Abstract
Pluripotent stem cells (PSCs) express telomerase and have unlimited proliferative potential. To study telomerase activation during reprogramming, 3 classes of embryonic stem cell (ESC)-like clones were isolated from mouse fibroblasts containing a transgenic hTERT reporter. Class I expressed few pluripotency markers, whereas class II contained many, but not Oct4, Nanog, and Sox2. Neither class of cells differentiated efficiently. Class III cells, the fully reprogrammed induced PSCs (iPSCs), expressed all pluripotency markers, formed teratomas indistinguishable from those of mESCs, and underwent efficient osteogenic differentiation in vitro. Interestingly, whereas the endogenous mTERT gene expression was only moderately increased during reprogramming, the hTERT promoter was strongly activated in class II cells and was further elevated in class III cells. Treatment of class II cells with chemical inhibitors of MEKs and glycogen synthase kinase 3 resulted in their further reprogramming into class III cells, accompanied by a strong activation of hTERT promoter. In reprogrammed human cells, the endogenous telomerase level, although variable among different clones, was dramatically elevated. Only in cells with the highest telomerase were telomeres restored to the lengths in hESCs. Our data, for the first time, demonstrated that the hTERT promoter was strongly activated in discrete steps, revealing a critical difference in human and mouse cell reprogramming. Because telomere elongation is crucial for self-renewal of hPSCs and replicative aging of their differentiated progeny, these findings have important implications in the generation and applications of iPSCs.—Mathew, R., Jia, W., Sharma, A., Zhao, Y., Clarke, L. E., Cheng, X., Wang, H., Salli, U., Vrana, K. E., Robertson, G. P., Zhu, J., Wang, S. Robust activation of the human but not mouse telomerase gene during the induction of pluripotency.
Keywords: pluripotent stem cells, reprogramming, promoter
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs), have the capacity for indefinite self-renewal and differentiation into all three germ layers. Induced pluripotent stem cells (iPSCs) are a new type of PSCs first generated by Shinya Yamanaka and colleagues (1) from mouse somatic cells via retroviral transduction of 4 transcription factors, Oct4, Sox2, Klf4, and c-Myc. Subsequently, similar reprogramming has been achieved in different types of mouse cells (2, 3) and human somatic cells (4,5,6,7). In addition to being a promising tool to generate patient-specific stem cells, iPSC technology offers a novel method to examine reprogramming-associated mechanisms.
Telomeres serve as essential protective caps of linear chromosomal ends in all eukaryotic cells, and their maintenance is indispensable for indefinite proliferative potential (8, 9). The telomeric TTAGGG repeats are replenished by telomerase, a ribonucleoprotein complex that consists of a catalytic reverse transcriptase protein subunit (TERT) (10,11,12), a template RNA (TERC) (13, 14), dyskerin (15), and other accessory proteins (16). In the vast majority of instances, TERT expression is limiting and correlates with telomerase activity (17, 18). Regulation of hTERT gene expression occurs primarily at the level of transcription (19,20,21), although contributions from post-transcriptional mechanisms have also been reported (22,23,24). In most human tissues, telomerase activity is either absent or expressed at extremely low levels (25, 26). Consequently, somatic cells suffer telomere attrition and undergo replicative senescence at the end of their life span, namely the Hayflick limit (27). In contrast, stem cells, most human tumors, and immortalized cell lines contain readily detectable telomerase (25, 28, 29). In fact, PSCs have been associated with high telomerase expression, which is viewed as a marker of undifferentiated ESCs (30, 31).
In contrast to the observations in humans, telomerase is readily detected in most mouse somatic tissues (17, 32, 33). Loss of telomeric DNA does not contribute to cellular senescence in mice, owing to the much longer telomeres (34). Indeed, these features have raised the question of whether replicative senescence exists at all in mice and some other animals (35, 36). Although mouse models have been exceptional tools for understanding a wide variety of human pathologies (37,38,39), telomere biology is one of the major distinctive features between mice and humans, and thus, caution may be necessary in interpreting behaviors of proliferating mouse cells as a model of the human condition (40).
The key to reprogramming of a differentiated cell is the resetting of transcriptional machinery and erasure of epigenetic marks in a somatic cell, including reactivation of telomerase and the restoration of telomere length. Because of the important role of telomeres and telomerase in the self-renewal of PSCs and given the fact that the telomere reservoir is limited in adult human somatic cells, telomerase activation and telomere maintenance in reprogrammed cells are thus critical for the potential practical applications of iPSCs in stem cell therapies. It has recently been reported that telomeres reacquire their ESC characteristics in mouse iPSCs (miPSCs) and that the generation of iPSCs from mouse embryonic fibroblasts (MEFs) of late-generation TERC−/− mice with critically short telomeres is impaired, highlighting a critical role for telomerase and telomere maintenance in reprogramming (41). Despite this, results on telomere restoration in cloned animals remain inconclusive. For example, although Dolly, the cloned sheep, survived to a reasonable age, her somatic cells were reported to have abnormally short telomeres, reflecting the age of the donor tissue and telomere attrition during in vitro culturing (42). Conversely, telomeres were restored in cloned calves and mice (43,44,45). The potentially conflicting reports on telomere maintenance in cloned animals raised the possibility that species-specific regulation of telomerase and telomere maintenance might contribute to these discrepancies.
In this report, we investigated the regulation of hTERT and mTERT promoters during reprogramming of mouse and human fibroblasts. Expression of both hTERT and mTERT genes were elevated during cellular reprogramming. However, whereas the level of mTERT mRNA increased modestly by <3-fold in miPSCs, transcription from the hTERT promoter is strongly activated during the reprogramming of both transgenic MEFs and human fibroblasts. The activation of the hTERT promoter occurred in multiple steps and correlated with the status of reprogramming. Surprisingly, despite increases in hTERT transcription and telomerase activity, telomere stabilization and elongation only occurred in a subset of human iPSCs (hiPSCs). Therefore, our results, for the first time, demonstrate species-specific activation of the TERT gene and telomere maintenance during iPSC generation and have important implications in therapeutic applications of these cells.
MATERIALS AND METHODS
Cell culture
Mouse embryonic fibroblasts (D-MEFs) were derived from a transgenic mouse line (D line) containing a bacterial artificial chromosome with a 162-kb human genomic DNA that included the entire hTERT gene, with a Renilla luciferase (Rluc) ORF inserted into its ATG codon (ref. 46 and unpublished results). HM1 mESCs and reprogrammed mouse cells were maintained on feeder layers of γ-irradiated wild-type MEFs in either mESC-serum medium (DMEM with 15% FBS (Atlanta Biologicals, Lawrenceville, GA, USA) and 1000 U/ml leukemia inhibitory factor (LIF; Chemicon, Temecula, CA, USA), or mESC-KSR medium [DMEM containing 15% knockout serum replacer (Invitrogen, Carlsbad, CA, USA) and LIF]. Human ESC lines, H1 and H9 (WiCell, Madison, WI, USA), and hiPSCs were cultured in DMEM-F12 with 20% KSR and 4 ng/ml bFGF. hiPSC-IMR90 (clone 1, passage 28) and hiPSC-FSC (clone 1, passage 22) cells were generously provided by Dr. James Thomson’s laboratory (University of Wisconsin, Madison, WI, USA) (5).
