Abstract
Rationale
Vascular calcification is highly associated with cardiovascular morbidity and mortality, especially in patients with chronic kidney disease. The nuclear receptor farnesoid X receptor (FXR) has been implicated in the control of lipid, carbohydrate and bile acid metabolism in several cell types. Although recent studies have shown that FXR is also expressed in vascular smooth muscle cells, its physiological role in vasculature tissue remains obscure.
Objective
Here, we have examined the role of FXR in vascular calcification.
Methods and Results
The FXR gene, a bile acid nuclear receptor, was highly induced during osteogenic differentiation of bovine calcifying vascular cells (CVC) and in the aorta of apolipoprotein E (ApoE)−/− mice with chronic kidney disease which are common tissue culture and mouse model, respectively, for aortic calcification. FXR activation by a synthetic FXR agonist, 6α-ethyl chenodeoxycholic acid (INT-747) inhibited phosphate induced-mineralization and triglyceride accumulation in CVC. FXR dominant negative expression augmented mineralization of CVC and blocked the anti-calcific effect of INT-747 whereas VP16FXR that is a constitutively active form reduced mineralization of CVC. INT-747 treatment also increased phosphorylated c-Jun N-terminal kinase (JNK). SP600125 (specific JNK inhibitor) significantly induced mineralization of CVC and ALP expression, suggesting that the anti-calcific effect of INT-747 is due to JNK activation. We also found that INT-747 ameliorates chronic kidney disease (CKD) induced-vascular calcification in 5/6 nephrectomized ApoE−/− mice without affecting the development of atherosclerosis.
Conclusions
These observations provide direct evidence for that FXR is a key signaling component in regulation of vascular osteogenic differentiation and, thus representing a promising target for the treatment of vascular calcification.
Keywords: farnesoid X receptor, vascular calcification, chronic kidney disease
Introduction
Vascular calcification is very common in subjects with chronic kidney disease (CKD) and is an independent predictor of cardiovascular mortality.1–6 Accumulation of calcium-phosphate complex in vascular wall decreases aortic elasticity and flexibility, which impairs cardiovascular hemodynamics, resulting in substantial morbidity and mortality. This process was considered to be passive. However, recent studies have shown that it is a highly orchestrated process that entrains a repertoire of transcription factors including msh homeo box 2(Msx2),7 Osterix 6 and runt-related transcription factor 2 (Runx2) 8 and involves the activation of an osteogenic program that recapitulates the molecular fingerprints seen in bone formation. Vascular calcified cells express many bone-related proteins, including alkaline phosphatase (ALP) and type I collagen (COL1A1). 5–7 In addition, in vitro and in vivo models of vascular calcification have implicated a variety of factors in the pathogenesis of calcification, including osteoprotegrin, osteopontin, osteocalcin (OCL), matrix γ-carboxyglutamic acid protein (MGP), phosphate, inflammatory cytokines, lipids and reactive oxygen species.6 Despite these insights, it is still not fully known how vascular calcification is regulated and potential treatment modalities for this disease remain elusive.
In addition to their detergent effects on dietary lipids and fat-soluble vitamins absorption, bile acids exert several biological functions via a number of nuclear and plasma membrane receptors, including farnesoid X receptor (FXR), TGR5, vitamin D receptor and pregnane X receptor. 8–11 Thus bile acids are signaling molecules governing not only bile acid synthesis, conjugation and transport, but also lipid, carbohydrate and energy metabolism.
FXR is a member of the nuclear receptor family of transcription factors activated by bile acids, the most potent endogenous ligand being chenodeoxycholic acid.12, 13 FXR is highly expressed in tissues in which bile acids are present at high concentration, such as liver, kidney and intestine. Analysis of FXR function using genetically deficient mice and synthetic agonists has established the important role of this receptor in the control of bile acid, lipid and carbohydrate metabolism14–16. Recent studies have also shown that functional FXR is expressed in vascular cells, including vascular smooth muscle cells and endothelial cells.17–19 FXR expression is also observed in the atherosclerotic lesions of human aorta.17 FXR directly and indirectly regulates the transcription of genes involved in lipoprotein metabolism such as SR-BI, ApoAI, ApoCII, ApoCIII, phospholipid transfer protein, sterol regulatory element binding protein (SREBP)-1c, and VLDL receptor.8, 12, 15, 16 Studies using FXR−/− mice have shown that FXR deficiency increases plasma triglycerides, cholesterol and HDL-cholesterol, while administration of FXR agonists reduces plasma triglycerides.20–23 However, the role of FXR in modulation of atherosclerosis is controversial24–28. In addition the role of FXR in vascular calcification development has not yet been studied.
In the present study, we have found that FXR is highly up-regulated during osteogenic differentiation in bovine calcifying vascular cells (CVC) and in the aorta of ApoE−/− mice with CKD. In addition, FXR activation with the specific agonist 6α-ethyl chenodeoxycholic acid (INT-747) blocks mineralization, lipid accumulation and osteogenic differentiation in CVC. We also have examined the effects of INT-747 administration to apolipoprotein E-deficient (ApoE−/−) mice with CKD induced by 5/6 nephrectomy. INT-747 treatment ameliorates CKD-induced vascular calcification in ApoE−/− mice without affecting the development of atherosclerosis. These observations suggest that FXR activation may represent a promising therapeutic agent for intervention in CKD-induced vascular calcification.
Methods
The expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org. 5/6 nephrectomized and sham-operated ApoE−/− mice were purchased from Jackson Laboratory. At 8 weeks of age, animals were fed a Western diet with or without INT-747 for 12 weeks. Bovine calcifying vascular cells (CVC) were cultured in DMEM containing 15% FBS with 2.0 mM phosphate.
