Abstract
Unlike mammals, bony fish have two type II interferons, IFNγ and IFNγrel, whose pro-inflammatory functions have not been fully characterized. To elucidate the distinct roles of these type II interferons of bony fish, we examined the effects of recombinant goldfish (rg) IFNγ and IFNγrel on the macrophage antimicrobial responses, immune gene expression, and their signaling pathways. Our findings indicate that rgIFNγ and rgIFNγrel possess unique capacities to mediate each of the above processes. Q-PCR analysis revealed similar expression of both cytokines in tissues and immune cell populations of the goldfish, although IFNγ mRNA levels were generally higher in most tissues and cell types. Whereas rgIFNγ had long-lasting effects on the priming of goldfish monocyte ROI production, the rgIFNγrel had relatively short-lived ROI priming potential and eventually down-regulated the priming of ROI production induced by rgIFNγ or rgTNFα2. Whereas rgIFNγ induced relatively modest phagocytic and nitric oxide responses of goldfish macrophages, rgIFNγrel induced significantly higher phagocytosis, iNOSA and iNOSB gene expression and nitric oxide production compared with rgIFNγ. The rgIFNγ and rgIFNγrel induced different gene expression profiles in goldfish monocytes. These differences included significantly higher induction of TNFα2, CXCL8, ceruloplasmin, and interferon regulatory factor (IRFs) expression after activation of monocytes with rgIFNγrel. The rgIFNγrel was more abundant in whole cell lysates compared with rgIFNγ. Both cytokines induced the phosphorylation of Stat1, while the nuclear localization of Stat1 was only observed following treatment of monocytes with rgIFNγ. Our findings suggest the presence of functional segregation of the induction of macrophage antimicrobial functions by type II interferons of bony fish.
Keywords: Cytokines Induction, Gene Expression, Innate Immunity, Interferon, Nitric Oxide, Phagocytosis, Reactive Oxygen Species (ROS), Fish, Interferon gamma, Interferon gamma-related Protein
Introduction
Interferon gamma (IFNγ)3 is a highly pleiotropic pro-inflammatory and anti-viral cytokine produced primarily by activated Th1 phenotype CD4+ cells (1) CD8+ cells (2) and natural killer (NK) cells (3). In addition to its weak antiviral activity (4–6), IFNγ is a central cytokine that regulates host defense against obligate and facultative intracellular pathogens (5, 7–9). For example, IFNγ gene knock-out mice are unable to control infections with Leishmania major (10), Listeria monocytogenes (11) and Mycobacteria (12), indicating that IFNγ is important for the regulation of macrophage antimicrobial responses (9, 13–16).
Homologues of the IFNγ have been identified in a number of bony fish (teleosts) including zebrafish (17), Japanese pufferfish (18), trout (19), Atlantic salmon (20), catfish (21), common carp (22), and goldfish (23). Of these fish species, zebrafish, catfish, common carp and goldfish have two isoforms of IFNγ, which differ markedly within each species in both sequence homology and expression in different tissues (17, 21, 22). Both isoforms contain IFNγ signature motifs (17, 21) and were initially named IFNγ1 and IFNγ2. IFNγ2 is structurally similar to mammalian IFNγ, whereas IFNγ1 is shorter and does not contain a C-terminal cationic residues required for IFNγ activity (19, 24). Consequently, the fish IFNγ1 and IFNγ2 are now referred to as IFNγ related (IFNγrel) and IFNγ, respectively. IFNγrel is expressed in LPS-stimulated common carp leukocytes enriched for B-cells (22). In grass carp increased mRNA levels are observed in immune organs following infection with reovirus, and stimulation with peptidoglycan, LPS, and poly(I:C) (25). However, there is conflicting evidence as to possible roles of fish type II IFNs in vivo (26, 27) and to date the functional roles of IFNγrel are not known.
The mammalian IFNγ mediates its biological effects by ligating interferon γ receptor 1 (IFNGR1), which then associates with IFNGR2, forming a signaling complex. Complex assembly leads to activation of Janus kinases (Jak) 1 and 2, associated with the receptor chains 1 and 2, respectively (28). These phosphotyrosine kinases then phosphorylate the IFNGR1-associated Stat1 (29) and to a lesser extent Stat2 (30) transcription factors. The activation of a plethora of other genes then ensues through homodimeric Stat1, heterodimeric Stat1:Stat2 as well as through the transcription factor complexes ISGF3 and Stat1-p48, composed of Stat1:Stat2:IRF-9 and Stat1:Stat1:IRF-9, respectively (30–32). The above transcription factors orchestrate gene regulation through recognition of IFNγ-activated sequences (GAS) in the promoter regions of target genes (33). Within the first 30 min of IFNγ signaling, an up-regulation in the expression of several interferon regulatory factors (IRFs) occurs, which then modulate subsequent waves of gene expression in the IFNγ signaling cascade (34). Several but not all of the genes in the IFNγ signaling pathway have been cloned (20, 35, 36) and an IFNγ-specific trout reporter cell line has been established (37). However, the consensus sequences of the GAS elements of the fish IFNγ-responsive promoters are less specific than their mammalian counterparts (38), and the precise mechanisms by which the fish IFNγ and IFNγrel signal are not known.
This report represents the first comprehensive functional characterization and direct comparison of the fish (rg)IFNγrel and rgIFNγ. Our findings indicate that rgIFNγrel and rgIFNγ possess distinct capacities to mediate specific pro-inflammatory responses of goldfish myeloid cells. The functional segregation of induction of macrophage antimicrobial functions by type II interferons of bony fish is different from the single Type II IFN system present in all other vertebrates examined.
EXPERIMENTAL PROCEDURES
Goldfish
Goldfish (Carassius auratus) were purchased from Mt. Parnell Fisheries Inc. (Mercersburg, PA) and maintained at the Aquatic Facility of the Department of Biological Sciences, University of Alberta. The fish were kept at 20 °C in a flow-through water system on a simulated natural photoperiod, and fed to satiation daily with trout pellets. The fish were acclimated to this environment for at least 3 weeks prior to use in experiments. All of the fish ranged from 10 to 15 cm in length and whenever possible an equal number of both sexes were used.
Macrophage Cultures
The procedures for the isolation and cultivation of primary kidney macrophages (PKM) and the medium (NMGFL-15) used for their cultivation have been described previously (39).
Isolation of Goldfish Splenocytes, Peripheral Blood Leukocytes (PBL), and Granulocytes
The isolation of goldfish splenocytes, PBLs, and kidney-derived granulocytes has been described previously (23).
Analysis of Goldfish IFNγ and IFNγrel Expression in Goldfish Tissues and Immune Cell Populations
Preparation of cDNA corresponding to goldfish tissues and immune cell populations and the Q-PCR thermocycling parameters were previously described (23). Goldfish specific IFNγ and IFNγrel primers were designed using Primer Express software (Applied Biosystems), and the expression was assessed relative to the endogenous control gene, elongation factor 1 α (EF-1α). Tissues from five goldfish (n = 5) and cell populations from four goldfish (n = 4) were used for the Q-PCR analysis carried out using 7500 Fast software (Applied Biosystems). Direct comparisons of IFNγ and IFNγrel expression was achieved by performing ddCT analysis using lowest expression (highest delta CT, IFNγrel: muscle and monocytes) as the standard for the expression for both cytokines. The RQ values were normalized against the lowest observed tissue and cell expression (IFNγrel, muscle, and monocytes, respectively). All primers used in this study are shown in supplemental Table S1.
Analysis of Immune Gene Expression of rgIFNγrel and rgIFNγ-stimulated Cells
Day 3 cultures enriched for monocytes and day 8 cultures abundant in mature macrophages (23, 29), were treated for 12 h with either medium only, 100 ng/ml of rgIFNγrel, 100 ng/mLof rgIFNγ, or 100 ng/ml rgIFNγrel + 100 ng/ml rgIFNγ. Each treatment group consisted of 1 × 106 cells in a final volume of 500 μl of complete medium using cells obtained from cultures established using kidney leukocytes isolated from individual fish (n = 5). Following indicated treatments, the total RNA was isolated from the cells using TRIzol and reverse transcribed into cDNA using the Superscript II cDNA synthesis kit according to manufacturer's directions. The genes examined included: IFNGR1–1 and IFNGR1–2; p40phox; p47phox; p67 phox; p22 phox; gp91phox; IL-1β-1; and IL-1β-2; TNFα1 and TNFα2; CXCL8; CCL1; iNOSA; and iNOSB, TGFβ and ceruloplasmin. Goldfish IRF expression analysis was performed by treating goldfish monocytes in a final volume of 500 μl for 0, 15, 30, or 90 min with 100 ng/ml of rgIFNγ or rgIFNγrel. RNA isolation and cDNA synthesis were performed as described above. Expression analysis of all genes was performed using the delta CT method relative to EF-1α and derived RQ values were normalized against respective untreated controls.
