Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is a common human neoplasia, of poor prognosis and survival, which frequently displays Akt overactivation. Previously, we reported that mice expressing high levels of constitutively Akt activity (myrAkt) in oral epithelia develop lesions and tumors in the oral cavity.
Materials and Methods
Functional genomics of primary keratinocytes from different transgenic mouse lines and immunostaining of mouse and human samples were performed in order to identify and validate putative biomarkers of oral cancer progression.
Results
The expression of KLF4 was found to be increased only in tumor prone samples from mice bearing overactivation of Akt. Such increased expression was confirmed in oral dysplasias and tumors arising in those mice. Tissue microarray analysis of human samples confirmed the association between active Akt and increased KLF4 expression.
Conclusion
These data support the notion that KLF4 is potentially a reliable marker of HNSCC, and that myrAkt transgenic mice are valuable tools for preclinical research of HNSCC.
Keywords: Akt, transgenic mouse, HNSCC, KLF4, microarray, Cancer
Head and neck squamous cell carcinoma (HNSCC) is a common type of human cancer worldwide associated with alcohol and/or tobacco abuse (1). In spite of using new therapeutic approaches (2–5), the improvement in overall survival in patients with HNSCC is still low. Therefore new targeted therapies are required for the management of this disease. HNSCC results from the accumulation of numerous genetic and epigenetic alterations, which occur in a multistep process, affecting multiple biochemical pathways. The major pathways involved in HNSCC development include the pRb and p53-dependent pathways, Epidermal growth factor receptor (EGFR), signal transducer and activator of transcription 3 (Stat3), Nuclear factor κB (NFκB) and Transforming growth factor β (TGFβ) (reviewed in (6–8)). These have provided several candidate genes of potential therapeutic relevance that are now being validated through in vitro analyses (6, 9, 10); however, these studies cannot recapitulate the complex nature of HNSCC tumors in vivo, and animal models of HNSCC will become essential tools for the evaluation of such therapeutic approaches. Nonetheless, there are few suitable genetically defined mouse models that fully recapitulate the molecular characteristics of human HNSCC in which to study the progression of this type of tumor under preclinical settings (6).
The Akt protein kinase regulates cell death and proliferation through phosphorylation of numerous targets and has been implicated in multiple human neoplasia (11). Several mouse models have recently shown that aberrant Akt signaling plays a predominant role in malignant transformation in vivo, either alone or in cooperation with other genetic alterations (12). We and others have provided evidence of the involvement of Akt activation in the development and progression of HNSCC (13, 14); indeed, molecular alterations in the PI3K/Akt/PTEN signaling pathway are found in about 50% of HNSCC cases (15), indicating that this is a plausible target for the treatment of this disease (16). More recently, to assess the functions of deregulated Akt activity in vivo, we have generated transgenic mice expressing wild-type Akt or myrAkt (myristoilated Akt, making the kinase constitutively active) in the basal layer of stratified epithelium (17). Importantly, we observed tha, besides developmental defects in ectodermal organs (18), the myrAkt mice developed multiple pretumoral oral lesions and tumors that, when combined with the ablation of Tp53 gene in the same cells, rapidly progress to overt oral tumors that phenocopy the molecular alterations previously found in human HNSCC (19). These characteristics make this model an excellent and unique preclinical tool for the therapeutic management of HNSCC at different steps. Here we use functional genomic approaches in these mice to analyze potential candidates of use as biomarkers of human HNSCC progression.
