Abstract
Background:
Obstructive sleep apnea (OSA) is associated with prothrombotic effects that could lead to venous thromboembolic disease. We performed a prospective cross-sectional study to determine the prevalence of snoring and risk of OSA in patients with acute pulmonary embolism (PE).
Methods:
We evaluated 270 consecutive patients who underwent a computed tomographic angiogram for suspected PE. Patients without PE served as a control group. Demographic and clinical characteristics were analyzed. The Berlin Questionnaire was used to determine the presence of snoring and the risk of OSA. A subset of patients also underwent formal nocturnal polysomnography.
Results:
PE was present in 71 (26%) of the 270 patients who underwent a computed tomographic angiogram. When compared with patients without PE, patients with PE had a significantly higher prevalence of snoring (75% vs 50%, odds ratio = 2.91, 95% confidence interval: 1.60, 5.33, P = 0.001) and an increased risk of having OSA, as defined by the Berlin Questionnaire (65% vs 36%, odds ratio = 3.25, confidence interval: 1.84, 5.72, P < 0.001). Results from the multivariate analysis showed that PE was independently associated with risk of OSA (OR = 2.78, P = 0.001).
Conclusions:
We found a higher prevalence of snoring and high risk of OSA in patients diagnosed with acute PE, in comparison with patients in whom PE was suspected but ruled out. This association might be independent of other risks factors common to both OSA and PE. Therefore, OSA may represent a risk factor for the development of PE.
Citation:
Epstein MD; Segal LN; Ibrahim SM; Friedman N; Bustami R. Snoring and the risk of obstructive sleep apnea in patients with pulmonary embolism. SLEEP 2010;33(8):1069-1074.
Keywords: Sleep apnea, pulmonary embolism, Berlin Questionnaire
THE ANNUAL INCIDENCE OF PULMONARY EMBOLISM (PE) EXCEEDS 1 PER 1000 IN THE UNITED STATES, WITH AN ASSOCIATED MORTALITY OF UP TO 40%.1 PE is thought to account for 5% to 10% of deaths in hospitalized patients.2,3 If untreated, approximately one third of those who survive an initial PE die of a future embolic episode. Although risk factors for the development of PE are well established, up to 30% of cases have no identifiable cause.4 Moreover, risk-factor assessment may have important implications for both prevention and treatment of PE.5
Obstructive sleep apnea (OSA) is characterized by episodes of recurrent upper airway obstruction during sleep, leading to repetitive sleep disruption, with or without concomitant oxygen desaturation.6 Recent studies estimate that OSA affects up to 25% of the adult population in the United States.7,8 OSA is increasingly recognized as a risk factor for cardiovascular disease.9–12 Some of the proposed mechanisms whereby OSA contributes to cardiovascular complications include hemodynamic alterations,13 sympathetic nervous system activation,14 oxidative stress,15 systemic inflammation,16 hypercoagulability,10 and vascular endothelial dysfunction.17 These same pathophysiologic derangements are prothrombotic and could promote the development of venous thromboembolic disease.
Several previous case reports and uncontrolled cohort studies have suggested a possible association between OSA and PE.18–23 We hypothesized that patients with PE might have an increased prevalence of OSA. We performed a prospective cross-sectional study to determine the prevalence of snoring and the risk of having OSA in patients suspected of having an acute PE.
METHODS
Study Population
We performed a prospective assessment for the presence of snoring and the risk of having OSA in consecutive patients older than 18 years of age who underwent a helical contrast enhanced computed tomographic scan of the chest (CT angiogram) for suspected PE. CT angiography was performed at the discretion of the patients' treating physicians. The probability of PE was based on the treating physicians' clinical suspicion (gestalt). All inpatients and outpatients were included. The study took place in a 650-bed, tertiary care teaching hospital. A board-certified radiologist determined the results of the CT angiogram. Patients in whom the CT angiogram was negative for PE comprised the control group. The research protocol was approved by the hospital's Institutional Review Board and registered with ClinicalTrials.gov #NCT00409045. All patients provided informed consent. The only exclusion criteria were an inability or unwillingness to participate or to provide consent.
