The molecular motors termed myosins are involved in transport of subcellular particles in diverse organisms from fungi to animals to plants. Here, we show that myosin-dependent transport is critical for the growth of plant cells and entire plants as well as for proper organization of the cell interior.
Abstract
The actomyosin system is conserved throughout eukaryotes. Although F-actin is essential for cell growth and plant development, roles of the associated myosins are poorly understood. Using multiple gene knockouts in Arabidopsis thaliana, we investigated functional profiles of five class XI myosins, XI-K, XI-1, XI-2, XI-B, and XI-I. Plants lacking three myosins XI showed stunted growth and delayed flowering, whereas elimination of four myosins further exacerbated these defects. Loss of myosins led to decreased leaf cell expansion, with the most severe defects observed in the larger leaf cells. Root hair length in myosin-deficient plants was reduced ∼10-fold, with quadruple knockouts showing morphological abnormalities. It was also found that trafficking of Golgi and peroxisomes was entirely myosin dependent. Surprisingly, myosins were required for proper organization of F-actin and the associated endoplasmic reticulum networks, revealing a novel, architectural function of the class XI myosins. These results establish critical roles of myosin-driven transport and F-actin organization during polarized and diffuse cell growth and indicate that myosins are key factors in plant growth and development.
INTRODUCTION
The actin cytoskeleton is conserved in diverse unicellular and multicellular organisms and is important for a variety of cellular processes (Embley and Martin, 2006). Due to their large size and compartmentalized organization, eukaryotic cells use active transport of macromolecules, vesicles, and organelles to coordinate cellular functions. Actomyosin-based transport is also a key feature of cellular structure and dynamics, including cell division, growth, and motility (Vale, 2003; Pollard and Cooper, 2009).
Although plants are sessile organisms, plant cells exhibit profoundly dynamic expansion and intracellular trafficking. Distinct types of plant cells expand via diffuse or polarized growth mechanisms, with cell sizes increasing up to 100-fold (Petricka and Benfey, 2008; Savaldi-Goldstein and Chory, 2008; Micol, 2009). Furthermore, in contrast with yeast or vertebrate cells, in which organelles move relatively slowly and primarily during cell division, plant cells continuously traffic Golgi stacks, mitochondria, peroxisomes, and other organelles with velocities of up to 10 μm/s. Using actin-specific inhibitors, it was established that both cell growth and organelle transport in plants rely on the actomyosin system (Shimmen and Yokota, 2004; Smith and Oppenheimer, 2005). In fact, the actin cytoskeleton, rather than microtubules, plays a principal role in the organization of the plant cell interior, including the endomembrane system and trafficking network (Smith and Oppenheimer, 2005; Szymanski and Cosgrove, 2009). Interestingly, plant cells exhibit F-actin dynamics characterized by microfilament growth and severance rates that are much higher than those in yeast or animal cells (Staiger et al., 2009).
Phylogenetic analysis of plant genomes identified two classes of myosins, VIII and XI, the latter of which is closely related to the fungal and animal class V myosins (Bezanilla et al., 2003; Foth et al., 2006; Avisar et al., 2008b). Extensive studies using yeast and vertebrate models have revealed that the functions of myosin V range from organelle segregation during cell division to mRNA transport to endosome recycling (Pruyne et al., 2004; Trybus, 2008; Valiathan and Weisman, 2008; Wang et al., 2008). These motor functions at the subcellular level underlie the essential roles of myosin V in yeast cell viability, as well as cell polarity, sensory adaptation, and memory formation in vertebrates.
By contrast, relatively little is known about the functions of myosins in plants. Class VIII myosins associate with endosomes and plasmodesmata, organelles involved in intercellular transport of proteins, RNA, and viruses (Reichelt et al., 1999; Avisar et al., 2008a; Golomb et al., 2008; Maule, 2008; Sattarzadeh et al., 2008). Extensive biophysical studies of class XI myosins revealed that they are the fastest known processive molecular motors (Tominaga et al., 2003). Due to their colocalization with organelles, it was generally assumed that organelle transport involved class XI myosins (Lee and Liu, 2004; Reisen and Hanson, 2007).
Recent studies using dominant-negative inhibition, RNA interference, and gene knockouts showed that class XI myosins indeed contribute to the rapid trafficking of Golgi stacks, mitochondria, and peroxisomes (Avisar et al., 2008b, 2009; Peremyslov et al., 2008; Prokhnevsky et al., 2008; Sparkes et al., 2008). These myosins were also implicated in endoplasmic reticulum (ER) streaming (Ueda et al., 2010) and remodeling (Sparkes et al., 2009a) as well as in plastid dynamics (Natesan et al., 2009; Sattarzadeh et al., 2009). In addition, some class XI myosins play a role in root hair elongation (Ojangu et al., 2007; Peremyslov et al., 2008; Prokhnevsky et al., 2008). Interestingly, whereas yeast and vertebrates possess only two or three class V myosins, the number of class XI myosins in plants is much higher, with 13 present in Arabidopsis thaliana (Reddy and Day, 2001). Myosin gene expression profiling (http://www.weigelworld.org/resources) showed that six myosin XI genes, XI-A, XI-B, XI-C, XI-D, XI-E, and XI-J, are expressed predominantly in male reproductive tissue (stamen and pollen), whereas the other seven (XI-1, XI-2, XI-F, XI-G, XI-H, XI-I, and XI-K) are expressed throughout the vegetative plant, suggesting that myosin evolution has involved functional specialization. On the other hand, analysis of double myosin knockout mutants revealed a degree of functional redundancy among the Arabidopsis myosins (Prokhnevsky et al., 2008).
Here, using several triple and quadruple knockout mutant combinations, we show that processive transport of Golgi stacks and peroxisomes during the vegetative phase of Arabidopsis development relies entirely on the four most highly expressed myosin motors (XI-K, XI-1, XI-2, and XI-I). We reveal a novel role of class XI myosins in the organization of the actin cytoskeleton and ER. Furthermore, we demonstrate that both the polarized elongation and diffuse growth of several plant cell types require myosin function. At the whole-plant level, we show that cessation of myosin-driven motility correlates with a dramatic reduction in plant size and delayed reproduction.
RESULTS
Developmental Defects in Myosin-Deficient Plants
To determine specific contributions of myosin motors to plant growth, we generated a series of triple and quadruple myosin knockout mutants (3KO and 4KO, respectively) of Arabidopsis. This has been done by crossing single and double knockout lines, in which RT-PCR analysis confirmed the lack of corresponding mRNAs (Peremyslov et al., 2008; Prokhnevsky et al., 2008). Each 3KO and 4KO line and its offspring was analyzed by PCR to demonstrate presence of mutant alleles, absence of wild-type alleles, and lack of segregation as described in Methods. The eliminated myosins included two pairs of closely related paralogs, myosins XI-K/XI-1 and XI-2/XI-B, as well as myosin XI-I, which has no such paralog (Avisar et al., 2008b). Of these five, the genes encoding myosins XI-K, XI-1, XI-2, and XI-I are the most highly expressed myosin XI genes throughout the entire Arabidopsis plant (http://www.weigelworld.org/resources).
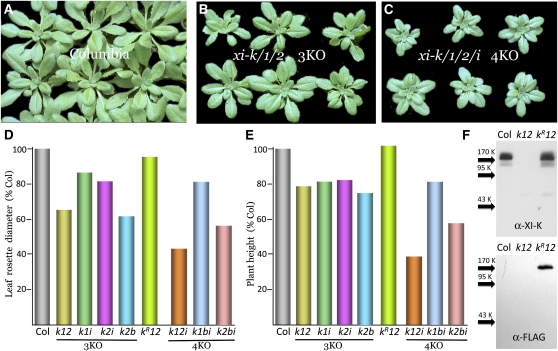
Investigation of the mutant phenotypes revealed a substantial reduction in the leaf rosette diameter in all three KOs, up to ∼65% of that in the wild type in the case of xi-k xi-1 xi-2 and xi-k xi-2 xi-b 3KOs (Figures 1A, 1B, and 1D). Interestingly, the greatest and most statistically significant changes (P < <0.001; see Supplemental Table 1 online) were observed in the xi-k xi-1 xi-2 and xi-k xi-2 xi-b 3KOs in which the paralogous myosin pairs XI-K/1 and XI-2/B, respectively, were inactivated (Figure 1D). The plant heights of the 3KO mutants were also reduced albeit to a more limited extent (Figure 1E; see Supplemental Table 2 online).
Figure 1.
Growth Phenotypes of the Arabidopsis Knockout Plants in Which Three or Four Myosin XI Genes Were Inactivated (3KO and 4KO, Respectively).
(A) to (C) Leaf rosettes of the groups of six plants sown on the same day.
(D) Mean width of the leaf rosettes; the absolute value for Columbia-0 is 81 mm; sample sizes are from 17 to 34.
(E) Mean plant heights; the absolute value for Columbia-0 is 174 mm; sample sizes are from 17 to 34. Each mutant variant is marked in an abbreviated manner; kR12, xi-k xi-1 xi-2 3KO transformed with the XI-KFLAG gene (R stands for rescue).
(F) Immunoblot analysis using the myosin XI-K–specific (top panel) or FLAG-specific (bottom panel) antibodies; arrows show protein marker positions. K, kD.
Columbia or Col, the parental Columbia-0 line.
