Abstract
Although pancreatic β-cell transplantation may serve as a potential cure for diabetes mellitus (DM), limited donor tissue availability poses a major challenge. Thus, there is a great demand to find new sources for pancreatic β-cells. Here, we present a lentiviral vector–based approach to achieve β-cell proliferation through the β-cell-specific activation of the hepatocyte growth factor (HGF)/cmet signaling pathway. The methodology is based on the β-cell-specific expression of a ligand-inducible, chimeric receptor (F36Vcmet), under transcriptional control of the promoter from the human insulin gene, and its ability to induce HGF/cmet signaling in the presence of a synthetic ligand (AP20187). High transduction efficiency of human pancreatic islets was achieved utilizing this approach with chimeric receptor expression confined to the β-cell population. In addition, specific proliferation of human pancreatic β-cells was induced utilizing this approach. Selective, regulated β-cell expansion may help to provide greater availability of cells for transplantation in patients with DM.
Introduction
The prevalence of diabetes mellitus (DM) continues to increase worldwide; there are an estimated 23.6 million children and adults (7.8% of the population) affected in the United States alone.1 Approximately 5–10% of these individuals have type 1 DM, which is caused by the autoimmune destruction of insulin-producing pancreatic β-cells.2 The ensuing lack of sufficient functional β-cells mandates lifelong therapy with exogenous insulin. Replacing damaged pancreatic β-cells with functional cells would represent a logical alternative approach for the long-term management of type 1 DM.
The success of the Edmonton protocol demonstrated that insulin independence can be achieved in selected patients with type 1 DM using allogeneic pancreatic islet transplantation.3 Despite the promising results, the procedure faces two major challenges: (i) Limited donor tissue availability and (ii) loss of insulin independence over time due to immunological rejection.4,5 Expanding donor-derived pancreatic β-cells ex vivo, prior to the transplantation procedure, might represent a rational approach to overcome some of these obstacles. It would not only increase the total number of β-cells, but might also allow islet transplantation with cells from a single donor, thereby reducing the risk of immunological sensitization and graft rejection.
Among the numerous molecules that have been proposed to induce β-cell expansion, hepatocyte growth factor (HGF) has received much attention. There is increasing evidence suggesting that HGF plays an important role in the proliferation and survival of pancreatic β-cells both in vitro and in vivo.6,7,8,9,10 In addition, multiple animal models have shown that HGF improves graft survival when administered systemically to transplant recipients11 or if it is overexpressed in the donor pancreatic β-cells prior to the procedure.12,13
The interpretation of some of the studies is challenging, however, due to the nature of HGF. Although its effects are initiated by a specific cell surface receptor called cmet,14,15 this HGF receptor is present on multiple cell types, and thus, conflicting results have been published regarding the ability of HGF to specifically induce pancreatic β-cell proliferation ex vivo.16,17
Herein, we present a novel approach aimed at activating the HGF/cmet signal transduction pathway exclusively in pancreatic β-cells. The approach utilizes the principle that dimerization of the cmet receptor promotes HGF/cmet signaling pathway activation and that the cytoplasmic domain of cmet is responsible to initiate the signaling event.18,19 In our system, the cytoplasmic signaling domain of cmet (amino acids 974–1,408) was fused downstream of the FK506-binding protein ligand-binding domain with a serine to valine substitution at amino acid 36 (F36V) that allows it to bind to a synthetic divalent ligand (AP20187) that acts as a chemical inducer of dimerization (CID) creating a ligand-inducible signaling protein. This F36Vcmet fusion protein remains functionally inert unless exposed to the CID that specifically bridges the drug-binding domain of two receptors. The ensuing proximity of two receptor signaling domains promotes receptor phosphorylation and thereby receptor activation.
This genetically engineered chimeric receptor was introduced into human islet preparations using a lentiviral vector in which a human insulin promoter fragment drives gene expression. Because transgene expression is confined to β-cells and receptor homodimerization only occurs in the presence of a synthetic ligand (CID or AP20187), specific and controlled β-cell expansion was achieved.
Results
Ligand-inducible signaling protein expression
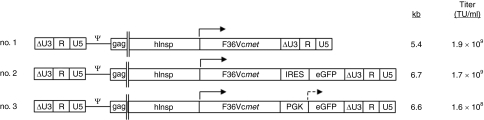
Three lentiviral vector constructs were initially prepared; all expressed the hemagglutinin- (HA) tagged ligand-inducible F36Vcmet chimeric receptor driven by the human insulin promoter fragment. Two vectors also contained the enhanced green fluorescent protein (eGFP) reporter gene with its transcription controlled by the internal ribosome entry site (IRES) in one of the vectors and the internal murine phosphoglycerate kinase (PGK) promoter in the other (Figure 1).
Figure 1.