Production and Purification of rgIFNγrel, rgIFNγ, and rgTNFα2
The production of rgTNFα2, rgIFNγ, and rgIFNγrel has been described previously (23, 40, 41).
Immunodetection of rgIFNγrel
The purified rgIFNγrel was used for generation of rabbit polyclonal α-rgIFNγrel IgG. The primary immunization was performed by combining equal volumes of rgIFNγrel (50 mg in 750 ml) with Freud's complete adjuvant (750 ml). Booster injections were carried out as above but using Freud's incomplete adjuvant. The IgG fraction was affinity-purified using HiTrap protein A HP column (Amersham Biosciences) in accordance with the manufacturer's protocol. The isolated α-rgIFNγrel IgG was filter-sterilized (0.22 mm filter, Milipore) and assessed for reactivity against rgIFNγrel using Western blot.
Respiratory Burst Assay
Goldfish monocytes were seeded into 96-well plates at a density of 3 × 105 cells per well. Cells were primed with either medium only, rgTNα2 (100 ng/ml), rgIFNγ (100 ng/ml), rgIFNγrel (0.001, 0.1, 10 ng/ml), rgTNFα2 (100 ng/ml) in combination with 0.001, 0.1, or 10 ng/ml of rgIFNγrel, or rgIFNγ (100 ng/ml) in combination with 0.001, 0.1, or 10 ng/ml of rgIFNγrel with or without α-rgIFNγrel IgG (5 μg/ml) in a total volume of 100 μl/well. All cultures were incubated for 1, 9, or 16 h after which phorbol ester (PMA) was used to trigger the ROI production. Medium-only-treated, PMA-triggered cells were negative controls. The nitroblue tetrazolium (NBT) assay was performed as described previously (23, 41).
Phagocytosis Assay
Monocytes from cultures established from kidney leukocytes isolated from individual fish (n = 5) were seeded into wells of 96-well plates at a density of 3 × 105 cells per well and were treated with either medium only, rgIFNγ (100 ng/ml), rgIFNγrel (1,10 or 100 ng/ml), a combination of rgIFNγ (100 ng/ml), and rgIFNγrel (1,10 or 100 ng/ml) with or without α-rgIFNγrel IgG (5 μg/ml). To each well fluorescent beads (2.0 μm diameter YG, Polysciences) were added at a ratio of 10 beads:1 cell, in a final volume of 100 μl. The phagocytosis assay was performed as described previously (23, 41).
Nitric Oxide Assay
8-day-old goldfish macrophage cultures established from kidney leukocytes of individual fish (n = 5) were seeded into wells of 96-well plates at a density of 3 × 105 cells/well and treated with either medium only, rgIFNγ (100 ng/ml), rgIFNγrel (1,10 or 100 ng/ml), and a combination of rgIFNγ (100 ng/ml) and rgIFNγrel (1, 10, or 100 ng/ml) with or without α-rgIFNγrel IgG (5 μg/ml). All cultures were incubated for 72 h before assessing nitrite production using the Griess reaction as described previously (23, 41).
Western Blot Analysis of Cell Lysates and Isolated Nuclei
Five million monocytes were incubated with either medium alone, rgIFNγrel, or rgIFNγ. For ligand association/internalization experiments, cells were incubated with 5 μg of each recombinant protein and assessed at 0, 15, 30, or 90 min after treatment. For phospho-Stat1 experiments, cells were treated with 100 ng/ml of IFNγrel or IFNγ and assessed at 0, 15, 30, 90 min. For all experiments, cells were pelleted by centrifugation and either immediately resuspended in Laemmli buffer and boiled at 95 °C or prepared for isolation of nuclei. The nuclei isolation protocol was adopted from Garcia et al. (42). Briefly, pelleted cells were flash frozen on dry ice-ethanol bath for 10 min and disrupted by resuspending them in hypotonic buffer (10 mm Hepes, 10 mm KCl, 1.5 mm MgCl2, 1 mm freshly added dithiothreitol, pH 7.9). Nuclei were recovered by centrifugation at 800 × g for 10 min in a cooled microcentrifuge, resuspended in Laemmli buffer and boiled at 95 °C. All samples were resolved on freshly cast 10% SDS gels, transferred onto nitrocellulose membranes, blocked for 1 h, and incubated overnight at 4 °C in appropriate primary antibody (α-polyhistidine, Sigma, and α-phospho-Stat1(Tyr), Cell Signaling Technology Inc.). The following day, the membranes were washed, incubated for 1 h with appropriate secondary antibody (goat-anti rabbit or goat anti-mouse IgG, BioRad), and developed using ECL developing substrate (Pierce).
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) and Tukey's test. A probability level of p < 0.05 was considered significant.
RESULTS
Analysis of IFNγrel and IFNγ Expression in Goldfish Tissues and Different Immune Cells
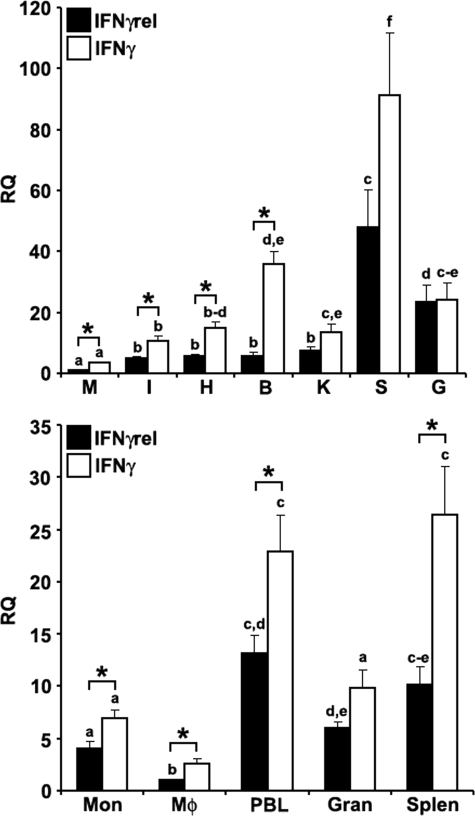
The expression analysis of goldfish IFNγ and IFNγrel revealed that the highest mRNA levels for both cytokines were in the spleen compared with other tissues and the lowest mRNA levels were in the muscle (Fig. 1A). However, significantly higher mRNA levels of IFNγ compared with IFNγrel were observed in most tissues (Fig. 1A).
FIGURE 1.
Quantitative expression analysis of goldfish IFNγ and IFNγrel in tissues and immune cell populations obtained from healthy fish. Top, goldfish IFNγrel tissue expression analysis. The tissues examined were: muscle (M), intestine (I), heart (H), brain (B), kidney (K), spleen (S), and gill (G). The expression of goldfish IFNγrel was assessed relative to endogenous control gene, elongation factor 1 α (EF-1α). Analyses of the relative tissue expression data are for tissues from five fish (n = 5). All results were normalized against the muscle IFNγrel expression levels. Bottom, goldfish IFNγrel expression in different immune cell populations. The cells examined were: monocytes (Mon), macrophages (Mϕ), peripheral blood leukocytes (PBL), granulocytes (Gran), and splenocytes (Splen). Immune cells populations were derived from four fish (n = 4) and the expression normalized against that of FACS-sorted macrophages. Direct comparisons of IFNγ and IFNγrel expression was achieved by performing ddCT analysis using lowest expression as the standard for the expression of both cytokines. The RQ values were normalized against the lowest observed tissue or cell expression (IFNγrel, muscle, and monocytes, respectively). Statistical analysis was performed using one-way ANOVA. Different letters above each bar denote significant differences (p < 0.05), the same letter indicate no statistical difference between groups.
The expression of IFNγ was significantly greater than that of IFNγrel in all goldfish immune cell population except granulocytes (Fig. 1B). The highest mRNA levels of both IFNγ and IFNγrel were observed in the PBLs and splenocytes (Fig. 1B). Lower mRNA levels were measured in monocytes and granulocytes, whereas the expression of both IFNγ and IFNγrel was lowest in mature macrophages (Fig. 1B).
