SUMMARY
Rhizopus oryzaeis the most common cause of mucormycosis, an angioinvasive fungal infection that causes more then 50% mortality rate despite first-line therapy. Clinical and animal model data clearly demonstrate that the presence of elevated available serum iron predisposes the host to mucormycosis. The high affinity iron permease gene (FTR1) is required for R. oryzae iron transport in iron-depleted environments. Here we demonstrate that FTR1 is required for full virulence of R. oryzae in mice. We show that FTR1 is expressed during infection in diabetic ketoacidotic (DKA) mice. In addition, we disrupted FTR1 by double cross-over homologous recombination, but multinucleated R. oryzae could not be forced to segregate to a homokaryotic null allele. Nevertheless, a reduction of the relative copy number of FTR1 and inhibition of FTR1 expression by RNAi compromised the ability of R. oryzae to acquire iron in vitro and reduced its virulence in DKA mice. Importantly, passive immunization with anti-Ftr1p immune sera protected DKA mice from infection with R. oryzae. Thus FTR1 is a virulence factor for R. oryzae, and anti-Ftr1p passive immunotherapy deserves further evaluation as a strategy to improve outcomes of deadly mucormycosis.
Keywords: Mucormycosis, R. oryzae, FTR1, virulence, iron, murine
INTRODUCTION
Mucormycosis (zygomycosis) is a life-threatening infection which occurs in patients with diabetes, in patients with increased available serum iron (e.g. from systemic acidosis or deferoxamine therapy), and in patients immunocompromised by neutropenia or medications (e.g. corticosteroids) (Ibrahim et al., 2003b, Kwon-Chung & Bennett, 1992, Spellberg et al., 2005). Rhizopus oryzae is the most common cause of mucormycosis, and is responsible for approximately 70% of such infections (Reed et al., 2008, Ribes et al., 2000, Roden et al., 2005, Spellberg et al., 2005). Due to the rising prevalence of diabetes, cancer, and organ transplantation in the aging United States population, the number of patients at risk for this deadly infection is significantly rising (Marr et al., 2002). Unfortunately, despite disfiguring surgical debridement and adjunctive antifungal therapy, the overall mortality of mucormycosis remains approximately 50%, and it approaches 100% in patients with disseminated disease, or persistent neutropenia (Gleissner et al., 2004, Kauffman, 2004, Kontoyiannis et al., 2000, Marr et al., 2002, Siwek et al., 2004, Spellberg et al., 2005). Clearly new strategies to prevent and treat mucormycosis are urgently needed.
The hypersusceptibility of patients with increased available serum iron to infection by R. oryzae, but not other pathogenic fungi (Ibrahim et al., 2003b, Sugar, 2005), highlights the central role of iron metabolism in the organism’s virulence. For example, the fungi that cause mucormycosis are normally unable to grow in serum due to the sequestration of iron by iron binding proteins. However, diabetic ketoacidosis (DKA) causes proton-mediated dissociation of iron from iron-sequestering proteins, and the increased levels of available iron enable enhanced growth of these fungi in serum (Artis et al., 1982, Ibrahim et al., 2006, Spellberg et al., 2005). Moreover, patients treated with deferoxamine are uniquely susceptible to mucormycosis (Boelaert et al., 1988, Boelaert et al., 1987, Boelaert et al., 1994). While deferoxamine is an iron-chelator from the perspective of the human host, Rhizopus spp. utilize deferoxamine as a xeno-siderophore to supply previously unavailable iron to the fungus (Boelaert et al., 1993, de Locht et al., 1994). Recently, we found that other iron chelators do not act as iron siderophores for Rhizopus. In fact, treatment of Rhizopus-infected mice with the iron chelators deferiprone (Ibrahim et al., 2006) or deferasirox (Ibrahim et al., 2007) markedly improved survival, reduced fungal burden in target organs, and enhanced the host inflammatory response to mucormycosis. These results further confirm the unique importance of iron in the pathogenesis of mucormycosis.
We have previously cloned the high-affinity iron permease of R. oryzae (FTR1), which is a primary effector of the organism’s ability to acquire iron in iron-limited environments (akin to those found in vivo during infection) (Fu et al., 2004). In the current study we sought to determine the role of FTR1 in the pathogenicity of R. oryzae by studying the expression of the gene and its product in vivo, elucidating the effect of gene disruption and gene silencing on the virulence of R. oryzae, and finally by determining the role of anti-Ftr1p antibodies in protecting DKA mice from R. oryzae infection.
RESULTS
FTR1 is expressed by R. oryzae during infection in DKA mice
For FTR1 to play a role in the pathogenesis of mucormycosis, it must be expressed during infection. We used quantitative real time PCR (qPCR) to investigate the expression of FTR1 in the brains of DKA mice infected intravenously with 105 spores of R. oryzae, an inoculum that causes a 100% mortality within 2–3 days (Ibrahim et al., 2005a). The brain was chosen for analysis because it is a primary target organ in this model (Ibrahim et al., 2005a). Expression of FTR1 from mice (n=5) sacrificed 24 h post infection increased by 4 fold [median (25th quartile, 75th quartile) = 4.12 (1.03, 0.27), p = 0.03 by Wilcoxon Rank Sum)] relative to the constitutive ACT1 gene. As expected, brains from uninfected mice did not show any expression of FTR1.
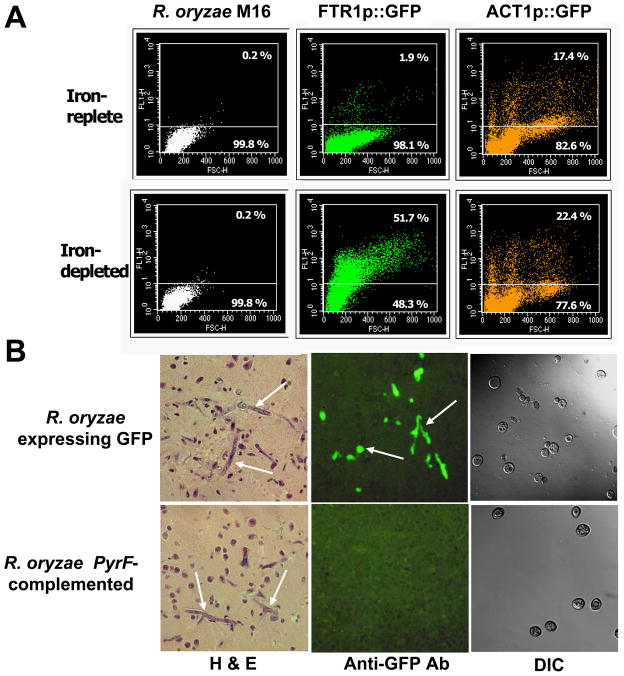
To directly visualize expression of FTR1 in vivo during infection, R. oryzae was transformed with a plasmid containing GFP under the control of the FTR1 promoter (strains described in Table 1). This strain fluoresced green when grown in iron-depleted but not iron-rich media in vitro, whereas R. oryzae transformed with GFP under the control of the constitutive actin promoter (positive control) fluoresced regardless of the iron concentration in the medium (Fig. 1A). DKA mice were infected with the GFP reporter strain or PyrF-complemented R. oryzae grown under iron-rich conditions to suppress GFP expression prior to infection. Twenty four or 48 h post infection brains were collected and processed for histopathology. Because the paraffin embedding process abrogated the intrinsic fluorescence of the GFP protein, the sections were stained with fluorescent anti-GFP antibody. Samples taken 24 h post infection did not show any fungal elements, which was expected since 48 h post infection is the earliest time point that fungal elements can be detected histopathologically in infected tissues (Ibrahim et al., 2005a). At 48 h of infection in the brain, the FTR1 reporter strain of R. oryzae expressed GFP, whereas the negative control, PyrF-complemented R. oryzae did not (Fig. 1B). Additionally, GFP expression was induced by low iron levels in the host environment since spores used for infecting mice were grown in iron-rich medium (condition that suppresses the expression of FTR1) and did not fluoresce green when used to infect mice (Fig. 1B, DIC overlaid with fluorescence).
Table 1.
Strains used in this study
| Strain | Genotype | Description and Source |
|---|---|---|
| R. oryzae 99–880 | Wild-type | Clinical isolate (Ibrahim et al., 2007) |
| R. oryzae M16 | pyrF205 | Uracil deficient (Skory & Ibrahim, 2007) |
| R. oryzae PyrF complemented | pyrF205::PyrF | M16 complemented with a wild-type copy of PyrF at its original locus, this work |
| R. oryzae GFP1 | M16 (pPFtr1-GFP) | M16 transformed with a plasmid containing an FTR1 promoter driven GFP (Ibrahim et al., 2007) |
| R. oryzae FTR1Ko | pyrF205, ftr1::PyrF | ftr1 knock out, this work |
| R. oryzae FTR1Inh | M16 (pFTRi-pdc intron) | FTR1 inhibited by RNAi, this work |
| R. oryzae Empty | M16 (pRNAi-pdc intron) | M16 transformed with empty plasmid, this work |
Fig. 1.
FTR1 is expressed in DKA mice infected intravenously with R. oryzae. (A) FACS analysis of R. oryzae transformed with plasmid containing the reporter gene GFP driven by either the FTR1 promoter or the constitutively expressed ACT1 promoter and grown in iron-rich or iron-depleted conditions. R. oryzae M16 transformed with an empty plasmid was used as a negative control. (B) FTR1 is expressed in the brains of DKA mice infected with R. oryzae expressing GFP under the control of FTR1p. For anti-GFP Ab stain, tissue section was stained with rabbit polyclonal antibody to GFP then counter stained with FITC conjugated anti-rabbit antibody. For DIC, confocal image showing non-fluorescent R. oryzae at the time of infection. Arrows denote fungal elements in infected brains. Magnification, X 400.