To confirm that the observed changes in plant stature were due to myosin deficiency rather than to potential effects on global gene regulation (e.g., via off-target RNA silencing induced by the multiple inserts), we performed a genetic rescue experiment. The xi-k xi-1 xi-2 3KO plants were transformed with a genomic clone of XI-K modified to accommodate the FLAG epitope tag (XI-KFLAG). The transformed plants were screened by immunoblotting using either XI-K- or FLAG-specific antibodies and a plant line producing nearly wild-type levels of the XI-KFLAG myosin was selected (Figure 1F, lane kR12). This line was found to be virtually indistinguishable from wild-type plants (Figures 1D and 1E; see Supplemental Tables 1 and 2 online) in agreement with the dominant role played by myosin XI-K in myosin-dependent processes (Peremyslov et al., 2008; Prokhnevsky et al., 2008; Ueda et al., 2010).
Our analysis of the 4KO mutants revealed even stronger stunting phenotypes. Among the three 4KO mutants, the xi-k xi-1 xi-2 xi-i combination, in which all four highly expressed myosins were eliminated, showed the most dramatic, ∼2.5-fold reduction in leaf rosette size and plant height (Figures 1C to 1E; see Supplemental Tables 1 and 2 online; P < 10−6). These results indicate that progressive myosin elimination results in an overall reduction in the growth of the aerial organs (Figures 1A to 1E). A similar tendency was observed in plant fecundity, with the xi-k xi-1 xi-2 xi-i 4KO mutant showing the most significant reduction in the mean number of seeds per silique (see Supplemental Figure 1 and Supplemental Table 3 online).
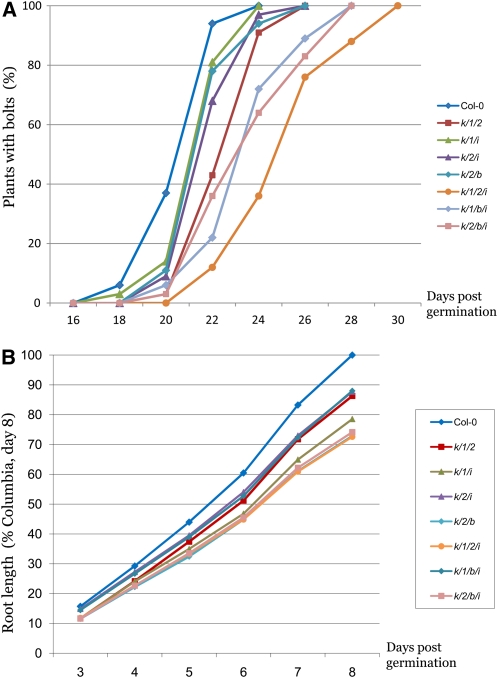
To further evaluate the effects of myosin elimination on plant reproduction, we monitored flowering time by counting the percentage of plants with bolts as a function of time postgermination. As shown in Figure 2A, flowering time of the 3KO lines was increased compared with Columbia and even more so in the 4KO lines. The mutant variant that showed the largest delay in bolt formation was, once again, the xi-k xi-1 xi-2 xi-i 4KO mutant. Therefore, inactivation of the highly expressed myosins substantially affects the onset of flowering.
Figure 2.
Changes in Flowering Time and Root Growth in the Multiple Knockout Mutants.
(A) Time course of plant flowering measured as percentage of bolted plants.
(B) Time course of root growth shown as percentage of root length of Columbia-0 (Columbia) at 8 d after germination (mean value 51 mm). Plant lines are color coded as shown at the right.
To evaluate the extent to which myosin elimination affected root growth, we measured root lengths over time. We found that, by and large, the 4KO variants showed slower root growth than the 3KO variants (Figure 2B; see Supplemental Table 4 online). However, the overall effect on roots was more limited than that on aerial parts, showing a <30% root length reduction in the most affected xi-k xi-1 xi-2 xi-i 4KO mutant (P < 0.0001).
Taken together, phenotypic analysis of the multiple myosin knockouts demonstrated an overall negative correlation between the number of inactivated myosins and plant growth and reproduction. Interestingly, development of the aerial organs was affected to a larger extent than root growth, indicating differential roles of the myosin-dependent processes in distinct plant organs. It should be noted that, apart from the overall reduction in size, no obvious morphological abnormalities were detected in the analyzed mutant lines.
Myosins Contribute to Diffuse Growth of the Leaf Epidermal and Mesophyll Cells
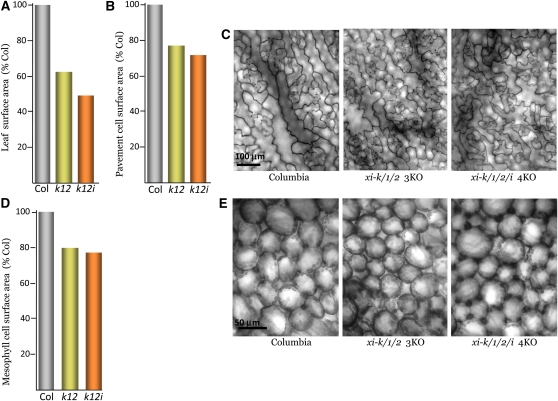
To determine the relationships between organ and cell growth in the myosin knockouts, we analyzed the sizes of several leaf cell types. Initially, we focused on the two principal cell types, pavement epidermal cells and spongy mesophyll cells. The mean leaf surface areas in the xi-k xi-1 xi-2 3KO and xi-k xi-1 xi-2 xi-i 4KO were 61 and 46% of that in Columbia, respectively (Figure 3A; P < 10−7; see Supplemental Table 5 online). The mean cell surface areas of pavement cells, which constitute most of the leaf surface in these mutants, were 76 and 71% of those in Columbia, respectively (Figures 3B and 3C; P < 0.001; see Supplemental Table 6 online). Therefore, the reduction in leaf surface area in the myosin mutants depended not only on a reduction in cell size, but also apparently involved a decrease in the number of leaf cells. This conclusion was further supported by analysis of spongy mesophyll cells. The mean surface area of mesophyll cells in a planar view was 79 and 76% of that in Columbia for the xi-k xi-1 xi-2 and xi-k xi-1 xi-2 xi-i mutants, respectively (Figures 3D and 3E; P < 0.0001; see Supplemental Table 7 online).
Figure 3.
Changes in Leaf and Cell Surface Areas in the Triple and Quadruple Knockout Mutants.
(A) Mean leaf surface areas in xi-k xi-1 xi-2 3KO and xi-k xi-1 xi-2 xi-i 4KO plants; sample size is from 29 to 31.
(B) and (C) Mean surface areas of the pavement cells of abaxial leaf epidermis and representative images used for measurements, respectively.
(D) and (E) Mean surface areas of the leaf mesophyll cells in a planar view and representative images used for measurements, respectively. The sample size for all cell measurements is 240. The mean values for Columbia-0 in (A), (B), and (D) are 295 mm2, 3538 μm2, and 1402 μm2, respectively.
Columbia or Col, the parental Columbia-0 line.
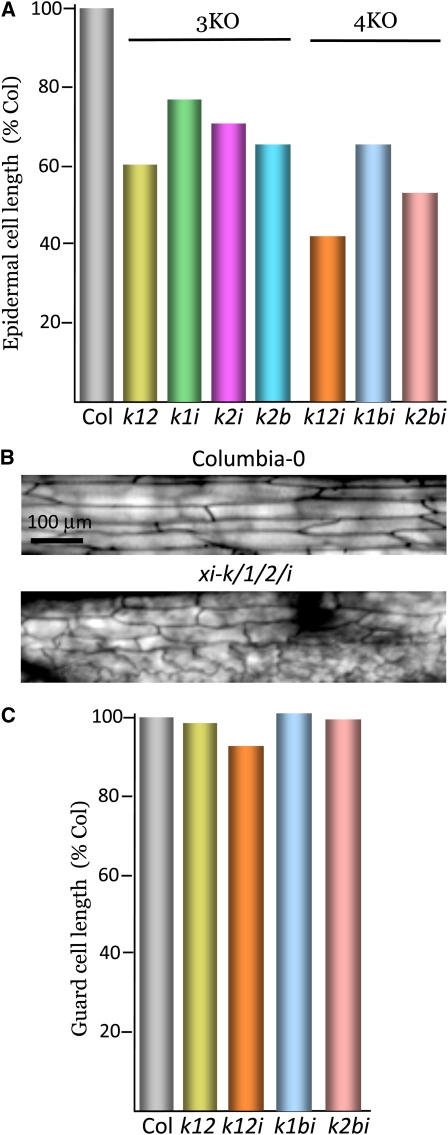
To gain further insight into the cellular determinants of the defective leaf growth in the multiple myosin knockouts, we measured the lengths of two additional contrasting cell types: epidermal cells of the midvein (Figures 4A and 4B; see Supplemental Table 8 online) that are oriented along the long leaf axis and the small, bean-shaped guard cells that are oriented randomly (Figure 4C; see Supplemental Table 9 online). Strikingly, the mean lengths of the midvein epidermis cells showed very high correlation with the rosette diameter in all seven multiple knockout lines (r = 0.9511; compare Figures 4A and 1D). Statistical analysis of the length differences between Columbia and each mutant showed high confidence levels for all variants (P < 0.00001; see Supplemental Table 8 online). By contrast, the lengths of the guard cells were very similar to those of Columbia for all tested myosin mutants (Figure 4C). Only xi-k xi-1 xi-2 xi-i 4KO showed a modest, albeit statistically significant, 5% reduction (see Supplemental Table 9 online). Collectively, these results revealed differential effects of myosin elimination on the diffuse growth of several types of leaf cells.