Expression Analysis of Immune Genes of Monocytes Treated with rgIFNγrel and rgIFNγ
To examine the immune gene expression in monocytes, cells were treated with either medium alone, rgIFNγrel, rgIFNγ, or with a combination of both cytokines and the mRNA levels of select immune genes measured using quantitative PCR. The following genes were examined: IFNGR1–1 and IFNGR1–2, components of the NADPH oxidase pathway, IL-1β isoforms 1 and 2, TNFα isoforms 1 and 2, the chemokines CXCL8 and CCL1, TGFβ, and ceruloplasmin.
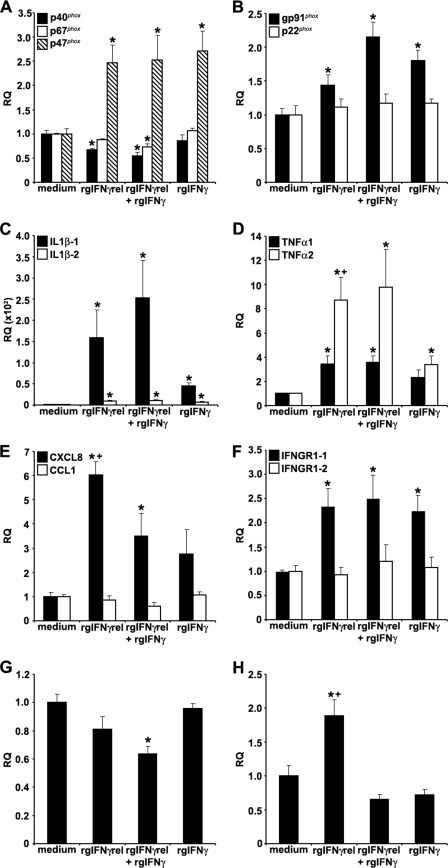
The expression of the NADPH oxidase components, after treatment of monocytes with rgIFNγrel or rgIFNγ was variable. For example, the expression of p47phox was significantly higher than that in medium-treated cells (Fig. 2A). The treatment of monocytes with rgIFNγrel alone or in combination with rgIFNγ caused a significant down-regulation in the expression of p40 phox, whereas treatment with rgIFNγ alone had no effect on the expression of this gene (Fig. 2A). Combined, but not individual treatments of monocytes with rgIFNγrel and rgIFNγ also resulted in decreased expression of p67phox (Fig. 2A). The expression of gp91phox, a gene that encodes a membrane-bound NADPH pathway component, was significantly elevated by all treatments, whereas the expression of p22phox did not change (Fig. 2B).
FIGURE 2.
Quantitative expression analysis of goldfish immune genes in monocytes stimulated with rgIFNγrel, rgIFNγ, or a combination of both cytokines. The reported expression was relative to EF-1α. The genes examined included: (A), p40phox, p47phox, p67phox; (B) gp91phox, p22phox; (C) IL-1β-1, IL-1β-2; (D) TNFα1, TNFα2; (E) CXCL8, CCL1; (F) IFNGR1–1, IFNGR1–2; (G) TGFβ; (E) ceruloplasmin. The expression data were normalized against those observed in medium treated cells, respectively for each gene. The results are mean ± S.E. RQ values for monocytes obtained from cultures established from individual fish (n = 5). Statistical analysis was performed using one-way ANOVA, and the results were deemed to be significant at p < 0.05. (*) denotes significantly different (p < 0.05) from the respective medium treated controls and (+) denotes significantly different (p < 0.05) from respective rgIFNγ-treated cells.
An increase in the expression of both IL-1β isoforms was observed after treatment with individual or combined rgIFNγrel and rgIFNγ (Fig. 2C). However, cells treated with rgIFNγrel alone or in combination with rgIFNγ had significantly higher IL-1β-1 mRNA levels than those treated with rgIFNγ alone (Fig. 2C).
Monocytes treated with rgIFNγrel and IFNγ had elevated TNFα1 and TNFα2 mRNA levels (Fig. 2D). Similar to the IL-1β expression, the mRNA levels of both TNF isoforms were substantially higher in cells treated with rgIFNγrel alone and in combination with rgIFNγ, compared with cells treated with rgIFNγ alone (Fig. 2D).
Although the treatment of monocytes with rgIFNγrel or rgIFNγ induced an up-regulation in the CXCL8 mRNA levels, the rgIFNγrel stimulation induced significantly higher increases in the expression of CXCL8 compared with that induced by rgIFNγ (Fig. 2E). Interestingly, the combined treatment of monocytes with both rgIFNγrel and rgIFNγ down-regulated the expression of CXCL8 compared with that induced by rgIFNγrel alone (Fig. 2E). In contrast, the expression of CCL1 in monocytes was not affected after treatment with either cytokine (Fig. 2E). Monocytes treated with either cytokine alone or in combination exhibited significantly elevated mRNA levels of IFNGR1–1 but not IFNGR1–2 (Fig. 2F).
When cells were treated with either rgIFNγrel or rgIFNγ, no significant changes were observed in the expression of the goldfish TGFβ gene (Fig. 2G). In contrast, monocytes treated with both rgIFNγrel and rgIFNγ significantly lower TGFβ mRNA levels compared with medium-treated cells (Fig. 2G).
In mammals, IFNγ stimulation of myeloid cells results in increased expression and production of the acute phase protein, ceruloplasmin. To address whether this up-regulation is also a feature of goldfish monocytes treated with rgIFNγrel and/or rgIFNγ, we cloned goldfish ceruloplasmin and design Q-PCR primers against it. To our surprise, treatment of monocytes with rgIFNγ alone or in combination with rgIFNγrel did not affect the expression of ceruloplasmin (Fig. 2F). However, the stimulation of monocytes with rgIFNγrel only, induced a significant up-regulation goldfish ceruloplasmin mRNA (Fig. 2F). The expression of select immune genes following exposure of mature macrophages to rgIFNγrel and rgIFNγ was similar to that observed for monocytes (data not shown).
Priming of Monocytes for ROI Production
We previously reported that goldfish monocytes derived from cultures of kidney leukocytes exhibited significant ROI production and phagocytosis after treatment with rgIFNγ (41). In contrast, activated mature macrophages have a robust nitric oxide response but drastically reduced ability to produce ROI (41).
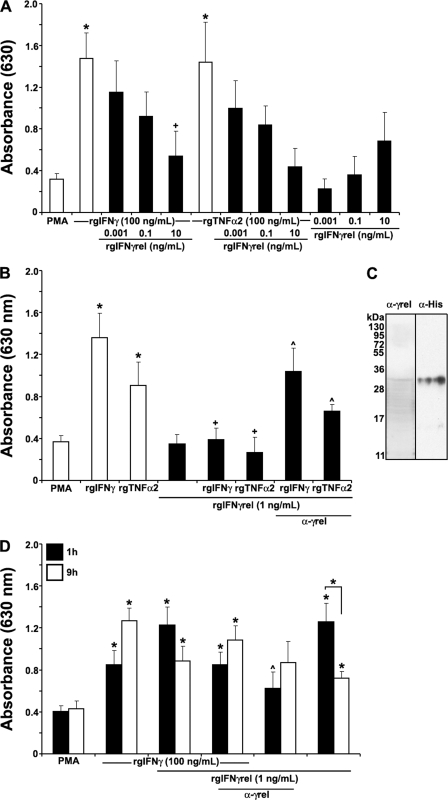
To determine whether rgIFNγrel primed goldfish monocytes for ROI production, we incubated cells with either medium alone, rgIFNγ, rgIFNγrel, rgTNFα2, a combination of rgIFNγrel+rgIFNγ, or rgIFNγrel+rgTNFα2. Surprisingly, when goldfish monocytes were treated with rgIFNγrel overnight (16 h) we did not observe significantly enhanced ROI production (Fig. 3A). Furthermore, when monocytes were primed overnight with either rgIFNγ or rgTNFα2 in conjunction with rgIFNγrel, the PMA-triggered ROI production by monocytes was substantially reduced compared with the ROI production of cells treated with either rgIFNγ or rgTNFα2 only (Fig. 3A). This down-regulation of the priming response and ultimately ROI production was evident when as little as 1 ng/ml of rgIFNγrel was added to the monocyte cultures (Fig. 3B). To ascertain whether rgIFNγrel was the cause of the decrease in rgIFNγ- or rgTNFα2-mediated ROI production, 5 μg/ml of α-rgIFNγrel affinity-purified rabbit IgG was added to monocyte cultures primed with rgIFNγ + rgIFNγrel or rgTNFα2 + rgIFNγrel (Fig. 3C). The addition of α-rgIFNγrel antibody partially restored the rgIFNγ or rgTNFα2-mediated ROI production (Fig. 3B). We did not observe further restoration of ROI production when higher concentrations of α-rgIFNγrel IgG were added to the cultures (data not shown).