Materials and Methods
Mice and histological procedures
The generation of Bk5myrAkt and Trp53F/F;K14cre mice and the protocols for genotyping have been previously described (17, 18, 20, 21). These mice were in an immunocompetent mixed C57/Bl6×DBA×FVB/n background. All the animal experiments were approved by the Animal Ethical Committee (CEEA) and conducted in compliance with Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT) Guidelines. For histological analysis, oral samples were fixed in formalin and embedded in paraffin prior to sectioning. Sections were stained and processed as described elsewhere(17–19). Antibodies were used as follows: anti-Ser473 phosphorylated Akt (Cell Signaling, Danvers, MA, USA)diluted 1/100; anti p53 (CM5 Novocastra, Newcastle, UK) diluted 1/500; anti p21 (Santa Cruz, Santa Cruz, CA, USA) diluted 1/100; anti-CycD1 (NeoMarkers, Fremont, CA, USA) diluted 1/100 and anti-KLF4 (Chemicon, Temecula, CA, USA)diluted 1/100. Biotin-conjugated secondary antibodies were purchased from Jackson ImmunoResearch, Palo Alyo, CA, USA and used at 1/1000. Signal was amplified using avidin-peroxidase (ABC elite kit Vector, Burlingame, CA, USA) and peroxidase was visualized using diaminobenzidine as a substrate (DAB kit Vector). Control slides were obtained by replacing primary antibodies with Phosphate Buffered Saline (PBS) or preimmune sera (data not shown). At least 5 different samples of each type (normal oral epithelium, dysplasia and Squamous cell carcinoma, SCC) from at least five different mice of each genotype (only non-lesional oral epithelium was observed in control mice) were analyzed.
Affymetrix mouse gene chip 430A analysis
Total RNA from primary keratinocytes was extracted with Trizol (Gibco-BRL,Gaithersburg, MD USA) and purified using RNAeasy columns (Qiagen, Valencia, CA, USA) following manufacturers recommendations. The integrity of the RNA populations was tested in a Bioanalyzer (Agilent, Santa Clara, CA, USA) showing 28S/18S ratios above 1.7. Total RNA were hybridized at the Genomic Facility of the Centro de Investigación del Cáncer (Salamanca, Spain). We exported .cel files from Affymetrix GCOS software, and performed background subtraction with RMA (22) using the GEPAS analysis suit (23). The signal intensity values of each probe set were log2 transformed and standarized. Further analyses were performed using MeV software (24). Statistical t-test was used to select the genes with differential expression between the L84 primary keratinocytes and the others mouse genotypes (p<0.002). A total number of 121 significant probe sets were selected.
Western blot analysis
Western blot analyses of oral keratinocytes were performed as previously described (17, 18) using the antibodies given above.
Tissue Microarray of human HNSCC
The construction of the tissue microarray containing human pretumoral and tumoral samples, and their histopathological characteristics have been previously described (25, 26). All specimens were obtained from patients diagnosed in the Hospital Universitario 12 de Octubre, Madrid, Spain. Informed consent was obtained from each patient and the study has been carried out with the correspondent ethical committee approval. Immunohistochemistry detection of Ser473 phosphorylated Akt and KLF4 was performed as for mouse samples. Statistical analyses were carried out with SSPS program, version 11. 5. (SSPS Inc, Chicago, IL, USA). Frequencies were compared by the χ2 contingency test.
Results
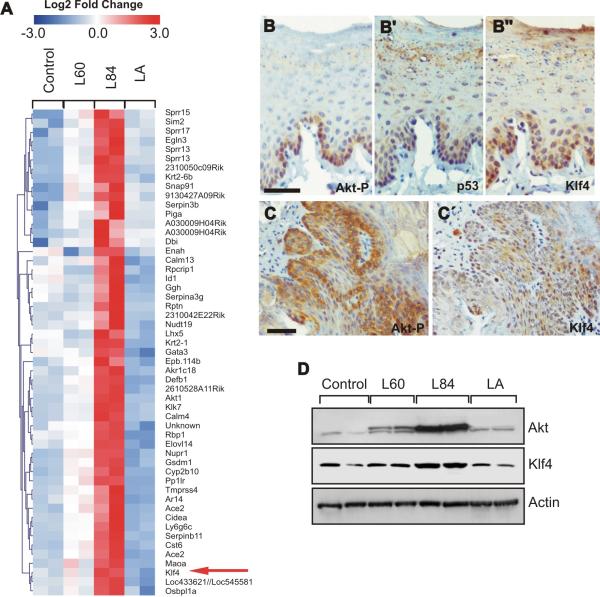
The activation of Akt has been previously involved in the development and progression of HNSCC (13–15, 27). In agreement, we have observed that the expression of high levels of myrAkt, rendering constitutively high Akt activity in oral epithelia, leads to the formation of dysplastic lesions that eventually proceed to oral SCC (19). Of note, these alterations were not observed in mice expressing wtAkt (wtAkt line LA) or low levels of myrAkt (myrAkt line L60), associated with lower Akt activity. To identify putative genes involved in oral cancer progression, we performed global expression profiling of paired RNA samples from primary keratinocyte extracts of transgenic (myrAkt L60, myrAkt L84, and wtAk tLA) and nontransgenic mice. The primary keratinocytes were selected to avoid the developmental defects observed in ectodermal tissues in these different mouse lines (18). As the pretumoral and tumoral lesions were only observed in myrAktL84 mice, the microarray data were processed to find specific clusters that included genes only upregulated in these primary keratinocytes compared with those derived from lines LA, L60 and controls (Figure 1A). Utilizing the Entrez gene database, we narrowed the dataset to those genes that were previously shown to be involved in cancer; this approach provides a list of 19 different genes (Table I). Most of them have been previously identified as being up-regulated in different types of cancer but their possible function in promoting carcinogenesis has not been evaluated. However, we also found up-regulation of some genes that have been previously suggested as cancer-promoting agents. Among them we found Pml, in agreement with our previous data (19), which accounts for the induction of p53 and premature senescence observed in tumoral samples derived from myrAkt L84 mice (19).
