Abstract
Background and Aims
Pediatric Crohn’s disease (CD) is associated with deficits in growth, lean mass (LM), and fat mass (FM). This study assessed changes in height and body composition in children and adolescents with CD following diagnosis, and identified determinants of these changes.
Methods
Whole body LM and FM were assessed using DXA in 78 CD subjects at diagnosis and 6, 12, and a median of 43 (range 24–63) months later. Race- and sex-specific Z-scores for lean mass (LM-ht-Z) and fat mass (FM-ht-Z) relative to height were derived using reference data in >900 controls. Serum cytokines and growth factors were measured and quasi-least squares regression was used to identify determinants of changes in height and body composition Z-scores.
Results
LM-ht-Z and FM-ht-Z (both p<0.005) improved significantly following diagnosis; however, females had persistent LM deficits vs. controls (−0.50 ± 1.02, p<0.05) at the final visit. Serum interleukin-6, TNF-α, and lipopolyscaccharide binding protein (LBP) decreased significantly (all p<0.001). Greater increases in LM-ht-Z were associated with infliximab therapy (p<0.05), increases in albumin (p<0.001), and decreases in ESR (p<0.05), interleukin-6 (p<0.005), and LBP (p<0.05). Greater increases in FM-ht-Z were associated with glucocorticoid, methotrexate, and infliximab therapy, and increases in albumin (p<0.05) and growth hormone binding protein (p<0.05). Overall, height-Z did not improve; however, greater increases in IGF-1 (p<0.05) and decreases in TNF-α (p<0.05), interleukin-6 (p<0.05) and LBP (p<0.05) levels were associated with increases in height-Z.
Conclusions
Immune-mediated mechanisms contribute to growth and body composition deficits in CD. Therapies should target these deficits.
Keywords: Crohn’s disease, body composition, growth, nutrition, children
INTRODUCTION
Childhood Crohn’s disease (CD) is associated with malnutrition, malabsorption, delayed puberty, decreased physical activity, glucocorticoid therapy and elevated inflammatory cytokines. Each of these may contribute to poor growth and alterations in lean mass (LM) and fat mass (FM). Growth and development are characterized by age-, sex-, maturation-, and race- specific increases in LM and FM. In adults, LM deficits are associated with demonstrable morbidity, including poor physical functioning, altered energy metabolism, and increased susceptibility to infections.1 In children, altered body composition is an indicator of poor nutritional status and overall health. Furthermore, LM deficits are associated with poor bone accrual.2
Prior cross-sectional studies of body composition in children and adults with inflammatory bowel disease were performed in small numbers of patients with conflicting results.3–14 We reported significant LM deficits in a prevalent cohort of 104 children and young adults with CD.15 Whole body LM was significantly lower in CD compared with controls, adjusted for age, height and maturation; however, FM was normal, consistent with inflammatory cachexia.16–18 In a cohort of 78 incident pediatric CD subjects, we noted cachexia (decreased LM) in males, and wasting (decreased LM and FM) in females at diagnosis.19
Prior longitudinal studies of body composition in children with incident CD are limited to two reports. In the first, whole body LM did not improve over a two year interval in 42 subjects; however, the study was limited by sparse reference data and did not address FM.20 In the second, we examined changes in regional LM and FM using tibia peripheral quantitative computed tomography (pQCT) in our incident pediatric CD cohort.21 Using robust reference data in over 650 controls, we demonstrated significant improvements in calf LM and FM over 12 months. The present study builds upon our prior studies in this cohort by including measures of whole body LM and FM, examining changes in cytokine and growth factor levels, and extending the period of observation to 2 to 5 years. The aims are (1) to assess changes in height, LM, and FM in children and adolescents with CD from the time of diagnosis through long-term follow up, and (2) to identify determinants of changes in height, LM, and FM in this cohort.
MATERIALS AND METHODS
Study Subjects
Incident CD subjects, ages 5 to 21 years, were recruited from the Children’s Hospital of Philadelphia (CHOP). Patients with other illnesses or medications affecting growth and development were excluded. Baseline visits were completed within two weeks of diagnosis, and follow-up visits 6 and 12 months later. A subset of 52 CD subjects enrolled in a subsequent ancillary study to complete an additional long term follow-up (LTFU) visit at a median of 43 months (range 24–63 months) after diagnosis. All CD subjects completed at least one follow-up visit. Baseline body composition and 12 month changes in pQCT outcomes have been reported in this cohort.19, 21
A total of 921 healthy control subjects recruited from pediatric clinics in the greater Philadelphia area were used to generate reference data for body composition measures. Control subjects were excluded for reported height or body mass index (BMI) below the third percentile at screening, and for chronic illnesses or medications affecting growth. A subset of controls (n=212), representative of the larger group in terms of age, race, and sex, enrolled in a longitudinal study with additional visits at 6 and 12 months.
The study protocol was approved by the CHOP Institutional Review Board. Informed consent was obtained from young adult subjects and the parents or guardians of subjects under 18 years of age. Assent was obtained from subjects seven to 18 years of age.
Crohn’s Disease Characteristics and Definitions
CD diagnosis was confirmed by radiographic, endoscopic, histologic, and clinical parameters, as previously described.19, 21 All 78 subjects were evaluated by colonoscopy, and 75 also underwent esophagoduodenoscopy. Site of disease was defined according to the Montreal classification.22 CD activity was assessed using the Pediatric Crohn Disease Activity Index (PCDAI) based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth data (20%), with scores ranging from 0 to 100.23 Growth data prior to diagnosis were obtained from the general pediatric medical record. Additionally, data on duration of symptoms, extraintestinal manifestations, dietary intake, medications, and nutritional supplements were collected.