Results
FXR is highly induced in the in vitro and in vivo models of vascular calcification
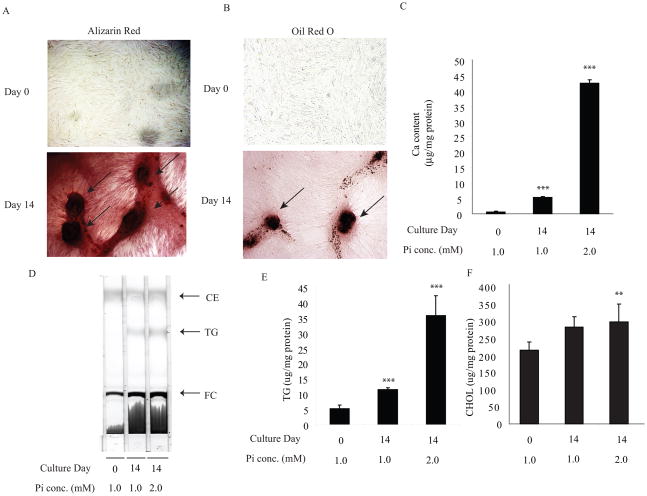
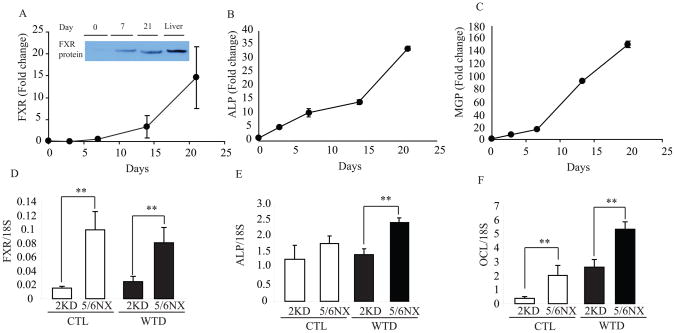
1. Members of the nuclear hormone receptor superfamily are transcription factors that regulate diverse pathways of metabolism. In addition to acting as molecular sensors of lipid and carbohydrate homeostasis, several members of the nuclear receptor family (e.g. eroxisome proliferator-activated receptor, retinoid X receptors and liver X receptors (LXR)) also exert beneficial pleiotropic effects to ameliorate atherosclerosis and its complications. However, the role of nuclear receptors in the development of vascular calcification is still unclear. To examine which nuclear receptors may be involved in vascular calcification and to identify nuclear receptor genes that were altered during osteogenic differentiation of vascular cells, we used a quantitative RT-PCR (qPCR) array to analyze all nuclear receptor genes with exception of EAR2, whose cDNA sequence is currently not available. To induce calcification, bovine vascular cells at 90–95% confluence were cultured with 2 mM phosphate (high phosphate) for 14 days. High phosphate treatment promoted mineralization (Figure 1A and 1C) consistent with previous reports. Interestingly, calcified nodules accumulate neutral lipids stained by Oil Red O (Figure 1B). Thin layer chromatography and quantitative analysis showed that triglycerides (Figure 1D and 1E) and total cholesterol (Figure 1F) levels were increased by 7.2-fold and 1.3-fold, respectively in CVC treated with 2 mM phosphate. RNA from these cells was subjected to qPCR analysis. We identified 15 nuclear receptor genes with distinct levels of expression, seven of which were markedly induced during osteogenic calcification of CVCs (Table I). The gene encoding mineralocorticoid receptor (MR) ranked the highest in this analysis, followed by FXR, HNF4α, COUP-TF1, RXRα, VDR, and RARγ gene. In this study, we focused on determining the role of FXR in the pathogenesis of vascular calcification because MR has already been reported to promote vascular calcification.29 FXR mRNA and protein levels were time-dependently induced during vascular osteogenic differentiation and increased by 14.5-fold in calcified cells at day 21 of culture compared to non-calcified cells (Figure 2A and 2A insert). As expected, osteogenic markers such as ALP (Figure 2B), MGP (Figure 2C) and OCL (data not shown) mRNAs were also induced during osteogenic differentiation.
Figure 1. The calcified nodules in bovine calcifying vascular cells (CVC) accumulate not only calcium but also neutral lipids.
CVC at 90–95 % confluence was treated with osteogenic medium containing 2.0 mM phosphate. After 14 days in culture, cells were stained with A) Alizarin Red and B) Oil Red O, which identify calcium mineral and neutral lipid as red, respectively. Arrows indicate calcified nodules. C) Ca content. D) TLC analysis. E) triglycerides and F) and Cholesterol levels.
Table 1.
Induced nuclear receptor genes during osteogenic differentiation of CVC
| Nomenclature | Gene | Abbreviation | Fold Change |
|---|---|---|---|
| 1. NR3C2 | Mineralocorticoid receptor | MR | 88.1 |
| 2. NR1H4 | Farnesoid X receptor | FXR | 11.3 |
| 3. NR2A | Hepatocyte nuclear factor 4α | HNF4α | 12.8 |
| 4. NR2A1 | Chiken ovalbumin upstream promoter TF1 | COUP-TF | 12.2 |
| 5. NR2B1 | Retinoid X receptor α | RXRα | 2.2 |
| 6. NR1I1 | Vitamin D receptor | VDR | 2.1 |
| 7. NR1B3 | Retinoic acid receptor γ | RARγ | 2.0 |
Bovine calcifying vascular cells were cultured for 14 days after confluency. The gene expression of nuclear receptor was determined by a customized PCR array. Data are presented as mRNA expression relative to Day 0 control. TF, transcription factor. The average Ct at day 14: MR; 29.32, FXR; 19.55, HNF4a; 27.24, COUP-TF1; 17.61, RXRa; 19.32, VDR; 18.95, RARg; 19.34
Figure 2. FXR expression is induced during osteogenic differentiation of CVC and in the aorta of ApoE−/− mice with chronic kidney disease.