Figure 1. KLF4 is up-regulated in myrAkt L84 mice.
A′,, Heatmap of genes specifically upregulated in myrAktL84 primary keratinocytes. B–C′, Examples of the immunohistochemical detection of phosphorylated Akt (B, C), p53 (B′) and KLF4 (B″, ′) in oral dysplasia (B, B′, B″) and oral SCC (C, C) in myrAkt L84 mice. Bars = 200μm. D, Western blot showing the expression of Akt1 and KLF4 in primary keratinocytes of the quoted lines. Actin was used as loading control.
Table I.
Upregulated genes in L84 primary keratinocytes
| Probe set ID | Gene symbol | Gene title | p Value | Fold change* |
|---|---|---|---|---|
| 1416868_at | Cdkn2c | Cyclin-dependent kinase inhibitor 2C (p18, inhibits CDK4) | 0.0013 | 4.38 |
| 1417040_a_at | Bok | Bcl-2-related ovarian killer protein | 0.0013 | 3.42 |
| 1417394_at | Klf4 | Kruppel-like factor 4 (gut) | 0.0009 | 3.72 |
| 1418649_at | Egln3 (PHD3) | EGL nine homolog 3 (C. elegans) | 0.0013 | 6.09 |
| 1419317_x_at | Sprrl1 (Eig3) | Small proline rich-like 1 | 0.0011 | 6.21 |
| 1419437_at | Sim2 /// LOC547289 /// LOC547335 | Single-minded homolog 2 (Drosophila) /// similar to single-minded 2 protein /// similar to single-minded 2 protein | 0.0002 | 5.93 |
| 1422470_at | Bnip3 (Nip3) | BCL2/adenovirus E1B 19kDa-interacting protein 1, NIP3 | 0.0019 | 4.69 |
| 1422588_at | Krt2-6b | Keratin complex 2, basic, gene 6b | 0.0015 | 5.65 |
| 1423542_at | Klk7 | Kallikrein 7 (chymotryptic, stratum corneum) | 0.0008 | 4.77 |
| 1424801_at | Enah | Enabled homolog (Drosophila) | 0.0015 | 2.09 |
| 1425711_a_at | Akt1 | Thymoma viral proto-oncogene 1 | 0.0009 | 4.28 |
| 1426047_a_at | Ptprr (PTP-SL) | Protein tyrosine phosphatase, receptor type, R | 0.0004 | 3.88 |
| 1435133_at | Ugcg (GCS) | UDP-glucose ceramide glucosyltransferase | 0.0019 | 2.74 |
| 1448756_at | S100a9 | S100 calcium binding protein A9 (calgranulin B) | 0.0012 | 5.99 |
| 1448886_at | Gata3 | GATA binding protein 3 | 0.0018 | 2.43 |
| 1448982_at | Prss18 (KLK6) | Protease, serine, 18 | 0.0009 | 4.69 |
| 1450633_at | Calm4 (Scarf) | Calmodulin 4 | 0.0004 | 4.65 |
| 1448757_at | Pml | PML:promyelocytic leukemia | 0.0006 | 5.13 |
| 1454159_a_at | Igfbp2 | Insulin-like growth factor binding protein 2 | 0.0002 | 4.87 |
Respect to non-transgenic keratinocytes
Interestingly, among the up-regulated ones, we also found Kruppel-like factor 4 (KLF4/GKLF/EZF). KLF 4 is a transcription factor that can both activate and repress genes that are involved in cell-cycle regulation and differentiation in epithelium (28). Different data have indicated that KLF4 may have tumor-suppressive or oncogenic functions in a tissue-specific manner (29). We confirmed the increased expression of KLF4 in parallel with phosphorylated Akt in oral tissues of the myrAkt transgenic mice including oral dyplasia and SCC (Figure 1B–C′) by immunohistochemistry, and in primary keratinocytes by Western blot (Figure 1D). Of note, in oral dysplasia of the transgenic mice, the constitutive activation of Akt (Figure 1B) also induces p53 expression (Figure 1B′), in agreement with the up-regulation of Pml (19).
