Abstract
Background & Aims
Visceral adipose tissue (VAT) is an important risk factor for the metabolic complications associated with obesity. Therefore, a reduction in VAT is considered an important target of obesity therapy. Therefore, we evaluated whether reducing VAT mass by surgical removal of the omentum, improves insulin sensitivity and metabolic function in obese patients.
Methods
We conducted: 1) a 12-month randomized controlled trial to determine whether reduction in VAT by omentectomy in 22 obese subjects enhances Roux-en-Y gastric bypass (RYGB) surgery-induced improvement in hepatic and skeletal muscle insulin sensitivity, assessed by using the hyperinsulinemic-euglycemic clamp technique, and 2) a 3-month longitudinal single-arm study to determine whether laparoscopic omentectomy alone in 7 obese subjects with type 2 diabetes mellitus (T2DM) improves insulin sensitivity, assessed by using the Frequently Sampled Intravenous Glucose Tolerance Test.
Results
The greater omentum, weighing 0.82 kg (95% CI: 0.67–0.97) was removed from subjects who had omentectomy in both studies. In study 1, muscle insulin sensitivity (relative increase in glucose disposal during insulin infusion) approximately doubled and hepatic insulin sensitivity increased by ~4-fold at 12 months after RYGB alone and RYGB plus omentectomy compared with baseline (P<0.001). There were no significant differences between groups (P>0.87) or group×time interactions (P>0.36). In study 2, surgery had no effect on insulin sensitivity (P=0.844) and use of diabetes medications.
Conclusions
These results demonstrate that decreasing VAT through omentectomy, whether performed alone or in combination with RYGB surgery, does not improve metabolic function in obese patients.
Keywords: intra-abdominal fat, portal hypothesis, insulin resistance, omentectomy
INTRODUCTION
Increased visceral adipose tissue (VAT) is an important risk factor for many of the metabolic complications of obesity, including insulin resistance, dyslipidemia, non-alcoholic fatty liver disease, type 2 diabetes (T2DM) and cardiovascular disease.1–4 These observations and the anatomical location of VAT, which releases free fatty acids (FFA) and adipokines directly into the portal vein for delivery to the liver, have led to the concept that VAT is a primary contributor to the metabolic abnormalities associated with obesity, particularly insulin resistance.5,6 Therefore, a reduction in VAT has become a key therapeutic goal in the management of obese patients.6,7 However, the association between VAT and insulin resistance does not prove causality, and it is possible that the factors responsible for obesity-related metabolic dysfunction also cause VAT accumulation.8
Surgical removal of the omentum provides an opportunity to directly evaluate the importance of VAT in the pathogenesis of insulin resistance. VAT is predominantly comprised of omental and mesenteric fat. Omental fat has the same or even greater potential for inducing metabolic dysfunction than mesenteric fat. Lipolytic activity is the same or greater in omental than mesenteric adipocytes9,10 and omental fat is more resistant to insulin-mediated suppression of lipolysis and has greater gene expression of most pro-inflammatory adipokines than mesenteric and subcutaneous fat.11,12 Data from studies conducted in animal models indicate that VAT resection improves insulin sensitivity.13,14 However, two studies conducted in obese patients undergoing bariatric surgery report contradictory results on the effect of omentectomy on insulin action.15,16 The reason for the differences between studies could be due to the confounding effect of greater weight loss in patients who had omentectomy in one study,15 or insensitive measures of insulin action in the other.16
The purpose of the present study was to determine whether surgical reduction in VAT through omentectomy has therapeutic metabolic effects in obese persons. Accordingly, we conducted: 1) a randomized controlled trial to test the hypothesis that removal of omental fat in obese subjects, in conjunction with Roux-en-Y gastric bypass surgery (RYGB), will cause a greater improvement in hepatic and skeletal muscle insulin sensitivity than RYGB surgery alone, and 2) a longitudinal single-arm study to test the hypothesis that removal of omental fat alone in obese subjects with T2DM will improve insulin sensitivity, β-cell function and glucose homeostasis.
METHODS
Participants
Obese men and women between 18 and 60 years old, with or without T2DM, with a body mass index (BMI) ≥40 kg/m2 or ≥35 kg/m2 plus a co-morbid complication (study 1) or between 30 and 50 kg/m2 and T2DM (study 2), were recruited from the Center for Surgical Weight Loss and the Endocrinology clinics at the Vanderbilt University Medical Center (VUMC), to participate in one of two study protocols conducted between March, 2005 and December, 2008. In both study protocols, the primary outcome was insulin sensitivity; secondary outcomes included cardiovascular risk factors and use of diabetes medications.
In study 1, 22 obese adults with a body mass index (BMI) between 39 and 69 kg/m2 were randomly assigned to undergo standard RYGB surgery (2 men and 9 women, 35–58 years old, 7 with T2DM, 9 Caucasian and 2 African-American) or RYGB surgery plus omentectomy (2 men and 9 women, 28–58 years old, 7 with T2DM, 9 Caucasian and 2 African-American), by using a computer-generated randomization code with a permuted block size of four (supplementary Figure 1). All recruited participants and researchers involved in the conduct of the study were blinded to treatment.