A) FXR gene and protein expression (insert), B) ALP, C) MGP gene expression in CVC. D) FXR, E) ALP and F) OCL in the aorta of ApoE−/− mice with 5/6 nephrectomy fed a western diet for 24 weeks. (A). Nuclear extract (100 μg) was subjected to SDS-PAGE and immunoblot analysis. Total liver protein lysate (10μg) was used as a positive control. (A–C) Data are presented as mRNA expression relative to Day 0 culture. (D–F) **p<0.001 vs. 2 kidney counterparts.
We then examined aortic FXR expression in mouse models of vascular calcification. Consistent with previous reports,30 5/6 nephrectomized ApoE−/− mice fed a western diet for 24 weeks exhibited a significant acceleration of atherosclerosis and vascular calcification compared to ApoE−/− mice with normal 2 kidneys. Atherosclerotic plaque size was increased by in 84% in 5/6 nephrectomized ApoE−/− (Online Figure IB) fed control and western diet, respectively. Aortic calcium content was increased by 2.7-fold (Online Figure IC) cmpared to ApoE−/− control fed western diet. Real-time PCR analysis showed that 5/6 nephrectomy induced aortic FXRα expression in ApoE−/− mice by 5.8-fold under control diet and 3.2-fold higher on western diet (Figure 2D). mRNA expression of the osteogenic markers ALP and OCL was significantly higher in 5/6-nephrectomized ApoE−/− mice fed with either western or control diet (Figure 2E and 2F).
FXR activation blocks phosphate induced-mineralization and triglyceride accumulation in bovine vascular cells
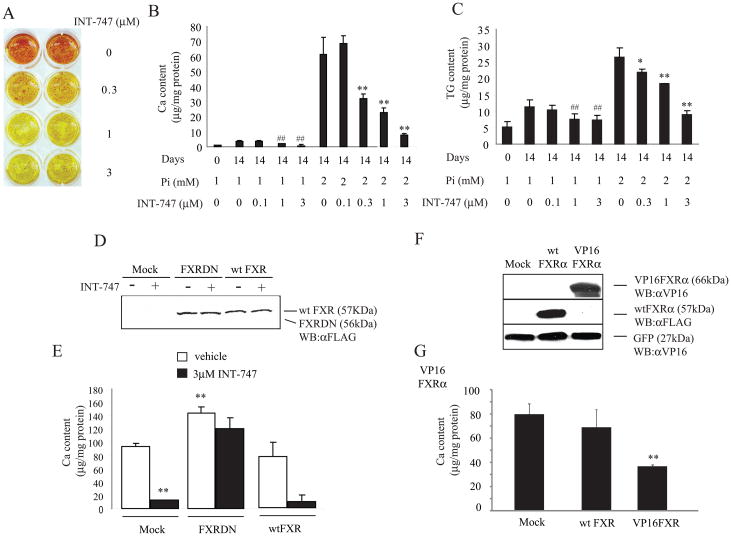
Since FXR mRNA was highly induced in both in vivo and in vitro vascular calcification models, we examined the effect of FXR activation in vascular calcification. CVC were treated with an FXR agonist INT-747 to test if FXR activation influences phosphate induced-mineralization. Alizarin red staining revealed that the treatment with INT-747 dose-dependently reduced phosphate induced-mineralization of CVC (Figure 3A). Calcium content of CVC was increased by 18.2-fold in response to 2 mM phosphate compared to 1 mM phosphate. Consistent with Alizarin red staining, INT-747 treatment dose-dependently decreased calcium content in CVC under both high and normal phosphate conditions. 3μM INT-747 reduced calcium content by 79% in normal phosphate and 83% in high phosphate conditions (Figure 3B). INT-747 treatment also reduced triglyceride content but not cholesterol content. At the 3μM concentration, triglyceride level was reduced by 38% and 64% in the presence of 1mM and 2mM phosphate, respectively (Figure 3C). To determine whether the anti-calcific effect of INT-747 is via FXR activation, we over-expressed FXR dominant negative (DN) 31 and wt FXR in CVC using Lenti-X lentiviral expression system. Figure 3D and 3E shows that FXR DN expression significantly increased calcium content in CVC but also blocked the anti calcific effect of INT-747. Wild-type FXR overexpression did not affect mineralization of CVC and the anti-calcific effect of INT-747 (Figure 3E and 3G). Since wild-type FXR overexpression was not effective to inhibit mineralization of CVC, we treated CVC with adenovirus expressing VP16FXR that is a constitutively active form. The overexpression of VP16FXR was able to reduce mineralization of CVC by 54% (Figure 3F and 3G) consistent with FXR activation by INT-747. We then analyzed genes encoding osteogenic markers such as ALP, COL1A1 and MGP to test if INT-747 affects osteogenic differentiation. As shown in Online Figure II, the gene expression of ALP, COL1A1 and MGP were reduced in CVC treated with 3μM INT-747 in the presence of high-phosphate for 14 days, suggesting that FXR activation inhibits osteogenic differentiation. In addition, INT-747 decreased mRNA levels of Msx2 and osterix but not Runx2 which are three major transcription factors involved in osteogenic differentiation (Online Figure II). Consistent with reduced triglyceride content (Figure 3B), INT-747 treatment decreased mRNA abundance of transcription factors and enzymes involved in lipogenesis including SREBP-1, SREBP-2, fatty acid synthase (FAS) and acetyl-CoA carboxylase1 (ACC1). As expected, long-term treatment (14 days) with 3 μM INT-747 significantly induced FXR target genes, small heterodimer partner (SHP) and angiotensin type II receptor (AT2R) 18, 32 (Online Figure II). In addition, short-term treatment (24 hours) with INT-747 dose-dependently increased SHP mRNA level (Online Figure III). FXRDN also completely inhibited the induction of SHP and the reduction of ALP, COL1A1, MGP, Msx2 and osterix by INT-747 treatment (Online Figure IV). Consistent with FXR activation by INT-747, VP16FXR expression significantly reduced Msx-2, Osterix, ALP and COL1A1 mRNA levels (Online Figure V)
Figure 3. FXR agonist, 6α-ethyl chenodeoxycholic acid (INT-747) reduced mineralization in CVC under normal and high phosphate conditions.