Figure 4.
Characterization of the Leaf Midvein Epidermal and Guard Cell Lengths in the Triple and Quadruple Knockout Mutants.
(A) and (B) Mean lengths of the elongated epidermal cells of the abaxial side of the midvein and representative images of these cells used for measurements, respectively. The sample sizes in (A) are ∼300 cells.
(C) Mean lengths of the guard cells; the sample sizes are ∼100 cells. The mean values for Columbia-0 (Col) in (A) and (C) are 368 and 22 μm, respectively.
Myosin-Dependent Organelle Trafficking
Our previous analysis of single and double gene knockouts revealed that three of the highly expressed Arabidopsis myosins (XI-K, XI-1, and XI-2) are involved in the trafficking of Golgi stacks, mitochondria, and peroxisomes in leaf and root hair cells (Peremyslov et al., 2008; Prokhnevsky et al., 2008). Among these, myosin XI-K provided dominant contributions, whereas myosins XI-2 and XI-1 were capable of partially rescuing organelle transport in plants lacking myosin XI-K. However, simultaneous elimination of two of these myosins did not completely abolish organelle trafficking (Prokhnevsky et al., 2008). The residual organelle motility in double gene knockouts could be due either to activity of the remaining myosins or to an alternative, microtubule-dependent transport mechanism. To distinguish between these possibilities, we investigated organelle trafficking in the 3KO and 4KO mutants.
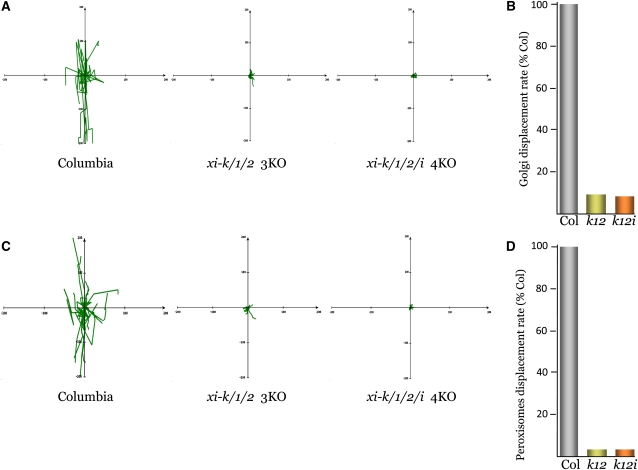
The xi-k xi-1 xi-2 3KO and xi-k xi-1 xi-2 xi-i 4KO plant lines were transformed with Golgi- and peroxisome-specific green fluorescent protein (GFP) reporters. In the latter mutant, all four highly expressed myosins were inactivated to account for a recently suggested role of the myosin XI-I in organelle transport (Avisar et al., 2009). The organelle trafficking patterns in the midvein epidermal cells were investigated by computing the tracks, velocities, and displacement rates of the hundreds of individual Golgi stacks and peroxisomes using time-lapse confocal laser scanning microscopy. It was found that in the xi-k xi-1 xi-2 3KO, most of the Golgi stacks and peroxisomes were immobile, with only a few organelles capable of moving away from the initial position. By contrast, many organelles covered substantial distances in the Columbia ecotype (Figures 5A and 5C; see Supplemental Movies 1 and 2 online). Conspicuously, virtually no Golgi stacks and peroxisomes displayed processive transport in xi-k xi-1 xi-2 xi-i 4KO (Figures 5A and 5C; see Supplemental Movie 3 online). These results showed that myosin XI-I is indeed capable of transporting organelles, albeit to a very limited extent.
Figure 5.
Motility of the Golgi Stacks and Peroxisomes in Leaf Epidermis Cells of the 3KO and 4KO Plants.
(A) and (C) Tracks of the Golgi stacks (A) and peroxisomes (C) present in one time series obtained using confocal laser scanning microscope and plotted to a common origin.
(B) and (D) Mean displacement rates of the organelles expressed as percentage of those in the parental Columbia-0 (Col) line; 0.86 μm/s and 1.05 μm/s for (B) and (D), respectively.
Columbia or Col, the parental Columbia-0 line.
Quantification of the mean Golgi and peroxisome displacement rates for both multiple knockout mutants revealed a dramatic reduction relative to those in Columbia (Figures 5B and 5D) with P < 2.1 × e−61 (see Supplemental Tables 10 and 11 online). However, comparison between the xi-k xi-1 xi-2 and xi-k xi-1 xi-2 xi-i variants showed only a marginal, ∼1% decrease in the latter relative to the former. This result reflects the fact that processive transport for most of the Golgi stacks and peroxisomes was eliminated in the 3KO variant. It should be noted that even with the cessation of detectable processive movement, the resulting displacement rates (∼8 and ∼3% of those in Columbia for Golgi and peroxisomes, respectively) do not amount to zero (Figures 5B and 5D). The residual wobbling of these organelles (see Supplemental Movie 3 online) could be due to a tug-of-war among the remaining molecular motors (that, however, fails to move Golgi or peroxisomes processively) to Brownian motion, or both. We conclude that elimination of the four myosins in the xi-k xi-1 xi-2 xi-i variant has effectively abolished transport of Golgi stacks and peroxisomes, demonstrating that trafficking of these organelles in plant cells relies primarily, if not entirely, on the highly expressed myosin motors.
There are apparent differences between these conclusions and other studies of organelle transport in plants. It was shown that overexpression of the myosin cargo binding tails (as dominant-negative inhibitors) of the same four myosins, XI-K, XI-1, XI-2, and XI-I, one at a time, reduces organelle transport (Sparkes et al., 2008; Avisar et al., 2009). However, these studies also proposed major transport roles for class XI-C myosins and XI-E. This contrasts with our work, in which Golgi and peroxisomes were effectively immobilized in the 4KO without inactivating either of the XI-C or XI-E myosins. The most likely explanation for this discrepancy is due to the different techniques used to study myosin functions. Whereas gene knockouts produce simple loss-of-function phenotypes, dominant-negative inhibition is less specific due to the potential for multiple effects of myosin tail overexpression. One such effect observed for myosin V is myosin inactivation via tail binding to the motor head (Li et al., 2006). Additionally, expression of both myosins XI-C and XI-E is pollen specific, whereas organelle transport was assayed in leaves in both studies. Finally, heterologous expression of Arabidopsis myosin tails caused distinct effects in two closely related Nicotiana species, further complicating the interpretation (Avisar et al., 2009). Thus, although dominant inhibition highlights the global importance of myosins in plant organelle movement, this technique may lack sufficient specificity to make it suitable for identifying the functions of individual myosins.
Myosins Are Involved in Organization of the Actin Cytoskeleton and ER Network
The differential growth response of distinct cell types to myosin XI inactivation is in contrast with a (nearly) uniform suppression of the organelle motility in these cells. This discrepancy could be explained by distinct levels of cell growth dependence on myosin transport or by involvement of other myosin-dependent processes. One intriguing possibility for such a process is the cytoskeletal remodeling described for some unconventional myosins (e.g., classes VI and X) but not to date for class V or class XI (Woolner and Bement, 2009). To determine if myosin elimination can affect F-actin organization, we focused again on midvein epidermis cells that showed the strongest growth response in the myosin knockouts (Figures 4A and 4B). Each of the xi-k xi-1 xi-2 and xi-k xi-1 xi-2 xi-i lines was transformed with the venusYFP-fABD2 reporter, a derivative of the GFP-fABD2 marker that faithfully labels F-actin without causing developmental defects in transgenic plants (Sheahan et al., 2004).
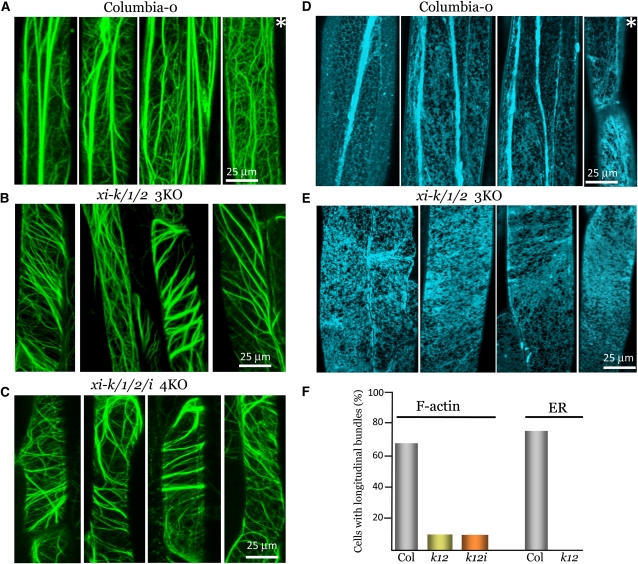
Analysis of F-actin organization revealed salient differences between the midvein epidermal cells of Columbia and the xi-k xi-1 xi-2 and xi-k xi-1 xi-2 xi-i mutants. As shown in Figures 6A and 6F, ∼70% of the cells in the transgenic Columbia line possessed thick, longitudinal F-actin bundles in addition to a complex network of thinner bundles. By contrast, a ∼90% majority of the cells in both 3KO and 4KO variants lacked such longitudinal thick bundles (Figures 6B, 6C, and 6F). Most of the bundles were thinner and were oriented at an angle, up to 90°, to the cell axis (Figures 6B and 6C). Although it cannot be excluded that at least some of the F-actin bundles seen in Columbia cells represent transvacuolar strands, the fact that single optical sections containing these bundles also exhibited an intricate F-actin network typical of the cortical cytoplasm rather than vacuole makes this interpretation unlikely.