In study 2, 10 obese adults with T2DM were screened and agreed to have laparoscopic omentectomy. However, only 7 of these participants (2 men and 5 women, 37–54 years old, 6 Caucasian and 1 Hispanic, BMI between 32 and 43 kg/m2) maintained a stable body weight (<5% change) and completed the follow-up metabolic studies at 3 months after surgery; 2 subjects lost and 1 gained more than 5% of body weight and were excluded from the study because of the confounding effect of this weight change on insulin action.
All participants completed a comprehensive medical evaluation. None had any history or evidence of metabolic acidosis, positive pregnancy test, or took medications that can affect metabolism other than diabetes medications. Participants who had T2DM were being treated with oral hypoglycemic agents; none were taking insulin. All subjects provided written informed consent before participating in this study, which was approved by the Health Science Committee of the Institutional Review Board at VUMC (Nashville, TN) and the Human Research Protection Office of Washington University School of Medicine (St. Louis, MO).
Study protocol
Study 1: RYGB surgery with or without omentectomy
Body composition analyses and hyperinsulinemic-euglycemic clamp procedures with isotope tracer infusion during the basal stage were performed in each subject 10–14 days before RYGB surgery, and within 11 days of the 6-month and 12-month post-surgery time points. Participants taking oral diabetes medications were asked to stop taking their medications for 5 days before admission to the Clinical Research Center (CRC) on each of the three study occasions. Participants were admitted to the CRC in the evening before the study and given a standard meal. The following morning, after they fasted overnight, body composition was assessed by using dual-energy X-ray absorptiometry (GE Lunar Prodigy, Madison, WI). Total fat mass and fat-free mass were extrapolated from half-body scans17 in subjects who were wider than the scanning area. A catheter was then inserted into a forearm vein to infuse high-performance liquid chromatography (HPLC)-purified [3-3H]glucose (PerkinElmer, Waltham, MA), potassium, dextrose and insulin, and a second catheter was inserted into a contralateral superficial forearm or hand vein, which was heated to 55°C, by using a thermostatically controlled box, to obtain arterialized blood samples. A primed, continuous infusion of [3-3H]glucose (0.14 μCi/min; priming dose: 33 μCi), dissolved in 0.9% NaCl solution, was started and maintained for 2.5 h, until the end of the basal period to evaluate basal glucose kinetics and hepatic insulin sensitivity. Thereafter, an insulin infusion was started and continued for 2 hours to evaluate muscle insulin sensitivity. The rate of insulin infusion during the clamp procedure performed before surgery was 2 mU/kg body weight per min (initiated with a priming dose of 4 mU/kg body weight per min for 8 min). To account for the surgical weight loss-induced decrease in basal plasma insulin concentration and increase in insulin clearance,18 the rate of insulin infusion during the clamp procedures performed after surgery was empirically adjusted upwards in an effort to achieve similar total insulin infusion rates and plasma insulin concentrations in all studies, in order to evaluate the effect of surgery on insulin sensitivity independent of changes in insulin concentrations. Insulin was infused at a rate of 2.75 mU/kg body weight per min (initiated with a priming dose of 5.5 mU/kg body weight per min for 8 min) at 6 months and 3.1 mU/kg body weight per min (initiated with a priming dose of 6.2 mU/kg body weight per min for 8 min) at 12 months after surgery. Euglycemia (~95 mg/dL if fasting blood glucose was ≤110 mg/dL or ~110 mg/dL if fasting blood glucose was >110 mg/dL) was maintained by a variable infusion of 20% dextrose. Blood samples were obtained every 5 min during the final 30 min of the basal period to determine plasma glucose specific activity (SA), and every 15 min (3 samples) during the last 30 min of each stage of the clamp to determine plasma substrate and insulin concentrations.
After completing the first clamp procedure, subjects underwent laparoscopic RYGB surgery. The study coordinator notified the surgeon of the randomization assignment (omentectomy or no omentectomy) at the beginning of the procedure. A small (~25 mL) gastric pouch was created by using a linear cutting stapler. The vagus nerve trunks were preserved. A Roux-en-Y gastrojejunostomy with a 30–50 cm biliopancreatic limb and a 100–200 cm Roux limb was constructed.
Study 2: Omentectomy alone
A frequently sampled intravenous glucose tolerance test (FSIVGTT) was performed in each subject before and 3 months after laparoscopic omentectomy. Subjects were asked to stop all medications used to treat lipid disorders and diabetes for 1 month and 4 days before the study, respectively, on each of the two study occasions. Participants were admitted to the CRC in the evening before the study and given a standard meal. The following morning, after an overnight fast, an intravenous catheter was inserted into each antecubital vein, and a bolus of glucose (0.3 g/kg body weight) was administered over 1 min. At 20 min after the glucose bolus, a bolus of regular insulin (0.03 U/kg) was given over 5 min. Blood samples were obtained at 15, 10, 5, and 1 min before and at 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 25, 30, 50, 70, 100, 140 and 180 min after the glucose bolus. Immediately after the first FSIVGTT, subjects were transported to the hospital and underwent laparoscopic omentectomy.
