Abstract
Arsenic has been a recognized contaminant and toxicant, as well as a medicinal compound throughout human history. Populations throughout the world are exposed to arsenic and these exposures have been associated with a number of human cancers. Not much is known about the role of arsenic as a human carcinogen and more recently its role in non-cancerous diseases, such as cardiovascular disease, hypertension and diabetes mellitus have been uncovered. The health effects associated with arsenic are numerous and the association between arsenic exposure and human disease has intensified the search for molecular mechanisms that describe the biological activity of arsenic in humans and leads to the aforementioned disease states. Arsenic poses a human health risk due in part to the regulation of cellular signal transduction pathways and over the last few decades, some cellular mechanisms that account for arsenic toxicity, as well as, signal transduction pathways have been discovered. However, given the ubiquitous nature of arsenic in the environment, making sense of all the data remains a challenge. This review will focus on our knowledge of signal transduction pathways that are regulated by arsenic.
Keywords: Arsenic, Arsenite, Arsenate, Signal transduction
Introduction
Humans have been exposed to arsenic throughout the course of history, and the toxicity and medicinal properties associated with arsenic have been recognized for more than two centuries (Zhu et al. 2002). Most often, humans are unknowingly exposed to arsenic by ingestion of contaminated ground water because arsenic is physically associated with geological formations and leaches into ground water supplies through natural geological processes. Without water purification systems or water monitoring procedures, human consumption of drinking water that has been contaminated with arsenic is the most common route of exposure, often due to the lack of alternative drinking water supplies. In addition to natural environmental contamination, human activity, such as the mining of precious metals has created a new mechanism for arsenic exposure, when human exposures are considered from a historical perspective. The smelting or enrichment procedures that are used to isolate the valuable metals from the impurities leave arsenic behind in mine tailings. Consequently, arsenic either leaches into the ground water or particulates in air are contaminated with arsenic due to erosion and wind near these mining sites. The burning of coal and the production of coal fly ash is another source of arsenic that contributes a small fraction of airborne arsenic (Chappell et al. 1999). Airborne particulates trap arsenic which is then inhaled by humans and likely contributes to the deleterious health effects associated with arsenic exposure. Regardless of the route of exposure, arsenic poses a human health risk due in part, to the activation or inhibition of cellular signal transduction pathways.
Human populations throughout the world are exposed to arsenic and these exposures have been associated with skin cancer and cancers of the bladder, kidney, liver and lung (Smith et al. 1998). In addition, arseniasis is manifested by other clinical conditions that include skin hyperpigmentation and depigmentation, palmoplantal hyperkeratosis, dermatitis, gastroenteritis, bronchitis, peripheral polyneuritis and polyneuropathy, hepatopathy, conjunctivitis, lens opacity, diabetes mellitus, mental retardation, ischemic heart disease, electrocardiographic abnormality, cerebrovascular accident, peripheral vascular disease leading to gangrene of the limb, microcirculation abnormality and hypertension (Chappell et al. 1999). Although the health effects associated with arsenic are numerous and even if some of these health effects are later found unrelated to arsenic exposure, the mere recognition of the association between arsenic exposure and human disease has intensified the search for molecular mechanisms that elucidate the biological activity of arsenic in humans. With the increasing difficulty, municipalities face to provide safe drinking water to their residents due to scarce resources and it is becoming increasingly more important for scientists and clinicians to provide sound and rational scientific evidence that effectively reassures the public of the risks associated with arsenic exposure.
Over the last few decades, some of the cellular mechanisms that account for arsenic toxicity, as well as the signal transduction pathways regulated by arsenic, have been uncovered. However, given the ubiquitous nature of arsenic in the environment, making sense of all the data remains a challenge. Although other review articles have been written on the subject of arsenic in recent years (Carter et al. 2003; Hughes 2009; Kitchin and Wallace 2008; Thomas et al. 2001, 2007) this review will focus on our knowledge of signal transduction pathways that are regulated by arsenic.
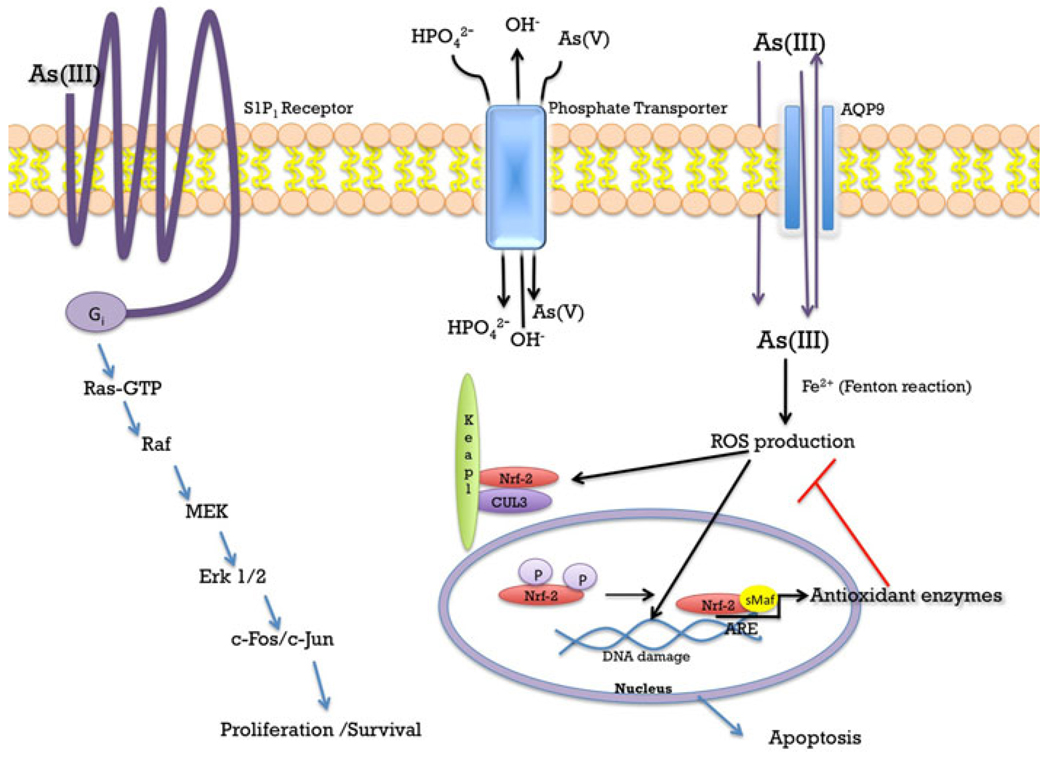
With regard to environmental human exposures, arsenate [H3AsO4] and arsenite [As(OH)3] are the principle arsenic species of concern. Recent review articles have discussed the chemical and metabolic aspects of arsenic and the reader is referred to those articles for further details on the subject (Carter et al. 2003; Thomas et al. 2001). Movement of arsenate into the cell occurs through phosphate transport proteins due to the fact that arsenate, with an oxidation state of (V), is chemically similar to phosphate (Bennett and Malamy 1970; Bun-ya et al. 1996). With serum concentrations of phosphorous at approximately 3.4 mg/dL [34,000 µg/L; de Boer et al. (2009)], an individual exposed to 50 µg/L (50 ppb) arsenate is likely to have very little cellular uptake of arsenate due to competition with the relatively higher concentration (680-fold) of phosphate in serum (Fig. 1). Therefore, when researchers study arsenic, arsenite is the species that is usually studied.
