Furthermore, both IL-2 and IL-4 can enhance AICD of effectors by initiating the degradation of the apoptosis inhibitor c-FLIP (20, 102). In vitro, IL-2 and TGFβ can synergize to protect Th1 but not Th2 effectors from AICD (86, 103), but it has not yet been established whether TGFβ plays a similar role in vivo. TNF, a cytokine that is produced by most T-cell subsets, has also been shown to enhance susceptibility to AICD (50, 104–106), but depending on the signaling pathway engaged by ligation of the TNF receptor, different biological responses such as resistance to AICD may ensue (107, 108). This in conjunction with many non-redundant AICD-inducing pathways may explain why, in some instances, TNF receptor-deficient mice have levels of AICD comparable to wildtype mice (109). Hence, the contribution of TNF to AICD may be dependent upon both the cytokine milieu in which responding effectors are present in addition to the model system employed, both of which may result in the triggering of unique intrinsic and extrinsic signaling pathways.
Fig. 4. Effector CD4+ T cells rapidly loose susceptibility to AICD.
(A) In vitro-generated Th1 effectors or effectors that rested for 3 days in vivo were either left unstimulated or restimulated for 20 h with peptide-pulsed antigen-presenting cells (APCs). Significantly fewer rested effectors underwent AICD, as determined by assessing a variety of markers associated with apoptotic death. (B) Naive HNT CD4+ T cells were compared to activated Th1 and Th2 effectors (generated in vitro), as well as memory cells (generated following adoptive transfer of in vitro effectors into antigen-free hosts). All populations were restimulated in vitro with antigen and APC, and CD4+ T-cell apoptosis in responding cultures was assessed by TUNEL staining.
IL-10 has also been demonstrated to enhance Fas-dependent T-cell death through IL-10-dependent upregulation of Fas ligand (110). During influenza infection, we observe that IL-10 levels are maximal in the lung and peak during the contraction phase at around day 7–8 (111). Furthermore, our results show that highly activated CD4+ T-cell effectors represent a major source of IL-10. To test if IL-10 contributes to contraction of CD4+ T cells responding against influenza, we transferred WT HNT donor cells to WT hosts or IL-10-deficient HNT donors to IL-10-deficient hosts infected with a sublethal dose of A/PR8, and enumerated donor cells at the peak of the response (day 7) and on day 14. We observed no difference in the number of donor cells responding in the presence or absence of IL-10 at either timepoint, suggesting a minimal role for IL-10 in the contraction of influenza-specific CD4+ T-cell effectors (Strutt, McKinstry, and Swain, unpublished observations).
Lack of role for IFNγ in CD4+ T-cell contraction in the influenza model
In addition to having a significant role in models of antigen-independent cell death of CD4+ T cells, to be discussed, several studies have implicated IFNγ as a major sensitizing factor to AICD (49, 112, 113). Both in vitro and in vivo models have shown IFNγ to impact susceptibility of CD4+ T cells to AICD. Given its central role in orchestrating apoptosis in responding CD4+ T cells, it is not surprising that aberrant contraction has been observed in vivo in the absence of IFNγ both in situations of chronic and acute infections, IFNγ-deficient cells appear resistant to contraction (49, 113). This is likely due to the fact that IFNγ globally regulates expression of a tremendous number of genes including several involved in intracellular apoptotic machinery, including signal transducer and activator of transcription-1 (STAT-1)-dependent upregulation of caspase-8 as well as several extracellular apoptosis-associated ligands (49, 112, 114, 115). Furthermore, given the early kinetics of IFNγ receptor expression on responding CD4+ T cells (116, 117), it is likely that the impact of IFNγ on contraction of CD4+ T-cell effectors occurs during the early priming phases of the response in a similar manner, as has been proposed for the action of IL-2. This mode of action might also help to explain why IFNγ-producing CD4+ T-cell effectors clearly accumulate during responses against many pathogens including influenza.
However, a pro-apoptotic role for IFNγ during CD4+ T-cell responses in vivo is not universal. In fact, studies employing LCMV show that IFNγ signaling is required for maximal antiviral CD4+ T-cell memory (118). Our studies with influenza suggest that IFNγ also plays a minimal role in coordinating the contraction of effector CD4+ T cells. To assess such a role, we transferred WT naive HNT CD4 T cells into WT hosts or IFNγ-deficient HNT CD4+ T cells into IFNγ-deficient hosts and infected with influenza. Both donor CD4+ T-cell populations reached similar peak numbers in both hosts on day 7 of the response in the lung as well as the spleen and draining lymph nodes. When assessed on day 14 post-infection, both populations were again present at similar numbers, suggesting a similar contraction program (Fig. 5) and its independence from autocrine or paracrine IFNγ. Thus, the impact of IFNγ on contraction of responding CD4+ T cells can be positive, negative, or negligible depending on the nature of the challenge, but certainly dramatic CD4+ T-cell contraction can occur without IFNγ.
Fig. 5. IFNγ does not appreciably impact contraction of responding CD4+ T cells during influenza infection.
Naive wildtype (WT) HNT CD4+ T cells were transferred to WT BALB/c hosts or naive IFNγ KO HNT cells transferred to IFNγ KO hosts. Mice were then inflected with a sublethal dose of influenza. Donor CD4+ T-cell numbers in the lung were determined at 7 and 14 days post-infection.
The impact of IFNγ on contraction could be differentially regulated in different experimental models in several ways. For example, it is possible that signals through the IFNγ receptor must synergize with other signals to induce maximal apoptosis in effectors, and the presence of or levels of these signals is likely to vary considerably depending on the nature of the challenge. It is also possible that factors produced by responding CD4+ T cells that counteract the IFNγ-mediated cell death, such as the immunity related GTPase Irgm1 (119), are differentially induced in responding IFNγ-producing populations.
