Abstract
Background
As drug abuse and addiction have been shown to decrease adherence to treatment of hepatitis C virus (HCV) or HIV, screening for substance use should be standard clinical practice in those undergoing an evaluation for these diseases.
Aims
To assess the effectiveness of the Kreek-McHugh-Schluger-Kellogg (KMSK) scale to quantify substance use and dependence among patients with viral hepatitis.
Methods
The KMSK scale, a validated instrument that quantifies lifetime use of alcohol, cocaine, heroin, and tobacco, was distributed to 161 consecutive patients referred to a hepatology clinic at an academic, tertiary-care center over a one-year period.
Results
Of the 159 patients who returned the KMSK scale, 62% reported illicit drug use and 30% met defined criteria for lifetime dependence on cocaine or heroin. We found that 15% of our population at some time had been co-dependent on cocaine and heroin. The KMSK scale identified significantly more cocaine, heroin, and alcohol use than that detected through the medical record (χ2=7.61, p<0.01, χ2=9.66, p<0.002, respectively). Cocaine dependence was significantly higher among HCV/HIV co-infected than among mono-infected patients (χ2=5.46, p<0.02).
Conclusions
The KMSK scale may be useful to diagnose drug and alcohol use and dependence among patients undergoing evaluation for treatment of viral hepatitis.
Keywords: Cocaine, co-dependency, hepatitis C/HIV co-infection, heroin, self-administered scales
Introduction
An estimated 170 million people worldwide, including at least 3 million Americans, have chronic hepatitis C virus (HCV) infection [1, 2]. The most common risk factor for HCV is injection drug use, followed by sexual transmission, surgery, and percutaneous injury [3]. Non-injection drug use has also been implicated as an important risk factor in HCV transmission [4].
Although recent guidelines have encouraged HCV treatment in drug users [5, 6], several studies have indicated that only 1 to 6% of participants from cohorts of drug users receive antiviral therapy [7, 8]. Historically, adherence concerns, particularly among active injection drug users (IDUs), have been one of the principal reasons for physician reluctance to prescribe HCV treatment in this population. HCV treatment efficacy in patients on methadone maintenance, however, is similar to those without a history of drug abuse [9, 10]. Treatment of opiate addiction with agonist pharmacotherapy, methadone or buprenorphine, mitigates drug seeking behavior and may improve HCV treatment adherence. Similarly, counseling for cocaine or alcohol dependence can decrease the use of these drugs and may lead to similar improvements in treatment adherence as those observed by individuals on pharmacotherapy for opiate dependence. Treatment of addiction and HCV or HIV concomitantly may reinforce each other as they may medically and psychosocially stabilize the patient and facilitate social support. For these reasons, accurate quantification of drug and alcohol abuse history may be important for guiding treatment for HCV infection.
Since illicit drug use is a sensitive and stigmatized topic, patients may be reluctant to disclose prior illicit drug use to healthcare providers. Patients suffering from drug or alcohol addiction are more likely to report feelings of stigma than those with other psychiatric diagnoses [11]. Similarly, aside from those directly involved in the treatment of addiction, many generalists do not approach the topic of drug or alcohol addiction with their patients and are reluctant to refer illicit drug or alcohol users for treatment of addiction [12–14]. In addition, several studies have demonstrated low rates of HCV referral, evaluation and treatment among HCV-infected drug users [7, 8, 15].
Three representative surveys of generalists from the United States found that although many physicians inquire about drug and alcohol use, screening is inadequate in terms of consistency, depth, and follow up [12–14]. In contrast, during the 1991 National Health Interview Study, only 39% and 23% of patients report screening for alcohol and drug use, respectively, during their most recent primary care visit [16]. In clinical practice, screening conventionally consists of direct physician questioning, which has been shown in at least one study to have a positive predictive value of only 52% [17]. In addition, severely constrained appointment duration and competing medical priorities have hampered physicians’ ability to inquire into these areas. As an alternative to direct physician questioning, brief, self-administered, standardized questionnaires that can be scanned into the electronic medical record for physician review during the medical encounter may be a reasonable alternative. Such procedures may reduce physicians’ time while improving the accuracy of the data obtained.
The Kreek-McHugh-Schluger-Kellogg (KMSK) scale quantifies lifetime exposure to alcohol, cocaine, opiates, and tobacco. As designed, the instrument is typically completed in less than five minutes when administered by a trained interviewer. The scale has been validated against the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [18], with very high sensitivity and specificity for opiates (100%, 99% respectively) and cocaine (97%, 94% respectively) [19]. These findings support the use of the KMSK scale when a history of drug abuse or dependency is suspected and its identification is clinically relevant, such as in the assessment of treatment-readiness among patients with HCV infection who are being evaluated for treatment with interferon-based therapy.