Patients were evaluated in the emergency department, during their hospitalization, or by telephone if already discharged from the hospital. Demographic and clinical characteristics were collected, including current and past medical history and known risk factors for PE.5,24,25 Hereditary risk factors included a family history of deep vein thrombosis (DVT) or PE. Acquired risk factors included a prior history of PE or DVT, oral contraceptive use, pregnancy, malignancy, immobility (hospitalization within the prior 90 days and/or prolonged travel in the prior 8 weeks), trauma, fractures, spinal cord injury, surgery (major or minor procedure within the preceding 30 days), and varicose veins. Laboratory investigation for thrombophilia was performed at the discretion of the patients' treating physicians.
Berlin Questionnaire
A Berlin Questionnaire (BQ) was completed for all study participants and was used to determine the risk of having OSA. The BQ consists of 10 questions divided into 3 categories, including snoring, daytime somnolence, and hypertension and obesity (defined as a body mass index [BMI] > 30 kg/m2).26 A high risk of having OSA is defined as a positive response in 2 or more of the following categories: persistent symptoms (≥ 3 times per week) in response to 2 or more questions about snoring; persistent symptoms (≥ 3 times per week) of daytime sleepiness or falling asleep while driving; and history of hypertension or obesity. We excluded patients for whom any of the BQ data were unknown or uncertain.
The BQ has been demonstrated to have good validity (Cronbach's alpha: 0.86 to 0.92) with a sensitivity of 86%, specificity of 77%, and a positive predictive value of 89% in a primary care setting.26 Subsequent studies have confirmed the accuracy in specific medical populations.27,28
Polysomnography
The accuracy of the BQ was validated in a subgroup of our study population who had completed both the BQ and formal nocturnal polysomnography. During the course of the study, nocturnal polysomnography was offered to 37 consecutive patients, 23 of whom underwent testing. Polysomnography included monitoring of the electroencephalogram, electrooculogram, electromyogram, electrocardiogram, thoracic and abdominal movements, nasal airflow, and pulse oximetry (Compumedics, Victoria, Australia). Sleep scoring was performed using the criteria of Rechtschaffen and Kales.29 An obstructive apnea was defined as an episode of complete airflow cessation lasting at least 10 seconds with persistent respiratory effort and an hypopnea was defined as a 30% or greater decrease in airflow from baseline lasting at least 10 seconds and a 4% or greater decrease in oxygen saturation from baseline.30
The sensitivity, specificity, and positive and negative predictive values of the BQ were determined using the polysomnographic findings as the gold standard. Polysomnography results were analyzed without knowledge of the patients' other clinical information. OSA was defined by criteria set forth by the American Academy of Sleep Medicine.31 An apnea-hypopnea index (AHI) of at least 15 was used to define a positive study. This threshold was chosen to avoid including patients with mild OSA because a higher AHI is associated with higher odds of the patient having cardiovascular comorbidity.32–34
Statistical Analysis
First, descriptive statistics were obtained comparing the PE and non-PE group in terms of the presence of OSA risk or snoring. The 2 groups were also compared in terms of various demographic and clinical variables. We evaluated risk factors for PE in 2 categories: hereditary and acquired. Since laboratory investigation for thrombophilia was not performed in the non-PE group, this factor was not examined in the model. Continuous variables are described using mean and SD while numbers and percentages are used to describe categorical variables.
Second, univariate logistic regression models for PE were performed to examine the unadjusted effect of each of the demographic and clinical variables on the risk of PE.
Adjusted analysis using a multivariate logistic regression model for PE was also performed to examine the independent effect of risk of having OSA after adjusting for other potential confounding factors. Factors that were significantly associated with PE in the univariate logistic regression analyses were included in the multivariate model, except for age, which was controlled for in the model, regardless of significance. The backward elimination procedure was used to obtain the final model, and factors that showed no further significant improvement to the model fit were eliminated. The validity of the model was assessed using the Hosmer and Lemeshow goodness-of-fit test.35
RESULTS
Study Population
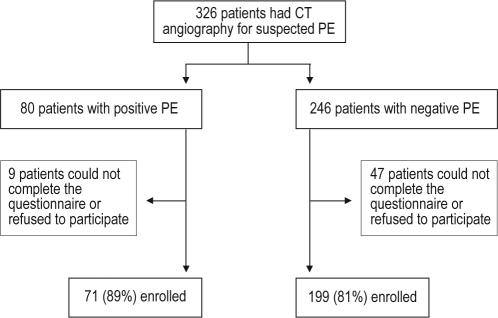
During the study period, 326 consecutive patients underwent CT angiography for suspected PE. There were 80 patients (25%) with PE and 246 patients (75%) with no evidence of PE on CT angiography. A total of 71 out of the 80 patients who had PE (89%) and 199 out of 246 patients who did not have PE (81%) were enrolled in the study (P = 0.11) (Figure 1). The remaining patients could not be contacted, could not complete the questionnaire, or refused to participate. The clinical signs and symptoms that led to suspicion for PE were similar among the PE and non-PE groups. There were no statistically significant differences between the different groups in terms of the reasons for nonparticipation in the study (data not shown).