Sample collection and analysis
Blood samples were collected in chilled tubes containing sodium ethylenediamine-tetra-acetate and placed on ice. Plasma was separated by centrifugation within 30 min of collection and stored at −80°C until final analyses were performed. Glucose was measured at bedside by using the glucose oxidase method (Beckman Glucose Analyzer, Fullerton, CA). Glycosylated hemoglobin was determined immediately after blood collection by using HPLC and the Variant II Hemoglobin Testing System (Bio-Rad Laboratories Diagnostic Group, Hercules, CA). Plasma concentrations of insulin and leptin were determined by radioimmunoassay (Millipore, St. Charles, MO). A multiplex immunoassay based on Luminex xMAP technology was used to measure plasma adiponectin (Millipore). C-reactive protein concentrations were determined by a high sensitivity enzyme-linked immunosorbent assay (Helica Biosystems, Fullerton, CA). FFA concentrations were quantified by using a colorimetric assay (Wako Chemicals, Richmond, VA). Serum total cholesterol, low-density lipoprotein and high-density lipoprotein cholesterol, and triglyceride concentrations were assayed immediately with ACE reagents and instrumentation (Alfa Wassermann, Caldwell, NJ). Plasma glucose SA ([3H]glucose/glucose) was determined by measuring glucose radioactivity in plasma on Symogyi filtrate (1:10 with 4.5% barium hydroxide and 4.5% zinc sulfate) after evaporation to remove radioactive water.
Calculations
The computerized, updated homeostasis model assessment, based on fasting plasma glucose and insulin concentrations and improved modeling algorithms, was used to provide an index of whole-body insulin resistance (HOMA2-IR).19
Study 1
Hepatic insulin sensitivity was assessed by using the Hepatic Insulin Sensitivity Index (HISI), as the inverse of the product of basal hepatic glucose production rate and fasting plasma insulin concentration, which has been used as a surrogate measure of insulin-mediated suppression of endogenous glucose production (EGP) during low-dose insulin infusion.20 Steady-states were achieved during the last 30 min of the basal period and during insulin infusion of the clamp procedure. During basal conditions, EGP, i.e., the rate of appearance of glucose into plasma, was calculated as tracer infusion rate/tracer SA.21 During insulin infusion, glucose disposal is reported as the glucose infusion rate (GIR) needed to maintain euglycemia, during the last 30 minutes of the clamp procedure. In addition, the M-value, (glucose disposal rate per kg of body weight), an insulin sensitivity index (M/I; M-value per unit of plasma insulin) and the metabolic clearance rate (MCR) of glucose (rate of glucose clearance from plasma) were also calculated to account for variations in body weight, steady-state insulinemia and glycemia, respectively. Skeletal muscle insulin sensitivity was determined as the increase in glucose disposal (above basal) induced by insulin infusion.22
Study 2
The insulin sensitivity index (Si), glucose effectiveness (Sg), and β-cell function were determined by minimal modeling of the data collected during the FSIVGTT (MINMOD Millennium, version 5.10; University of Pennsylvania School of Veterinary Medicine, Kennett Square, PA).23
Statistical analysis
Study data were collected and managed by using REDCap, a research electronic data capture tool.24 Based on in-house data on the intra-individual variability of glucose kinetics in the basal state and during insulin infusion in obese persons, a sample size of 11 subjects per group in Study 1 and 7 subjects in Study 2 would allow us to detect a difference of 22–25% (effect size: 0.42–0.48) in skeletal muscle insulin sensitivity (i.e., relative increase in glucose disposal during insulin infusion) and a difference of 20–23% (effect size: 0.47–0.54) in hepatic insulin sensitivity (i.e., HISI), with an alpha value of 0.05 and power of 80% (beta = 0.2) for two-sided tests. A difference of this magnitude represents the lower end of the clinically meaningful observed effect of current treatment strategies for insulin resistance, such as moderate weight loss25–28 and thiazolidinedione therapy29–31 which increase hepatic and skeletal muscle insulin sensitivity by 25–50%.
Analyses were carried out with Predictive Analytics Software (PASW), version 18.0.0 (SPSS, Chicago, IL). All data sets were tested for normality according to the Kolmogorov-Smirnov procedure. Non-normally distributed variables were log-transformed to achieve normality before all subsequent analyses. Results are reported as means with 95% confidence intervals. A P value < 0.05 was considered statistically significant.
The effect of omentectomy in study 1 was assessed by using two-way repeated measures analysis of variance for fixed effects; group served as the between-subjects factor (RYGB alone vs. RYGB plus omentectomy) and time served as the within-subjects factor (before vs. 6 vs. 12 months after surgery); differences between time points were assessed with statistical contrasts. To ensure data were missing at random, key variables and covariates were evaluated in completers and non-completers at baseline, 6 months and 12 months; no variables were significantly different between completers and non-completers and the values were similar in both groups (supplementary Tables 1–3). All drop-outs were due to personal life issues, and were not related to treatment group, weight loss or clinical outcome, supporting the assumption that data were missing at random. The effect of omentectomy in study 2 was evaluated by using Student’s t test for paired samples.