Retrovirus preparation
The pMX-based retroviral expression vectors containing mouse and human Oct4, Sox2, c-Myc, and Klf4, and lentiviral constructs encoding human Nanog and LIN28 were obtained from Addgene (Cambridge, MA, USA) (1, 4, 5). The vector pBMN-NIG was constructed from pBMN-I-GFP. Briefly, a 999-bp BglII-SmaI fragment from pSV2-Neo carrying the Neomycin resistance marker was inserted into the BamHI/StuI sites before the IRES sequence in pBMN-I-GFP. The vector pLXSN-ET, encoding the mouse ecotropic retroviral receptor, was obtained by subcloning a 2.3-kb EcoRI fragment from the vector pJET (47) into the EcoRI site in pLXSN. Ecotropic and amphotropic recombinant retroviruses were packaged in Phoenix-E and -A cells, respectively, as described previously (1, 48). Lentiviruses were prepared by two separate cotransfections of 293FT cells with a mix of one lentiviral vector and the packaging constructs psPAX2 and pMD2.G (Addgene) using Lipofectamine LTX (Invitrogen).
iPSC induction
For mouse cells, D-MEFs of passages 1–3 were infected twice with virus supernatants containing 4 Yamanaka factors at 24 and 36 h postseeding (1). At 48 h after the first infection, cells were trypsinized and replated in feeder-covered plates with fresh mESC-serum medium. The culture was then maintained and observed for the appearance of colonies. For human cells (4, 5), normal human fibroblasts (NHFs) of passage 5 were infected with amphotropic LXSN-ET viruses and selected with G418 for 3 passages to obtain NHF-ET cells. These cells were then infected with a combination of ecotropic retroviral human Oct4, Sox2, c-Myc, and Klf4 and lentiviral Nanog and LIN28. At 2 d postinfection, cells were trypsinized and plated on to a 10-cm plate with irradiated SNL feeder cells (a mouse fibroblast cell line transformed with neomycin resistance and murine LIF genes) (49), maintained in hESC medium, and observed for colony development. Clones 5–10, 5–11, and 5–38 at passages 12, 7, and 5, respectively, were used for further characterization.
Characterization of iPSCs
For alkaline phosphate (AP) staining, cells were fixed for 30–45 s with 3.7% formaldehyde in methanol and stained with 240 μg/ml Fast Violet B Salt and 4% Napthol AS-MX AP solution (Sigma). Luciferase activities were measured using a dual luciferase reporter assay system (Promega, Madison, WI, USA). Semiquantitative RT-PCR was performed as described previously (46, 50). Equal amounts of total RNA were treated with RNase-free DNase I (Roche Diagnostics, Indianapolis, IN, USA) and subjected to reverse transcription and PCR analysis. Quantitative PCR analyses were performed in triplicate using FastStart Real-Time Master Mix (Roche) on an Applied Biosystems StepOnePlus PCR system (Applied Biosystems, Foster City, CA, USA), and data were normalized to 18S ribosomal RNA. Sequences of probes and primers are provided in Supplemental Table 1.
Conversion of partially reprogrammed cells
As noted in Results, during isolation of iPSCs, we identified cell colonies that appear to be only partially reprogrammed. We therefore utilized one such clonal line (II-25 cells) to investigate their ability to be fully converted to iPSCs. II-25 cells in mESC-KSR medium were treated with 10 μM U0126 and 3 μM CHIR99021 (Stemgent, Cambridge, MA, USA). The cells were subsequently passaged a number of times, and multiple aliquots were collected for RNA isolation and luciferase assays at each passage. The chemical inhibitors were present in the medium for 28 d and removed from the cells thereafter.
Differentiation
For teratoma formation, nude mice (Harlan Laboratories, Indianapolis, IN, USA) were anesthetized and injected subcutaneously with 106 cells in 100 μl into the dorsal flank. The tumor progression was monitored over a 40-d period, followed by surgical dissection. Paraffin-embedded sections were stained with hematoxylin and eosin (H&E) and evaluated in a double-blinded manner by multiple pathologists. Osteogenic differentiation of reprogrammed cells was performed as we have previously described (46). Briefly, reprogrammed cells were allowed to differentiate into embryoid bodies (EBs) in the absence of LIF. The developing 2-d-old EBs were dispersed and cultured under osteogenic conditions. Bone nodules were stained 3 wk later by silver nitrate.
Epigenetic analyses
CpG methylation was initially determined by bisulfite sequencing, and later by the pyrosequencing method when the instrument became available. Briefly, genomic DNAs were treated using the EpiTect bisulfite kit (Qiagen, Valencia, CA, USA). Amplified products were cloned into pCR2.1-TOPO (Invitrogen), and 8 random clones were sequenced with M13 forward and reverse primers. For pyrosequencing, bisulfite-treated genomic DNA samples were PCR amplified one round with primers mNPyF3 and mNPyR3. The products were used for a second round of PCR with mNPyR3 and UniBiotin primers. The resultant PCR product was subjected to pyrosequencing using mNPyS3 in a PyroMark Q24 machine, and the data were analyzed using the PyroMarkQ24 analysis software (Qiagen). The primer sequences are listed in Supplemental Table 1. Chromatin immunoprecipitation (ChIP) analysis was performed as described previously, using primers and a probe specific for the transgenic hTERT promoter (51). ChIP-grade antibodies against H3K4Me2 (07–030) and H3K9Me3 (07–442) were purchased from Upstate Biotechnology (Lake Placid, NY, USA).
Reverse transcription and PCR analyses
Semiquantitative and real-time RT-PCR analyses (qRT-PCR) were performed as described previously (46, 51). To specifically detect the endogenous Oct4, Klf4, Sox2, and c-Myc mRNAs, downstream primers in the 3′ UTRs were used, which were not included in the pMXs cDNA clones used for iPSC induction. For specific detection of the transgenic transcripts, the upstream primer was in the viral LTR sequence of the pMXs vector, whereas the downstream primer for each gene was in its cDNA sequence. PCR primers and Taqman probes are listed in Supplemental Table 1.
Telomerase activity and telomere length analyses
Telomerase activities were assessed by the telomeric repeat amplification protocol (TRAP), and telomere length was determined by Southern blot analysis, as we have described previously (21).
RESULTS
Isolation of reprogrammed cells
To investigate the regulation of mTERT and hTERT genes during reprogramming in the same cellular setting, we tried to generate iPSCs from mouse fibroblasts via retroviral transduction of the 4 factors, Oct4, Sox2, Klf4, and c-Myc (1). MEFs from a transgenic mouse line (D line) containing an integrated hTERT BAC reporter, referred to as D-MEFs, were used. This reporter contains three human loci, the hCRR9, hTERT, and hXtrp2, and was modified by insertion of an Rluc expression cassette into the hTERT ATG codon. The expression pattern of this luciferase reporter recapitulated that of the native hTERT gene in adult human tissues (unpublished data), consistent with a previously published report (33).
We initially attempted to isolate reprogrammed colonies solely on the basis of morphology (52). The kinetics of appearance of ESC-like colonies following retroviral transduction of the 4 factors closely resembled previous reports. Subsequent to an initial rapid proliferation obvious within 72 h of infection, colonies began to appear at 6–7 d and were large enough to be isolated by 18–20 d post-transduction. The appearance of colonies in the initial plates was heterogeneous (Fig. 1Aa, b, top panels). When the ESC-like colonies were isolated and expanded after 20 d post-transduction, most colonies gave rise to a relatively homogeneous ESC-like morphology within 4 to 5 passages (Fig. 1Aa, b, middle panels). However, detailed examination revealed that the resultant cells were characterized by marked heterogeneity in the up-regulation of pluripotency markers and lack of retroviral silencing. By isolation exclusively on the basis of ESC-like morphology, we did not obtain fully reprogrammed iPSCs, which would have expressed the endogenous pluripotency markers Oct4, Sox2, and Nanog, with silenced retroviral transgenes. Hence, we considered these colonies of cells partially reprogrammed.
Figure 1.