A) Alizarin staining: CVC were treated with different concentration of INT-747 for 14 days in the presence of high (2.0 mM) phosphate and stained with Alizarin Red. B) Calcium content: C) Triglyceride content was analyzed by enzymatic analysis. D) Western blot analysis of FXR DN and wild-type (wt) FXR. FXR DN and wt FXR proteins were detected using anti-FLAG antibody. E) FXR DN but not wt FXR expression augments mineralization of CVC and inhibits the anti-calcific effect of INT-747. CVC were pretreated overnight with lentivirus expressing Mock (empty), FXR DN and wt FXR at 10 MOI and incubated with INT-747 (3 μM) for 14 days. F) Western blot analysis of wt FXR and VP16FXR and G) Ca content in VP16FXR-treated CVC. Wt FXR and VP16FXR protein were detected using anti-Flag and anti-VP16 antibody, respectively. CVC were pre-treated overnight with adenoviruses expressing Mock (empty), wt FXR and VP16FXR at 40 MOI and incubated for 14 days in the presence of high-phosphate.
The anti-calcific effect of INT-747 is due to activation of c-Jun N-terminal kinase (JNK)
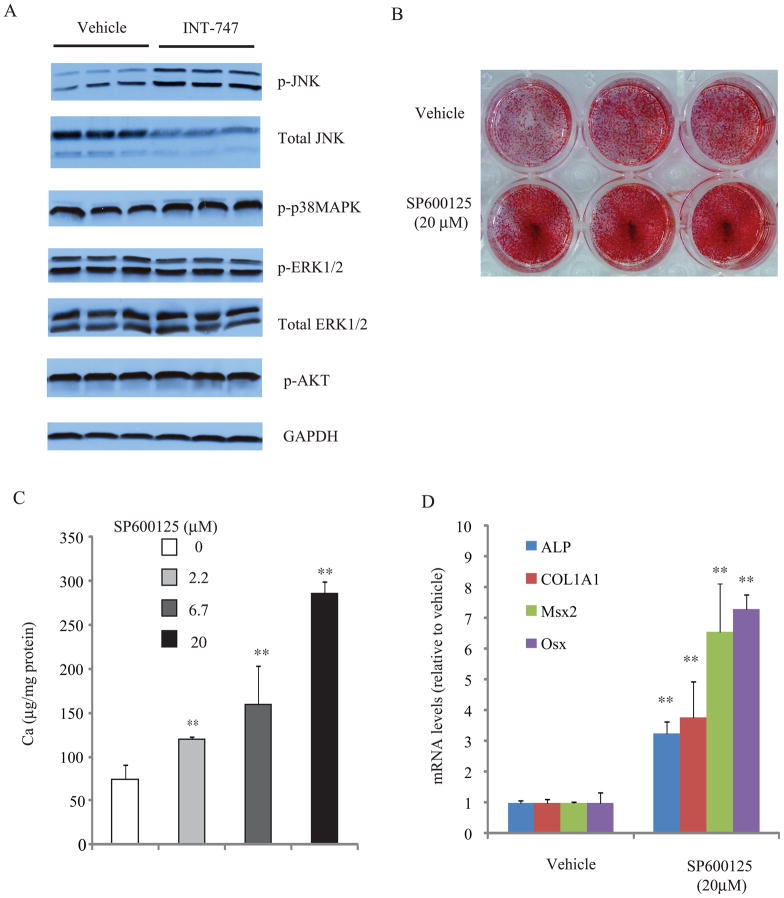
To understand the mechanisms by which FXR activation blocks mineralization of CVC, we examined if FXR activation influences a cell signaling involved in vascular calcification. Previous studies have shown that MAPK and AKT pathways are involved in osteogenic differentiation and mineralization of vascular cells.11, 33 INT-747 treatment significantly increased phosphorylated (p) JNK level by 3.1-fold. Interestingly total JNK protein was significantly lowered in INT-747 treated-CVC; p-ERK, total ERK, p-p38 MAPK, p-Akt and GAPDH were not different (Figure 4A). We therefore hypothesized that the activation of JNK contributes the anti-calcific effect of INT-747. Consistent with our hypothesis, A JNK-specific inhibitor SP600125 dose-dependently induced phosphate-induced mineralization of CVC (Figure 4B and 4C). The treatment with SP60012 at 20μM for 14 days also induced ALP, COL1A1, Msx2 and osterix expression by 3.2-, 3.8-, 5.6- and 7.3, respectively (Figure 4D). The 24 hours treatment also induced ALP, COL1A1 and Msx2 by 11-, 39- and 4.5-fold, respectively, whereas osterix expression was not altered (data not shown).