Isolation of a homokaryotic ftr1 null could not be achieved in multinucleated R. oryzae despite integration of the disruption cassette at the FTR1 locus
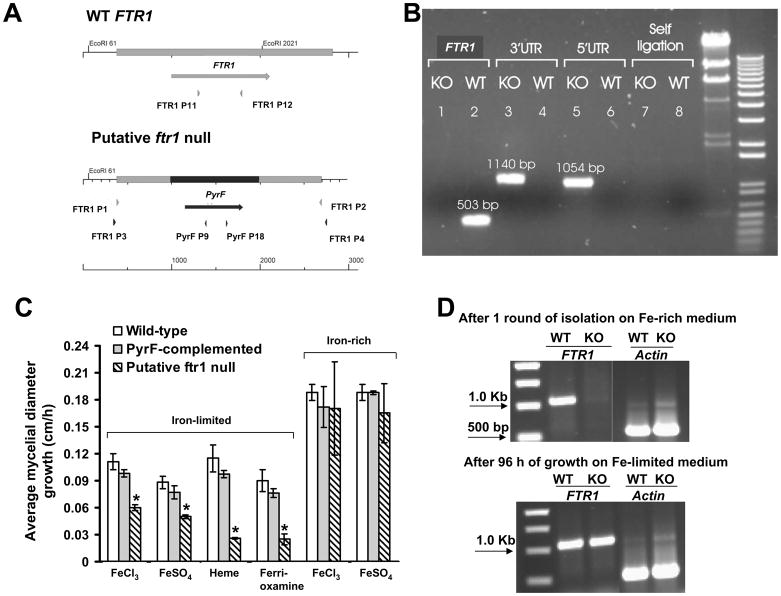
The expression of FTR1 during active infection suggested a role for FTR1 in the pathogenicity of R. oryzae. We sought to study the effect of FTR1 gene disruption on the ability of R. oryzae to take up iron in vitro and cause disease in vivo. Isolates obtained from two separate transformations were purified with one round of sporulation and single colony isolation. To achieve single colony isolation, transformants were grown on chemically defined medium (YNB+CSM-URA) supplemented with 1 mM FeCl3 (iron rich) to favor the segregation of the ftr1 null allele, since FTR1 is poorly expressed in concentrations ≥ 350 μM of FeCl3 (Fu et al., 2004). Isolates were screened for integration of the disruption cassette with PCR primer pairs FTR1-P3/PyrF-P9 (expected 1054 bp) and PyrF-P18/FTR1-P4 (expected 1140 bp). Disruption of the FTR1 locus was tested by the absence of a PCR amplification product using primers FTR1-P11/FTR1-P12 (expected 503 bp), which amplified a segment from the ORF of FTR1 (Table 2 and Fig. 2A). PCR confirmed integration of the disruption cassette in the FTR1 locus, and absence of FTR1 ORF from several putative null mutant strains (Fig. 2B). Furthermore, these amplification products were also sequenced to demonstrate that the disruption cassette had integrated into the FTR1 locus by homologous recombination (data not shown). Finally, integration of the disruption cassette in the FTR1 locus was confirmed by Southern blotting (see below).
Table 2.
Oligonucleotides used in this study.
| Primers | Sequence | Description |
|---|---|---|
| Primers used for detecting in vivo expression of FTR1 | ||
| FTR1-RT5′ | GGTGGTGTCTCCTTGGGTAT | 5′ primer |
| FTR1-RT3′ | AAGGAAACCGACCAAACAAC | 3′ primer |
| 18S-RT5′ | CCAGACTGGCTTGTCTGTAATC | 5′ primer annealing to rRNA |
| 18S-RT3′ | AAGTCAAATTGTCGTTGGCA | 3′ primer annealing to rRNA |
| ACT1-RT5′ | TGAACAAGAAATGCAAACTGC | 5′ primer |
| ACT1-RT3′ | CAGTAATGACTTGACCATCAGGA | 3′ primer |
| Primers used for making the ftr1 disruption cassette and confirming integration in the FTR1 locus | ||
| FTR1 P1 | TTCGAAAAGACCGTCAGGATTAGC | Annealing to FTR1-5′ UTR |
| FTR1 P2 | GAGGGACACAAGCAAGCAGAAAGT | Annealing to FTR1-3′ UTR |
| FTR1 P3 | CACTTACGGCCATTTTCCATTGAC | Annealing to FTR1-5′ UTR upstream of the disruption cassette |
| FTR1 P4 | CGCGCTAAATGAACAAAGAAT | Annealing to FTR1-3′ UTR downstream of the disruption cassette |
| FTR1 P5 | ATGTCTCAAGATCTCTTCAACCGTACC | 5′ primer testing for the entire FTR1 ORF (1100 bp) |
| FTR1 P6 | TTAAGCCTTAATAGCATCAGATTCG | 3′ primer testing for the entire FTR1 ORF (1100 bp) |
| FTR1 P11 | GATCACTGCCATGGGTCTTGCTAT | 5′ primer to test for 503 bp of FTR1 ORF |
| FTR1 P12 | TATCATGTTGGCTTCTGGGTCTC | 3′ primer to test for 503 bp of FTR1 ORF |
| PyrF P9 | GCCGTGGCGCAGACAAGAG | 3′ primer annealing to pyrF |
| PyrF P18 | GTGCCGAAATCGCTCCAGA | 5′ primer annealing to pyrF |
| ACT1 P1 | GTCTTTCCTTCTATTGTTGGTC | 5′ primer to test for functional template DNA (600bp) |
| ACT1-P2 | CCATCAGGAAGTTCATAAGAC | 3′ primer to test for functional template DNA (600bp) |
| Primers used in making PyrF-complemented R. oryzae | ||
| PyrF P11 | CAAAGCCAATTCAGCCTCAAATG | 5′ primer to amplify partial PyrF (815 bp) |
| PyrF P13 | CTTGGATCAGGGTGGACTCGTAG | 3′ primer to amplify partial PyrF (815 bp) |
| Primers used to determine FTR1 wild-type or ftr1 mutant copy number | ||
| FTR1 P9 | CCAACAGTGAAAAGTCATCCTTT | 5′ primer to amplify FTR1 (150 bp) |
| FTR1 P10 | GCAATAGGAATTGATTTTCCTTG | 3′ primer to amplify FTR1 (150 bp) |
| ftr1 P1 | TTCAACCCTTTTCTCCAAGG | 5′ primer annealing to UTR of FTR1 to amplify ftr1 mutant allele (100 bp) |
| ftr1 P2 | TTGATCAAGGGCACAATGA | 3′ primer annealing to PyrF to amplify ftr1 mutant allele (100 bp) |
| ACT1 P3 | TATCGTTCTTGACTCTGGTGATG | 5′ primer to amplify actin (150 bp) |
| ACT1 P4 | GAAAGAGTGACCACGTTCAGC | 3′ primer to amplify actin (150 bp) |
| Primers used for making RNAi strain | ||
| PyrF14 | CTCGAGGCTTTAGGTCAAATTGTGG | 5′ primer to amplify 1641bp of PyrF to clone in pRNAi-pdc intron |
| PyrF15 | CCCGGGTTATTGCTTGATACCATATTGTG | 3′ primer to amplify 1641bp of PyrF to clone in pRNAi-pdc intron |
| FTR1 P7 | GCGGCCGCGCTAGCGCATGCATGTCTCAAGATCTCTTCAACGTACCGATC | 5′ primer to amplify 450bp of FTR1to clone in pRNAi-pdc |
| FTR1 P8 | GACGTCCCGCGGGGCGCGCCGG TGATAAAAGGCAAGACAAAGAACGCGTA | 3′ primer to amplify 450bp of FTR1 to clone in pRNAi-pdc |
| 18S rRNA P1 | CATGGTTGAGATTGTAAGATAG | 5′ primer to amplify 18S rRNA |
| 18S rRNA P2 | AGTCAATGGACGTGGAGTC | 3′ primer to amplify 18S rRNA |
| Primers used for making synthetic FTR1p in E. coli | ||
| SynFtr1p P5 | CATCACCATGGGATCAAAAGAATGTTTAATACTGAATCTCCA | 5′ primer to amplify synthetic Ftr1p |
| SynFtr1p P6 | CTAATTAAGCTTGGCTTAAGCTTTAATAGCATCAGATTCAATTTTTTC | 3′ primer to amplify synthetic Ftr1p |
Fig. 2.
Disruption cassette integrates in FTR1 locus but complete elimination of FTR1 could not be achieved. (A) A diagram summarizing the strategy used to achieve FTR1 disruption. PyrF (998 bp) was used as a selectable marker flanked by 606 and 710 bp fragments of FTR1-5′ UTR and FTR1-3′ UTR, respectively. (B) Gel electrophoresis showing integration of the disruption cassette in a representative putative ftr1 null mutant (KO) but not in the wild-type (WT) (see 5′UTR and 3′UTR). Primers FTR1 P11 and FTR1 P12 were used to amplify 503 bp from the FTR1 ORF only from the wild-type but not from the putative ftr1 null mutant (see FTR1). Primers PyrF P9 and PyrF P18 to test for possible reciculization of the transformed plasmid with expected band of 2094 bp were also used (see self ligation). (C) Comparison of growth rate of R. oryzae wild-type, R. oryzae PyrF-complemented, or putative ftr1 null mutants grown on different sources of iron on iron-limited or iron-rich media. Growth was measured after 48 h for media containing 10 or 1000 μM of FeCl3 or FeSO4 or 100 μM of ferrioxamine, while growth was measured after 72 h for medium supplemented with 100 μM heme. Values (n=12 from four independent transformants with their growth measured in three experiments with similar results) are expressed as increase in mycelial diameter growth on solid growth medium in cm/h. * P<0.05 compared to wild-type or R. oryzae PyrF-complemented strains. (D) Gel electrophoresis showing lack of amplification of FTR1 after one round of purification of the putative null mutants on iron-rich medium (1000 μM FeCl3) and amplification of the FTR1 from the same isolate following growth on iron-depleted medium (i.e. 100μM ferrioxamine) for 96 h. Amplification of actin (600 bp) was used to control for DNA loading.
We previously found that FTR1 is expressed in vitro in iron-depleted conditions (FeCl3 concentration between 0–50 μM) and suppressed in iron replete media (FeCl3 concentrations of ≥350 μM) (Fu et al., 2004). To investigate if FTR1 disruption had an effect on the ability of R. oryzae to grow in media with different sources and concentration of iron, we compared growth of several putative null mutant strains to growth of wild-type or PyrF-complemented R. oryzae. Growth was compared on media (CSM-URA) which had been previously chelated for iron and then supplemented with defined concentrations of free iron (i.e. FeCl3 or FeSO4) or iron complexed to deferoxamine [ferrioxamine] or heme. Compared to wild-type or PyrF-complemented R. oryzae, putative ftr1 null mutant strains had significantly less growth at 48 h in iron-depleted media (i.e. free iron at 10 μM) (Fig. 2C). Ferrioxamine or iron complexed with heme at 100 μM (relatively depleted because iron is complexed) supported the growth of the wild-type and PyrF-complemented strains better than the putative ftr1 null mutant. However, free iron at 1000 μM (iron-rich media) supported the growth of all strains equally (Fig. 2C) consistent with our previous findings that ftr1 is primarily expressed in iron-depleted environments.