Figure 1.
Trial profile
Table 1 lists demographic and clinical characteristics in both the PE and non-PE groups, as well as results from univariate logistic regression models for the risk of PE. Average age was similar for both groups. There was a higher prevalence of men in the PE group versus the non-PE group (61% vs 37% respectively, odds ratio [OR] = 2.65, P = 0.001). Weight was also greater among patients with PE than among those without PE (86 ± 23 kg vs 78 ± 20 kg, respectively, OR = 1.18 per 10-kg increase, P = 0.008), but BMI was not significantly different between those with and without PE (28.7 ± 7.0 kg/m2 vs 27.9 ± 6.7 kg/m2, respectively, OR = 1.02, P = 0.38).
Table 1.
Demographic, clinical characteristics, snoring and risk of OSA of the study populationa
| Factor | PE |
OR (95%CI) | P-value | |
|---|---|---|---|---|
| Positive (n = 71) | Negative (n = 199) | |||
| Demographics | ||||
| Age, y | 60.0 ± 15.7 | 61.3 ± 17.2 | 0.96 (0.81,1.12) | 0.58 |
| Men | 43 (61) | 73 (37) | 2.65 (1.52,4.62) | 0.001 |
| Weight, kg | 86.1 ± 23.6 | 78.0 ± 20.3 | 1.18 (1.04,1.34) | 0.008 |
| BMI, kg/m2 | 28.7 ± 7.0 | 27.9 ± 6.7 | 1.02 (0.98, 1.06) | 0.38 |
| Clinical Characteristics | ||||
| PE risk factors | ||||
| Hereditarya | 10 (14) | 6 (3) | 5.27 (1.84, 15.10) | 0.002 |
| Acquiredb | 53 (75) | 128 (64) | 1.63 (0.89, 3.00) | 0.11 |
| Hypertension | 31 (44) | 106 (53) | 0.68 (0.39, 1.17) | 0.17 |
| CHF | 4 (6) | 30 (15) | 0.34 (0.11, 0.99) | 0.048 |
| MI | 4 (6) | 19 (10) | 0.57 (0.19, 1.72) | 0.32 |
| Smoking | 13 (18) | 39 (20) | 0.92 (0.46, 1.84) | 0.81 |
| Pacemaker/central line | 10 (14) | 16 (8) | 1.88 (0.81, 4.35) | 0.14 |
| IBD | 2 (3) | 3 (2) | 1.89 (0.31, 11.57) | 0.49 |
| Nephrotic syndrome | 0 (0) | 3 (2) | N/A | 1.00 |
| Snoring and risk of OSA | ||||
| Snoring | 53 (75) | 100 (50) | 2.91 (1.60, 5.33) | 0.001 |
| Risk of OSA | 46 (65) | 72 (36) | 3.25 (1.84, 5.72) | <0.001 |
Data are shown as mean ± SD or number (%). OR refers to odds ratio from univariate logistic regression analyses for pulmonary embolism (PE); OSA, obstructive sleep apnea; CI, confidence interval; BMI, body mass index; CHF, congestive heart failure; MI, myocardial infarction; IBD, inflammatory bowel disease.
Family history of deep vein thrombosis (DVT) or PE.
Personal history of DVT/PE, oral contraceptive use, pregnancy, malignancy, immobility, trauma, fractures, spinal cord injury, surgery, or varicose veins.