Figure 6.
Organization of F-Actin Bundles and ER Network in the Cells of Midvein Epidermis.
(A) to (C) Confocal images of midvein epidermal cells in plants stably transformed to express the F-actin marker venusYFP-fABD2. Note preferential longitudinal organization of the thick F-actin bundles in (A) but not (B) and (C).
(D) and (E) Images of midvein epidermal cells in plants stably transformed to express the ER marker ER-CFP. Note the thick longitudinal ER strands in (D) but not (E).
(F) Proportion of cells with thick longitudinal bundles of F-actin or ER as indicated. The number of screened cells in each data set was (from left to right) 24, 31, 21, 22, and 22, respectively. Col, the parental Columbia-0 line.
Asterisks in (A) and (D) mark cells that do not show thick longitudinal F-actin or ER bundles.
To determine if rearrangement of the actin cytoskeleton is a common feature of distinct cell types in the multiple myosin gene knockouts, we imaged polymorphic leaf pavement cells and root epidermal cells. The F-actin bundles in neither of these cell types were oriented in a discernibly regular manner, and no differences were apparent between cells of Columbia versus the xi-k xi-1 xi-2 3KO or xi-k xi-1 xi-2 xi-i 4KO lines (see Supplemental Figure 2 online). While these observations indicated that the myosin-dependent F-actin remodeling is cell type specific, the multidirectional orientation of the F-actin in these cells made the analysis less conclusive. It also cannot be ruled out that the role of myosin in F-actin organization in these cells is limited to a specific developmental phase (e.g., during leaf initiation and primordial growth).
Because the ER network in plants is supported primarily by the actin cytoskeleton (Sparkes et al., 2009b), we also examined ER architecture in transgenic plants that expressed an ER-targeted cyan fluorescent protein (CFP) reporter. Strikingly, the differences in ER organization between the Columbia and xi-k xi-1 xi-2 lines closely mimicked the differences in F-actin organization. In Columbia, thick, longitudinal ER cables were present in ∼80% of cells, in addition to a typical reticulate network (Figures 6D and 6F), whereas in the xi-k xi-1 xi-2 line, no cells with such cables were found (Figures 6E and 6F). These results revealed a novel activity of the highly expressed class XI myosins in shaping both the actin cytoskeleton and the ER network in a particular cell type. It will be interesting to determine if the myosins expressed at lower levels play similar roles in other cell types.
Polarized Elongation of Root Hairs Is Arrested in the Multiple Myosin Gene Knockouts
Root hairs are a powerful model cell type to study polarized cell growth (Smith and Oppenheimer, 2005; Cole and Fowler, 2006; Szymanski and Cosgrove, 2009). To further address the roles of class XI myosins in this process, we characterized the root hairs of all seven multiple knockout mutant combinations.
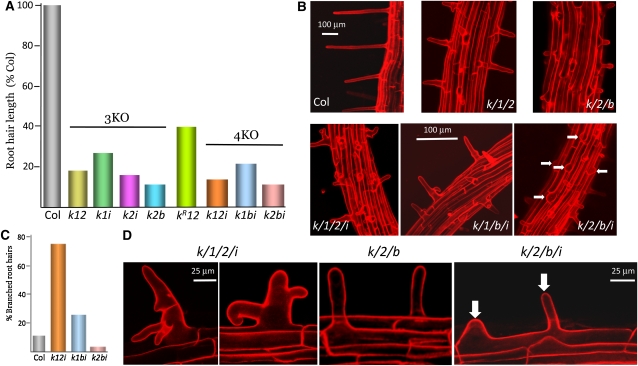
Among the 3KOs, the xi-k xi-2 xi-b variant, in which all three of the myosins previously implicated in root hair elongation (Ojangu et al., 2007; Peremyslov et al., 2008; Prokhnevsky et al., 2008) were lacking, was affected the most. The root hairs in this mutant were ∼10-fold shorter than in Columbia with P < 7.3 × e−71 (Figure 7A; see Supplemental Table 12 online). Despite this immense reduction in length, root hair morphology, orientation perpendicular to the root, and root hair initiation in the files of trichoblast cells were not affected in the triple gene knockouts xi-k xi-2 xi-b or xi-k xi-1 xi-2 (Figures 7B and 7D). Transformation of the xi-k xi-1 xi-2 3KO plants with the myosin XI-KFLAG construct partially rescued root hair elongation. The length of the root hairs in a resulting xi-kR xi-1 xi-2 variant was restored to ∼40% of that in Columbia (Figure 7A), similar to the root hair length in the xi-2 single knockout mutant (Peremyslov et al., 2008). This was an expected outcome given that myosin XI-1 has little if any role in root hair elongation (Prokhnevsky et al., 2008).
Figure 7.
Characterization of the Root Hairs in the Triple and Quadruple Knockout Mutants.
(A) Mean root hair length in the 3KO and 4KO mutant plant lines; the absolute value for Columbia-0 (Col) is 414 μm. The sample sizes are ≥206. Designations and color codes are the same as in Figure 1D.
(B) Representative confocal images of root hairs in 3KO and 4KO mutant plants. Arrows in the bottom right image mark bulged root hairs that failed to elongate.
(C) Percentages of branched root hairs in Columbia-0 and three 4KO mutant plants.
(D) Representative confocal images of branched, misshaped root hairs in xi-k xi-1 xi-2 xi-i 4KO, morphologically normal root hairs in xi-k xi-2 xi-b 3KO, and two root hairs originating from the same trichoblast in xi-k xi-2 xi-b xi-i 4KO (white arrows).
Analysis of root hairs in the 4KO mutants revealed more complex and intriguing morphological changes. Although both the xi-k xi-2 xi-b 3KO and xi-k xi-2 xi-b xi-i 4KO plants had on average equally short root hairs (Figure 7A), the shape of many cells in the latter but not the former mutant resembled swollen buds (Figures 7B and 7D). Furthermore, some of the trichoblasts in the xi-k xi-2 xi-b xi-i 4KO mutant exhibited abnormal initiation of an additional root hair near the middle of the cell (arrows in Figure 7D). These results indicated that class XI myosins contribute to the proper initiation of root hair growth and in the transition from bud swelling to tip growth.
The salient defects in root hair morphology were observed in the xi-k xi-1 xi-2 xi-i 4KO mutant. Instead of the normally straight, single-pointed cells, almost 80% of the cells in this mutant were misshaped and branched (Figures 7B to 7D). This aberrant morphology indicates that inactivation of the four highly expressed myosins caused a multipolar disorder, an inability to form a unique growing tip that is critical for normal polarized elongation.
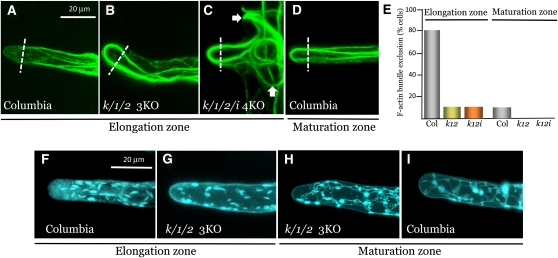
An important aspect of myosin function in root hair growth was revealed by analysis of F-actin organization. It is known that in wild-type root hairs located in the root elongation zone, thick F-actin bundles are oriented longitudinally and are excluded from the tip-growing cell apex (Smith and Oppenheimer, 2005; Cole and Fowler, 2006; Kost, 2008). Our analysis showed that at least 80% of the growing root hairs in Columbia exhibited clear apical exclusion of F-actin bundles (Figures 8A and 8E). By contrast, such exclusion was lacking in >90% of root hairs in the elongation zone of the xi-k xi-1 xi-2 3KO and the xi-k xi-1 xi-2 xi-i 4KO plants (Figure 8E). The mutant root hairs harbored abundant, thick F-actin bundles projecting into the cell apices (Figures 8B and 8C). In the 4KO mutant, misshaped and often twisted bundles filled the entire space within the abnormally branched root hairs (Figure 8C). Therefore, the mechanism responsible for the exclusion of longitudinal F-actin bundles from the rapidly growing root hair tips is myosin dependent and is dysfunctional in the myosin-deficient plants.
Figure 8.
Organization of F-Actin Bundles and ER Network in the Root Hairs.
(A) to (D) Confocal images of root hairs in plants stably transformed to express the F-actin marker venusYFP-fABD2. Note the exclusion of thick F-actin bundles from the wild-type cell apex (demarcated by dotted line) in (A), but not in (B) to (D), and the presence of additional root hair branches (white arrows) in (C).
(E) Proportion of the root hairs in which the thick longitudinal bundles of F-actin are excluded from the cell apex. The number of screened hairs in each data set was (from left to right) 19, 9, 9, 21, 9, and 9, respectively.