FIGURE 3.
Recombinant goldfish IFNγrel temporally regulates the priming of the monocyte reactive oxygen production. A, rgIFNγrel reduces the ROI production mediated by rgIFNγ and rgTNFα2. Cells were treated with medium, rgTNFα2 (100 ng/ml), rgIFNγ (100 ng/ml), rgIFNγrel (0.001, 0.1, 10 ng/ml), or a combination of rgTNFα2 (100 ng/ml), or rgIFNγ (100 ng/ml), and 0.001, 0.1, or 10 ng/ml of rgIFNγrel. B, application of an anti-rgIFNγrel partially restored the reactive oxygen production down-regulated by rgIFNγrel. Cells were treated with medium, rgTNFα2 (100 ng/ml), rgIFNγ (100 ng/ml), rgIFNγrel (1 ng/ml) or a combination of rgTNFα2 (100 ng/ml) or rgIFNγ (100 ng/ml), and 1 ng/ml of rgIFNγrel, alone or in conjuction with 5 μg/ml of α-rgIFNγrel polyclonal IgG. C, Western blot detection of rgIFNγrel using α-rgIFNγrel IgG and α-His IgG. D, rgIFNγrel elicits a short-lived priming effect for monocyte ROI production. Cells were treated with medium, rgIFNγ (100 ng/ml), rgIFNγrel (1 ng/ml), or a combination of rgIFNγ (100 ng/ml), and 1 ng/ml of rgIFNγrel, alone or in conjuction with 5 μg/ml of α-rgIFNγrel IgG. All experiments were conducted as described above using monocytes from cultures established from individual fish (n = 5). Statistical analysis was performed using one-way ANOVA, and the results were deemed to be significant at p < 0.05. (*) denotes significantly different (p < 0.05) from the respective medium-treated controls and (+) denotes significantly different from respective treatments (rgIFNγ or rgTNFα2) without rgIFNγrel (p < 0.05). (^) denotes significantly different from respective treatments without antibody application.
To address whether rgIFNγrel had the capacity to down-regulated the priming for ROI production at shorter incubation times, we treated monocytes with rgIFNγrel alone or in combination with rgIFNγ for 1 or 9 h (Fig. 3D). To our surprise, monocytes treated with as little as 1 ng/ml of rgIFNγrel alone for 1 h, primed the cells for significant ROI production (Fig. 3D). The combined treatment of monocytes for 1 h with rgIFNγrel and rgIFNγ resulted in ROI production similar to that induced by treatment of cells with rgIFNγrel alone (Fig. 3D). As expected, the rgIFNγrel-mediated priming for ROI production was significantly reduced after addition of 5 μg/ml of α-rgIFNγrel IgG to the monocyte cultures (Fig. 3D). The addition of higher amounts of rgIFNγrel did not result in further increases in ROI production (data not shown).
When monocytes were treated longer (9 h) with rgIFNγrel, a significant decrease in the production of ROI was observed (Fig. 3D). In contrast, prolonged treatment of monocytes with rgIFNγ for 9 h resulted in a further increase of ROI production, compared with that by cells treated for 1 h (Fig. 3D). Interestingly, a 9-hour incubation of monocytes with a combination of rgIFNγ and rgIFNγrel caused a decreased ROI response compared with that induced by rgIFNγ alone (Fig. 3D).
Recombinant Goldfish IFNγrel Induces Higher Phagocytosis Than rgIFNγ
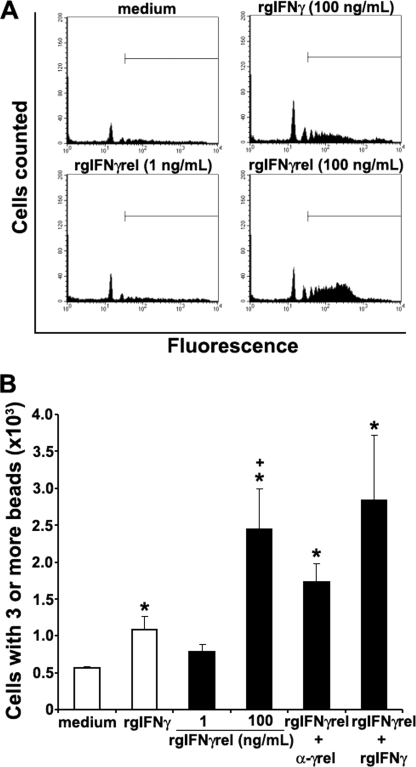
We previously reported that rgIFNγ enhanced the phagocytosis of fluorescent latex beads by monocytes (23). To determine whether rgIFNγrel-activated monocytes to ingest fluorescent latex beads, cells were treated with either medium alone, rgIFNγ (100 ng/ml), rgIFNγrel (1 or 100 ng/ml), or a combination of both cytokines. The capacity of monocytes to engulf fluorescent latex beads was determined using flow cytometry. As seen in FACS plots of monocyte cultures from a representative fish, treatment of cells with 100 ng/ml of rgIFNγ induced a modest increase in the uptake of latex beads (Fig. 4A). In contrast, monocytes obtained from the same fish and treated with 100 ng/ml of rgIFNγrel exhibited substantially higher phagocytic activity compared with those treated with rgIFNγ (Fig. 4A).
FIGURE 4.
Recombinant goldfish IFNγrel induces higher monocyte phagocytic responses compared with rgIFNγ. Goldfish monocyte cultures were treated with medium, rgIFNγ (100 ng/ml), or rgIFNγrel (1, 100 ng/ml) and phagocytosis assessed by FACS. A, representative phagocytosis histogram plots of cells from an individual fish treated with medium, rgIFNγ, or rgIFNγrel. B, mean + S.E. phagocytic response of monocytes obtained from cultures established from individual fish (n = 5) that have ingested 3 or more beads following treatment with medium, rgIFNγ (100 ng/ml), rgIFNγrel (1, 100 ng/ml), a combination of rgIFNγ (100 ng/ml) and rgIFNγrel (100 ng/ml), or rgIFNγrel (100 ng/ml) in conjuction with 5 μg/ml of α-rgIFNγrel IgG. Statistical analysis was done using one-way ANOVA. (*) denotes statistically different (p < 0.05) from medium control. (+) denotes statistically significant (p < 0.05) from rgIFNγ-induced phagocytosis values.
The flow cytometry-based phagocytosis assay allows for analysis of discrete populations of monocytes that have ingested 1, 2, 3, or more beads. We previously reported that enhanced phagocytic activity of activated monocytes was related to the uptake of 3 or more beads (23). Treatment of monocytes with 100 ng/ml rgIFNγ or rgIFNγrel resulted in a significant increase in phagocytosis of activated monocytes (Fig. 4B), where rgIFNγrel induced significantly higher phagocytosis compared with rgIFNγ (Fig. 4B). The addition of α-rgIFNγrel IgG to the monocyte cultures partially decreased the phagocytic activity of monocytes induced by rgIFNγrel (Fig. 4B).
Recombinant Goldfish IFNγrel Induces iNOS Gene Expression and Nitric Oxide Response of Goldfish Macrophages
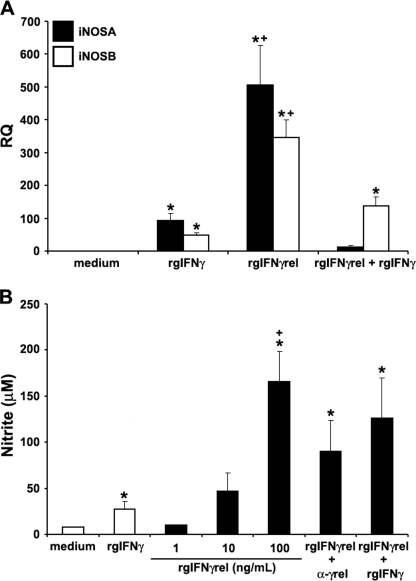
We previously reported that activated mature goldfish macrophages exhibit significant nitric oxide responses after treatment with pro-inflammatory cytokines (23, 41). To compare the ability of rgIFNγrel and rgIFNγ to induce a nitric oxide response, 8-day-old macrophage cultures were treated with these two cytokines either individually or in combination and the expression of iNOS isoforms A and B measured by Q-PCR (Fig. 5A). An increase in the expression of both iNOSA and iNOSB was observed when macrophages were treated with either rgIFNγ or rgIFNγrel; however, mRNA levels of iNOSA and iNOSB were significantly higher after rgIFNγrel stimulation (Fig. 5A). Interestingly, treatment of macrophages with rgIFNγ + rgIFNγrel caused a substantial down-regulation of the expression of both iNOS isoforms (Fig. 5A).