Growth factor and antigen withdrawal in contraction of CD4+ T cells
Changes in the environment in which CD4+ T cells are responding can play a central role in orchestrating contraction. In contrast to mechanisms of AICD that are induced by antigen recognition, just discussed, withdrawal of antigen and the coincident removal of growth and survival factors may induce contraction especially in activated effector cells that already express substantial levels pro-apoptotic proteins and caspases (21, 23, 71). Both TCR signaling and signals from IL-7 and other IL-2Rγ-binding cytokines can induce the upregulation of anti-apoptotic proteins such as Bcl-2 and Bcl-XL (120–124) and the downregulation of proapoptotic components such as Bim and Puma (125–127), which prevent the Bcl-2 family members from blocking Bad and Bax that directly induce caspases (128, 129). Studies in several different models show that Bim plays a central role coordinating cell death and that its expression level is a critical regulator of T-cell contraction (73, 74, 130). Since the pro- and anti-apoptotic molecules exist in equilibrium, the expressed level in an effector is critical to its fate. Those levels are often determined during initial antigen encounter and effector differentiation (27). Below we briefly discuss how antigen withdrawal and changes in the inflammatory environment, often referred to as 'death by neglect' or 'passive cell death', can impact the contraction of effector CD4+ T cells. How distinct effector populations respond to these signals can differ dramatically.
Perhaps the most obvious factor that influences contraction of effector T cells in vitro is the removal of antigen. After antigen withdrawal from highly activated in vitro-generated CD4+ T-cell effector cultures, a significant number of cells undergo apoptosis within 2–3 days (53, 54, 96). Similar kinetics of contraction occur when highly activated in vivo-generated effectors are isolated and plated in vitro without antigen (131). Similar kinetics of contraction are observed when antigen levels wane in vivo; as shown in Fig. 1 and discussed above, numbers of CD4+ T-cell effectors drop dramatically during the period of viral clearance and thereafter following influenza challenge. Such similar patterns of contraction within effector populations both in vitro and in vivo suggest that some common programmed cell death pathways are initiated upon antigen withdrawal, although growth factors are removed as well in most models.
IL-2 signaling during antigen-specific priming of CD4+ T cells both in vitro and in vivo (132, 133) has been shown to significantly enhance the long-term survival of effectors after antigen withdrawal (134, 135). A similar impact on CD4+ T-cell survival after antigen clearance has also been reported for CD28 signaling during priming (64, 136–138). The enhanced survival of effectors primed in the presence of IL-2 signals has been shown to be due in some cases to IL-2-dependent upregulation of IL-7 receptor (CD127) (139). This mechanism is consistent with earlier findings showing that access to IL-7 promotes optimal survival of effector CD4+ T cells during the transition to memory, when growth factor signals such as those delivered through CD25 are no longer available (124, 140). However, other factors must also come into play, as recent studies have shown IL-7 is not likely to be the limiting factor during the transition CD4+ T-cell contraction in a response to vaccinia virus (141). IL-15 could represent such a factor, as studies suggest that IL-15 signaling can rescue responding T cells from AICD (142) and promote survival of CD4+ and CD8+ T-cell memory (36, 143).
Paradoxically, other studies have suggested that IL-2 drives susceptibility to programmed death as well as AICD (97–99). This apparent discrepancy can be resolved by considering the status of the cells in question. IL-2 is critical in vitro for the full differentiation of both Th1 and Th2 subsets (51, 144), including the induction of many of the components of death pathways (99–101). This explains, at least in part, why it is those differentiated effectors that are susceptible to programmed death. Layered on top of this susceptibility due to expression of death pathway components is induction of expression of receptors for survival factors that will prevent death, if these factors are available (139). Thus, IL-2-dependent mechanisms can both enhance and moderate the degree of programmed death seen.
To test if IL-2 production by CD4+ T cells regulates contraction during responses against influenza, we transferred naive WT or IL-2-deficient CD4+ T cells specific for OVA to unprimed WT hosts and infected with a sublethal dose of A/PR8-OVA. Similar to observations in numerous other models (133), WT and IL-2-deficient expanded to a similar degree, with similar kinetics in all organs tested by day 7 post-infection (McKinstry, Strutt, and Swain, unpublished observations). However, when donor populations were assessed at day 14 post-infection, significantly fewer IL-2-deficient donor cells were detected compared to WT donors. Given the fact that IL-2-deficient donor CD4+ T cells were responding in WT mice, these results suggest that autocrine IL-2 production by responding CD4+ T cells during influenza challenge has an important role in regulating contraction.
