Abstract
Chromatophore organs are complex and unique structures responsible for the variety of body coloration patterns used by cephalopods to communicate and camouflage. They are formed by a pigment-containing cytoelastic sacculus, surrounded by muscle fibers directly innervated from the brain. Muscle contraction and relaxation are responsible for expansion and retraction of the pigment-containing cell. Their functioning depends on glutamate and Phe-Met-Arg-Phe-NH2-related peptides, which induce fast and slow cell expansion, respectively, and 5-hydroxytryptamine, which induces retraction. Apart from these three substances and acetylcholine, which acts presynaptically, no other neuroactive compounds have so far been found to be involved in the neuroregulation of chromatophore physiology, and the detailed signaling mechanisms are still little understood. Herein, we disclose the role of nitric oxide (NO) as mediator in one of the signaling pathways by which glutamate activates body patterning. NO and nitric-oxide synthase have been detected in pigment and muscle fibers of embryo, juvenile, and adult chromatophore organs from Sepia officinalis. NO-mediated Sepia chromatophore expansion operates at slower rate than glutamate and involves cGMP, cyclic ADP-ribose, and ryanodine receptor activation. These results demonstrate for the first time that NO is an important messenger in the long term maintenance of the body coloration patterns in Sepia.
Keywords: Cyclic GMP (cGMP); Glutamate Receptors Ionotropic (AMPA, NMDA); Immunochemistry; Nitric Oxide; Nitric-oxide Synthase; Chromatophore; Glutamate; Mollusk; Neuroregulation; Sepia
Introduction
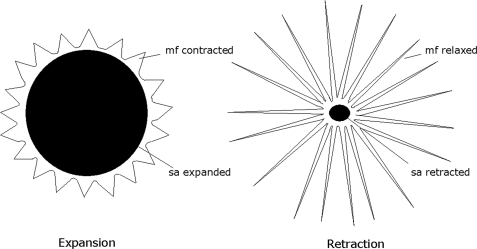
Chromatophore organ expansion is one of the many mechanisms underlying the multifaceted body patterning that Sepia and other cephalopods display for camouflage, in response to threatening situations, or for courtship (1–3). This mechanism is the result of the coordinated contraction of the radial striated muscle fibers attached to the margins of the chromatophore organs. With expansion, the pigment-containing cytoelastic sacculus is stretched, thus causing the visible color changes that characterize body patterns (4). After the original stimulus has ceased, relaxation of chromatophore muscles results in retraction of sacculus whereby the basal (resting) color pattern is restored (Fig. 1). Apart from extreme contraction/expansion situations, a range of intermediate states is possible as well as rapid mini-contraction/relaxation cycles, like those responsible for the “flickering” behavior, which stand witness to the unique dynamic features of the chromatophore system, which is under direct neural control.
FIGURE 1.
Schematic illustration of a chromatophore organ highlighting the pigment-containing sacculus and the radial muscle fibers. When muscle fibers contract, the cytoplasm with the pigment is forced into a thin layer, resulting in chromatophore expansion. When muscle fibers are relaxed, the pigment sacculus is lenticular in shape resulting in chromatophore retraction. mf, muscle fiber; sa, sacculus.
The pigment imparts color to the chromatophore organ: yellow, orange, red, brown, or black. The chromatophores of different species differ in color. Thus, for example, Loligo opalescens and Sepia officinalis have only yellow, red, and brown chromatophores, whereas Octopus vulgaris has yellow, orange, red, brown, and black chromatophores (1, 5). It is generally believed that chromatophore pigments are ommochromes, deriving from the oxidation of tryptophan along the kynurenine pathway in which the first and rate-limiting step is the conversion of tryptophan to N-formylkynurenine, catalyzed by the enzyme tryptophan-2,3-dioxygenase (6, 7). However, the presence of melanin has also been hypothesized in the chromatophore pigments of some cephalopods (2).
