Abstract
Cytokines play an emerging role as neurotransmitters, neuromodulators, and neurohormones in the brain. This paradigm shift in cytokine function offers a new framework to understand their roles in ameliorating neurodegenerative disorders, such as Alzheimer’s disease (AD). Molecular adjuvant therapy of AD animal models with glatiramer acetate induces anti-inflammatory responses and therapeutic effects. Although these effects are potentially mediated through anti-inflammatory cytokine signaling, the exact molecular identities and pathways are poorly understood. Here, we show that virus-mediated expression of the mouse interleukin (IL)-4 gene in β-amyloid precursor protein + presenilin-1 (APP+PS1) bigenic mice attenuates AD pathogenesis. Introduction of an adeno-associated viral (AAV) vector encoding IL-4 into the hippocampus resulted in sustained expression of IL-4, reduced astro/microgliosis, amyloid-β peptide (Aβ) oligomerization and deposition, and enhanced neurogenesis. Moreover, increased levels of IL-4 improved spatial learning, promoted phosphorylation of N-methyl-d-aspartate receptor subunit 2B at Tyr 1472, and enhanced its cell surface retention both in vivo and in vitro. Our data suggest that neuronal anti-inflammatory cytokine signaling may be a potential alternative target for non-Aβ-mediated treatment of AD.—Kiyota, T., Okuyama, S., Swan, R. J., Jacobsen, M. T., Gendelman, H. E., Ikezu, T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J.
Keywords: IL-4, AAV therapy, memory formation, neurogenesis, NMDA receptor
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder in the world (1). Accumulation and extracellular deposition of aggregated amyloid-β peptide (Aβ) has been suggested as the root of the disease. Aβ immunization of transgenic mice expressing a β-amyloid precursor protein (APP) mutant resulted in significant clearance of Aβ deposition (2), as well as restored cognitive function (3). Passive Aβ immunotherapies utilizing antibodies against Aβ were also proven to be effective in Aβ clearance in the brain (4, 5). Therefore, Aβ immunotherapy has been an emerging therapeutic approach to combat AD (6). However, recent clinical studies report somewhat disappointing results for Aβ immunotherapy (7). This suggests that Aβ may not be an effective therapeutic target after the onset of later-stage pathology, as demonstrated by neurofibrillary tangle formation and neurodegeneration. Thus, alternative non-Aβ therapy to ameliorate secondary disease progression must be investigated.
Recent advancements in neuroimmunological studies of AD animal models include, but are not limited to, non-Aβ vaccination therapy of AD animal models, beneficial effects of nonsteroidal anti-inflammatory drugs, and the critical role of regulatory cytokines (such as transforming growth factor-β) on β-amyloidosis (Aβ aggregation and deposition in the brain), and neurodegeneration (8, 9). Although Aβ triggers the accumulation of astrocytes and microglia that produce proinflammatory cytokines and chemokines causing chronic neuroinflammation (10), glatiramer acetate (GA)-treated animals develop Th2 cells, which suppress the inflammatory response and secrete anti-inflammatory cytokines such as IL-4 (11, 12). In a mouse model of AD, GA with specific adjuvants induces clearance of Aβ deposition in the APP mouse brain (13, 14). GA immunotherapy especially leads to improved memory function, enhanced neurogenesis, reduced β-amyloidosis, and increased numbers of CD11c+ dendritic cells, thus demonstrating the anti-inflammatory effect of GA against AD pathogenesis (13, 15). Treatment with IL-4, an anti-inflammatory cytokine, mirrors these effects (15), suggesting that anti-inflammatory cytokines play an important role in mediating the cellular pathology.
IL-4 has been characterized as a potential modulator of neuronal activities in the brain. IL-4 receptors are expressed in the hippocampus, and down-regulation of IL-4 causes aging-related deficits of hippocampal long-term potentiation (LTP) (16). Treatment of rat hippocampus with IL-4 attenuates Aβ-induced LTP deficiency, as well as the expression of proinflammatory IL-1β in rat hippocampus (17). Moreover, IL-4 stimulation of human macrophages or microglia enhances Aβ degradation (18, 19). These findings suggest that the expression of anti-inflammatory cytokines, such as IL-4, may have direct effects on the regulation of neuroinflammation and β-amyloidosis-related pathology. Therefore, we hypothesize that anti-inflammatory cytokine signaling efficiently enhances Aβ clearance, synaptic transmission, and amelioration of AD progression.
For this purpose, we have employed our established adeno-associated viral (AAV) 1/2 hybrid recombinant gene delivery system to induce the neuronal expression of murine IL-4 in mouse hippocampi (20). We analyzed the effect of IL-4 expression in double transgenic (Tg) mice expressing familial AD mutants of APP (21) and presenilin-1 (22) (APP+PS1 Tg), which exhibit accelerated Aβ deposition and memory impairment as compared to APP Tg mice (23).
MATERIALS AND METHODS
Animals
All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee of University of Nebraska Medical Center. Tg2576 mice expressing the Swedish mutation of human APP695 were obtained from Dr. George Carlson (McLaughlin Research Institute, Great Falls, MT, USA) and Dr. Karen Hsiao-Ashe (University of Minnesota, Minneapolis, MN, USA) through the Mayo Clinic (Rochester, MN, USA) (21). PS1 mutant mice (M146L line 6.1) were provided by Dr. Karen Duff (Columbia University, New York, NY, USA) through the University of South Florida (Tampa, FL, USA) and maintained as PS1 transgene homozygotes (22). Tg2576 and PS1 mice were crossed to generate APP+PS1 bigenic mice in the B6/129 F1 strain, and genotyping was performed as previously described (20). Age-matched non-Tg mice in the matched B6/129 F1 strain (Jackson laboratory, Bar Harbor, ME, USA) were maintained by intercrossing in the same facility.
AAV-IL-4 gene construction, AAV1/2 hybrid virus generation, and purification
To construct an AAV vector for expressing the mouse IL-4 gene, PCR primers (5′-AAAGGATCCGCCACCATGGGTCTCAACCCCCAGCT-3′ and 5′-AAACTCGAGTCAGTACTACGAGTAATCCATTTGC-3′) and a cDNA template (ATCC pCD-2A-E3) were used for proofreading PCR amplification of the 453-bp cDNA region. The PCR amplicon was digested with BamHI-XhoI and subcloned into the corresponding restriction sites of pAAV2-MCS-WPRE (20) to develop pAAV2-MCS-WPRE-IL-4. The PCR-amplified region was entirely DNA sequenced prior to AAV virus generation. For AAV-GFP, pGFP vector was used (provided by Dr. Ronald Klein, Louisiana State University, Shreveport, LA, USA) (24). Recombinant AAV virus expressing IL-4 or GFP was generated, purified, and titrated as described previously (20).
Stereotactic injection
AAV viruses were bilaterally injected into mouse hippocampi as described previously (20). Briefly, mice at 3 mo of age received an intraperitoneal injection of ketamine/xylazine anesthesia (100 mg/kg ketamine and 20 mg/kg xylazine). After mice were immobilized in a stereotactic apparatus (Stoelting, Wood Dale, IL, USA), a linear skin incision was made over the bregma, and a 1-mm burr hole was drilled in the skull 2.1 mm posterior and 1.8 mm lateral to the bregma on both sides using a hand-held driller. Two microliters of saline containing AAV-GFP or AAV-IL-4 (2×109 VP) was injected into the hippocampus 1.8 mm below the surface of the skull using a 10-μl Hamilton syringe.