Regulation of programmed cell death by Th polarization
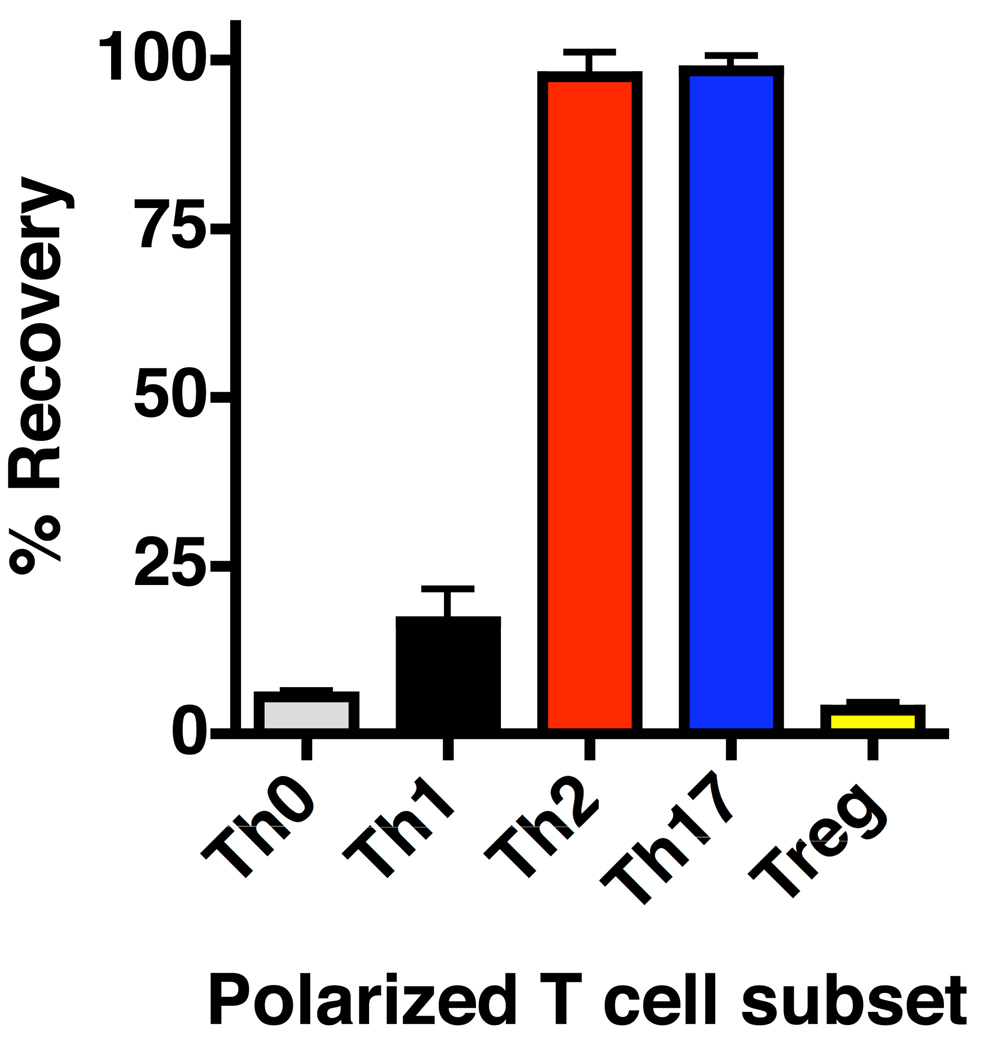
While some factors, such as decreased Bmi1 expression (145), seem to negatively regulate survival of effectors upon antigen withdrawal independently of Th-polarization, several observations demonstrate that the degree of programmed contraction varies significantly between different effector populations specific for the same antigen. For example, well-polarized Th1, Th2, Th17, and unpolarized effectors generated from naive TCR transgenic cells in vitro in the presence of IL-2 have different rates of apoptotic death following antigen withdrawal. Compared to Th1 and especially unpolarized (Th0) populations, significantly more Th2 and Th17 survive 3 days post antigen withdrawal (Fig. 6). Thus, availability of polarizing cytokines and APC populations during priming that impact Th-polarization contribute to the sensitivity of CD4+ T-cell effectors to apoptotic death following resolution of infection. This may be an important consideration in optimizing the design of non-replicating vaccination strategies in which antigen is cleared rather rapidly, since the number of memory T cells may be significantly influenced by routes of immunization (146) in addition to adjuvants that favor particular Th subset survival (147). These same considerations also influence the susceptibility to AICD, as already discussed.
Fig. 6. Th-polarization impacts antigen-independent contraction of CD4+ T-cell effectors.
Naive HNT cells were cultured with antigen and antigen presenting cells under Th0, Th1, Th2, Th17, or Treg polarizing conditions in vitro. After 4 days, well-polarized effectors were rested for three days in the absence of antigen and cytokines and the number of live cells determined (depicted as percentage of effector cells).
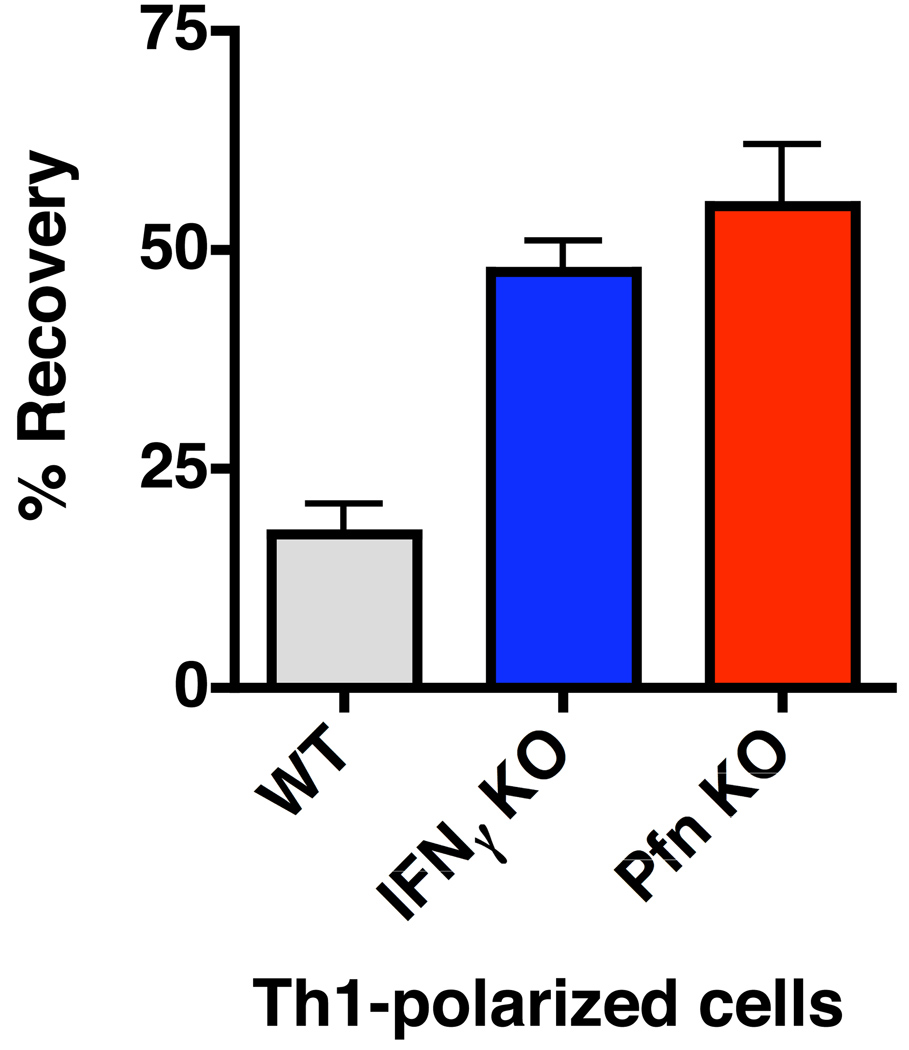
Cytokines produced by effectors and other Th-specific effectors can also significantly affect the degree of contraction observed upon antigen withdrawal. For example, in vitro Th1-polarized effector cultures generated from IFNγ-deficient TCR transgenic CD4+ T cells, while reaching similar peak numbers, undergo significantly less contraction following antigen withdrawal compared to those from WT cultured cells (Fig. 7). The enhanced survival of IFNγ-deficient cells is observed even when high concentrations of exogenous IFNγ are added to the priming milieu, suggesting that IFNγ signals during initial priming do not substantially impact passive cell death upon antigen withdrawal. Similarly, Th1-polarized effectors generated from perforin-deficient cells display a distinct survival advantage compared to WT cells of the same specificity upon antigen withdrawal.
Fig. 7. In vitro contraction of Th1-polarized effector populations is regulated by effector molecules.
Naive WT, IFNγ KO, or perforin KO HNT CD4+ T cells cultured under Th1-polarizing conditions in vitro. After 4 days, resulting effectors were rested for three days in the absence of antigen and cytokines and the number of live cells determined (depicted as percentage of effector cells).