The variation of body coloration patterns is generated by the central nervous system and in particular by the posterior chromatophore lobe, which directly innervates the chromatophore organs in the skin of the mantle (8). Thus far, three neuroactive substances have been demonstrated in chromatophore neuroregulation: l-glutamate, Phe-Met-Arg-Phe-NH2 (FMRFamide)3-related peptides and 5-hydroxytryptamine (5-HT). l-Glutamate is the excitatory transmitter of the chromatophore and acts directly on the postsynaptic membrane, inducing a depolarization, an increase in cytoplasmic calcium levels and contraction of the radial muscle fibers resulting in chromatophore expansion. In Sepia, FMRFamide-related peptides act like glutamate by inducing chromatophore expansion but leading to a slower and more sustained response. Thus, it appears that the glutamatergic pathway is the primary mediator activated during fast production of transient body patterns, whereas the slow pathway is involved in the long term maintenance of the pattern (9–13). In the absence of excitatory motoneuron activity, 5-HT facilitates chromatophore retraction (4, 12) by suppressing calcium release from ryanodine-sensitive stores (14, 15). Apart from these three substances and acetylcholine, which acts presynaptically, no other neuroactive compounds have so far been found to be involved in the neuroregulation of chromatophore physiology, and the detailed signaling mechanisms are still little understood.
In the frame of our continuing program on the physiologic roles of the nitric oxide (NO) signaling pathway in S. officinalis (16) we recently focused our attention on the occurrence of this pluripotent messenger in the chromatophore organ of this cephalopod. This study was motivated by considerations of the peculiar properties of this highly diffusible messenger molecule, which is known to elicit fast and transient responses in relation to glutamate receptor stimulation, and the recent localization of nitric oxide synthase (NOS) in several areas of Sepia central nervous system deputed to control different behavioral responses, such as defense, motor control, equilibrium system, vision, feeding, learning, olfaction, and taste (17, 18). Herein, we report that NO is generated in chromatophore organs and acts on the muscle fibers as a result of glutamate signaling, functioning as an important messenger in the long term maintenance of the body coloration patterns necessary for camouflage or other adaptive purposes.
EXPERIMENTAL PROCEDURES
Animals
Adult specimens of S. officinalis and Loligo vulgaris were collected in the Bay of Naples and kept in tanks with circulating seawater (SW) at 20 °C until use. Fertilized eggs, obtained directly from the sea or in tanks from adult specimens, were kept in tanks, and their development was followed. All procedures were performed after anesthesia with 0.1% ethanol in SW.
Chemicals
l-Glutamic acid, diethylamine (DEA), ruthenium red, cADP ribose, 8-bromo-cyclic ADP-ribose (8-Br-cADP ribose), 5-HT, 1,3,7-trimethylxanthine (caffeine), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), N-methyl-d-aspartic acid (NMDA), glycine, β-NADPH, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were purchased from Sigma. [2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3oxide] (c-PTIO), 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA/NO), 1-(2-trifluormethylphenyl)imidazole (TRIM) were from Alexis. Nitro blue tetrazolium was obtained from Roche. 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM-DA) was from Molecular Probes. 8-Bromo-cyclic GMP (8-Br-cGMP) was from Calbiochem. d-(−)-2-Amino-5-phosphonopentanoic acid (d-AP5) was from Tocris.
Isolation of Chromatophore Organs
The dorsal mantle of decapitated juveniles (1 week old, 10-mm length) or a 1-cm2 piece of medial dorsal skin from adult was removed, and chromatophore organs were isolated following the protocol previously reported, with some modifications (13). The treatments with trypsin and papain/collagenase-P were carried out for 5 and 15 min, respectively. The isolated chromatophore organs were used for NADPH diaphorase staining, immunocytochemistry, NO detection, and pharmacological bioassays.
NADPH Diaphorase Histochemistry
Embryos, peeled of egg envelopes and chorion, were fixed overnight at 4 °C in 4% paraformaldehyde, 0.1 m MOPS, pH 7.5, 0.5 m NaCl. Isolated chromatophore organs from juvenile and adult were fixed in the same solution for 1 h at room temperature, in poly-l-lysine-coated Petri dishes. After washing with 0.1 m Tris-HCl buffer, pH 8, permeabilization was performed by incubation in 0.1 m Tris-HCl, pH 8, 0.3% Triton X-100 overnight at 4 °C for embryos and for 30 min for isolated juvenile and adult chromatophores. Samples were incubated for up to 1 h at 37 °C, in the dark in 2 mm β-NADPH, 0.2% nitro blue tetrazolium in 0.1 m Tris-HCl, pH 8, 0.3% Triton X-100. After washings, the staining was examined by using a Zeiss AxioImager M1 microscope.