Bromodeoxyuridine (BrdU) administration and tissue preparation
The cell-proliferation marker BrdU was intraperitoneally injected (50 mg/kg of body weight) twice daily every 12 h for 2.5 d (5 total injections) to label proliferating cells as described previously (15). Three weeks after the first BrdU injection, mice were deeply anesthetized with isoflurane and transcardially perfused with 25 ml of ice-cold phosphate-buffered saline (PBS; pH 7.0). The brain was rapidly removed; the left hemisphere was immediately frozen in dry ice for biochemistry, and the right hemisphere was immersed in freshly depolymerized 4% paraformaldehyde for 48 h at 4°C and cryoprotected by successive 24-h immersions in 15% and 30% sucrose in 1× PBS. Fixed, cryoprotected brains were frozen and sectioned coronally using a Cryostat (Leica, Bannockburn, IL, USA) with sections collected serially and stored at −80°C before performing immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed using coded section slides in a masked procedure, as described previously (20). Briefly, immunohistochemistry was performed using specific antibodies (Abs) to identify pan-Aβ (rabbit polyclonal antibody, pAb, 1:100; Zymed, San Francisco, CA, USA), glial fibrillary acidic protein (GFAP; astrocyte marker, rabbit pAb, 1:2000; Dako, Carpenteria, CA, USA), ionized calcium binding adaptor molecule 1 (IBA1; microglia marker, rabbit pAb, 1:1000; Wako, Richmond, VA, USA), and doublecortin (Dcx; premature neuronal marker, goat pAb, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunodetection was visualized using Envision Plus (Dako) with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) for Aβ, GFAP, and IBA1 staining. For Dcx staining, biotin-conjugated anti-goat secondary IgG and Vectastain ABC Elite kit (Vector Laboratories) were used. Compact plaques were stained with 1% thioflavin S (TS) (Sigma, St. Louis, MO, USA) in 50% ethanol. For immunofluorescence, sections were incubated with anti-BrdU (6 μg/ml) (mouse mAb; Roche Diagnostics, Indianapolis, IN, USA), followed by incubation with Alexa Fluor488-conjugated anti-mouse IgG (1:1000; Invitrogen, Carlsbad, CA, USA). The sections were then incubated with biotin-conjugated anti-NeuN (neuron-specific nuclear protein, mouse mAb, 1:500; Millipore, Billerica, MA, USA), followed by incubation with Streptavidin-Alexa Fluor568 (1:1000) (Invitrogen). For quantification analysis, the areas of Aβ loads and TS-positive (TS+) plaques were analyzed using image analysis software [ImageJ, National Institutes of Health (NIH), Bethesda, MD, USA] at 300-μm intervals in ten 30-μm thickness coronal sections from each mouse. Five mouse brains per group were analyzed. The numbers of GFAP+ astrocytes and IBA1+ microglia around Aβ plaques in the hippocampus were counted at 300-μm intervals in ten 30-μm thickness coronal sections from each mouse. Three TS+ plaques were randomly chosen per section, and 5 mouse brains/group were analyzed (30 plaques/animal) for counting the number of astrocytes and microglia surrounding the plaques by 2 personnel in a masked procedure, according to the published method (20). The numbers of Dcx+ and BrdU+NeuN+ cells in the subgranular zone (SGZ) of the dentate gyrus were calculated by a stereological method based on the Cavalieri principle as described previously (25). Ten 30-μm thickness coronal sections at 300-μm intervals from each mouse were used for the quantification.
Two-day radial arm water maze (RAWM)
The RAWM task was run as described previously, with minor modifications (20, 26). Animals were introduced into the perimeter of a circular water-filled tank, 110 cm in diameter and 91 cm in height (San Diego Instruments, San Diego, CA, USA) with triangular inserts placed in the tank to produce 6 swim paths radiating out from a central area. Spatial cues for mouse orientation were present on the walls of the tank. At the end of one arm, a 10-cm circular Plexiglas platform was submerged 1 cm deep—hidden from the mice. On d 1, 15 trials (12 trials with visible platform followed by 3 trials with hidden platform) were run in 5 blocks of 3. A cohort of 4 mice was run sequentially for each block. After each 3-trial block, a second cohort of mice was run, creating an extended rest period before mice were exposed to the second block. The target arm location remained constant for a given mouse throughout the test. Each trial lasted 1 min, and an error was scored each time the body of the mouse, excluding tail, entered the wrong arm, entered the arm with the platform but did not climb on it, or did not make a choice for 20 s. Each trial ended when the mouse climbed onto and remained on the hidden platform for 10 s. The mouse was given 20 s to rest on the platform between each trial. On d 2, the mice were run in exactly the same manner as d 1, except that the platform was hidden for all trials. Between blocks 4 and 5 on d 1, and blocks 9 and 10 on d 2, an additional break of 30 min was given to each mouse to test short-term memory recall. The errors on each block were averaged and used for statistical analysis.
Enzyme-linked immunosorbent assay
Protein extraction was performed as described previously (n=3/group) (27). IL-4 was measured using a commercially available enzyme immunoassay (mouse IL-4 enzyme-linked immunosorbent assay set, BD OptEIA; BD Biosciences, San Jose, CA, USA), according to manufacturer’s instruction.
Primary neuronal culture
Primary neurons were cultured as described previously (28) and treated with recombinant mouse IL-4 at a concentration of 0, 0.5, 1, 2, or 10 ng/ml for indicated periods. Cells were lysed with lysis buffer (10 mM Tris-Cl, pH 7.4; 100 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1% Triton X-100; 10% glycerol; 0.1% SDS; 0.5% deoxycholate; 1× protease inhibitor cocktail (Sigma); 1 mM NaF; 20 mM Na4P2O7; and 2 mM Na3VO4), and subjected to immunoblot analysis.
Surface biotinylation assay
The surface of neurons was biotinylated as described previously, with a minor modification (29). Primary neurons were plated at 1.6 × 106 cells/well in plastic 6-well plates. Neurons were treated with recombinant mouse IL-4 (2 ng/ml) and/or oligomeric Aβ42 (1 μM) for 30 min. Oligomeric Aβ42 was prepared from synthetic human Aβ1-42 peptide (Invitrogen), as described previously (30). After treatment, neurons were placed on ice and rinsed in ice-cold PBS (pH 8.0), incubated in PBS (pH 8.0) containing 1.5 mg/ml sulfo-NHS-LC-biotin (Pierce, Rockford, IL, USA) for 20 min at 4°C, washed 3 times with PBS + 100 mM glycine, and then lysed in 200 μl of lysis buffer [PBS with 0.1% SDS, 1% Triton X-100, 1× protease inhibitor cocktail (Sigma), 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4]. To isolate biotinylated proteins, 80% of the cell lysate was incubated with 50 μl NeutrAvidin agarose (Pierce) for 1 h with rotations. The agarose was washed 3 times with ice-cold PBS, boiled in SDS-PAGE sample buffer to break the cross-linker, and subjected to immunoblot analysis (n=3/group).