(F) to (I) Images of root hairs in plants stably transformed to express the ER marker ER-CFP. Note the presence of abundant spindle-shaped ER bodies and diffuse ER material in (F) and (G), whereas in (H) and (I), the ER appears as a condensed reticulate network.
Columbia or Col, the parental Columbia-0 line.
Interestingly, analysis of full-grown root hairs in the maturation zone revealed that F-actin bundles are extended to the apical region in >90% of cells in Columbia and 100% of the observed root hairs in 3KO and 4KO mutants (Figures 8D and 8E). These results demonstrated that the myosin-dependent exclusion of the F-actin bundles from the apical zone of root hairs is developmentally regulated.
The ER organization in root hairs (Figures 8F to 8I) was strikingly distinct from that in leaf epidermal cells (Figures 6D and 6E). In elongating root hairs, most of the ER-specific CFP marker was present in motile ER bodies (Figures 8F and 8G; see Supplemental Movie 4 online). These spindle-shaped ER derivatives harbor β-glucosidase and are found in plants from the order Brassicales (Hara-Nishimura et al., 2004; Yamada et al., 2008). Consistent with the roles of myosins XI-K, XI-1, and XI-2 in ER transport (Ueda et al., 2010), the ER bodies were motionless in xi-k xi-1 xi-2 3KO plants (see Supplemental Movie 5 online). Despite the differences in F-actin organization in the apical cell areas between elongating root hairs in Columbia control versus xi-k xi-1 xi-2 3KO mutant plants (Figures 8A and 8B), no detectable changes in the ER organization in these variants were observed (Figures 8F and 8G). The ER organization in mature root hairs was distinct from that seen in growing root hairs (Figures 8H and 8I), as most of the ER marker was present in a less dynamic ER network composed of cisternae interconnected by strands.
DISCUSSION
Arabidopsis has emerged as an excellent model for investigating the roles of myosin motors in multicellular eukaryotes. The value of this model is underscored by heavy reliance of plant cell dynamics on myosins XI, which are structurally similar to the myosins V of fungi and animals (Foth et al., 2006; Li and Nebenfuhr, 2007; Avisar et al., 2008b). A recent crop of publications on plant myosins XI listed below has dramatically increased appreciation of this area of plant cell biology.
Using single gene knockouts in Arabidopsis, it was found that myosins XI-K and XI-2 function in root hair elongation and transport of Golgi stacks, mitochondria, and peroxisomes (Ojangu et al., 2007; Peremyslov et al., 2008). Analysis of double gene knockouts unmasked contributions of myosins XI-1 and XI-B to organelle transport and root hair growth that were not apparent in the xi-1 and xi-b single knockouts, due to functional overlap with myosins XI-K and XI-2 (Prokhnevsky et al., 2008). Furthermore, this analysis revealed the first, albeit moderate, plant growth phenotype in response to elimination of one pair of myosin paralogs, XI-K/1 (Prokhnevsky et al., 2008). Although these and other recent publications (Reisen and Hanson, 2007; Avisar et al., 2009; Sattarzadeh et al., 2009; Sparkes et al., 2009a) provided an intriguing glimpse into myosin functions, they also made it clear that myosin redundancy could conceal the full biological significance of myosin-dependent processes.
Here, we employed a straightforward approach of solving the redundancy problem by generating multiple gene knockout plants. We assumed that the highly expressed genes encoding myosins XI-K, XI-1, XI-2, and XI-I account for the majority of myosin-dependent processes in vegetative cells. The myosin XI-2 paralog, XI-B, was also included in our analysis due to its prominent role in root hair elongation. In contrast with previous myosin studies, which focused on isolated aspects of cell growth or dynamics, we conducted extensive analysis of the mutant phenotypes at three levels: whole plant, plant organs, and cellular, including several distinct cell types that expand via diffuse or polarized growth. We also investigated the trafficking of Golgi and peroxisomes and organization of the F-actin and ER networks in myosin-deficient cells. The following novel findings of this work, which were unattainable in previous studies, amounted to a conceptual step change in the field of plant myosin research.
Our first conceptual advancement is the demonstration that progressive elimination of the highly expressed class XI myosins results in an increasingly strong dwarfing phenotype and delayed plant development. The leaf rosette diameters and plant heights in the xi-k xi-1 xi-2 xi-i 4KO plants are ∼2.5-fold lower than those in Columbia and are more severe than the modest 30% change described for the xi-k/1 mutant (Prokhnevsky et al., 2008). Interestingly, root growth in multiple knockout plants is affected to a much lesser extent, attesting to a particular role of these myosins in aerial organ growth. In agreement with this, the flowering time in the 3KO and 4KO mutant plants is also progressively delayed, showing that myosins are required for normal plant development. Therefore, our work advances myosin-dependent motility as a novel factor involved in determination of plant organ size, in addition to the genetic and phytohormonal factors described earlier (Mizukami, 2001).
Our second significant finding is that the gradual “freezing” of myosin-dependent motility in 3KO and 4KO mutant plants correlates with a progressive reduction in cell expansion. In contrast with previous work limited to a single cell type (Prokhnevsky et al., 2008), here, we investigated four distinct leaf cell types that expand by diffuse growth. Strikingly, we reveal that the extent of size reduction is cell specific. We found that the size reduction is proportional to the normal cell size. In the myosin 4KOs, this extent varied from negligible in the smallest (∼20 μm) guard cells (Figure 4C), to a moderate ∼30% reduction in the medium-size (∼150 μm) mesophyll cells, to a drastic 2.5-fold reduction in the long (∼350 μm) midvein epithelial cells (Figure 4B). In comparison, only a marginal 15% size reduction in the latter cell type was described previously (Prokhnevsky et al., 2008). Therefore, the functional significance of the actomyosin motility increases with the distances at which it operates, making myosin motors a novel important factor for diffuse cell growth. It remains to be seen if the less-highly expressed myosins XI that were not considered here contribute to the growth of specific cell types, such as guard cells.
The strong correlation between the length of leaves and of the midvein epidermis cells in the myosin-deficient plants (cf. Figures 1D and 4A) presents the intriguing possibility of a causal relationship between the two. This possibility resonates with a pivotal role of epidermal cell layers in the entire program of leaf development (Savaldi-Goldstein et al., 2007; Savaldi-Goldstein and Chory, 2008).
The third important conclusion of our work is that the significance of myosin activity for polarized cell expansion is even more critical than its significance for diffuse cell growth. Our analysis demonstrates that the class XI myosins are essential for polarized elongation of root hairs, as root hair growth is nearly abolished in the triple and quadruple myosin gene knockout plants. Furthermore, myosins contribute to proper root hair initiation, transition from bulging to tip growth, selection of the growing tip, and organization of the F-actin in the elongating root hairs. Indeed, profound defects in the growing tip formation were observed in the xi-k xi-1 xi-2 xi-i and xi-k xi-2 xi-b xi-i variants (Figure 7). The swollen bud phenotype of the latter 4KO was reminiscent of the kojak mutant, except that the myosin mutant does not exhibit bursting of the root hair bud (Favery et al., 2001). This difference suggests that, unlike kojak, in which a wall polysaccharide deficiency apparently compromises the integrity of the bud cell wall, bulk delivery of the building materials to the tip was significantly affected in the myosin-deficient cells. On the other hand, trichoblasts with multiple root hair initiation sites, as seen in the xi-k xi-2 xi-b xi-i variant (Figure 7D), were also observed in Arabidopsis mutants with perturbed Rho GTPase-based control of root hair growth (Jones et al., 2002; Carol et al., 2005; Kost, 2008). This similarity indicates that class XI myosins may be involved in proper targeting of Rho or its cofactors.
It is important to emphasize that although the previous studies have implicated class XI myosins in root hair growth (Ojangu et al., 2007; Peremyslov et al., 2008; Prokhnevsky et al., 2008), they did not establish the full significance of myosin motors in this process. By contrast, this study demonstrates that myosins are essential for root hair elongation. Furthermore, it places myosin function downstream from the initiation of tip growth. This latter conclusion is based on the fact that the transition from bulge formation to rapid elongation, but not the initiation of the root hair bulge itself, is suppressed in several myosin knockouts.
Our fourth novel finding is that inactivation of the four highly expressed myosin XI genes leads to cessation of processive transport of Golgi stacks and peroxisomes (Figure 5). Therefore, we demonstrate that the mechanism of this transport is entirely myosin dependent, ending a long-standing controversy on the potential involvement of microtubule-associated motors (Lee and Liu, 2004). In comparison, only a reduction in the mean organelle velocity was reported previously for the double myosin knockout plants (Prokhnevsky et al., 2008).
It should be pointed out that we used the Golgi stacks and peroxisomes as a proxy to characterize a broader myosin role in endomembrane trafficking. As shown elsewhere, mitochondrial and ER transport in plant cells relies on class XI myosins (Prokhnevsky et al., 2008; Ueda et al., 2010). It seems likely that at least some pathways of vesicular trafficking in plants are also myosin dependent, by analogy with yeast (Bretcher, 2003). This theory is supported by examination of the myosin mutants using time-lapse differential interference contrast microscopy that reflects bulk movement of the cellular endomembranes, including small organelles, ER, and vesicular aggregates traditionally defined as cytoplasmic streaming (Shimmen and Yokota, 2004). As shown in Supplemental Movies 6 to 8 online, both xi-k xi-1 xi-2 3KO and the xi-k xi-1 xi-2 xi-i 4KO exhibit dramatic suppression of this rapid, long-distance trafficking of endomembranes.