As psychiatric disorders and drug addiction have been regarded as factors that negatively impact adherence to treatment for HCV, their assessment as part of HCV evaluation is critically important. Additionally, as alcohol has a synergistic effect with HCV on hepatic fibrosis progression, its assessment is particularly important in patients with viral hepatitis [20]. Assessment of depression, through the use of instruments, such as the Beck Depression Inventory [21], is frequently included as part of the HCV evaluation prior to initiation of pegylated interferon and ribavirin. In contrast, although drug and alcohol addiction have similarly been shown to have a negative effect on treatment adherence [22] and disease progression, their assessment is not presently part of the routine evaluation to determine treatment readiness. We hypothesized that the KMSK scale, when self-administered, would identify more patients with a history of drug use in comparison to that noted in the medical record. In addition, the KMSK scale systematically differentiates between drug use and dependence, procedures that are facilitated by a standardized questionnaire that could be easily utilized as part of routine patient management. To assess the potential usefulness of the KMSK scale as a self-administered instrument to identify patients with a lifetime history of addiction, the KMSK scale was distributed to all consecutive patients evaluated in a hepatology clinic during a one-year period.
Materials and Methods
Study subjects
During a one-year period, we systematically assessed the prevalence of drug use and dependence by distributing KMSK scales to all patients in the Hepatology Clinic at Weill Cornell Medical College. The clinic receives referrals from the entire New York City Metropolitan area and serves an ethnically diverse population. The scale was systematically distributed to all patients by the receptionist upon registration. In the majority of cases, patients were able to complete the scale while awaiting their appointment with the physician. If not, patients were permitted to complete it after their examination. If a patient did not complete the KMSK scale during the initial administration, he/she was given the opportunity to complete the scale on subsequent visits. As we desired to minimize input by health care personnel in the administration of the questionnaire, we did not assess patients’ reasons for failing to complete the KMSK. At the end of the clinic encounter, the scale was returned to the receptionist, was subsequently reviewed by the physician, and scanned into the electronic medical record (Epic, Verona, WI, USA) for subsequent patient management. The electronic medical record at our institution has essentially supplanted paper records; medical records preceding implementation of the electronic medical record have been scanned into and are accessible from the electronic medical record.
The study was performed consistent with the ethical guidelines of the 1975 Helsinki Declaration (6th revision, 2008) as reflected in a priori approval by the Weill Cornell Medical College Institutional Review Board. At the end of the study period, all retrievable KMSK scales were obtained from the electronic medical record and were reviewed by study personnel for completion. Patients without a record of having been evaluated by the physician (n = 1) or for whom the medical record could not be located (n = 1) were excluded from the analysis.
All physician notes in the electronic medical record written on or before the date of KMSK administration were reviewed by the investigators for specific mention of lifetime cocaine or heroin use, as well as information about the route of administration. Notes in the electronic medical record were also reviewed for demographic, medical, disease status, and laboratory parameters. All laboratory measurements, including HCV RNA, HCV genotype, HIV RNA, lymphocyte subsets, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were performed at New York-Presbyterian Hospital (NYPH) or reference laboratories contractually associated with the hospital. In subjects who had a liver biopsy, necroinflammation and fibrosis were assessed by NYPH staff pathologists using the Scheuer system [23]. In five (3%) subjects, grade and stage were derived from a noninvasive index of hepatic histology (Fibrosure, Laboratory Corporation of America, Research Triangle Park, NC, USA). In seven (4%) subjects, a diagnosis of cirrhosis was made based upon clinical or laboratory evidence of hepatic decompensation.
KMSK scale description and scoring
The KMSK scale assesses lifetime drug use of four separate substances – alcohol, cocaine, heroin and tobacco - based upon responses to three separate questions. Only one version of the scale has been validated [11]. Since its initial validation, sections assessing the use of other illicit drugs and a scale assessing use of each drug during the past 30 days have been added. As the goal of this study was to assess the prevalence of use and dependence among patients seeking treatment for viral hepatitis, we decided to use the validated form of the KMSK that assesses lifetime use.
A total score for each substance is calculated based upon the sum of responses to three individual questions: duration of use, frequency of use and amount used. If a patient fails to respond to all three questions in a section, drug use cannot be quantified and thus, dependence cannot be calculated. A score of 11 or more indicates alcohol or cocaine dependence, and a score of 9 or more indicates heroin dependence [19]. KMSK scale determinations have not been established for tobacco dependence as criteria for tobacco dependence do not exist in the most recent version of the DSM- IV [18, 19]. A section was considered complete if the patient recorded an answer for all three questions in that section. The KMSK scale as a whole was considered complete if all four sections were completed.
For 14 patients, more than one KMSK scale was obtained. When a discrepancy existed between the two administrations (six for alcohol, seven for cocaine, and six for heroin), the highest score was used. As we believe that patients had minimal incentive to exaggerate their drug use history and many reasons to minimize it, we determined that the highest score was likely to be the most accurate representation of the patients’ history.