These findings show that beyond signals experienced during priming, autocrine or paracrine signals experienced during the expansion phase can directly impact an effector cell’s fitness after the removal of antigen and growth factors. The strong impact of IFNγ on programmed cell death in vitro (Fig. 7) is seemingly at odds with the negligible role for IFNγ on the contraction of effectors during influenza infection (Fig. 5). These results might suggest that passive cell death pathways are not as critical as antigen-dependent mechanisms in regulating death of CD4+ T cells responding to influenza. More importantly, these results again highlight the difficulty of applying in vitro observations of contraction, where levels of antigen and inflammatory constituents can be controlled, to cell death in vivo during pathogen challenge, where the relative impact of individual pro-apoptotic signals are more difficult to define and are likely to flux.
Programmed cell death and residual antigen
Antigen clearance following the resolution of a primary immune response can set in motion the contraction of effector CD4+ T cells that is influenced both by imprinting during priming by signals that ultimately generate different subsets of CD4+ effectors, and also by growth and survival factors present at the effector stage. Since different sites, such as the site of infection and peripheral non-infected secondary lymphoid sites, are populated by distinct effector populations (28, 148) and since different sites likely have different antigen loads (46, 47) and cytokine environments, it is not surprising that contraction of effectors is more dramatic in the infected site than in secondary lymphoid tissues. While control of antigen is easily manipulated in vitro, its availability to T-cell populations in different sites in vivo is complex and less well understood. For example, residual antigen presentation has been observed following the resolution of several acute infections (41–44), including our own studies with influenza (41, 45). One unresolved question is whether there is sufficient antigen available to trigger autocrine cytokine production or only to generate cytokine-independent division? Another issue regarding persisting antigen is whether access to residual antigen is restricted to certain anatomical or cellular sites and if only certain T-cell subsets are able to migrate to those sites (76). Clearly, more analysis is needed to evaluate whether persisting antigen not associated with live pathogen plays a role in some aspects of T-cell contraction.
In most infectious models, it is difficult to ascertain the contribution of antigen withdrawal to contraction of effector CD4+ T-cell populations in vivo, because there is a coordinated downregulation of inflammation coincident with clearance (71). As many elements of inflammation can promote T-cell proliferation and survival (149–152), an unknown fraction of contraction may be dependent on growth factor and/or survival factor withdrawal (27, 73). Indeed, as mentioned earlier, responding effectors and the resting memory cells derived from them depend on IL-7R and IL-2Rγ-mediated survival signals so the level of CD127 expression influences the extent of contraction (122, 124, 139), illustrating the importance of access to proper cytokine signals. It will be important to determine whether any other cytokines or chemokines associated with inflammatory sites influence effector and/or memory survival, as there is some evidence that bystander inflammatory environments can prolong the survival of effector T-cell populations (153).