Figure 4. FXR activation reduced phosphate-induced JNK activation.
A) CVCs were pretreated with INT-747 (3 μM) for 24 hours in the presence of high phosphate. Parallel immunoblots were run from same cell lysates using antibodies against the phosphorylated JNK (p-JNK), total JNK, p38 MAPK (p-p38MAPK), phosphorylated ERK (p-ERK), total ERK, phosphorylated phosphorylated AKT (p-AKT) and GAPDH. Data are representative of two experiments with similar results. B) Alizarin staining and C) Ca content. CVC were treated with SP600125 at the indicated concentration for 14 days in the presence of high-phosphate. D) ALP, COL1A1, Msx2 and osterix expression in CVC treated with SP600125 at 20 μM for 14 days. **p<0.01 vs. vehicle.
Msx2 and osterix augments mineralization and osteogenic differentiation of CVC
To determine if Msx2 and osterix (Osx) mediate phosphate induced-calcification, Msx2 and osterix were over-expressed using adenoviral expression system. CVC were pretreated adenovirus expressing Msx2 and osterix at 40 MOI for overnight and then cultured for 7 days in the presence of high-phosphate. Msx2 and osterix overexpression (Online Figure VI) increased calcium levels in CVC by 9.3-fold and 3.7-fold, respectively (Online Figure VI B&C). In addition, Msx2 and osterix overexpression induced ALP expression by 10.7-fold and 5.7-fold, respectively and COL1A1 by 8.0-fold and 7.4-fold, respectively (Online Figure VI D).
FXR activation prevents the development of vascular calcification in ApoE−/− with chronic kidney disease
In order to examine if FXR activation protects against in vivo vascular calcification, we treated 5/6 nephrectomized ApoE−/− mice with INT-747 at either 10 mg/kg (mpk) or 20 mg/kg (mpk) for 12 weeks. There were no significant differences in body weight and food intake among the experimental groups. Blood cholesterol, triglyceride and phosphorus levels were significantly higher in ApoE−/− mice compared to C57BL/6 control mice. Blood urea nitrogen and serum phosphorus levels were higher in ApoE−/− mice with 5/6 nephrectomy than 2 kidney control, confirming that ApoE−/− mice developed CKD induced by 5/6 nephrectomy. BUN and phosphorus levels were unaffected by INT-747 treatment. Twelve week treatment with INT-747 at either dose reduced both serum and hepatic triglycerides in ApoE−/− mice with CKD. Hepatic cholesterol was reduced in the liver of ApoE−/− mice with CKD treated with INT-747 at the higher 20 mpk concentration. Serum cholesterol, serum BUN, serum phosphorus, serum calcium, serum glucose and aspartate aminotransferase levels were unaffected by INT-747 treatment (Online Table I).
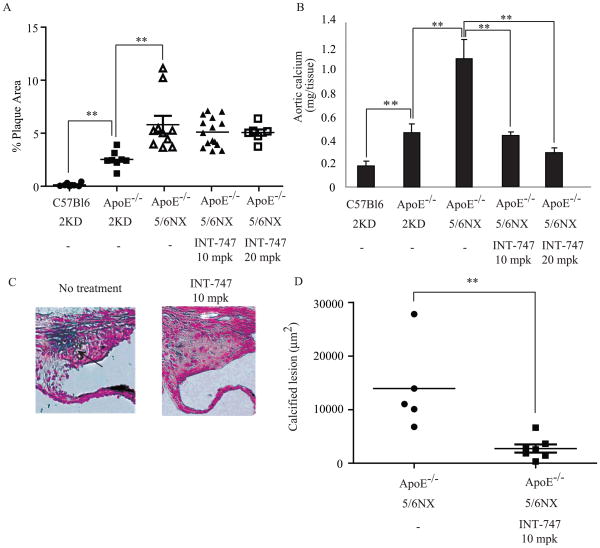
Atherosclerotic lesions in male ApoE−/− mice were quantified by en face analysis of aortas after 12 weeks on a western diet in the presence or absence of the FXR agonist. Quantification of Sudan IV-stained en face preparations of aortas showed that atherosclerosis developed in ApoE−/− mice with 2 kidneys and was accelerated by 5/6 nephrectomy. No atherosclerotic plaque formation was observed in C57BL/6 mice. Unexpectedly, however, INT-747 treatment at both 10 mpk and 20 mpk doses had no effect on atherosclerotic plaque formation in ApoE−/− mice with CKD (Figure 5A). In contrast, 5/6 nephrectomized ApoE−/− mice receiving the LXR agonist GW3965 at 5mg/kg as a positive control exhibited a statistically significant 84% reduction in average lesion area (unpublished data, S.M. M.L and M.M). In order to examine the effectiveness of INT-747 in influencing aortic calcification, the calcium content in the whole aorta was analyzed. Consistent with Figure S1, Figure 5B shows that aortic calcium content was significantly increased in ApoE−/− mice and was further increased by CKD. The groups receiving both 10 mpk and 20 mpk INT-747 showed a statistically significant 61% and 66% reduction in aortic calcium content compared to untreated ApoE−/− mice with CKD. To further demonstrate the anti-vascular calcific effect, aortic sinus lesions from ApoE−/− mice with CKD were analyzed with Von Kossa staining. Quantification of calcified lesion revealed that a significant 81 % reduction in calcified lesion area in mice treated with 10 mpk INT-747 compared with untreated controls (Figure 5C and 5D). No significant difference was observed in atherosclerotic lesion between vehicle and 10 mpk INT-747 groups, consistent with en face analysis (1704582 ± 61545 vs. 1733145 ± 53813 μm2).