Statistical analysis
Descriptive statistics were calculated for all demographic variables. To evaluate the possible differences in self-reported drug use found in the KMSK or in the patients’ medical records, Chi-square analysis was used for each illicit substance: cocaine and heroin. We investigated the relationship between self-reported drug use (as reflected in each subscale and in total scale scores for alcohol, cocaine, and heroin) and virologic and histologic measures using Pearson correlation. Statistical analysis was performed using Prism Software (Version 5.0, GraphPad Software, Inc, La Jolla, CA, USA), and the level of significance was set at α = 0.05.
Results
KMSK scale completion
To determine the prevalence of addiction in a referral hepatology practice, we distributed KMSK scales to 161 patients over a one-year period. Of the 159 eligible patients to whom KMSK scales were distributed, 145 (91%) patients answered at least one question. As illustrated in Table 1a, 133 (84%) completed the alcohol section, 113 (71%) completed the cocaine section, 108 (68%) completed the heroin section, and 131 (82%) completed the tobacco section. Overall, a total of 120 (75%) patients completed at least three sections of the instrument. Table 1b illustrates the total number of sections completed per patient.
Table 1.
| Table 1a. Number of participants that completed each of the four subsets of the Kreek-McHugh-Schluger-Kellogg instrument by the 159 subjects included in this study. | ||||
|---|---|---|---|---|
| Alcohol | Cocaine | Heroin | Tobacco | |
| Complete | 133 (84%) | 113 (71%) | 108 (68%) | 131 (82%) |
| Partially Complete | 10 (6%) | 18 (11%) | 17 (11%) | 7 (5%) |
| No information entered | 16 (10%) | 28 (18%) | 34 (21%) | 21 (13%) |
| Table 1b. Number of subsets of the Kreek-McHugh-Schluger-Kellogg completed by participants. | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Subsets of KMSK completed N (%)(n=159) | 18 (11%) | 1 (1%) | 21 (13%) | 34 (21%) | 85 (53%) |
Information provided by the KMSK scale in comparison with the medical record for a diagnosis of illicit drug use
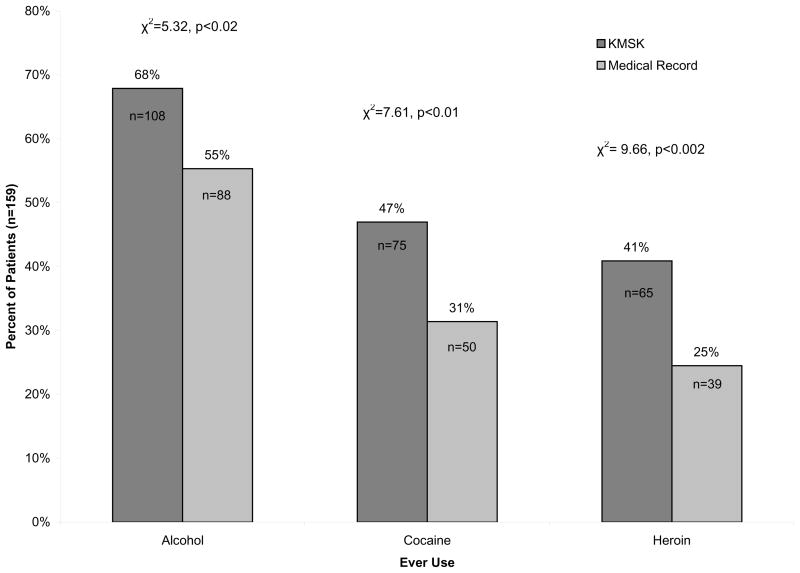
Figure 1 shows a comparison of the percentage of individuals recording a history of drug use as indicated by the KMSK scale in comparison with the medical record. We observed that the KMSK scale identified significantly more patients that used cocaine, heroin, and alcohol than was indicated in the medical record (χ2=5.32, p<0.02, χ2=7.61, p<0.01 & χ2=9.66, p<0.002, respectively).
Figure 1.
Lifetime drug use ascertained by the Kreek-McHugh-Schluger-Kellogg (KMSK) or extracted from the medical record. The use of the KMSK scale identified lifetime alcohol, cocaine, and heroin use among a significantly increased percentage of individuals as compared with the medical record (χ2=5.32, p<0.02, χ2=7.61, p<0.01 & χ2=9.66, p<0.002, respectively).
Demographic, virologic and histologic characteristics
Table 2 contains the demographic, virologic and histologic characteristics of the sample. The median age of the patients in this study was 48 (range: 19–67) years. A total of 141 (89%) patients were HCV antibody positive: 58 of whom were infected only with HCV, 77 were HCV/HIV co-infected, 1 was HCV/HBV co-infected and 5 were HCV/HIV/HBV co-infected. Of the 18 patients without HCV, 2 were HIV-infected, 2 were HBV-infected, 7 were HIV/HBV co-infected and 7 did not have a viral infection.
Table 2.