Interestingly, growth of the putative ftr1 null mutants increased to levels similar to the wild-type and PyrF-complemented strains after 96 h on iron-depleted media (data not shown). Furthermore, PCR analysis of these cultures after 96 h of growth confirmed that the FTR1 ORF was once again detectable in all of the putative ftr1 null mutant transformants (Fig. 2D). Similar results were obtained with five other putative ftr1 null mutants and it was concluded that one round of sporulation and single colony isolation was not sufficient to purify an ftr1 homokaryotic null allele strain.
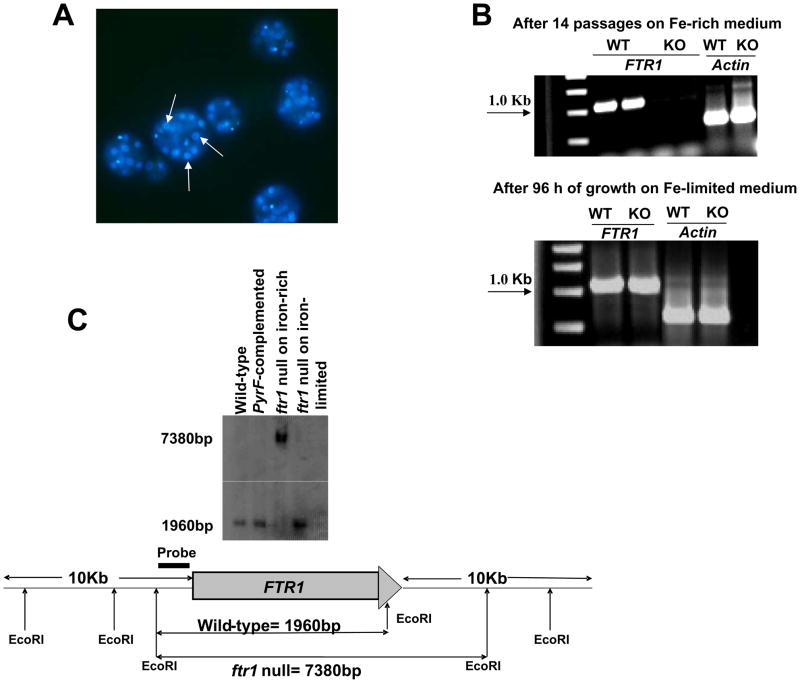
R. oryzae is known to be coenocytic and it is generally presumed that sporangiospores are multinucleated, although the number of nuclei has not been previously described (Ma et al., 2009). We hypothesized that gene disruption was complicated by the presence of heterokaryotic nuclei in both the mycelium and sporangiospores, and sought to determine the number of nuclei present in swollen spores using DAPI staining. We found that R. oryzae strain M16 had at least > 10 nuclei per swollen spore (Fig. 3A). Given the high number of nuclei present, we performed 14 rounds of sporulation and single colony isolation on four independent putative ftr1 null mutants on iron-rich medium (i.e. medium containing 1000 μM of FeCl3) to segregate the null alleles by relieving the selective pressure for maintaining FTR1 (since FTR1 is poorly expressed in iron-rich conditions) (Fu et al., 2004). PCR analysis of the putative null mutants after 14 rounds of selection demonstrated lack of amplification of FTR1 ORF (Fig. 3B). Similar to the results in Fig. 2C, the null mutant had defective growth on iron limited sources for the first 48 h compared to wild-type or PyrF-complemented strains. However, after growth of the same putative null mutants in iron-depleted environment (100 μM ferrioxamine), the FTR1 ORF was once again amplified by PCR. Similar results were obtained with 12 other independent putative ftr1 null transformants. Further, these results were confirmed with Southern blot analysis (Fig. 3C). The Southern blot demonstrated almost complete elimination of the FTR1 band (1960 kb) from gDNA of the putative ftr1 null mutants grown on iron-rich medium, but return of the FTR1 band after growth of the same strain on iron-depleted medium (Fig. 3C). Additionally, Southern hybridization analysis confirmed the site-specific integration of the disruption cassette into the ftr1 locus only when the putative ftr1 null mutant was grown in iron-rich medium. Finally, there was no evidence of ectopic integration or extrachromosomal replication, consistent with the fact that the relative copy number of the ftr1 null allele was dependent on the ratio of heterokaryotic nuclei.
Fig. 3.
Confirmation of the lack of complete disruption of FTR1 in the multinucleated R. oryzae. (A) DAPI stain of swollen R. oryzae spores showing the presence of multiple nuclei with a single spore. Arrows denote nuclei. Original magnification, x1000. (B) Gel electrophoresis showing lack of amplification of FTR1 after 14 passages of the putative null mutants on iron-rich medium (1000 μM FeCl3) and amplification of the FTR1 from the same isolate following growth on iron-depleted medium (i.e. 100 μM ferrioxamine) for 96 h. Amplification of actin (600 bp) was used to confirm the integrity of DNA used as template and the absence of PCR inhibitors. (C) Southern blot confirming the integration of the disruption cassette in the putative ftr1 (7380 bp band is present only in DNA sample extracted from putative ftr1 grown in iron-rich medium) and almost complete elimination of the FTR1 copy (lack of 1960 bp in DNA sample extracted from putative ftr1 grown in iron-rich medium).
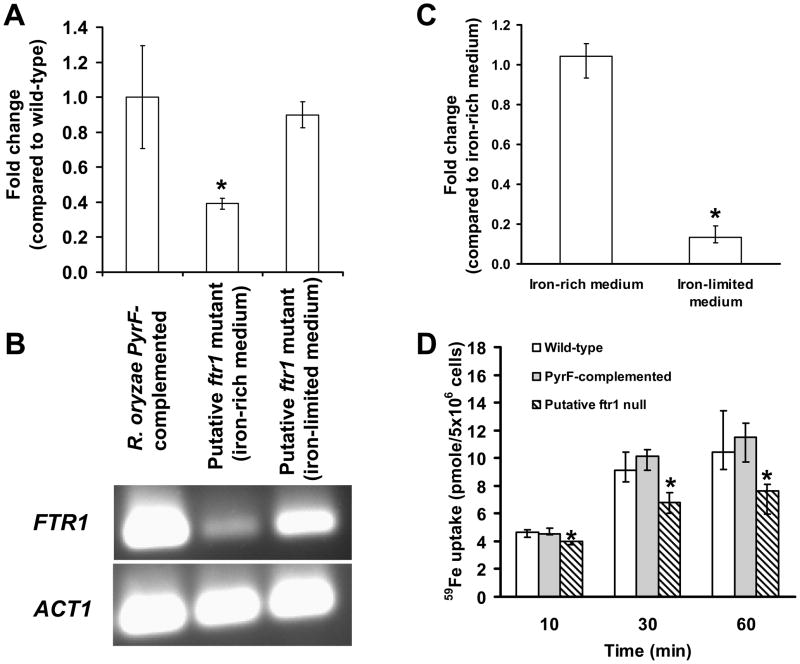
We next used the more sensitive method of qPCR to quantify the copy numbers of wild-type FTR1 or disrupted ftr1 in the putative ftr1 null mutant. The mutant was passaged through 14 rounds of sporulation and single colony isolation on iron-rich then grown on iron-depleted media. The putative ftr1 null mutant strain grown in iron-rich media had a 60% reduction in the relative copy number of the wild-type functional FTR1 (normalized to ACT1 gene) compared to the same strain grown in iron-depleted media or to the R. oryzae PyrF-complemented strain (Fig. 4A and 4B). Thus, while it was possible to significantly decrease the relative copy number of functional FTR1 in multinucleated R. oryzae, we were unable to obtain a homokaryotic isolate of this mutant allele. Additionally, the disrupted ftr1 allele demonstrated a 90% decrease in copy number when the putative ftr1 null mutant was grown on iron-depleted medium following the 14 rounds of sporulation and single colony isolation on iron-rich medium (Fig. 4C). These results are consistent with the Southern blot analysis which showed almost complete elimination of the disrupted allele (Fig 3C). Thus while it is possible to almost completely select for the wild-type phenotype (i.e. pyrF/FTR1) on iron-limited CSM-URA medium, small number of nuclei harboring PyrF/ftr1 genotype remain, thereby explaining the growth of the these cells on CSM-URA medium.
Fig. 4.
Reduced copy number results in compromised ability of R. oryzae to take up iron. (A) qPCR demonstrating reduced copy number of the wild-type FTR1 in the putative ftr1 null mutant compared to R. oryzae PyrF-complemented strain or to the same mutant grown in iron-depleted medium. (B) Gel electrophoresis of samples taken from the qPCR tube showing the amplification specificity for the FTR1 product. (C) qPCR demonstrating reduced copy number of the disrupted ftr1 allele in the putative ftr1 null when grown in iron-limited medium following passing in iron-rich medium (n=9 samples from two independent transformants). (D) The putative ftr1 mutant demonstrated reduced ability to acquire 59Fe compared to R. oryzae wild-type or R. oryzae PyrF-complemented strains. 59Fe uptake by wild-type, R. oryzae PyrF-complemented, or putative ftr1 mutant. Germinated spores were incubated with 0.1 μM 59FeCl3 (a concentration in which high-affinity iron permeases are induced (Fu et al., 2004)). *P <0.05 when compared with R. oryzae wild-type, R. oryzae PyrF-complemented strains, or the same strain grown on different concentrations of iron. Data (n= 9 from three separate experiments using three independent transformants) are expressed as medians + interquartile ranges.
We hypothesized that FTR1 is required for sporangiospore germination, since we could not isolate a homokaryon cell. If FTR1 was required for germination, any sporangiospores containing only homokaryotic ftr1 null alleles would be incapable of growth. Therefore, we attempted to circumvent sporangiospore germination and relied on multiple hyphal tip transfers on medium containing 1 mM FeCl3 (iron-rich conditions). Again, we were unable to obtain a homokaryotic isolate of the FTR1 null allele (data not shown), suggesting the necessity of FTR1 even in iron-rich growth conditions.