Fig. 1.
Arsenic signaling. Arsenate [As(V)] may enter the cell through phosphate anti-transporters located at the cell membrane, which normally transport phosphate into the cell and hydroxide out of the cell. Arsenite [As(III)] may enter the cell via cell diffusion or through aquaporin transporters (AQP9) located the cell membrane. There is evidence that AQP9 may transport arsenite out of the cell. At physiological pH arsenate is reduced to arsenite, which may undergo a Fenton reaction and produce reactive oxygen species (ROS). ROS may interact with NF-E2 related factor 2 (Nrf2) which would then cause Nrf2 to disassociate from the Keap1-Cul3 complex and translocate to the nucleus. In the nucleus, Nrf2 binds to a small MAF protein and to the antioxidant response element (ARE) located at the promoter region of phase II and antioxidant genes, resulting in the transcription of anti-oxidant enzymes such as NQO1, GST, and HO-1. Arsenite activates the G-protein-coupled receptor, S1P1. Activation of the S1P1 receptor has been shown to activate the Ras-Raf pathway leading to cell proliferation and survival
Arsenate is reduced to arsenite [As(V) → As(III)] and at physiological pH, arsenite is an uncharged species. As such, arsenite can gain entry into the cell by diffusion, making arsenite potentially much more toxic than arsenate. Moreover, arsenite can enter cells via transport proteins. Mammalian aquaglyceroporin (AQP9) has been reported to transport arsenite into cells when expressed in yeast (Liu et al. 2002) and lack of AQP9 expression in mice leads to increased toxicity, suggesting that arsenite export from cells is also mediated by AQP9 (Carbrey et al. 2009) (Fig. 1). The latter study re-emphasizes that arsenite uptake also occurs through AQP9-independent mechanisms which have not been characterized to date. Once inside the cell, arsenite may be metabolized to methylated species through reduction and methylation reactions. In cells, such as adipocytes and myocytes, for example, arsenite is not metabolized while arsenite is metabolized to dimethylarsinic acid [DMAs(V)] in hepatocytes. Undoubtedly, these differences in arsenic metabolism will result in the regulation of unique signal transduction pathways.
In vascular endothelial or liver sinusoidal endothelial cells, arsenite stimulates the sphingosine-1-phosphate receptor (S1P1), which is a G protein-coupled receptor (Straub et al. 2009). Given that arsenic exposure typically occurs via ingestion of contaminated drinking water, it is particularly disconcerting to know that arsenic functions as a sphingosine-1-phosphate receptor mimetic (Fig. 1). The treatment of endothelial cells with pertussis toxin, which is a selective inhibitor of the Go and Gi family of G proteins, abrogated arsenite-dependent responses further re-enforcing the fact that arsenite is acting through an extracellular receptor. Moreover, knocking down sphingosine-1-phosphate receptor expression by siRNA resulted in the loss of arsenite-dependent Rac1 GTPase activity. These results clearly demonstrate that arsenite at environmentally relevant doses is capable of stimulating signaling pathways that elicit physiological responses. Of greater concern is the fact that Rac signaling is essential in cell survival and cell motility, which are signaling events that are hallmarks of tumorigenic transformation and metastasis (Sun et al. 2006). Knowing that arsenite can activate Rac through the sphingosine-1-phosphate receptor may help in explaining how arsenite contributes to the development of cancer and may identify the sphingosine-1-phosphate receptor as a therapeutic target in cancer prevention.
In liver sinusoidal endothelial cells, extensive fenestrations and weak junctional connections between cells enables liver hepatocytes to pass nutrients and macromolecular waste for efficient metabolism under normal physiological conditions. In the presence of arsenite, pathological defenestration and capillarization of sinusoidal endothelial cells induced the expression of junctional platelet endothelial cell adhesion molecule (PECAM)-1 (Straub et al. 2008). In addition, the NADPH oxidase system is required for capillarization of sinusoidal endothelial cells, which indicates that superoxide and reactive oxygen species (ROS) are also part of the pathological signaling mechanism. In another study investigating the role of the NADPH oxidase pathway, Suzuki et al. used apocynin, an inhibitor of NADPH oxidase, and demonstrated decreased cytotoxicity of rat bladder epithelial cells (Suzuki et al. 2009). Because the Rac1-GTPase is activated by arsenite through stimulation of the sphingosine-1-phosphate receptor, at least in endothelial cells and possibly epithelial cells, arsenite regulates a signal transduction pathway that begins with a G protein-coupled receptor, G protein, and effector enzyme (i.e. NADPH oxidase) that is capable of increasing PECAM-1 gene transcription just like other canonical G protein-coupled receptor pathways. These results confirm what many in the signaling Weld believed all along, that is, arsenic is not merely a cellular toxicant, but has pleiotropic effects because it is also capable of activating cellular responses by stimulating protein activity (Fig. 1).
Arsenic and diabetes
Historically, cancer has been the public health concern typically associated with arsenic exposure. However, the discovery of unique populations in Bangladesh, Taiwan, and Chile that have been chronically exposed to arsenic, usually in their drinking water, has allowed epidemiologists to investigate other clinical conditions associated with arsenic exposure. More recently, researchers have evaluated epidemiological data suggesting a causal link between arsenic exposure and diabetes (Navas-Acien et al. 2008, 2009; Steinmaus et al. 2009). Although lacking consensus on the issue, there is biochemical data to support the idea that arsenic can adversely affect glucose homeostasis and the insulin signaling pathway, thus providing evidence that arsenic could cause or exacerbate the development of diabetes mellitus.