Immunohistochemistry/Cytochemistry
Embryos were fixed overnight at 4 °C in 4% paraformaldehyde, 0.1 m MOPS, pH 7.5, 0.5 m NaCl. Isolated juvenile and adult chromatophore organs were fixed in the same solution for 45 min at room temperature. After washing in PBS, permeabilization was performed on embryos in 0.5% Triton X-100 in PBS for 48 h at 4 °C, and on isolated chromatophore organs in 0.3% Triton X-100 in PBS for 40 min at room temperature. Nonspecific binding was blocked by incubating the embryos in blocking buffer I (PBS, 0.5% Triton X-100, 5% normal goat serum) for 2 h at room temperature and then in blocking buffer II (PBS, 0.5% Triton X-100, 1% bovine serum albumin) overnight at 4 °C. For isolated chromatophore organs, blocking buffer III (PBS, 0.1% Triton X-100, 1% bovine serum albumin) was used for 1 h. Embryos and isolated chromatophore organs were then incubated with anti-uNOS (Affinity Bioreagents, Inc.) at a dilution of 1:1,000 at 4 °C for 48 h in blocking buffer II and overnight in blocking buffer III, respectively. Staining was performed using the Vectastain alkaline phosphatase ABC-AP kit and visualized by a Zeiss AxioImager M1 microscope.
NO Detection
NO detection was performed using DAF-FM-DA. Embryos or isolated juvenile and adult chromatophore organs were incubated in the dark with 12.5 μm or 2.5 μm DAF-FM-DA (5 mm stock in dimethyl sulfoxide), respectively, in filtered SW for 20 min. Subsequently, they were incubated in SW for 30 min to allow complete deesterification of intracellular diacetates. The fluorescence was visualized with a Zeiss AxioImager M1 microscope equipped with a filter λEXC = 470 ± 40 nm, λEM = 525 ± 50 nm. Control experiments were performed pretreating the embryos with 1 mm c-PTIO for 5 h or 1 mm TRIM for 1 h and isolated chromatophore organs with 0.4 mm c-PTIO for 10 min or 5 mm TRIM for 3 h.
Pharmacological Bioassays
Biossays were performed at 25 °C using a 1-cm2 piece of skin removed from the medial dorsal mantle of adult Sepia, with the epidermis arranged uppermost, according to a modified previously reported procedure (9). The skin was continuously perfused with ASW except during the application of test drugs, when the perfusion was stopped. DEA/NO, DEA, or glutamate was added in a window cut in the epidermis. Test solutions were washed out by restarting perfusion. DEA/NO (20 mm stock in 0.01 m NaOH) spontaneously dissociates in a pH-dependent, first-order process with a half-life of 16 min at 22–25 °C, pH 7.4 (19). The effect was recorded using a DEC-18 Digital Eyepiece Camera (World Precision Instruments) mounted on dissecting microscope (Zeiss). Acquired video frames were processed using ImageJ (National Institutes of Health). Reversed images were created, and changes in the size of all chromatophores in the field were monitored as the mean of gray value: chromatophore expansion corresponded to an increase in the gray value and vice versa. To investigate the NO signaling, bioassays were performed on isolated juvenile chromatophore organs monitoring chromatophore expansion and NO levels. For chromatophore expansion, test drugs (DEA/NO, DEA, ODQ, 8-Br-cGMP, ruthenium red, cADP-ribose, and 8-Br-cADP ribose) were added separately, and when necessary, chromatophore organs were preincubated for 5 min with ruthenium red or ODQ or 8-Br-cADP ribose before DEA/NO treatment. The effect was visualized by acquiring time-lapse images (30-s interval, 20-min duration) with a Zeiss AxioImager M1 microscope equipped with Axio Vision Release 4.7 software (Zeiss). The frames were analyzed using ImageJ. Changes in the cytoelastic sac size were monitored by setting a region of interest and measuring transmitted light variation: chromatophore expansion corresponded to a decrease in transmitted light and vice versa. Relative transmitted light was expressed as R(T/T0), where T is the value during experiments and T0 is the value before application of drugs. For NO detection, isolated chromatophore organs were loaded with DAF-FM-DA as described above. Subsequently, they were treated with glutamate in ASW or in Ca2+-free ASW containing EGTA, caffeine in Ca2+-free ASW, NMDA in ASW, NMDA + glycine in Mg2+-free ASW (460 mm NaCl, 10 mm KCl, 11 mm CaCl2, 10 mm Hepes, pH 7.8). When necessary, chromatophore organs were preincubated for 10 min with CNQX or d-AP5 in ASW and for 4 min with 5-HT in Ca2+-free ASW. All concentrations are reported in the figure legends. The effect was visualized by acquiring time-lapse images with a Zeiss AxioImager M1 fluorescence microscope as reported above. The region of interest containing the chromatophore organs was set, and relative NO fluorescence was expressed as R(F/F0), where F is a background-subtracted fluorescence image of chromatophore organs during experiments and F0 is a background-subtracted fluorescence image just before application of drugs.