Finally, because gene disruption studies were performed in R. oryzae harboring a point mutation that renders the PyrF null, we hypothesized that lack of our ability to generate a homokaryotic ftr1 null could have been complicated by correction of the point mutation of pyrF in nuclei that has FTR1 intact. If this were the case, the selection pressure of growth on CSM-URA media would be lost, and there would be no way to select for growth of cells bearing nuclei with intact PyrF/FTR1, separating them from PyrF/ftr1 null nuclei. To test this hypothesis, the wild-type allele of PyrF was amplified from transformants passaged 14 times on iron-rich medium without uracil or from transformants that have been grown on iron-depleted medium without uracil. The sequence of the PyrF from both was proven to still harbor the point mutation of G to A nucleotide transversion at nt +205 (Skory & Ibrahim, 2007) (data not shown). Therefore, it is clear that lack of obtaining a homokaryotic null is due to the inability of the homokaryotic null to grow under the conditions used in this study.
Reduction of the FTR1 copy number attenuates iron uptake in vitro and reduces virulence in vivo
Although we could not achieve complete disruption of FTR1 we hypothesized that reduction of the relative copy number of functional FTR1 in R. oryzae would be sufficient to decrease iron uptake and therefore reduce virulence. The putative ftr1 null mutant had a ~35% reduction in 59Fe uptake compared to wild-type or R. oryzae PyrF-complemented strain (Fig. 4D).
Uracil auxotrophy has been shown to affect in vivo virulence of several fungal infections (Kirsch & Whitney, 1991, Varma et al., 1992, D’Enfert et al., 1996). However, in contrast to other fungi, uracil auxotrophy did not affect the virulence of R. oryzae in the DKA mouse model (Fig. 5A) (p=0.36 by Log Rank test). These results indicate that the fungus can obtain uracil from infected mice and therefore, any defect in virulence of mice infected with putative ftr1 null would be due to reduced FTR1 copy number rather than a defect in uracil synthesis. Next, we compared the virulence of the putative ftr1 null mutant to the R. oryzae wild-type or PyrF-complemented strains during infection in mice with DKA. Mice were infected intravenously (i.v.) or intranasally (i.n.) with strains that demonstrated similar growth in vitro on iron-rich environment of YPD or CSM-URA (0.185±0.005 or 0.257±0.003 cm/h for the putative ftr1 null vs. 0.188 ± 0.008 or 0.260±0.0051cm/h for the PyrF-complemented on iron rich CSM-URA or YPD medium, respectively) (Fig. 5B). In both models, the putative ftr1 null mutant showed reduced virulence compared to the wild-type or PyrF-complemented strain (54% vs. 100% mortality for mutant vs. control strains in mice with disseminated infection, and 44% vs. 75% mortality for mutant vs. control strains in the intranasal model) (Fig. 5C,D).
Fig. 5.
Reduction of FTR1 copy number reduces R. oryzae virulence in the DKA mouse models. (A) Survival of DKA mice (n=6 for wild-type and 5 for pyrF null mutant) infected with R. oryzae wild-type (3.3 × 103) or pyrF null mutant (2.6 × 103). (B) A representative of the putative ftr1 null mutant demonstrated comparable growth to R. oryzae PyrF-complemented strain on YPD or CSM-URA media. (C) Survival of mice (n=13 per group from two experiments with similar results) infected i.v. with R. oryzae wild-type (average inoculum of 4.3 × 103 spores), R. oryzae PyrF-complemented strain (average inoculum of 4.2 × 103 spores) or with putative ftr1 null mutant (average inoculum of 3.2 × 103 spores). *, P<0.0005 compared to wild-type or PyrF-complemented strains. (D) Survival of mice (n= 9) infected intranasally with R. oryzae wild-type (4.3 × 103 spores), R. oryzae PyrF-complemented strain (5.1 × 103 spores) or putative ftr1 null mutant (5.3 × 103 spores). *, P=0.04 compared to wild-type or PyrF-complemented strains.
By qPCR, brains from moribund mice (n = 5) had 40% fewer copies of the mutated ftr1 and 78% increased copies of the wild-type FTR1 alleles compared to surviving mice. Thus, reduction in copy number of wild type FTR1 reduced virulence of the organism, and in vivo restoration of the wild type allele restored virulence. Additionally, in both models the PyrF-complemented strain had similar virulence to the wild-type R. oryzae, confirming that restoration of the PyrF gene in its original locus does not affect virulence.
Inhibition of FTR1 gene expression by RNAi reduces iron uptake and diminishes virulence of R. oryzae
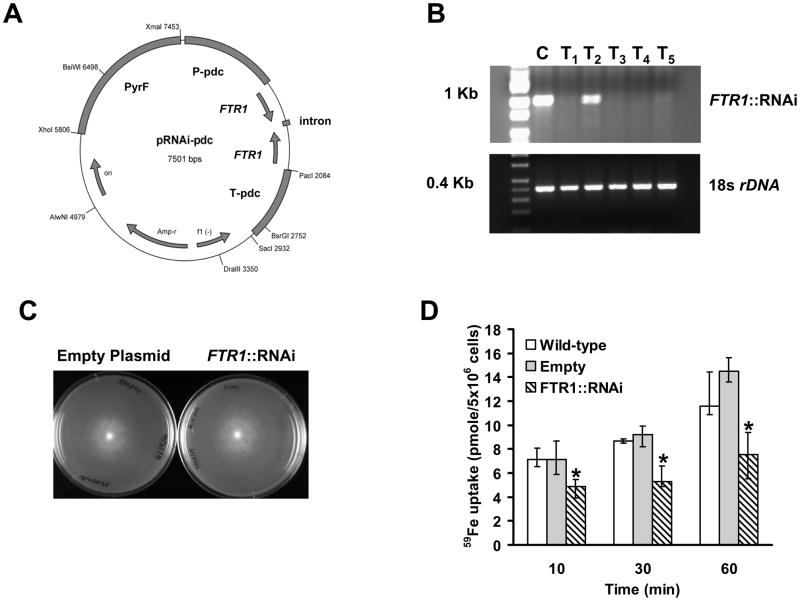
To confirm the phenotypes seen after gene disruption, we used RNA interference (RNAi) to diminish FTR1 expression in R. oryzae (Fig. 6A). Southern blot analysis (data not shown) revealed that all RNAi transformants maintained the transformed plasmid extrachromosomally, consistent with the fact that we did not linearize the plasmid during transformation (Skory, 2002). FTR1 expression was compared by end-point RT-PCR in five transformants vs. the control strain (i.e. R. oryzae pyrf null mutant [M16] transformed with empty-plasmid). FTR1 expression was almost completely blocked in 4 transformants, while readily detected in the control strain (Fig. 6B). Amplification of 18s rDNA with the same RT templates demonstrated the integrity of the starting sample and the lack of PCR inhibitors. A representative RNAi transformant demonstrated similar growth to control strain when grown on CSM-URA media (0.193±0.082 cm/h for the transformant vs. 0.201 ± 0.087 cm/h for the control strain) (Fig. 6C).
Fig. 6.
Inhibition of FTR1 expression reduces R. oryzae ability to take up 59Fe in vitro. (A) Plasmid pRNAi-pdc intron used to generate FTR1::RNAi strains. (B) RT-PCR showing lack of expression of FTR1 in R. oryzae transformed with RNAi plasmid (T1 and T3-T5) compared to R. oryzae transformed with empty plasmid (C, control). Primers amplifying the 18s rDNA served as a control to demonstrate the integrity of starting sample and lack of PCR inhibitors. (C) A representative of the RNAi transformants demonstrated comparable growth to the R. oryzae M16 transformed with empty plasmid on CSM-URA media. (D) 59Fe uptake by wild-type, R. oryzae M16 transformed with the empty plasmid, or one of the RNAi transformants. Germinated spores were incubated with 0.1 μM 59FeCl3 (a concentration in which high-affinity iron permeases are induced (Fu et al., 2004)). *P <0.05 when compared with R. oryzae wild-type or R. oryzae M16 transformed with empty plasmid. Data (n= 9 from three separate experiments) are expressed as medians ± interquartile ranges.
The ability to take up iron was tested in vitro of the transformant with near complete inhibition of FTR1 expression and similar growth to the control strain. Interestingly, RNAi decreased 59Fe uptake by R. oryzae more effectively than did gene disruption, with ~50% inhibition of iron uptake at all times tested (Fig. 6D).
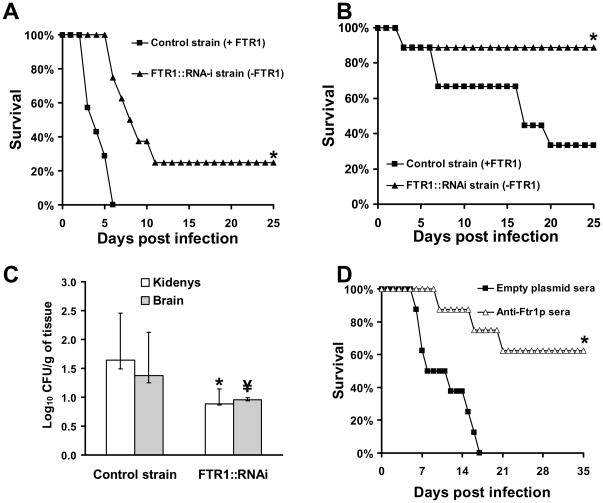
Next, the virulence of the RNAi transformant was compared to the control strain in the DKA mouse models of hematogenously disseminated or intranasal mucormycosis. The RNAi transformant demonstrated reduced virulence compared to the control strain in both models (75% vs. 100% mortality for RNAi transformant vs. control strain in mice with disseminated infection, and 11% vs. 67% mortality for RNAi transformant vs. control in the intranasal model, p<0.02 for both comparisons by Log Rand test) (Fig. 7A and 7B). Interestingly, strains recovered from kidneys of mice that died of infection with the RNAi transformant had lost the RNAi plasmid as evident by growth of R. oryzae colonies on YPD plates and not YNB+CSM-URA (data not shown), and hence had regained ability to express FTR1. In contrast, strains recovered from kidneys of mice that survived the infection through day 25, when the experiment was terminated, had not lost their RNAi plasmid. Additionally, mice infected intravenously with the RNAi transformant had ~ 6- and 3-fold reduction in kidney and brain fungal burden compared to mice infected with the control strain, respectively (Fig. 7C). These data demonstrate that the FTR1 gene product is an important virulence factor for R. oryzae in DKA mice.