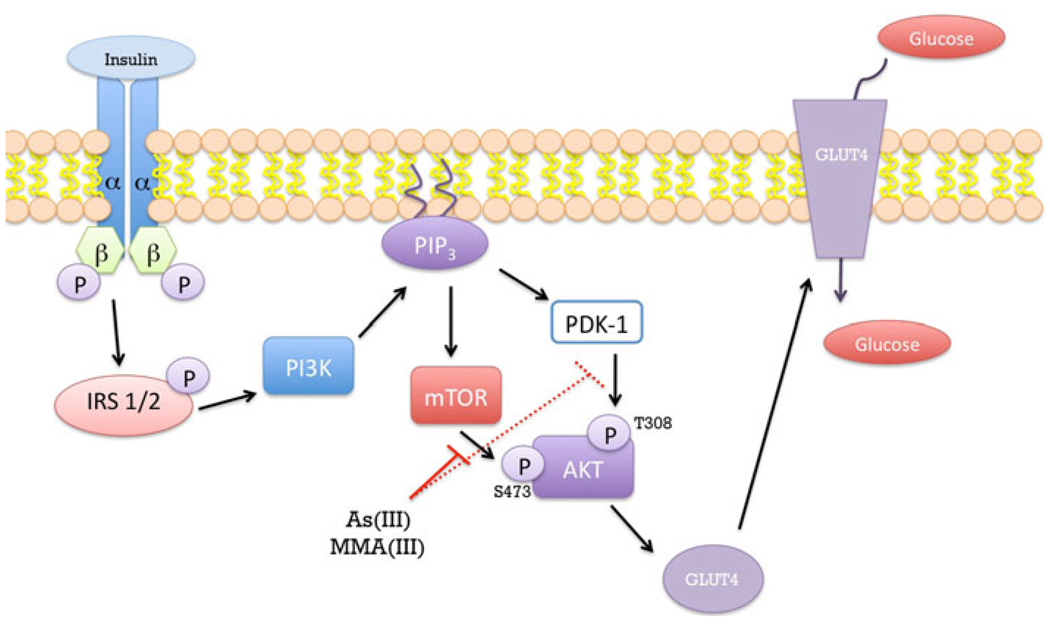
Disruption of glucose homeostasis can involve impaired glucose utilization or insulin resistance by peripheral tissues, such as skeletal muscle and adipose tissue. In C57BL/6 mice that were exposed to 50 ppb As(III) in the drinking water, the mice had high expression of hexokinase II indicative of dysregulation of glucose homeostasis (Pysher et al. 2007). In adipocytes, 50 µM arsenite inhibited insulin-dependent glucose uptake with an estimated IC50 of 25 µM (Paul et al. 2007). In contrast, the monomethylated form of arsenite, monomethylarsonous acid [MMA(III)] inhibited insulin-dependent glucose uptake with an IC50 of 4 µM, demonstrating the greater potency of MMA(III) over arsenite. Both arsenite and MMA(III) inhibited GLUT4 recruitment to the plasma membrane of insulin-stimulated adipocytes. Further analysis of the proteins in the insulin signaling pathway demonstrated a loss of phosphorylation of Akt at Thr308 within the activation loop and Ser473 in the hydrophobic motif of the carboxyl terminus. Akt is required for GLUT4 translocation and glucose transport (Kohn et al. 1996), and loss of phosphorylation at essential sites required for Akt activation suggests that arsenite and MMA(III) act upstream of Akt (Fig. 2).
Fig. 2.
Mechanisms of arsenic-induced insulin resistance. Under normal conditions insulin binds to the insulin receptor located at the cell membrane. Activated insulin receptor autophosphorylates and trans-phosphorylates the β subunits of the receptor located at the innermembrane. This is followed by phosphorylation of the insulin receptor substrate (IRS) causing activation of the phosphatidylinositol 3-kinase (PI3K) pathway leading to phosphorylation of AKT at serine 473 by mTOR. This phosphorylation allows for phosphorylation of AKT at threonine 308 by PDK-1. These phosphorylations of AKT at serine 473 and threonine 308 allow for the stimulation of GLUT4 from cellular vesicles to translocate to the cell membrane and allows for glucose to enter the cell. It is postulated that arsenite and its metabolite MMA(III) directly inhibit the phosphorylation of AKT at serine 473 by mTOR (continuous red line) and indirectly inhibit phosphorylation of threonine 308 by PDK-1 (broken red line) thereby preventing the translocation of the GLUT4 transporter to the cell membrane and leading to insulin resistance
It seems unlikely that both arsenite and MMA(III) inhibit two different enzymes since the implication is that PDK and rictor-mTOR have the same “arsenic” binding site. Phosphoinositide-dependent kinase (PDK)-1 is known to phosphorylate Akt at Thr308 (Alessi et al. 1997) and the rictor-mTOR (mammalian target of rapamycin) complex phosphorylates Ser473 (Sarbassov et al. 2005). If one considers the molecular mechanism for the dual phosphorylation of Akt, a more viable explanation emerges. Scheid et al. demonstrated that PDK-dependent phosphorylation of Thr308 of Akt requires the prior phosphorylation of Ser473 by rictor-mTOR (Scheid et al. 2002). Inhibition of GLUT4 recruitment to the plasma membrane probably occurs through inhibition of rictor-mTOR since inhibition of rictor-mTOR would prevent phosphorylation of Ser473 and indirectly inhibit PDK-dependent phosphorylation of Thr308 (Fig. 2). Therefore, loss of dual phosphorylation of Akt could occur by inhibition of only rictor-mTOR.
mTOR is a central protein in two distinct multi-protein complexes consisting of mTOR complex 1 (mTORC1) and mTORC2 [reviewed in (Laplante and Sabatini 2009)]. mTORC1 consists of five proteins while mTORC2 has six proteins. Both mTORC1 and mTORC2 regulate cell metabolism, growth, proliferation and survival. Given the pleiotropic effects of arsenic in cell biology and toxicology, it may not be surprising to learn in the future that arsenic either inhibits or activates specific protein components in either of the mTOR signaling networks. However at this time, there are no reports evaluating mTOR activity in the presence of arsenite or MMA(III), but as the protein components of the mTOR pathways are further elucidated, studies evaluating the role of arsenite or MMA(III) in the mTOR pathways will become feasible.
Arsenic and ROS
Arsenite is known to chemically modify cysteine residues in proteins, with a preference for dithiols (Delnomdedieu et al. 1993). In bacteria, the arsRDABC operon confers resistance to arsenite. The arsD and arsR genes encode the ArsD and ArsR proteins, respectively. The function of the ArsD and ArsR proteins is to repress arsRDABC transcription. When arsenite concentrations increase in the environment of the bacteria, the ArsD and ArsR proteins bind arsenite which de-represses transcription of the arsRDABC operon because ArsD and ArsR are released from the arsR-DABC promoter DNA (Li et al. 2001). For ArsR, dithiols from cysteines 32 and 34 are needed for activity (Shi et al. 1996), while vicinal dithiols from cysteines 12–13 and 112–113 were needed for activity for ArsD (Li et al. 2001). These data demonstrate that arsenite affects protein activity through what appears to be covalent modification of cysteine residues in proteins. The preference for modification of cysteine also suggests that arsenite can affect the redox status within the cell.
It has been demonstrated that arsenite has the ability to induce the formation of ROS in a wide variety of cells including human vascular smooth muscle cells, human epithelial bladder cells (Eblin et al. 2007), human-hamster hybrid cells, vascular endothelial cells and Chinese hamster ovary cells to name a few (Leonard et al. 2004; Qian et al. 2003; Shi et al. 2004). In the presence of Fe2+, arsenite undergoes a Fenton reaction and produces ROS resulting in cellular damage (Felix et al. 2005; Leonard et al. 2004; Shi et al. 2004). DNA damage is one of the most prominent toxic effects produced by ROS in cells exposed to arsenite (Chen et al. 1998; Felix et al. 2005; Gurr et al. 1998), which frequently results in apoptosis (Chen et al. 1998; de la Fuente et al. 2002; Felix et al. 2005; Jiang et al. 2001; Roboz et al. 2000). Gupta et al. showed that chronic exposure of arsenite (300 µg/L) in rats resulted in depletion of glutathione and an increased oxidized glutathione and lipid peroxidation in the brain (Chattopadhyay et al. 2002; Chaudhuri et al. 1999).