Statistical Analysis
The data were expressed as mean ± S.E. The significance of the difference of between means was analyzed by the least significant difference test of analysis of variance. p values < 0.05 were considered as significant and marked with *; p values < 0.01 were considered as highly significant and marked with **.
RESULTS
NOS Detection in Chromatophore Organs of S. officinalis
The presence of NOS in embryo, isolated juvenile, and adult chromatophore organs was investigated using NADPH diaphorase histochemistry combined with immunohistochemistry/cytochemistry. S. officinalis shows a direct development without larva stage and with 30 embryonic stages (20). At stage 30, hatching occurs, leading to a juvenile strikingly similar to the adult. Some yellow-orange chromatophores begin to appear at stage 26 in the dorsal and lateral mantle. At stage 27, the chromatophores are more numerous and dark orange in color. At hatching, the juvenile skin is completely covered by chromatophores also in the ventral region. In this study, we considered stage 27 as an embryo stage and specimens 1 week after hatching as juvenile.
NADPH diaphorase histochemistry is a widely used technique indicative of NOS activity. Its specificity resides in the ability of NOS to retain its activity after aldehyde fixation, unlike other diaphorases (21). In embryos, NADPH diaphorase staining is visible in the cytoelastic sacculus (Fig. 2A). In the juvenile, NOS activity is localized in both the pigment sacculus and in the muscle fibers (Fig. 2B). In the adult stage, the staining is clearly visible at the level of muscle fibers (Fig. 2C), whereas any possible signal in the sacculus is probably masked by the pigment visible under control conditions (Fig. 2F). Interestingly, after treatment with the Triton-containing buffer used for washings and incubations in the NADPH diaphorase protocol, embryo and juvenile chromatophores do not show any pigment, probably because of pigment solubilization (Fig. 2, D and E). On the contrary, the pigment at these stages is clearly visible when the chromatophores are kept in SW or in PBS buffer (supplemental Fig. S1).
FIGURE 2.
NOS detection in chromatophore organs of S. officinalis. A–C, NADPH diaphorase reaction. A, embryo chromatophore organs. The NADPH diaphorase staining is visible in the cytoelastic sacculus, which is not fully pigmented. B, isolated juvenile chromatophore organ showing the staining in the fully pigmented sacculus and in the relaxed and contracted muscle fibers. C, isolated adult chromatophore organ with the reaction localized in contracted muscle fibers. D–F, controls of embryo, juvenile, and adult chromatophore organs with omission of β-NADPH showing loss of reaction and solubilization of embryo and juvenile chromatophore pigment (D and E). G–I, immunohistochemistry/cytochemistry reaction. G, embryo chromatophore organs. The immunopositivity is localized in the cytoelastic sacculus. H, isolated juvenile chromatophore organ showing the immunoreactivity in the pigment sacculus and in the contracted and partially relaxed muscle fibers. I, Sepia isolated adult chromatophore organ. The immunopositivity is visible in contracted and partially relaxed muscle fibers. Scale bars, 20 μm. mf, muscle fiber; sa, sacculus.
NOS immunohistochemistry/cytochemistry was performed using an antibody generated to a peptide of a conserved region in all known animal NOSs. NOS-like immunoreactivity is localized in the cytoelastic sacculus of embryo chromatophore organs (Fig. 2G). In isolated juvenile chromatophore organs a strong immunoreactivity is observed both at the level of the cytoelastic sacculus and the muscle fibers (Fig. 2H). In the adult stage, the immunopositivity is clearly visible at the level of muscle fibers (Fig. 2I). As in NADPH diaphorase histochemistry, the pigment in embryo and juvenile chromatophore organs is solubilized after permeabilization with Triton X-100, whereas it is insoluble at adult stage (data not shown).
NO Detection in Chromatophore Organs of S. officinalis
Localization of endogenous NO in embryo, isolated juvenile, and adult chromatophore organs was performed using DAF-FM-DA. This is a most sensitive cell-permeable and nonfluorescent reagent that develops intense fluorescence after reaction with NO in air to form a benzotriazole derivative (22). In embryo, juvenile, and adult chromatophore organs, NO is present in the muscle fibers (Fig. 3, A–C). In the embryo, NO is also present in the cytoelastic sacculus as shown by the fluorescence visible in the central region of the sacculus that lacks the pigment (Fig. 3A). In the juvenile and adult chromatophores the cytoelastic sacculus is fully pigmented (Fig. 3, E and F), thus preventing the detection of any possible fluorescence in this structure (Fig. 3, B and C). No fluorescence is detected in the presence of the NO scavenger, c-PTIO, as well as the NOS inhibitor TRIM (supplemental Figs. S2 and S3).