Immunoblotting
Protein extraction of extracellular, intracellular, and membrane-enriched fractions and immunoblot analysis for Aβ oligomers were performed as described previously (31). Briefly, 30 μg of extracellular enriched protein samples (n=6/group) were analyzed by electrophoresis on 16% SDS-polyacrylamide Tris-Tricine gels (32) and electroblotted to 0.2-μm pore size polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories). For the other immunoblotting, membrane-enriched protein samples (n=6/group) or lysates from mouse primary neuron cultures (n=3/group) were run on Tris-glycine polyacrylamide gel and electroblotted to 0.45-μm pore size PVDF membranes (Immobilon-P; Millipore, Billerica, MA, USA). Membranes were blocked in 5% skim milk/TBST (Tris-buffered saline-Tween 20), and incubated with Aβ mAb (6E10, 1:1000; Covance, Emeryville, CA, USA), NR2B goat pAb (1:1000; Santa Cruz Biotechnology), phospho-Tyr1472 NR2B rabbit pAb (1:1000, Invitrogen), or β-actin mAb (Sigma). HRP-conjugated anti-mouse, rabbit, or goat secondary IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was used. Immunoreactive bands were detected with SuperSignal West Pico or Femto Chemiluminescent substrate (Pierce) and quantitatively analyzed by normalizing band intensities to the controls on scanned films by ImageJ software (NIH).
Statistics
All data were normally distributed and presented as mean ± se values of measurement. In the case of a single mean comparison, data were analyzed by Student’s t test. In case of multiple mean comparisons, the data were analyzed by 1-way ANOVA and Newman-Keuls posttest or 2-way repeated measures ANOVA, followed by Bonferroni multiple-comparison test using statistics software (Prism 4.0; GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were regarded as a significant difference.
RESULTS
AAV-mediated expression of mouse IL-4 in the brain
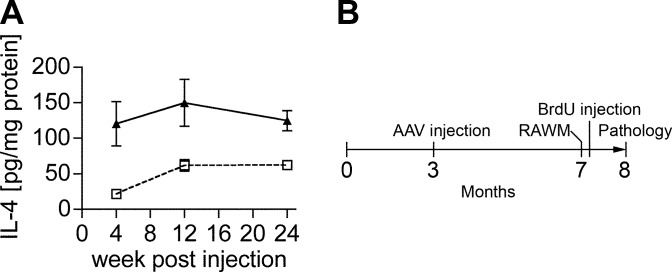
We first tested the time course of recombinant IL-4 protein expression in non-Tg mice after stereotactic hippocampal injection of the AAV serotype 1/2 hybrid, which shows long-term neuronal expression of recombinant proteins in hippocampal neurons, including granular neurons in the dentate gyrus (20). We determined the appropriate number of viral particles (VPs) by dose-response of the AAVs in non-Tg mice using AAV-IL-4 or negative control AAV-GFP. The AAV injections themselves did not induce gliosis in mouse brain (20). AAV-IL-4 produced IL-4 expression of 120.4, 149.8, and 124.7 pg/mg at 4, 12, and 24 wk, respectively, which is significantly higher than AAV-GFP-injected mouse hippocampus (21–63 pg/mg of IL-4 over the same time period, Fig. 1A), demonstrating long-term expression of the recombinant protein.
Figure 1.
AAV injection and strategy. A) AAV-mediated somatic gene transfer of IL-4. Non-Tg mice at 3 mo of age received bilateral hippocampal injection of AAV-GFP (2×109 viral particles (VP)/hippocampus, dotted line) or AAV-IL-4 (2×109 VP/hippocampus, solid line), were sacrificed 4, 12, and 24 wk postinjection. The amounts of IL-4 in mouse hippocampal protein extracts were quantified by enzyme-linked immunosorbent assay, showing sustained expression of IL-4. Error bars represent se (n=3). B) Experimental design. APP+PS1 mice received bilateral hippocampal injection of AAV-GFP or AAV-IL-4 at 3 mo of age and were tested by 2-d RAWM task at 7 mo of age. Non-Tg mice served as a positive control for the spatial learning task. Mice were intraperitoneally injected with BrdU 5 times for 2.5 d, 21 d before being sacrificed at 8 mo of age.
IL-4 suppresses gliosis and β-amyloidosis in APP+PS1 Tg mice
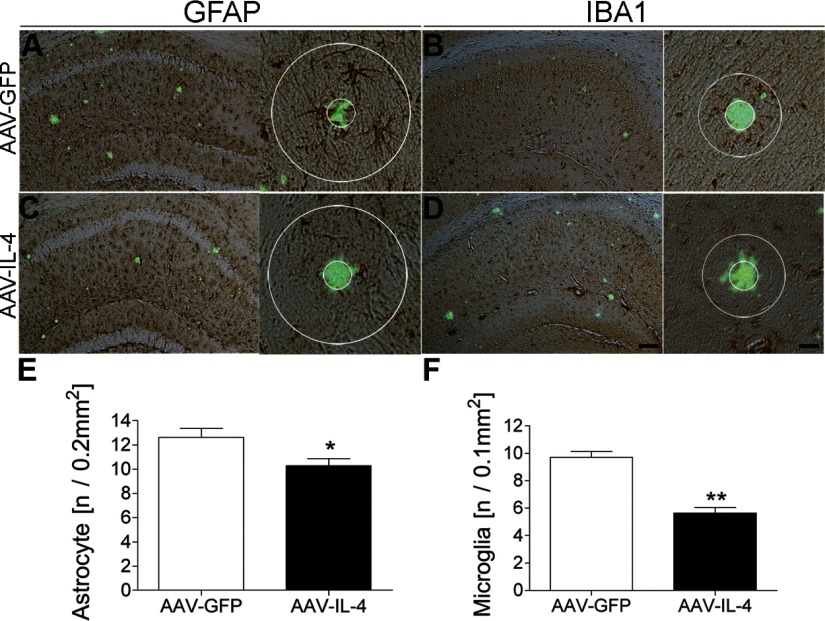
Aβ peptides and deposition cause a reactive gliosis and subsequent neuroinflammation as another aspect of AD-like neuropathogenesis (28, 33, 34). To understand the effect of anti-inflammatory cytokines on β-amyloidosis, we injected AAV-IL-4 or control AAV-GFP virus into bilateral hippocampal regions of APP+PS1 mice at 3 mo of age with neuropathological analysis at 8 mo of age (Fig. 1B). AAV-IL-4-injection significantly reduced astrogliosis (18.7% reduction vs. AAV-GFP-injected group, Fig. 2A, C, E) and microglial accumulation around TS+ compact plaques (41.8% reduction vs. AAV-GFP-injected group, Fig. 2B, D, F). These results suggest that anti-inflammatory cytokine IL-4 can attenuate neuroinflammation induced by Aβ (35, 36).
Figure 2.