Anthropometry and Pubertal Development
Weight and height were measured using a digital scale to the nearest 0.1 kg (Scaltronix, White Plains, NY) and a wall-mounted stadiometer to the nearest 0.1 cm (Holtain Ltd., Croswell, Crymych, United Kingdom), respectively. Pubertal status was assessed with a validated self-assessment questionnaire in subjects greater than eight years of age24 and classified according the method of Tanner.25
Body Composition Measures
Whole body LM (kg) and FM (kg) were assessed by dual energy x-ray absorptiometry (DXA) using a Hologic Delphi densitometer (Bedford, MA) with a fan beam in the array mode (software version 12.4), excluding the head.16, 26 LM was calculated as fat free mass minus bone mineral content. DXA is a precise (CV 1–4%)27 method used extensively to describe age-, sex-, race-, and pubertal maturation-related variability in body composition.28–31 The instrument was calibrated daily using an hydroxyapatite phantom and weekly with a whole body phantom.
Laboratory studies
Laboratory studies included serum hematocrit (mg/dL), erythrocyte sedimentation rate (ESR) (mm/h), and albumin (g/dL) in CD subjects; assays were performed using standard techniques in the CHOP clinical laboratories and were obtained at all four study visits. The following cytokine and growth factor measurements were obtained at baseline, 6 months, and LTFU. Serum tumor necrosis-alpha (TNF-α) (sensitivity: 0.05 pg/mL, inter-assay variation: 8.3%), interleukin-6 (IL-6 ) (sensitivity: 0.10 pg/mL, inter-assay variation: 7%), and interleukin -1β (IL-1β) (sensitivity: 0.06 pg/mL, inter-assay variation: 8.8%) were measured using the Luminex™ platform and the human cytokine six-plex high sensitivity antibody bead kit (Millipore, Billerica, MA). Serum insulin-like growth factor-1 (IGF-1) (sensitivity: 1.9 ng/mL, inter-assay variation: 7%, Immunodiagnostic Systems, Tyne & Wear, UK), growth hormone binding protein (GHBP) (sensitivity: 1.69 pmol/L, inter-assay variation: 6.3%, DSL, Webster, TX), and intact insulin-like growth factor binding protein-3 (IGFBP-3) (sensitivity: 0.05 ng/mL, inter-assay variation: 6.4%, (R&D Systems, Minneapolis, MN) were measured by enzyme-linked immunosorbant sandwich assay (ELISA) , using commercially available kits. Serum lipopolysaccharide binding protein (LBP) (sensitivity: 4.4 ng/mL) was determined by solid-phase ELISA (Hycult Biotechnology, Uden, Netherlands).
Statistical Analysis
Analyses were conducted using STATA 10.0 (Stata Corporation, College Station, TX). A two-tailed p-value <0.05 was the criterion for statistical significance. Differences in means were assessed using Student’s t test and Wilcoxon’s rank sum test. Correlations between anthropometric and body composition parameters and continuous variables were assessed by Pearson product moment correlations or Spearman rank correlation tests, where appropriate.
Height and BMI were converted to age- and sex-specific standard deviation scores (Z-scores) in the CD subjects and controls using national reference data.32 CD was associated with significantly lower height Z-scores compared with controls, and body composition variables were highly correlated with height (LM: R = 0.94, p < 0.001; FM: R = 0.57, p < 0.001). Therefore, whole body LM and FM were assessed as race- and sex-specific Z-scores relative to height, as previously described.16, 19, 26 The distributions of LM and FM relative to height demonstrated significant heteroscedasticity and skew. Therefore, LM for height (LM-Ht) and FM for height (FM-Ht) Z-scores were generated in CD subjects and controls based on the control population utilizing the LMS method.33 Race-, and sex-specific estimates of the distribution median (M), coefficient of variation (S) and degree of skewness (L) in LM and FM relative to height were obtained by a maximum-likelihood curve-fitting technique.
Quasi-least squares (QLS) regression using the xtqls function in STATA 10.0 assessed (1) changes over time in disease activity, anthropometry and body composition Z-scores and (2) determinants of changes in these Z-scores in CD subjects.34 QLS models allow for a variable number of measurements per subject and the implementation of the Markov correlation structure, which is appropriate for modeling associations among measurements that are unequally spaced in time. Signed rank tests assessed changes between the baseline and the LTFU visit cytokine and growth factor values. Because height, BMI, LM-ht, and FM-ht Z-scores did not increase linearly during the study period, variables for time and (time)2 were added to the models. The initial models to assess determinants of changes from baseline in Z-scores for height, LM-ht, and FM-ht included baseline Z-score, race, and pubertal status for the following reasons: (1) the large influence of baseline Z-score on the magnitude of change in Z-score, (2) significant differences in anthropometry and body composition in blacks and whites, and (3) delays in the onset and progression through puberty in children and adolescents with CD. Tanner stage was categorized as prepuberty to early puberty (Tanner stages 1 and 2) vs. middle to late puberty (Tanner stages 3 to 5). Analyses involving changes in height Z-score were limited to those subjects in Tanner stages 1–4. Sex was not a significant covariate in these multivariate models. Multiplicative interaction terms did not reveal interactions between Tanner stage and sex or race. All models involving changes in variables other than those expressed as Z-scores (e.g., laboratory values) used absolute changes from baseline, adjusted for baseline values.
Medication use changed significantly between study visits; therefore, models examining the impact of medications on changes in height, LM-ht, and FM-ht Z-scores used interval change (the change in LM-ht or FM-ht Z-score between consecutive visits) rather than change from baseline. Models for interval change in each Z-score included covariates for the interval baseline Z-score (i.e., the Z-score at the beginning of an interval) and interval medication use (expressed as mean mg/kg/day for glucocorticoids, and as a categorical yes/no variable for other medications) in addition to race and Tanner stage.
Last, we previously reported that greater FM-ht Z-scores were associated with greater LM-ht Z-scores in healthy children.15 In order to determine if CD subjects had the expected increases in LM-ht Z-score relative to increases in FM-ht Z-score, analyses were performed within the CD subjects and the subset of controls that completed 6 or 12 month visits. These models used generalized estimating equations since the intervals between study visits were constant during the first 12 months.
RESULTS
Subject and Disease Characteristics at Baseline
Table 1 summarizes subject characteristics in CD subjects and controls. The sex and racial distribution of the CD subjects is consistent with the demographics of the disease.35 Although the proportions of prepubertal subjects in CD subjects and controls were comparable, CD subjects had significantly greater age for Tanner stage and sex compared with controls (p < 0.001), consistent with delayed puberty. CD subjects had significantly lower height and BMI Z-scores compared with controls. Height and BMI Z-scores did not differ according to sex within the CD subjects. Baseline disease characteristics are summarized in Table 2; the majority of CD subjects presented with growth failure, moderate to severe disease activity, anemia, and ileocolonic disease..