Schematic diagram of the novel lentiviral vector constructs. Three sets of self-inactivating lentiviral vectors were generated, all encoding the HA-tagged, ligand-inducible chimeric receptor F36Vcmet under control of a human insulin promoter 1.4 kb fragment. Two of the vectors (nos. 2 and 3) also expressed enhanced green fluorescent protein (eGFP) either utilizing internal ribosomal entry site (IRES) or driven by the phosphoglycerate kinase promoter (PGK). The approximate size of the vector genomes is expressed in kilobase pairs (kb), and the titer of the vectors, as quantified by qPCR, is also indicated. δU3-R-U5 indicates components of the self-inactivating (SIN) long-terminal repeats; hInsp, human insulin promoter fragment; PGK, phosphoglycerate kinase promoter. The titers of the vectors made and measured simultaneously are indicated as transducing units per milliliter (TU/ml).
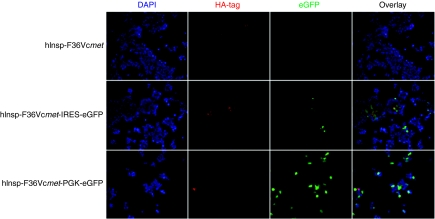
To compare the performance of these vector constructs, protein expression was initially assessed using immunohistochemistry in a murine insulinoma cell line (NIT-1). As shown in Figure 2, significant differences were noted in vector performance. Although eGFP was efficiently expressed by the dual-promoter construct (Insp-F36Vcmet-PGK-eGFP), fusion protein synthesis was barely detectable by HA-tag staining. The second vector, Insp-F36Vcmet-IRES-eGFP, only provided moderate expression of both proteins. The single promoter vector (Insp-F36Vcmet) was found to be the most efficient in expressing the protein of interest, F36Vcmet. Based on these initial results, the single promoter construct, Insp-F36Vcmet, was selected for further studies and its transduction efficiency was next evaluated in primary human pancreatic β-cells.
Figure 2.
Protein expression in murine insulinoma cell line. The murine insulinoma cell line, NIT-1, was transduced with the three different vector constructs at multiplicity of infection of 5, and protein expression was analyzed 7 days post-transduction using immunohistochemistry. Chimeric receptor expression was detected by HA-tag staining (red), and eGFP expression appears in green. DAPI stains the cell nuclei (blue). eGFP, enhanced green fluorescent protein; HA, hemagglutinin.
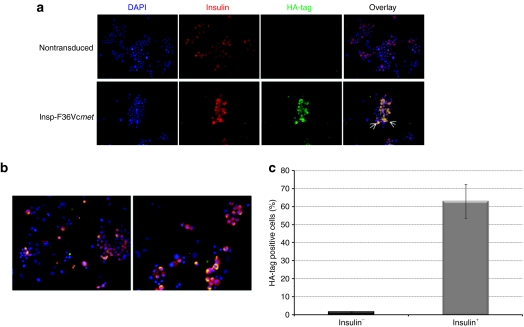
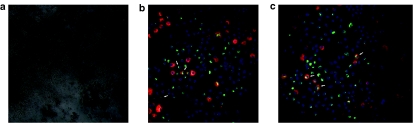
As evidenced by HA-tag staining (Figure 3a), transduced human pancreatic β-cells from isolated islets expressed the fusion protein very efficiently, whereas, as expected, no F36Vcmet was detected in the nontransduced, control islets. Overlay images are shown in Figure 3b. As islet preparations represent a heterogeneous cell population, it was important to examine whether fusion protein expression is specific for β-cells. Our results show that 65% cells are positive for insulin stained with HA-tag antibody versus merely 1.5% of the insulin-negative cells (Figure 3c). These data demonstrate the high specificity of the Insp-F36Vcmet vector for the β-cell population.
Figure 3.
F36Vcmet protein expression in human pancreatic islets. (a) Immunohistochemical analysis of nontransduced and transduced human pancreatic islets 7 days post-transduction (DAPI: blue; insulin: red; HA-tag staining: green). (b) Additional immunofluorescent images of human pancreatic islets transduced with F36Vcmet (DAPI: blue; insulin: red; HA-tag staining: green). (c) Proportion of insulin-negative (black column) and insulin-positive (gray column) cells that stained positive for the F36Vcmet protein, assessed by HA-tag immunohistochemical staining. HA, hemagglutinin.
Ex vivo expansion of human pancreatic β-cells
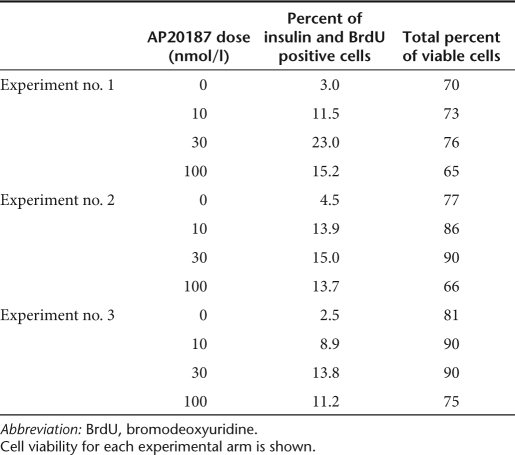
In order to successfully stimulate F36Vcmet protein dimerization and thus to expand pancreatic β-cells ex vivo, the optimal working concentration of the CID molecule had to be determined. Based on three sets of experiments evaluating cell viability and β-cell expansion at day 7, the synthetic drug applied at a concentration of 30 nmol/l was deemed to be optimal (Table 1). This concentration of CID prompted the greatest increase in the proportion of proliferating β-cells with cell viability ranging between 76 and 90% as determined by Trypan blue exclusion. Based on these results, AP20187 was utilized at a final concentration of 30 nmol/l in all subsequent experiments.