Gene delivery of IL-4 suppresses glial accumulation in APP+PS1 mice. A–D) APP+PS1 mice injected with AAV-GFP (A, B) or AAV-IL-4 (C, D) at 3 mo of age were sacrificed at 8 mo of age. Hippocampal frozen sections were immunostained for GFAP (astrocyte; A, C) or IBA1 (microglia; B, D), and counterstained by TS. Scale bars = 200 μm (low magnification; left); 40 μm (high magnification; right). E, F) Quantification of GFAP+ (E) or IBA1+ (F) cells found within the circle surrounding TS+ Aβ plaques. Radii of outer concentric circles in GFAP+ cells were 100 μm greater than the inner circles that surrounded the compact plaques (A, C), and 50 μm greater in IBA1+ cells (B, D). Error bars = se (n=5/group, 10 sections/brain). *P < 0.05, **P < 0.01 vs. AAV-GFP group; Student’s t test.
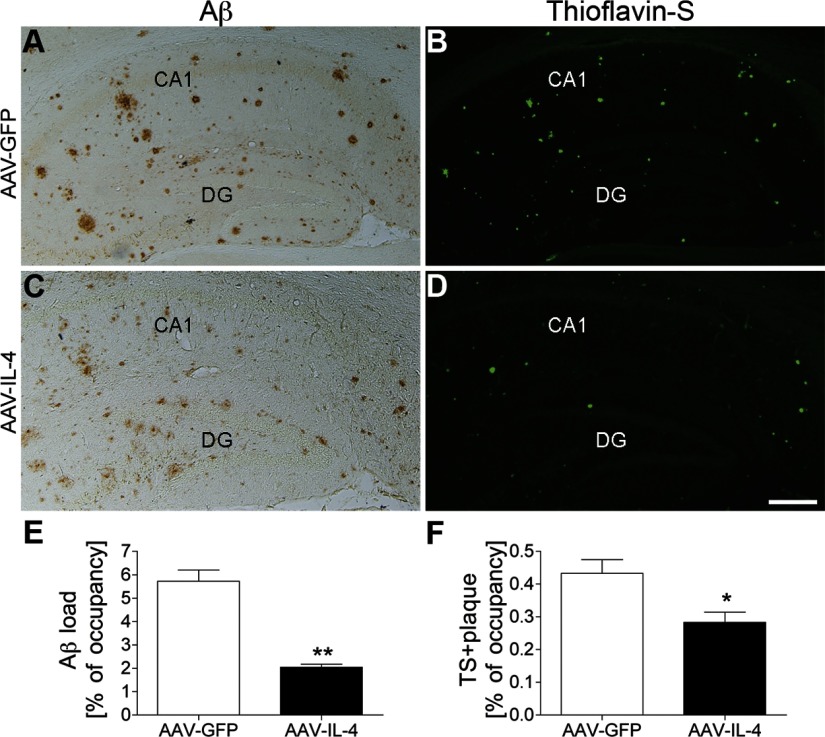
Since GA treatment significantly induces the anti-inflammatory response and attenuates β-amyloidosis (15), we examined whether IL-4 expression affects β-amyloidosis. The AAV-IL-4-injected group exhibited a significantly reduced hippocampal Aβ load (74.0% less than AAV-GFP-injected group, Fig. 3A, C, E) and fewer TS+ compact plaques (50.0% less than AAV-GFP group, Fig. 3B, D, F), demonstrating the anti-β-amyloidosis effect of IL-4.
Figure 3.
Aβ deposition in the hippocampal region of gene-delivered APP+PS1 mouse brain. A–D) Frozen brain sections of APP+PS1 mice injected with AAV-GFP (A, B) or AAV-IL-4 (C, D) were immunostained with anti-Aβ antibody (A, C) and counterstained by TS for compact plaque (B, D). E, F) Total Aβ load (E) and TS+ area (F) in hippocampal regions were quantified in APP+PS1 mice injected with AAV-GFP (GFP) or AAV-IL-4 (IL-4). Error bars = se (n=5/group, 10 sections/brain). *P < 0.05, **P < 0.01 vs. AAV-GFP group; Student’s t test. Scale bar = 500 μm.
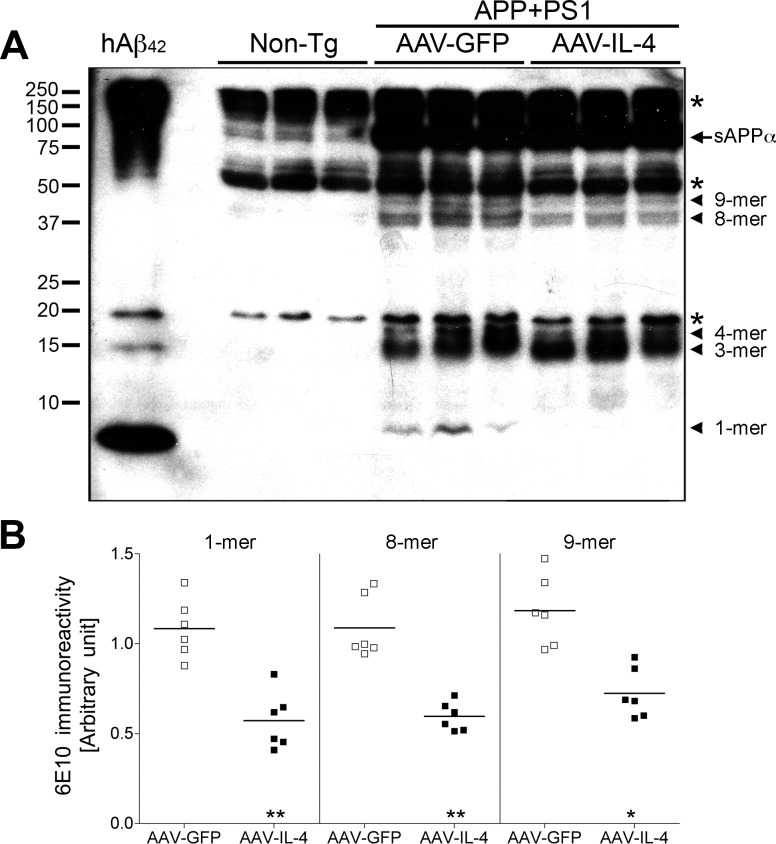
Since oligomeric forms of Aβ produce greater Aβ-induced neurophysiological impairment (37, 38), hippocampal degeneration (39), and memory impairment (31, 40), we examined the effect of AAV-IL-4 on the generation of Aβ oligomeric species by SDS-PAGE and immunoblotting of extracellular enriched mouse hippocampal fractions (Fig. 4A) as described previously (31). Although the amounts of trimer (3-mer) and tetramer (4-mer) were unchanged between AAV-GFP and AAV-IL-4-injected groups (Supplemental Fig. 1), those of monomer (1-mer), octamer (8-mer), and nonamer (9-mer) were significantly decreased in the AAV-IL-4-injected group (52.7, 54.7, 61.1, and 59.4% of the AAV-GFP-injected group, respectively) (Fig. 4B). We could not detect other Aβ oligomeric forms corresponding to dimer, hexamer, or dodecamer in these experiments.
Figure 4.