FIGURE 5.
Recombinant goldfish IFNγrel induces higher macrophage iNOS gene expression and nitric oxide production compared with rgIFNγ. A, Q-PCR analysis of gene expression of iNOS isoforms A and B in goldfish macrophages treated with medium, rgIFNγrel (100 ng/ml), rgIFNγ (100 ng/ml), or both recombinant cytokines (100 ng/ml of each protein). Gene expression was performed using the delta CT method against the endogenous control, elongation factor 1 α (EF-1α). The results are mean ± S.E. RQ values for macrophages obtained from cultures established from individual fish (n = 5) and normalized against the RQ values from medium-treated cells. B, nitrite production by cytokine stimulated goldfish macrophages. Macrophages were obtained from cultures established from individual fish (n = 5) and were treated with medium, rgIFNγ (100 ng/ml), rgIFNγrel (1, 10, 100 ng/ml) or a combination of rgIFNγ (100 ng/ml) and rgIFNγrel (100 ng/ml), or rgIFNγrel (100 ng/ml) in conjunction with 5 μg/ml of α-rgIFNγrel IgG. Nitric oxide production was determined using the Griess reaction and nitrite concentration was calculated using a nitrite standard curve. The results are mean ± S.E. μm nitrite. Statistical analysis was done using one-way ANOVA. (*) denotes statistically different (p < 0.05) from medium controls. (+) denotes significant difference (p < 0.05) from rgIFNγ-treated cells.
We then examined the ability of goldfish macrophages to produce nitrite after treatment with rgIFNγrel and/or rgIFNγ using Griess reaction assay. The treatment of macrophage cultures with rgIFNγ induced significantly elevated nitrite production, compared with medium-treated cells (Fig. 5B). The addition of rgIFNγrel to macrophage cultures resulted in significantly higher nitrite production compared with that induced by rgIFNγ (Fig. 5B). The addition of α-rgIFNγrel IgG partially decreased the nitrite production of macrophages (Fig. 5B).
Analysis of Cellular Association of rgIFNγrel and rgIFNγ
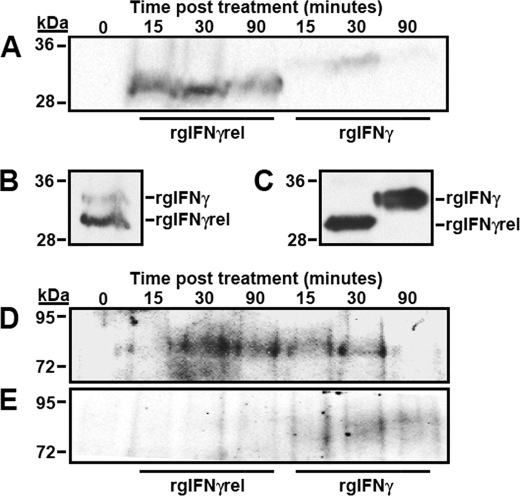
Monocytes were treated for 0, 15, 30, or 90 min with either rgIFNγrel or rgIFNγ and the cellular association of the two proteins determined using Western blot. Analysis of the whole cell lysates revealed that more of rgIFNγrel was associated with the cells compared with rgIFNγ (Fig. 6A). Increased association of rgIFNγrel was evident as early as 15 min and persisted during the observation period (90 min). (Fig. 6A). In contrast, most of rgIFNγ was present in whole cell lysates at 30 min (Fig. 6A). The incubation of monocytes with rgIFNγ + rgIFNγrel for 30 min did not alter the association of either cytokine with the cells (Fig. 6B). Because monocytes were incubated with equal amounts of either rgIFNγ or rgIFNγrel (Fig. 6C), it appears that more rgIFNγrel was associated with the cells (Fig. 6A).
FIGURE 6.
Analysis of rgIFNγrel and rgIFNγ cellular association, Stat1 tyrosine phosphorylation and phospho-(Y)-Stat1 nuclear accumulation in monocytes treated with rgIFNγrel or rgIFNγ. Five million monocytes were incubated with either medium alone, 5 μg of rgIFNγrel or 5 μg of rgIFNγ for 0, 15, 30, or 90 min. Whole cell lysates (A) were assayed by Western blot using α-polyHis antibody. Cells were also co-incubated with 5 μg of rgIFNγrel and 5 μg of rgIFNγ for a half-hour (B). The relative amounts of rgIFNγrel and rgIFNγ added to cells can be seen in C. Five million monocytes were incubated with either medium, 100 ng/ml of rgIFNγrel, or 100 ng/ml of rgIFNγ for 0, 15, 30, or 90 min. Whole cell lysates (D) or isolated nuclei (E) were assessed by Western blot with an α-phospho-(Tyr)-Stat1 antibody.
Analysis rgIFNγrel- and rgIFNγ-mediated Stat1-(Y) Phosphorylation and Nuclear Accumulation
Western blot analyses of rgIFNγrel- and rgIFNγ-treated monocytes using an α-phospho-(Y)-Stat1 antibody were performed (Fig. 6, D and F). When cells were treated with rgIFNγrel, Stat1 tyrosine phosphorylation was evident at 30 and 90 min after stimulation (Fig. 6D). In contrast, rgIFNγ treatment resulted in substantial Stat1-(Y) phosphorylation as early as 15 and 30 min after stimulation, which was then substantially reduced by 90 min (Fig. 6D).
To determine whether rgIFNγrel or rgIFNγ stimulation resulted in nuclear accumulation of phospho-(Y)-Stat1, nuclei were isolated from monocyte at 0, 15, 30, or 90 min after treatment with either rgIFNγrel or rgIFNγ (Fig. 6E). Nuclear accumulation of phospho-(Y)-Stat1 was evident 30 and 90 min after treatment with rgIFNγ. (Fig. 6E). In contrast, no phospho-(Y)-Stat1 was detected in the nuclei isolated from rgIFNγrel-treated cells (Fig. 6E).
Expression Analysis of Interferon Regulatory Factors in Monocytes Treated with rgIFNγrel and rgIFNγ
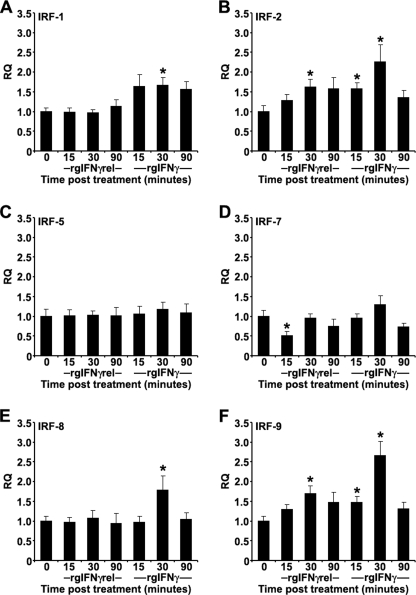
In mammals, the first wave of IFNγ-induced gene activation, including those that encode interferon regulatory factors (IRFs) occurs 15–30 min after treatment of cells with IFNγ (34). The IRFs then help to regulate the next wave of gene transcription in the IFNγ signaling cascade. To compare the signaling pathways of rgIFNγrel and rgIFNγ, we measured the expression of IRFs after stimulation with the two cytokines. In addition to goldfish IRF-1 and IRF-7 sequences, available in the NCBI data base, we cloned the goldfish IRF-2, IRF-5, IRF-8, and IRF-9 and designed specific Q-PCR primers (supplemental Table S1). Monocytes were treated with 100 ng/ml of rgIFNγrel or rgIFNγ for 0, 15, 30, and 90 min, and the expression levels of different IRFs determined (Fig. 7, A–F).