Arsenite produces alterations in intracellular oxidation/reduction reactions that activate signaling pathways that regulate early response genes. The activation of these early response genes is thought to be in response to arsenite-induced cellular stress via changes in intracellular redox status (Felix et al. 2005; Kapahi et al. 2000). Stress response transcription factors, such as activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB) play an important role in these early responses and regulate the expression of a variety of downstream target genes, such as pro-inflammatory genes that are involved in cellular antioxidant defense mechanisms (Kapahi et al. 2000). Activation of these stress response transcription factors is arsenic concentration and cell type dependant. For example, high concentrations of arsenite (>10 µM) have been shown to generally inhibit NF-κB activation (Felix et al. 2005; Qian et al. 2003), whereas treatment with low, sub-cytotoxic doses (<10 µM) of arsenite generally induced NF-κB and AP-1. The molecular mechanisms by which arsenite affect the activation of the NF-κB pathway have not yet been fully identified although some evidence has come forth that arsenite may activate NF-κB independently of the degradation of IκB in airway epithelial cells (Qian et al. 2003) implying that there may be an alternative mechanism for arsenic-induced activation of NF-κB.
Nrf2 is another transcription factor of interest in arsenic-dependent signal transduction pathways. Nrf2 is a central regulator of a variety of antioxidant enzymes such as, NQO-1, HO-1, GCLC, CAT, SOD and many others. The transcription of these enzymes is carefully controlled through antioxidant response elements (AREs) located within their promoter regions (Pi et al. 2007; Wang et al. 2008). ROS have the ability to activate signal transduction pathways as part of normal system physiology. For example, in pancreatic β-cells, glucose increases the intracellular accumulation of H2O2, which then results in the secretion of insulin (Pi et al. 2007). β-cells express low levels of antioxidant enzymes, thereby rendering them more susceptible to oxidative damage and present an interesting model for the study of ROS as signaling molecules. Pi et al. found that the accumulation of H2O2 produced in response to increased levels of glucose was H2O2 specific and that other ROS producing molecules, such as arsenite suppressed the secretion of insulin and induced the nuclear accumulation of Nrf-2 and the induction of its target genes, such as HO-1, NAD(P)H and γ-glutamate cysteine ligase catalytic subunit (Pi et al. 2007; Wang et al. 2008). The activity of Nrf2 itself is tightly regulated by Keap1 at multiple levels: (1) Keap1 senses disturbances in cellular redox conditions and modulates the Nrf2 response accordingly; (2) Keap1 in a complex with Cul3, functions as a E3 ubiquitin ligase and constantly targets Nrf2 for ubiquination and degradation; and (3) When Nrf2 is activated, E3 ubiquitin ligase activity is inhibited leading to an increase in Nrf2 levels and increased translocation of Nrf2 into the nucleus (Wang et al. 2008). Wang et al. showed that arsenite activates the Nrf2 pathway in UROtsa cells. In addition, they showed that arsenite enhanced the interaction between Keap1 and Cul3, which resulted in impaired dynamic assembly/disassembly of the E3 ubiquitin ligase for Nrf2 and thus decreased Nrf2 degradation. Interestingly, the researchers also found that induction of Nrf2 by arsenite is independent of the Cys151 residue in Keap1 that is required for Nrf2 activation by other environmental stressors, such as tert-butylhydroquinone and sulforaphane (Wang et al. 2008). This novel finding provides evidence for distinct mechanisms of Nrf2 activation by arsenite and may provide a molecular target for future therapeutics although much more research is needed in this area (Wang et al. 2008).
Oxidative stress has also been associated with increased cellular proliferation after exposure to low levels of arsenite treatment (Yang et al. 2007). In human embryonic lung fibroblasts (HELF) cells, it has been shown that low levels of arsenite (0.5 µM) stimulated cellular proliferation, while higher concentrations (5–10 µM) actually inhibited proliferation and cell growth. These observations correlated positively with ROS levels and arsenite concentration (Yang et al. 2007). In low level arsenite-treated groups, the activity of the antioxidant enzyme superoxide dismutase (SOD) was significantly increased over non-treated cells, but inhibited in the cells treated with the higher concentration of arsenite, thereby providing evidence that there is a concentration-dependent relationship between arsenite and ROS production (Eblin et al. 2008; Yang et al. 2007).
Although it has been shown that arsenicals, such as As(III) and MMA(III) are capable of activating the MAPK signaling pathways in a variety of cells (Drobna et al. 2003; Eblin et al. 2007, 2008; He et al. 2007; Liu et al. 1996; Luster and Simeonova 2004; Simeonova et al. 2002), it has not been shown whether the activation of MAPK signaling is caused directly by arsenite, activated by arsenite-induced ROS or activated by another indirect mechanism. Eblin et al. set out to answer this question in UROtsa cells. They reported that ROS production is a viable mechanism for the signaling alterations seen in UROtsa cells exposed to arsenic. To determine the importance of ROS in the MAPK signaling cascade and the downstream induction of Src and COX-2, Eblin et al. employed the use of specific ROS antioxidants and used these concomitantly with low levels of arsenic (1 µM). They found that COX-2 protein and mRNA levels were more influenced by altering levels of ROS in cells and that the addition of the antioxidant enzyme SOD effectively blocked As(III)-associated COX-2 induction. These data strongly suggest that signaling pathways that are sensitive to changes in ROS may be more responsive to low level arsenic exposure. The duration of arsenic exposure may also be a critical component of signaling mechanisms that significantly contributes to the biological response associated with arsenic exposure.
Malignant transformation
Chronic, low dose exposure to arsenite is known to cause cancer in humans, but the mechanism for arsenite-induced cancer is still unknown. To explore this question, Zhao et al. (1997) exposed a rat liver cell line, TRL 1215, to 500 nM sodium arsenite for at least 18 weeks. Even though TRL 1215 cells are diploid and non-tumorigenic, chronic exposure to arsenite in cell culture and subsequent injection into nude mice resulted in a marked increase in tumor incidence. In addition, genomic DNA hypomethylation increased along with tumor incidence. A further evaluation of the phenotypic properties of transformed TRL 1215 cells indicated that arsenic-induced apoptosis was diminished in the transformed cells relative to passage matched cells (Qu et al. 2002). Characterization of MAPK activity demonstrated that JNK activity from arsenic-transformed cells was less than the non-transformed cells, while the ERK and p38 MAPKs responded similarly in both cell types. Although these data provide some mechanistic insight that begins to elucidate how arsenic-induced malignant transformation occurs, it is still unclear how JNK phosphorylation is selectively diminished in arsenic-transformed cells.
In terms of malignant transformation, it is interesting to note that even though MMA(III) is found in human urine samples (Le et al. 2000) and is more cytotoxic than arsenite (Petrick et al. 2000; Styblo et al. 2000), it is arsenite and not MMA(III) that is mutagenic. Toby Rossman’s group showed that a low dose of arsenite (i.e. 0.1 µM) was three times more potent at inducing anchorage-independent cell growth of HOS human osteosarcoma cells than 0.2 µM arsenite (Mure et al. 2003). Transformation of HOS cells required at least 30 generations of continuous arsenite exposure suggesting that an accumulation of molecular events is required before malignant transformation occurs. Although arsenite-induced apoptosis was not measured in this study, the results suggest that HOS cells treated with 0.1 µM arsenite evade apoptotic cell death, while cells treated with 0.2 µM arsenite cross a threshold of cellular damage that is, in part, managed by cell death mechanisms such that damaged cells are not allowed to accumulate mutations which get passed on to subsequent generations after cell division. If this mechanism were proven correct, then it would support the need to further assess low dose arsenic responses, since the low dose biological responses could have greater deleterious health effects.