RESULTS
Study 1: RYGB surgery with or without omentectomy
Subjects in the RYGB plus omentectomy group had their greater omentum resected, which weighed 774 g (570–979 g) or 1.2% (0.6–1.8%) of total body fat.
Body weight and composition
Body weight, BMI, and fat mass decreased after surgery in both groups, but there were no significant differences between groups (Table 1). Weight loss from baseline was 27% (25–29%) in both groups at 6 months, and 34% (30–37%) and 34% (29–39%) in the RYGB alone and RYGB plus omentectomy groups, respectively, at 12 months.
Table 1.
Effect of Roux-en Y gastric bypass surgery with or without omentectomy on body composition and metabolic variables
| Omentectomy | Before surgery | 6 months after surgery | 12 months after surgery | P values | |||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | Group | G×T | Time | |
| Weight (kg) | 134 (117–151) | 134 (124–144) | 97 (85–110)* | 98 (89–106)* | 89 (76–103)*† | 88 (80–96)*† | 0.960 | 0.860 | <0.001 |
| Body mass index (kg/m2) | 47.6 (42.7–52.6) | 47.8 (43.1–52.4) | 34.6 (30.8–38.5)* | 34.8 (31.2–38.3)* | 31.7 (27.6–35.9)*† | 31.4 (28.0–34.9)*† | 0.998 | 0.885 | <0.001 |
| Fat mass (kg) | 61 (58–64) | 63 (56–70) | 37 (31–44)* | 40 (34–45)* | 29 (23–36)*† | 30 (25–35)*† | 0.561 | 0.705 | <0.001 |
| Body fat (%) | 48 (44–53) | 47 (42–52) | 41 (34–48)* | 42 (36–47)* | 35 (27–42)*† | 35 (29–40)*† | 0.994 | 0.674 | <0.001 |
| Glucose (mg/dL)a | 130 (106–159) | 123 (102–150) | 92 (84–101)* | 98 (88–110)* | 90 (82–97)* | 102 (82–127)* | 0.557 | 0.207 | <0.001 |
| Insulin (mU/L) | 26 (18–35) | 24 (17–30) | 8 (5–11)* | 8 (6–11)* | 7 (5–8)* | 7 (4–9)* | 0.731 | 0.555 | <0.001 |
| HOMA2-IR | 4.6 (3.1–6.1) | 4.0 (3.1–5.0) | 1.3 (0.8–1.8)* | 1.4 (1.0–1.9)* | 1.1 (0.9–1.4)* | 1.1 (0.8–1.5)* | 0.713 | 0.465 | <0.001 |
| Glycosylated hemoglobin (%) | 6.5 (5.9–7.0) | 7.0 (5.8–8.2) | 5.5 (5.0–6.0)* | 5.9 (5.4–6.3)* | 5.6 (5.3–5.9)* | 6.2 (5.3–7.0)* | 0.218 | 0.768 | 0.001 |
| Free fatty acids (mmol/L) | 0.70 (0.60–0.79) | 0.70 (0.52–0.87) | 0.58 (0.51–0.64)* | 0.56 (0.49–0.64)* | 0.53 (0.45–0.62)* | 0.56 (0.47–0.64)* | 0.929 | 0.882 | 0.032 |
| Total cholesterol (mg/dL) | 197 (177–218) | 175 (148–203) | 146 (126–166)* | 148 (126–169)* | 157 (135–180)* | 150 (129–172)* | 0.463 | 0.290 | <0.001 |
| LDL-cholesterol (mg/dL) | 117 (99–135) | 94 (74–114) | 83 (64–102)* | 84 (65–104)* | 91 (70–112)* | 84 (64–104)* | 0.354 | 0.202 | 0.015 |
| HDL-cholesterol (mg/dL) | 45 (38–52) | 47 (42–53) | 43 (37–49) | 46 (40–53) | 49 (45–54)† | 50 (44–57)† | 0.491 | 0.594 | 0.002 |
| Total triglyceride (mg/dL) | 178 (125–231) | 170 (104–236) | 98 (76–121)* | 102 (73–130)* | 83 (67–100)*† | 83 (61–104)*† | 0.922 | 0.904 | <0.001 |
| C-reactive protein (μg/mL)a | 3.0 (1.0–8.5) | 3.1 (0.9–10.5) | 1.5 (0.9–2.4) | 1.8 (0.8–4.3) | 1.1 (0.7–1.6)*† | 0.9 (0.4–2.0)*† | 0.928 | 0.294 | 0.003 |
| Leptin (ng/mL)a | 39 (28–54) | 33 (25–43) | 18 (12–26)* | 13 (10–17)* | 15 (9–25)*† | 8 (5–13)*† | 0.113 | 0.371 | <0.001 |
| Total adiponectin (μg/mL) | 8.6 (3.8–13.3) | 8.5 (3.3–13.8) | 9.8 (6.5–13.1) | 10.5 (7.4–13.5) | 18.8 (11.2–26.3)*† | 15.0 (9.5–20.4)*† | 0.675 | 0.403 | <0.001 |
| Basal EGP (mg/min)a | 145 (120–175) | 146 (125–170) | 101 (91–112)* | 113 (105–123)* | 101 (95–108)* | 110 (97–125)* | 0.293 | 0.582 | <0.001 |
| Basal EGP (mg/kg·min) | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.2) | 1.2 (1.1–1.3) | 1.2 (1.0–1.3) | 1.3 (1.1–1.5) | 0.328 | 0.589 | 0.108 |