Aβ oligomer formation in the hippocampus of gene-delivered APP+PS1 mouse brain. A) Immunoblotting using 6E10 antibody showed specific Aβ oligomers in the extracellular-enriched fraction of AAV-injected APP+PS1 mouse hippocampus. Arrowheads indicate respective migration positions of multimers. Arrow indicates sAPPα fragments processed from full-length APP. Asterisk indicates nonspecific band. B) Band luminescent intensities for 1-, 8-, and 9-mers were quantified by Image J software. Bars represent average values (n=6/group). *P < 0.05, **P < 0.01 vs. AAV-GFP group; Student’s t test.
Enhanced neurogenesis in AAV-IL-4-injected APP+PS1 mice
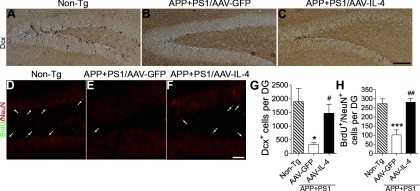
Next, we examined the effect of AAV-IL-4 on neurogenesis and neuronal differentiation, since previous GA immunotherapy of APP mice showed significant enhancement of neurogenesis in the SGZ of the dentate gyrus (15). Additionally, Aβ oligomers potently induce loss of synapses and impair neuronal differentiation (40, 41). We examined the expression of Dcx, a marker for newly generated premature neurons, in the SGZ (Fig. 5A–C, G) (42). The number of Dcx+ cells in the dentate gyrus of AAV-GFP-injected APP+PS1 mice was significantly reduced (83.3% less than non-Tg mice, Fig. 5A, B, I). However, the AAV-IL-4-injected APP+PS1 mice show significantly increased Dcx+ cells in the SGZ (465.2% increase vs. AAV-GFP-injected APP+PS1 mice, Fig. 5C, G), suggesting enhanced neuronal differentiation or overall neuronal proliferation in response to IL-4 expression. To determine the effect of IL-4 on neuronal proliferation and differentiation, the animals were temporally injected with BrdU to track cell proliferation 3 wk prior to euthanasia, and the neuronal differentiation of BrdU-incorporated cells in the SGZ was evaluated by double immunofluorescence of BrdU and NeuN, a differentiated neuronal marker (Fig. 5D–F, H). The number of BrdU+NeuN+ cells was significantly increased in the AAV-IL-4-injected APP+PS1 mice (277.2% increase vs. AAV-GFP-injected APP+PS1 mice, Fig. 5F, H). These data demonstrate that neuronal expression of IL-4 can enhance the number of newly synthesized neurons, which may be involved in memory recovery in APP+PS1 mice.
Figure 5.
Gene delivery of IL-4 enhances neurogenesis in SGZ of APP+PS1 mice. A–C) Representative images of Dcx staining in the dentate gyrus of non-Tg (A) or APP+PS1 mice injected with AAV-GFP (B) or AAV-IL-4 (C) into the hippocampus at 3 mo of age, and intraperitoneally injected with BrdU 3 wk prior to euthanasia at 8 mo of age. D–F) Immunofluorescence of BrdU (green) and NeuN (red) in the dentate gyrus of non-Tg (D) or APP+PS1 mice injected with AAV-GFP (E) or AAV-IL-4 (F). G, H) Quantification of Dcx+ (G) or BrdU+Neu+ cells (H) in SGZ. Error bars = se (n=5/group, 10 sections/brain). *P < 0.05, ***P < 0.001 vs. non-Tg group; #P < 0.05, ##P < 0.01 vs. AAV-GFP group; 1-way ANOVA and Newman-Keuls posttest. Scale bars = 200 μm (C); 100 μm (F).
IL-4 inhibits spatial learning impairment in APP+PS1 mice
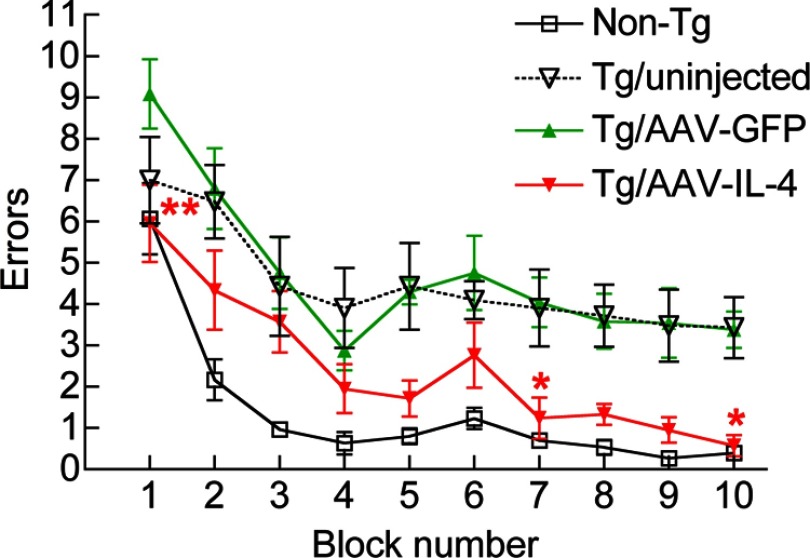
Enhanced neurogenesis and reduced β-amyloidosis strongly suggest a beneficial effect of IL-4 on cognitive function of APP+PS1 mice. For that purpose, we subjected the non-Tg and APP+PS1 mice with various treatments to a 2-d RAWM task at 7 mo of age (Figs. 1B and 6) according to the established protocol (20, 26). Day 1 consisted of 4 visible platform tests (T1–T4), followed by one hidden platform test (T5) after a short-term (30 min) break. Day 2 consisted of 5 hidden platform tests (T6–T10) with a short-term break (30 min) between T9 and T10. Error counts at T7–T9 reflect established memory, and those at T6 and T10 reflect short-term memory recall after overnight (T6) or 30-min breaks (T10). The age-matched non-Tg mouse group modeled the wild-type learning curve. In contrast, AAV-GFP-injected and uninjected APP+PS1 groups showed significantly higher errors than the non-Tg group throughout the trials, indicating impaired spatial memory acquisition and recall. On the other hand, the AAV-IL-4-injected groups showed significantly reduced error numbers compared to the AAV-GFP-injected or uninjected group, indicating secured memory acquisition and recall in this experimental paradigm. The average swimming speeds in the open water were unchanged among tested groups, ruling out the possibility of differences in their swimming abilities distorting the data (Supplemental Fig. 2).
Figure 6.
Gene delivery of IL-4 improves memory function of APP+PS1 mice. APP+PS1 mice received bilateral hippocampal injection of AAV-GFP or AAV-IL-4 at 3 mo of age and were tested by the 2-d RAWM task at 7–8 mo of age. Non-Tg and uninjected APP+PS1 mice (Tg/uninjected group) serve as positive/negative controls for the spatial learning task. Error bars = se (n=8/group). *P < 0.05, **P < 0.01 vs. AAV-GFP group; 2-way repeated-measures ANOVA and Bonferroni post hoc test.