The effector to memory transition of CD4+ T cells
Coincident with the contraction phase of activated CD4+ T cells is the initiation of the transition of surviving effectors to resting memory cells. Both our laboratory and others have shown that antigen withdrawal marks a critical starting point in this transition process and that few other signals are required (53, 54, 96). A remarkable feature of the effector to memory transition is its rapid kinetics. Employing in vitro-generated populations, we found that effectors rested for 3 days in the absence of antigen and cytokine, closely resemble long-term memory cells as judged by cell-surface phenotype, key functional attributes, and by gene expression profiles (53). A similar rapid transition is seen when we transfer effectors to antigen-free hosts (53, 96, 140). More recently, we have also shown that 3 day rested effectors respond similarly to influenza challenge, as virus-specific memory cells generated through heterosubtypic priming (111, our unpublished observations). Another striking element of this transition program is that Th1-, Th2-, Th0-, and Th17-polarized effector populations all give rise, with identical kinetics, to corresponding memory populations. Similarly, we have shown that 3 days rest in vitro is sufficient for highly activated CD4+ T-cell effectors responding to influenza in the lung to assume phenotypic and functional criteria associated with long-term memory cells (131). Thus, beyond the rapid contraction of large activated effector populations that can pose a risk to the host, surviving effector cells rapidly transition to rest and the more benign memory mode during the termination of immune responses.
As the effector to memory transition often occurs during the period of contraction, it is important to ask which effector cells survive. The answer to this question remains unclear. Our own results and those of others (53, 154, 155) suggest that memory cells arise stochastically from effectors progressing along a linear differentiation pathway. However, it seems clear that effectors can reach a terminally differentiated state (156) and that certain molecules capable of influencing cell death, such as IFNγR, can be differentially expressed on dividing T cells at very early phases of the response (157).
Contraction during secondary responses: role of IL-2
While contraction following primary CD4+ T-cell responses has been analyzed in several different model systems, contraction of T cells during recall responses is less well studied. As phenotypic, functional, and gene expression differences distinguish memory from naive CD4+ T cells (131), the signals impacting the contraction phase could also be distinct. Furthermore, significant heterogeneity has been observed in both human and mouse memory T-cell populations, as defined by phenotypic and functional criteria (158–160). In particular, several recent studies have found differences in the ability of memory CD4+ T cells to produce IL-2 in addition to other effector cytokines (56, 161–165), and memory CD4+ T cells producing IL-2 in addition to IFNγ have been correlated with improved protection against a variety of pathogens compared to memory cells producing only IFNγ (166). Given the central role of IL-2 in both sensitizing responding CD4+ T cells to AICD and in rescuing them from antigen-independent as well as dependent apoptosis, it is possible that IL-2-positive versus -deficient memory populations specific for the same antigen would have different survival characteristics following in vivo antigen restimulation. This could have important consequences for vaccine design, especially when involving prime-boost regiments.
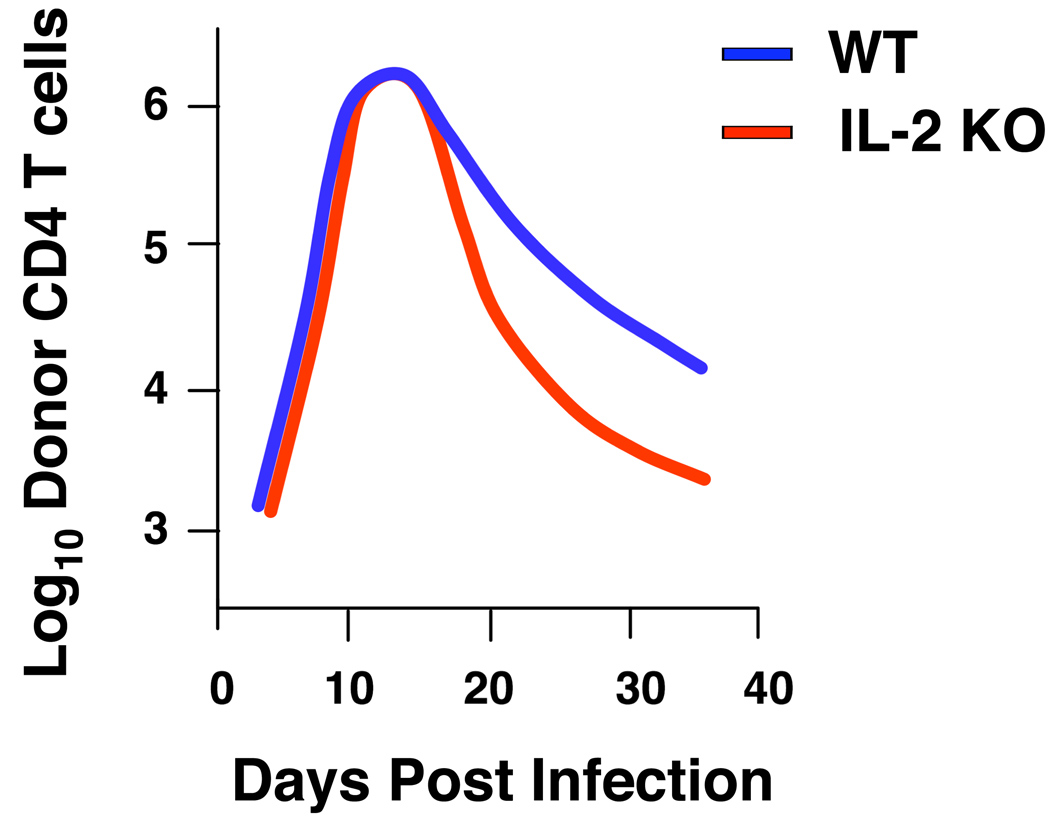
To test this hypothesis, we generated memory CD4+ T cells specific for influenza in vitro from wildtype or IL-2-deficient TCR Tg CD4+ T cells. We generated effectors in the presence of high amounts of exogenous IL-2, so that both populations were exposed to IL-2 during priming. Indeed, we observed similar expansion of both populations after 4 days of culture and also similar numbers of memory cells following a period of in vitro rest (authors’ unpublished observations). We transferred equal numbers of wildtype or IL-2-deficient memory CD4+ T cells to naive wildtype mice. We then infected with influenza and observed, surprisingly, that both populations reached similar peak numbers in the lung, at levels significantly above those observed in primary responses. This finding suggests that autocrine IL-2 production is not involved in driving enhanced memory CD4+ T-cell responses in vivo. After expansion, the wildtype memory effector cells underwent extensive contraction, confirming that secondary effectors contract much like those generated during primary responses. However, mirroring our observations with naive IL-2-deficient responders (authors’ unpublished observations), IL-2-deficient memory CD4+ T cells underwent significantly enhanced contraction (Fig. 8). This finding suggests that not all memory CD4+ T cells are equivalent in terms of resistance to apoptosis during the contraction phase of recall responses and that ability to produce IL-2 may substantially enhance resistance to multiple mechanisms of cell death in responding memory CD4+ T-cell populations. Whether memory populations respond to other signals impacting antigen-dependent and -independent apoptosis pathways in a similar or different manner than during primary responses will require further study.