An additional hallmark of cell transformation is increased proliferation. Chronic arsenite exposure (5 µM) for 30 weeks to a non-tumorigenic, human prostate epithelial cell line, RWPE-1, resulted in malignant transformation (Benbrahim-Tallaa et al. 2005). The cells were observed to overexpress K-Ras. Although K-Ras was not mutated at the canonical codons 12, 13 and 61 and changes in cell proliferation were not reported, increased ERK activity was reported and correlated with K-Ras expression suggesting that chronic arsenite exposure might increase cell proliferation in prostate epithelial cells (Benbrahim-Tallaa et al. 2007). In addition, arsenic-transformed RWPE-1 cells overexpressed prostate-specific antigen (PSA) in the absence of androgen, suggesting that chronic arsenite exposure could exacerbate prostate cancer progression to more aggressive forms of the disease.
In addition to K-Ras expression, arsenite exposure activates the ERK MAP kinases which function downstream of the Ras proteins. The p53 binding protein, Mdm2 (mouse double minute 2), is up-regulated in response to arsenite in 53-null MEFs via the ERK MAP kinases (Huang et al. 2008). Mdm2 is an ubiquitin E3 ligase that targets p53 for ubiquitination and degradation by the 26S proteasome or nuclear export. In cells pre-exposed to 1 µM arsenite for 24 h, the p53 response to genotoxic stress was lost, presumably due to up-regulation of Mdm2 which resulted in the cytoplasmic localization of p53. Once in the cytoplasm, p53 loses the ability to function as a transcription factor and is unable to induce an apoptotic response to DNA damaging agents. Consequently, by incapacitating the p53 response arsenite promotes malignant cellular transformation through the accumulation of mutations and genomic instability.
Epidemiological studies have provided compelling evidence that inorganic arsenic is a human carcinogen (Smith et al. 1998). However, it has been difficult to recapitulate the results from human exposures in adult experimental animals. Waalkes et al. (2004) altered their experimental design such that they treated mice in utero and then assessed tumor incidence in the adults. They showed that in utero exposure to arsenite in the drinking water of pregnant mice for only 10 days was sufficient to increase tumor incidence which was manifested during the adult lives of the mice. Gestational arsenite exposure in mice resulted in liver, ovary, lung and adrenal gland tumors. It is interesting to note that the liver and lung are target organs typically associated with carcinogenesis in humans (Zhu et al. 2002). In a similar experimental design, Lantz et al. (2009) exposed pregnant mice with arsenite and assessed lung function. They discovered that lung function was compromised when arsenite exposure occurred in utero. These results demonstrate that the timing of arsenite exposure can have profound effects depending on when the exposure occurs during development.
Arsenite is also known to target the skin in humans, and the gestational model for arsenite-induced carcinogenesis in mice did not result in skin tumors unless the mice were also exposed to a tumor promoter like TPA (Waalkes et al. 2004). These results support the study of Rossman et al. who showed that the oral exposure of inorganic arsenite to adult mice had no effect unless the mice also received ultraviolet irradiation (Rossman et al. 2001). Although the mechanism is still unknown, pretreatment of keratinocytes with arsenite and subsequent exposure to UVB irradiation resulted in a decreased pro-apoptotic response (Chen et al. 2005) suggesting that arsenite promotes cell survival signaling pathways that permit mutagenic events to propagate within the genome of the skin epithelium. In support of this conclusion, Pi et al. (2005) showed that chronic arsenite exposure to keratinocytes resulted in increased stability of activated Akt and apoptotic resistance.
Arsenite covalently reacts with Cys179 of the IκB kinase (IKK) which inhibits the catalytic activity of IKK (Kapahi et al. 2000). As a result of IKK inhibition, cellular responses that require NF-κB transcriptional activity can be inhibited by arsenite. In melanoma cells, NF-κB is highly expressed and leads to an anti-apoptotic response (Ueda and Richmond 2006). However, in EGF-R-positive melanoma cells, moderate treatment (5–10 µM) with arsenite decreases NF-κB levels and induces PARP cleavage and apoptosis (Ivanov and Hei 2005). Although IKK inhibition was not reported, Ivanov and Hei demonstrated a decrease in NF-κB DNA binding activity suggesting that arsenite might target IKK in melanoma cells. These results also suggest that arsenite might have clinical utility in treating melanoma. In fact, EGF-R inhibitors when combined with arsenite increased the apoptotic response in melanoma cells (Ivanov and Hei 2005). Thus, a combination of an EGF-R inhibitor with arsenite increased the apoptotic response in melanoma cells while pretreatment of keratinocytes with arsenite when combined with UVB irradiation, a known inducer of apoptosis, resulted in a decreased apoptotic response. These examples illustrate the tissue-specific responses associated with arsenic exposure. All together, these results demonstrate that arsenite likely has distinct mechanisms of action that are highly dependent on the exposed tissue, as well as the timing of arsenite exposure.
Arsenic is also used as a cancer therapeutic for example, in melanoma cells and the use of arsenite when combined with menadione increased the cell death response (Chowdhury et al. 2009). While in non-malignant cells, arsenite and menadione failed to elicit a cytotoxic response, suggesting that this drug combination may prove useful in the treatment of melanoma. In malignant melanoma cells, the apoptotic response was accompanied with ROS production, activation of the p38 MAPK, phosphorylation of Ser46 of p53 which is indicative of apoptotic cells, and increased expression of p21 and p27. The cyclin-dependent kinase (CDK) inhibitor, p21, and its relative, p27, regulate cell the cycle check point at G1 by binding to CDK/cyclin complexes.
Melanoma cells are typically resistant to apoptosis which is one reason why this form of skin cancer is so hideous. However, an increase in cell death in melanoma cells in response to arsenite and menadione, another ROS inducer, may offer a new therapeutic approach in the treatment of a deadly disease. Arsenite is approved for the treatment of acute promyelocytic leukemia because it induces an apoptotic response (Chen et al. 1997; Sun et al. 1992). The apoptotic response may be due to a caspase-dependent loss of Akt (Mann et al. 2008). Similarly, arsenite induces an apoptotic response in melanoma cells, but is not approved for the treatment of melanoma.
Arsenite is a complete carcinogen in mice after gestational exposure (Waalkes et al. 2004). Using a known human carcinogen-like arsenite to derive a positive as opposed to a negative health outcome suggests that the timing of arsenite exposure is critical when evaluating an arsenite-dependent biological response. In contrast, the use of arsenite to treat a disease-like melanoma which is typically associated with adolescents and adults, and not children under 14 years of age (Berg and Lindelof 1997) and may provide a therapeutic benefit in the treatment of melanoma.
The seemingly opposing carcinogenic and apoptotic responses associated with arsenic suggest that arsenic affects signaling pathways differently during gestational and adult periods of life. The diametrically opposing physiological phenotypes associated with arsenic exposure indicate that a careful comparison of apoptotic signaling pathways between neonates and adults might identify some important mechanistic signaling pathways that are uniquely affected by arsenic. Moreover, the results from such studies might identify periods of enhanced arsenic susceptibility during a lifetime. It has been known for over 40 years that sunlight exposure in young children predisposes them to melanoma later in life, and now we are beginning to appreciate that arsenic exposure may have deleterious consequences when exposures occur in neonates or young children, but may have therapeutic benefits later in life.