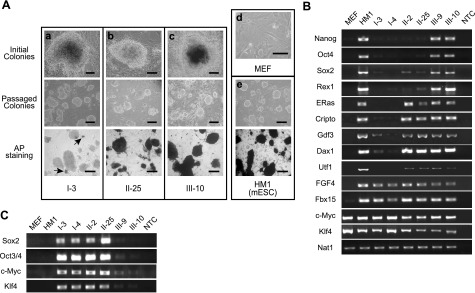
Characterization of reprogrammed cells from D-MEFs. A) Morphological analysis of reprogrammed clones. a–c) I-3 (a), II-25 (b), and III-10 (c) are representative clones from classes I, II, and III, respectively. Top panel: initial appearance of colonies. Middle panel: passaged culture of each clone. Bottom panel: AP staining. d, e) MEFs (d) and HM1 mouse ESCs (e, top panel) and their AP staining (e, bottom panel) are shown as controls. Initial colonies were imaged using an ×40 objective, the rest with an ×10 objective. Scale bars = 100 μm. B, C) Expression levels of pluripotency markers (B) and retroviral transgenes (C) in representative clones of each class of reprogrammed cells, as determined by RT-PCR. Nat1 was used as an internal control. NTC, no template control.
On the basis of the parameters relevant to reprogramming, colonies isolated from these initial experiments could be broadly classified into two classes. Class I cells were characterized by forming very compact and raised colonies that closely resembled mESCs. They were generally stained negative for alkaline phosphatase (AP), but often displayed the presence of a few AP-positive cells within the colonies as indicated by arrowheads in the lower panel of Fig. 1Aa. However, repeated subcloning attempts failed to generate any homogeneous AP-positive colonies. RT-PCR analysis clearly showed the expression of FGF4 and Fbx15, accompanied by modest activation of Gdf3, in class I cells, whereas all these three genes were expressed in HM1 mESCs (Fig. 1B). Nonetheless, the retroviral transgenes were expressed at high levels in class I cells (Fig. 1C).
Class II colonies tended to be less compact but were clearly AP positive (Fig. 1Ab, bottom panel). Although these cells expressed more markers than class I cells, they did not express the critical pluripotency markers, such as Oct4, Nanog, and Sox2 (Fig. 2B, II-2 and II-25). In addition, they were also deficient in silencing the retroviral transgenes (Fig. 2C).
Figure 2.
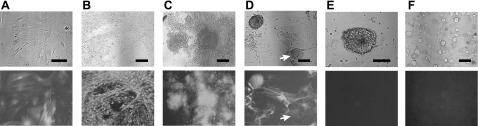
Isolation of fully reprogrammed clones (class III) in D-MEFs. A) D-MEFs infected with pBMN-NIG viruses and selected by neomycin for 7 d. B) D-MEFs at 6 d after transduction by the 4 viruses. Rapid cell proliferation and formation of patches of apparently transformed cells are visible. C) Formation of colonies that were initially all GFP-positive. D) Appearance of a GFP-silenced colony, as indicated by arrows. E) GFP-negative colony, III-10, after initial isolation. F) Morphology of clone III-10 grown on MEF feeder after 3 passages. Top panels: phase-contrast fields. Bottom panels: green fluorescence fields. Scale bars = 100 μm.
To isolate fully reprogrammed iPSCs, both class I and II cells were seeded at lower densities, and subcloning of individual cells was attempted. However, none of the isolated subclones had characteristics of greater reprogramming than the respective original cultures, indicating that these cells were most likely trapped in stable partially reprogrammed states (data not shown).
Silencing of retroviral transgenes as an indicator of complete reprogramming
Because silencing of retroviral transgenes occurred in all PSCs (53), we exploited this feature to identify fully reprogrammed cells. To this end, pBMN-NIG, a recombinant retroviral vector containing a neomycin resistance gene and a GFP coding sequence, separated by an internal ribosome entry site, was used. Early passage D-MEFs were transduced with pBMN-NIG retroviruses and selected with G418 for 2 passages. Postselected D-MEFs displayed homogenous GFP expression (Fig. 2A) and were therefore used as the starting cells for iPSC induction using the 4 reprogramming factors. The kinetics of colony appearance was similar to those of the previous experiments. Patches of rapidly proliferating and transformed-looking cells appeared immediately following transduction (Fig. 2B). Subsequently, colonies began to develop and were initially GFP-positive (Fig. 2C). GFP-negative colonies started to emerge by d 17 post-transduction (Fig. 2D). Less than 10% of the colonies had silenced GFP by d 21, when the GFP-negative colonies were isolated (Fig. 2E). Upon further passaging, these GFP-negative colonies gave rise to homogeneous ESC-like cultures with no evident expression of GFP thereafter (Fig. 2F). In addition, silencing of the retroviral GFP transgene was associated with the activation of endogenous pluripotency markers, Oct4, Nanog, and Sox2, and the cessation of expression from the 4 viral iPSC-inducing factors (Fig. 1B, C; III-9 and III-10). These clones also showed elevation of all ESC-associated genes (Fig. 1B). Taken together, these results suggested that the GFP-negative clones, named as class III cells, were fully reprogrammed cells.
Pluripotency of reprogrammed cells
Pluripotency status of reprogrammed cells can be evaluated by their ability to form teratomas and their contribution to the development of tissues from all three germ layers. Two representative clones from each of the 3 classes were injected subcutaneously into nude mice, and all formed tumors. To detect differences in the efficiencies of teratoma formation by the different classes of cells, 106 cells were injected per site and kinetics of teratoma development were determined by measuring tumor sizes at various time points (Fig. 3A). HM1 mESCs developed teratomas rapidly, and class III clones III-9 and III-10 developed tumors at a rate comparable to HM1. Surprisingly, class I clones I-3 and I-4 formed tumors at a rate similar to that of class III cells, whereas class II clones II-2 and II-25 were much slower at developing tumors.
Figure 3.
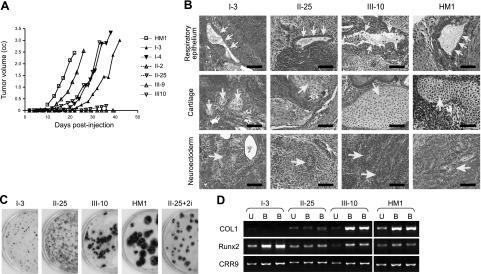
Characterization of differentiation potentials of reprogrammed cells. A) Kinetics of teratoma development following subcutaneous injection of cells into nude mice. Each clone was injected at 2 subcutaneous sites (106 cells/site). Once palpable, the volume of each tumor (length × breadth × thickness) was measured using calipers every 3 or 4 d. Data are from 1 injection site of each cell type. The other site differed in absolute tumor volume, but kinetics was the same. MEFs were injected as control, which did not develop any tumors (data not shown). B) Histological analysis of teratomas derived from reprogrammed cells. Tumors were removed about 1 mo postinjection and subjected to H&E staining. Respiratory epithelium (top panels), immature cartilage (middle panels), and neuroectoderm (bottom panels) represent differentiated cells that belong to the 3 germ layers, endoderm, mesoderm, and ectoderm, respectively. All images were photographed at ×400. Arrows indicate respective tissues as labeled at left. Scale bars = 100 μm. C) Osteogenic differentiation of reprogrammed cells. Differentiated bone colonies formed under osteogenic culture conditions were stained dark brown with silver nitrate in 6-cm plates, whereas nonbone colonies were counterstained with methylene blue. II-25+2i, II-25 cells treated with CHIR99021 and U0126 for 4 wk (see Fig. 6). D) Expression of osteogenic markers, as analyzed by RT-PCR. U, undifferentiated cells; B, bone colony; COL1, type I collagen.
Histological examination revealed that the class III teratomas formed differentiated tumors identical to those formed by HM1 mESCs (Fig. 3B). Examination of H&E-stained slides could not distinguish between tumors formed by class III cells and HM1 mESCs. Differentiation into all 3 germ layers, including neural tissues, cartilage, skeletal muscle, and glandular epithelium, could be recognized. Both the fast-growing class I and the slow-growing class II tumors contained structures of all three germ layers in teratomas. However, a characteristic difference between classes I and II on the one hand and class III on the other hand could be identified by double-blinded examination: the presence of a large number of morphologically immature cells in the former group. Although the presence of immature cells could be identified even in tumors formed by HM1 ESCs and class III cells, the proportion constituted by these underdifferentiated cells was much lower. The higher proportion of immature blastema-like cells in class I tumors than those of class II may provide a possible explanation for the surprisingly faster growth of class I tumors. This suggested that class I cells, albeit less reprogrammed, had been driven to a more transformed state, allowing them to generate larger tumors composed mainly of immature cells, with smaller pockets of differentiated tissues.