Hereditary risk factors for PE were significantly more prevalent in the PE group than in the non-PE group (14% vs 3%, OR = 5.27, P = 0.002), whereas the effect of acquired risk factors for PE did not reach statistical significance (75 % vs 64%, OR 1.63, P = 0.11). Furthermore, results from the evaluation of each individual factor grouped as acquired risk factors showed no statistically significant association with PE (data not shown).
There was a lower prevalence of congestive heart failure in the PE group than in the non-PE group (6% vs15%, OR = 0.34, P = 0.048). There was no difference in the prevalence of hypertension, smoking, or myocardial infarction between groups. Other known risk factors for PE (pacemaker/central line, inflammatory bowel disease, and nephrotic syndrome) showed no significant difference.
Prevalence of snoring and risk of OSA in patients suspected of having a PE
Table 1 demonstrates that patients with PE had a significantly higher prevalence of snoring, compared with patients without PE (75% vs 50%, respectively). The OR for snoring was 2.91 (95% confidence interval: 1.60, 5.33, P = 0.001). Similarly, patients with PE had a significantly higher prevalence of risk of having OSA, as defined by a positive BQ (65% vs 36%). Results from the univariate analyses showed that PE was significantly associated with risk of having OSA as defined by the BQ (OR = 3.25, P < 0.001).
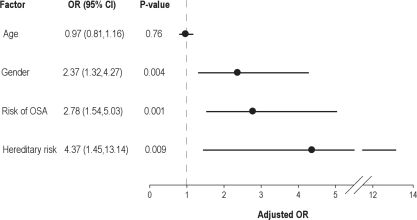
The association of snoring and the risk of having OSA with the presence of PE was further evaluated with a multivariate logistic regression model that considered several demographic and clinical factors, as previously described (Figure 2). Factors examined in the final model included age, gender, hereditary risk factors, and risk of having OSA (BQ). PE was significantly associated with male gender (OR = 2.37, P = 0.004) and the presence of hereditary PE risk factors (OR = 4.37, P = 0.009).
Figure 2.
Multivariate logistic regression model for pulmonary embolism (N = 270). Model includes groups of individual risk factors. OR refers to odds ratio; CI, confidence interval; OSA, obstructive sleep apnea.
Although weight was significantly greater in the PE-positive versus the PE-negative group, (Table 1), the effect of weight (not shown) was not statistically significant in the multivariate model (OR = 1.04, per 10-kg increase, P = 0.61). Similarly, despite the association between PE and absence of CHF in the univariate analysis, this association was not significant in the multivariate model (OR = 0.38, P = 0.10).
PE was significantly associated with the risk of having OSA, as determined by a positive BQ (OR = 2.78; 95% confidence interval = 1.54, 5.03; P = 0.001). In contrast, snoring (not shown) was not significantly associated with PE in the multivariate model (OR = 1.32, P = 0.58). This suggests that the association between snoring and PE, as shown in the univariate analysis, becomes insignificant once it is examined in the multivariate model that includes the risk of OSA. This can be explained by the fact that snoring is already incorporated in the BQ from which the risk of OSA was determined.
BQ Validation
Twenty-three consecutive patients, 19 of whom were positive for PE, underwent polysomnography in addition to completing the BQ. An AHI of at least 15 was used to define a positive study. The BQ was positive in 13 patients, 12 of whom had a positive nocturnal polysomnogram. BQ was negative in 10 patients, all of whom had a negative nocturnal polysomnogram. When compared with results of polysomnography as the gold standard, the BQ performed with a sensitivity of 100% and specificity of 91%. The positive predictive value was 92%, and the negative predictive value was 100%.
DISCUSSION
We found a significantly higher prevalence of snoring and risk of having OSA in patients with acute PE, in comparison with patients in whom PE was suspected but ruled out. Our data also suggest that this association might be independent of other risks factors common to both PE and OSA. A relationship between sleep disordered breathing and PE was first suggested more than 30 years ago in case reports of PE in patients with the so-called Pickwickian syndrome.18–20 Several more recent studies have examined the prevalence of OSA in uncontrolled case series of patients with acute PE.21,22 A small, uncontrolled, prospective cohort study of patients with newly diagnosed OSA found an increased incidence of venous thromboembolism over 3 years following OSA diagnosis.23 To our knowledge, the present investigation is the first controlled study to demonstrate an association between OSA and PE.