Demographic Characteristics
| Parameter | Values | |
|---|---|---|
| Age (years) (n=159) | Median (range) | 48 (19–67) |
| > 40 | N (%) | 126 (79%) |
| ≤ 40 | 33 (21%) | |
| Gender, N (%) (n=159) | Male | 99 (62 %) |
| Female | 60 (38 %) | |
| Infection Status, N (%) (n=159) | HCV infected | 58 (36%) |
| HCV/HIV co-infected | 77 (48%) | |
| HCV/HBV co-infected | 1 (1%) | |
| HCV/HIV/HBV co-infected | 5 (3%) | |
| HIV infected | 2 (1%) | |
| HIV/HBV infected | 7 (4%) | |
| HBV infected | 2 (1%) | |
| No Infection | 7 (4%) | |
| HCV antibody, N (%) (n=159) | Positive | 141 (89 %) |
| Negative | 17 (11%) | |
| Not determined | 1 (1 %) | |
| HCV RNA (IU/ml) | Median | 1,575,518 |
| ≤ 1,000,000 | N (%) (n=141) | 62 (44%) |
| >1,000,000 | 76 (54%) | |
| Not determined | 3 (2%) | |
| HCV Genotype 1 | N (%) (n=141) | 112 (79%) |
| Non-1 | 16 (11%) | |
| Not determined | 13 (9%) | |
| HIV Positive, N (%) (n=159) | Positive | 91 (57%) |
| Negative | 68 (43%) | |
| HIV RNA, N (%) (n=91) | Detectable | 39 (43%) |
| Undetectable | 51 (56%) | |
| Not Found | 1 (1%) | |
| CD4+ (cells/ml) (n=92) | Median (range) | 457 (24–1092) |
| ≤ 200 | N (%) (n=92) | 14 (15%) |
| 200–500 | 43 (47%) | |
| ≥ 500 | 35 (38%) | |
| HBsAg, N (%)(n=159) | Positive | 15 (9%) |
| Negative | 129 (81%) | |
| Undetermined | 15 (9%) | |
| Hepatic Fibrosis Stagea N (%) (n=159) | Advanced (≥ 2) | 93 (58%) |
| Mild (< 2) | 30 (19%) | |
| None (0) | 3 (2%) | |
| Not determined | 33 (21%) | |
| Inflammatory Gradea N (%) (n=159) | Severe (≥ 2) | 84 (53%) |
| Mild (< 2) | 31 (19%) | |
| Not determined | 44 (28%) | |
Stage and grade assessed using the Scheuer system [23]
Abbreviations: HCV, hepatitis C virus; HBV, hepatitis B virus; HBsAg, hepatitis B virus surface antigen; HIV, human immunodeficiency virus; IU, international units.
A total of 91 patients (57%) were HIV-infected. Among these individuals, 15 (16%) had <200 CD4+ T-cells/mm3; 39 (43%) had undetectable HIV RNA. Of the 126 patients who underwent liver biopsy, 93 (73%) had severe hepatic fibrosis (stage ≥ 2) and 84 (67%) had severe hepatic inflammation (grade ≥ 2).
With regard to hepatitis B virus (HBV) infection, 15 (9%) of the patients were hepatitis B surface antigen positive, 129 (81%) were negative, and 15 (9%) were of unknown status. Eighty-two (52%) individuals were positive for HBV core antibody, 46 (29%) were negative and 31 (19%) were undetermined. Thirty-five patients were HBV core antibody positive and negative for both HBV surface antigen and antibody, suggesting possible occult HBV infection [24].
Prevalence of abuse and dependence as assessed by the KMSK
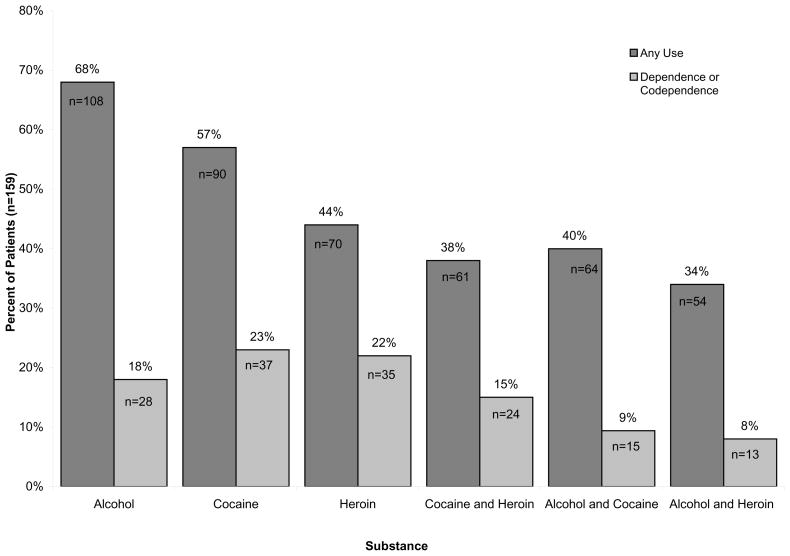
Of the four substances assessed by the KMSK scale, alcohol use was most prevalent with 108 (68%) reporting ever having used and 28 (18%) reporting dependence, as shown in Figure 2. A total of 99 (62%) patients reported either cocaine or heroin use of whom 48 (30%) met dependence criteria for either or both drugs. When each illicit substance was assessed individually, more patients reported cocaine use while dependence among the two drugs was comparable (90 [57%] versus 70 [44%] for use; 37 [23%] versus 35 [22%] for dependence, respectively). A total of 61 (38%) subjects reported use of both cocaine and heroin and 24 (15%) individuals were co-dependent on both cocaine and heroin. For tobacco, 105 (66%) reported prior usage. Tobacco dependence was not assessed as dependence criteria have not been published in the DSM-IV [18, 19].