Table 1.
Baseline Characteristics of Incident Crohn’s Disease Subjects and Healthy Controls
| Variable | Crohn Disease | Controls | p |
|---|---|---|---|
| N | 78 | 921 | |
| Age,years | 12.7 ± 2.8 | 11.8 ± 3.9 | 0.03 |
| Range | 5.5,18.0 | 5.0,22.0 | |
| Sex,% Male | 56 | 48 | 0.1 |
| Race,% Black | 10 | 44 | <0.001 |
| Tanner Stage 1–5, n | 24, 18, 13, 18, 5 | 332, 110, 122, 188, 163 | |
| Pre- to early Pubertal,% Tanner 1–2 | 54 | 48 | 0.3 |
| Height Z-score | −0.26 ± 1.1 | 0.26 ± 0.9 | <0.001 |
| Range | −3.2,2.2 | −2.6,3.3 | |
| BMI Z-score | −0.55 ± 1.1 | 0.36 ± 1.0 | <0.001 |
| Range | −2.7,2.2 | −3.4,3.0 |
Continuous variables presented as mean ± SD
Table 2.
Crohn’s Disease Characteristics at the Baseline Visit
| Variable | Value |
|---|---|
| Duration of symptoms prior to diagnosis (months), median(range) | 7.1(0.5,52.6) |
| Family history of Crohn’s disease, n(%) | 22(30%) |
| History of growth failure, n(%) | 62(84%) |
| PCDAI, Mean ± SD | 38.4±18.0 |
| No active disease (≤10) | 3(4%) |
| Mild (11–30) | 28(38%) |
| Moderate to Severe (>30) | 43(58%) |
| Albumin (g/dL), median(range) | 3.5(1.8,4.7) |
| ESR (mm/h), median,(range) | 21(0,128) |
| Anemia, n(%) | 60(77%) |
| Site of Disease, n(%) | |
| Isolated ileal disease | 2(3%) |
| Isolated colonic disease | 7(9%) |
| Ileo-colonic disease | 46(59%) |
| Isolated upper GI tract disease | 5(7%) |
| Perianal Involvement | 28(36%) |
PCDAI, Pediatric CD Activity Index; ESR, erythrocyte sedimentation rate; GI, gastrointestinal.
Table 3 summarizes growth factor and cytokine values in CD subjects at diagnosis compared with controls. CD subjects had significantly greater IL-6, TNF-α, and LBP levels compared with controls. These differences remained significant (IL-6, p<0.01; TNF-α p<0.05; LBP, p<0.001) after adjustment for sex, age, and Tanner stage. CD subjects also had lower IGF-1 and greater IGFBP-3 and GHBP levels compared with controls, and these findings remained significant (all p<0.001) after adjustment for sex, age, and Tanner stage.
Table 3.
Cytokine and Growth Factor Levels in Crohn’s Disease Subjects at Baseline and Controls
| CD | n | Controls | n | p | |
|---|---|---|---|---|---|
| IL-6 | 21.6 (4.48,182.81) | 58 | 9.31 (0.35,102.74) | 154 | <0.0001 |
| TNF-α | 8.23 (2.5,18.50) | 58 | 6.15 (0.80,73.47) | 154 | 0.0001 |
| IL-1β | 1.16 (0.03,7.53) | 58 | 0.76 (0.03,34.05) | 154 | 0.84 |
| LBP | 39.9 (4.3,302.8) | 58 | 19.4 (0.8,114.3) | 75 | <0.0001 |
| IGF-1 | 221.0 (2.5,500.0) | 57 | 272.57 (30.96,793.84) | 154 | <0.005 |
| IGFBP3 | 3508.1 (1108.2,5313.1) | 58 | 2675.55 (818.19,5820.25) | 154 | <0.0001 |
| GHBP | 775.62 (48.42,3179.96) | 58 | 606.49 (37.09,3147.55) | 154 | <0.05 |
All values are expressed as median (range) in pg/mL.
Changes in Therapies, Disease Activity, and Laboratory Parameters
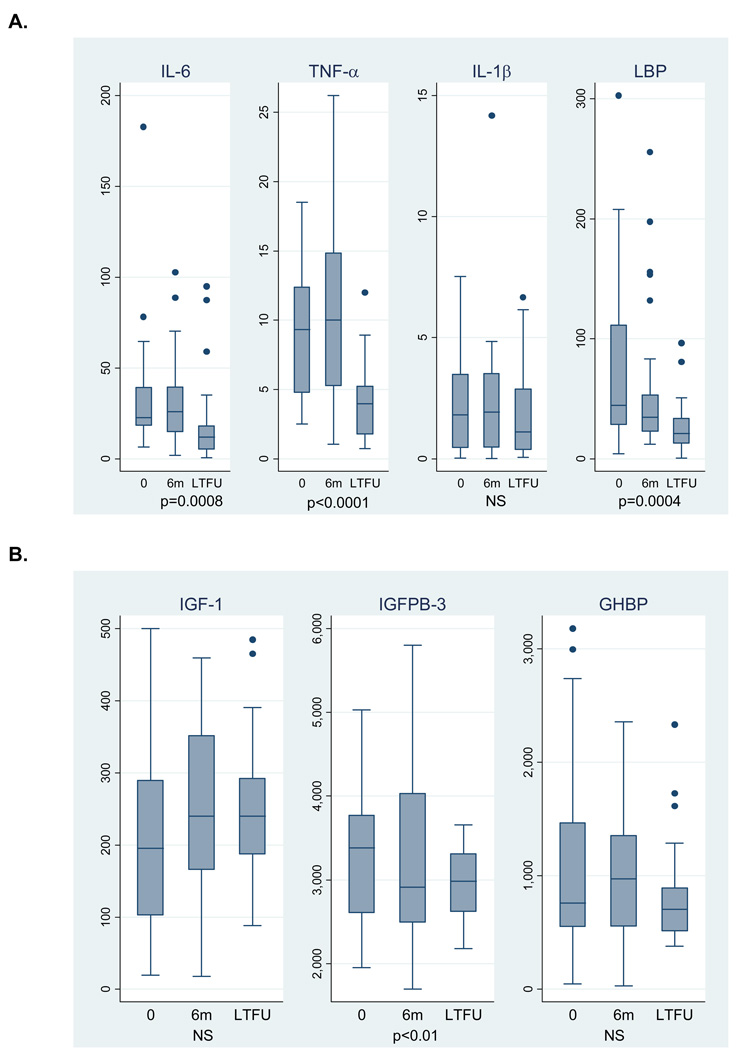