Fig. 7.
Inhibition of FTR1 expression reduces virulence of R. oryzae in the DKA mouse models and passive immunization with anti-Ftr1p sera protects DKA mice from R. oryzae infection. (A) Survival of mice (n=8) infected i.v. with R. oryzae transformed with empty plasmid (control strain, 2.9 × 103 spores) or with RNAi plasmid targeting expression of FTR1 (FTR1-i, 4.1 × 103 spores). *, P<0.001. (B) Survival of mice (n=9) infected intranasally with R. oryzae transformed with empty plasmid (control strain, 2.8 × 103 spores) or with RNAi plasmid targeting expression of FTR1 (FTR1-i, 7.6 × 103 spores). *, P<0.02. (C) Kidney or brain Fungal burden of mice (n=8) infected i.v. with R. oryzae transformed with empty plasmid (control strain, 4.2 × 103 spores) or with RNAi plasmid targeting expression of FTR1 (FTR1-i, 5.1 × 103 spores). *, P<0.0006 and \, P<0.04 compared to control strain. Data are expressed as medians + interquartile ranges. The y-axes reflect lower limits of detection of the assay. (D) Survival of mice (n=8) infected intranasally with R. oryzae (intended inoculum of 2.5 × 107 spores and actual inhaled inoculum of 9 × 103 spores) and treated with serum collected from mice immunized with either Ftr1p or proteins collected form empty plasmid clone. *, P<0.007.
Passive immunization with sera collected from mice vaccinated with Ftr1p protects mice from R. oryzae infection
Because FTR1 was required for full virulence of R. oryzae, we hypothesized that passive immunization targeting FTR1 would protect against mucormycosis. To generate immune serum for passive immunization, mice were immunized by SQ injection of Ftr1p mixed with complete Freund’s adjuvant (CFA) followed by a boost with another dose of the antigen with incomplete Freund’s adjuvant (IFA) at day 21, and bled for serum collection two weeks later. Pooled sera collected from vaccinated mice had anti-Ftr1p IgG titers of >1:800,000, whereas pooled sera collected from negative control mice vaccinated with purified supernatant from an empty plasmid transformed clone had an anti-Ftr1p IgG titer of 1:200. Administration of immune sera to DKA mice subsequently infected intranasally with R. oryzae significantly improved survival compared to mice treated with control serum (63% vs. 0% survival for immune sera vs. non-immune sera, p<0.001) (Fig. 7D).
Discussion
Genetic manipulation of R. oryzae has long been hampered by the strong tendency of transformed plasmids to replicate in high-molecular-weight concatenated structures which rarely integrate into the chromosome (Skory, 2002). Even linearized plasmids tend to replicate autonomously following a non-homologous end joining event (Skory, 2005). It has been shown that introducing a double-strand break within the homologous regions on a linearized plasmid can significantly improve the likelihood of integration in R. oryzae, with up to 20% of the transformants showing homologous integration (Skory, 2002, Skory, 2005). However, this recombination of transformed plasmids happens only through a single cross over event or through a double strand break repair mechanism that corrects for point mutations (Skory, 2005). The standard double cross over event which is widely used to disrupt genes in other bacteria and fungi has not been described previously to occur in R. oryzae. We report for the first time that targeted gene disruption via a double cross over event is feasible in R. oryzae.
Previously, we unsuccessfully tried to disrupt genes through amplification of a gene disruption cassette in E. coli. This gene disruption cassette contained a 2.1 Kb PyrF fragment as a selectable marker flanked by up to 3 kb upstream and downstream sequences of FTR1. The disruption cassette was transformed into R. oryzae M16 after releasing it with restriction enzymes or as whole plasmid. In this study, we amplified a gene disruption cassette of ~1000 kb fragment of pyrF flanked by ~700 bp of upstream and downstream sequences of FTR1. The assembled disruption cassette was amplified by PCR instead of using E. coli. The current success in gene disruption may be due to trimming the selectable marker, or due to lack of methylation resulting from PCR amplification, in contrast to plasmid amplification in E. coli which results in DNA methylation. These possibilities are currently under active investigation.
We confirmed that the disruptant cassette was integrated into the appropriate locus and that growth in high iron concentrations selected for nuclei containing the ftr1 null allele. However, we were unable to obtain a homokaryotic isolate even with 14 rounds of sporulation and single colony isolations or hyphae passaging on medium containing 1 mM FeCl3, which exerted a diminished selective pressure to maintain FTR1. It is imperative to emphasize that this phenomenon of not obtaining a homokaryotic null is specific to FTR1 since we were able to obtain a homokaryotic his3 null in the background of R. oryzae M16 (unpublished data). Unlike other fungi, such as Candida albicans (Ramanan & Wang, 2000), Saccharomyces cerevisiae (Dix et al., 1994), Cryptococcus neoformans (Jung et al., 2008), and Fusarium graminearum (Park et al., 2006), R. oryzae lacks a low affinity iron permease and lacks a homolog for FTR1, which might support the hypothesis that FTR1 is an essential gene for the organism. That a single gene can be essential for growth has been described in fungi (Pierson et al., 2004, Sanyal & Carbon, 2002). The possibility that FTR1 is an essential gene in R. oryzae is currently under active investigation. These studies will include the possibility of using FTR1 gene controlled by a regulatory promoter or by generating conditional mutants of FTR1.
Even though we were unable to obtain a homokaryotic isolate of the FTR1 null allele, reduction in the relative copy number of functional FTR1 significantly reduced iron uptake and significantly abrogated virulence in vivo. Although we could not construct an FTR1-complemented strain due to the undeveloped state of genetic manipulation of R. oryzae (e.g. lack of dominant selection marker and the absence of auxotrophic marker other than pyrF), we were able to use RNAi-mediated reduction of FTR1 expression levels to confirm the role of FTR1 in iron uptake and pathogenesis. Furthermore, anti-Ftr1p polyclonal antibody resulted in significant protection against mucormycosis infection in DKA mice. Finally, several reports have demonstrated that altered URA3 expression due to integration in different loci when used as a selection marker in C. albicans affects virulence when constructing null mutants (Brand et al., 2004, Lay et al., 1998, Sundstrom et al., 2002). However, the diminished virulence of R. oryzae ftr1 putative null mutant or RNAi transformant cannot be attributed to altered PyrF expression since pyrF auxotrophy had no effect on virulence of R. oryzae in the DKA mouse model. Collectively, these data confirm that FTR1 is a virulence factor for the organism in vivo, and indicate that targeting of FTR1 by immunotherapy is of great promise in improving outcomes of these infections.
Fungi can acquire iron from the environment by three mechanisms (Ibrahim et al., 2008). These include: 1) reduction of the ferric form of iron into the more soluble ferrous form, followed by subsequent transport of iron via a high or low affinity iron permeases (reductive/permease system) that function in iron-limited or iron-rich environments, respectively (Dix et al., 1994, Eck et al., 1999, Knight et al., 2002, Ramanan & Wang, 2000); 2) a siderophore permease that facilitates the uptake of siderophore-sequestered iron (Heymann et al., 2002, Lesuisse et al., 2002, Lesuisse et al., 1998); and 3) release of ferric iron through degradation of heme by heme oxygenase (Santos et al., 2003). Pathogenic fungi utilize one or more of these systems to acquire iron from the host during infection. For example, the reductive/permease system has been shown to be critical for pathogenicity of C. albicans and C. neoformans through mediating iron uptake from transferrin (Jung et al., 2008, Knight et al., 2005). Additionally, heme oxygenase was shown to be involved in iron uptake from heme and hemoglobin by C. albicans (Pendrak et al., 2004, Santos et al., 2003) through binding to the cell surface mannoproteins encoded by RBT5 and RBT51 (Weissman & Kornitzer, 2004, Weissman et al., 2008). C. neoformans also utilizes heme and hemoglobin as a source of iron (Jung et al., 2008), however the receptor(s) that mediate binding of the fungus to these molecules are yet to be determined. In contrast, it was shown that the siderophore system and not the reductive/permease is needed for virulence in mice infected with Aspergillus fumigatus (Hissen et al., 2004, Hissen et al., 2005, Schrettl et al., 2004). Recently the genome sequencing project of R. oryzae revealed the presence of a single copy of the high affinity iron permease, several copies of putative siderophore permeases, and two copies of heme oxygenases (Ma et al., 2009). We found that the putative ftr1 null mutant had lagging ability to grow on FeCl3 or FeSO4 compared to wild-type or PyrF-complemented strains only in iron-depleted concentrations, confirming the importance of FTR1 in iron uptake from iron-limited but not iron-rich environments. Additionally, previous studies on iron uptake from ferrioxamine by Rhizopus showed that the organism efficiently liberates ferric iron from the siderophore extracellularly through an energy dependent step that requires the reduction of Fe3+ to Fe2+ prior to transporting the iron intracellularly, suggesting the involvement of the reductase/high affinity permease system (de Locht et al., 1994). Concordant with this hypothesis is our finding of lagging growth of the ftr1 putative null mutant compared to wild-type or PyrF-complemented strain when ferrioxamine is utilized as the source of iron. Moreover, we found the putative ftr1 null mutant had lagging growth on media supplemented with heme as well. Similar to bacteria (Allen & Schmitt, 2009, Liu et al., 2008, Stojiljkovic & Hantke, 1994, Zhu et al., 2008), FTR1 in R. oryzae may act as a cytoplasmic membrane permease that facilitates intracellular heme uptake which is followed by release of Fe3+ iron through degradation with heme oxygenases intracellularly. In fact, a greater growth defect was found when the putative ftr1 null mutant was grown on medium supplemented with ferrioxamine or heme compared to the putative ftr1 null mutant grown on FeCl3- or FeSO4-depleted medium. These results suggest that FTR1 is primarily responsible for the intracellular transport of iron from these two sources more than from iron salt sources.