Figure 2.
Characterization of drug dependence. The percentage of all subjects (n=159) who met criteria for use or dependence on each of the three domains of the Kreek-McHugh-Schluger-Kellogg (KMSK) scale. “Alcohol and Cocaine” indicates the total number of unique individuals who reported any use or co-dependence to both alcohol and cocaine. “Cocaine or Heroin” indicates the total number of unique individuals who reported use or dependence to either or both substances. “Cocaine and Heroin” indicate the percentage of individuals who reported use of or who were co-dependent on heroin and cocaine. As DSM-IV criteria for tobacco dependence do not exist, the KMSK could not be validated for tobacco dependence; thus, the percentage of individuals using tobacco is not illustrated.
Association between illicit drug use and dependence and other covariates
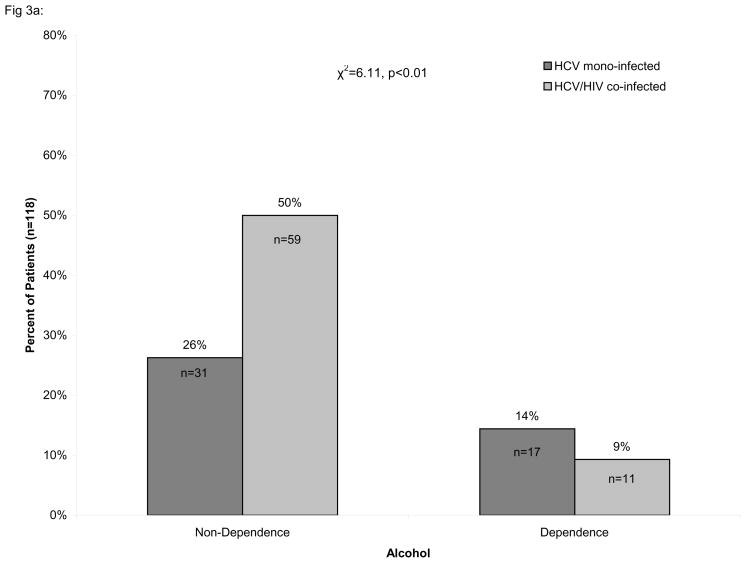
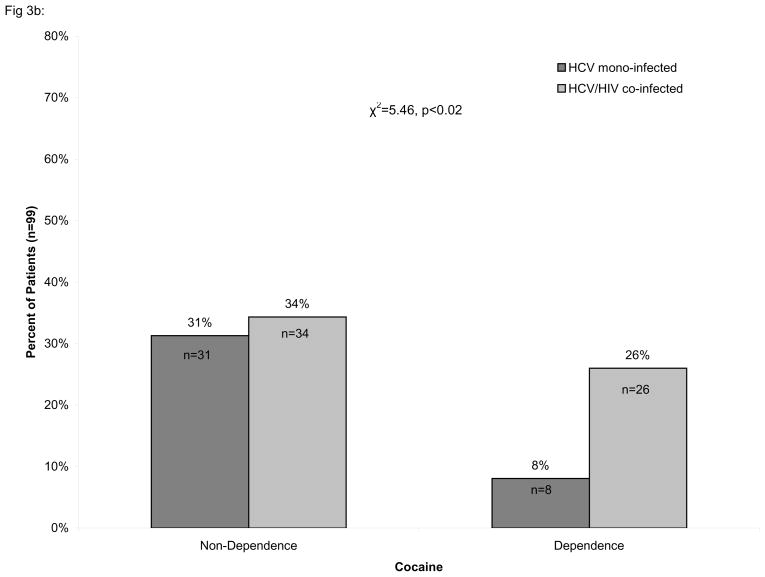
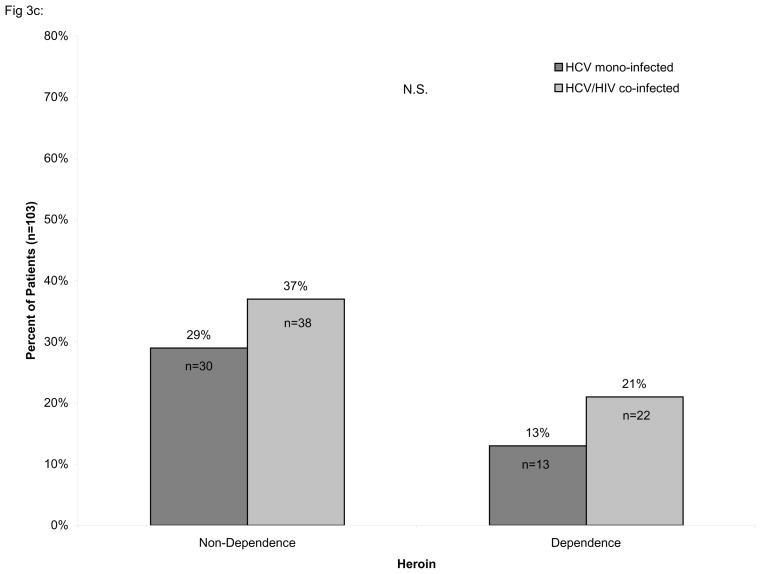
Consistent with early work from our laboratory [25], we found that cocaine dependence was significantly more prevalent amongst HIV/HCV co-infected individuals than in HCV mono-infected individuals (χ2=5.46, p<0.02) while the prevalence of heroin dependence did not differ significantly between those with HCV mono-infection and HIV/HCV co-infection, as shown in Figure 3. In contrast, alcohol dependence was significantly less prevalent amongst HIV/HCV co-infected individuals than in HCV mono-infected (χ2=6.11, p<0.01).