Table 1. Effect of incremental AP20187 concentrations on the percentage of insulin and BrdU double-positive cells.
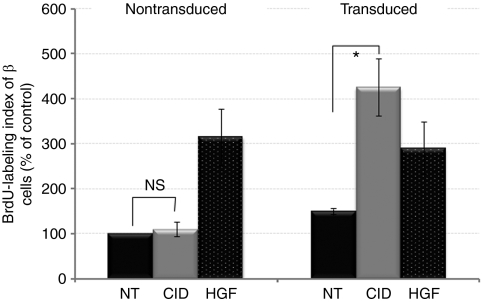
To assess the potential of the ligand-inducible F36Vcmet signaling to induce β-cell proliferation, the proportion of insulin and bromodeoxyuridine (BrdU) double-positive cells were determined at treatment day 7 in the Insp-F36Vcmet transduced and nontransduced cell populations (Figures 4 and 5). On average, transduced insulin-positive cells showed a fourfold increase in BrdU-labeling index in the presence of AP20187 when compared to the control groups that were either nontransduced but treated with CID or transduced but not treated with CID. In contrast, recombinant HGF protein only induced a threefold increase in BrdU-labeling index, independent of viral transduction. BrdU-labeling index for nontreated controls and for nontransduced but AP20187 treated cells were similar suggesting that fusion protein expression is a prerequisite for the synthetic drug to induce cell proliferation. Interestingly, we documented a minimal increase of the BrdU-labeling index (1.18-fold) for β-cells that were transduced but were not treated with CID, possibly indicating some leaky signaling by F36Vcmet in the absence of the CID ligand.
Figure 4.
Microscopic images of human pancreatic islets. (a) Light microscopic image showing the monolayer of human pancreatic islets on HTB9 matrix. (b,c) Immunohistochemical images of human pancreatic islets incorporating bromodeoxyuridine (BrdU) 7 days after CID treatment. White arrows indicate examples of insulin and BrdU double-positive cells representing proliferating pancreatic β-cells (DAPI: blue; insulin: red; BrdU: green).
Figure 5.
Proliferation of human pancreatic β-cells. Relative BrdU-labeling index of pancreatic β-cells for each experimental arm. Values are shown as the percentage of the nontransduced, nontreated control group. Each bar graph represents the average of six separate experiments with at least 1,000 cells counted for each. NT, nontreated cells (black); CID, treatment with AP20187 at 30 nmol/l (gray); HGF, treatment with hepatocyte growth factor at 50 ng/ml (dotted bars). NS, difference not significant; *P < 0.05. BrdU, bromodeoxyuridine.
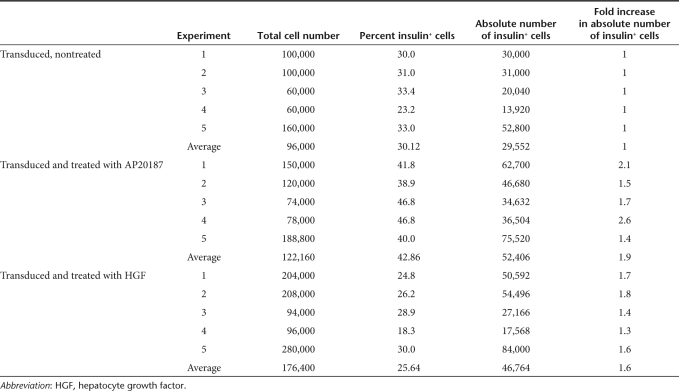
The absolute number and proportion of insulin-positive cells were next determined at day 7 in each experimental group that was previously transduced with the Insp-F36Vcmet vector (Table 2). The total number of cells in the islets that were transduced and treated with CID increased by an average of 1.3-fold, compared to the transduced islets not exposed to CID. Additionally, the proportion of insulin-positive cells increased 1.4-fold with CID treatment by day 7, when compared to the nontreated groups. The resultant increase in the absolute number of insulin-positive cells was 1.9-fold. These results are critical and confirm the selective expansion of insulin-positive β-cells by our approach. Although a 1.6-fold increase in the absolute number of insulin-positive cells was achieved with HGF by day 7, it actually prompted a reduction in the proportion of insulin-positive cells in the cultures. This finding agrees well with previous studies and suggests the nonspecific nature of HGF that induces the proliferation of multiple cell types in islets.