Our fifth finding of broad significance to plant cell biology is that class XI myosins play a novel role in F-actin organization. We show that myosin XI elimination in two types of elongated cells, leaf midvein epidermal cells and root hairs, leads to profound and distinct changes in F-actin architecture (Figures 6 and 8). This work and a parallel study of the cotyledonary petiole cells (Ueda et al., 2010) cement the case for a dual function of plant myosins XI, in motility along F-actin tracks and in controlling the distribution of these tracks. Important corollaries of myosin activity in structuring F-actin are concomitant contributions to the overall ER architecture (Figure 6), motility (Ueda et al., 2010), and intricate arrangement of the ER components (Sparkes et al., 2009a). It will be intriguing to see if the newly discovered role of myosins XI in plastid dynamics (Natesan et al., 2009; Sattarzadeh et al., 2009) involves myosin-driven F-actin reorganization, transport of the plastid stromules, or both.
The mechanism whereby myosins contribute to F-actin organization is a fascinating problem for future study. It seems likely that myosin-dependent formation of thick longitudinal F-actin cables in epidermal cells involves promotion of F-actin bundling as proposed for the mammalian class X myosins (Tokuo et al., 2007). Stiffening of the F-actin network due to myosin-generated forces can also increase actin bundling (Mizuno et al., 2007; Szymanski and Cosgrove, 2009). An unexpected role of myosins in the exclusion of F-actin bundles from the apical zone of root hairs (Figure 8) requires a distinct interpretation. It is known that the transition from root hair bulge to polarized growth involves apical F-actin rearrangement (Kandasamy et al., 2009; Szymanski and Cosgrove, 2009). A similar process governed by F-actin remodeling proteins operates during the polarized growth of moss cells (Vidali et al., 2009). Our work shows that the class XI myosins play a major role in the dynamic F-actin organization required for polarized growth. Because the myosin-dependent exclusion of F-actin bundles is seen in the apical zone of growing, but not mature root hairs, this role is developmentally regulated (Figure 8).
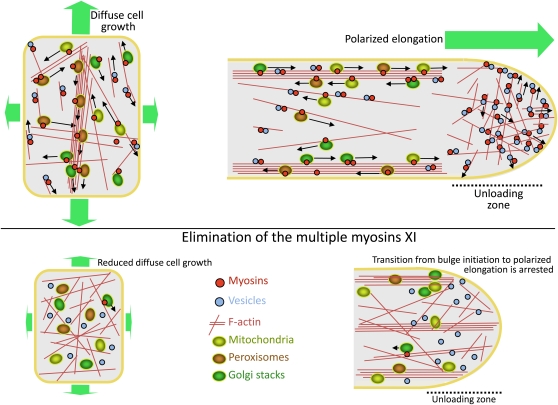
Taken together, our findings allow us to advance a general concept of myosin functions in plants. This concept proposes that myosin-driven cell interior dynamics are required for cell expansion that, in turn, translates into proper plant growth and development. Mechanistically, cell growth defects in response to myosin elimination are likely a cumulative result of the abolished motility of organelles and endomembrane vesicles and F-actin restructuring. A corresponding working model is presented in Figure 9. In addition to delivering organelles and vesicles to their destinations, myosin-driven motility increases cytosolic circulation and metabolite redistribution (Brangwynne et al., 2008). Consequently, the elevated metabolic status of the cells leads to more rapid cell growth and division (Crickmore and Mann, 2008; Edgar and Kim, 2009). Cessation of the myosin-dependent motility reduces diffuse cell growth and arrests polarized elongation (Figure 9). At the whole-plant level, this loss of motility results in stunting and a developmental impediment evident from delayed flowering (Figures 1 and 2).
Figure 9.
Hypothetical Model of the Mechanisms Whereby Myosins Contribute to Diffuse Cell Growth and Polarized Elongation.
Top: Myosin-dependent transport elevates the metabolic status of a cell via increased diffusion and delivers building materials to the cell periphery (left) or growing tip (right). Bottom: Inactivation of the highly expressed myosins ceases cytosolic stirring and targeted delivery of vesicles. Note the distinct patterns of F-actin reorganization in response to myosin inactivation in cells growing via diffuse or polarized mechanisms.
Intriguingly, primitive land plants, such as mosses, have only two or three class XI myosins and exhibit relatively slow organelle movement, as well as a small organism size. It seems possible that the myosin proliferation in higher plants allowed for more robust intracellular dynamics and larger organism size. Therefore, we advance the data-based hypothesis that addresses the biological role of a prominent and unique aspect of higher plant cell biology; strong actomyosin-powered intracellular dynamics. We posit that the increased intracellular motility gives higher plants a critical evolutionary advantage of faster growth and increased productivity.
In addition, our work sheds light on the evolution of the multigene myosin family by proposing that myosin proliferation in plants is dominated by gene duplication followed by gradual subfunctionalization. Two pairs of closely related myosin paralogs, XI-K/1 and XI-2/B, are the case in point. Myosin XI-K plays critical roles in the transport of Golgi, peroxisomes, mitochondria, and ER, as well as polarized elongation of root hairs, whereas myosin XI-1 contributes to organelle transport, but not to root hair growth. Myosin XI-2 is involved in transport of Golgi and peroxisomes, but not mitochondria; it also plays a role in root hair elongation. By contrast, myosin XI-B does not contribute to organelle transport but is specialized in polarized growth. Finally, myosin XI-I plays only marginal roles in organelle motility and cell growth, and these become apparent only when other highly expressed myosins are eliminated. Therefore, plant myosins XI exhibit varying degrees of functional redundancy and specialization.
METHODS
T-DNA Insertion Mutants
The single knockout lines of Arabidopsis thaliana ecotype Columbia-0, homozygous for inactivated genes encoding myosins XI-K, XI-1, XI-2, XI-B, and XI-I were described earlier (Peremyslov et al., 2008). These lines and the corresponding homozygous double knockout mutants xi-k xi-1, xi-k xi-2, and xi-2 xi-b (Prokhnevsky et al., 2008) were used for crossing to generate 3KO and 4KO lines. The progeny plants were screened by PCR for the presence of three or four mutant alleles and for the absence of corresponding wild-type alleles to generate four homozygous 3KO lines (xi-k xi-1 xi-2, xi-k xi-2 xi-b, xi-k xi-1 xi-i, and xi-k xi-2 xi-i) and three 4KO lines (xi-k xi-1 xi-2 xi-i, xi-k xi-1 xi-b xi-i, and xi-k xi-2 xi-b xi-i). The offspring of each line were again screened by PCR to demonstrate a lack of segregation for each of the mutant loci. For the genetic rescue experiments, a 12.3-kb genomic fragment encompassing the XI-K (At5g20490) gene together with the upstream 1.2-kb and downstream 0.8-kb flanking sequences (positions 6938542 to 6926216 on chromosome 5) was amplified using a Qiagen Long Range PCR Kit and cloned into a binary vector pMDC32 (Curtis and Grossniklaus, 2003) using cloning sites AscI and MauBI. The resulting plasmid was modified to delete the 35S promoter and leader sequence between sites SbfI and AscI. Coding sequence of the FLAG epitope (5′-GACTACAAGGACGACGATGACAAG-3′, DYKDDDDK) was inserted by overlapping PCRs between nucleotides 6927066 and 6927067, resulting in the in-frame addition of the FLAG epitope to the C terminus of myosin XI-K. The xi-k xi-1 xi-2 3KO plants were transformed with this genomic clone; the resulting line was designated xi-kR xi-1 xi-2. A transgenic line expressing a nearly wild-type level of the XI-KFLAG myosin was selected by immunoblot assay using a XI-K–specific rabbit polyclonal antiserum diluted 1:5000 (Peremyslov et al., 2008) or a FLAG-specific mouse monoclonal antibody (GenScript). SDS-PAGE was done using a 10% resolving gel. The selected protein markers shown in Figure 1F were from the Prestained Protein Ladder PageRuler (Fermentas).
Plant Phenotypes
Plants were grown in the greenhouse with a 16/8-h light/dark cycle (temperatures of ∼24°C/daytime and 18°C/nighttime with a variation of ±2°C; typical light intensity of ∼400 μmol m−2 s−1 photosynthetic photon flux) in 72-well flats and used for phenotypic characterization (Prokhnevsky et al., 2008). Typically, 20 to 30 plants of each line were used for measurements of plant height and leaf rosette diameter at 6 weeks after sowing (Figures 1D and 1E; see Supplemental Tables 1 and 2 online). Leaf rosette diameter was measured as the greatest distance between the apices of two opposite leaves. For determination of flowering time, groups of 36 plants were monitored every 2 d starting at day 16 until all of the plants developed bolts (Figure 2A). Bolt appearance was registered when the internode between the upper cauline leaf and inflorescence became visible. Root lengths were determined for groups of 20 plants growing on vertical plates between 3 and 8 d postgermination (Figure 2B). Mean leaf surfaces were determined using ImageJ software (National Institutes of Health) by scanning and measuring surface areas of the two largest leaves from each of 10 rosettes (Figure 3A). Mean surface areas and/or lengths of the abaxial pavement cells (Figures 3B and 3C), mesophyll cells (Figures 3D and 3E), elongated epidermal cells of the lower side of midvein (Figures 4A and 4B), and guard cells (Figure 4C) were determined using 20 images for each variant. Leaves for imaging were cleared overnight in ethanol, rehydrated in 0.1 M K-citrate buffer, pH 4.4, and stained with 0.01% Toluidine blue in the same buffer. Cell images were acquired with a Leica DMRB microscope equipped with a CCD camera and measured using Image-Pro (Media Cybernetics). For each experimental variant, the cell areas in μm2 were determined for 240 pavement or leaf mesophyll cells using ImageJ software.