We found a higher prevalence of PE in men, consistent with previous reports.36,37 The lower prevalence of PE in patients with congestive heart failure may be explained by the fact that the presenting symptoms (most commonly shortness of breath and chest pain) in the negative PE patients were more likely due to cardiovascular disease. Obesity constitutes a well-known risk factor for OSA, although its association with PE has been debated.5,38 Weight was higher in the PE group, perhaps because the majority of patients with PE were men, but body mass index was similar between the 2 groups. Interestingly, it has been hypothesized that sleep apnea may be one of the mechanisms whereby obesity contributes to the development of cardiovascular disease.39 It is possible that this same relationship could exist between obesity and PE.
Utilizing the BQ, the 2005 National Sleep Foundation poll of adults in the United States estimated a prevalence of OSA of approximately 25%.8 Furthermore, the prevalence of OSA in hospitalized patients with cardiovascular disease has been reported to be as high as 65.7%.40,41 The higher risk of having OSA in our cohort (36% in the non-PE group and 65% in the PE group) may reflect the fact that the majority of patients in our study were hospitalized.
Thromboemboli arise in the setting of venous stasis, endothelial injury and hypercoagulability (Virchow's triad). Each of these conditions may develop in the setting of OSA. OSA-related hemodynamic alterations may lead to acute reductions in venous return and chronic venous stasis.13 OSA is associated with an increase in circulating thrombogenic factors, including fibrinogen, von Willebrand Factor, platelet activation, plasminogen activator inhibitor-1, and D-dimer levels.10,15,42–44 There is evidence that OSA directly impairs vascular endothelial function: both an increase in endothelin-1 levels, which may lead to vasoconstriction, and a reduction in nitric oxide, which may impair vasodilatation, have been described.17 Furthermore, OSA is associated with sympathetic nervous system activation14 and an increase in inflammatory mediators, catecholamines, cellular and vascular endothelial adhesion molecules, and oxidative stress, all of which have prothrombotic effects.12,15,16,45 Hence, OSA may be sufficient, in and of itself, to fulfill the conditions of Virchow's triad. Our study results provide clinical evidence to further support an association between OSA and PE.
Although we found a high prevalence of snoring and risk of having OSA in patients with acute PE, our results must be interpreted in light of several limiting factors. First, the risk of OSA was determined by the BQ, rather than by polysomnography, which is the gold standard for diagnosis. Although some of the questions entail a degree of subjectivity, we excluded patients for whom any of the BQ data were unknown or uncertain (e.g., perception of snoring in subjects living alone). The BQ has been shown to be very accurate in predicting OSA in an outpatient primary care setting, as well as in other patient populations.26–28 The BQ was also highly accurate in our validation study, but the sample size was small and the majority of those studied were positive for PE. Patients with PE were more prevalent in our validation subgroup, probably due to a greater willingness to undergo polysomnography. This may reflect the fact that the patients who had PE were more concerned about knowing whether OSA could have contributed to their PE.
Second, the effect of risk factors on the association between PE and OSA should be interpreted with caution. Laboratory testing for thrombophilia was performed at the discretion of the treating physicians and was not obtained in the majority of patients. Other known risk factors for PE that did not reach statistical significance (probably due to small sample size) were not included in the multivariate logistic regression model. Immobility, defined as hospitalization within the prior 90 days or prolonged travel in the prior 8 weeks, was considered as an acquired risk factor, which has been associated with PE.5,24,46,47 However, we found no statistical difference for immobility in the PE versus the non- PE group. A reduced level of physical activity has also been shown to correlate with the severity of OSA.48,49 However, these data were based on retrospective analyses, and the methodology and definitions have not been well standardized or well validated. Although it is possible that relative physical inactivity, as a result of OSA, might also predispose a patient to the development of PE, we were not able to accurately quantify the level of physical activity in our patient population.
Third, the decision to obtain CT angiography was based on the clinical gestalt of the treating physician. There is conflicting evidence regarding the use of standardized clinical decision rules versus clinical gestalt in the diagnosis of PE. However, in general, these two approaches are felt to have comparable accuracy among experienced physicians.50–54 Additionally, and in accordance with prior reports,55 the prevalence of clinical signs and symptoms that led to the suspicion of PE showed no statistically significant difference among the PE and non-PE groups in our study.