Figure 5. FXR activation ameliorates vascular calcification in ApoE−/− mice with CKD without affecting the development of atherosclerosis.
A) En face analysis of atherosclerosis in 5/6 nephrectomized ApoE−/− mice treated with INT-747. Mice were fed western diet supplemented with INT-747 for 12 weeks. B) Aortic calcium content in 5/6 nephrectomized ApoE−/− mice treated with INT-747. C) Calcified area stained by Von Kossa in aortic sinus of 5/6 nephrectomized ApoE−/− mice treated with vehicle (left) or 10 mg/kg INT-747 (right). D) Quantification of calcified lesion area in the aortic sinus. The arrow indicates calcified lesion.
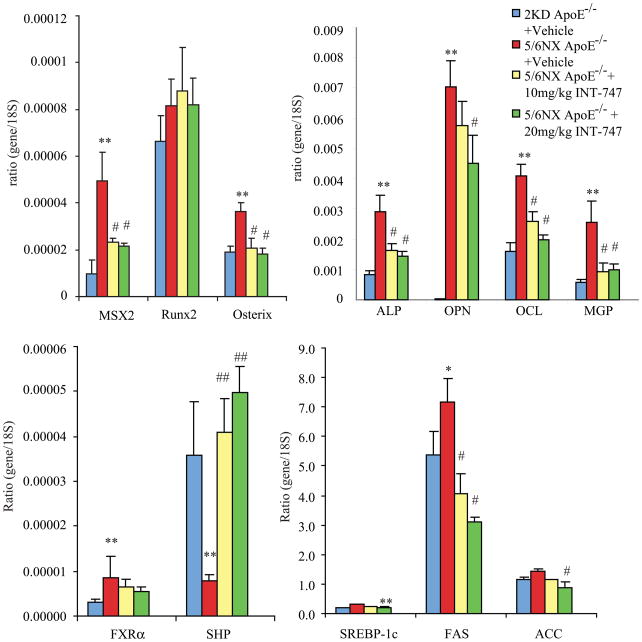
Since vascular calcification is known to be associated with increased expression of genes involved in osteogenesis, we examined whether INT-747 affects aortic osteogenic gene expression in ApoE−/− mice with CKD. Consistent with previous reports, CKD induced osteogenic marker genes such as ALP, osteopotin, OCL, MGP and genes encoding osteogenic transcription factors including Msx2 and Osterix.5 Treatment with INT-747 reduced aortic mRNA levels of osteogenic markers (ALP, OPN, OCL and MGP) and osteogenic transcription factors (Msx2 and osterix). Aortic mRNA level of bone morphogenetic protein 2 was unchanged by INT-747 treatment (data not shown). The FXR target gene SHP was induced by INT-747 treatment, whereas SREBP-1c and its target genes including FAS and ACC1 were reduced in ApoE−/− mice treated with INT-747 (Figure 6).
Figure 6. INT-747 reduced aortic gene expression of osteogenic transcription factors, osteogenic markers and lipogenic enzyme genes in ApoE−/− mice with CKD.
Male mice were fed a western diet for 12 weeks. A) osteogenic transcription factors; Msx2, Runx2 and Osterix, B) osteogenic markers; ALP, OPN, OCL and MGP, C) FXR and its target SHP and D) lipogenic genes; SREBP-1c, FAS and ACC1.
Discussion
Members of the nuclear hormone receptor superfamily are transcription factors that regulate diverse pathways of metabolism. In addition to acting as molecular sensors of lipid, carbohydrate and mineral homeostasis, several members of the nuclear receptor family (e.g. PPARs and LXRs) also exert beneficial pleiotropic effects to ameliorate atherosclerosis and its complications.34 In this study, we examined which nuclear receptor(s) is involved in the development of vascular calcification, a major complication in subjects with CKD and a strong predictor of cardiovascular mortality. We first analyzed gene expression of 47 nuclear receptors in CVC, an in vitro model of vascular calcification. We found that 8 genes encoding nuclear receptors, including MR and FXR, were dramatically induced during osteogenic differentiation of CVC (Table I). Since it has been already reported that activation of MR with aldosterone promotes vascular osteogenic differentiation and mineralization of CVC, 29 we focused our studies to determine the role of FXR activation in the development of vascular calcification. This study demonstrates that a synthetic FXR agonist, INT-747 inhibits mineralization and osteogenic differentiation of cultured vascular cells. The anti-calcific effect of INT-747 was blocked in CVC expressing FXR DN (Figure 3D). Consistent with FXR activation by INT-747 treatment, VP16FXR expression also inhibits mineralization of CVC. In addition, FXR activation increased phospho-JNK in CVC. SP600125 (JNK-specific inhibitor) significantly increased phosphate induced-mineralization of CVC. These results suggest that the anti-calcific effect of INT-747 is due to JNK activation. We also found that FXR activation prevents the development of CKD induced-vascular calcification in ApoE−/− mice without affecting the development of accelerated atherosclerosis. These observations suggest that FXR mediates inhibitory signaling in vascular calcification, similar to known inhibitory signaling factors such as osteocalcin, osteoprotegrin and osteopontin that are also increased in the osteogenic process.