FIGURE 7.
Quantitative expression analysis of goldfish IRFs in monocytes treated with medium, 100 ng/ml of rgIFNγrel or 100 ng/ml of rgIFNγ for 0, 15, 30, or 90 min. The reported expression was relative to EF-1α. The genes examined included: (A) IRF-1; (B) IRF-2; (C) IRF-5; (D) IRF-7; (E) IRF-8; (F) IRF-9. The expression data were normalized against expression of respective IRFs at the 0 min time point. The results are mean ± S.E. RQ values for monocytes obtained from cultures established from individual fish (n = 5). Statistical analysis was performed using one-way ANOVA, and the results were deemed to be significant at p < 0.05. (*) denotes significantly different (p < 0.05) from the respective 0 time point control.
Treatment of monocytes with rgIFNγ caused increased expression of IRF-1, IRF-2, IRF-8, and IRF-9, while treatment with rgIFNγrel caused increased expression of IRF-2 and IRF-9 and decreased expression (at 15 min) of IRF-7 (Fig. 7, A–F). No changes in expression of IRF-5 were observed after treatment of monocytes with either cytokine (Fig. 7C).
DISCUSSION
Unlike mammals, bony fish have two Type II interferons, IFNγ and IFNγrel, whose pro-inflammatory functions have not been fully characterized. We previously reported that rgIFNγ primed monocytes for ROI production, and induced increased phagocytosis and nitrite production of mature macrophages (23). In this study, we report that rgIFNγrel elicited a robust and relatively short-lived priming of monocytes for ROI production, and that it down-regulated the priming potential of other pro-inflammatory cytokines (rgIFNγ and rgTNFα2). To our knowledge this is the first report that a type II interferon can down-regulate antimicrobial response of macrophages.
Our results indicate differences in the signaling pathways of bony fish rgIFNγ and rgIFNγrel. In mammals it has been documented that Stat1 activation and concomitant IRF-1 production after IFNγ stimulation, determine the differentiation and fate of the activated cells (43, 44). Our findings indicate that while rgIFNγ induced both Stat1 nuclear translocation and increased IRF-1 expression, rgIFNγrel did not mediate Stat1 nuclear translocation and did not affect IRF-1 expression. Indeed the two cytokines induced very unique profiles of functional responses in the goldfish monocytes and macrophages. For example, rgIFNγ exhibited long-lasting priming effects for induction of ROI production of monocytes, compared with rgIFNγrel whose priming effect for ROI production was short-lived, and was followed by a down-regulation of the priming for the monocyte ROI production. When compared with rgIFNγ, rgIFNγrel induced significantly higher phagocytosis, increased iNOS gene expression and nitrite production in monocytes and macrophages, respectively. Schroder et al. (45) reported that in general ROI responses are better suited to deal with phagocytosed extracellular pathogens and that nitric oxide responses evolved for more efficient destruction of obligate intracellular pathogens. We previously reported that fish macrophages mounted sequential antimicrobial responses following stimulation with macrophage activating factors (MAF) contained in mitogen-induced cell supernatants (46). The ROI response was selectively deprogrammed once maximal induction has occurred without affecting the nitric oxide response of activated macrophages. The ability of the host to selectively deactivate ROI production may play an important role in host defense, because the regulation of the duration and intensity of the ROI response would minimize tissue damage at an inflammatory site, in an otherwise futile attempt to eliminate ROI resistant pathogens. It is likely that MAF contain both IFNγrel and IFNγ. Consequently, the differences in the induction of antimicrobial responses of monocytes/macrophages by rgIFNγrel and rgIFNγ may have evolved to regulate the intensity and the duration of specific antimicrobial functions during an inflammatory response.
Treatment of monocytes with rgIFNγrel caused a significant decrease in the p67phox mRNA while the addition of rgIFNγrel + rgIFNγ induced significant decreases of both p67phox and p40 phox mRNA. The p67 phox domain of NADPH oxidase is essential for electron transfer through flavocytochrome b centers (47, 48) and p40phox participates in the activation of NADPH oxidase (49–51). Humans suffering from chronic granulomotous disease have dysfunctional p67phox and exhibit a concomitant decrease in p40 phox expression (52, 53). It is possible that the observed down-regulation of monocyte ROI production by rgIFNγrel may be at least partially due to the transcriptional decreases of p67phox and p40phox.
Monocytes and macrophages treated with rgIFNγrel and/or rgIFNγ exhibited distinct expression of select immune genes. The mammalian IFNγ has been documented to up-regulate the gene expression of NADPH oxidase components p67 phox (14, 54) and gp91 phox (55). In contrast, goldfish rgIFNγ and/or rgIFNγrel were found to up-regulate only gp91phox and p47phox. It is possible that the mechanisms that regulate NADPH oxidase activation may be different between mammals and fish.
We previously reported that goldfish monocytes derived from cultures of kidney leukocytes exhibited significant ROI production and phagocytosis after treatment with rgIFNγ (41). In contrast, activated mature macrophages have a robust nitric oxide response but drastically reduced ability to produce ROI (23, 41). Our results indicate that iNOS A and B gene expression was significantly reduced after stimulation of goldfish macrophages with rgIFNγ and rgIFNγrel, when compared with rgIFNγrel alone, supporting distinct biological roles for the two cytokines in activation of antimicrobial functions of macrophages.
Human IFNγ has been shown to increase the expression of the gene encoding the acute phase protein, ceruloplasmin, and also differentially affect the translation of this protein (56–58). This enhanced mRNA levels of ceruloplasmin correlated with increase in protein level shortly following activation (56), however, significant inhibition of translation of ceruloplasmin was reported at later times after treatment (57, 58). Interestingly, rgIFNγrel, which is structurally less related to the mammalian IFNγ, significantly increased the expression of goldfish ceruloplasmin, while the more related rgIFNγ did not. Unfortunately, goldfish recombinant ceruloplasmin and α-goldfish ceruloplasmin antibodies are currently not available, preventing us from examining the relationship between fish type II IFNs and ceruloplasmin at the protein level.
Within the first 30 min of treatment of mammalian cells with IFNγ, the expression of specific IRF transcription factors is up-regulated and these IRFs participate in further signaling events (34). In this study, we examined the expression of goldfish IRFs after treatment of monocytes with IFNγ or IFNγrel. The expression of IRF-1 is dependent on Stat1 activation (59, 60) while the expression of IRF-8 is strictly induced by IFNγ but not by Type I IFNs (61, 62). Our results indicate that like the mammalian IFNγ, the goldfish rgIFNγ also induced phospho-Stat1 nuclear translocation and up-regulation in IRF-1 and IRF-8 expression. In contrast, IFNγrel did not induce phospho-Stat1 nuclear translocation or IRF-1 and IRF-8 gene up-regulation, suggesting that it this cytokine signals through a different signaling pathway. Interestingly IRF-2, which primarily serves as a transcriptional repressor of IRF-1 and ISGF3 (63), was up-regulated in fish monocytes treated with either rgIFNγrel or rgIFNγ. It is possible that consistent down-regulation of goldfish IRFs at 90 min post-stimulation may be due to increased IRF-2 protein levels.
The changes in gene expression of both IRF-5 (64) and IRF-7 (65) are believed to be controlled by Type I but not Type II IFNs. This is consistent with our observations that the expression of the goldfish IRF-5 and IRF-7 is not up-regulated in monocyte stimulated with either rgIFNγ or rgIFNγrel.
In addition to transcriptional regulation as a homodimer, IFNγ-activated Stat1also regulates gene expression by forming the interferon signaling gene factor (ISGF3) complex composed of Stat1, Stat2 and IRF-9 (30, 31) as well as a different complex composed of a Stat1 homodimer and IRF-9 (32). Our results indicate that both rgIFNγ and rgIFNγrel induced an up-regulation in the expression of goldfish IRF-9.
We recently showed that zebrafish and goldfish have two distinct IFNGR1 genes (40). Most vertebrate species have a single IFNGR1 gene, and it is reasonable to speculate that these distinct fish genes arose from a gene duplication of a single ancestral gene. As such it is important to emphasize that the two zebrafish IFNGR1 isoforms have not been evolutionarily retained on a single chromosome, but instead reside on distinct chromosomes, each with some but not all homologs of genes that are syntenic to the single mammalian IFNGR1 gene (40). Because gene synteny is suggestive of biological relationships between respective genes, the lack of synteny between the zebrafish IFNGR1 genes, support the hypothesis that the genes encoding these receptors have evolved to mediate distinct biological functions.