Of the 78 subjects originally enrolled in the study, 74, 67, and 52 completed 6-month, 12-month, and LTFU study visits, respectively. There were no baseline differences in sex, race, PCDAI, and height Z-score in subjects who did vs. did not complete a LTFU visit. Table 4 summarizes the proportions of subjects treated with CD therapies during each study interval, including the median glucocorticoid dose in those treated with glucocorticoids. Among the 46 subjects who completed all 4 study visits, the proportions who received the following medications during any interval in the study were: systemic steroids 34 (74%), methotrexate 11 (24%), 6-mercaptopurine 36 (78%), azathioprine 5 (11%), infliximab 13 (28%), and enteral nutrition 9 (20%). Of note, no subject received exclusive enteral nutritional therapy. Among these 46 subjects, PCDAI improved significantly over the study interval (p<0.001), with a median score of 40 at baseline, 15 at 6 months, 10 at 12 months, and 5 at LTFU. Figure 1 summarizes changes in growth factors and cytokines. CD subjects had significant reductions in IL-6 (p=0.0008), TNF-α (<0.0001), LBP (p=0.0004), and IGFBP-3 (p<0.01) over the study interval.
Table 4.
Crohn’s Disease Therapies During Each Interval
| Baseline to 6 Months n=72 |
6 to 12 Months n=67 |
12 months to LTFU n=52 |
|
|---|---|---|---|
| Systemic Glucocorticoids | 44 (61%) | 21 (31%) | 16 (31%) |
| Median (range) mg/kg/day | 0.19 (0.02,0.86) | 0.15 (0.05,0.36) | 0.04 (0.003,0.19) |
| Methotrexate | 4 (6%) | 6 (9%) | 11 (21%) |
| 6-Mercaptopurine | 28 (39%) | 33 (49%) | 31 (60%) |
| Azathioprine | 4 (6%) | 5 (7%) | 3 (6%) |
| Infliximab | 5 (7%) | 9 (13%) | 13 (25%) |
| Enteral feeds | 4 (6%) | 3 (4%) | 8 (15%) |
Medication use expressed as n (%)
Figure 1.
Cytokine (A) and growth factor (B) levels following disease diagnosis in incident Crohn’s Disease subjects, expressed as pg/mL. Data presented are limited to the 30 subjects with data at each study visit. P-values are based on results of a signed rank test between baseline and LTFU values.
Changes in Anthropometry and Body Composition Parameters in Crohn’s Disease Subjects
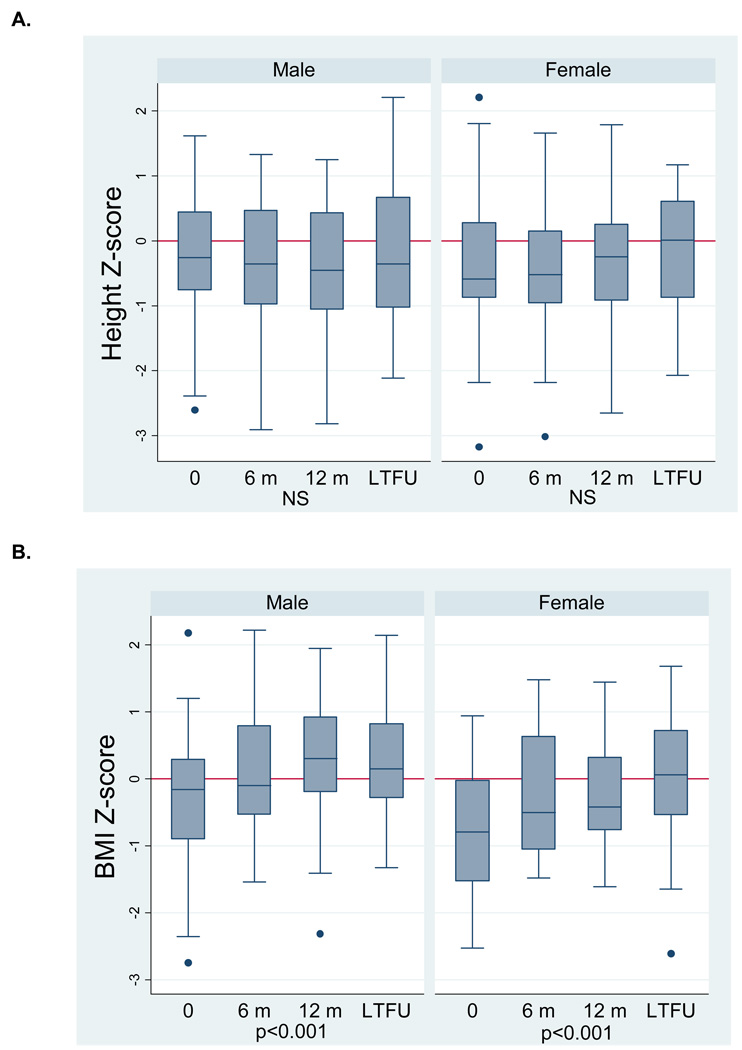
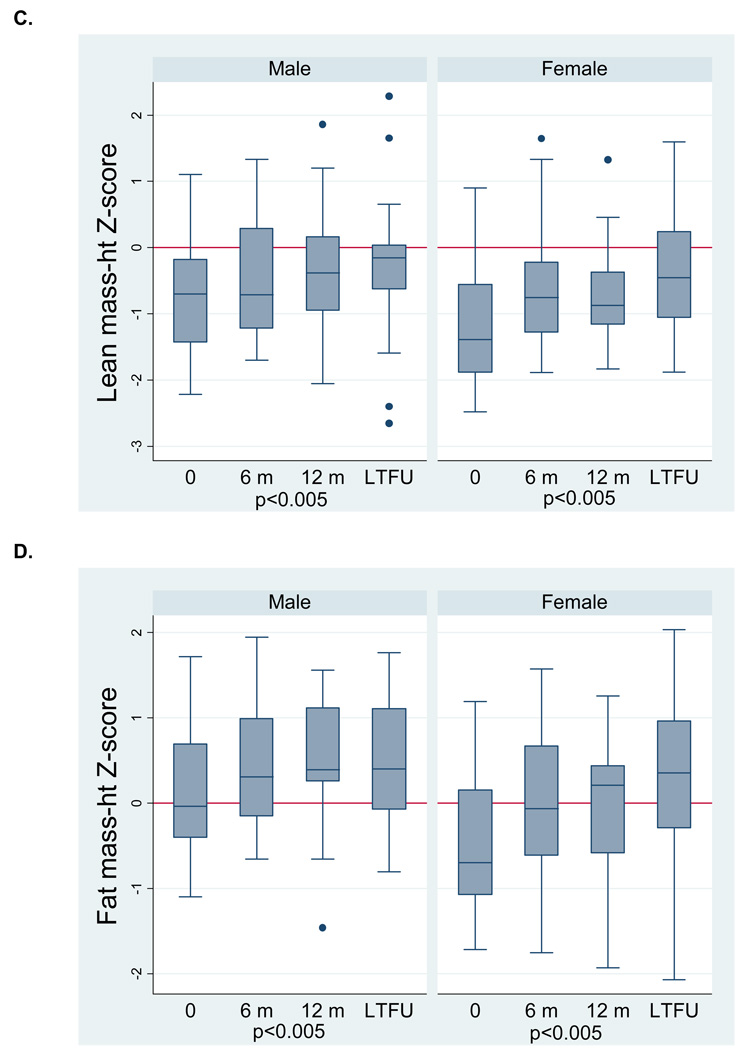
We previously reported sex differences in baseline body composition deficits in this cohort; male CD subjects presented with LM deficits (cachexia), and females presented with LM and FM deficits (wasting).19 Therefore, the graphical displays of longitudinal anthropometry and body composition measures are stratified by sex. Height z-score did not change significantly (Figure 2A) for either sex. However, both male and female CD subjects showed significant improvements (Figure 2B) in BMI Z-score (both, p<0.001). Figures 2C and 2D illustrate the significant improvements in LM-ht (both, p<0.005) and FM-ht (both, p<0.005) Z-scores in both males and females. LM-ht Z-scores at LTFU were significantly greater than those at 12 months, indicating continued improvement beyond the first year of therapy. The body composition Z-scores at the LTFU visit in CD were also compared with controls. In the males with CD, LM-ht Z-scores were comparable to controls, and FM-ht Z-scores were significantly greater than controls (0.56 ± 0.72, p<0.005). Female CD subjects had LM deficits at LTFU (−0.50 ± 1.02, p<0.05) and FM-ht Z-scores were comparable to controls.