Figure 3.
Percentage of HIV/HCV co-infected individuals with or without dependence. The percentage of alcohol (A), cocaine (B), and heroin (C) dependent and non-dependent individuals is stratified by hepatitis C virus (HCV) infection and HIV/HCV co-infection. The number of alcohol dependent individuals was found to be significantly lower among HCV/HIV co-infected individuals (χ2=6.11, p<0.01) while the number of cocaine dependent individuals was significantly greater among HIV/HCV co-infected individuals than in HCV mono-infected individuals (χ2=5.46, p<0.02). No association was found for heroin dependent individuals. Only individuals with complete information on the Kreek-McHugh-Schluger-Kellogg (KMSK) scale are shown in the figure.
We next investigated the association between scores on each substance assessed by the KMSK scale and virologic and histologic factors. A higher score on the alcohol section of the KMSK scale was weakly associated with a higher level of HCV RNA (r=0.214, p<0.02). A similar association was found for each individual component of the questions pertaining to alcohol with the strongest association found for the question of duration of usage (r=0.233, p<0.01).
Similar analyses were performed for cocaine and heroin use and dependence. An elevated score on the heroin section of the KMSK was weakly associated with higher CD4+ cell counts (r=0.229, p<0.01). A similar association was found for each individual question pertaining to heroin usage with the strongest association found in the amount (r=0.363, p<0.005). A weak association was also observed between heroin use and stage of liver fibrosis (r=0.24, p<0.05).
Discussion
In this study, we assessed the ability of the KMSK scale, when self-administered, to assess the prevalence of lifetime drug use and dependence in a large number of patients with viral hepatitis. The use of a standardized questionnaire identified prior or current drug use in a significantly higher percentage of our patients than that recorded by health care professionals in the medical record. We found that approximately two-thirds of patients reported prior alcohol and tobacco use. The finding that one-third of individuals reported lifetime dependence to heroin or cocaine and that one-sixth were co-dependent on both substances, is quite important because of the adverse effect that drug use has on adherence to anti-HCV medication. The finding that approximately one-fifth of patients reported prior alcohol dependence is also significant because alcohol has been shown to synergize with HCV as a promoter of fibrogenesis, to reduce the sensitivity to interferon, and to decrease adherence to anti-viral therapy [20]. The finding of such a high level of dependence in a hepatology clinic is a surprise; heretofore, addiction has not been identified to this extent as a concomitant disease, which is often deserving of treatment. These results argue for active screening for addiction among patients with viral hepatitis.
Integration of HCV and HIV treatment with addiction management is important as each can reinforce compliance with the other. As approximately three-fourths of our patients had advanced hepatic fibrosis, HCV treatment was strongly indicated in the majority of our study subjects. Active screening for addiction among HCV-infected patients under therapy should be pursued as active drug use may compromise compliance with the therapeutic regimen, and treatment of addiction might increase the efficacy of antiviral agents. Opiate replacement therapy, methadone or buprenorphine, has been shown to prevent “relapse” in rodent models [26, 27] and humans [28]. Furthermore, it has been shown to normalize the hypothalamus-pituitary-adrenal axis in humans [29]. Although effective pharmacologic therapy does not presently exist for cocaine addiction, abstinence-based programs are recommended [30]. When the addicted patient is stabilized, HCV treatment efficacy has been shown to be equivalent to that of a nonaddicted population [31, 32]. In contrast, those who relapse to active drug use demonstrate significantly lower levels of adherence [33], which potentially could compromise treatment efficacy [34].
Assessment of illicit drug use conventionally relies on direct patient questioning during a medical evaluation, a process that may be inaccurate because health care providers may be reluctant to inquire about drug use and because patients may be hesitant to discuss this topic. Alternatively, drug use assessment via a standardized questionnaire, such as the KMSK scale, has several advantages. As we illustrate, the scale can detect drug use in significantly more individuals than were detected through the medical record. The scale could be completed by patients while awaiting their clinic appointment and subsequently scanned into the electronic medical record. The medical record provides a permanent repository that permits instantaneous information retrieval and restricts access to maintain patient confidentiality.