IL-4 restores NR2B expression in neurons of the hippocampus in APP+PS1 mice
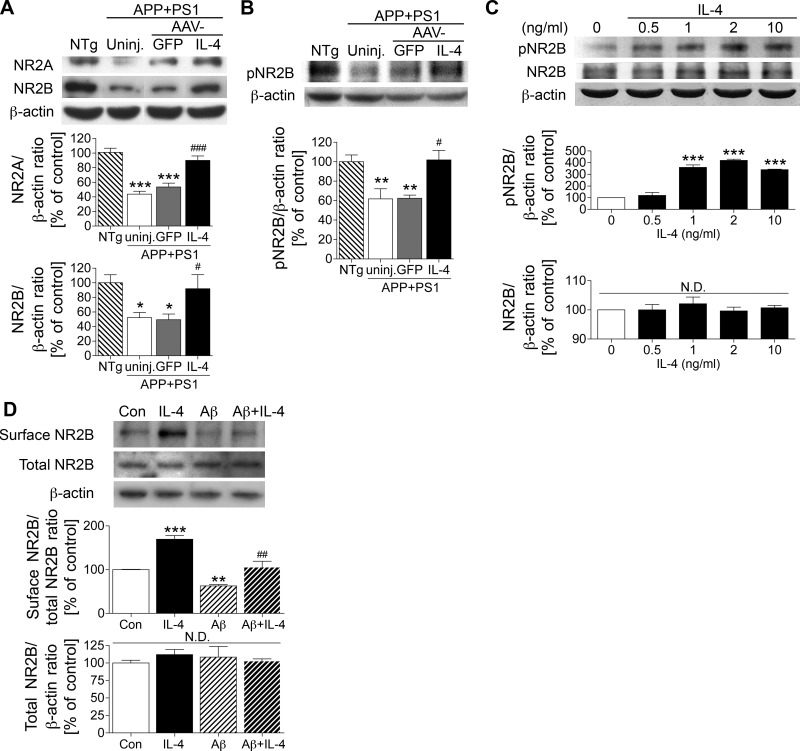
Aβ-induced loss of synaptic transmission is one potential mechanism of memory impairment in APP+PS1 mice. For example, Aβ decreases expression of N-methyl-d-aspartic acid (NMDA) receptor (NR) subunits on the cell surface and suppresses NMDA-evoked currents in pyramidal neurons (29). Using membrane-enriched fractions of hippocampal tissues, we could also confirm that expression of NR2A and NR2B, components of the NMDA receptor channel, was significantly reduced in the hippocampus of uninjected or AAV-GFP-injected APP+PS1 mice as compared to non-Tg mouse hippocampus (43.4 or 53.1% of non-Tg mice for NR2A and 52.3 or 49.4% for NR2B, Fig. 7A). However, AAV-IL-4 injection rescued expression of both NR2A and NR2B (91.9 and 102.0% of non-Tg mice, Fig. 7A). We next examined the phosphorylation of the NR2B subunit at Tyr1472, which is critically involved in the synaptic insertion of the receptor (29, 43). We found that the Tyr1472 phosphorylation is significantly reduced in the hippocampus of uninjected or AAV-GFP-injected APP+PS1 mouse hippocampus as compared to those of non-Tg mice (61.8 or 62.5% of non-Tg mice, Fig. 7B). However, AAV-IL-4 injection also restored the Tyr1472 phosphorylation to non-Tg level (102.0% of non-Tg mice, Fig. 7B).
Figure 7.
IL-4 promotes the expression and phosphorylation of NR2B. A) Immunoblotting (top) and quantification (bottom) of NR2A and NR2B in the membrane-enriched fraction of the mouse hippocampus in non-Tg (NTg), uninjected APP+PS1 (uninj.), APP+PS1 mice injected with AAV-GFP (GFP), and APP+PS1 mice injected with AAV-IL-4 (IL-4). B) Immunoblotting (top) and quantification (bottom) of phospho-Tyr1472 NR2B (pNR2B) in the same fraction of the hippocampus. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NTg group; #P < 0.05, ###P < 0.001 vs. AAV-GFP group; 1-way ANOVA and Newman-Keuls post hoc test. Error bars = se (n=6/group). C) Immunoblotting (top) and quantification (bottom) of pNR2B, NR2B, and β-actin in IL-4-treated primary cultured neurons. D) Immunoblotting (top) and quantification (bottom) of biotinylated NR2B at the cell surface level, total NR2B in IL-4 and/or oligomeric Aβ42-treated primary cultured neurons. **P < 0.01, ***P < 0.001 vs. control; ##P < 0.01 vs. Aβ42 group; 1-way ANOVA and Newman-Keuls post hoc test. Error bars represent se (n=3/group). Con, control; N.D., no significant difference.
The effect of IL-4 on NR2B phosphorylation in vivo was confirmed with IL-4 treatment of mouse primary neuron culture in vitro. We found that IL-4 treatment increased the phosphorylation of NR2B at Tyr1472 in a dose-dependent manner (up to 320% increase over basal level, Fig. 7C), although the amount of total NR2B was unchanged by IL-4 treatment. To determine whether IL-4 enhances expression of NR2B on the cell surface, the IL-4-treated mouse primary neurons were subjected to cell surface biotinylation, followed by affinity precipitation of biotinylated molecules and immunoblotting for NR2B. IL-4 increased cell surface NR2B expression (169.7% increase over basal level, Fig. 7D). Consistent with a previous report (29), treatment with oligomeric Aβ reduced the surface NR2B expression, but coincubation with oligomeric Aβ and IL-4 partially rescued NR2B expression (62.7 and 105.7% increase over basal level, Fig. 7D). These data suggest that IL-4 enhances NR2B phosphorylation and its cell surface expression in neurons.
DISCUSSION
Anti-inflammatory cytokines, such as IL-4, potently polarize T cells and macrophages to type-2 (Th2 and M2) cells (44,45,46,47) and lead to the suppression of proinflammatory responses in macrophages, microglia, T cells, and astrocytes (44, 48,49,50,51,52). However, the potential effects of IL-4 on neurons have been poorly investigated in vivo. We have shown that glial accumulation was partially suppressed by AAV-IL-4 treatment of APP+PS1 mice in the hippocampal regions, which was also associated with restoration of impaired spatial learning. IL-4 gene delivery also reduced Aβ deposition and oligomer formation. This suggests that the anti-inflammatory cytokine IL-4 has broader implications in AD therapy, including β-amyloidosis.
Previous studies reported that subcutaneous Aβ immunization with IL-4, rather than without, results in boosted antibodies against Aβ and decreased Aβ burden in the brain, demonstrating promoted Aβ clearance by IL-4 (53, 54). Our system does not utilize Aβ immunization, which may not be an effective treatment in Aβ immunotherapy (7). Nonetheless, we also showed that AAV-mediated IL-4 expression alone reduces the Aβ burden, compared to the AAV-GFP group. Our finding suggests that IL-4 alone is sufficient to ameliorate β-amyloidosis in the brain of AD. One potential mechanism is that IL-4 promotes Aβ degradation in mononuclear phagocytes. We have previously reported differences in Aβ clearance after IL-4 and other cytokine treatments of human monocyte-derived macrophages (MDM) (19). IL-4 stimulation can enhance Aβ degradation in MDM (19), which is potentially mediated by the expression of scavenger receptor CD36 and enhanced insulin-degrading enzyme activity (18). In addition, IL-4 introduction into the APP+PS1 mouse brain can reduce levels of monomeric Aβ, as well as some Aβ oligomeric species. The significant effect of IL-4 on Aβ reduction may also partially explain the repair of β-amyloidosis-related dysfunction in APP+PS1 mice, including NR2B expression and neurogenesis.