Table 2. Proportion and absolute number of insulin-positive cells in human pancreatic islet cultures, 7 days post-transduction (vector construct used: hInsp-F36Vcmet).
Expansion of human pancreatic β-cells over time
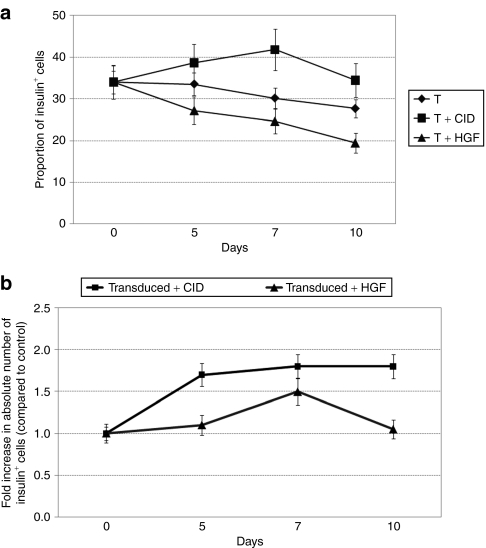
To accurately assess their expansion, the proportion of β-cells in the cultures was determined on days 5, 7, and 10 post-transduction, and results were compared to baseline values. Data for three experimental sets are shown in Figure 6a. Extra attention was paid to compare data only from β-cell cultures with similar initial purity. Consistent with previous findings, the proportion of insulin-positive cells decreased over time in cultures that were treated with HGF and in cultures that were not treated, independent of their transduction status. Although the decrease in the nontreated groups was ~20% by day 10 of the study, it reached as high as 43% when cells were exposed to HGF. In contrast, we observed an increase in the proportion of insulin-positive cells in the transduced and CID-treated cultures reaching as high as 29% on day 7. These results confirm the successful expansion of functional β-cells with our approach. Besides determining the proportion of β-cells, we also followed the change in their absolute number within the cultures for 10 days with data recorded at days 5, 7, and 10. Results are expressed as fold increase, in comparison to the transduced and nontreated control (Figure 6b). Interestingly, different dynamic changes were observed in the effects of F36Vcmet and HGF treatment. In the Insp-F36Vcmet-transduced and CID-treated group, an early, 1.8-fold increase was noted in the absolute number of insulin-positive cells by day 5 that remained stable over the follow-up period. In contrast, HGF elicited its peak proliferative effect at day 7, which was followed by a significant drop in the absolute number of viable β-cells by day 10.
Figure 6.
Change in proportion and number of insulin-positive cells over time. (a) Change in the proportion of β-cells over time in cultures that were transduced with F36Vcmet and were either left untreated or were treated with CID or HGF. (b) Fold increase in the absolute number of transduced insulin-positive cells over a 10-day follow-up period; cultures were either treated with CID or HGF. Cultures of transduced but not-treated cells serve as the basis for evaluation. CID, chemical inducer of dimerization; HGF, hepatocyte growth factor.
Functional tests
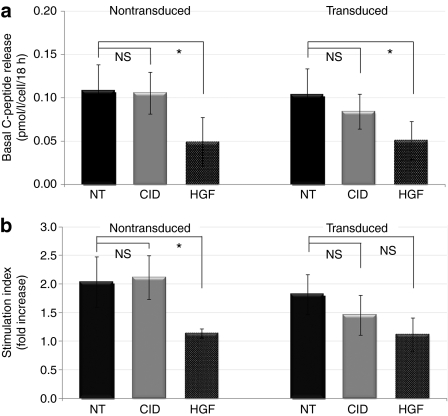
Basal and glucose-induced C-peptide releases were determined in order to examine the functionality of the expanded β-cells. As shown in Figure 7a, 18-hour basal C-peptide release was compared in nontransduced and in Insp-F36Vcmet-transduced β-cell cultures in the presence or absence of AP20187 or HGF. Transduction itself had no significant effect on β-cell function. AP20187 treatment of cells transduced with the Insp-F36Vcmet vector prompted a moderate decrease in their synthetic activity. In contrast, treatment with HGF reduced basal C-peptide release in both groups to values that were significantly below control.
Figure 7.
Functional analysis of human pancreatic β-cells. The functionality of the β-cells was assessed 7 days after HGF or AP20187 treatment by quantifying C-peptide release into the culture medium. (a) Basal C-peptide release during an 18-hour incubation period with glucose concentration at physiological levels (5.5 mmol/l). (b) Glucose-induced C-peptide release into the medium (commonly referred to as stimulation index). NT, nontreated (black bars); CID, treatment with AP20187 (gray bars); HGF, hepatocyte growth factor (dotted bars). Each bar represents the average of six separate experiments. NS, difference not significant; *P < 0.05.