The differentiation potentials of each class of the reprogrammed cells were also examined by their abilities to differentiate toward the osteogenic lineage. Cells were first allowed to differentiate into EBs, which were dispersed and cultured in osteogenic medium for 4 to 5 wk, followed by staining with silver nitrate (46). Cells of clones I-3 and II-25, representatives of classes I and II, respectively, formed small colonies that were mostly counterstained with methylene blue (Fig. 3C). In contrast, cells of clone III-10, a representative of class III cells, formed large colonies that were stained brownish by silver nitrate, resembling those derived from HM1 mESCs. The brownish color was silver nitrate staining for mineral deposits secreted by differentiated osteogenic cells. In addition, RT-PCR analysis revealed that III-10-derived colonies had elevated mRNA levels of type I collagen and Runx2, two markers of bone cell differentiation (Fig. 3D). These results demonstrated that III-10 cells, but not I-3 and II-25 cells, underwent efficient differentiation into cells of the osteogenic lineage under in vitro cultural conditions.
Modest increases of mTERT expression during reprogramming
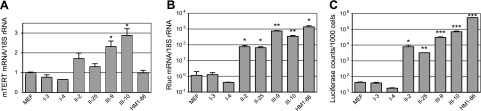
To determine the TERT regulation in association with the states of reprogramming, transcription from the TERT promoters was examined. First, mTERT transcription among all 3 classes of reprogrammed cells was compared using quantitative RT-PCR (qRT-PCR) analysis. Class III cells showed highest mTERT expression. However, the degree of mTERT activation was limited. The mTERT level in class III cells (clones III-9 and III-10) increased by <3-fold compared to that in parental D-MEFs, whereas class I cells (I-3 and I-4) had essentially the same level of mTERT transcription as D-MEFs (Fig. 4A). Although class II cells (II-2 and II-25) had a trend toward higher mTERT transcription, it was not significantly different from that of MEFs. Interestingly, the mTERT mRNA level in HM1 mESCs was similar to that in MEFs but significantly less than that in class III reprogrammed cells. These results indicated that mTERT transcription was easily detected in MEFs, and its increase in pluripotent cells was modest. Indeed, significant mTERT expression was also detected in many adult somatic tissues in mice (ref. 33 and data not shown). The disparate genetic backgrounds likely contributed to the different levels of mTERT expression in class III cells and HM1 ESCs.
Figure 4.
Expression of the endogenous mTERT gene and transgenic hTERT reporter in reprogrammed cells. A, B) Levels of mTERT (A) and Rluc (B) mRNAs in reprogrammed cells, determined by qRT-PCR (Taqman assay). Data are normalized to 18S ribosomal RNA and presented as relative values to those of MEFs. C) Rluc activities in reprogrammed cells, as determined by luciferase assays and presented for every 1000 cells. All experiments were performed at least twice and in triplicate. Data are averages of triplicates from one representative experiment. *P < 0.05, **P < 0.01, ***P < 0.001 vs. MEFs; Student’s t test.
Robust activation of hTERT promoter during reprogramming
To determine the changes of hTERT promoter activity during reprogramming, expression from the transgenic hTERT reporter gene in D-MEFs and their reprogrammed derivatives was determined by both luciferase assays and qRT-PCR. Transcription from the hTERT promoter underwent a much more striking increase than that of mTERT on reprogramming (Fig. 4B, C). Whereas the hTERT promoter remained silenced in class I cells, class II cells activated its transcription by almost 2 orders of magnitude compared to the parental D-MEFs. Class III cells exhibited a further 10-fold increase in hTERT promoter activation. To compare these levels of hTERT promoter activity to that of ESCs, we utilized a stable line of HM1, HM1–86, which contains a chromosomally integrated hTERT reporter, 117B23-cFtRvSVP, a similar BAC as the transgenic reporter in D-MEFs except that a firefly luciferase was inserted into the CRR9 locus. Thus, the hTERT promoter activity can be monitored by Rluc level in these mESCs. As shown in Fig. 4B, C, both Rluc mRNA and activity levels in HM1–86 mESCs were severalfold higher than those in class III cells, mostly likely owing to the higher copy number (∼10 copies) of the integrated BAC reporter, as compared to two copies in D-MEFs and their derivatives (data not shown). Thus, these results demonstrated that the transgenic hTERT promoter was dramatically activated during reprogramming and its activity in class III cells was similar to that in mESCs, further supporting our conclusion that class III cells were the products of complete reprogramming.
Epigenetic status of TERT promoters
Promoter methylation plays an important role in transcriptional silencing, and demethylation is a key mechanism to cellular reprogramming. Hypomethylation of promoters of pluripotency genes like Nanog is a defining feature of ESCs and iPSCs. Thus, bisulfite sequencing analysis was carried out using genomic DNAs from representative clones of all 3 classes. As shown in Fig. 5A, the Nanog promoter was methylated at ∼50% of the CpG sites in D-MEFs. This level of CpG methylation increased further in I-3 and II-25 cells. In III-10 cells, however, CpG methylation at the Nanog promoter almost completely disappeared. Similarly, the Nanog promoter in HM1–86 mESCs was also hypomethylated. These data provided another line of evidence that, whereas class I and II cells were partially reprogrammed, class III cells were fully reprogrammed iPSCs. The observation that both classes of partially reprogrammed cells maintained Nanog promoter in a hypermethylated state was in accordance with previous reports that DNA demethylation was an inefficient step during reprogramming (53, 54).
Figure 5.
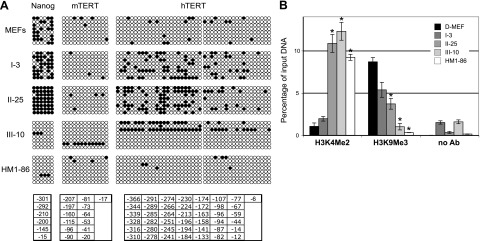
Epigenetic analyses of Nanog and TERT promoters in reprogrammed cells. A) Analysis of CpG methylation of endogenous Nanog and mTERT promoters and transgenic hTERT promoter. Genomic DNAs were subjected to bisulfite conversion, PCR cloning, and sequencing. Six CpG sites immediately upstream of the Nanog ATG codon, and 13 and 43 CpGs immediately upstream of the mTERT and hTERT TSSs, respectively, are shown. Open circles indicate unmethylated CpG sites; solid circles denote methylated sites. Coordinates of Cs listed at bottom are relative to the Nanog ATG codon, and mTERT and hTERT TSSs. B) ChIP analysis of hTERT promoter. H3K4Me2 and H3K9Me3 are antibodies against dimethylated lysine 4 and trimethylated lysine 9 residues of histone H3, respectively. Precipitated DNA fragments were analyzed by quantitative PCR using primers and a probe specific for the transgenic hTERT promoter and normalized to input DNA. *P < 0.01 vs. D-MEFs; Student’s t test.
Although methylation of the hTERT promoter in many cell lines has been previously reported, a clear correlation between its transcriptional activity and methylation profile has not been established (55, 56). Thus, the TERT promoters were analyzed to determine whether activation of the TERT promoters during reprogramming was associated with an altered methylation profile. For the 43 CpG sites in a 366-bp region immediately upstream of the hTERT transcription start site (TSS), the level of CpG methylation was generally low in D-MEFs and all reprogrammed clones, although it was higher in clones I-3 and II-25, likely due to accumulation from in vitro culturing (Fig. 5A). These results indicated that promoter methylation played a minimal role in the repression of hTERT promoter in D-MEFs, and its demethylation was not directly involved in hTERT activation in reprogrammed cells. Similarly, the mTERT promoter had a low level of methylation in all types of cells examined, and there was no discernible difference between HM1 ESCs and clones I-3, II-25, or III-10 cells (Fig. 5A).