Rhizopus is also known to secrete rhizoferrin, a siderophore that belongs to the polycarboxylate family (Thieken & Winkelmann, 1992). This siderophore supplies Rhizopus with iron through a receptor-mediated, energy dependant process (de Locht et al., 1994, Thieken & Winkelmann, 1992). However, it is not currently known whether rhizoferrin transports iron by release of iron extracellularly or if the siderophore is internalized prior to releasing iron in the cytoplasm. What is known is that rhizoferrin is inefficient in obtaining iron from serum (Boelaert et al., 1993, de Locht et al., 1994), and therefore the contribution of the organism’s endogenous siderophores to its virulence in a mammalian host is likely minimal. The lack of rhizoferrin ability to take up iron from serum is also highlighted by the adaptation of the organism to utilize xeno-siderophores such as deferoxamine, which are more efficient in obtaining iron from the host. Instead rhizoferrin and siderophore permeases are possibly required for iron uptake in environments other than mammalian hosts and likely explain the lack of total abrogation of iron uptake in the putative null and RNAi mutants in vitro compared to control strains (Fig. 4D and 6D).
In summary, we report for the first time the successful use of a double cross over event to integrate a gene disruption cassette in R. oryzae. While we successfully targeted the disruption cassette to the chromosome, it was not possible to obtain a homokaryon of the FTR1 null allele from multinucleated R. oryzae despite the fact that several independently constructed putative null mutants were tested. We found that FTR1 was required for full pathogenesis in vivo. Abrogation of FTR1 function resulted in diminished iron uptake and diminished virulence in vivo, and passive immunization with anti-Ftr1p antibody significantly improved survival in infected mice. These data contribute to the understanding of the critical role of iron uptake in mucormycosis pathogenesis. Finally, the use of passive immunotherapy against FTR1 is worthy of investigation as a strategy to improve outcomes of these deadly infections.
Experimental Procedures
Organisms and culture conditions
R. oryzae strains used in this study are listed in Table 1. Organisms were grown on potato dextrose agar (PDA) or on YPD plates [1% yeast extract (Difco Laboratories), 2% bacto-peptone (Difco), and 2% D-glucose] for 4 days at 37°C. For R. oryzae M16 (a pyrF null mutant that is unable to synthesize its own uracil), PDA was supplemented with 100 μg/ml uracil. We used an 815 bp partial pyrF PCR fragment (pyrF P11/P13) to restore R. oryzae M16 to prototrophy. This fragment overlaps the pyrF mutation present in M16 (i.e. point mutation at nt +205 of G to A)(Skory & Ibrahim, 2007) and is capable of restoring functionality through gene replacement. In some experiments, R. oryzae was starved for iron by growth on yeast nitrogen base (YNB) (Difco/Becton Dickinson, Sparks, MD) supplemented with complete supplemental media without uracil (CSM-URA) (Q-Biogene), (YNB+CSM-URA) [formulation/100ml, 1.7g YNB without amino acids, 20g glucose, 0.77g CSM-URA] in the presence of 1 mM of ascorbic acid and ferrozine. The sporangiospores were collected in endotoxin free PBS containing 0.01% Tween 80, washed with PBS, and counted with a hemacytometer to prepare the final inocula.
In vitro and in vivo expression of FTR1
To study the expression of FTR1, we utilized GFP as a reporter system for FTR1 promoter expression. R. oryzae M16 was transformed with a plasmid containing the reporter gene GFP driven by the FTR1 promoter (R. oryzae GFP1) as previously described (Ibrahim et al., 2007). GFP was also cloned under the constitutively expressed actin promoter (Act1p) then transformed into R. oryzae M16 to serve as a positive control. Prior to studying the expression of FTR1 in vivo we examined the expression of FTR1 in vitro using FACS analysis. Briefly, R. oryzae transformed with either GFP driven by Ftr1p or Act1p were grown in YNB+CSM-URA with (iron-limited conditions) or without (iron-rich conditions) 1 mM of ascorbic acid and ferrozine at 37°C for 12 h. These conditions produced germlings of R. oryzae rather than hyphae. Fluorescence of 1 ml of germlings was determined using a FACSCaliber (Becton Dickinson) instrument equipped with an argon laser emitting at 488 nm. Fluorescence emission was read with 515/40 bandpass filter. Fluorescence data were collected with logarithmic amplifiers. The mean fluorescence intensities of 104 events were calculated using CELLQUEST software.
For in vivo expression, BALB/c male (>20g) mice were rendered diabetic in ketoacidosis with a single i.p. injection of 210 mg/kg streptozotocin in 0.2 ml citrate buffer 10 days prior to infection with R. oryzae (Ibrahim et al., 2003a, Ibrahim et al., 2006, Ibrahim et al., 2007). Glycosuria and ketonuria were confirmed in all mice 7 days after streptozotocin treatment. Mice were infected via the tail vein with 2 × 106 spores of R. oryzae GFP1. Mice were sacrificed 24 or 48 h post infection and infected organs were collected and fixed in 10% zinc formalin, embedded in paraffin, and 5 mm sections, were stained with hematoxylin and eosin (H&E) to detect R. oryzae hyphae (Ibrahim et al., 2007). To detect GFP expression, anti-GFP rabbit polyclonal antibody (Novus) was used to stain the histopathological samples then counter stained with FITC conjugated anti-rabbit antibody.
To quantify the expression of FTR1 in infected tissues, brains of BALB/c mice infected with R. oryzae wild-type (99–880) through tail vein injection were collected 24 or 48 hr post infection and immediately flash frozen in liquid nitrogen prior to grinding and RNA extraction with phenol. Brains collected from uninfected DKA mice were processed in parallel and served as negative controls. Frozen brains were then ground under liquid nitrogen and total RNA was then isolated using the hot phenol method (Gravelat et al., 2008). Contaminating genomic DNA was removed from RNA samples by treatment with 1 μl of Turbo-DNase (Ambion) for 30 min at room temperature. DNase was then removed using an RNA Clean-Up kit (Zymo Research). First-strand cDNA synthesis was performed using the Retroscript first-strand synthesis kit (Ambion). FTR1 specific primers (listed in Table 2) were designed with the assistance of online primer design software (Genscript). The amplification efficiency was determined by serial dilution experiments, and the resulting efficiency coefficient was used for the quantification of the products (Pfaffl, 2001).
Gene expression was analyzed by an ABI Prism 7000 Sequence Detection System (Applied Biosystems) using the QuantiTect Sybr Green PCR kit (Qiagen). PCR conditions were 10 min at 90°C and 40 cycles of 15 s at 95°C and 1 min at 60°C. Single PCR products were confirmed with the heat dissociation protocol at the end of the PCR cycles. The amount of FTR1 expression in infected brains was normalized to either 18S rRNA or ACT1 (Table 2) and the quantified using the 2(−ΔΔC(T)) method (Livak & Schmittgen, 2001). All reactions were performed in duplicate, and the mixture included a negative no-reverse transcription (RT) control in which reverse transcriptase was omitted.
FTR1 disruption
To disrupt the FTR1, we constructed a gene disruption cassette encompassing a functional PyrF copy (998 bp) amplified from R. oryzae wild-type flanked by 606 and 710 bp fragments of FTR1-5′ UTR and FTR1-3′ UTR, respectively (Fig. 2A). The gene disruption construct was PCR amplified using primers FTR1 P1/P2 (Table 2) in order to obtain a 2.3 kb disruption fragment containing only the PyrF flanked by homologous FTR1 UTR sequence (Fig. 2A). This was then used to transform R. oryzae M16 (pyrF mutant) with biolistic bombardment (Skory, 2002). The disruption cassette replaces the entire FTR1 coding region from −16 to the stop codon, with the PyrF gene fragment. Isolates obtained from three separate transformations were purified with one round of sporulation and single colony isolation on chemically defined medium (YNB+CSM-URA) supplemented with 1 mM FeCl3 (iron-rich) to favor the segregation of the FTR1 null allele, since FTR1 expression in this iron concentration is suppressed (Fu et al., 2004). Isolates were tested for integration of the disruption cassette with PCR primer pairs FTR1-P3/PyrF-P9 (expected 1054 bp) and PyrF-P18/FTR1-P4 (expected 1140 bp). Disruption of FTR1 was confirmed by the absence of a PCR amplification product using primers FTR1-P11/FTR1-P12 to amplify 503 bp, primers FTR1-P5/FTR1-P6 to amplify the entire ORF of FTR1 and by Southern blot analysis. In an effort to obtain a homokaryotic isolate containing the FTR1 null allele, transformants with confirmed integration in the FTR1 locus were further taken through 14 rounds of sporulation and single colony isolation on YNB+CSM-URA supplemented with 1 mM FeCl3.
Nuclear staining
To determine the number of nuclei present in R. oryzae spores, we pregerminated spores in YPD medium for 2 h at 37°C. Swollen spores were washed once with cold PBS then suspended at a concentration of 5 × 105/ml in PBS. One μl of 50 μg/ml of 4′6-diamidino- 2-phenylindole (DAPI, Sigma) were added and the cells were electroporated (BioRad) according to the manufacturer instructions. The swollen spores were washed five times using cold PBS prior to resuspending in 100 μl PBS. Ten μl sample was placed on a glass slide and covered with a coverslip. The stained cells were visualized using an epifluorescence microscope.
Determination of the FTR1 wild-type or ftr1 mutant allele copy number
To compare the copy number of FTR1 wild-type or ftr1 mutant allele in the putative ftr1 null mutant grown on iron-rich or iron-limited media or to those of PyrF-complemented strain, we used qPCR. Briefly, genomic DNA was extracted with the OmniPrep lysis buffer (GBiosciences) from PyrF-complemented R. oryzae grown in YNB+CSM-URA supplemented with 1mM FeCl3 or putative ftr1 null mutant grown in either YNB+CSM-URA supplemented with 1 mM FeCl3 or 100 μM ferrioxamine. FTR1 wild-type or ftr1 mutant allele copy number in each sample was determined by qPCR using an ABI Prism 7000 Sequence Detection System (Applied Biosystems) and amplification products were detected with Power Sybr Green Cells-to-CT™ kit (Applied Biosystems). PCR conditions were as follows: denaturing at 95°C for 15 s min and amplification 40 cycles with annealing/extension carried out at 60°C for 1 min. FTR1 wild-type or ftr1 mutant allele copy numbers were then normalized to R. oryzae ACT1, and relative copy number was estimated using the formula 2−ΔΔ CT, where ΔCT= [Cttarget gene-CtACT1] and ΔΔCT= [ΔCT of mutant-ΔCT of PyrF-complemented strain].