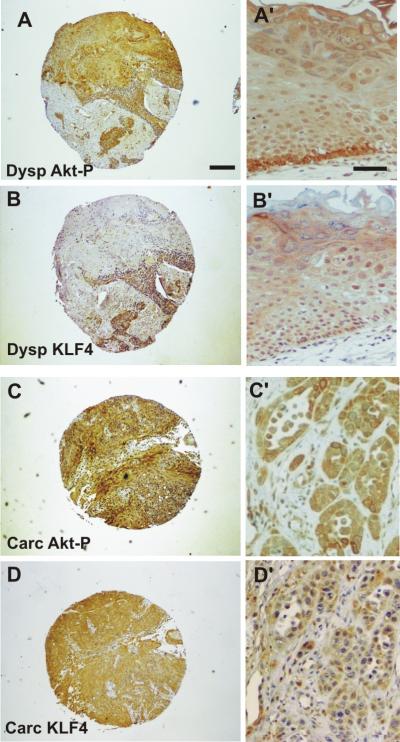
Elevated KLF4 levels have also been linked to the early stages of human oral squamous-cell carcinomas development (30) and the ectopic KLF4 expression in transgenic mice has been shown to induce squamous epithelial dysplasia (31, 32). These data, together with our observations, may suggest that KLF4 could represent a potential biomarker associated with Akt activation for oral dysplasias and SCCs. In order to test this hypothesis, the expression of phosphorylated Akt was studied in parallel with KLF4 expression in tissue microarrays containing 84 HNSCCs and 59 oral dysplasias from human patients (25, 26). Of these, 59 dysplasias and 80 tumors were informative (Table II; examples of positive staining are provided in Figure 2). We found a significant number of dysplasias and tumors showing increased Akt activity, indicating that Akt activation is an early event during human HNSCC development in agreement with our previous data (14, 17, 19). Similarly, the expression of KLF4 was prominent in both types of samples (Figure 2 and Table II) in agreement with others (30). Of note, there was a significant correlation between active, phosphorylated Akt and KLF4 expression in dysplasias (p≤ 0.01) and HNSCC (p≤0. .027) (Table II). These data confirmed our observations in oral samples from myrAkt L84 mice and support the notion that KLF4 can be used as a surrogate marker of Akt activation during human oral carcinogenesis.
Table II.
Summary of tissue microarray analysis.
| Oral dysplasia | Akt-P | Total | ||
|---|---|---|---|---|
| Negative | Positive | |||
| KLF4 | Negative | 10 | 7 | 17 |
| Positive | 7 | 35 | 42 | |
| Total | 17 | 42 | 59 | |
| HNSCC | Akt-P | Total | ||
|---|---|---|---|---|
| Negative | Positive | |||
| KLF4 | Negative | 28 | 15 | 43 |
| Positive | 15 | 26 | 41 | |
| Total | 43 | 41 | 80 | |
p≤0.001
p≤0.027
Figure 2. Examples of tissue microarray analysis.
Low magnification (A–D) and details (A′–D′) of human oral dysplasia (A–B′) and human HNSCC (C–D′) stained for phosphorylated Akt (A, A', C, C') and KLF4 (B, B', D, D) Bar in A= mm; in B=200 μm.