Covalent modifications of histones are critical for the regulation of gene expression. To determine whether the reprogramming process is accompanied by changes of histone modifications at the transgenic hTERT promoter, we performed a ChIP experiment to examine the status of dimethylation of the lysine 4 residue (H3K4Me2) and trimethylation of the lysine 9 residue (H3K9Me3) of histone H3, correlating with active and repressive states of gene transcription, respectively (57, 58, 59). As shown in Fig. 5B, H3K4Me2 at the transgenic hTERT promoter was very low in D-MEFs and I-3 cells but was significantly elevated in II-25, III-10, and HM1–86 cells. On the other hand, H3K9Me3, initially abundant in D-MEFs, decreased progressively during reprogramming, and largely disappeared in fully reprogrammed III-10 cells and HM1–86 ESCs. These results are consistent with our findings that the transgenic hTERT promoter was activated in class II and III cells but not in class I cells. Therefore, activation of hTERT transcription during the induction of pluripotency was accompanied by the acquisition of active histone marks at the promoter.
Complete reprogramming of II-25 cells induced by chemical inhibitors
It has recently been shown that blocking inductive differentiation stimuli by modulating intracellular signaling with inhibitors of mitogen-activated protein (MAP) kinase and glycogen synthase kinase-3 (GSK3) pathways can facilitate complete reprogramming of partially reprogrammed cells (60). To determine whether partially reprogrammed cells could be induced to undergo further reprogramming, II-25 cells were subjected to treatment with the MEK inhibitor U0126 and the GSK3 inhibitor CHIR99021. In order to use retroviral silencing as a marker for complete reprogramming, cells were first transduced with pBMN-NIG retroviruses and selected with G418 so that all pretreated cells expressed GFP (Fig. 6B). Given the marked difference in hTERT promoter activities among different classes of cells, reprogramming was also monitored by measuring Rluc activity transcribed from the transgenic hTERT promoter.
Figure 6.
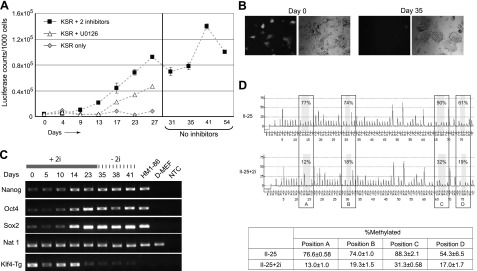
Conversion of partially reprogrammed class II cells to fully reprogrammed cells by chemical inhibitors. Class II-25 cells were infected with pBMN-NIG retroviruses and selected with neomycin. These cells were then cultured for 4 wk in the presence of both MEK inhibitor U0126 (10 μM) and GSK3 inhibitor CHIR99021 (3 μM), or U0126 alone. Cells treated with both inhibitors (II-25+2i) were cultured for another 26 d in the absence of chemicals. A) Luciferase activities from the transgenic hTERT reporter, determined at each passage. B) Expression of retroviral GFP in II-25 cells. Day 0, pretreated cells; day 35, post-treated cells. Left panel: green fluorescence field. Right panel: phase-contrast field. C) Expression of pluripotency markers induced by chemical inhibitors in II-25 cells, as determined by RT-PCR. Klf4-Tg, transgenic retroviral Klf4; +2i, cells treated with both U0126 and CHIR99021; −2i, cells were cultured in medium without the two inhibitors; NTC, no template control. D) Pyrosequencing analysis of Nanog promoter. Representative pyrograms from pretreated II-25 (top panel) and II-25+2i (lower panel) cells are shown. Numbers in boxes indicate percentage of methylated residue at each CpG site. Table at bottom presents average methylation percentage at each CpG site in II-25 and II-25+2i cells, calculated from 3 independent pyrosequencing reactions. Positions A–D correspond to nucleotides −412, −434, −491, and −541 with respect to the Nanog TSS, respectively.
II-25 cells were cultured in the presence of both inhibitors in mESC-KSR medium, in which serum was replaced with KSR to preclude any serum-derived prodifferentiation stimuli. Such cultural conditions resulted in a gradual and steady rise in Rluc activity (Fig. 6A). After 4 wk of treatment, the Rluc activity plateaued at a level that was 10-fold higher than in the initial II-25 cells and as high as that in class III cells. The high Rluc activity was maintained steady for at least another month when the culture was grown in the absence of any inhibitors; this culture was designated II-25+2i. Interestingly, this rise of hTERT promoter activity, as indicated by the Rluc activity, was also associated with a progressive increase of cells that had silenced retroviral GFP expression. After the 4-wk treatment, the culture had completely silenced the GFP expression (Fig. 6B). Treatment of II-25 cells with U0126 alone led to a slower increase of Rluc activity and a heterogeneous population in terms of GFP expression (Fig. 6A and data not shown). In contrast, similar treatment of class I cells led to slow and modest, albeit inconsistent, increases of Rluc activity, which was not accompanied by the silencing of retroviruses (data not shown).
Additional analyses were performed to confirm that the changes in II-25+2i cells resulted from nuclear reprogramming. First, whereas class II cells originally displayed little activation of the endogenous pluripotency markers, Oct4, Nanog, and Sox2, treatment of II-25 cells with the two inhibitors, as shown by RT-PCR analysis, led to progressively increased expression of these markers. Thus, II-25+2i cells with stably high hTERT promoter activity expressed the pluripotency markers at levels as high as the HM1 mESCs (Fig. 6C). Second, compared to pretreated II-25 cells, the Nanog promoter in II-25+2i cells was hypomethylated at CpG sites, as determined by pyrosequencing analysis (Fig. 6D). Finally, unlike II-25 cells, II-25+2i cells differentiated into osteogenic cells as efficiently as class III cells and HM1–86 mESCs (Fig. 3C). These results indicated that inhibition of the MAP kinase and GSK3 pathways facilitated reprogramming of II-25 cells into a state similar to that of class III cells and mESCs. Taken together, our data demonstrated that nuclear reprogramming was accompanied by a stepwise increase of hTERT promoter activation. The difference in hTERT promoter transcription between class II and III cells marked two different stages of reprogramming toward iPSCs.
Activation of endogenous hTERT in reprogrammed human cells
To determine the regulation of endogenous hTERT gene during the induction of PSCs, reprogrammed human cells were generated from NHFs by retroviral transduction of 6 factors, Oct4, Sox2, c-Myc, Klf4, Nanog, and Lin28 (4, 5). Colonies that morphologically resembled hESCs were isolated, of which 3 representative clones, NHF5–10, NHF5–11, and NHF5–38, were subjected to further analysis. In addition, 2 previously reported iPSC clones, hiPSC-IMR90, and hiPSC-FSC, derived from human embryonic lung fibroblast IMR90 and foreskin cells, respectively, were obtained from Dr. James Thomson’s laboratory and used in this study. To determine the reprogramming status of these cells, a set of pluripotency markers was examined by RT-PCR, using the constitutively expressed CRR9 mRNA as a control (46). Similar to H1 and H9 hESCs, NHF5–10, NHF5–11, hiPSC-FSC, and hiPSC-IMR90 cells expressed all endogenous pluripotency markers: Oct4, Nanog, Sox2, and Lin28 (Fig. 7A). In NHF5–38 cells, the Oct4 expression was not elevated, but the expression levels of Nanog, Sox2, and Lin28 were significantly increased, indicating that this clone was incompletely reprogrammed. Klf4 and c-Myc were expressed in all cells before and after reprogramming. Furthermore, all reprogrammed human clones stained positive for AP (data not shown). Together, these results suggested that these morphologically ESC-like clones had undergone extensive reprogramming. In the previous report (5), hiPSC-IMR90 (clone 1) and hiPSC-FSC (clone 1) cells demonstrated capability of multilineage differentiation in both EBs and teratomas, indicating that these two clones were fully reprogrammed iPSCs.