Figure 2.
Anthropometry (A,B) and body composition (C,D) Z-scores following disease diagnosis in incident Crohn’s disease subjects. Data for Figure 2A are limited to the 27 subjects in Tanner stages 1–4 at each study visit. Data for Figure 2B – D are limited to the 46 subjects with data at each study visit. P-values for time and (time)2 are reported jointly to capture the non-linear relations.
Using the longitudinal data available in a subset of controls at baseline, 6, and 12 months, we assessed if CD subjects demonstrated the expected increases in LM-ht Z-scores relative to concurrent increases in FM-ht Z-scores during the first 12 months after diagnosis. Female CD subjects had greater increases in LM-ht z-score relative to increases in FM-ht z-score, compared to controls, but there was no group difference in male CD subjects vs. male controls. That is, the improvement in LM in boys was commensurate with the gains in FM, relative to controls. In girls, however, the gain in LM was significantly greater than expected for the concurrent gain in FM (p<0.01).
Crohn’s Disease-Specific Determinants of Changes in Body Composition and Height
Lean Mass
The multivariable QLS base model of determinants of changes from baseline in LM-ht Z-score included baseline LM-ht Z-score, race, and pubertal status. Each of the variables included in Tables 2 through 4, was assessed individually within the multivariate base model, and results below are expressed as [β-coefficient, (95% confidence interval), p-value]. Greater increases in LM-ht Z-score were associated with greater increases in FM-ht Z-score [0.52,(0.37, 0.66),p<0.001] and albumin [0.39,(0.17,0.60), p<0.001], and decreases in ESR [−0.01,(−0.02,−0.0001), p<0.05], IL-6 [−0.01(−0.02,−0.004), p<0.005], and LBP [−0.01(−0.002,−0.004), p<0.05]. Concurrent infliximab therapy [0.30, (0.02,0.58), p<0.05] was associated with greater increases in LM-ht Z-score over the corresponding interval.
Fat Mass
Similar to the LM base model above, the multivariable QLS base model to assess determinants of changes from baseline in FM-ht Z-score included baseline FM-ht Z-score, race, and pubertal status. Greater increases in FM-ht Z-score were associated with increases in albumin [0.19,(0.02,0.36), p<0.05] and GHBP [0.0003,(0.0001,0.0006), p<0.05], as well as presentation with weight loss at CD diagnosis [0.35,(0.11, 0.60), p<0.005]. Greater increases in FM-ht Z-score at the end of any given interval were significantly associated with cumulative glucocorticoid exposure [0.69,(0.12,1.26), p<0.05], methotrexate [0.30,(0.04,0.56), p<0.05], and infliximab [0.29, (0.06, 0.52), p<0.05]. When all three medications were included in the same model, the glucocorticoid and methotrexate effects remained significant, and the infliximab effect was attenuated [0.19 (−0.04,0.41), p=0.10].