Our observations from in vivo and in vitro experiments suggest that direct effects of FXR agonist on vascular smooth muscle cells in the artery wall are responsible for the anti-vascular calcific effects. Consistent with this hypothesis, we found that reduction of mineralization by INT-747 correlates with the reduction of genes involved in osteogenic differentiation. INT-747 also reduces crucial transcription factors involved in osteogenic differentiation such as Msx2 and osterix in the both CVC and aorta of ApoE−/− mice. These results suggest that these transcription factors are targets of FXR and that the reduction of these osteogenic transcription factors may contribute to the anti-calcific effects of INT-747. Consistent with the reduction of mineralization and osteogenic transcription factor expression, other osteogenic markers including ALP, OCL, MGP and COL1A1 were reduced in both aorta of CKD-ApoE−/− mice and CVC treated with INT-747. In addition, SP600125 treatment induced Msx2 and osterix expression, suggesting that JNK activation mediates the reduction of Msx2 and osterix expression by INT-747. Taken together, these results suggest that FXR is a key regulator in regulation of osteoblastic differentiation of vascular cells.
Inflammatory cytokines and oxidative stress induced by macrophages are also known to contribute to the development of vascular calcification and atherosclerosis.11, 35–37 ApoE−/− mice with CKD show increased macrophage infiltration (CD68 and F4/80) and inflammatory maker gene (TNFα and IL-1β) expression compared to normal ApoE−/− mice (Online Figure VII). However, INT-747 treatment did not affect macrophage infiltration or inflammatory cytokine expression induced by CKD. In addition to the evidence showing absence of expression of FXR in macrophages, 24, 27 these results support our hypothesis that INT-747 directly inhibits osteogenic differentiation in vascular smooth muscle cells. We cannot completely exclude the possibility that FXR activation in tissues other than vascular smooth muscle cells (i.e., liver, small intestine, and kidney) contributes to the anti-calcific effect of INT-747. In fact, we found that INT-747 treatment reduced hepatic neutral lipids (triglyceride and cholesterol) and altered hepatic (SHP, CYP7Al and SREBP-1c) gene and intestinal gene (FGF-15) expression, consistent with previous reports12–15, 21. The reduction in vascular calcification is also accompanied by a reduction in circulating triglycerides but not cholesterol. Since circulating triglyceride level is a risk factor for vascular diseases, the reduction of circulating triglycerides may be involved in the reduction of vascular calcification. Studies with tissue-specific FXR knock out and transgenic mice would be of considerable interest to determine the contribution of systemic effects of alterations in serum lipid levels versus the direct effect of FXR in the vasculature.
This is the first report showing that calcified vascular cells accumulate not only minerals but also neutral lipids (Figure 1). Quantitative lipid analysis showed that the accumulated neutral lipid was triglyceride, which was reduced by INT-747 treatment. The data suggests that the accumulation of lipids contributes to mineralization and osteogenic differentiation of CVC. Furthermore, in both CVC and aorta of ApoE−/− mice with CKD, INT-747 treatment increased expression of a major FXR target gene, SHP, which is required for the anti-lipogenic and the triglyceride-lowering effect of FXR agonists in the liver by interacting with LXRs.21 We also found that INT-747 reduced SREBP-1 gene and its target lipogenic genes including FAS and ACC in CVC and also in aorta of ApoE−/− mice consistent with reduced triglyceride level. A recent study reported that LXR activation by synthetic LXR agonists accelerates protein kinase A induced-mineralization in mouse vascular smooth muscle cells.38 Consistent with this report, we also found that LXR activation increased SREBP-1c expression and triglyceride level in CVC, thereby accelerating phosphate-induced mineralization of CVC (unpublished data, S.M-A and M.M). The inhibition of LXR-SREBP1c pathway, thus anti-lipogenic program by FXR agonist may also be playing an important role in the anti-calcific effect. Additional studies to ascertain the involvement of SHP and SREBP-1 in the anti-calcific effect of FXR agonist and the development of vascular calcification are currently underway.
The influence of FXR on the development of atherosclerosis has been controversial. In male ApoE−/− mice, FXR deficiency increased atherosclerotic lesions in mice fed a Western diet containing 1.25% cholesterol.25 In contrast to males, female FXR−/−; ApoE−/− double mutant mice fed a high fat cholesterol-free diet showed a significant reduction of atherosclerotic lesion formation.24 In LDLR−/− mice, loss of FXR reduced atherosclerotic lesion size in male but not in female mice.27 In addition, two recent publications showed that FXR activation by synthetic FXR agonists including INT-747 prevents atherosclerotic lesion formation in LDLR−/− and ApoE−/− mice.26, 28 Males were used in this study because males are more sensitive to CKD-induced vascular diseases than females and the anti-atherogenic effect of INT-747 was previously studied in males.26, 28 In the present study, INT-747 treatment did not prevent CKD-induced atherosclerosis in ApoE−/− mice. The differential response of INT-747 to atherosclerosis between this study and the study by Mencarelli et al is probably due to the differences in kidney function, although the administration method (oral gavage vs. dietary administration) is also different.26 A recent study has shown that FXR activation improves diabetic nephropathy.39 These data suggest that the normal kidney function may be required for the anti-atherosclerotic effect of FXR agonists and that FXR activation is not effective in preventing CKD induced-atherosclerosis.
In summary, FXR expression is increased during osteogenic differentiation of vascular cells which likely plays an inhibitory role in vascular calcification. Indeed, we have provided evidence that FXR activation inhibits aortic calcification in ApoE−/− with CKD without affecting the development of atherosclerosis. FXR activation also directly inhibits mineralization of vascular cells. Clinical studies have demonstrated that more than half of patients with chronic kidney disease die of cardiovascular diseases, including advanced calcific arterial disease2,4. However no pharmacological therapies have yet been shown to prevent disease progression. Therefore, the present data will be valuable in directing future efforts in the development of FXR agonists to treat CKD induced-vascular calcification.
Novelty and Significance.
What Is Known?
Vascular calcification is the leading cause of death in patients with chronic kidney disease (CKD).