Although arsenic might have a therapeutic benefit in treating melanoma in adults, arsenic exposure in drinking water from artesian wells in Taiwan was associated with prostate cancer mortality in humans (Chen et al. 1988). Similarly, when looking at the role of arsenic in drinking water and prostate cancer in the US, Lewis et al. (1999) concluded that there was a significant elevation of prostate cancer mortality associated with arsenic in drinking water. However, it is difficult to know from these studies whether gestational, adolescent, adult, or a cumulative lifetime of arsenic exposure contributed to the development of prostate cancer.
Chronic arsenic exposure to prostate epithelial cells is sufficient to induce a malignant phenotype in vitro. Human prostate epithelial cells, RWPE-1, were exposed to 5 µM arsenic for 30 weeks and inoculation of the cells into immunocompromised mice resulted in tumor growth, whereas the passage matched cells were not tumorigenic (Achanzar et al. 2002). In the arsenic-treated RWPE-1 cells, DNA hypomethylation was observed as well as over-expression of unmutated K-Ras (Benbrahim-Tallaa et al. 2005). Although DNA hypomethylation of the promoter regions of some genes is often associated with overexpression of the cognate protein, K-Ras overexpression was not associated with DNA hypomethylation as the promoter region of both the arsenic-treated and passage matched RWPE-1 cells was essentially unmethylated. Nevertheless, overexpression of K-Ras resulted in increased phosphorylation of MEK1/2, a dual-specificity kinase as well as the ELK transcription factor, suggesting that K-Ras overexpression correlated with activation of downstream signaling proteins (Benbrahim-Tallaa et al. 2007). In addition, when arsenic-transformed RWPE-1 cells were inoculated in immunocompromised mice, the cells expressed matrix metalloproteinase 9 (MMP-9) and human PSA, which are features also common to human prostate cancers (Achanzar et al. 2002). In summary, depending on the cellular context, arsenic when combined with another ROS-inducer can promote apoptosis of melanoma cells suggesting that arsenic may provide a therapeutic benefit, while in prostate epithelial cells chronic arsenic exposure induces a malignant phenotype. Regardless of the cell type, it is clear that arsenic is capable of regulating signaling pathways that range from apoptosis to cell proliferation and survival.
Arsenic and disease susceptibility
A study by Kozul et al. (2009) investigated the co-morbidity of arsenic exposure combined with H1N1 influenza virus infection in C57BL/6J male mice. A prior 5-week exposure to 100 ppb of arsenite in the drinking water followed by influenza virus infection resulted in a severe morbidity, while the viral infection or arsenic alone had essentially no effect. To assess the role of arsenite in cardiovascular disease, Srivastava et al. exposed ApoE−/− mice to 49 ppm arsenite in drinking water (Srivastava et al. 2009). Although plasma triglycerides and large VLDL particles unexpectedly decreased in response to arsenite, atherosclerotic lesions increased in aortic valves and the aortic arch. Consistent with these results is the study by Hays et al. (2008) who demonstrated decreases in the expression of genes associated with the vascular matrix in the hearts of C57BL/6 mice in response to 50 ppb As(III). Hence, both human population and animal studies provide some compelling evidence that the combined effect of arsenic exposure with some other factor can yield serious health consequences, however, researchers have yet to attribute these health consequences to a specific signal transduction pathway.
Future perspectives
One of the major difficulties associated with studying signal transduction pathways regulated by arsenic is that to mimic environmentally relevant exposures in the laboratory requires chronic administration of arsenic or arsenic metabolites at low doses and such experiments are feasible in animal studies, but somewhat more difficult in cell culture. Nevertheless, Bredfeldt et al. (2006) used an immortalized human bladder cell line (UROtsa) and exposed the cells to 50 nM MMA(III) for up to 52 weeks. The investigators determined that MMA(III) transformed the cells after 24 weeks of chronic MMA(III) exposure, while the cells became hyperproliferative in as little as 12 weeks. These results demonstrate that MMA(III) can cause a gradual progression of cellular phenotype, moving from immortalized urothelial cells to malignant transformation. Therefore, clearly even a low dose of an arsenic metabolite is sufficient to affect cellular phenotype, but identifying a specific signaling pathway or pathways that mediates the change in cellular phenotype remains elusive. In a similar type of study, Zhao et al. (1997) dosed rat liver epithelial cells with arsenite and the cells displayed DNA hypomethylation, but when injected into nude mice the cells developed malignant tumors. At this time, it is difficult to know whether the differences in DNA methylation observed in the two studies are due to cell type differences in bladder and liver cells or differences in responses due to MMA(III) and arsenite (Jensen et al. 2009; Zhao et al. 1997).
A recent study by Jensen et al. (2009) may provide some insight as to how arsenicals could change cellular phenotype. An analysis of DNA methylation from MMA(III) transformed UROtsa bladder cells demonstrated an increase in both DNA hypermethylation and hypomethylation in gene promoter regions. Of the genes analyzed, those genes with increased DNA methylation of cytosine in CpG islands in promoter regions had progressively less gene expression as the duration of MMA(III) exposure persisted indicating that epigenetic changes in promoter methylation correlated with a more malignant phenotype. Hundreds of genes had aberrant DNA methylation due to MMA(III) exposure in UROtsa cells. The Holy Grail will be to identify which gene or genes are necessary to acquire a malignant phenotype.
With regard to MMA(III) and presumably arsenite, DNA methyltransferases (DNMT3A and 3B) and demethylases may be the common denominators in the signaling mechanisms that affect cell phenotype. Although the general phenomenon of DNA methylation is a well-recognized mechanism that leads to transcriptional silencing and loss of gene expression (Jones et al. 1998; Nan et al. 1998), the regulatory mechanisms associated with DNA methylation at specific CpG islands still remain unclear. To add to the complexity of DNA hypermethylation and hypomethylation, even less is known about the molecular mechanisms associated with DNA demethylation (Ooi and Bestor 2008). With regard to DNA hypermethylation, methylation of the inducible nitric oxide synthase (iNOS) promoter in the region known to associate with the NF-κB transcription factor inhibits transcription of the iNOS gene (Yu and Kone 2004). Although the NF-κB binding motif within the promoter of the iNOS gene is methylated and needed for transcription, it is not known what regulates the DNA methyltransferase at the iNOS promoter. Moreover to make the issue even more complicated, one can not ignore the proteins that bind methylated cytosines in CpG islands. These proteins are referred to as methyl-CpG domain (MBD) proteins and their binding to methylated CpG islands serves to repress gene transcription (Bird 2002). Although MBD proteins play a role in regulating gene expression, DNA methylation is still the essential element in the regulatory mechanism.
At the very least, arsenicals are capable of affecting the epigenetic landscape in the nucleus which is highly likely to affect signal transduction pathways in the cell. Given the diverse clinical manifestations associated with arsenic exposure, it may not be surprising to discover that modulation of the epigenome occurs in a cell type-specific manner. Using arsenic trioxide to induce apoptosis of leukemic cells and treat patients with acute promyelocytic leukemia is probably the best evidence for a cell type-specific response associated with arsenic exposure (Zhu et al. 2002).