Glucose-induced C-peptide release was next determined with the results plotted in Figure 7b. Interestingly, a pattern similar to basal C-peptide release was observed: β-cells expanded using the Insp-F36Vcmet vector construct and AP20187 retained their responsiveness to high glucose concentration to a greater extent than those treated with HGF. However, the difference did not reach statistical significance in these set of experiments.
Discussion
Replacing pancreatic β-cells via islet transplantation represents a novel approach to manage and treat type 1 DM, an increasingly frequent disease with the primary pathology of complete β-cell destruction by autoimmune processes. The success of the methodology is, however, hindered by the immunological rejection of the transplanted cells and the critical shortage of donor tissue available for the procedure. Thus, it has been a long-standing goal to identify novel ways to improve tissue supply and to perform islet transplantation ideally from a single source.
HGF has been used in the past by multiple research groups to expand human β-cells ex vivo. Their success was limited, however, and conflicting results have been reported as to what extent and how specifically HGF is able to induce β-cell proliferation ex vivo.6,16 The controversy arises from the fact that islet preparations are never entirely pure and always contain a significant number of cells other than β-cells that may also respond to HGF treatment.20,21 In addition, HGF has been shown to prompt a loss of overall insulin release into the medium when compared to cultures not treated with the growth factor. This accelerated loss of function may reflect cellular dedifferentiation secondary to induced proliferation.
Herein, we present a novel methodology aimed at the selective and controlled expansion of β-cells in cultures of human adult pancreatic islets. We have shown that by expressing a novel chimeric receptor, F36Vcmet, specifically in β-cells and treating the cultures with a synthetically designed, receptor-specific ligand (AP20187, CID), the selective expansion of pancreatic β-cells can be achieved. In addition, the synthetic activity of the cell population managed with this approach was superior to those treated with HGF, as assessed by overall C-peptide release into the culture medium.
To achieve chimeric receptor expression restricted to pancreatic β-cells, a series of lentiviral vectors were designed with a human insulin promoter fragment driving their expression. Among the tested vectors, the single promoter construct provided superior performance with F36Vcmet expression confined almost exclusively to the target cell population, the insulin-expressing β-cells. Following transduction, 65% of β-cells were positive for the chimeric receptor on average, whereas merely 1.5% of insulin-negative cells expressed the protein. The rare occurrences of F36Vcmet protein expression in the non-β-cell population may reflect vector leakiness or, alternatively, dedifferentiation of previously insulin-positive cells.
Selective proliferation of pancreatic β-cells was induced with this approach, as proven by two distinct methodologies. First, BrdU-labeling index of β-cells at day 7 showed a consistent fourfold increase in the proportion of proliferating β-cells expanded with the Insp-F36Vcmet vector and CID when compared to the nontreated controls. The effect was dependent on both chimeric receptor (F36Vcmet) expression and the presence of AP20187, as no increased cell proliferation was registered if nontransduced cells were treated with AP20187 or if transduced cells were left untreated. The extent of the proliferative response exceeded that achieved by HGF treatment (threefold). Second, determination of the absolute number of insulin-positive cells in the cultures revealed a twofold increase between those expanded with our approach and the control cultures. We hypothesize that the inherent differences between the two detection techniques may explain this discrepancy in the magnitude of the effect (fourfold versus twofold): although BrdU labeling accurately reflects the proportion of cells in the S-phase of the cell cycle, it does not address whether these cells indeed complete a successful cell division.
The fourfold increase in β-cell proliferation upon CID treatment might not completely explain the twofold increase in the absolute number of insulin-positive cells observed at 1 week in the transduced and CID-treated group versus the control group. We speculate that an alternative mechanism may also have contributed to the increase in the number of insulin-positive cells in the T+CID group. One potential, but likely insignificant mechanism may be the differentiation of transduced progenitor cells into β-cells upon CID treatment. It has been suggested previously that HGF may drive the differentiation of pancreatic progenitor cells into the β-cell lineage.22,23,24 Although our vector construct was highly specific for the insulin-positive cell population, we are not able to entirely exclude the possibility that some cells harboring the F36Vcmet vector construct were indeed pancreatic progenitor cells. Activation of the HGF signaling through the F36Vcmet receptor in the progenitor population may have driven the differentiation of these cells toward the β-cell lineage. This would contribute to the observed increase in the number of insulin-positive cells. Alternatively, it is tempting to speculate that insulin-positive cells were under positive selection in the T+CID group as the insulin promoter was driving F36Vcmet expression. Alternatively, the activity of the F36Vcmet construct may have provided survival advantage for transduced cells treated with CID. Improved cell survival is recognized as an effect of active HGF signaling in many cell types including pancreatic islets owing likely to antiapoptotic mechanisms.8,9,25,26 The potential antiapoptotic effect of the novel vector construct has not been tested in the present study.