IL-4 introduction into the mouse brain significantly enhanced the number of Dcx+ and BrdU+NeuN+ newly synthesized neurons in the SGZ of APP+PS1 mice. It was suggested that IL-4 promotes neurogenesis in adult mouse neural progenitor cells cocultured with IL-4-stimulated microglia in the presence of Aβ (15). To the best of our knowledge, this is the first report to show that CNS expression of IL-4 can directly enhance neurogenesis, suggesting that IL-4 is likely to enhance neuronal progenitor cell proliferation, as well as neuronal differentiation. Potential direct mechanisms of IL-4 induced cell proliferation may include IL-4-mediated activation of extracellular signaling-regulated kinases, as well as p70 ribosomal protein S6 kinase-survival signaling pathways and insulin-response substrate 1 and 2 (IRS-1/-2) pathways, protecting neurons from apoptosis though the activation of phosphatidylinositol-3-kinase and Akt (a survival-promoting serine-threonine protein kinase) (55). Similarly, insulin-like growth factor 1 also promotes the survival of motor neurons through the activation of IRS-1 (56). Therefore, IL-4 induction may also contribute to neuroprotection via the IRS-1/-2 signaling pathway in the hippocampus of APP+PS1 mice.
NR2B is a major subunit of the NMDA receptor channel, which modulates the calcium influx and is essential for glutamatergic synaptic transmission (43). Aβ potently down-regulates cell surface expression of NR2B by causing its endocytosis, which is enhanced by dephosphorylation and activation of striatum-enriched protein tyrosine phosphatase (STEP) via the Aβ/a7 nicotinic ACh receptor/calcineurin pathway (29, 43). Our data also demonstrate that NR2B expression was significantly reduced in the membrane-rich fraction of APP+PS1 mouse brain in vivo. Synaptic insertion of NR2B is tightly regulated by the phosphorylation status of the Tyr1472 residue of NR2B, which is controlled by Fyn tyrosine kinase-mediated phosphorylation and STEP-mediated dephosphorylation (29, 43). Our study suggests that IL-4 can restore NR2B expression, potentially through enhanced Tyr1472 phosphorylation of NR2B, indicating that IL-4 may induce Fyn kinase activation or STEP inactivation. IL-4 and IL-13 can activate Janus kinase (JAK)-2 with its association and phosphorylation of Fyn kinase (57,58,59). These studies suggest that the IL-4-induced activation of the JAK-2 / Fyn kinase signaling pathway may contribute to the Tyr1472 phosphorylation of NR2B. Regarding the possibility of IL-4-induced inactivation of STEP, one potential mechanism is that IL-4 induces accumulation of cAMP (60), which activates protein kinase A (PKA) and subsequently phosphorylates and inactivates STEP (61). We examined whether IL-4 enhances PKA activation in primary mouse neurons using PKA substrate antibody blotting, which detects all the phosphorylated substrates of PKA, and saw no changes (unpublished observation). Thus, we ruled out the possibility of IL-4-induced STEP inactivation via the PKA pathway in the primary mouse neuron system.
In summary, we demonstrated that neuronal expression of the anti-inflammatory cytokine IL-4 restores the spatial learning function of APP+PS1 mice through its distinct effect on Aβ reduction, glial activation, NR2B expression, and neurogenesis. This work has also shown that anti-inflammatory cytokines can function as neuromodulators or neurohormones in the brain and creates a novel approach to treat neurodegenerative disorders through anti-inflammatory signaling cascades in the brain.
Supplementary Material
Acknowledgments
The authors thank Dr. Karen Hsiao-Ashe (University of Minnesota, Minneapolis, MN, USA) for providing Tg2576 mice; Dr. Karen Duff (Columbia University, New York, NY, USA) for providing M146L PS1 mice; Dr. Dave Morgan (University of South Florida, Tampa, FL, USA) for RAWM test training and consultation; Dr. Ronald Klein (Louisiana State University, Shreveport, LA, USA) for pGFP plasmid; the University of Pennsylvania Gene Therapy Program for recombinant AAV1 vectors; Dr. Sylvain Lesne (University of Minnesota, Minneapolis, MN, USA) for the Aβ oligomer immunoblotting protocol; and Tim Smith, Scott Andrews, and Meg Marquardt for editing of the manuscript. This work is supported by the Vada Kinman Oldfield Alzheimer’s Research Fund (T.K., T.I.); UNMC Brain Bank Core Fund (T.I.); and NIH grants P01 NS043985 (H.E.G., T.I.), R01 MH083523 (T.I.), and R21 AG032600 (T.I.).
References
- Selkoe D. J. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Liao Z., Lieberburg I., Motter R., Mutter L., Soriano F., Shopp G., Vasquez N., Vandevert C., Walker S., Wogulis M., Yednock T., Games D., Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Morgan D., Diamond D. M., Gottschall P. E., Ugen K. E., Dickey C., Hardy J., Duff K., Jantzen P., DiCarlo G., Wilcock D., Connor K., Hatcher J., Hope C., Gordon M., Arendash G. W. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Bard F., Barbour R., Cannon C., Carretto R., Fox M., Games D., Guido T., Hoenow K., Hu K., Johnson-Wood K., Khan K., Kholodenko D., Lee C., Lee M., Motter R., Nguyen M., Reed A., Schenk D., Tang P., Vasquez N., Seubert P., Yednock T. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C., Konietzko U., Streffer J. R., Tracy J., Signorell A., Muller-Tillmanns B., Lemke U., Henke K., Moritz E., Garcia E., Wollmer M. A., Umbricht D., de Quervain D. J., Hofmann M., Maddalena A., Papassotiropoulos A., Nitsch R. M. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Brody D. L., Holtzman D. M. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham K., Frantz S. Set back to Alzheimer vaccine studies. Nat Med. 2002;8:199–200. doi: 10.1038/nm0302-199b. [DOI] [PubMed] [Google Scholar]
- Tesseur I., Zou K., Esposito L., Bard F., Berber E., Can J. V., Lin A. H., Crews L., Tremblay P., Mathews P., Mucke L., Masliah E., Wyss-Coray T. Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad S. C., Miller D. R., Kowall N. W., Felson D. T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfara D., Lifshitz V., Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R., Meshorer A., Sela M., Arnon R. Oral treatment of mice with copolymer 1 (glatiramer acetate) results in the accumulation of specific Th2 cells in the central nervous system. J Neuroimmunol. 2002;126:58–68. doi: 10.1016/s0165-5728(02)00053-x. [DOI] [PubMed] [Google Scholar]