Height
Height Z-score did not change significantly during the study interval overall; although some subjects demonstrated increases in height Z-scores. The median change in height Z-score between baseline and LTFU in Tanner 1–4 subjects was 0.13 (interquartile range −0.40, 0.71). Subject race, gender, age, baseline disease severity, and height Z-score did not differ between those with increases vs. decreases in height z-score over the study interval. QLS models similar to those used for LM-ht and FM-ht Z-scores were used to assess determinants of changes in height Z-score. Greater increases in height Z-score were associated with increases in IGF-1 [0.0008 (0.0001,0.0015), p<0.05], and with decreases in PCDAI [−0.007 (−0.011,−0.003), p<0.001], TNF-α [−0.0215(−0.0385,−0.0045), p<0.05], IL-6 [−0.007 (−0.0135,−0.0018), p<0.05], and LBP [−0.0008(−0.0016,−0.00001), p<0.05]. Greater decreases in height Z-score at the end of any given interval between consecutive visits were significantly associated with greater concurrent glucocorticoid use [−0.63(−1.00,−0.28), p=0.0005].
DISCUSSION
This study demonstrated significant improvements in body composition parameters in incident CD subjects from diagnosis throughout a period of extended follow-up. Additionally, the study identified the following determinants of improvements in LM-ht Z-score following diagnosis: increases in FM-ht Z-score and albumin; decreases in ESR, IL-6, and LBP; and infliximab therapy. Increases in albumin and GHBP, presentation with weight loss at diagnosis; and use of glucocorticoids, methotrexate, and infliximab were associated with greater increases in FM-ht Z-score. The data reported here differ significantly from prior reports and also provide new findings regarding determinants of changes in body composition in pediatric CD from diagnosis. First, our study is the first longitudinal study to show improvements in body composition parameters. Second, the inclusion of a robust contemporary reference population allowed for sex- and race-specific assessment of body composition relative to height, with further adjustment for Tanner stage. Additionally, the inclusion of the subset of controls with baseline, 6- and 12-month body composition data allow for the assessment of changes in LM relative to FM during the first 12 months of therapy in CD subjects. Third, to our knowledge, our study is the first to show improvements in LM-ht Z-score associated with improvements in IL-6 and LBP, as well as infliximab use.
A prior study of changes in body composition during two years after CD diagnosis, limited by a small cross-sectional reference population (n=81), did not show improvements in LM, did not note sex differences in body composition changes, and did not include measures of FM.20 In male CD subjects, we noted cachexia at baseline, with complete recovery of LM deficits at LTFU. In fact, increases in FM in males resulted in significantly greater FM in male CD subjects at LTFU compared with controls. In contrast, female CD subjects presented with LM and FM deficits at baseline and demonstrated complete recovery of FM deficits but persistent LM deficits despite improvement in LM-ht Z-scores at LTFU. The patterns of progressing from cachexia to excess adiposity in males and from wasting to cachexia in females over the study interval are not captured by examining differences in BMI Z-score (Figure 2B). These findings highlight the importance of assessing lean and fat compartments individually as indicators of nutritional status.
Although improvements in PCDAI were not associated with improvements in LM-ht Z-score, improvements in albumin and ESR, components of the PCDAI, and reductions in IL-6 and LBP, were associated with improvements in LM-ht Z-score. In fact, serum cytokines remained elevated six months following diagnosis, despite improvements in clinical disease activity, suggesting that therapies used to induce remission were not immediately effective in suppressing systemic inflammation. We were able to demonstrate a relationship between improvements in height Z-scores and reductions in PCDAI , IL-6, TNF-α, and LBP, though not infliximab therapy. The lack of an association between infliximab therapy and improvement in height Z-score is consistent with a similar study in an inception cohort of children with Crohn’s disease initiating infliximab therapy over a varied interval following disease diagnosis.36 Two prior studies that reported an association between infliximab therapy and changes in height Z-score were based on subjects’ enrollment at the time of initiation of infliximab therapy and did not include untreated Crohn’s disease controls.37–38
Recent studies using murine models of CD have elucidated the roles of IL-6 in growth failure.39–40 Although it is well recognized that sarcoactive cytokines41–42 induce myoblast apoptosis43 and stimulate protein degradation with resultant decreases in muscle mass, the association between improvements in LM deficits and IL-6 in incident pediatric CD has not been previously reported. LBP is an acute phase protein that is upregulated in the liver in response to endotoxin and IL-6 exposure. Additionally, a recent study of cross-sectional measures of LBP in a prevalent pediatric CD cohort demonstrated an association between elevated LBP and lower height Z-scores, suggesting that increased exposure to endotoxin results in immune-mediated growth hormone resistance.44 The study reported here is the first to examine changes in LBP and associated improvements in height and LM-ht Z-scores. Although TNF-α decreased significantly from baseline to LTFU, these changes were not associated with improvements in LM-ht Z-score. However, interval increases in LM-ht Z-score were associated with infliximab therapy. Together, the associations between improvements in LM deficits, reductions in IL-6 and LBP, and infliximab use emphasize the role of inflammation as an etiology for LM deficits.