Growth comparisons
Growth of the putative ftr1 disruption mutants were compared to R. oryzae wild-type or R. oryzae PyrF-complemented strains by growing on plates YNB+CSM-URA supplemented with 10 or 1000 μM of FeCl3, FeSO4, or with 100 μM of heme, or ferrioxamine as a source of iron. Additionally, putative ftr1 null or RNAi mutants were compared to their corresponding control strains for their growth on YPD or chemically defined medium (i.e. YNB+CSM-URA). Briefly, 10 μl of 105 spores of R. oryzae spores were spotted in the center of plates and the mycelial diameter was measured after 48 h of growth for medium containing FeCl3, FeSO4, or ferrioxamine or for 72 h for plates supplemented with heme. The experiment was repeated three times on different days and growth rate was expressed as average increase in mycelial diameter of the fungus per hour.
RNAi of FTR1
We used RNAi technology to inhibit the expression of FTR1 in R. oryzae. The RNAi construct was established in plasmid pRNAi-pdc intron (Fig 6A) which was generated using expression vector pPdcA-Ex (Mertens et al., 2006) as a backbone plasmid. Approximately a 2.0 kb fragment representing the PyrG was excised from plasmid pRNAi-pdc intron and replaced by 1641 bp of PyrF by digesting with XhoI and XmaI. The generated plasmid contained a pdc promoter for constitutive expression and an intron segment from Rhizopus pdcA gene (Skory, 2003) to serve as a linker for stabilization of the intended dsRNA structure (Nakayashiki et al., 2005, Wesley et al., 2001). We PCR amplified the first 450 bp fragment of FTR1 ORF containing the REGLE motif believed to interact with iron during uptake (Stearman et al., 1996). The amplified fragment was cloned in plasmid pRNAi-pdc intron down stream of the pdc promoter following digestion with SphI and AscI to yield pRNAi-FTR1partial. A reverse complement sequence of the 450 bp fragment was PCR amplified and cloned down stream of the intron segment following digestion with SacII and NheI to yield pRNAi-FTR1complete. The generated plasmid was transformed into R. oryzae pyrF mutant using the biolistic delivery system (BioRad) and transformants were selected on minimal medium lacking uracil (Skory, 2003).
To detect the effect of the RNAi construct on the expression of FTR1 in vitro, R. oryzae transformed with pRNAi-FTR1 complete or empty plasmid were grown in potato dextrose broth (PDB) at 37°C for 18 h. The mycelia were collected by filtration and transformed to fresh PDB containing 2 mM deferasirox dissolved in 2% DMSO for 1 h to induce iron starvation and FTR1 expression (Ibrahim et al., 2007). RNA samples were extracted using the RNeasy Plant Mini kit (Qiagen) and first-strand cDNA were generated with oligo(dT) primer using the SuperScript First-Strand Synthesis System (Invitrogen). The products were diluted and used in PCR to detect the expression of FTR1 of R. oryzae and 18S rDNA. The PCR products were separated on a 2% agarose gel containing 0.1 μg/ml ethidium bromide.
Iron uptake assay
To characterize the effect of FTR1 manipulation on the ability of R. oryzae to take up iron in vitro, ftr1 putative disruption mutants or RNAi mutants were compared to wild-type or R. oryzae PyrF-complemented strains in their ability to accumulate intracellular 59FeCl3 (Amersham Pharmacia Biotech) using a modification of our published method (Fu et al., 2004). Briefly, spores were pre-germinated for 3 h in YPD medium supplemented with 1mM ferrozine and 1 mM ascorbic acid at 37°C with shaking. Cells were harvested by centrifugation, washed twice with ice cold assay buffer pH 6.1 (minimal medium + 10 mM 4-morpholinepropanesulfonic acid + 1 mM ferrozine), and then resuspended in assay buffer without any ferrozine to give a concentration of 108 cells per ml. To measure uptake of 59Fe, 50 μl of the cell suspension was added to 450 μl of chilled assay buffer without ferrozine but supplemented with 0.1μM 59FeCl3, and incubated in a shaking water bath at 30°C. After selected time points, the assay samples were chilled on ice, vortexed, vacuum filtered through Whatman GF/C filters and washed with 10 ml ice cold SSW (1 mM EDTA, 20 mM Na3-citrate pH 4.2, 1 mMKH2PO4, 1 mM CaCl2, 5 mM MgSO4, and 1 mM NaCl). Filters were removed and placed in glass scintillation vials containing 10 ml scintillation fluid (Filter-count). Cell-associated 59Fe was counted in a Packard 2200CA liquid scintillation counter (Packard Instrument Co., Downers Grove, Il). Nonspecific uptake due to cell surface adsorption was determined by preparing parallel assays that were held on ice for 10 min before filtration and washing. The background levels of 59Fe were subtracted before calculation of uptake rates. The experiment was carried out in triplicate and repeated three times on different days. The data is presented as specific uptake in pmole/5× 106 germinated spores.
Pathogenesis studies
For in vivo infection, BALB/c male mice (≥20g) were rendered diabetic with a single i.p. injection of 190 mg/kg streptozotocin in 0.2 ml citrate buffer 10 days prior to fungal challenge.(Ibrahim et al., 2003a) Glycosuria and ketonuria were confirmed in all mice 7 days after streptozotocin treatment. DKA mice were infected with fungal spores by tail vein injection with a target inoculum of 5 × 103 spores. To confirm the inoculum, dilutions were streaked on PDA plates containing 0.1% triton and colonies were counted following a 24 h incubation period at 37°C. For the intranasal infection, 107 spores in 20 μl of 0.01% Tween 80 in PBS were placed on the nostrils of ketamine (100 mg/kg) sedated mice (Waldorf et al., 1984). To confirm the inoculum, mice were sacrificed immediately after inhaling R. oryzae spores, and lungs were homogenized, plated on PDA containing 0.1% triton and colonies were counted following incubation at 37°C. For both models, the primary efficacy endpoint was time to death. In some experiments, as a secondary endpoint, brain and kidney fungal burden (primary target organs) (Ibrahim et al., 2005a) was determined by homogenization by rolling a pipette on organs placed in Whirl-Pak bags (Nasco, Fort Atkinson, WI) containing 1 ml saline. The homogenate was serially diluted in 0.85% saline and then quantitatively cultured on PDA plates containing 0.1% triton. Values were expressed as log10 cfu g−1 tissue.
Expression and purification of a synthetic recombinant Ftr1p
Initially we expressed Ftr1p in E. coli using a Qiagen expression system (QIAexpressionist), however the yield was low. To enhance protein production we synthesized (Genscript) a gene encoding a more hydrophilic protein by removing the signal peptide and 6 transmembrane domains (Stearman et al., 1996, Fu et al., 2004) which direct localization of the protein to the cell membrane. While the synthesized gene had sequence elements removed, none of the remaining sequence was altered, so as to avoid altering potential epitopes in the exposed, hydrophilic regions of the protein. The synthetic gene also included a 6X-His-tag to affinity purify the expressed protein. This gene was cloned into pQE32 expression vector and transformed into E. coli. Log phase bacterial cells were induced with IPTG and the cells were harvested and the recombinant protein was purified over a Ni-agarose affinity column according to the manufacturer instructions (Qiagen). The generated protein was used to raise murine antibodies as described below.
Passive immunization
To generate immune serum for passive immunization, BALB/c mice were immunized by SQ injection of synthetic recombinant Ftr1p (20 μg) mixed with CFA at day 0, boosted with another dose of the antigen with IFA at day 21, and bled for serum collection two weeks later. Another set of mice were vaccinated with supernatants collected from E. coli transformed with empty plasmid to generate non-immune control serum. Antibody titers were determined using ELISA plates coated with 5 μg of synthetic recombinant Ftr1p as we previously described (Ibrahim et al., 2005b). Immune or control sera (0.25 ml) were administered i.p. to DKA recipient mice 2 h before intranasal infection with 2.5 × 107 R. oryzae 99–880 spores. Sera doses were repeated 3 days post infection and survival of mice was followed for 35 days post infection.
All animal experiments described in this study were approved by the institutional animal use and care committee, following the National Institutes of Health guidelines for animal housing and care.
Statistical analysis
The non-parametric log-rank test was used to determine differences in survival time, whereas differences in kidney fungal burden, iron uptake, growth rate and in vivo FTR1 expression were compared by the non-parametric Wilcoxon Rank Sum test.
Acknowledgments
The authors gratefully acknowledge the helpful discussions with Mingfu Liu and the technical assistance of May Abdallah and Mary Collins. Research described in this manuscript was conducted in part at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. This work was supported by Public Health Service grant R01 AI063503 and R21 AI082414 to A.S.I.
Footnotes
The authors have no conflict of interest to declare.