The bile acid nuclear receptor, FXR controls bile acid, lipid and glucose metabolism and is expressed in vascular cells including vascular smooth muscle cells and endothelial cells.
FXR activation by synthetic agonists protects mice from a variety of metabolic diseases such as liver steatosis, insulin resistance, hyperlipidemia, cholestasis, diabetic nephropathy and atherosclerosis; however, its role in vascular calcification is unknown.
What New Information Does This Article Contribute?
Vascular FXR is highly induced during vascular calcification.
Pharmacologic activation of FXR reduces mineralization of vascular cells as well as vascular calcification in hyperlipidemic mice with CKD.
Activation of FXR reduces osteogenic differentiation of vascular cells by reducing expression of osteogenic transcription factors and activating c-Jun N-terminal kinase (JNK).
Although more than half of the death in patients with CKD is due to cardiovascular diseases, particularly vascular calcification, the efficient treatment and the etiology for this disease is unknown. Farnesoid X Receptor plays a central role in regulation of bile acid, cholesterol and glucose metabolism but its specific role in vascular diseases in CKD has not been studied. In this study, we have identified that FXR is highly induced in vascular calcification of both cell culture and animal models. In addition, FXR activation by genetic and pharmacological modification blocks vascular calcification in vitro and in vivo. We also found that JNK activation and reduced osteogenic transcription factor such as Msx2 and Osterix contributes to the anti-calcific effect of the FXR activation. This is the first study to demonstrate that FXR plays an important role in a feedback loop of vascular calcification. Furthermore, FXR activation might be a promising tool for prevention and treatment for vascular calcification in CKD.
Supplementary Material
Acknowledgments
We thank Drs Linda Demer and Yin Tintut (University of California, Los Angeles) for calcifying vascular cells and Drs Luciano Adorini and Mark Pruzanski (Intercept Pharmaceuticals) for providing us with INT-747. We also thank Dr. Dagmar Kratky (the Medical University of Graz, Austria) for valuable advices.
Sources of Funding
This work is supported by School of Medicine, University of Colorado-Denver, Colorado Clinical Nutrition Research Unit (NIH 5P30DK048520), UCHSC Diabetes and Endocrinology Research Center (NIH P30DK57516), NIH U01 DK076134, NIH R01 AG026529 and VA Merit Review Grant. Adelheid Kratzer was supported by a Mobility fellowship of the Austrian Federal Ministry of Science and Research (Genomforschung Austria Project G.O.L.D.II, Genomics of Lipid-associated Disorders II).
Non-standard abbreviations and non-standard acronyms
- ACC
acetyl-CoA carboxylase
- ALP
alkaline phosphatase
- Apo
apolipoprotein
- FXR
farnesoid X receptor
- CKD
chronic kidney disease
- COL1A1
type I collagen
- CVC
bovine calcifying vascular cells
- DN
dominant negative
- FAS
fatty acid synthase
- INT-747
6α-ethyl chenodeoxycholic acid
- JNK
c-Jun N-terminal kinase
- LXR
liver X receptor
- Msx2
msh homeo box 2
- MGP
matrix γ-carboxyglutamic acid protein
- mpk
mg/kg
- OCL
osteocalcin
- qPCR
quantitative RT-PCR
- Runx2
runt-related transcription factor 2
- SREBP
sterol regulatory element binding protein
- SHP
small heterodimer partner
Footnotes
Disclosures
None.
References
- 1.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 2.Giachelli CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–722. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 4.Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- 5.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 6.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 7.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 9.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 10.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. Embo J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Edwards PA. FXR signaling in metabolic disease. FEBS Lett. 2008;582:10–18. doi: 10.1016/j.febslet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Cariou B, Staels B. FXR: a promising target for the metabolic syndrome? Trends Pharmacol Sci. 2007;28:236–243. doi: 10.1016/j.tips.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23:443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- 16.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 17.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, He F, Kuruba R, Gao X, Wilson A, Li J, Billiar TR, Pitt BR, Xie W, Li S. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res. 2008;77:560–569. doi: 10.1093/cvr/cvm068. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, Zhang LM, Pitt BR, Xie W, Li S. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. 2008;77:169–177. doi: 10.1093/cvr/cvm016. [DOI] [PubMed] [Google Scholar]
- 20.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo GL, Santamarina-Fojo S, Akiyama TE, Amar MJ, Paigen BJ, Brewer B, Jr, Gonzalez FJ. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim Biophys Acta. 2006;1761:1401–1409. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. 2005;46:2595–2604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol. 2009;296:H272–281. doi: 10.1152/ajpheart.01075.2008. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 28.Hartman HB, Gardell SJ, Petucci CJ, Wang S, Krueger JA, Evans MJ. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J Lipid Res. 2009;50:1090–1100. doi: 10.1194/jlr.M800619-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 30.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, Szumilak D, Mothu N, Phan O, Daudon M, Lacour B, Drueke TB, Muntzel MS. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 31.Kocarek TA, Shenoy SD, Mercer-Haines NA, Runge-Morris M. Use of dominant negative nuclear receptors to study xenobiotic-inducible gene expression in primary cultured hepatocytes. J Pharmacol Toxicol Methods. 2002;47:177–187. doi: 10.1016/S1056-8719(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 32.Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 33.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98:727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 34.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 35.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 36.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 37.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 38.Hsu JJ, Lu J, Huang MS, Geng Y, Sage AP, Bradley MN, Tontonoz P, Demer LL, Tintut Y. T0901317, an LXR agonist, augments PKA-induced vascular cell calcification. FEBS Lett. 2009;583:1344–1348. doi: 10.1016/j.febslet.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.