For root hair analysis, seeds were grown on vertical plates containing 0.5× Murashige and Skoog medium, 5 mM MES, pH 5.8, 2% sucrose, and 0.6% Phytogel. Root hairs of 5-d-old seedlings were photographed and measured as described (Peremyslov et al., 2008). At least 200 root hairs were measured to determine the mean lengths for each plant line shown in Figure 7. To image the root hairs, 4-d-old roots were incubated in 20 mM propidium iodide (MP Biomedical) for 10 min and subjected to microscopy using a LSM 510 laser scanning confocal microscope (Zeiss) and the 543-nm line of the HeNe laser for excitation and 620-nm long-pass filter for emission detection.
All data sets were analyzed in a Microsoft Excel spreadsheet. Statistical analyses included determination of the mean values, standard errors of the mean, and t tests (see Supplemental Tables 1 to 12 online).
Organelle Trafficking
To analyze organelle motility, each plant line was stably transformed with the Golgi marker NAG-EGFP (the transmembrane stem region of Arabidopsis N-acetylglucosaminyl transferase I) (Grebe et al., 2003) or the peroxisome marker CSY2-GFP containing the targeting signal of Arabidopsis peroxisomal citrate synthase 2 (Pracharoenwattana et al., 2005). The resulting T1 plant lines exhibiting hygromycin resistance were grown, and the transgenic lines expressing organelle markers were selected using epifluorescent microscopy. Time-lapse confocal microscopy was performed by acquiring consecutive images at 1-s intervals (Avisar et al., 2008b). Fluorescent Golgi stacks and peroxisomes were observed in single confocal planes of the leaf epidermal cells using an LSM 510 laser scanning confocal microscope (Zeiss) equipped with a krypton/argon laser at 488-nm excitation line, 488 dichroic mirror, and 500 to 530 band-pass filter settings. Tracking and measurements of displacement rates of individual organelles was performed using Volocity 3.7.0 Classification software (Improvision, Image Processing and Vision Company), which allows for simultaneously tracking of multiple moving objects (Figures 5A and 5C). As described earlier (Prokhnevsky et al., 2008), organelle tracks acquired by software were edited manually to exclude infrequent erroneous tracks. At least 200 individual organelles were analyzed for each experimental variant.
F-Actin and ER Organization
To visualize F-actin, plants were transformed to stably express venusYFP-fABD2, a marker containing actin binding domain 2 of Arabidopsis fimbrin (Sheahan et al., 2004) fused to an enhanced YFP variant Venus (Nagai et al., 2002). Analogously, ER was visualized using an ER-targeted CFP derived from an original marker containing the N-terminal signal peptide of the Arabidopsis vacuolar basic chitinase and the C-terminal amino acid sequence HDEL (Haseloff et al., 1997) via replacement of GFP with CFP. Both markers were subcloned into the pCB302 minibinary plasmid (Xiang et al., 1999) and modified by adding the cauliflower mosaic virus 35S promoter and terminator sequences (Prokhnevsky et al., 2002). Selection of transgenic plants was performed on plates supplemented with 0.1 mM glufosinate ammonium (Crescent Chemical). Confocal imaging of F-actin in epidermal cells (Figures 6A to 6C and 6F) or root hairs (Figures 8A to 8E) was performed as described above for the organelles except that the 514-nm excitation line and the 530- to 600-nm band-pass filter were used to collect images. For ER-CFP visualization, the 405-nm excitation line of the diode laser and the 470- to 500-nm band-pass filter were used. Images shown in Figures 6D and 6E are stacks of three consecutive optical planes of a total depth of 6 μm that primarily represent the cortical cytoplasm.
Accession Numbers
The T-DNA insertion lines and the accession numbers for the following myosin XI genes were as follows: XI-K (SALK_067972; At5g20490), XI-1 (SALK_019031; At1g17580), XI-2 (SALK_055785; At5g43900), XI-B (SALK_113062; At1g04160), and XI-I (SALK_082443; At4g33200).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Plant Fecundity Expressed as the Number of Seeds per Silique (Percentage of the Columbia-0 Control) Is Reduced in the Triple (3KO) and Quadruple (4KO) Myosin Knockout Mutants.
Supplemental Figure 2. Confocal Images of the Cells in Plants Stably Transformed to Express F-Actin Marker VenusYFP-fABD2.
Supplemental Table 1. Data for Figure 1D: Leaf Rosette Diameter (mm).
Supplemental Table 2. Data for Figure 1E: Plant Height (mm).
Supplemental Table 3. Data for Supplemental Figure 1: Number of Seeds per Silique.
Supplemental Table 4. Data for Supplemental Figure 2B: Root Length (mm).
Supplemental Table 5. Data for Figure 3A: Leaf Surface Area (mm2).
Supplemental Table 6. Data for Figure 3B: Pavement Cell Surface Area (μm2).
Supplemental Table 7. Data for Figure 3C: Mesophyll Cell Surface Area (μm2).
Supplemental Table 8. Data for Figure 4A: Epidermal Cell Length (μm).
Supplemental Table 9. Data for Figure 4C: Guard Cell Length (μm).
Supplemental Table 10. Data for Figure 5B: Golgi Displacement Rate (μm/s).
Supplemental Table 11. Data for Figure 5D: Peroxisome Displacement Rate (μm/s).
Supplemental Table 12. Data for Figure 7A: Root Hair Length (μm).
Supplemental Movie 1. Time-Lapse Confocal Scanning Microscopy of the Leaf Midvein Epidermis Cells Stably Expressing Golgi-Specific Reporter NAG-EGFP in the Columbia-0 Plants.
Supplemental Movie 2. Time-Lapse Confocal Scanning Microscopy of the Leaf Midvein Epidermis Cells Stably Expressing Golgi-Specific Reporter NAG-EGFP in the xi-k xi-1 xi-2 3KO Plants.
Supplemental Movie 3. Time-Lapse Confocal Scanning Microscopy of the Leaf Midvein Epidermis Cells Stably Expressing Golgi-Specific Reporter NAG-EGFP in the xi-k xi-1 xi-2 xi-i 4KO Plants.
Supplemental Movie 4. Time-Lapse Confocal Scanning Microscopy of the Elongating Root Hairs Stably Expressing ER-Specific YFP Reporter in the Columbia-0 Plants.
Supplemental Movie 5. Time-Lapse Confocal Scanning Microscopy of the Elongating Root Hairs Stably Expressing ER-Specific YFP Reporter in the xi-k xi-1 xi-2 3KO Plants.
Supplemental Movie 6. Time-Lapse Differential Interference Contrast Microscopy of the Leaf Midvein Epidermis Cells in the Columbia-0 Plants.
Supplemental Movie 7. Time-Lapse Differential Interference Contrast Microscopy of the Leaf Midvein Epidermis Cells in the xi-k xi-1 xi-2 3KO Plants.
Supplemental Movie 8. Time-Lapse Differential Interference Contrast Microscopy of the Leaf Midvein Epidermis Cells in the xi-k xi-1 xi-2 xi-i 4KO Plants.
Supplemental Movie Legends.
Acknowledgments
We thank John Fowler, Ikuko Hara-Nishimura, Amy Klocko, Eugene Koonin, and Daniel Szymanski for critical reading of the manuscript, and Ben Scheres, David McCurdy, and Steven Smith for providing organelle-specific reporters. This work was supported by National Institutes of Health American Recovery and Reinvestment Act Award GM087658 to V.V.D.