As mentioned above, KLF4 can act as a tumor suppressor or an oncogene in a tissue-specific manner (29). KLF4 expression is induced during epithelial maturation in vivo (30, 33) and its repression in proliferating cells seems to occur through posttranscriptional mechanisms (31). Nonetheless, the ectopic expression of KLF4 is sufficient to drive skin hyperplasia, dysplasia and SCC in epidermis of transgenic mice (31, 32). One possible explanation for the dual role of KLF4 as a tumor suppressor or an oncogene relies on the findings of Rowland et al. (34), which identified KLF4 as a gene that can bypass ras-induced senescence, but whose expression in untransformed cells causes cell proliferation arrest (34). The mechanism underlying these effects appears to be mediated by the different effects on p53 and p21 expression and requires cyclin D1 (34). Notably, in myrAkt transgenic mice, the progression to overt oral SCC is prevented by p53 induction and thus premature senescence (19). Indeed, we observed the induction of p53 (Figure 1B′) in the same cells that display induced KLF4 (Figure 1B′′) as a consequence of Akt activation (Figure 1B).
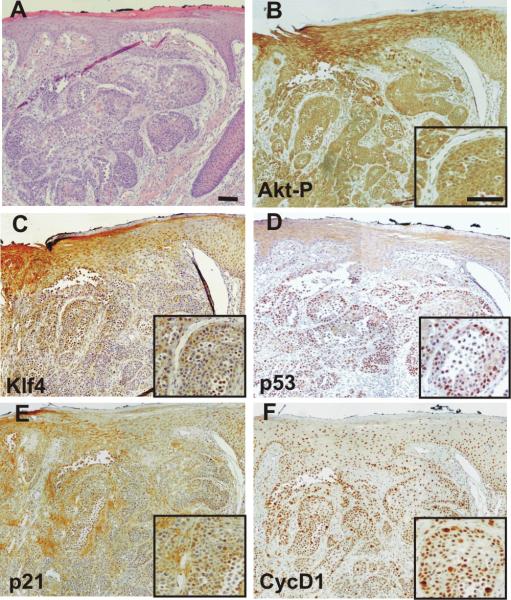
To explore this aspect in detail, human HNSCC previously characterized as displaying active Akt expression (14) were analyzed by immunohistochemistry for the expression of active Akt, p53, p21, cyclin D1 and KLF4 (Figure 3). We observed that tumoral cells expressing active Akt (Figure 3B) and subsequently KLF4 (Figure 3C), also displayed strong staining for p53 (Figure 3C), p21 (Figure 3D) and cyclin D1 (Figure 3E). Of note, p53-positive reactivity of human cancer samples has been correlated with inactivating mutations in this tumor suppressor gene. Nonetheless, as KLF4 transcriptionally represses p53 gene expression regardless of its mutational status, these findings indicate that in human HNSCC samples, the induction of KLF4 is not sufficient to completely abrogate p53 expression. This may explain why, even upon KLF4 induction, constitutive Akt expression in transgenic mice leads to premature senescence mediated by the induction of p53 (Figure 1B and B′; see also (19). The observed induction of p21, which is in agreement with the data obtained in cultured cells (19, 34)}, might suggest reduced proliferation; however, the induction of cyclin D1, which is also mediated by Akt activation (14, 35) can counteract the possible antiproliferative effects of such p21 induction.
Figure 3. Analyses of KLF4-related pathways in HNSCC.
A, Example of hematoxylin-eosin-stained section of human oral SCC. B–F, Consecutive sections stained for phosphorylated Akt (B), KLF4 (C), p53 (D), p21 (E) and cyclin D1 (F). Insets in B, C, D, E, F denote the same area of the tumor at higher magnification. Bars =200 μm.