References
- Allen CE, Schmitt MP. HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae. J Bacteriol. 2009;191:2638–2648. doi: 10.1128/JB.01784-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31:1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. Journal of Clinical Investigation. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert JR, Van Cutsem J, de Locht M, Schneider YJ, Crichton RR. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney International. 1994;45:667–671. doi: 10.1038/ki.1994.89. [DOI] [PubMed] [Google Scholar]
- Boelaert JR, van Roost GF, Vergauwe PL, Verbanck JJ, de Vroey C, Segaert MF. The role of desferrioxamine in dialysis-associated mucormycosis: report of three cases and review of the literature. Clinical Nephrology. 1988;29:261–266. [PubMed] [Google Scholar]
- Boelaert JR, Vergauwe PL, Vandepitte JM. Mucormycosis infection in dialysis patients [letter] Annals of Internal Medicine. 1987;107:782–783. doi: 10.7326/0003-4819-107-5-782_2. [DOI] [PubMed] [Google Scholar]
- Brand A, MacCallum DM, Brown AJ, Gow NA, Odds FC. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell. 2004;3:900–909. doi: 10.1128/EC.3.4.900-909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Enfert C, Diaquin M, Delit A, Wuscher N, Debeaupuis JP, Huerre M, Latge JP. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect Immun. 1996;64:4401–4405. doi: 10.1128/iai.64.10.4401-4405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochemical Pharmacology. 1994;47:1843–1850. doi: 10.1016/0006-2952(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ. The FET4 gene encodes the low affinity Fe (II) transport protein of Saccharomyces cerevisiae. J Biol Chem. 1994;269:26092–26099. [PubMed] [Google Scholar]
- Eck R, Hundt S, Hartl A, Roemer E, Kunkel W. A multicopper oxidase gene from Candida albicans: cloning, characterization and disruption. Microbiology. 1999;145(Pt 9):2415–2422. doi: 10.1099/00221287-145-9-2415. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lee H, Collins M, Tsai HF, Spellberg B, Edwards JE, Jr, Kwon-Chung KJ, Ibrahim AS. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol Lett. 2004;235:169–176. doi: 10.1016/j.femsle.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45:1351–1360. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- Gravelat FN, Doedt T, Chiang LY, Liu H, Filler SG, Patterson TF, Sheppard DC. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect Immun. 2008;76:3632–3639. doi: 10.1128/IAI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann P, Gerads M, Schaller M, Dromer F, Winkelmann G, Ernst JF. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect Immun. 2002;70:5246–5255. doi: 10.1128/IAI.70.9.5246-5255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissen AH, Chow JM, Pinto LJ, Moore MM. Survival of Aspergillus fumigatus in serum involves removal of iron from transferrin: the role of siderophores. Infect Immun. 2004;72:1402–1408. doi: 10.1128/IAI.72.3.1402-1408.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect Immun. 2005;73:5493–5503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Avanessian V, Spellberg B, Edwards JE., Jr Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob Agents Chemother. 2003a;47:3343–3344. doi: 10.1128/AAC.47.10.3343-3344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Bowman JC, Avanessian V, Brown K, Spellberg B, Edwards JE, Jr, Douglas CM. Caspofungin inhibits Rhizopus oryzae 1,3-beta-D-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob Agents Chemother. 2005a;49:721–727. doi: 10.1128/AAC.49.2.721-727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Edwards JE, Jr, Fu Y, Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother. 2006;58:1070–1073. doi: 10.1093/jac/dkl350. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Edwards JEJ, Filler SG. Zygomycosis. In: Dismukes WE, Pappas PG, Sobel JD, editors. Clinical mycology. New York: Oxford University Press; 2003b. pp. 241–251. [Google Scholar]
- Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE, Spellberg BJ. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg B, Edwards J., Jr Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE., Jr Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect Immun. 2005b;73:999–1005. doi: 10.1128/IAI.73.2.999-1005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Sham A, Lian T, Singh A, Kosman DJ, Kronstad JW. Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 2008;4:e45. doi: 10.1371/journal.ppat.0040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman CA. Zygomycosis: reemergence of an old pathogen. Clin Infect Dis. 2004;39:588–590. doi: 10.1086/422729. [DOI] [PubMed] [Google Scholar]
- Kirsch DR, Whitney RR. Pathogenicity of Candida albicans auxotrophic mutants in experimental infections. Infect Immun. 1991;59:3297–3300. doi: 10.1128/iai.59.9.3297-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SA, Lesuisse E, Stearman R, Klausner RD, Dancis A. Reductive iron uptake by Candida albicans: role of copper, iron and the TUP1 regulator. Microbiology. 2002;148:29–40. doi: 10.1099/00221287-148-1-29. [DOI] [PubMed] [Google Scholar]
- Knight SA, Vilaire G, Lesuisse E, Dancis A. Iron acquisition from transferrin by Candida albicans depends on the reductive pathway. Infect Immun. 2005;73:5482–5492. doi: 10.1128/IAI.73.9.5482-5492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis DP, V, Wessel C, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ, Bennett JE. Medical Mycology. Philadelphia: Lea & Febiger; 1992. Mucormycosis; pp. 524–559. [Google Scholar]
- Lay J, Henry LK, Clifford J, Koltin Y, Bulawa CE, Becker JM. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun. 1998;66:5301–5306. doi: 10.1128/iai.66.11.5301-5306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuisse E, Knight SA, Camadro JM, Dancis A. Siderophore uptake by Candida albicans: effect of serum treatment and comparison with Saccharomyces cerevisiae. Yeast. 2002;19:329–340. doi: 10.1002/yea.840. [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Simon-Casteras M, Labbe P. Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology. 1998;144(Pt 12):3455–3462. doi: 10.1099/00221287-144-12-3455. [DOI] [PubMed] [Google Scholar]
- Liu M, Tanaka WN, Zhu H, Xie G, Dooley DM, Lei B. Direct hemin transfer from IsdA to IsdC in the iron-regulated surface determinant (Isd) heme acquisition system of Staphylococcus aureus. J Biol Chem. 2008;283:6668–6676. doi: 10.1074/jbc.M708372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, Elias M, Idnurm A, Lang BF, Sone T, Abe A, Calvo SE, Corrochano LM, Engels R, Fu J, Hansberg W, Kim JM, Kodira CD, Koehrsen MJ, Liu B, Miranda-Saavedra D, O’Leary S, Ortiz-Castellanos L, Poulter R, Rodriguez-Romero J, Ruiz-Herrera J, Shen YQ, Zeng Q, Galagan J, Birren BW, Cuomo CA, Wickes BL. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Mertens JA, Skory CD, Ibrahim AS. Plasmids for expression of heterologous proteins in Rhizopus oryzae. Archives of microbiology. 2006;186:41–50. doi: 10.1007/s00203-006-0121-9. [DOI] [PubMed] [Google Scholar]
- Nakayashiki H, Hanada S, Nguyen BQ, Kadotani N, Tosa Y, Mayama S. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet Biol. 2005;42:275–283. doi: 10.1016/j.fgb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Park YS, I, Choi D, Kang CM, Ham MS, Kim JH, Kim TH, Yun SH, Lee YW, Chang HI, Sung HC, Yun CW. Functional identification of high-affinity iron permeases from Fusarium graminearum. Fungal Genet Biol. 2006;43:273–282. doi: 10.1016/j.fgb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Pendrak ML, Chao MP, Yan SS, Roberts DD. Heme oxygenase in Candida albicans is regulated by hemoglobin and is necessary for metabolism of exogenous heme and hemoglobin to alpha-biliverdin. J Biol Chem. 2004;279:3426–3433. doi: 10.1074/jbc.M311550200. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson CA, Jia N, Mo C, Lees ND, Sturm AM, Eckstein J, Barbuct R, Bard M. Isolation, characterization, and regulation of the Candida albicans ERG27 gene encoding the sterol 3-keto reductase. Med Mycol. 2004;42:461–473. doi: 10.1080/1369378032000141471. [DOI] [PubMed] [Google Scholar]
- Ramanan N, Wang Y. A high-affinity iron permease essential for Candida albicans virulence. Science. 2000;288:1062–1064. doi: 10.1126/science.288.5468.1062. [DOI] [PubMed] [Google Scholar]
- Reed C, Bryant R, Ibrahim AS, Edwards J, Jr, Filler SG, Goldberg R, Spellberg B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Santos R, Buisson N, Knight S, Dancis A, Camadro JM, Lesuisse E. Haemin uptake and use as an iron source by Candida albicans: role of CaHMX1-encoded haem oxygenase. Microbiology. 2003;149:579–588. doi: 10.1099/mic.0.26108-0. [DOI] [PubMed] [Google Scholar]
- Sanyal K, Carbon J. The CENP-A homolog CaCse4p in the pathogenic yeast Candida albicans is a centromere protein essential for chromosome transmission. Proc Natl Acad Sci U S A. 2002;99:12969–12974. doi: 10.1073/pnas.162488299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, Jr, Haynes K, Haas H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med. 2004;200:1213–1219. doi: 10.1084/jem.20041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek GT, Dodgson KJ, de Magalhaes-Silverman M, Bartelt LA, Kilborn SB, Hoth PL, Diekema DJ, Pfaller MA. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis. 2004;39:584–587. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]
- Skory CD. Homologous recombination and double-strand break repair in the transformation of Rhizopus oryzae. Mol Genet Genomics. 2002;268:397–406. doi: 10.1007/s00438-002-0760-8. [DOI] [PubMed] [Google Scholar]
- Skory CD. Induction of Rhizopus oryzae pyruvate decarboxylase genes. Curr Microbiol. 2003;47:59–64. doi: 10.1007/s00284-002-3933-0. [DOI] [PubMed] [Google Scholar]
- Skory CD. Inhibition of Non-Homologous End Joining and integration of DNA upon transformation of Rhizopus oryzae. Mol Genet Genomics. 2005;274:373–383. doi: 10.1007/s00438-005-0028-1. [DOI] [PubMed] [Google Scholar]
- Skory CD, Ibrahim AS. Native and modified lactate dehydrogenase expression in a fumaric acid producing isolate Rhizopus oryzae 99–880. Curr Genet. 2007;52:23–33. doi: 10.1007/s00294-007-0135-0. [DOI] [PubMed] [Google Scholar]
- Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RD, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Sugar AM. Agents of Mucormycosis and Related Species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier; 2005. p. 2979. [Google Scholar]
- Sundstrom P, Cutler JE, Staab JF. Reevaluation of the role of HWP1 in systemic candidiasis by use of Candida albicans strains with selectable marker URA3 targeted to the ENO1 locus. Infect Immun. 2002;70:3281–3283. doi: 10.1128/IAI.70.6.3281-3283.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieken A, Winkelmann G. Rhizoferrin: a complexone type siderophore of the Mucorales and entomophthorales (Zygomycetes) FEMS Microbiol Lett. 1992;73:37–41. doi: 10.1016/0378-1097(92)90579-d. [DOI] [PubMed] [Google Scholar]
- Varma A, Edman JC, Kwon-Chung KJ. Molecular and genetic analysis of URA5 transformants of Cryptococcus neoformans. Infect Immun. 1992;60:1101–1108. doi: 10.1128/iai.60.3.1101-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. Journal of Clinical Investigation. 1984;74:150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman Z, Kornitzer D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol. 2004;53:1209–1220. doi: 10.1111/j.1365-2958.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- Weissman Z, Shemer R, Conibear E, Kornitzer D. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans. Mol Microbiol. 2008;69:201–217. doi: 10.1111/j.1365-2958.2008.06277.x. [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 2001;27:581–590. doi: 10.1046/j.1365-313x.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xie G, Liu M, Olson JS, Fabian M, Dooley DM, Lei B. Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J Biol Chem. 2008;283:18450–18460. doi: 10.1074/jbc.M801466200. [DOI] [PMC free article] [PubMed] [Google Scholar]