| GIR (mg/min) | 591 (451–732) | 612 (486–738) | 697 (589–804)* | 794 (714–874)* | 795 (723–867)*† | 825 (702–948)*† | 0.276 | 0.582 | 0.005 |
| M (mg/kg·min) | 4.6 (3.4–5.8) | 4.6 (3.6–5.7) | 7.2 (6.3–8.0)* | 8.3 (7.2–9.3)* | 9.1 (8.3–9.9)*† | 9.4 (8.1–10.7)*† | 0.359 | 0.315 | <0.001 |
| M/I (mg·mL/kg·min·mU)a | 14 (9–22) | 16 (11–24) | 28 (21–38)* | 33 (25–43)* | 31 (24–40)*† | 38 (28–53)*† | 0.307 | 0.948 | <0.001 |
| Glucose MCR (mL/kg·min) | 4.8 (3.5–6.2) | 4.7 (3.6–5.9) | 8.0 (6.5–9.5)* | 8.6 (7.4–9.7)* | 9.9 (9.0–10.8)*† | 10.1 (8.6–11.6)*† | 0.746 | 0.742 | <0.001 |
Values are shown as means with 95% confidence intervals for n=11 per group; P values for the main effects of group and time and their interaction (G×T) are reported; symbols represent statistical contrasts:
P≤0.02 vs. value before surgery and
P≤0.01 vs. value at 6 months after surgery.
Data were log-transformed for analysis.
Abbreviations: HOMA2-IR, updated homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein; EGP, endogenous glucose production rate; GIR, glucose infusion rate; MCR, metabolic clearance rate.
To convert the values for glucose to mmol/L, multiply by 0.05551. To convert the values for insulin to pmol/L, multiply by 6. To convert the values for cholesterol to mmol/L, multiply by 0.0259. To convert the values for triglyceride to mmol/L, multiply by 0.0113.
Metabolic variables and insulin sensitivity
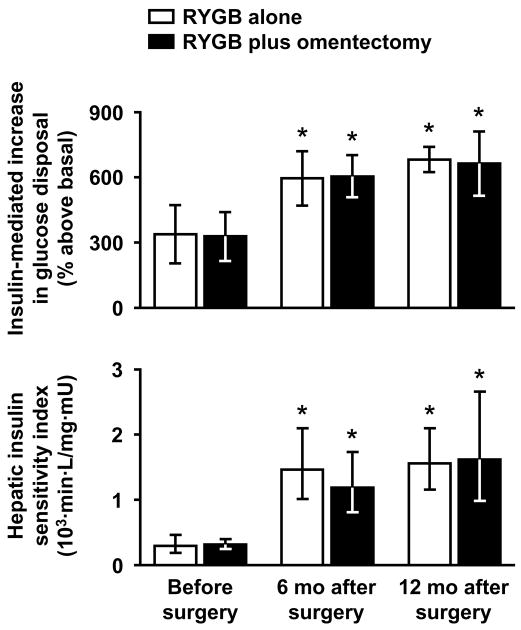
All metabolic variables improved after RYGB surgery in both groups, but there were no differences between groups or group×time interactions (Table 1). No significant differences between groups or group×time interactions were detected with respect to glucose kinetics and insulin sensitivity measures (Table 1 and Figure 1). Compared with baseline, hepatic insulin sensitivity (HISI) increased ~4-fold, and skeletal muscle insulin sensitivity (insulin-mediated glucose disposal and the relative increase in glucose disposal) approximately doubled at 12 months after surgery in both groups (all P<0.001), with most of the changes being already evident by 6 months (Table 1 and Figure 1). Plasma insulin concentration during the clamp procedure averaged 281 mU/L (257–304 mU/L) among all studies, and was similar in studies performed before and at 6 and 12 months after surgery (P=0.508) and in both groups (P=0.369), with no significant interaction (P=0.836).
Figure 1. Effects of bariatric surgery with or without omentectomy on skeletal muscle and hepatic insulin sensitivity.
The relative increase (above basal) in glucose disposal during insulin infusion and the hepatic insulin sensitivity index were determined at baseline (before surgery), and at 6 and 12 months after Roux-en-Y gastric bypass (RYGB) surgery alone (n=11) or RYGB surgery plus omentectomy (n=11) in obese subjects with and without type 2 diabetes, by using the hyperinsulinemic glucose clamp procedure with radioactive tracer infusion. Data for hepatic insulin sensitivity were log-transformed for analysis. Values are shown as means with 95% confidence intervals. There were significant main effects of time on skeletal muscle and hepatic insulin sensitivity (both P<0.001) but no significant differences between groups (P=0.897 and P=0.874, respectively) or group×time interactions (P=0.959 and P=0.361, respectively); *P<0.00002 vs. value before surgery.
All diabetes medications were discontinued after RYGB surgery-induced weight loss, with or without omentectomy, in all subjects with diabetes.