Figure 7.
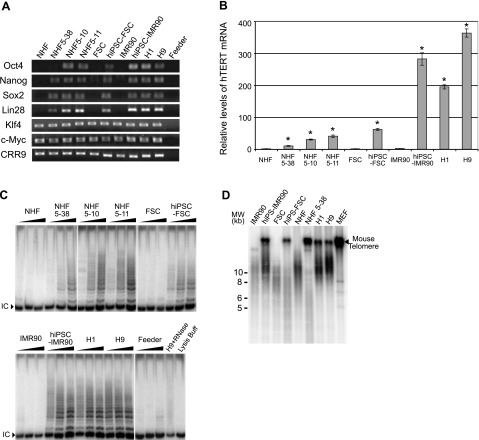
Telomerase expression and telomere maintenance in reprogrammed human cells. A) Expression of endogenous pluripotency markers, as determined by RT- PCR analysis. B) qRT-PCR analysis of the hTERT mRNA, normalized to those of 18S ribosomal RNA and plotted relative to hTERT expression level in NHFs. Data are averages of triplicates; assays were repeated at least twice. *P < 0.0001 vs. all fibroblasts; Student’s t test. C) Telomerase activities as determined by TRAP assay; 10, 100, and 1000 ng of cell extracts were used in each set of assays. IC, internal control. D) Assessment of telomere lengths by Southern blot analysis of telomeric restriction fragments. DNA size markers (kb) are shown at left. Mouse telomere signals in reprogrammed human cells and hESCs are from feeder layers. Feeder, irradiated MEFs used as feeder layers for culturing hESCs and reprogrammed human cells.
The endogenous hTERT expression in reprogrammed human cells was assessed by qRT-PCR analysis. As shown in Fig. 7B, the hTERT mRNA level in H1 and H9 hESCs was 200- and 360-fold higher than in fibroblasts, respectively. hiPSC-IMR90 cells expressed 280-fold more hTERT mRNA than the parental IMR90 cells, comparable to those in hESCs. hiPSC-FSC cells had ∼60-fold higher hTERT expression than the parental foreskin cells, although this level was significantly less when compared to hESCs. In addition, reprogrammed NHF clones, 5–38, 5–10, and 5–11, expressed ∼10-, 30-, and 40-fold of hTERT mRNA compared to their parental NHFs and other fibroblasts, respectively. Figure 7C shows the telomerase activities in these cells as determined by TRAP assay. Whereas telomerase was undetectable in fibroblasts, the reprogrammed human cells contained significant levels of telomerase activity, which correlated with their hTERT mRNA levels. hiPSC-IMR90 expressed telomerase activity comparable to that in hESCs, while hiPSC-FSC and reprogrammed NHF clones contained less telomerase activities. These data indicated that, although all reprogrammed cells had activated hTERT and telomerase expression, their levels varied significantly.
Next, telomere lengths, measured by telomeric restriction fragments (TRFs), were determined by Southern blot analysis (Fig. 7D). Whereas IMR90 cells (population doubling 25–30) had an average TRF of ∼10 kb, hiPSC-IMR90 cells contained longer telomeres similar to those of H1 and H9 hESCs, indicating that activation of the endogenous hTERT gene in these reprogrammed cells led to a high level of telomerase activity and telomere restoration. Surprisingly, average TRFs in hiPSC-FSC and NHF5–38 cells were much shorter than those in hESCs, and even their respective parental fibroblasts. These results demonstrated that the levels of hTERT expression and telomerase activities in these cells were insufficient to sustain their telomere length. Hence, although activation of telomerase and the hTERT gene occurs during reprogramming of human cells, restoration of telomere length requires a very high level of hTERT expression (61), which only occurs in a subset of reprogrammed cells. In addition to a required high telomerase expression, it is also possible that telomere elongation during reprogramming is a relatively slow process and requires more extensive proliferation of reprogrammed cells. Thus, it remains to be determined whether the levels of hTERT mRNA and telomerase activity and the lengths of telomeres will alter upon further passaging of the reprogrammed clones.
DISCUSSION
PSCs, such as ESCs, are immortal cells that express telomerase and maintain their telomeres. Here, we demonstrated that, during somatic cell reprogramming, the expression of TERT gene, encoding the limiting subunit of telomerase, was strongly activated in human cells, but only moderately increased in mouse cells. Using transgenic MEFs containing an hTERT reporter as a model, we revealed a multistep process of hTERT promoter activation during the formation of iPSCs. The activation of the hTERT promoter directly correlated with the state of reprogramming and was accompanied by a robust increase of telomerase activity in human cells. Despite increased hTERT expression and telomerase activity, telomeres were shorter in many reprogrammed human cells as compared to their parental fibroblasts, except in hiPSC-IMR90 cells with the highest telomerase expression, indicating that a threshold of telomerase activity is required for telomere elongation.
The important interspecies difference of TERT activation was demonstrated in mouse cells containing a transgenic hTERT BAC reporter, which offered several advantages for the study of hTERT regulation. First, the transgenic reporter recapitulated the native hTERT promoter. It was highly active in mESCs, silenced in differentiated cells (46), and strongly activated during nuclear reprogramming. Second, mouse PSCs grow more rapidly and are easier to manipulate as compared to their human counterparts. Finally, the transgenic system allowed us to investigate the hTERT and mTERT promoter regulation in the same cellular setting and to study the intermediate steps of hTERT activation in partially reprogrammed cells because stronger telomerase activation might be required for human cell reprogramming due to their shorter telomeres.
Partially reprogrammed mouse cells have been isolated in several laboratories and are important for studying the molecular mechanisms of reprogramming (1, 54, 60). The partially reprogrammed clones isolated in this study can be divided into two broad categories, based on the expression of AP and pluripotency markers. Class I clones expressed little AP and only a few pluripotency markers. These cells formed compact colonies, similar to the MCV6 clones reported by Meissner’s group (54). Class II clones were more extensively reprogrammed, stained positive for AP, and expressed a broader set of markers, except for Oct4, Nanog, and Sox2. Many of the initial Fbx15-selected iPSC clones would likely fall into this group (1), as did the previously reported preiPSCs (60) and MCV8 cells (54).
Both class I and II clones had attained a certain degree of pluripotency, because differentiated tissues derived from all 3 germ layers were identified in teratomas formed by these cells. However, the teratomas, especially those of class I cells, contained many immature cells, and both class I and II cells failed to differentiate into osteogenic cells in vitro, indicating that their differentiation was inefficient. Class III cells, in contrast, formed teratomas that were histologically indistinguishable from those derived from mESCs and underwent efficient in vitro osteogenic differentiation. These cells also contained a complete set of fully activated pluripotency markers, including Oct4, Nanog, and Sox2, epigenetic reprogramming of the Nanog promoter, and silencing of retroviral transgenes. Therefore, although we have not examined their potentials for germline transmission, class III cells are likely true iPSCs, comparable to previously reported iPSCs in every aspect that has been examined.
Class I cells formed tumors at a rate similar to that of class III cells, but the mechanisms that drove the oncogenic potentials of these cells might be very different. It is likely that class I cells were trapped in partially transformed states due to the transforming potentials of reprogramming factors c-Myc and Oct4. Nevertheless, tumors formed by class I cells did contain small pockets of fully differentiated cells. Thus, it is possible that these cells underwent additional reprogramming during in vivo tumorigenesis, as they contained high levels of ectopic reprogramming factors. On the other hand, the ability of class III cells to form teratomas was presumably due to the limitless self-renewal potential acquired upon complete reprogramming, since these cells had ceased expressing the viral transgenes.