Fig. 8. IL-2 regulates contraction of memory-effectors.
Naive WT or IL-2 KO CD4+ T cells were cultured for 4 days under Th1-polarizing, including exogenous IL-2 conditions and then rested for 3 days in the absence of antigen and cytokines to generate memory. WT and IL-2 KO memory cells were transferred to WT hosts, then inflected with influenza, and donor CD4+ T-cell numbers were determined between.
Concluding remarks
How the death of activated T cells is regulated in vivo remains unclear. It is evident that signals impacting the contraction of CD4+ T cells occur both during the effector phase of immune responses and also during priming, where cells are programmed to different fates. The evidence so far suggests that both antigen-dependent and antigen-independent pathways drive cell-death during the termination of productive CD4+ T-cell responses (Fig. 9). Thus, multiple mechanisms have the potential to shape contraction of CD4+ T-cell populations, but determining the relative contribution of discrete pathways during in vivo responses against pathogens is difficult. This is compounded by the fact that the influence of signals impacting contraction can differ dramatically depending on the pathogen challenge.
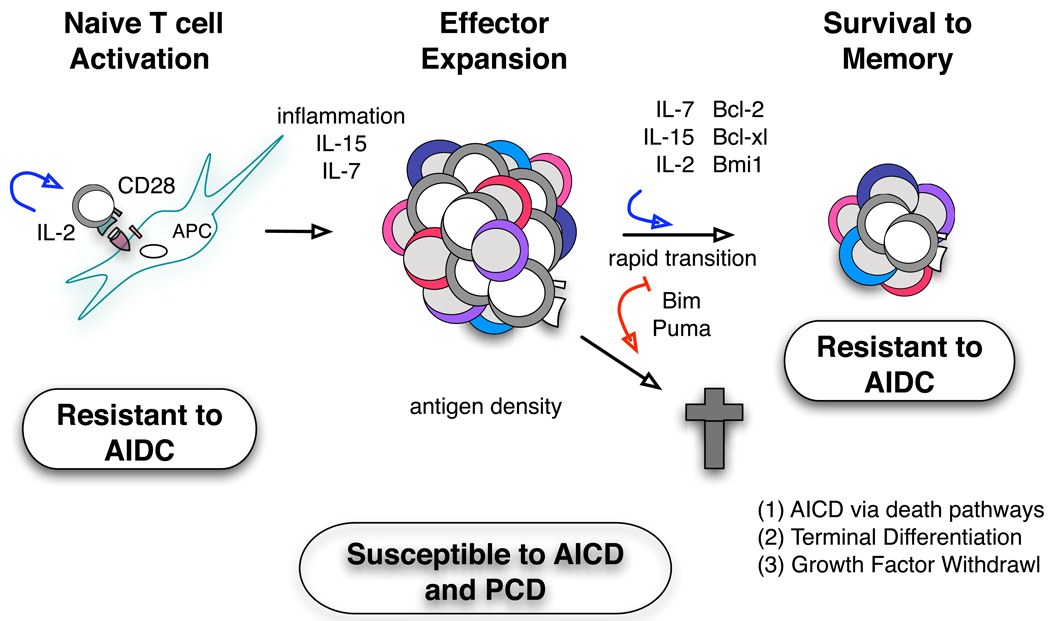
Fig. 9. Signals impacting contraction of CD4+ T cells in vivo.
In the schematic, we point out key roles for factors that influence contraction at different stages of response (red arrows denote increased contraction, and blue arrows denote reduced contraction). First, in regulating the programming of effectors to susceptibility to contraction by inducing maximal differentiation and migration to sites of infection (to the lung in the influenza model). Second, at the effector stage where availability of antigen, and autocrine and paracrine cytokines can further modulate the extent of death and degree of contraction, determining what fraction of the population survives to memory.
We feel that influenza infection provides an excellent model in which to study the regulation of contraction of CD4+ T cells for several reasons. First, a tremendous degree of contraction occurs during the period of just a few days. Second, antigen is confined largely to the lung, and antigen levels fall precipitously during the period of contraction. Third, contraction can be assessed in different organs in which cohorts of activated CD4+ T cells are exposed to very different stimuli in terms of levels of antigen and inflammation. Finally, the mouse model of influenza infection allows the implementation of powerful tools for the manipulation of critical signals impacting cell death.
Acknowledgements
The authors and experiments were supported by NIH grants: to Swain R01AI76534 and P01AI46530 and RNS061014 to Cory Teuscher.