Self-administration of the KMSK scale has several limitations and advantages. As the scale was originally designed and validated to be administered during an interview, the validity and reproducibility of patients’ responses and cutoff scores for a diagnosis of dependence when the instrument is self-administered have not been determined. Additional limitations include the availability of the KMSK scale in other languages, i.e., only Hebrew and Spanish, failure to distribute or to collect the scale by the clinic staff, or incomplete or partial responses to individual items. While the majority of our sample (53%) completed all four sections of the KMSK scale, a significant minority (10%) did not complete any section. The completion rate for each KMSK section may also reflect the degree of stigma attached to the use of a particular substance. As we observed, for example, the completion rate for alcohol and tobacco was markedly higher than for the illicit drugs, cocaine and heroin. This suggests that part of the explanation for the incomplete KMSK scales that we received might result from an individual’s fear of stigmatization because of the prior use of illicit substances. Advantages of self-administration include potential distribution to large numbers of patients in diverse venues, such as community-based clinics or sexually transmitted diseases clinics, where the scale could be utilized as a surveillance instrument to identify those at high-risk for HCV infection.
While we used the shorter, validated lifetime version of the KMSK scale in this study, other forms of the scale have been developed, and their clinical relevance should be assessed in future studies. In addition to lifetime use, subsequent iterations of the scale assess heroin, cocaine, alcohol and tobacco use and dependence in the past 30 days, and sections covering illicit benzodiazepene, amphetamine, and marijuana use and dependence have been added. Separate sections of the scale can be administered individually tailored to a specific purpose. For example, assessment of alcohol dependence in a hepatology clinic may afford an opportunity to discuss the detrimental effects of alcohol on hepatic fibrogenesis. Although assessment of tobacco use may be more frequently assessed in a drug treatment facility than in a hepatology practice, counseling by any health care provider about the dangers of tobacco use may augment cessation [35]. Selecting the sections of the KMSK scale most relevant to the clinical venue and the disease under evaluation will streamline its administration while maximizing the utility of the information gathered for the clinician or the researcher.
We also investigated the relationship between the use of specific drugs and demographic, virologic, and patient-derived variables. We found significant associations between cocaine use and HIV/HCV co-infection, alcohol use and elevated HCV RNA levels, heroin use and increased CD4+ cell counts, and heroin use and higher stages of hepatic fibrosis.
Investigation of the epidemiology of drug use-related infectious diseases may identify high-risk populations that could benefit from targeted screening. The association we observed between cocaine dependence and HIV/HCV co-infection suggests that noninjection drug use may be an important route of HIV transmission [25] and that cocaine users should be screened for these infections. Although injection drug use has conventionally been the more important route of HCV transmission [3], a higher percentage of HCV-infected patients reported cocaine use, suggesting that this route of self-administration could be important for HCV transmission. These drugs can also have pleiotropic effects on the immune system that may underlie the associations described in the present work. Since the early days of the AIDS epidemic, heroin use in the absence of HIV-1 infection has been known to be associated with increased number of T-cells, including CD4+ cell counts [36], along with reduced numbers and function of natural killer cells. Additionally, the immunosuppressive effect of alcohol may lead to diminished immune control of viral replication resulting in significantly higher HCV RNA levels as we observed in this study [37, 38].
In summary, we have demonstrated that a standardized scale identifies a high percentage of patients with a prior history of cocaine and heroin use attending a hepatology clinic. Furthermore, the KMSK scale identified an unexpectedly high percentage of patients with dependence to cocaine or heroin and co-dependence to both substances. Given the high prevalence of use and dependence to licit and illicit drugs, routine screening for addiction should be integrated into the clinical management of patients with viral hepatitis.
Supplementary Material
Acknowledgments
We acknowledge the contribution of the office and clinical staff of the Hepatology Clinic at Weill Cornell-New York Hospital Medical Center and Annette Ocasio, Hepatology Practice Administrator, for assistance with the administration of the KMSK instrument. We also acknowledge Alain Litwin, MD, MPH for helpful discussions.
Declaration of Funding Interests
This study was funded in part by the Greenberg Medical Research Foundation (AHT), by a commitment made at the Clinton Global Initiatives (AHT), the Adelson Medical Research Foundation (MJK), and National Institutes of Health, NIDA P60DA05130 (to MJK), and NIAID K23 AI065319 (KMM). No commercial support was provided for the conduct of this study, data analysis, or writing of the manuscript.
Footnotes
Presentation: A preliminary version of this work was presented at the 71st annual meeting of the College on the Problems of Drug Dependence; 2009 June 20–25, Reno, NV; abstract # 255.