Telomerase expression is essential for self-renewal of PSCs. Previously reported data demonstrated that both mTERT mRNA and telomerase activity were found in most mouse somatic tissues (17, 32, 62). Consistent with these previous reports, our current study showed that the mTERT gene was expressed in MEFs and that its expression was only moderately increased by <3-fold during the entire reprogramming process. Nevertheless, this moderate increase might be sufficient to restore the ESC characteristics of telomeres in miPSCs (41). In contrast, the hTERT promoter was activated several 100-fold in the completely reprogrammed cells, such as class III miPSCs and hiPSC-IMR90. Human cells have much shorter telomeres than mouse cells, and transcription of the hTERT gene is much more tightly regulated in somatic cells. Thus, it is conceivable that a dramatic increase of hTERT transcription is crucial for human cell reprogramming during which extensive cell divisions incur. Accordingly, all human ESC-like clones had elevated levels of hTERT mRNA and telomerase activity. Nonetheless, telomeres were only elongated in the clone with highest hTERT expression, namely hiPSC-IMR90. In all other clones, telomeres were shorter than their parental fibroblasts. Association of telomerase expression, but not telomere lengthening, with extended proliferative potential has been reported previously (63, 64), indicating that, in addition to telomere synthesis, telomerase might provide a telomere-capping function and allow cells with short telomeres to survive and proliferate (65). Indeed, many telomerase-positive tumor cells and immortal cell lines, contains shorter telomeres than their normal counterparts (21, 66).
Telomerase expression and telomere restoration in reprogrammed cells are of particular interest because telomere length is an important determinant of cellular life span and proliferative potential of differentiated cells derived from PSCs. In normal human development, telomere length is reset during germline development (67, 68) and maintained during embryonic development due to the expression of hTERT gene and telomerase (69). In somatic tissues, telomeres shorten with each cell division, owing to the repression of the telomerase enzyme and, in doing so, become the mitotic clock that determines proliferative life span (70). However, the germline development is bypassed during the induction of iPSCs. The proliferation and survival of hiPSCs with shorter telomeres are unlikely to be affected due to their high expression of telomerase, which protects and maintains short telomeres. However, because hTERT transcription dramatically decreases immediately following the initiation of differentiation and becomes silenced in differentiated cells (46), cells derived from hiPSCs with short telomeres may have severely limited ability for proliferation and survival. As a result, long-term survival of such cells would depend on the initial telomere lengths in their parental PSCs. Besides telomere elongation, increased telomerase activity enhances self-renewal ability and differentiation efficiency of mESCs over-expressing TERT (71), implicating noncanonical telomere-independent functions of telomerase. Hence, our study suggests that telomere length and telomerase expression should be considered to be an important benchmark for characterizing hiPSCs and evaluating their potential uses in regenerative medicine.
Our studies also revealed several important aspects of hTERT regulation. First, the hTERT core promoter was minimally methylated in fibroblasts, indicating that CpG methylation at this core promoter region was not essential for hTERT silencing in these cells. Yet, CpG methylation in regions further upstream and downstream of the core promoter have been reported in a number of cell lines (72, 73). Thus, more extensive efforts will be needed to determine whether DNA methylation outside of the core promoter plays a role in hTERT silencing in normal fibroblasts and its activation in iPSCs. Second, although c-Myc was previously proposed to be an important activator of the hTERT promoter, its role in hTERT expression during reprogramming appeared to be minimal. Whereas the endogenous c-Myc was expressed in the parental fibroblasts and all iPSC clones, retroviral c-Myc was only expressed in class I and II cells, but not class III iPSCs, which had the highest level of hTERT promoter activity. Thus, c-Myc overexpression was neither necessary nor sufficient for hTERT expression in reprogrammed cells. Finally, it was not anticipated that several hiPSC clones with reactivated hTERT expression had shorter telomeres. Although it remains to be determined whether extended passaging will lead to increased telomere lengths, these hiPSCs did not exhibit longer telomeres after limited passaging for a couple of months (data not shown).
In summary, we have demonstrated an important difference of TERT activation during the induction of human and mouse PSCs. Because telomere length restoration occurred only in a subset of hiPSC clones with high levels of hTERT expression and telomerase activity, the hTERT expression level and telomere length should be important parameters for determining whether a hiPSC line is completely reprogrammed, in addition to the expression of pluripotency markers, retroviral silencing, and promoter demethylation.
Supplementary Material
Acknowledgments
The authors thank Dr. James Thomson (University of Wisconsin, Madison, WI, USA) for the generous gift of hiPSC clones. The authors are also grateful to the Carrel Laboratory at Penn State College of Medicine for their technical help with pyrosequencing. The work was supported in part by National Institutes of Health (NIH) grant GM071725-S1 (J.Z.), a Penn State Cancer Institute Experimental Therapeutic research initiative (J.Z.), and a Dean’s feasibility grant (S.W.). J.Z. was a research scholar of the American Cancer Society (RSG-04-197-01-MGO). A.S., G.P.R., and K.E.V. were supported by NIH grants R03 CA142060-01, CA-127892-01A, and GM38931-S1, respectively. H.W. was supported by grants from the Chinese National Natural Science Foundation (30871786) and the Chinese National Programs for Fundamental Research and Development (973 plan, 2009CB941002).
References
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors [see comment] Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Aoi T., Yae K., Nakagawa M., Ichisaka T., Okita K., Takahashi K., Chiba T., Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- Hanna J., Wernig M., Markoulaki S., Sun C. W., Meissner A., Cassady J. P., Beard C., Brambrink T., Wu L. C., Townes T. M., Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors [see comment] Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., Slukvin I. I., Thomson J. A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Park I. H., Zhao R., West J. A., Yabuuchi A., Huo H., Ince T. A., Lerou P. H., Lensch M. W., Daley G. Q. Reprogramming of human somatic cells to pluripotency with defined factors [see comment] Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Lowry W. E., Richter L., Yachechko R., Pyle A. D., Tchieu J., Sridharan R., Clark A. T., Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- Lingner J., Hughes T. R., Shevchenko A., Mann M., Lundblad V., Cech T. R. Reverse transcriptase motifs in the catalytic subunit of telomerase [see comments] Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- Meyerson M., Counter C. M., Eaton E. N., Ellisen L. W., Steiner P., Caddle S. D., Ziaugra L., Beijersbergen R. L., Davidoff M. J., Liu Q., Bacchetti S., Haber D. A., Weinberg R. A. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Harrington L., McPhail T., Mar V., Zhou W., Oulton R., Bass M. B., Arruda I., Robinson M. O. A mammalian telomerase-associated protein [see comments] Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Functional evidence for an RNA template in telomerase. Science. 1990;247:546–552. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- Blasco M. A., Funk W., Villeponteau B., Greider C. W. Functional characterization and developmental regulation of mouse telomerase RNA. Science. 1995;269:1267–1270. doi: 10.1126/science.7544492. [DOI] [PubMed] [Google Scholar]
- Cohen S. B., Graham M. E., Lovrecz G. O., Bache N., Robinson P. J., Reddel R. R. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- Venteicher A. S., Abreu E. B., Meng Z., McCann K. E., Terns R. M., Veenstra T. D., Terns M. P., Artandi S. E. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. A., Allsopp R. C., Chin L., Morin G. B., DePinho R. A. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Meyerson M., Eaton E. N., Ellisen L. W., Caddle S. D., Haber D. A., Weinberg R. A. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- Greenberg R. A., O'Hagan R. C., Deng H., Xiao Q., Hann S. R., Adams R. R., Lichtsteiner S., Chin L., Morin G. B., DePinho R. A. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene. 1999;18:1219–1226. doi: 10.1038/sj.onc.1202669. [DOI] [PubMed] [Google Scholar]