References
- Avisar D., Prokhnevsky A.I., Dolja V.V. (2008a). Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog. J. Virol. 82: 2836–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avisar D., Prokhnevsky A.I., Makarova K.S., Koonin E.V., Dolja V.V. (2008b). Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. 146: 1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avisar D., Abu-Abied M., Belausov E., Sadot E., Hawes C., Sparkes I.A. (2009). A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol. 150: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Horton A.C., Sevener H.C., Quatrano R.S. (2003). Phylogenetic analysis of new plant myosin sequences. J. Mol. Evol. 57: 229–239 [DOI] [PubMed] [Google Scholar]
- Brangwynne C.P., Koenderink G.H., MacKintosh F.C., Weitz D.A. (2008). Cytoplasmic diffusion: Molecular motors mix it up. J. Cell Biol. 183: 583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretcher A. (2003). Polarized growth and organelle segreation in yeast: The tracks, motors, and receptors. J. Cell Biol. 160: 811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol R.J., Takeda S., Linstead P., Durrant M.C., Kakesova H., Derbyshire P., Drea S., Zarsky V., Dolan L. (2005). A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Cole R.A., Fowler J.E. (2006). Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 9: 579–588 [DOI] [PubMed] [Google Scholar]
- Crickmore M.A., Mann R.S. (2008). The control of size in animals: Insights from selector genes. Bioessays 30: 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B.A., Kim K.J. (2009). Sizing up the cell. Science 325: 158–159 [DOI] [PubMed] [Google Scholar]
- Embley T.M., Martin W. (2006). Eukaryotic evolution, changes and challenges. Nature 440: 623–630 [DOI] [PubMed] [Google Scholar]
- Favery B., Ryan E., Foreman J., Linstead P., Boudonck K., Steer M., Shaw P., Dolan L. (2001). KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev. 15: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth B.J., Goedecke M.C., Soldati D. (2006). New insights into myosin evolution and classification. Proc. Natl. Acad. Sci. USA 103: 3681–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb L., Abu-Abied M., Belausov E., Sadot E. (2008). Different subcellular localizations and functions of Arabidopsis myosin VIII. BMC Plant Biol. 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe M., Xu J., Mobius W., Ueda T., Nakano A., Geuze H.J., Rook M.B., Scheres B. (2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13: 1378–1387 [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I., Matsushima R., Shimada T., Nishimura M. (2004). Diversity and formation of endoplasmic reticulum-derived compartments in plants. Are these compartments specific to plant cells? Plant Physiol. 136: 3435–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Siemering K.R., Prasher D.C., Hodge S. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.A., Shen J.J., Fu Y., Li H., Yang Z., Grierson C.S. (2002). The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M.K., McKinney E.C., Meagher R.B. (2009). A single vegetative actin isovariant overexpressed under the control of multiple regulatory sequences is sufficient for normal Arabidopsis development. Plant Cell 21: 701–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. (2008). Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol. 18: 119–127 [DOI] [PubMed] [Google Scholar]
- Lee Y.-R.J., Liu B. (2004). Cytoskeletal motors in Arabidopsis. Sixty-one kinesins and seventeen myosins. Plant Physiol. 136: 3877–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.D., Jung H.S., Mabuchi K., Craig R., Ikebe M. (2006). The globular tail domain of myosin Va functions as an inhibitor of the myosin Va motor. J. Biol. Chem. 281: 21789–21798 [DOI] [PubMed] [Google Scholar]
- Li J.F., Nebenfuhr A. (2007). The tail that wags the dog: The globular tail domain defines the function of myosin V/XI. Traffic 9: 1–9 [DOI] [PubMed] [Google Scholar]
- Maule A.J. (2008). Plasmodesmata: Structure, function, and biogenesis. Curr. Opin. Plant Biol. 11: 680–686 [DOI] [PubMed] [Google Scholar]
- Micol J.L. (2009). Leaf development: Time to turn over a new leaf? Curr. Opin. Plant Biol. 12: 9–16 [DOI] [PubMed] [Google Scholar]
- Mizukami Y. (2001). A matter of size: Developmental control of organ size in plants. Curr. Opin. Plant Biol. 4: 533–539 [DOI] [PubMed] [Google Scholar]
- Mizuno D., Tardin C., Schmidt C.F., Mackintosh F.C. (2007). Nonequilibrium mechanics of active cytoskeletal networks. Science 315: 370–373 [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E.S., Kubota M., Mikoshiba K., Miyawaki A. (2002). A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20: 87–90 [DOI] [PubMed] [Google Scholar]
- Natesan S.K., Sullivan J.A., Gray J.C. (2009). Myosin XI is required for actin-associated movement of plastid stromules. Mol. Plant 2: 1262–1272 [DOI] [PubMed] [Google Scholar]
- Ojangu E.L., Jarve K., Paves H., Truve E. (2007). Arabidopsis thaliana myosin XIK is involved in root hair as well as trichome morphogenesis on stems and leaves. Protoplasma 230: 193–202 [DOI] [PubMed] [Google Scholar]
- Peremyslov V.V., Prokhnevsky A.I., Avisar D., Dolja V.V. (2008). Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol. 146: 1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka J.J., Benfey P.N. (2008). Root layers: Complex regulation of developmental patterning. Curr. Opin. Genet. Dev. 18: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D., Cooper J.A. (2009). Actin, a central player in cell shape and movement. Science 326: 1208–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I., Cornah J.E., Smith S.M. (2005). Arabidopsis peroxisomal citrate synthase is required for fatty acid respiration and seed germination. Plant Cell 17: 2037–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky A.I., Peremyslov V.V., Dolja V.V. (2008). Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc. Natl. Acad. Sci. USA 105: 19744–19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky A.I., Peremyslov V.V., Napuli A.J., Dolja V.V. (2002). Interaction between long-distance transport factor and Hsp70-related movement protein of beet yellows virus. J. Virol. 76: 11003–11011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretcher A. (2004). Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20: 559–591 [DOI] [PubMed] [Google Scholar]
- Reddy A.S., Day I.S. (2001). Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2: RESEARCH0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt S., Knight A.E., Hodge T.P., Baluska F., Samaj J., Volkmann D., Kendrick-Jones J. (1999). Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J. 19: 555–567 [DOI] [PubMed] [Google Scholar]
- Reisen D., Hanson M.R. (2007). Association of six YFP-myosin XI-tail fusions with mobile plant cell organelles. BMC Plant Biol. 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattarzadeh A., Franzen R., Schmelzer E. (2008). The Arabidopsis class VIII myosin ATM2 is involved in endocytosis. Cell Motil. Cytoskeleton 65: 457–468 [DOI] [PubMed] [Google Scholar]
- Sattarzadeh A., Krahmer J., Germain A.D., Hanson M.R. (2009). A myosin XI tail domain homologous to the yeast myosin vacuole-binding domain interacts with plastids and stromules in Nicotiana benthamiana. Mol. Plant 2: 1351–1358 [DOI] [PubMed] [Google Scholar]
- Savaldi-Goldstein S., Chory J. (2008). Growth coordination and the shoot epidermis. Curr. Opin. Plant Biol. 11: 42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S., Peto C., Chory J. (2007). The epidermis both drives and restricts plant shoot growth. Nature 446: 199–202 [DOI] [PubMed] [Google Scholar]
- Sheahan M.B., Staiger C.J., Rose R.J., McCurdy D.W. (2004). A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells. Plant Physiol. 136: 3968–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmen T., Yokota E. (2004). Cytoplasmic streaming in plants. Curr. Opin. Plant Biol. 16: 68–72 [DOI] [PubMed] [Google Scholar]
- Smith L.G., Oppenheimer D.G. (2005). Spatial control of cell expansion by the plant cytoskeleton. Annu. Rev. Cell Dev. Biol. 21: 271–295 [DOI] [PubMed] [Google Scholar]
- Sparkes I., Runions J., Hawes C., Griffing L. (2009a). Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21: 3937–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I.A., Frigerio L., Tolley N., Hawes C. (2009b). The plant endoplasmic reticulum: A cell-wide web. Biochem. J. 423: 145–155 [DOI] [PubMed] [Google Scholar]
- Sparkes I.A., Teanby N.A., Hawes C. (2008). Truncated myosin XI tail fusions inhibit peroxisome, Golgi, and mitochondrial movement in tobacco leaf epidermal cells: A genetic tool for the next generation. J. Exp. Bot. 59: 2499–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger C.J., Sheahan M.B., Khurana P., Wang X., McCurdy D.W., Blanchoin L. (2009). Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J. Cell Biol. 184: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski D.B., Cosgrove D.J. (2009). Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 19: R800–R811 [DOI] [PubMed] [Google Scholar]
- Tokuo H., Mabuchi K., Ikebe M. (2007). The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J. Cell Biol. 179: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M., Kojima H., Yokota E., Orii H., Nakamori R., Katayama E., Anson M., Shimmen T., Oiwa K. (2003). Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J. 22: 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K.M. (2008). Myosin V from head to tail. Cell. Mol. Life Sci. 65: 1378–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Yokota E., Kutsuna N., Shimada T., Tamura K., Shimmen T., Hasezawa S., Dolja V.V., Hara-Nishimura I. (2010). Myosin-dependent ER motility and F-actin organization in plant cells. Proc. Natl. Acad. Sci. USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R.D. (2003). The molecular motor toolbox for intracellular transport. Cell 112: 467–480 [DOI] [PubMed] [Google Scholar]
- Valiathan R.R., Weisman L.S. (2008). Pushing for answers: Is myosin V directly involved in moving mitochondria? J. Cell Biol. 181: 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., van Gisbergen P.A.C., Guerin C., Franco P., Li M., Burkart G.M., Augustine R.C., Blanchoin L., Bezanilla M. (2009). Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc. Natl. Acad. Sci. USA 106: 13341–13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Edwards J.G., Riley N., Provance D.W., Karcher R., Li H.-D., Davison I.G., Ikebe M., Mercer J.A., Kauer J.A., Ehlers M.D. (2008). Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135: 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner S., Bement W.M. (2009). Unconventional myosins acting unconventionally. Trends Cell Biol. 19: 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C., Han P., Lutziger I., Wang K., Oliver D.J. (1999). A mini binary vector series for plant transformation. Plant Mol. Biol. 40: 711–717 [DOI] [PubMed] [Google Scholar]
- Yamada K., Nagano A.J., Nishina M., Hara-Nishimura I., Nishimura M. (2008). NAI2 is an endoplasmic reticulum body component that enables ER body formation in Arabidopsis thaliana. Plant Cell 20: 2529–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]