Discussion
Molecular targeted therapies are promising in HNSCC management, and are now being validated through in vitro analyses (6, 9, 10). The relevance of Akt/PTEN pathway in these malignancies (8, 13–15, 27) supports its potential use in such therapies (16, 36). Therefore, in vivo systems aimed at the analysis of these therapies are necessary. These approaches have been largely hindered by a lack of appropriate animal models mimicking these tumors at both the pathological and molecular levels. We have generated transgenic mice expressing constitutively active Akt in the basal layer of stratified epithelium, including that of the oral cavity (17, 18). These mice, besides displaying some developmental defects in ectodermal organs (18), also display spontaneous tumor development and increased sensitivity to chemical carcinogenesis protocols (17). However, in spite of the development of oral pretumoral lesions with a complete penetrance, few of these lesions progress to overt tumors due to the induction of Pml and, subsequently, of a p53-dependent premature senescence (19). Indeed, the somatic ablation of Trp53 tumor suppressor gene in the same cells that express myrAkt leads to oral SCCs, which recapitulate the molecular features of human HNSCCs (19). These transgenic mice provide novel and plausible mouse models of human oral cancer, which, besides their possible use to test targeted therapies, may allow the consecutive steps involved in tumor initiation and progression to be studied and, consequently, novel biomarkers of progression of this disease to be identified.
Here, we used functional genomics to identify possible markers that may explain the tumor susceptibility in the oral cavity displayed by myrAkt L84 mice. We focused on those genes expressed selectively in myrAkt L84 primary keratinocytes because only in these transgenic mice did we find oral lesions (19). Moreover, we used primary cells to avoid the representation of genes involved in ectodermal development previously identified that can mask other possible pathways (18). This approach rendered a list of possible relevant genes that was further manually assessed in the search for genes previously involved in carcinogenesis. We thus obtain a list of 19 genes of potential use as possible biomarkers.
Due to its previous implication in human tumors (29, 37), here we focused our studies on KLF4. This transcription factor has been characterized as a tumor suppressor in some types of human gastrointestinal cancers (37–39), but it was also found to be overexpressed in human skin, oral cavity and breast cancers (30, 31, 40).
The use of tissue microarrays containing human pretumoral and tumoral samples not only allowed us to establish the relevance of Akt and KLF4 in this type of human pathologies, but also to determine a possible functional linkage between these two proteins. The possible molecular mechanisms of this functional connection are unknown but our data from myrAkt L84 mice may indicate that KLF4 is downstream of Akt signaling. However, there are few data about the transcriptional regulation of KLF4 (41, 42) that may explain this possibility and further studies are required. On the other hand, from our studies in human samples that KLF4 may lie upstream of Akt in human premalignat lesions can not be excluded.
The functions of KLF4 as an oncogene or tumor suppressor have been correlated with its different activities in p53 and p21 expression. Indeed KLF4 represses p53 whereas it also activates p21 (29, 34). The repression of p53 may justify why KLF4 can also cause chromosomal instability (43, 44). We observed that KLF4 is coexpressed with p53, thus suggesting that in oral tumors, KLF4 is not sufficient to completely abolish the expression of p53. These data, suggesting that oncogenic activities of KLF4 are not solely mediated by p53 repression, are further supported by the functional cooperation between the ectopic expression of KLF4 and p53 absence observed in transgenic mice (32).
Collectively, our observations, besides reinforcing the possible use of myrAkt mice in preclinical intervention and prevention studies, also highlight its application in characterizing possible biomarkers, such as KLF4 reported here, and their related molecular mechanisms, for the analysis of HNSCC progression.
Acknowledgements
We want to express our gratitude to Jesús Martínez and the personnel of the animal facility of CIEMAT for the excellent care of the animals, and to Pilar Hernández (CIEMAT) for the histological preparations.
This work was partially supported by Grants: SAF2008-00121 (MICIN), Oncocycle (S2006/BIO-0232) from CAM, PS-090100-2006-3 from MICINN and ISCIII-RETIC RD06/0020 (MSC) to JMP and by NIH grant CA37111, NIEHS Center grant ES00784 and Cancer Center Support Grant CA16672 to JD.