A recent report using morpholino knockdowns in zebrafish embryos showed that zebrafish IFNγrel appears to be essential for clearance of Escherichia coli (27). The knockdown of both IFNγrel and IFNγ had a more drastic effect on embryo mortality during the course of the infection compared with that caused by the knockdown of either cytokine alone (27). It should be noted that the injection of zebrafish with recombinant IFNγ failed to protect fish against viral and bacterial infections possibly due to high rate of clearance of the recombinant protein by injected animals (26).
We previously reported that rgIFNγrel (∼rgIFNγ1) and rgIFNγ (∼rgIFNγ2) each bound to one but not the other IFNGR1 isoform (40). In silico analyses revealed that the two zebrafish and the two goldfish IFNGR1 isoforms had putative and evolutionarily conserved docking sites for both Jak1 and Stat1 (40). In the present study, both rgIFNγrel and rgIFNγ induced Stat1 tyrosine phosphorylation, suggesting a role for Stat1 in their signaling pathways. It should be noted that nuclear translocation of phospho-Stat1 was observed only after monocyte stimulation with rgIFNγ but not with rgIFNγrel and that only goldfish IFNγ has the nuclear localization signal sequence (NLS).
The leading model for mammalian IFNγ signaling, as proposed by Subramaniam et al. (66), suggests that following ligation of IFNγ to its receptor complex, Stat1 is delivered into the nucleus via the IFNγ NLS in a complex consisting of Stat1:IFNGR1:IFNγ. This NLS is made up of a positively charged stretch of residues at the C-terminal end of the protein (supplemental Fig. S1). While treatment of monocytes with either rgIFNγ or rgIFNγrel resulted in the presence of tyrosine-phosphorylated Stat1 in the whole cell lysates, phospho-(Y)-Stat1 was observed in the nuclei of IFNγ but not IFNγrel-treated cells. We used the α-phospho-(Y)-Stat1 antibody because, as ascertained by protein alignments, the epitope recognized by this antibody has been evolutionarily conserved. This is not the case with other fish Stat proteins, since they do not have a high sequence homology with the mammalian Stat proteins. Our attempts to immunodetect goldfish Stat2 and Stat3 using antibodies raised against mammalian proteins were not successful. The findings of this study and our previous work (23) indicate that goldfish and mammalian IFNγ are structurally more related and may signal through similar pathways, In contrast, IFNγrel which induces a plethora of significant biological effects in goldfish monocytes and macrophages, does not appear to signal through Stat1, because phospho-(Y)-Stat1 was not detected in nuclei of monocytes. A more thorough investigation of signaling mechanisms used by the fish Type II IFNs and in particular IFNγrel must await the generation of fish-specific reagents.
Recently, Type I IFNs of teleosts have been identified and grouped into two groups based on structural similarities, with one group found in all teleost and the other present only in relatively primitive fish species (67). As with the fish Type II IFNs, these also appear to possess functionally distinct properties (26, 67). Using morpholino knockdowns in the context of embryo reactivity to a zebrafish type I IFN, Levrad et al. (68) identified potential candidates for IFNAR1 and IFNAR2. However similar studies have not been performed with other recently identified Type I IFNs. It is possible that like Type II fish interferons, teleost Type I IFNs may mediate biological events through distinct receptor/ligand complexes.
Our findings suggest the presence of a functional segregation in the induction of monocyte and macrophage antimicrobial functions by type II interferons of bony fish. This is different from the single Type II IFN systems present in all other vertebrates examined thus far. Given the importance of innate immunity in host defense of bony fish, it is perhaps not surprising that they have evolved a more elaborate cytokine-regulated induction of macrophage antimicrobial responses. However, the precise evolutionary as well as practical advantage for a more elaborate Type II interferon system in bony fish remains to be fully elucidated.
Supplementary Material
This work was supported by the Natural Sciences and Engineering Council of Canada (NSERC) (to M. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and Table S1.
- IFNγ
- interferon γ
- FACS
- fluorescence-activated cell sorter
- GAS
- γ-IFN-activated sequences
- IFNAR
- interferon α receptor
- IFNγrel
- interferon γ-related
- IFNGR1
- interferon γ receptor 1
- iNOS
- inducible nitric-oxide synthase
- IL-1β
- interleukin-1 β
- IRF
- interferon regulatory factor
- ISGF3
- interferon-stimulated gene factor 3
- MAF
- macrophage-activating factor
- NLS
- nuclear localization signal
- Jak
- janus kinase
- NO
- nitric oxide
- PBLs
- peripheral blood leukocytes
- PMA
- phorbol myristate acetate
- Q-PCR
- quantitative-polymerase chain reaction
- rg
- recombinant goldfish
- RQ
- relative quantification
- ROI
- reactive oxygen intermediates
- Stat
- signal transducer and activator of transcription
- TGFβ
- transforming growth factor β
- TNFα
- tumor necrosis factor α
- ANOVA
- analysis of variance.
REFERENCES
- 1.Mosmann T. R., Coffman R. L. (1989) Annu. Rev. Immunol. 7, 145–173 [DOI] [PubMed] [Google Scholar]
- 2.Sad S., Marcotte R., Mosmann T. R. (1995) Immunity 2, 271–279 [DOI] [PubMed] [Google Scholar]
- 3.Perussia B. (1991) Curr. Opin. Immunol. 3, 49–55 [DOI] [PubMed] [Google Scholar]
- 4.Wheelock E. F. (1965) Science 149, 310–311 [PubMed] [Google Scholar]
- 5.Staeheli P. (1990) Adv. Virus. Res. 38, 147–200 [DOI] [PubMed] [Google Scholar]
- 6.Kerr I. M., Stark G. R. (1992) J. Interferon Res. 12, 237–240 [DOI] [PubMed] [Google Scholar]
- 7.Stevenson M. M., Tam M. F., Belosevic M., van der Meide P. H., Podoba J. E. (1990) Infect. Immun. 58, 3225–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. (1996) J. Immunol. 143, 266–274 [PubMed] [Google Scholar]
- 9.Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. (1988) J. Immunol. 141, 890–896 [PubMed] [Google Scholar]
- 10.Wang Z. E., Reiner S. L., Zheng S., Dalton D. K., Locksley R. M. (1994) J. Exp. Med. 179, 1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. (1993) Science 259, 1742–1745 [DOI] [PubMed] [Google Scholar]
- 12.Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. (1993) J. Exp. Med. 178, 2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berton G., Zeni L., Cassatella M. A., Rossi F. (1986) Biochem. Biophys. Res. Commun. 138, 1276–1282 [DOI] [PubMed] [Google Scholar]
- 14.Cassatella M. A., Bazzoni F., Flynn R. M., Dusi S., Trinchieri G., Rossi F. (1990) J. Biol. Chem. 265, 20241–20246 [PubMed] [Google Scholar]
- 15.Fertsch D., Vogel S. N. (1984) J. Immunol. 132, 2436–2439 [PubMed] [Google Scholar]
- 16.Martin E., Nathan C., Xie Q. W. (1994) J. Exp. Med. 180, 977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igawa D., Sakai M., Savan R. (2006) Mol. Immunol. 43, 999–1009 [DOI] [PubMed] [Google Scholar]
- 18.Zou J., Yoshiura Y., Dijkstra J. M., Sakai M., Ototake M., Secombes C. (2004) Fish Shellfish Immunol. 17, 403–409 [DOI] [PubMed] [Google Scholar]