Fourth, we enrolled only patients who underwent a CT angiogram of the chest, and the concomitant presence of DVT was not routinely ascertained. Even though DVT is considered part of the spectrum of venous thromboembolism, our study did not address whether OSA is also associated with DVT. Finally, we did not include patients in whom diagnostic testing for PE was performed with ventilation/perfusion scanning. We specifically sought to define our study population (both cases and controls) based on a single test. CT angiography was chosen based on its superior specificity and the lack of need to assess clinical pretest probability, as is required for ventilation/perfusion scanning.56–58
In summary, we found a high prevalence of snoring and high risk of having OSA in patients diagnosed with acute PE. Based on a growing body of evidence suggesting pathophysiologic plausibility, our findings support the concept that OSA may represent an independent risk factor for the development of PE. Given the high prevalence of OSA and the high morbidity and mortality associated with PE, additional studies are warranted to address this relationship.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Institution at which the work was performed: Morristown Memorial Hospital, Atlantic Health System, Morristown, New Jersey.
Footnotes
A commentary on this article appears in this issue on page 1009.
REFERENCES
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Baglin TP, White K, Charles A. Fatal pulmonary embolism in hospitalised medical patients. J Clin Pathol. 1997;50:609–10. doi: 10.1136/jcp.50.7.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356:1438–44. doi: 10.1056/NEJMcp067264. [DOI] [PubMed] [Google Scholar]
- 4.Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost. 2002;28(Suppl 2):3–13. doi: 10.1055/s-2002-32312. [DOI] [PubMed] [Google Scholar]
- 5.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 6.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–97. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the National Sleep Foundation Sleep in America 2005 Poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 9.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 10.von Kanel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–67. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 11.Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109:951–7. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 13.Marrone O, Bonsignore MR. Pulmonary haemodynamics in obstructive sleep apnoea. Sleep Med Rev. 2002;6:175–93. doi: 10.1053/smrv.2001.0185. [DOI] [PubMed] [Google Scholar]
- 14.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Kanel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–9. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 16.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 17.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3:409–15. [PMC free article] [PubMed] [Google Scholar]
- 18.MacGregor MI, Block AJ, Ball WC., Jr Topics in clinical medicine: serious complications and sudden death in the Pickwickian syndrome. Johns Hopkins Med J. 1970;126:279–95. [PubMed] [Google Scholar]
- 19.Godfrey S, Meltzer E, Shabbath T. Pulmonary embolism and the Pickwick syndrome. Br J Dis Chest. 1972;66:155–61. [PubMed] [Google Scholar]
- 20.Hasan FM, Auchincloss JH, Jr, Gilbert R. Thromboembolic disease and cardiorespiratory syndrome of obesity. N Y State J Med. 1976;76:272–5. [PubMed] [Google Scholar]
- 21.Hasegawa R, Shiomi T, Sasanabe R, et al. Sleep apnea syndrome in patients with pulmonary thromboembolism. Psychiatry Clin Neurosci. 2000;54:342–3. doi: 10.1046/j.1440-1819.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 22.Arnulf I, Merino-Andreu M, Perrier A, Birolleau S, Similowski T, Derenne JP. Obstructive sleep apnea and venous thromboembolism. JAMA. 2002;287:2655–6. doi: 10.1001/jama.287.20.2655. [DOI] [PubMed] [Google Scholar]
- 23.Ambrosetti M, Lucioni A, Ageno W, Conti S, Neri M. Is venous thromboembolism more frequent in patients with obstructive sleep apnea syndrome? J Thromb Haemost. 2004;2:1858–60. doi: 10.1111/j.1538-7836.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- 24.Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245–8. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 25.Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037–52. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 26.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 27.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 28.Chung F, Ward B, Ho J, Yuan H, Kayumov L, Shapiro C. Preoperative identification of sleep apnea risk in elective surgical patients, using the Berlin questionnaire. J Clin Anesth. 2007;19:130–4. doi: 10.1016/j.jclinane.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Rechtschaffen, Kales . A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 30.Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med. 2001;163:398–405. doi: 10.1164/ajrccm.163.2.9808132. [DOI] [PubMed] [Google Scholar]