In addition to cell type specificity to arsenic exposure, there is also individual variation to arsenic exposure. For example, in a study of a Mexican population that was exposed to arsenic in the drinking water, there were variations in arsenic methylation efficiency among individuals (Gomez-Rubio et al. 2009). In another study of Bangladeshi adults whose diets were deficient in folate, it was found that variability in nutritional status may be a risk factor for arsenic toxicity (Gamble et al. 2005). Folate is needed for the biochemical pathway of one-carbon metabolism which is essential for methylation of arsenic metabolites as well as DNA. As the signal transduction pathways associated with arsenicals continue to emerge, it may not be long before concrete themes evolve regarding arsenic biology and toxicology that will help in shaping future regulatory, and possibly, dietary standards for human arsenic exposures worldwide.
Acknowledgments
The research herein was made possible by the NIEHS Superfund Basic Research Program (ES 04940) and the Southwest Environmental Health Sciences Center (ES 06694).
References
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, Waalkes MP. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-Ras oncogene activation. Toxicol Appl Pharmacol. 2005;206:288–298. doi: 10.1016/j.taap.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Webber MM, Waalkes MP. Mechanisms of acquired androgen independence during arsenic-induced malignant transformation of human prostate epithelial cells. Environ Health Perspect. 2007;115:243–247. doi: 10.1289/ehp.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RL, Malamy MH. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun. 1970;40:496–503. doi: 10.1016/0006-291x(70)91036-3. [DOI] [PubMed] [Google Scholar]
- Berg P, Lindelof B. Differences in malignant melanoma between children and adolescents. A 35-year epidemiological study. Arch Dermatol. 1997;133:295–297. [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Jagadish B, Eblin KE, Mash EA, Gandolfi AJ. Monomethylarsonous acid induces transformation of human bladder cells. Toxicol Appl Pharmacol. 2006;216:69–79. doi: 10.1016/j.taap.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr Genet. 1996;29:344–351. [PubMed] [Google Scholar]
- Carbrey JM, Song L, Zhou Y, Yoshinaga M, Rojek A, Wang Y, Liu Y, Lujan HL, DiCarlo SE, Nielsen S, Rosen BP, Agre P, Mukhopadhyay R. Reduced arsenic clearance and increased toxicity in aquaglyceroporin-9-null mice. Proc Natl Acad Sci USA. 2009;106:15956–15960. doi: 10.1073/pnas.0908108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Chappell WR, Abernathy CO, Calderon RL. Arsenic exposure and health effects. Proceedings of the 3rd international conference on arsenic exposure and health effects; San Diego, CA. 12–15 July 1998; New York: Chapman & Hall, Amsterdam; 1999. [Google Scholar]
- Chattopadhyay S, Bhaumik S, Purkayastha M, Basu S, Nag Chaudhuri A, Das Gupta S. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol Lett. 2002;136:65–76. doi: 10.1016/s0378-4274(02)00282-5. [DOI] [PubMed] [Google Scholar]
- Chaudhuri AN, Basu S, Chattopadhyay S, Das Gupta S. Effect of high arsenic content in drinking water on rat brain. Indian J Biochem Biophys. 1999;36:51–54. [PubMed] [Google Scholar]
- Chen CJ, Kuo TL, Wu MM. Arsenic and cancers. Lancet. 1988;1:414–415. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chen PH, Lan CC, Chiou MH, Hsieh MC, Chen GS. Effects of arsenic and UVB on normal human cultured keratinocytes: impact on apoptosis and implication on photocarcinogenesis. Chem Res Toxicol. 2005;18:139–144. doi: 10.1021/tx049834b. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Chowdhury S, Roychoudhury P, Mandal C, Chaudhuri K. Arsenic induced apoptosis in malignant melanoma cells is enhanced by menadione through ROS generation, p38 signaling and p53 activation. Apoptosis. 2009;14:108–123. doi: 10.1007/s10495-008-0284-8. [DOI] [PubMed] [Google Scholar]
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente H, Portales-Perez D, Baranda L, Diaz-Barriga F, Saavedra-Alanis V, Layseca E, Gonzalez-Amaro R. Effect of arsenic, cadmium and lead on the induction of apoptosis of normal human mononuclear cells. Clin Exp Immunol. 2002;129:69–77. doi: 10.1046/j.1365-2249.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ. Transfer of arsenite from glutathione to dithiols: a model of interaction. Chem Res Toxicol. 1993;6:598–602. doi: 10.1021/tx00035a002. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Jaspers I, Thomas DJ, Styblo M. Differential activation of AP-1 in human bladder epithelial cells by inorganic and methylated arsenicals. Faseb J. 2003;17:67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ. Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: role in arsenic-induced carcinogenesis. Toxicol Sci. 2007;95:321–330. doi: 10.1093/toxsci/kfl160. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Hau AM, Jensen TJ, Futscher BW, Gandolfi AJ. The role of reactive oxygen species in arsenite and monomethylarsonous acid-induced signal transduction in human bladder cells: acute studies. Toxicology. 2008;250:47–54. doi: 10.1016/j.tox.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix K, Manna SK, Wise K, Barr J, Ramesh GT. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol. 2005;19:67–77. doi: 10.1002/jbt.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Meza-Montenegro MM, Cantu-Soto E, Klimecki WT. Genetic association between intronic variants in AS3MT and arsenic methylation efficiency is focused on a large linkage disequilibrium cluster in chromosome 10. J Appl Toxicol. 2009 doi: 10.1002/jat.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr JR, Liu F, Lynn S, Jan KY. Calcium-dependent nitric oxide production is involved in arsenite-induced micronuclei. Mutat Res. 1998;416:137–148. doi: 10.1016/s1383-5718(98)00076-x. [DOI] [PubMed] [Google Scholar]
- Hays AM, Lantz RC, Rodgers LS, Sollome JJ, Vaillancourt RR, Andrew AS, Hamilton JW, Camenisch TD. Arsenic-induced decreases in the vascular matrix. Toxicol Pathol. 2008;36:805–817. doi: 10.1177/0192623308323919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XQ, Chen R, Yang P, Li AP, Zhou JW, Liu QZ. Biphasic effect of arsenite on cell proliferation and apoptosis is associated with the activation of JNK and ERK1/2 in human embryo lung fibroblast cells. Toxicol Appl Pharmacol. 2007;220:18–24. doi: 10.1016/j.taap.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhang J, McHenry KT, Kim MM, Zeng W, Lopez-Pajares V, Dibble CC, Mizgerd JP, Yuan ZM. Induction of cytoplasmic accumulation of p53: a mechanism for low levels of arsenic exposure to predispose cells for malignant transformation. Cancer Res. 2008;68:9131–9136. doi: 10.1158/0008-5472.CAN-08-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF. Arsenic methylation, oxidative stress and cancer—is there a link? J Natl Cancer Inst. 2009;101:1660–1661. doi: 10.1093/jnci/djp437. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Hei TK. Combined treatment with EGFR inhibitors and arsenite upregulated apoptosis in human EGFR-positive melanomas: a role of suppression of the PI3K-AKT pathway. Oncogene. 2005;24:616–626. doi: 10.1038/sj.onc.1208125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TJ, Novak P, Wnek SM, Gandolfi AJ, Futscher BW. Arsenicals produce stable progressive changes in DNA methylation patterns that are linked to malignant transformation of immortalized urothelial cells. Toxicol Appl Pharmacol. 