Study 2: Omentectomy alone
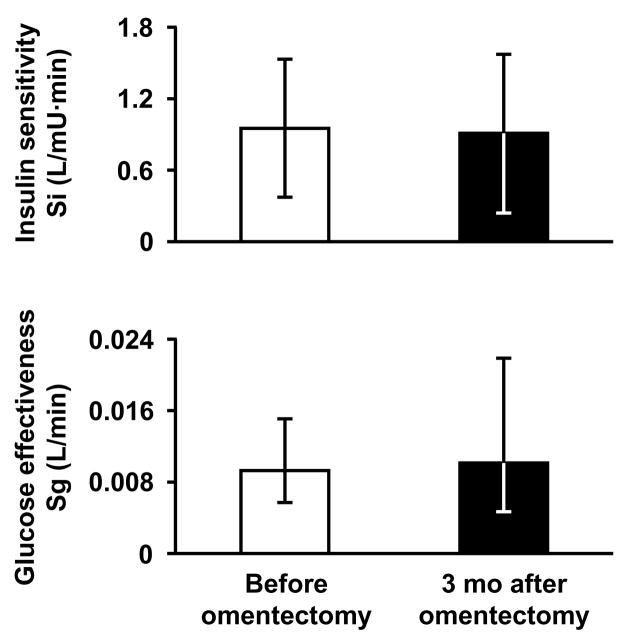
The greater omentum was successfully resected in all subjects and weighed 887 g (593–1181 g). Body weight, metabolic variables and minimal model-derived indices of insulin sensitivity, glucose effectiveness and β-cell function did not change significantly 3 months after omentectomy compared with baseline (Table 2 and Figure 2). All subjects were being treated with diabetes medications before surgery, including metformin, sulfonylureas, and thiazolidinediones, and remained on the same therapy for 3 months after surgery before the follow-up metabolic studies were performed.
Table 2.
Effect of laparoscopic omentectomy alone on body weight and metabolic variables
| Before omentectomy | 3 months after omentectomy | P value | |
|---|---|---|---|
| Weight (kg) | 111 (102–120) | 110 (99–121) | 0.662 |
| Body mass index (kg/m2) | 38.0 (33.9–42.2) | 37.9 (33.3–42.4) | 0.641 |
| Glucose (mg/dL) | 164 (128–200) | 174 (121–228) | 0.534 |
| Insulin (mU/L) | 21 (2–39) | 19 (3–34) | 0.197 |
| HOMA2-IRa | 1.9 (1.0–3.9) | 1.9 (1.0–3.5) | 0.811 |
| Glycosylated hemoglobin (%) | 7.0 (6.0–7.9) | 7.4 (6.3–8.5) | 0.287 |
| Free fatty acids (mmol/L) | 0.56 (0.43–0.69) | 0.61 (0.47–0.75) | 0.412 |
| Total cholesterol (mg/dL) | 210 (175–246) | 191 (153–228) | 0.122 |
| LDL-cholesterol (mg/dL) | 119 (87–151) | 110 (87–134) | 0.475 |
| HDL-cholesterol (mg/dL) | 45 (40–50) | 41 (38–45) | 0.135 |
| Triglyceride (mg/dL) | 233 (141–325) | 196 (115–277) | 0.076 |
| C-reactive protein (μg/mL) | 3.1 (0.8–5.4) | 3.9 (1.1–6.8) | 0.067 |
| Total adiponectin (μg/mL) | 9.7 (4.9–14.4) | 9.2 (4.0–14.4) | 0.457 |
| β-Cell function (mU/mM)a | 48 (11–206) | 40 (10–159) | 0.186 |
Values are shown as means with 95% confidence intervals for n=7.
Data were log-transformed for analysis.
Abbreviations: HOMA2-IR, updated homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
To convert the values for glucose to mmol/L, multiply by 0.05551. To convert the values for insulin to pmol/L, multiply by 6. To convert the values for cholesterol to mmol/L, multiply by 0.0259. To convert the values for triglyceride to mmol/L, multiply by 0.0113.
Figure 2. Effects of omentectomy alone on insulin sensitivity and glucose effectiveness.
Insulin sensitivity (Si) and glucose effectiveness (Sg) were determined by using the frequently sampled intravenous glucose tolerance test and minimal model in obese subjects with type 2 diabetes (n=7) before and 3 months after laparoscopic omentectomy. Data for glucose effectiveness were log-transformed for analysis. Values are shown as means with 95% confidence intervals. There were no significant differences in Si (P=0.844) or Sg (P=0.663).
DISCUSSION
Although an accumulation of VAT is linked with multiple cardiometabolic risk factors, particularly insulin resistance,1–3,6 a cause-and-effect relationship has never been established. Surgical removal of the greater omentum provided an opportunity to test the hypothesis that omental fat itself contributes to insulin resistance. We found that omentectomy did not enhance weight loss-induced improvement in hepatic or skeletal muscle insulin sensitivity in obese subjects who underwent RYGB surgery, and did not improve insulin sensitivity, glucose effectiveness or β-cell function in obese subjects with T2DM who remained weight stable. Therefore, the results from the present study suggest that increased VAT mass is not a major cause of metabolic dysfunction.