- 31.AASM. The international classification of sleep disorders: diagnostic & coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 32.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 33.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 35.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. Second ed. New York: 2000. [Google Scholar]
- 36.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–80. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350:2558–63. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 38.Hansson PO, Eriksson H, Welin L, Svardsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the study of men born in 1913”. Arch Intern Med. 1999;159:1886–90. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 39.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173:7–15. doi: 10.1164/rccm.200509-1478OE. [DOI] [PubMed] [Google Scholar]
- 40.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–95. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 41.Skinner MA, Choudhury MS, Homan SD, Cowan JO, Wilkins GT, Taylor DR. Accuracy of monitoring for sleep-related breathing disorders in the coronary care unit. Chest. 2005;127:66–71. doi: 10.1378/chest.127.1.66. [DOI] [PubMed] [Google Scholar]
- 42.Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W. Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:648–52. doi: 10.1034/j.1399-3003.2000.16d14.x. [DOI] [PubMed] [Google Scholar]
- 43.Olson LJ, Olson EJ, Somers VK. Obstructive sleep apnea and platelet activation: another potential link between sleep-disordered breathing and cardiovascular disease. Chest. 2004;126:339–41. doi: 10.1378/chest.126.2.339. [DOI] [PubMed] [Google Scholar]
- 44.Shitrit D, Peled N, Shitrit AB, et al. An association between oxygen desaturation and D-dimer in patients with obstructive sleep apnea syndrome. Thromb Haemost. 2005;94:544–7. [PubMed] [Google Scholar]
- 45.Ohga E, Nagase T, Tomita T, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87:10–4. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 46.ten Wolde M, Kraaijenhagen RA, Schiereck J, et al. Travel and the risk of symptomatic venous thromboembolism. Thromb Haemost. 2003;89:499–505. [PubMed] [Google Scholar]
- 47.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal L, Bishop C, Guido P, et al. The sleep/wake habits of patients diagnosed as having obstructive sleep apnea. Chest. 1997;111:1494–9. doi: 10.1378/chest.111.6.1494. [DOI] [PubMed] [Google Scholar]
- 49.Endeshaw YW, Unruh ML, Kutner M, Newman AB, Bliwise DL. Sleep-disordered breathing and frailty in the Cardiovascular Health Study Cohort. Am J Epidemiol. 2009;170:193–202. doi: 10.1093/aje/kwp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chunilal SD, Eikelboom JW, Attia J, et al. Does this patient have pulmonary embolism? JAMA. 2003;290:2849–58. doi: 10.1001/jama.290.21.2849. [DOI] [PubMed] [Google Scholar]
- 51.Runyon MS, Webb WB, Jones AE, Kline JA. Comparison of the unstructured clinician estimate of pretest probability for pulmonary embolism to the Canadian score and the Charlotte rule: a prospective observational study. Acad Emerg Med. 2005;12:587–93. doi: 10.1197/j.aem.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Chagnon I, Bounameaux H, Aujesky D, et al. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002;113:269–75. doi: 10.1016/s0002-9343(02)01212-3. [DOI] [PubMed] [Google Scholar]
- 53.Kabrhel C, Camargo CA, Goldhaber SZ. Clinical gestalt and the diagnosis of pulmonary embolism: does experience matter? Chest. 2005;127:1627–30. doi: 10.1378/chest.127.5.1627. [DOI] [PubMed] [Google Scholar]
- 54.Kabrhel C, McAfee AT, Goldhaber SZ. The contribution of the subjective component of the Canadian Pulmonary Embolism Score to the overall score in emergency department patients. Acad Emerg Med. 2005;12:915–20. doi: 10.1197/j.aem.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 55.PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis (PIOPED) JAMA. 1990;263:2753–9. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 56.Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298:2743–53. doi: 10.1001/jama.298.23.2743. [DOI] [PubMed] [Google Scholar]
- 57.Trowbridge RL, Araoz PA, Gotway MB, Bailey RA, Auerbach AD. The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism. Am J Med. 2004;116:84–90. doi: 10.1016/j.amjmed.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Schoepf UJ, Goldhaber SZ, Costello P. Spiral computed tomography for acute pulmonary embolism. Circulation. 2004;109:2160–7. doi: 10.1161/01.CIR.0000128813.04325.08. [DOI] [PubMed] [Google Scholar]