2009;241:221–229. doi: 10.1016/j.taap.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho CH, Lai KC, Lin MC, Kung HF, Lam SK. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer. 2001;91:173–179. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1039>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Wallace K. The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J Inorg Biochem. 2008;102:532–539. doi: 10.1016/j.jinorgbio.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect. 2009;117:1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz RC, Chau B, Sarihan P, Witten ML, Pivniouk VI, Chen GJ. In utero and postnatal exposure to arsenic alters pulmonary structure and function. Toxicol Appl Pharmacol. 2009;235:105–113. doi: 10.1016/j.taap.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le XC, Lu X, Ma M, Cullen WR, Aposhian HV, Zheng B. Speciation of key arsenic metabolic intermediates in human urine. Anal Chem. 2000;72:5172–5177. doi: 10.1021/ac000527u. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107:359–365. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen Y, Rosen BP. Role of vicinal cysteine pairs in metalloid sensing by the ArsD As(III)-responsive repressor. Mol Microbiol. 2001;41:687–696. doi: 10.1046/j.1365-2958.2001.02546.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guyton KZ, Gorospe M, Xu Q, Lee JC, Holbrook NJ. Differential activation of ERK, JNK/SAPK and P38/CSBP/RK map kinase family members during the cellular response to arsenite. Free Radic Biol Med. 1996;21:771–781. doi: 10.1016/0891-5849(96)00176-1. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster MI, Simeonova PP. Arsenic and urinary bladder cell proliferation. Toxicol Appl Pharmacol. 2004;198:419–423. doi: 10.1016/j.taap.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Mann KK, Colombo M, Miller WH., Jr Arsenic trioxide decreases AKT protein in a caspase-dependent manner. Mol Cancer Ther. 2008;7:1680–1687. doi: 10.1158/1535-7163.MCT-07-2164. [DOI] [PubMed] [Google Scholar]
- Mure K, Uddin AN, Lopez LC, Styblo M, Rossman TG. Arsenite induces delayed mutagenesis and transformation in human osteosarcoma cells at extremely low concentrations. Environ Mol Mutagen. 2003;41:322–331. doi: 10.1002/em.10164. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Rejoinder: arsenic exposure and prevalence of type 2 diabetes: updated findings from the National Health Nutrition and Examination Survey, 2003–2006. Epidemiology. 2009;20:816–820. doi: 10.1097/EDE.0b013e3181afef88. (discussion e1–e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Paul DS, Harmon AW, Devesa V, Thomas DJ, Styblo M. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007;115:734–742. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pi J, He Y, Bortner C, Huang J, Liu J, Zhou T, Qu W, North SL, Kasprzak KS, Diwan BA, Chignell CF, Waalkes MP. Low level, long-term inorganic arsenite exposure causes generalized resistance to apoptosis in cultured human keratinocytes: potential role in skin co-carcinogenesis. Int J Cancer. 2005;116:20–26. doi: 10.1002/ijc.20990. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- Pysher MD, Sollome JJ, Regan S, Cardinal TR, Hoying JB, Brooks HL, Vaillancourt RR. Increased hexokinase II expression in the renal glomerulus of mice in response to arsenic. Toxicol Appl Pharmacol. 2007;224:39–48. doi: 10.1016/j.taap.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Castranova V, Shi X. New perspectives in arsenic-induced cell signal transduction. J Inorg Biochem. 2003;96:271–278. doi: 10.1016/s0162-0134(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Qu W, Bortner CD, Sakurai T, Hobson MJ, Waalkes MP. Acquisition of apoptotic resistance in arsenic-induced malignant transformation: role of the JNK signal transduction pathway. Carcinogenesis. 2002;23:151–159. doi: 10.1093/carcin/23.1.151. [DOI] [PubMed] [Google Scholar]
- Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP, Jr, Rafii S. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525–1530. [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Dong J, Scott RA, Ksenzenko MY, Rosen BP. The role of arsenic-thiol interactions in metalloregulation of the ars oper-on. J Biol Chem. 1996;271:9291–9297. doi: 10.1074/jbc.271.16.9291. [DOI] [PubMed] [Google Scholar]
- Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Wang S, Hulderman T, Luster MI. c-Src-dependent activation of the epidermal growth factor receptor and mito-gen-activated protein kinase pathway by arsenic role in carcinogenesis. J Biol Chem. 2002;277:2945–2950. doi: 10.1074/jbc.M109136200. [DOI] [PubMed] [Google Scholar]
- Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Vladykovskaya EN, Haberzettl P, Sithu SD, D’Souza SE, States JC. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE−/− mice. Toxicol Appl Pharmacol. 2009;241:90–100. doi: 10.1016/j.taap.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Liaw J, Smith AH. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology. 2009;20:807–815. doi: 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- Straub AC, Clark KA, Ross MA, Chandra AG, Li S, Gao X, Pagano PJ, Stolz DB, Barchowsky A. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J Clin Invest. 2008;118:3980–3989. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Klei LR, Stolz DB, Barchowsky A. Arsenic requires sphingosine-1-phosphate type 1 receptors to induce angiogenic genes and endothelial cell remodeling. Am J Pathol. 2009;174:1949–1958. doi: 10.2353/ajpath.2009.081016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Sun H, Ma L, Hu XC, Zhang TD. Ai-Lin I treated 32 cases of acute promyelocytic leukemia. Chin J Integrat Chinese Western Med. 1992;12:170–172. [Google Scholar]
- Sun D, Xu D, Zhang B. Rac signaling in tumorigenesis and as target for anticancer drug development. Drug Resist Updat. 2006;9:274–287. doi: 10.1016/j.drup.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Arnold LL, Pennington KL, Kakiuchi-Kiyota S, Cohen SM. Effects of co-administration of dietary sodium arsenite and an NADPH oxidase inhibitor on the rat bladder epithelium. Toxicology. 2009;261:41–46. doi: 10.1016/j.tox.2009.04.042. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: inorganic arsenic is a transplacental carcinogen in mice. Toxicol Appl Pharmacol. 2004;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Chen W, Li Y, Villeneuve NF, Zhang DD. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1–C151: enhanced Keap1-Cul3 interaction. Toxicol Appl Pharmacol. 2008;230:383–389. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, He XQ, Peng L, Li AP, Wang XR, Zhou JW, Liu QZ. The role of oxidative stress in hormesis induced by sodium arsenite in human embryo lung fibroblast (HELF) cellular proliferation model. J Toxicol Environ Health A. 2007;70:976–983. doi: 10.1080/15287390701290832. [DOI] [PubMed] [Google Scholar]
- Yu Z, Kone BC. Hypermethylation of the inducible nitric-oxide synthase gene promoter inhibits its transcription. J Biol Chem. 2004;279:46954–46961. doi: 10.1074/jbc.M407192200. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci USA. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen Z, Lallemand-Breitenbach V, de The H. How acute promyelocytic leukaemia revived arsenic. Nat Rev Cancer. 2002;2:705–713. doi: 10.1038/nrc887. [DOI] [PubMed] [Google Scholar]