- 19.Zou J., Carrington A., Collet B., Dijkstra J. M., Yoshiura Y., Bols N., Secombes C. (2005) J. Immunol. 175, 2484–2494 [DOI] [PubMed] [Google Scholar]
- 20.Robertsen B. (2006) Fish. Shellfish Immunol. 20, 172–191 [DOI] [PubMed] [Google Scholar]
- 21.Milev-Milovanovic I., Long S., Wilson M., Bengten E., Miller N. W., Chinchar V. G. (2006) Immunogenetics 58, 70–80 [DOI] [PubMed] [Google Scholar]
- 22.Stolte E. H., Savelkoul H. F., Wiegertjes G., Flik G., Lidy Verburg-van Kemenade B. M. (2008) Dev. Comp. Immunol. 32, 1467–1481 [DOI] [PubMed] [Google Scholar]
- 23.Grayfer L., Belosevic M. (2009) Dev. Comp. Immunol. 33, 235–246 [DOI] [PubMed] [Google Scholar]
- 24.Bader T., Weitzerbin J. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 11831–11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W. Q., Xu Q. Q., Chang M. X., Zou J., Secombes C. J., Peng K. M., Nie P. (2009) Vet. Immunol. Immunopathol. 134, 199–207 [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Munoz A., Roca F. J., Meseguer J., Mulero V. (2009) J. Immunol. 182, 3440–3449 [DOI] [PubMed] [Google Scholar]
- 27.Sieger D., Stein C., Neifer D., van der Sar A. M., Leptin M. (2009) Dis. Model. Mech. 2, 571–581 [DOI] [PubMed] [Google Scholar]
- 28.Ihle J. N., Kerr I. M. (1995) Trends Genet. 11, 69–74 [DOI] [PubMed] [Google Scholar]
- 29.Darnell J. E., Jr., Kerr I. M., Stark G. R. (1994) Science 264, 1415–1421 [DOI] [PubMed] [Google Scholar]
- 30.Takaoka A., Mitani Y., Suemori H., Sato M., Yokochi T., Noguchi S., Tanaka N., Taniguchi T. (2000) Science 288, 2357–2360 [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M., Tanaka N., Harada H., Kimura T., Yokochi T., Kitagawa M., Schindler C., Taniguchi T. (1999) Biol. Chem. 380, 699–703 [DOI] [PubMed] [Google Scholar]
- 32.Bluyssen H. A., Muzaffar R., Vlieststra R. J., van der Made A. C., Leung S., Stark G. R., Kerr I. M., Trapman J., Levy D. E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 5645–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaoka A., Yanai H. (2006) Cell Microb. 8, 907–922 [DOI] [PubMed] [Google Scholar]
- 34.Young H. A., Hardy K. J. (1995) J. Leuk. Biol. 58, 373–381 [PubMed] [Google Scholar]
- 35.Collet B., Ganne G., Bird S., Collins C. M. (2009) Dev. Comp. Immunol. 33, 821–829 [DOI] [PubMed] [Google Scholar]
- 36.Holland J. W., Bird S., Williamson B., Woudstra C., Mustafa A., Wang T., Zou J., Blaney S. C., Collet B., Secombes C. J. (2008) Mol. Immunol. 46, 269–285 [DOI] [PubMed] [Google Scholar]
- 37.Castro R., Martin S. A., Zou J., Secombes C. J. (2010) Fish Shellfish Immunol. 28, 312–319 [DOI] [PubMed] [Google Scholar]
- 38.Castro R., Martin S. A., Bird S., Lamas J., Secombes C. J. (2008) Mol. Immunol. 45, 3454–3462 [DOI] [PubMed] [Google Scholar]
- 39.Neumann N. F., Barreda D. R., Belosevic M. (2000) Fish Shellfish Immunol. 10, 1–20 [DOI] [PubMed] [Google Scholar]
- 40.Grayfer L., Belosevic M. (2009) Mol. Immunol. 46, 3050–3059 [DOI] [PubMed] [Google Scholar]
- 41.Grayfer L., Walsh J. G., Belosevic M. (2008) Dev. Comp. Immunol. 32, 532–543 [DOI] [PubMed] [Google Scholar]
- 42.García-García E., Rosales C. (2007) J. Immunol. Methods 320, 104–118 [DOI] [PubMed] [Google Scholar]
- 43.Bernabei P., Allione A., Rigamonti L., Bosticardo M., Losana G., Borghi I., Forni G., Novelli F. (2001) Europ. Cytokine Net. 12, 6–14 [PubMed] [Google Scholar]
- 44.Bernabei P., Coccia E. M., Rigamonti L., Bosticardo M., Forni G., Pestka S., Krause C. D., Battistini A., Novelli F. (2001) J. Leuk. Biol 70, 950–960 [PubMed] [Google Scholar]
- 45.Schroder K., Hertzog P. J., Ravasi T., Hume D. A. (2004) J. Leuk. Biol. 75, 163–189 [DOI] [PubMed] [Google Scholar]
- 46.Neumann N. F., Belosevic M. (1996) Dev. Comp. Immunol. 20, 427–439 [DOI] [PubMed] [Google Scholar]
- 47.Han C. H., Freeman J. L., Lee T., Motalebi S. A., Lambeth J. D. (1998) J. Biol. Chem. 273, 16663–16668 [DOI] [PubMed] [Google Scholar]
- 48.Nisimoto Y., Motalebi S., Han C. H., Lambeth J. D. (1999) J. Biol. Chem. 274, 22999–23005 [DOI] [PubMed] [Google Scholar]
- 49.He R., Nanamori M., Sang H., Yin H., Dinauer M. C., Ye R. D. (2004) J. Immunol. 173, 7462–7470 [DOI] [PubMed] [Google Scholar]
- 50.Suh C. I., Stull N. D., Li X. J., Tian W., Price M. O., Grinstein S., Yaffe M. B., Atkinson S., Dinauer M. C. (2006) J. Exp. Med. 203, 1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian W., Li X. J., Stull N. D., Ming W., Suh C. I., Bissonnette S. A., Yaffe M. B., Grinstein S., Atkinson S. J., Dinauer M. C. (2008) Blood 112, 3867–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsunawaki S., Mizunari H., Nagata M., Tatsuzawa O., Kuratsuji T. (1994) Biochem. Biophys. Res. Commun. 199, 1378–1387 [DOI] [PubMed] [Google Scholar]
- 53.Wientjes F. B., Hsuan J. J., Totty N. F., Segal A. W. (1993) Biochem. J. 296, 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newburger P. E., Ezekowitz R. A., Whitney C., Wright J., Orkin S. H. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 5215–5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta J. W., Kubin M., Hartman L., Cassatella M., Trinchieri G. (1992) Cancer Res. 52, 2530–2537 [PubMed] [Google Scholar]
- 56.Mazumder B., Mukhopadhyay C. K., Prok A., Cathcart M. K., Fox P. L. (1997) J. Immunol. 159, 1938–1944 [PubMed] [Google Scholar]
- 57.Mazumder B., Fox P. L. (1999) Mol. Cell. Biol. 19, 6898–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampath P., Mazumder B., Seshadri V., Fox P. L. (2003) Mol. Cell. Biol. 23, 1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durbin J. E., Hackenmiller R., Simon M. C., Levy D. E. (1996) Cell 84, 443–450 [DOI] [PubMed] [Google Scholar]
- 60.Meraz M. A., White J. M., Sheehan K. C., Bach E. A., Rodig S. J., Dighe A. S., Kaplan D. H., Riley J. K., Greenlund A. C., Campbell D., Carver-Moore K., DuBois R. N., Clark R., Aguet M., Schreiber R. D. (1996) Cell 84, 431–442 [DOI] [PubMed] [Google Scholar]
- 61.Driggers P. H., Ennist D. L., Gleason S. L., Mak W. H., Marks M. S., Levi B. Z., Flanagan J. R., Appella E., Ozato K. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3743–3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson N., Kanno Y., Hong C., Contursi C., Fujita T., Fowlkes B. J., O'Connell E., Hu-Li J., Paul W. E., Jankovic D., Sher A. F., Coligan J. E., Thornton A., Appella E., Yang Y., Ozato K. (1996) J. Immunol. 156, 3711–3720 [PubMed] [Google Scholar]
- 63.Harada H., Takahashi E., Itoh S., Harada K., Hori T. A., Taniguchi T. (1994) Mol. Cell. Biol. 14, 1500–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taniguchi T., Ogasawara K., Takaoka A., Tanaka N. (2001) Annu. Rev. Immunol. 19, 623–655 [DOI] [PubMed] [Google Scholar]
- 65.Zhang L., Pagano J. S. (1997) Mol. Cell. Biol. 17, 5748–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subramaniam P. S., Torres B. A., Johnson H. M. (2001) Cytokine 15, 175–187 [DOI] [PubMed] [Google Scholar]
- 67.Zou J., Tafalla C., Truckle J., Secombes C. J. (2007) J. Immunol. 179, 3859–3871 [DOI] [PubMed] [Google Scholar]
- 68.Levraud J. P., Boudinot P., Colin I., Benmansour A., Peyrieras N., Herbomel P., Lutfalla G. (2007) J. Immunol. 178, 4385–4394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.