REFERENCES
- 1.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 2.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 4.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maitre A, Pajak TF, Poulsen MG, O'Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay JH, Pinto LH, Fallai C, Fu KK, Sylvester R, Pignon JP. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368(9538):843–54. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, Gascon P, Amellal N, Harstrick A, Eckardt A. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5568–77. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 6.Lu SL, Herrington H, Wang XJ. Mouse models for human head and neck squamous cell carcinomas. Head Neck. 2006;28(10):945–54. doi: 10.1002/hed.20397. [DOI] [PubMed] [Google Scholar]
- 7.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5(4):311–6. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 8.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2008 doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Ordonez B, Beauchemin M, Jordan RC. Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol. 2006;59(5):445–53. doi: 10.1136/jcp.2003.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimeno A, Kulesza P, Wheelhouse J, Chan A, Zhang X, Kincaid E, Chen R, Clark DP, Forastiere A, Hidalgo M. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br J Cancer. 2007;96(6):952–9. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4(4):257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 13.Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, Sauk J, Sausville EA, Molinolo AA, Gutkind JS. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10(12 Pt 1):4029–37. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 14.Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, Leis H, Garcia-Escudero R, Martinez-Cruz AB, Martinez-Palacio J, Hernandez P, Ballestin C, Paramio JM. Molecular determinants of Akt-induced keratinocyte transformation. Oncogene. 2006;25(8):1174–85. doi: 10.1038/sj.onc.1209155. [DOI] [PubMed] [Google Scholar]
- 15.Pedrero JM, Carracedo DG, Pinto CM, Zapatero AH, Rodrigo JP, Nieto CS, Gonzalez MV. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer. 2005;114(2):242–8. doi: 10.1002/ijc.20711. [DOI] [PubMed] [Google Scholar]
- 16.Moral M, Paramio JM. Akt pathway as a target for therapeutic intervention in HNSCC. Histol Histopathol. 2008;23(10):1269–78. doi: 10.14670/HH-23.1269. [DOI] [PubMed] [Google Scholar]
- 17.Segrelles C, Lu J, Hammann B, Santos M, Moral M, Cascallana JL, Lara MF, Rho O, Carbajal S, Traag J, Beltran L, Martinez-Cruz AB, Garcia-Escudero R, Lorz C, Ruiz S, Bravo A, Paramio JM, DiGiovanni J. Deregulated Activity of Akt in Epithelial Basal Cells Induces Spontaneous Tumors and Heightened Sensitivity to Skin Carcinogenesis. Cancer Res. 2007;67(22):10879–10888. doi: 10.1158/0008-5472.CAN-07-2564. [DOI] [PubMed] [Google Scholar]
- 18.Segrelles C, Moral M, Lorz C, Santos M, Lu J, Cascallana JL, Lara MF, Carbajal S, Martinez-Cruz AB, Garcia-Escudero R, Beltran L, Segovia JC, Bravo A, Digiovanni J, Paramio JM. Constitutively Active Akt Induces Ectodermal Defects and Impaired Bone Morphogenetic Protein Signaling. Mol Biol Cell. 2008;19(1):137–149. doi: 10.1091/mbc.E07-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moral M, Segrelles C, Lara MF, Martinez-Cruz AB, Lorz C, Santos M, Garcia-Escudero R, Lu J, Kiguchi K, Buitrago A, Costa C, Saiz C, Rodriguez-Peralto JL, Martinez-Tello FJ, Rodriguez-Pinilla M, Sanchez-Cespedes M, Garin M, Grande T, Bravo A, DiGiovanni J, Paramio JM. Akt activation synergizes with Trp53 loss in oral epithelium to produce a novel mouse model for head and neck squamous cell carcinoma. Cancer Res. 2009;69(3):1099–108. doi: 10.1158/0008-5472.CAN-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29(4):418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Cruz AB, Santos M, Lara MF, Segrelles C, Ruiz S, Moral M, Lorz C, Garcia-Escudero R, Paramio JM. Spontaneous squamous cell carcinoma induced by the somatic inactivation of retinoblastoma and Trp53 tumor suppressors. Cancer Res. 2008;68(3):683–92. doi: 10.1158/0008-5472.CAN-07-3049. [DOI] [PubMed] [Google Scholar]