Conflict of Interest
No conflicts of interest exist between any of the authors and the sources of funding for this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. [accessed August 15, 2009];Hepatitis C. Fact Sheet No. 164. 2000 Available at: http://www.who.int/mediacentre/factsheets/fs164/en/
- 2.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wasley A, Miller JT, Finelli L. Surveillance for acute viral hepatitis--United States, 2005. MMWR Surveill Summ. 2007;56:1–24. [PubMed] [Google Scholar]
- 4.Martinez A, Talal AH. Noninjection drug use: an under-appreciated risk factor for hepatitis C virus transmission. Liver Int. 2008;28:757–760. doi: 10.1111/j.1478-3231.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 5.Kreek MJ, Talal AH, Piccolo P. Treating chronic hepatitis C in recovering opiate addicts: yes, we can. Dig Liver Dis. 2009;41:308–310. doi: 10.1016/j.dld.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16:352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylvestre DL. Treating hepatitis C in methadone maintenance patients: an interim analysis. Drug Alcohol Depend. 2002;67:117–123. doi: 10.1016/s0376-8716(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37:32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link BG, Struening EL, Rahav M, et al. On stigma and its consequences: evidence from a longitudinal study of men with dual diagnoses of mental illness and substance abuse. J Health Soc Behav. 1997;38:177–190. [PubMed] [Google Scholar]
- 12.Friedmann PD, McCullough D, Saitz R. Screening and intervention for illicit drug abuse: a national survey of primary care physicians and psychiatrists. Arch Intern Med. 2001;161:248–251. doi: 10.1001/archinte.161.2.248. [DOI] [PubMed] [Google Scholar]
- 13.Millstein SG, Marcell AV. Screening and counseling for adolescent alcohol use among primary care physicians in the United States. Pediatrics. 2003;111:114–122. doi: 10.1542/peds.111.1.114. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico EJ, Paddock SM, Burnam A, et al. Identification of and guidance for problem drinking by general medical providers: results from a national survey. Med Care. 2005;43:229–236. doi: 10.1097/00005650-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Fishbein DA, Lo Y, Reinus JF, et al. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr. 2004;37:1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- 16.Deitz D, Rohde F. Prevalence of screening for alcohol use by physicians during routine physical examinations. Alcohol Health & Research World. 1994;18:162–168. [PMC free article] [PubMed] [Google Scholar]
- 17.Brown RL, Leonard T, Saunders LA, et al. A two-item conjoint screen for alcohol and other drug problems. J Am Board Fam Pract. 2001;14:95–106. [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. revised ed. [Google Scholar]
- 19.Kellogg SH, McHugh PF, Bell K, et al. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- 20.Gitto S, Micco L, Conti F, et al. Alcohol and viral hepatitis: a mini-review. Dig Liver Dis. 2009;41:67–70. doi: 10.1016/j.dld.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. Beck depression inventory manual. 2. New York: Psychological Corp; 1996. [Google Scholar]
- 22.Dore GJ, Hellard M, Matthews G, et al. Effective treatment of injecting drug users with recently acquired Hepatitis C virus infection. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 24.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 25.Novick DM, Trigg HL, Des Jarlais DC, et al. Cocaine injection and ethnicity in parenteral drug users during the early years of the human immunodeficiency virus (HIV) epidemic in New York City. J Med Virol. 1989;29:181–185. doi: 10.1002/jmv.1890290307. [DOI] [PubMed] [Google Scholar]
- 26.Leri F, Tremblay A, Sorge RE, et al. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- 27.Kosten TR, Rounsaville BJ, Kleber HD. A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry. 1986;43:733–738. doi: 10.1001/archpsyc.1986.01800080019003. [DOI] [PubMed] [Google Scholar]
- 28.Leri F, Zhou Y, Goddard B, et al. Steady-state methadone blocks cocaine seeking and cocaine-induced gene expression alterations in the rat brain. Eur Neuropsychopharmacol. 2009;19:238–249. doi: 10.1016/j.euroneuro.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schluger JH, Borg L, Ho A, et al. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- 30.National Institute of Drug Abuse. Principles of drug addiction treatment: A research-based guide. 2. Bethesda: NIH publication No 09-4180; 2009. [Google Scholar]
- 31.Backmund M, Meyer K, Von Zielonka M, et al. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer M, Heinz A, Backmund M. Treatment of chronic hepatitis C in patients with drug dependence: time to change the rules? Addiction. 2004;99:1167–1175. doi: 10.1111/j.1360-0443.2004.00821.x. [DOI] [PubMed] [Google Scholar]
- 33.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- 34.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 35.West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Health Education Authority Thorax. 2000;55:987–999. doi: 10.1136/thorax.55.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novick DM, Ochshorn M, Ghali V, et al. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J Pharmacol Exp Ther. 1989;250:606–610. [PubMed] [Google Scholar]
- 37.Ochshorn-Adelson M, Bodner G, Toraker P, et al. Effects of ethanol on human natural killer cell activity: in vitro and acute, low-dose in vivo studies. Alcohol Clin Exp Res. 1994;18:1361–1367. doi: 10.1111/j.1530-0277.1994.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 38.Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.