Cell cultures expanded with Insp-F36Vcmet transduction and CID or with HGF were followed prospectively in a series of experiments, and changes in the absolute number and the proportion of insulin-positive cells were registered regularly. We found that expanding the cells with the two approaches produced similar results when comparing the absolute number of β-cell at day 7: 1.9× expansion with F36Vcmet and CID versus 1.6× using HGF. In contrast, significant differences were observed when comparing the proportion of insulin-positive cells. Although the percentages of insulin-positive cells in the cultures increased with F36Vcmet and CID compared to the control, HGF treatment prompted an accelerated decline in the proportion of insulin-positive cells. As also suggested by previous publications, this latter observation may reflect the coexpansion of non-β-cells by HGF in the primary islet cultures, most likely ductal cells.16 In contrast, the F36Vcmet and CID approach preferentially expanded cells that retained insulin positivity because the chimeric receptor utilized in the present study is expressed from the insulin promoter, which should only be active in β-cells that maintain their differentiated state. In addition, minor differences in the downstream signaling initiated by HGF versus the method utilizing F36Vcmet and AP20187 cannot be excluded. Further studies will be needed to clarify the exact underlying mechanisms of these observations.
It is important to emphasize that the functional properties of the β-cells expanded using the F36Vcmet and CID approach were better preserved, as determined by measuring C-peptide secretion, compared to the effects of HGF. This may again be explained by the fact that the insulin promoter drives the chimeric receptor expression; thereby, the receptor will preferentially be synthesized in cells that are viable, retain differentiation and are able to produce insulin. An alternative explanation for the observed difference between the function of cells expanded with HGF as opposed to CID might be that HGF also increased the proliferation of non-β-cells thereby leading to decreased C-peptide release per islet mass. Thus, underestimation of basal C-peptide release in cultures expanded with HGF relative to the CID group is a possibility. However, this would not affect the registered difference in glucose-induced C-peptide release presented as stimulation index. In addition, minor differences in downstream signaling events as well as differences in the cross talk between various signaling pathways initiated by HGF versus the novel approach cannot be entirely excluded. This may also provide partial explanation for the observed functional difference of cells expanded with HGF versus the novel vector construct.
Although we demonstrate that this approach using transduction with a ligand-inducible signaling molecule led to improved expansion of β-cells, the effects were modest, on the order of two- to fourfold. Nevertheless, the principle is illustrated that specific manipulation of signaling pathways may have useful applications. Combining this approach with additional factors, such as other growth factors, extracellular matrices, and bioreactor devices may lead to clinically useful methods to expand the supply of β-cells available for transplantation to benefit patients with type 1 DM.
Materials and Methods
Lentiviral vector preparations. The plasmid encoding the ligand-inducible chimeric receptor, F36Vcmet, with an influenza HA tag at its C-terminus, was a generous gift of Blau and Lieber at the University of Washington.27 Third-generation lentiviral vector constructs were produced in which expression of F36Vcmet (Figure 1) was directed from the human insulin gene promoter fragment (the 1.4 kb Bcl I to Hind III fragment from pFOXCAT1.4; kindly provided by German, University of California at San Francisco).28 Two of the vector constructs also expressed eGFP (BD Biosciences, Mountain View, CA) downstream of the fusion protein either under control of the IRES or the murine PGK promoter. All vectors were based on the pCCL self-inactivating vector backbone developed by Naldini29 and were packaged using the VSV-G envelope pseudotype and the p8.9 plasmid. Vector particles were generated via the transient transfection of 293T cells and were concentrated by ultrafiltration and ultracentrifugation as described previously.30 Quantitative PCR analysis was used to determine vector titers on HT29 cells that ranged between 1.6 × 108 and 1.9 × 109.
Cell lines. Human colorectal adenocarcinoma (HT29) and murine pancreatic insulinoma (NIT-1) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The HT29 cell line was maintained in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Omega Scientific, Tarzana, CA), 2 mmol/l -glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gemini Bio-Products, West Sacramento, CA). The NIT-1 cell line was cultured in F12K medium (ATCC) supplemented with 10% fetal bovine serum, -glutamine, penicillin, and streptomycin. Cell lines were kept at 37 °C and in a humidified carbon dioxide (5%) incubator.
Cell line transduction. Cell line transduction was performed as described previously.30 Briefly, NIT-1 cells (105 cells/ml in 1 ml) were transduced with a vector concentration of 5 × 105 (multiplicity of infection = 5) in the presence of 8 µg/ml Polybrene (Sigma-Aldrich, St Louis, MO).
Human adult pancreatic islet culture and transduction. Human adult pancreatic islets were obtained from the National Disease Research Interchange and the Islet Cell Resource Center Basic Science Human Islet Distribution Program. Islets from 10 different donors (mean age: 43.1 years ranging from 30 to 55 years) were used for the experiments with the islet purity ranging between 65 and 90% (based on reports from the islet providers). Pancreatic islet preparations were mechanically dissociated into smaller cell clusters upon their receipt and were transduced with a final vector concentration of 1 × 107 transducing units/ml in the presence of 4 µg/ml Polybrene. Cells were then distributed into the wells of a 96-well plate with ~75 islets per well. Islets were cultured as monolayers on HTB9 matrix as described previously31 and were maintained in RPMI-1640 medium containing 5.5 mmol/l glucose, 10% fetal bovine serum, 2 mmol/l -glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin with daily medium changes.