We demonstrated decreased IGF-1 in CD subjects compared with controls but did not show significant increases over time or a relationship with LM deficits. However, increases in height Z-score were associated with increases in IGF-1. The predominant source of circulating IGF-1 is the liver, and recent murine studies have shown that hepatic IGF-1 is dispensable for many aspects of postnatal growth.45 Therefore, local IGF-1 action, not reflected in serum measurements, may be sufficient for attainment of LM.46 Under normal conditions, IGFBP-3 is produced by hepatic Kupffer cells in association with IGF-1 production by hepatocytes. We observed increased IGFBP-3 in CD subjects compared with controls at baseline, which normalized during long-term follow-up. This was unexpected, as serum IGFBP-3 is typically reduced during catabolism. However, IL-6 has been shown to induce IGFBP-3 secretion by Kupffer cells in vitro; this may explain the increase observed in the serum at earlier time points when IL-6 was elevated.47 The net effect of an increase in total IGF-1, and decrease in IGFBP-3, during LTFU would have been to increase the relative amount of free IGF-1 available to promote accrual of LM. GHBP is produced in humans from cleavage of the extra-cellular domain of the growth hormone receptor and release into the serum.48 Under normal conditions, serum GHBP is positively associated with FM.49 Therefore, it was surprising to observe increased in GHBP at baseline in CD patients, compared with controls. This may reflect an increase in GHR cleavage under inflammatory conditions, as we recently demonstrated in a murine model of acute endotoxin exposure.50 We did observe the expected positive association between increases in FM-ht Z-score and GHBP during therapy.49
Our study has some limitations, including the lack of dietary and physical activity data. For example, the associations between infliximab therapy and lean mass may be mediated by improved physical activity and dietary intake. There was loss to follow-up during the study interval, but subjects who did and did not complete all 4 study visits were no different in terms of demographics, disease activity, anthropometry, or body composition at baseline, minimizing potential bias. In addition, mucosal, as opposed to serum, levels of inflammatory cytokines such as TNF-α, were not available and may have correlated better with body composition outcomes. Finally, the biologic and clinical significance of the observed relations between the changes in cytokines and growth factors and associated changes in growth and body composition is unknown; however, our findings support the hypothesis that alterations in these biomarkers may directly impact growth and body composition.
In summary, this study demonstrated significant improvements in body composition over a median of 43 months following CD diagnosis. These observational data suggest that biologic therapies targeting inflammatory cytokines and the intestinal microbiome (e.g. LBP) may benefit patients presenting with linear growth failure, cachexia or wasting at diagnosis. However, randomized clinical trials are needed to determine if early use of such therapies will affect growth and body composition in pediatric Crohn’s disease.
Acknowledgments
Grant Support: NIH R01DK60030, K23 DK082012, K24 DK076808, and R01 DK068164; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Crohn’s and Colitis Foundation of America, and The Children’s Hospital of Philadelphia Clinical Translational Research Center UL1 RR024134
Abbreviations
- CD
Crohn’s disease
- LM
lean mass
- FM
fat mass
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author roles:
Study concept and design: MT, JS, BSZ, RNB, MBL
Acquisition of Data: MT, BSZ, KMH, AR, MBL
Analysis and Interpretation: MT, LAD, JS, JMB, RNB, BSZ, MBL
Drafting of Manuscript: MT and MBL
Critical revision of manuscript: MT, LAD, JS, BSZ, JMB, RNB, MBL
Statistical analysis: MT, JS, BSZ, JMB, MBL
Obtained funding: MT, LAD, RNB, MBL
Technical/Material Support: KMH, AR
Study Supervision: MBL
Disclosures: No disclosures.
No conflicts of Interest
Transcript Profiling: N/A
Writing Assistance: N/A
REFERENCES
- 1.Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutrition Reviews. 1991;49:163–175. doi: 10.1111/j.1753-4887.1991.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 2.Schonau E, Werhahn E, Schiedermaier U, et al. Influence of muscle strength on bone strength during childhood and adolescence. Hormone Research. 1996;45 Suppl 1:63–66. doi: 10.1159/000184834. [DOI] [PubMed] [Google Scholar]
- 3.Boot AM, Bouquet J, Krenning EP, et al. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42:188–194. doi: 10.1136/gut.42.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capristo E, Addolorato G, Mingrone G, et al. Effect of disease localization on the anthropometric and metabolic features of Crohn's disease. Am J Gastroenterol. 1998;93:2411–2419. doi: 10.1111/j.1572-0241.1998.00696.x. [DOI] [PubMed] [Google Scholar]
- 5.Capristo E, Mingrone G, Addolorato G, et al. Metabolic features of inflammatory bowel disease in a remission phase of the disease activity. J Intern Med. 1998;243:339–347. doi: 10.1046/j.1365-2796.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Mingrone G, Benedetti G, Capristo E, et al. Twenty-four-hour energy balance in Crohn disease patients: metabolic implications of steroid treatment. Am J Clin Nutr. 1998;67:118–123. doi: 10.1093/ajcn/67.1.118. [DOI] [PubMed] [Google Scholar]
- 7.Haderslev KV, Jeppesen PB, Sorensen HA, et al. Body composition measured by dual-energy X-ray absorptiometry in patients who have undergone small-intestinal resection. Am J Clin Nutr. 2003;78:78–83. doi: 10.1093/ajcn/78.1.78. [DOI] [PubMed] [Google Scholar]
- 8.Azcue M, Rashid M, Griffiths A, et al. Energy expenditure and body composition in children with Crohn's disease: effect of enteral nutrition and treatment with prednisolone. Gut. 1997;41:203–208. doi: 10.1136/gut.41.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahnsen J, Falch JA, Mowinckel P, et al. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2003;98:1556–1562. doi: 10.1111/j.1572-0241.2003.07520.x. [DOI] [PubMed] [Google Scholar]
- 10.Geerling BJ, Lichtenbelt WD, Stockbrugger RW, et al. Gender specific alterations of body composition in patients with inflammatory bowel disease compared with controls. Eur J Clin Nutr. 1999;53:479–485. doi: 10.1038/sj.ejcn.1600780. [DOI] [PubMed] [Google Scholar]
- 11.Schneeweiss B, Lochs H, Zauner C, et al. Energy and substrate metabolism in patients with active Crohn's disease. J Nutr. 1999;129:844–848. doi: 10.1093/jn/129.4.844. [DOI] [PubMed] [Google Scholar]