- 22.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 23.Vaquerizas JM, Conde L, Yankilevich P, Cabezon A, Minguez P, Diaz-Uriarte R, Al-Shahrour F, Herrero J, Dopazo J. GEPAS, an experiment-oriented pipeline for the analysis of microarray gene expression data. Nucleic Acids Res. 2005;33(Web Server issue):W616–20. doi: 10.1093/nar/gki500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34(2):374–8. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Pinilla M, Rodriguez-Peralto JL, Hitt R, Sanchez JJ, Ballestin C, Diez A, Sanchez-Verde L, Alameda F, Sanchez-Cespedes M. Cyclin A as a predictive factor for chemotherapy response in advanced head and neck cancer. Clin Cancer Res. 2004;10(24):8486–92. doi: 10.1158/1078-0432.CCR-04-0771. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Pinilla M, Rodriguez-Peralto JL, Hitt R, Sanchez JJ, Sanchez-Verde L, Alameda F, Ballestin C, Sanchez-Cespedes M. beta-Catenin, Nf-kappaB and FAS protein expression are independent events in head and neck cancer: study of their association with clinical parameters. Cancer Lett. 2005;230(1):141–8. doi: 10.1016/j.canlet.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Molinolo AA, Hewitt SM, Amornphimoltham P, Keelawat S, Rangdaeng S, Meneses Garcia A, Raimondi AR, Jufe R, Itoiz M, Gao Y, Saranath D, Kaleebi GS, Yoo GH, Leak L, Myers EM, Shintani S, Wong D, Massey HD, Yeudall WA, Lonardo F, Ensley J, Gutkind JS. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13(17):4964–73. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 28.McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29(6):549–57. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6(1):11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 30.Foster KW, Ren S, Louro ID, Lobo-Ruppert SM, McKie-Bell P, Grizzle W, Hayes MR, Broker TR, Chow LT, Ruppert JM. Oncogene expression cloning by retroviral transduction of adenovirus E1A-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zinc finger protein GKLF. Cell Growth Differ. 1999;10(6):423–34. [PubMed] [Google Scholar]
- 31.Huang CC, Liu Z, Li X, Bailey SK, Nail CD, Foster KW, Frost AR, Ruppert JM, Lobo-Ruppert SM. KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia. Cancer Biol Ther. 2005;4(12):1401–8. doi: 10.4161/cbt.4.12.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster KW, Liu Z, Nail CD, Li X, Fitzgerald TJ, Bailey SK, Frost AR, Louro ID, Townes TM, Paterson AJ, Kudlow JE, Lobo-Ruppert SM, Ruppert JM. Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24(9):1491–500. doi: 10.1038/sj.onc.1208307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22(4):356–60. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 34.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7(11):1074–82. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- 35.Leis H, Segrelles C, Ruiz S, Santos M, Paramio JM. Expression, localization, and activity of glycogen synthase kinase 3beta during mouse skin tumorigenesis. Mol Carcinog. 2002;35(4):180–5. doi: 10.1002/mc.10087. [DOI] [PubMed] [Google Scholar]
- 36.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65(21):9953–61. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 37.Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006;27(1):23–31. doi: 10.1093/carcin/bgi243. [DOI] [PubMed] [Google Scholar]
- 38.Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, Wu TT, Huang S, Xie K. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65(7):2746–54. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 39.Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, Yang VW. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67(15):7147–54. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W, Lobo-Ruppert SM, Ruppert JM. Bad things happen in the basal layer: KLF4 and squamous cell carcinoma. Cancer Biol Ther. 2008;7(5):783–5. doi: 10.4161/cbt.7.5.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehlermann J, Pfisterer P, Schorle H. Dynamic expression of Kruppel-like factor 4 (Klf4), a target of transcription factor AP-2alpha during murine midembryogenesis. Anat Rec A Discov Mol Cell Evol Biol. 2003;273(2):677–80. doi: 10.1002/ar.a.10089. [DOI] [PubMed] [Google Scholar]
- 42.Behr R, Kaestner KH. Developmental and cell type-specific expression of the zinc finger transcription factor Kruppel-like factor 4 (Klf4) in postnatal mouse testis. Mech Dev. 2002;115(1–2):167–9. doi: 10.1016/s0925-4773(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 43.Yoon HS, Ghaleb AM, Nandan MO, Hisamuddin IM, Dalton WB, Yang VW. Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene. 2005;24(25):4017–25. doi: 10.1038/sj.onc.1208576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon HS, Yang VW. Requirement of Kruppel-like factor 4 in preventing entry into mitosis following DNA damage. J Biol Chem. 2004;279(6):5035–41. doi: 10.1074/jbc.M307631200. [DOI] [PMC free article] [PubMed] [Google Scholar]