Drug treatment. Lyophilized AP20187 (kindly provided by ARIAD Pharmaceuticals, Cambridge, MA) was reconstituted in 100% ethanol according to the manufacturer's instructions to prepare a stock solution with a concentration of 100 µmol/l. Aliquots of the stock solution were stored at ‐20 °C until use. Working solutions with a final concentration of 3–100 nmol/l were prepared via the half-log serial dilution of the stock with cell culture medium. Human recombinant HGF (hrHGF; R&D Systems, Minneapolis, MN) was reconstituted at 50 ng/ml using cell culture medium. Depending on the experimental arm, cells were either treated with the carrier: ethanol (untreated experimental arms), received AP20187 or hrHGF with their medium throughout the experiments.
Immunohistochemical analysis. For immunohistochemical analysis, cytospin slides were prepared. Cells were fixed with 4% paraformaldehyde for 15 minutes and were washed three times (5 minutes each) using phosphate-buffered saline (PBS) with 0.05% Tween-20 (PBS-T; Sigma-Aldrich). Blocking and permeabilization were performed in PBS-T containing 10% (vol/vol) normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.1% Triton X-100 (Sigma-Aldrich). Slides were incubated overnight at 4 °C in the presence of a polyclonal guinea-pig anti-insulin antibody (1:200 dilution; Dako, Carpinteria, CA) and HA-probe, a murine monoclonal antibody to HA (F-7; Santa Cruz Biotechnology, Santa Cruz, CA). Following the overnight incubation, all slides were washed three times with PBS-T and were incubated for 45 minutes at room temperature in the presence of the following secondary antibodies: Cy3-conjugated donkey anti-guinea-pig antibody (1:250 dilution; Jackson ImmunoResearch) and Alexa-488-conjugated donkey anti-mouse antibody in 1:500 dilution (Invitrogen, Carlsbad, CA). Slides were washed multiple times with PBS-T and mounted using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Fluorescent images were obtained with a Leica DM RXA (Leica Microsystems, Heidelberg, Germany) upright fluorescent microscope utilizing EasyFISH (Applied Spectral Imaging, Vista, CA) software. Image quantification was performed using MetaMorph version 6.1 software (Molecular Devices, Sunnyvale, CA).
Cell proliferation assay. Proliferation of the β-cell population was assessed by double-staining the cells for insulin and BrdU, followed by fluorescent microscopic evaluation. BrdU labeling and detection were performed with a commercially available kit (BrdU In Situ Detection Kit; BD Biosciences, Franklin Lakes, NJ) with minor modifications to the manufacturer's suggested protocol. In brief, islet preparations were incubated with BrdU at 0.1 mmol/l for 24 hours. Cytospin slides were prepared, fixed and washed three times (5 minutes each). Following a 30-minute permeabilization step, antigen retrieval was performed by treating the slides with 2N HCl for 30 minutes. After an extensive washing cycle, slides were incubated with the following primary antibodies for 1 hour at room temperature: polyclonal guinea-pig anti-insulin antibody in 1:200 dilution and biotinylated anti-BrdU antibody in 1:10 dilution (provided in the kit). Cells were washed three times using PBS-T (Sigma-Aldrich) and were stained for 45 minutes at room temperature with the following secondary antibodies: Cy3-conjugated donkey anti-guinea-pig antibody in 1:250 dilution and Alexa-488-conjugated streptavidin in 1:500 dilution. Slides were washed multiple times with PBS-T and mounted using Vectashield mounting medium with DAPI. Fluorescent images were obtained with a Leica DM RXA upright fluorescent microscope and were analyzed using the EasyFISH software. Image quantification was performed using Metamorph version 6.1 software.
Functional assay. An ultrasensitive, human-specific C-peptide enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden) was used for the quantitative evaluation of pancreatic β-cell function. After the initial determination of basal C-peptide release (5.5 mmol/l glucose exposure for 18 hours), cultures were sequentially exposed to low (1.6 mmol/l) and high (16.6 mmol/l) glucose concentrations, each for 1 hour at 37 °C. A stimulation index was calculated as the ratio of C-peptide release in the high and low glucose containing media.
Statistical analysis. All data are expressed as mean ± SD. Two-tailed, paired Student's t-test was used to determine statistical significance. The difference between the experimental arms was considered significant, in case the P value was <0.05.
Acknowledgments
We thank Denise A. Carbonaro-Sarracino and Xingchao Wang for their technical assistance. We acknowledge the Cellular Imaging Core Facility of Childrens Hospital Los Angeles. We thank the ICR Basic Science Islet Distribution Program and the National Disease Research Interchange (NDRI) for providing human pancreatic islet preparations. ARIAD Pharmaceuticals provided the CID compound, and Blau and Lieber from the University of Washington the F36Vcmet gene. E.P. is a recipient of fellowships from the California Institute for Regenerative Medicine (CIRM) and The Saban Research Institute of Childrens Hospital Los Angeles.