- 12.Rocha R, Santana GO, Almeida N, et al. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr. 2009;101:676–679. doi: 10.1017/S0007114508032224. [DOI] [PubMed] [Google Scholar]
- 13.Lee N, Radford-Smith GL, Forwood M, et al. Body composition and muscle strength as predictors of bone mineral density in Crohn's disease. J Bone Miner Metab. 2009;27:456–463. doi: 10.1007/s00774-009-0059-5. [DOI] [PubMed] [Google Scholar]
- 14.Schneider SM, Al-Jaouni R, Filippi J, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflamm Bowel Dis. 2008;14:1562–1568. doi: 10.1002/ibd.20504. [DOI] [PubMed] [Google Scholar]
- 15.Burnham JM, Shults J, Semeao E, et al. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19:1961–1968. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 16.Burnham JM, Shults J, Semeao E, et al. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr. 2005;82:413–420. doi: 10.1093/ajcn.82.2.413. [DOI] [PubMed] [Google Scholar]
- 17.Roubenoff R, Roubenoff RA, Ward LM, et al. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. Journal of Rheumatology. 1992;19:1505–1510. [PubMed] [Google Scholar]
- 18.Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 2004;43:1219–1223. doi: 10.1093/rheumatology/keh321. [DOI] [PubMed] [Google Scholar]
- 19.Thayu M, Shults J, Burnham JM, et al. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn's disease. Inflamm Bowel Dis. 2007;13:1121–1128. doi: 10.1002/ibd.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sylvester FA, Leopold S, Lincoln M, et al. A two-year longitudinal study of persistent lean tissue deficits in children with Crohn's disease. Clin Gastroenterol Hepatol. 2009;7:452–455. doi: 10.1016/j.cgh.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 24.Morris NN, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 25.Tanner JM. Growth at Adolescence. Blackwell Scientific Publication; 1962. [Google Scholar]
- 26.Foster BJ, Shults J, Zemel BS, et al. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr. 2004;80:1334–1341. doi: 10.1093/ajcn/80.5.1334. [DOI] [PubMed] [Google Scholar]
- 27.Ellis KJ, Shypailo RJ, Abrams SA, et al. The reference child and adolescent models of body composition. A contemporary comparison. Ann N Y Acad Sci. 2000;904:374–382. doi: 10.1111/j.1749-6632.2000.tb06486.x. [DOI] [PubMed] [Google Scholar]
- 28.Ellis KJ. Body composition of a young, multiethnic, male population. Am J Clin Nutr. 1997;66:1323–1331. doi: 10.1093/ajcn/66.6.1323. [DOI] [PubMed] [Google Scholar]
- 29.Goulding A, Taylor RW, Gold E, et al. Regional body fat distribution in relation to pubertal stage: a dual-energy X-ray absorptiometry study of New Zealand girls and young women. Am J Clin Nutr. 1996;64:546–551. doi: 10.1093/ajcn/64.4.546. [DOI] [PubMed] [Google Scholar]
- 30.Ogle GD, Allen JR, Humphries IR, et al. Body-composition assessment by dual-energy x-ray absorptiometry in subjects aged 4–26 y. Am J Clin Nutr. 1995;61:746–753. doi: 10.1093/ajcn/61.4.746. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd T, Chinchilli VM, Eggli DF, et al. Body composition development of adolescent white females: the Penn State Young Women's Health Study. Arch Pediatr Adolesc Med. 1998;152:998–1002. doi: 10.1001/archpedi.152.10.998. [DOI] [PubMed] [Google Scholar]
- 32.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 34.Shults J, Ratcliffe SJ, Leonard MB. Improved generalized estimating equation analysis via xtqls for implementation of quali-least squares in Stata. The STATA Journal. 2007;7:147–166. [Google Scholar]
- 35.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. doi: 10.1016/s0889-8553(05)70056-x. vii. [DOI] [PubMed] [Google Scholar]
- 36.Pfefferkorn M, Burke G, Griffiths A, et al. Growth abnormalities persist in newly diagnosed children with crohn disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr. 2009;48:168–174. doi: 10.1097/MPG.0b013e318175ca7f. [DOI] [PubMed] [Google Scholar]
- 37.Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn's disease. Inflamm Bowel Dis. 2007;13:424–430. doi: 10.1002/ibd.20069. [DOI] [PubMed] [Google Scholar]
- 38.Thayu M, Leonard MB, Hyams JS, et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn's disease: results of the REACH study. Clin Gastroenterol Hepatol. 2008;6:1378–1384. doi: 10.1016/j.cgh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Denson L, Held M, Arnold D, et al. Interleukin 6 inhibits growth hormone signaling via induction of cis and socs-3. Gastroenterology. 2002;122:A42–A362. [Google Scholar]
- 40.Sawczenko A, Azooz O, Paraszczuk J, et al. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the -174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci U S A. 2005;102:13260–13265. doi: 10.1073/pnas.0503589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langen RC, Schols AM, Kelders MC, et al. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. Faseb J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 42.Langen RC, Schols AM, Kelders MC, et al. Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am J Physiol Cell Physiol. 2002;283:C714–C721. doi: 10.1152/ajpcell.00418.2001. [DOI] [PubMed] [Google Scholar]
- 43.Stewart CE, Newcomb PV, Holly JM. Multifaceted roles of TNF-alpha in myoblast destruction: a multitude of signal transduction pathways. J Cell Physiol. 2004;198:237–247. doi: 10.1002/jcp.10387. [DOI] [PubMed] [Google Scholar]
- 44.Pasternak BA, D'Mello S, Jurickova II, et al. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn's disease and murine colitis. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohlsson C, Mohan S, Sjogren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148:1489–1497. doi: 10.1210/en.2006-1431. [DOI] [PubMed] [Google Scholar]
- 47.Lelbach A, Scharf JG, Ramadori G. Regulation of insulin-like growth factor-I and of insulin-like growth factor binding protein-1, -3 and -4 in cocultures of rat hepatocytes and Kupffer cells by interleukin-6. J Hepatol. 2001;35:558–567. doi: 10.1016/s0168-8278(01)00170-2. [DOI] [PubMed] [Google Scholar]
- 48.Alele J, Jiang J, Goldsmith JF, et al. Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology. 1998;139:1927–1935. doi: 10.1210/endo.139.4.5906. [DOI] [PubMed] [Google Scholar]
- 49.Fisker S, Hansen B, Fuglsang J, et al. Gene expression of the GH receptor in subcutaneous and intraabdominal fat in healthy females: relationship to GH-binding protein. Eur J Endocrinol. 2004;150:773–777. doi: 10.1530/eje.0.1500773. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Jiang J, Warram J, et al. Endotoxin-induced proteolytic reduction in hepatic growth hormone (GH) receptor: a novel mechanism for GH insensitivity. Mol Endocrinol. 2008;22:1427–1437. doi: 10.1210/me.2007-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]