- Stern J. N., Keskin D. B., Zhang H., Lv H., Kato Z., Strominger J. L. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc Natl Acad Sci U S A. 2008;105:5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D., Maron R., Burt D. S., Weiner H. L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears β-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- Butovsky O., Koronyo-Hamaoui M., Kunis G., Ophir E., Landa G., Cohen H., Schwartz M. Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2006;103:11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher F. O., Nolan Y., Lynch M. A. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Lyons A., Downer E. J., Crotty S., Nolan Y. M., Mills K. H., Lynch M. A. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E., Kawahara K., Kajizono M., Sawada M., Nakayama H. IL-4-induced selective clearance of oligomeric β-amyloid peptide(1–42) by rat primary type 2 microglia. J Immunol. 2008;181:6503–6513. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kiyota T., Walsh S. M., Liu J., Kipnis J., Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-beta peptide degradation by human mononuclear phagocytes. J Immunol. 2008;181:3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T., Yamamoto M., Schroder B., Jacobsen M. T., Swan R. J., Lambert M. P., Klein W. L., Gendelman H. E., Ransohoff R. M., Ikezu T. AAV1/2-mediated CNS gene delivery of dominant-negative CCL2 mutant suppresses gliosis, beta-amyloidosis, and learning impairment of APP/PS1 mice. Mol Ther. 2009;17:803–809. doi: 10.1038/mt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D., Morgan D., Gordon M. N., Holcomb L., Refolo L., Zenk B., Hardy J., Younkin S. Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Holcomb L., Gordon M. N., McGowan E., Yu X., Benkovic S., Jantzen P., Wright K., Saad I., Mueller R., Morgan D., Sanders S., Zehr C., O'Campo K., Hardy J., Prada C. M., Eckman C., Younkin S., Hsiao K., Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Klein R. L., Hamby M. E., Gong Y., Hirko A. C., Wang S., Hughes J. A., King M. A., Meyer E. M. Dose and promoter effects of adeno-associated viral vector for green fluorescent protein expression in the rat brain. Exp Neurol. 2002;176:66–74. doi: 10.1006/exnr.2002.7942. [DOI] [PubMed] [Google Scholar]
- West M. J. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Wilcock D. M., Rojiani A., Rosenthal A., Subbarao S., Freeman M. J., Gordon M. N., Morgan D. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Horiba M., Buescher J. L., Huang D., Gendelman H. E., Ransohoff R. M., Ikezu T. Overexpression of monocyte chemotactic protein-1/CCL2 in β-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kiyota T., Horiba M., Buescher J. L., Walsh S. M., Gendelman H. E., Ikezu T. Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., Greengard P. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Knowles J. K., Rajadas J., Nguyen T. V., Yang T., LeMieux M. C., Vander Griend L., Ishikawa C., Massa S. M., Wyss-Coray T., Longo F. M. The p75 neurotrophin receptor promotes amyloid-beta(1–42)-induced neuritic dystrophy in vitro and in vivo. J Neurosci. 2009;29:10627–10637. doi: 10.1523/JNEUROSCI.0620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Tan J., Town T., Crawford F., Mori T., DelleDonne A., Crescentini R., Obregon D., Flavell R. A., Mullan M. J. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- He P., Zhong Z., Lindholm K., Berning L., Lee W., Lemere C., Staufenbiel M., Li R., Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanik A. M., Funes S., Petko W., Ringheim G. E. IL-4, IL-10 and IL-13 modulate A β(1–42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113:49–62. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- Lyons A., Griffin R. J., Costelloe C. E., Clarke R. M., Lynch M. A. IL-4 attenuates the neuroinflammation induced by amyloid-β in vivo and in vitro. J Neurochem. 2007;101:771–781. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary J. P., Walsh D. M., Hofmeister J. J., Shankar G. M., Kuskowski M. A., Selkoe D. J., Ashe K. H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Chafekar S. M., Hoozemans J. J., Zwart R., Baas F., Scheper W. Aβ 1–42 induces mild endoplasmic reticulum stress in an aggregation state-dependent manner. Antioxid Redox Signal. 2007;9:2245–2254. doi: 10.1089/ars.2007.1797. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Shetty A. K. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Braithwaite S. P., Paul S., Nairn A. C., Lombroso P. J. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Howard M., O'Garra A., Ishida H., de Waal Malefyt R., de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Lalani I., Bhol K., Ahmed A. R. Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469–483. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- Opal S. M., Wherry J. C., Grint P. Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin Infect Dis. 1998;27:1497–1507. doi: 10.1086/515032. [DOI] [PubMed] [Google Scholar]
- Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Brown M. A., Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E., Arevalo-Martin A., Castrillo A., Bosca L., Vela J. M., Guaza C. Interleukin-4 and interleukin-10 modulate nuclear factor κB activity and nitric oxide synthase-2 expression in Theiler’s virus-infected brain astrocytes. J Neurochem. 2002;81:1242–1252. doi: 10.1046/j.1471-4159.2002.00925.x. [DOI] [PubMed] [Google Scholar]
- Koeberle P. D., Gauldie J., Ball A. K. Effects of adenoviral-mediated gene transfer of interleukin-10, interleukin-4, and transforming growth factor-β on the survival of axotomized retinal ganglion cells. Neuroscience. 2004;125:903–920. doi: 10.1016/S0306-4522(03)00398-1. [DOI] [PubMed] [Google Scholar]
- Marques C. P., Hu S., Sheng W., Cheeran M. C., Cox D., Lokensgard J. R. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47:358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- DaSilva K., Brown M. E., Westaway D., McLaurin J. Immunization with amyloid-β using GM-CSF and IL-4 reduces amyloid burden and alters plaque morphology. Neurobiol Dis. 2006;23:433–444. doi: 10.1016/j.nbd.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Frazer M. E., Hughes J. E., Mastrangelo M. A., Tibbens J. L., Federoff H. J., Bowers W. J. Reduced pathology and improved behavioral performance in Alzheimer’s disease mice vaccinated with HSV amplicons expressing amyloid-β and interleukin-4. Mol Ther. 2008;16:845–853. doi: 10.1038/mt.2008.39. [DOI] [PubMed] [Google Scholar]
- Lin S. J., Chang C., Ng A. K., Wang S. H., Li J. J., Hu C. P. Prevention of TGF-β-induced apoptosis by interleukin-4 through Akt activation and p70S6K survival signaling pathways. Apoptosis. 2007;12:1659–1670. doi: 10.1007/s10495-007-0085-5. [DOI] [PubMed] [Google Scholar]
- Vincent A. M., Mobley B. C., Hiller A., Feldman E. L. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Murata T., Noguchi P. D., Puri R. K. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–2978. [PubMed] [Google Scholar]
- Sayeski P. P., Ali M. S., Safavi A., Lyles M., Kim S. O., Frank S. J., Bernstein K. E. A catalytically active Jak2 is required for the angiotensin II-dependent activation of Fyn. J Biol Chem. 1999;274:33131–33142. doi: 10.1074/jbc.274.46.33131. [DOI] [PubMed] [Google Scholar]
- Ip W. K., Wong C. K., Lam C. W. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin Exp Immunol. 2006;145:162–172. doi: 10.1111/j.1365-2249.2006.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adao-Novaes J., Guterres Cde. C., da Silva A. G., Campello-Costa P., Linden R., Sholl-Franco A. Interleukin-4 blocks thapsigargin-induced cell death in rat rod photoreceptors: involvement of cAMP/PKA pathway. J Neurosci Res. 2009;87:2167–2174. doi: 10.1002/jnr.22026. [DOI] [PubMed] [Google Scholar]
- Paul S., Snyder G. L., Yokakura H., Picciotto M. R., Nairn A. C., Lombroso P. J. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.