An increase in circulating FFA inhibits the ability of insulin to stimulate muscle glucose uptake and suppress hepatic glucose production.32 Accordingly, it has been hypothesized that FFA released from VAT, which are delivered directly to the liver, are responsible for insulin resistance, particularly impaired hepatic insulin action, in patients with abdominal obesity.6,33 However, only about 20% and 14% of total FFA delivered to the liver and skeletal muscle, respectively, originate from lipolysis of VAT in obese adults.34,35 Most of the FFA in the portal vein are derived from lipolysis of subcutaneous fat, which ultimately enter the portal circulation after passage through the splanchnic bed.36 Therefore, VAT is not a major source of FFA in either the portal or systemic circulations, and is unlikely to cause insulin resistance by an FFA-mediated mechanism. These findings are consistent with our observation that omentectomy does not improve insulin sensitivity or other metabolic variables. The mechanism responsible for the relationship between VAT and metabolic dysfunction is not known.
We are aware of two other studies conducted in obese persons that evaluated whether removal of omental fat can enhance the metabolic benefits of bariatric surgery-induced weight loss. One study showed no additional effect of omentectomy, compared with RYGB surgery alone, on the prevalence of hyperglycemia and hyperinsulinemia, evaluated at 24 months after surgery.16 However, insulin sensitivity was not assessed, so it is possible that a beneficial effect could have been missed within the overall robust effect of weight loss. The second study found that compared with subjects who underwent open adjustable gastric banding (AGB) alone, those who had open AGB plus omentectomy had a much greater improvement in insulin sensitivity, assessed by using an oral glucose tolerance test and an intravenous insulin tolerance test before and 24 months after surgery.15 In fact, removal of ~600 g of omental fat was associated with more than a 50% greater increase in insulin sensitivity.15 However, the group who had AGB plus omentectomy experienced more than a 30% greater weight loss than the group who had AGB alone,15 which could have been responsible for the additional benefit on insulin action. The present studies were designed to overcome the limitations of previous investigations in order to resolve the controversy regarding the potential therapeutic effects of omentectomy. In the first study protocol, weight loss was carefully matched 12 months after surgery in subjects randomized to RYGB surgery alone and those randomized to RYGB surgery plus omentectomy. In addition, isotopically labeled glucose tracer infusion and the hyperinsulinemic-euglycemic clamp procedure were used to provide a sensitive evaluation of hepatic and skeletal muscle insulin sensitivity. In the second study protocol, the possibility that weight loss might mask a subtle effect of omentectomy was addressed by evaluating subjects who maintained a stable body weight for 3 months after the procedure.
An important limitation of our study is that we were only able to remove the greater omentum, which weighed approximately 0.8 kg. Therefore, it is possible that removing a greater amount of VAT could have resulted in beneficial metabolic effects. However, we believe this is unlikely because our results do not even show a slight improvement in metabolic function after omentectomy, whereas only 15–25% diet-induced reduction in VAT is associated with 25–50% increased insulin sensitivity in skeletal muscle and liver.26–28 Moreover, removing more VAT than the greater omentum is technically difficult and potentially dangerous. It is also possible that removal of mesenteric fat, rather than omental fat, would have resulted in a therapeutic metabolic effect. However, data from studies conducted in adipose tissue obtained from different intra-abdominal depots during surgery suggest this is unlikely, because lipolytic activity is the same or greater in omental than in mesenteric adipocytes,9,10 lipolysis is more resistant to suppression by insulin in omental than mesenteric fat, and gene expression of many pro-inflammatory adipokines is greater in omental fat than mesenteric and subcutaneous fat.11,12 Therefore, omental fat has the same or even greater potential for inducing metabolic dysfunction than mesenteric fat. Another potential limitation of our study is the high insulin infusion rate during the clamp procedure, which resulted in near-maximal stimulation of glucose disposal; hence we cannot rule out the possibility that differences in insulin sensitivity might manifest at submaximal insulin infusion rates.
In conclusion, our data demonstrate that reducing VAT by omentectomy is not a useful approach for improving insulin sensitivity or the metabolic complications associated with obesity or diabetes. Our results challenge the notion that the small decrease in VAT that occurs with weight loss is an important direct contributor to the observed improvement in insulin sensitivity.6 These results suggest that increased VAT mass is not an important cause of metabolic dysfunction in obese persons.
Supplementary Material
Acknowledgments
Funding: This study was supported by National Institutes of Health grants DK 70860, DK 37948, UL1 RR024975 (Vanderbilt Clinical and Translational Science Award), DK20593 (Vanderbilt Diabetes Research and Training Center), DK058404 (Vanderbilt Digestive Disease Research Center), DK 56341 (Washington University Clinical Nutrition Research Unit), RR024992 (Washington University Clinical and Translational Science Award), and grants from Covidien (Mansfield, MA) and Ethicon Endo-Surgery (Cincinnati, OH).
We thank Erik N. Hansen, MD, MPH, James M. Isbell, MD, Jabbar Saliba, MD, Marcy Buckley, RN, Joan Kaiser, RN, MS, and Kareem Jabbour, BS, for acquisition of data, and Julia P. Dunn, MD, and Nadine Saliba, BS, for technical support. We also thank the volunteers and patients who took part in these studies.