REFERENCES
- National Center for Health Statistics, Centers for Disease Control and Prevention, Division of Diabetes Translation (2008) Long-term trends in diabetes . < www.cdc.gov/diabetes/statistics >.
- DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, et al. Current status of clinical islet transplantation. Transplantation. 2005;79:1289–1293. doi: 10.1097/01.tp.0000157273.60147.7c. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Cirulli V, Lopez AD., and, Hayek A. Ex vivo expansion of human pancreatic endocrine cells. J Clin Endocrinol Metab. 1997;82:1852–1856. doi: 10.1210/jcem.82.6.4009. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, et al. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes. 2002;51:3435–3439. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Beattie GM, Rubin JS, Lopez AD, Baird A., and, Hayek A. Hepatocyte growth factor/scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes. 1994;43:947–953. doi: 10.2337/diab.43.7.947. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Cirulli V, Beattie M, Mally MI, Soto G, Rubin JS, et al. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic beta-cell growth. Endocrinology. 1996;137:3131–3139. doi: 10.1210/endo.137.7.8770939. [DOI] [PubMed] [Google Scholar]
- Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC., and, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- Nakano M, Yasunami Y, Maki T, Kodama S, Ikehara Y, Nakamura T, et al. Hepatocyte growth factor is essential for amelioration of hyperglycemia in streptozotocin-induced diabetic mice receiving a marginal mass of intrahepatic islet grafts. Transplantation. 2000;69:214–221. doi: 10.1097/00007890-200001270-00004. [DOI] [PubMed] [Google Scholar]
- Rao P, Cozar-Castellano I, Roccisana J, Vasavada RC., and, Garcia-Ocaña A. Hepatocyte growth factor gene therapy for islet transplantation. Expert Opin Biol Ther. 2004;4:507–518. doi: 10.1517/14712598.4.4.507. [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch NM, Berman DM, Sicari BM, Takane KK, Garcia-Ocaña A, Ricordi C, et al. Hepatocyte growth factor enhances engraftment and function of nonhuman primate islets. Diabetes. 2008;57:2745–2754. doi: 10.2337/db08-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Lefebvre VH, Otonkoski T, Ustinov J, Huotari MA, Pipeleers DG., and, Bouwens L. Culture of adult human islet preparations with hepatocyte growth factor and 804G matrix is mitogenic for duct cells but not for beta-cells. Diabetes. 1998;47:134–137. doi: 10.2337/diab.47.1.134. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Itkin-Ansari P, Cirulli V, Leibowitz G, Lopez AD, Bossie S, et al. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes. 1999;48:1013–1019. doi: 10.2337/diabetes.48.5.1013. [DOI] [PubMed] [Google Scholar]
- Bolanos-Garcia VM. MET meet adaptors: functional and structural implications in downstream signalling mediated by the Met receptor. Mol Cell Biochem. 2005;276:149–157. doi: 10.1007/s11010-005-3696-6. [DOI] [PubMed] [Google Scholar]
- Vigna E, Naldini L, Tamagnone L, Longati P, Bardelli A, Maina F, et al. Hepatocyte growth factor and its receptor, the tyrosine kinase encoded by the c-MET proto-oncogene. Cell Mol Biol (Noisy-le-grand) 1994;40:597–604. [PubMed] [Google Scholar]
- Liu N, Furukawa T, Kobari M., and, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am J Pathol. 1998;153:263–269. doi: 10.1016/S0002-9440(10)65567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilá MR, Nakamura T., and, Real FX. Hepatocyte growth factor is a potent mitogen for normal human pancreas cells in vitro. Lab Invest. 1995;73:409–418. [PubMed] [Google Scholar]
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O., and, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB., and, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M, Yamasaki K, et al. Hepatocyte growth factor prevents endothelial cell death through inhibition of bax translocation from cytosol to mitochondrial membrane. Diabetes. 2002;51:2604–2611. doi: 10.2337/diabetes.51.8.2604. [DOI] [PubMed] [Google Scholar]
- Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF., and, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau CA, Peterson KR, Drachman JG., and, Spencer DM. A proliferation switch for genetically modified cells. Proc Natl Acad Sci USA. 1997;94:3076–3081. doi: 10.1073/pnas.94.7.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odagiri H, Wang J., and, German MS. Function of the human insulin promoter in primary cultured islet cells. J Biol Chem. 1996;271:1909–1915. doi: 10.1074/jbc.271.4.1909. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KL, Pais E, Ge S, Hardee C, Skelton D, Hollis RP, et al. Lentiviral vectors with amplified beta cell-specific gene expression. Gene Ther. 2009;16:998–1008. doi: 10.1038/gt.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie GM, Rubin JS, Mally MI, Otonkoski T., and, Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996;45:1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]