- Gunes C., Lichtsteiner S., Vasserot A. P., Englert C. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by Mad1. Cancer Res. 2000;60:2116–2121. [PubMed] [Google Scholar]
- Wang S., Zhu J. Evidence for a relief of repression mechanism for activation of the human telomerase reverse transcriptase promoter. J Biol Chem. 2003;278:18842–18850. doi: 10.1074/jbc.M209544200. [DOI] [PubMed] [Google Scholar]
- Liu K., Hodes R. J., Weng N. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol. 2001;166:4826–4830. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- Ulaner G. A., Hu J. F., Vu T. H., Giudice L. C., Hoffman A. R. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- Aisner D. L., Wright W. E., Shay J. W. Telomerase regulation: not just flipping the switch. Curr Opin Genet Dev. 2002;12:80–85. doi: 10.1016/s0959-437x(01)00268-4. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer [see comments] Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Masutomi K., Yu E. Y., Khurts S., Ben-Porath I., Currier J. L., Metz G. B., Brooks M. W., Kaneko S., Murakami S., DeCaprio J. A., Weinberg R. A., Stewart S. A., Hahn W. C. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- Hayflick L., Moorhead P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Lansdorp P. M. Telomeres, stem cells, and hematology. Blood. 2008;111:1759–1766. doi: 10.1182/blood-2007-09-084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. Embryonic stem cell lines derived from human blastocysts [see comment] Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. 1827 (erratum) [DOI] [PubMed] [Google Scholar]
- Armstrong L., Lako M., Lincoln J., Cairns P. M., Hole N. mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mech Dev. 2000;97:109–116. doi: 10.1016/s0925-4773(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Prowse K. R., Greider C. W. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa I., Chiang Y. J., Patterson T., Feigenbaum L., Leem S. H., Michishita E., Larionov V., Hodes R. J., Barrett J. C. Differential cis-regulation of human versus mouse TERT gene expression in vivo: identification of a human-specific repressive element. Proc Natl Acad Sci U S A. 2005;102:18437–18442. doi: 10.1073/pnas.0508964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- Forsyth N. R., Elder F. F., Shay J. W., Wright W. E. Lagomorphs (rabbits, pikas and hares) do not use telomere-directed replicative aging in vitro. Mech Ageing Dev. 2005;126:685–691. doi: 10.1016/j.mad.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Peters L. L., Robledo R. F., Bult C. J., Churchill G. A., Paigen B. J., Svenson K. L. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- Van Dyke T., Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Herzig M., Christofori G. Recent advances in cancer research: mouse models of tumorigenesis. Biochim Biophys Acta. 2002;1602:97–113. doi: 10.1016/s0304-419x(02)00039-2. [DOI] [PubMed] [Google Scholar]
- Rangarajan A., Hong S. J., Gifford A., Weinberg R. A. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6:171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Marion R. M., Strati K., Li H., Tejera A., Schoeftner S., Ortega S., Serrano M., Blasco M. A. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141–154. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Shiels P. G., Kind A. J., Campbell K. H., Waddington D., Wilmut I., Colman A., Schnieke A. E. Analysis of telomere lengths in cloned sheep. Nature. 1999;399:316–317. doi: 10.1038/20580. [DOI] [PubMed] [Google Scholar]
- Lanza R. P., Cibelli J. B., Blackwell C., Cristofalo V. J., Francis M. K., Baerlocher G. M., Mak J., Schertzer M., Chavez E. A., Sawyer N., Lansdorp P. M., West M. D. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- Wakayama T., Shinkai Y., Tamashiro K. L., Niida H., Blanchard D. C., Blanchard R. J., Ogura A., Tanemura K., Tachibana M., Perry A. C., Colgan D. F., Mombaerts P., Yanagimachi R. Cloning of mice to six generations. Nature. 2000;407:318–319. doi: 10.1038/35030301. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Ferrier P., Aslam S., Burl S., Denning C., Wylie D., Ross A., de Sousa P., Wilmut I., Cui W. Proliferative lifespan is conserved after nuclear transfer. Nat Cell Biol. 2003;5:535–538. doi: 10.1038/ncb992. [DOI] [PubMed] [Google Scholar]
- Wang S., Hu C., Zhu J. Transcriptional silencing of a novel hTERT reporter locus during in vitro differentiation of mouse embryonic stem cells. Mol Biol Cell. 2007;18:669–677. doi: 10.1091/mbc.E06-09-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Zhu J., Woods D., McMahon M., Bishop J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhu J. The hTERT gene is embedded in a nuclease-resistant chromatin domain. J Biol Chem. 2004;279:55401–55410. doi: 10.1074/jbc.M411352200. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang S., Popova E. Y., Grigoryev S. A., Zhu J. Rearrangement of upstream sequences of the hTERT gene during cellular immortalization. Genes Chromosomes Cancer. 2009;48:963–974. doi: 10.1002/gcc.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A., Wernig M., Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M., Maherali N., Breault D. T., Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T. S., Hanna J., Zhang X., Ku M., Wernig M., Schorderet P., Bernstein B. E., Jaenisch R., Lander E. S., Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessain S. K., Yu H., Reddel R. R., Beijersbergen R. L., Weinberg R. A. Methylation of the human telomerase gene CpG island. Cancer Res. 2000;60:537–541. [PubMed] [Google Scholar]
- Devereux T. R., Horikawa I., Anna C. H., Annab L. A., Afshari C. A., Barrett J. C. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 1999;59:6087–6090. [PubMed] [Google Scholar]
- Rice J. C., Briggs S. D., Ueberheide B., Barber C. M., Shabanowitz J., Hunt D. F., Shinkai Y., Allis C. D. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- Peters A. H., Kubicek S., Mechtler K., O'Sullivan R. J., Derijck A. A., Perez-Burgos L., Kohlmaier A., Opravil S., Tachibana M., Shinkai Y., Martens J. H., Jenuwein T. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- Bernstein B. E., Kamal M., Lindblad-Toh K., Bekiranov S., Bailey D. K., Huebert D. J., McMahon S., Karlsson E. K., Kulbokas E. J., 3rd, Gingeras T. R., Schreiber S. L., Lander E. S. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Silva J., Barrandon O., Nichols J., Kawaguchi J., Theunissen T. W., Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofari G., Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadeneau C., Siegel P., Harley C. B., Muller W. J., Bacchetti S. Telomerase activity in normal and malignant murine tissues. Oncogene. 1995;11:893–898. [PubMed] [Google Scholar]
- Counter C. M., Botelho F. M., Wang P., Harley C. B., Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994;68:3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Wang H., Bishop J. M., Blackburn E. H. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening [see comments] Proc Natl Acad Sci U S A. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Chan S., Chang J., Fulton T. B., Krauskopf A., McEachern M., Prescott J., Roy J., Smith C., Wang H. Molecular manifestations and molecular determinants of telomere capping. Cold Spring Harb Symp Quant Biol. 2000;65:253–263. doi: 10.1101/sqb.2000.65.253. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomerase activation One step on the road to cancer? Trends Genet. 1999;15:109–112. doi: 10.1016/s0168-9525(98)01681-3. [DOI] [PubMed] [Google Scholar]
- Kozik A., Bradbury E. M., Zalensky A. Increased telomere size in sperm cells of mammals with long terminal (TTAGGG)n arrays. Mol Reprod Dev. 1998;51:98–104. doi: 10.1002/(SICI)1098-2795(199809)51:1<98::AID-MRD12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Piatyszek M. A., Rainey W. E., Byrd W., Shay J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bekaert S., Derradji H., Baatout S. Telomere biology in mammalian germ cells and during development. Dev Biol. 2004;274:15–30. doi: 10.1016/j.ydbio.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Armanios M., Greider C. W. Telomerase and cancer stem cells. Cold Spring Harb Symp Quant Biol. 2005;70:205–208. doi: 10.1101/sqb.2005.70.030. [DOI] [PubMed] [Google Scholar]
- Liu L., Saldanha S. N., Pate M. S., Andrews L. G., Tollefsbol T. O. Epigenetic regulation of human telomerase reverse transcriptase promoter activity during cellular differentiation. Genes Chromosomes Cancer. 2004;41:26–37. doi: 10.1002/gcc.20058. [DOI] [PubMed] [Google Scholar]
- Renaud S., Loukinov D., Abdullaev Z., Guilleret I., Bosman F. T., Lobanenkov V., Benhattar J. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007;35:1245–1256. doi: 10.1093/nar/gkl1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.