Abbreviations
- AGB
adjustable gastric banding
- BMI
body mass index
- CRC
Clinical Research Center
- EGP
endogenous glucose production
- FFA
free fatty acid
- FSIVGTT
frequently sampled intravenous glucose tolerance test
- GIR
glucose infusion rate
- HISI
hepatic insulin sensitivity index
- HOMA2-IR
updated homeostasis model assessment of whole-body insulin resistance
- HPLC
high-performance liquid chromatography
- MCR
metabolic clearance rate
- RYGB
Roux-en-Y gastric bypass surgery
- SA
specific activity
- T2DM
type 2 diabetes
- VAT
visceral adipose tissue
- VUMC
Vanderbilt University Medical Center
Footnotes
Clinical trial registration: The studies are registered with ClinicalTrials.gov, numbers NCT00212160 (study 1) and NCT00270439 (study 2).
Conflicts of interest: There are no financial conflicts with the subject matter or materials discussed in this manuscript with any of the authors.
Author contributions: EF and RAT contributed equally to this work. NNA had full access to all of the data and takes full responsibility for the veracity of the data and analysis. NNA and SK conceived the studies and designed the study protocols. RAT, PAM, AWE and WOR performed the studies, obtained data, and offered administrative, technical, and/or material support. FM performed statistical analyses, and EF, FM, SK and NNA interpreted data. EF and FM drafted the manuscript, and all authors contributed to critical revision of the manuscript for important intellectual content. NNA and SK obtained funding and supervised the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray GA, Jablonski KA, Fujimoto WY, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87:1212–1218. doi: 10.1093/ajcn/87.5.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 3.Despres JP. Intra-abdominal obesity: an untreated risk factor for Type 2 diabetes and cardiovascular disease. J Endocrinol Invest. 2006;29:77–82. [PubMed] [Google Scholar]
- 4.Jakobsen MU, Berentzen T, Sorensen TI, et al. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77–87. doi: 10.1093/epirev/mxm002. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14:16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 7.Hansen E, Hajri T, Abumrad NN. Is all fat the same? The role of fat in the pathogenesis of the metabolic syndrome and type 2 diabetes mellitus. Surgery. 2006;139:711–716. doi: 10.1016/j.surg.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frayn KN. Visceral fat and insulin resistance--causative or correlative? Br J Nutr. 2000;83:S71–77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- 9.Fried SK, Leibel RL, Edens NK, et al. Lipolysis in intraabdominal adipose tissues of obese women and men. Obes Res. 1993;1:443–448. doi: 10.1002/j.1550-8528.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 10.Rebuffe-Scrive M, Anderson B, Olbe L, et al. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism. 1990;39:1021–1025. doi: 10.1016/0026-0495(90)90160-e. [DOI] [PubMed] [Google Scholar]
- 11.Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34:317–327. doi: 10.1016/j.diabet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 13.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lottati M, Kolka CM, Stefanovski D, et al. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obesity (Silver Spring) 2009;17:674–680. doi: 10.1038/oby.2008.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorne A, Lonnqvist F, Apelman J, et al. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 16.Csendes A, Maluenda F, Burgos AM. A prospective randomized study comparing patients with morbid obesity submitted to laparotomic gastric bypass with or without omentectomy. Obes Surg. 2009;19:490–494. doi: 10.1007/s11695-008-9660-2. [DOI] [PubMed] [Google Scholar]
- 17.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 18.Camastra S, Manco M, Mari A, et al. β-Cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes. 2005;54:2382–2389. doi: 10.2337/diabetes.54.8.2382. [DOI] [PubMed] [Google Scholar]
- 19.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Steele R, Wall JS, De Bodo RC, et al. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 22.Korenblat KM, Fabbrini E, Mohammed BS, et al. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boston RC, Stefanovski D, Moate PJ, et al. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley DE, Wing R, Buonocore C, et al. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 26.Kirk E, Reeds DN, Finck BN, et al. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiikkainen M, Bergholm R, Rissanen A, et al. Effects of equal weight loss with orlistat and placebo on body fat and serum fatty acid composition and insulin resistance in obese women. Am J Clin Nutr. 2004;79:22–30. doi: 10.1093/ajcn/79.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 29.Schrauwen-Hinderling VB, Mensink M, Hesselink MK, et al. The insulin-sensitizing effect of rosiglitazone in type 2 diabetes mellitus patients does not require improved in vivo muscle mitochondrial function. J Clin Endocrinol Metab. 2008;93:2917–2921. doi: 10.1210/jc.2008-0267. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Tonelli J, Kishore P, et al. Insulin-sensitizing effects of thiazolidinediones are not linked to adiponectin receptor expression in human fat or muscle. Am J Physiol Endocrinol Metab. 2007;292:E1301–1307. doi: 10.1152/ajpendo.00312.2006. [DOI] [PubMed] [Google Scholar]
- 31.Coletta DK, Sriwijitkamol A, Wajcberg E, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52:723–732. doi: 10.1007/s00125-008-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 33.Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- 34.Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basso LV, Havel RJ. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970;49:537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.