References
- 1.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999;11:163–171. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins MK, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Openshaw P, et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Strutt TM, McKinstry KK, Swain SL. Functionally diverse subsets in CD4 T cell responses against influenza. J Clin Immunol. 2009;29:145–150. doi: 10.1007/s10875-008-9266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain SL, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 13.Elson CO, et al. Experimental models to study molecular mechanisms underlying intestinal inflammation. Ann NY Acad Sci. 1998;859:85–95. doi: 10.1111/j.1749-6632.1998.tb11113.x. [DOI] [PubMed] [Google Scholar]
- 14.Swain SL, et al. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 15.Croft M, Duncan DD, Swain SL. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubey C, Croft M, Swain SL. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- 17.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 18.Green DR. Overview: apoptotic signaling pathways in the immune system. Immunol Rev. 2003;193:5–9. doi: 10.1034/j.1600-065x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 19.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66:52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Lenardo M, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 22.Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 23.Arch RH, Thompson CB. Lymphocyte survival--the struggle against death. Annu Rev Cell Dev Biol. 1999;15:113–140. doi: 10.1146/annurev.cellbio.15.1.113. [DOI] [PubMed] [Google Scholar]
- 24.Li C, et al. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 25.Scott B, et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman E, et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell TJ, et al. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clin Immunol. 2004;113:89–100. doi: 10.1016/j.clim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Doherty PC, Topham DJ, Tripp RA. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 33.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 34.De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171:3928–3935. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- 35.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 38.Gray D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 39.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 41.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray PM, Reiner SL, Smith DF, Kaye PM, Scott P. Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major. J Immunol. 2006;177:925–933. doi: 10.4049/jimmunol.177.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fazilleau N, et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 44.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 46.Eichelberger MC, Wang ML, Allan W, Webster RG, Doherty PC. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72:1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XZ, Stepp SE, Brehm MA, Chen HD, Selin LK, Welsh RM. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity. 2003;18:631–642. doi: 10.1016/s1074-7613(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 49.Li X, McKinstry KK, Swain SL, Dalton DK. IFN-gamma acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J Immunol. 2007;179:939–949. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller S, et al. Soluble TNF-alpha but not transmembrane TNF-alpha sensitizes T cells for enhanced activation-induced cell death. Eur J Immunol. 2009;39:3171–3180. doi: 10.1002/eji.200939554. [DOI] [PubMed] [Google Scholar]
- 51.Rogers PR, Huston G, Swain SL. High antigen density and IL-2 are required for generation of CD4 effectors secreting Th1 rather than Th0 cytokines. J Immunol. 1998;161:3844–3852. [PubMed] [Google Scholar]
- 52.Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL. Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med. 2005;201:1101–1112. doi: 10.1084/jem.20041852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harbertson J, Biederman E, Bennett KE, Kondrack RM, Bradley LM. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J Immunol. 2002;168:1095–1102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- 55.Foulds KE, et al. IFN-gamma mediates the death of Th1 cells in a paracrine manner. J Immunol. 2008;180:842–849. doi: 10.4049/jimmunol.180.2.842. [DOI] [PubMed] [Google Scholar]
- 56.Darrah PA, et al. Multifunctional T(H)1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 57.Panus JF, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific T helper cell function: differential cytokine expression in primary and memory responses. J Exp Med. 2000;192:1301–1316. doi: 10.1084/jem.192.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kristensen NN, Christensen JP, Thomsen AR. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J Gen Virol. 2002;83:2123–2133. doi: 10.1099/0022-1317-83-9-2123. [DOI] [PubMed] [Google Scholar]
- 59.Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J Immunol. 2001;166:4627–4633. doi: 10.4049/jimmunol.166.7.4627. [DOI] [PubMed] [Google Scholar]
- 60.Somma F, Tuosto L, Gilardini Montani MS, Di Somma MM, Cundari E, Piccolella E. Engagement of CD4 before TCR triggering regulates both Bax- and Fas (CD95)-mediated apoptosis. J Immunol. 2000;164:5078–5087. doi: 10.4049/jimmunol.164.10.5078. [DOI] [PubMed] [Google Scholar]
- 61.Algeciras A, Dockrell DH, Lynch DH, Paya CV. CD4 regulates susceptibility to Fas ligand- and tumor necrosis factor-mediated apoptosis. J Exp Med. 1998;187:711–720. doi: 10.1084/jem.187.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foger N, Marhaba R, Zoller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol. 2000;30:2888–2899. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 63.He YW, Bevan MJ. High level expression of CD43 inhibits T cell receptor/CD3-mediated apoptosis. J Exp Med. 1999;190:1903–1908. doi: 10.1084/jem.190.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. [PubMed] [Google Scholar]
- 65.Weinberg AD, Vella AT, Croft M. OX-40: life beyond the effector T cell stage. Semin Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- 66.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Brunner T, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 68.Ju ST, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 69.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 70.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Lohman BL, Razvi ES, Welsh RM. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol. 1996;70:8199–8203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hildeman DA, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 74.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 77.Mount AM, Smith CM, Kupresanin F, Stoermer K, Heath WR, Belz GT. Multiple dendritic cell populations activate CD4+ T cells after viral stimulation. PLoS One. 2008;3:e1691. doi: 10.1371/journal.pone.0001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 80.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 Regulates Survival and Memory Development in Th1 Cells. Immunity. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma V, Delgado M, Ganea D, Granzyme B. a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176:97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- 83.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 84.Hildeman DA, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe N, et al. Th1 and Th2 subsets equally undergo Fas-dependent and -independent activation-induced cell death. Eur J Immunol. 1997;27:1858–1864. doi: 10.1002/eji.1830270807. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X, et al. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi G, et al. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devadas S, et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Yu Y, et al. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 91.Kataoka T, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 92.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 93.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Li QS, Tanaka S, Kisenge RR, Toyoda H, Azuma E, Komada Y. Activation-induced T cell death occurs at G1A phase of the cell cycle. Eur J Immunol. 2000;30:3329–3337. doi: 10.1002/1521-4141(200011)30:11<3329::AID-IMMU3329>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 95.Riou C, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu H, Huston G, Duso D, Lepak N, Roman E, Swain SL. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol. 2001;2:705–710. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- 97.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 98.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 99.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 100.Xiao S, et al. FasL promoter activation by IL-2 through SP1 and NFAT but not Egr-2 and Egr-3. Eur J Immunol. 1999;29:3456–3465. doi: 10.1002/(SICI)1521-4141(199911)29:11<3456::AID-IMMU3456>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 101.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, et al. IL-4 potentiates activated T cell apoptosis via an IL-2-dependent mechanism. J Immunol. 2003;170:3495–3503. doi: 10.4049/jimmunol.170.7.3495. [DOI] [PubMed] [Google Scholar]
- 103.Genestier L, Kasibhatla S, Brunner T, Green DR. Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–239. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 105.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 106.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 107.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 108.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 109.Nguyen LT, McKall-Faienza K, Zakarian A, Speiser DE, Mak TW, Ohashi PS. TNF receptor 1 (TNFR1) and CD95 are not required for T cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur J Immunol. 2000;30:683–688. doi: 10.1002/1521-4141(200002)30:2<683::AID-IMMU683>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 110.Barreiro R, Luker G, Herndon J, Ferguson TA. Termination of antigen-specific immunity by CD95 ligand (Fas ligand) and IL-10. J Immunol. 2004;173:1519–1525. doi: 10.4049/jimmunol.173.3.1519. [DOI] [PubMed] [Google Scholar]
- 111.McKinstry KK, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haring JS, Harty JT. Aberrant contraction of antigen-specific CD4 T cells after infection in the absence of gamma interferon or its receptor. Infect Immun. 2006;74:6252–6263. doi: 10.1128/IAI.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 115.Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- 116.Bach EA, et al. Ligand-induced autoregulation of IFN-gamma receptor beta chain expression in T helper cell subsets. Science. 1995;270:1215–1218. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- 117.Pernis A, et al. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 118.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 119.Feng CG, et al. The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-gamma-induced cell death. Nat Immunol. 2008;9:1279–1287. doi: 10.1038/ni.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 121.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 122.Li XC, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 123.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 124.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 126.Erlacher M, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bauer A, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci USA. 2006;103:10979–10984. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bouillet P, Strasser A. Bax and Bak: back-bone of T cell death. Nat Immunol. 2002;3:893–894. doi: 10.1038/ni1002-893. [DOI] [PubMed] [Google Scholar]
- 129.Wei MC, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McKinstry KK, Strutt TM, Swain SL. The effector to memory transition of CD4 T cells. Immunol Res. 2008;40:114–127. doi: 10.1007/s12026-007-8004-y. [DOI] [PubMed] [Google Scholar]
- 132.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 133.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 134.Malek TR, Yu A, Scibelli P, Lichtenheld MG, Codias EK. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol. 2001;166:1675–1683. doi: 10.4049/jimmunol.166.3.1675. [DOI] [PubMed] [Google Scholar]
- 135.Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. J Immunol. 2004;172:5973–5979. doi: 10.4049/jimmunol.172.10.5973. [DOI] [PubMed] [Google Scholar]
- 136.Dooms H, Abbas AK. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol Rev. 2006;211:23–38. doi: 10.1111/j.0105-2896.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 137.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 138.Vella AT, et al. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J Immunol. 1997;158:4714–4720. [PubMed] [Google Scholar]
- 139.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7Ralpha-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting Edge: Limiting amounts of IL-7 do not control contraction of CD4+ T cell responses. J Immunol. 2007;178:4027–4031. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamashita M, et al. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J Exp Med. 2008;205:1109–1120. doi: 10.1084/jem.20072000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mitchell TC, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 148.Baumgarth N, Kelso A. Functionally distinct T cells in three compartments of the respiratory tract after influenza virus infection. Eur J Immunol. 1996;26:2189–2197. doi: 10.1002/eji.1830260934. [DOI] [PubMed] [Google Scholar]
- 149.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174:4761–4767. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 151.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–5796. [PubMed] [Google Scholar]
- 152.Marrack P, et al. T-cell survival. Immunol Rev. 1998;165:279–285. doi: 10.1111/j.1600-065x.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 153.Mitchell T, Kappler J, Marrack P. Bystander virus infection prolongs activated T cell survival. J Immunol. 1999;162:4527–4535. [PubMed] [Google Scholar]
- 154.Lohning M, et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 156.Wu CY, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 157.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 158.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 159.Kassiotis G, Stockinger B. Anatomical heterogeneity of memory CD4+ T cells due to reversible adaptation to the microenvironment. J Immunol. 2004;173:7292–7298. doi: 10.4049/jimmunol.173.12.7292. [DOI] [PubMed] [Google Scholar]
- 160.Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol. 2007;179:64–70. doi: 10.4049/jimmunol.179.1.64. [DOI] [PubMed] [Google Scholar]
- 161.Aagaard CS, Hoang TT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol. 2009;183:2659–2668. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- 162.Beveridge NE, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huaman MC, Mullen GE, Long CA, Mahanty S. Plasmodium falciparum apical membrane antigen 1 vaccine elicits multifunctional CD4 cytokine-producing and memory T cells. Vaccine. 2009;27:5239–5246. doi: 10.1016/j.vaccine.2009.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trumpfheller C, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]