FIGURE 3.
DAF fluorescence in chromatophore organs of S. officinalis. A–C, fluorescence images. D–F, bright-field images of the same field. A, Sepia embryo chromatophore organs. The fluorescence is visible in muscle fibers and in the cytoelastic sacculus. In the sacculus, the fluorescence is detectable in the central region of the sacculus which lacks the pigment. B, isolated Sepia juvenile chromatophore organ showing a strong fluorescence in contracted muscle fibers. C, isolated Sepia adult chromatophore organ with a strong fluorescence in relaxed muscle fibers. Scale bars, 20 μm. mf, muscle fiber; sa, sacculus.
NO Detection in Chromatophore Organs of L. vulgaris
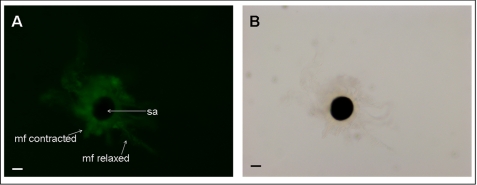
To ascertain that the presence of NO is not only restricted to cuttlefish, isolated adult chromatophore organs of L. vulgaris were examined for the presence of NO. Fluorescence is visible in the muscle fibers (Fig. 4A). As in Sepia, the pigment is not soluble in Tris, thus hampering the detection of any possible reaction at sacculus level (Fig. 4B).
FIGURE 4.
DAF fluorescence in chromatophore organs of L. vulgaris. A, fluorescence image. B, bright-field image of the same chromatophore organ. A, isolated Loligo adult chromatophore organ showing fluorescence in relaxed and contracted muscle fibers. mf, muscle fiber; sa, sacculus.
Biological Role of NO in Chromatophore Organs
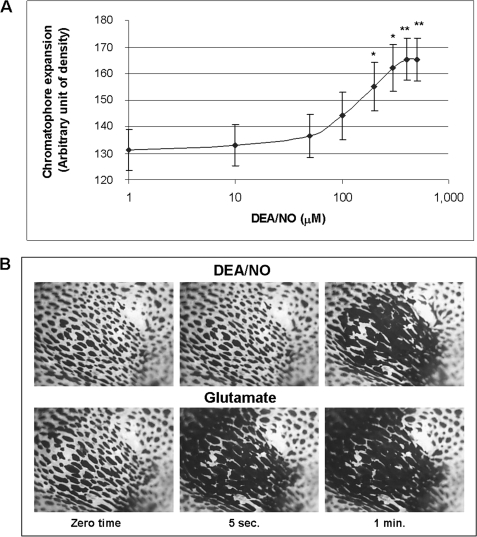
To get an insight into the biological role of NO in chromatophore organs, pharmacological bioassays were performed on adults using DEA/NO as the NO donor at various concentrations. Treatment with DEA/NO resulted in chromatophore expansion in a concentration-dependent manner (Fig. 5A). A plot of the maximum expansions reached as a function of DEA/NO concentration gave a sigmoidal curve with an EC50 value of 136 μm. Up to 300 μm concentration, the effect was reversible, i.e. restarting perfusion the chromatophores return to their original retracted state in about 4 min. The plateau was reached at 500 μm concentration, and at this concentration the process was irreversible.
FIGURE 5.
NO-induced versus glutamate-induced chromatophore expansion. A, different concentrations of the NO donor DEA/NO were applied on a window cut in the epidermis of a piece of dorsal skin, removed from the medial dorsal mantle of adult cuttlefish. The maximum chromatophore expansion was reported as a function of DEA/NO concentration. NO causes chromatophore expansion in a concentration-dependent manner. Results are shown as mean ± S.E. (error bars). Statistical analysis was performed as reported under “Experimental Procedures.” *, p < 0.05; **, p < 0.01 with respect to the value corresponding to 1 μm DEA/NO; n = 8 experiments. B, time course of chromatophore expansion after addition of DEA/NO (200 μm) or glutamate (100 μm). Images are taken at zero time, 5 s, and 1 min. Chromatophore expansion, visualized by darkening, appears earlier with glutamate (5 s) relative to DEA/NO (1 min) and denotes a faster response based on muscle contraction.
NO-induced chromatophore expansion was compared with that caused by glutamate, the classical transmitter reported to be effective in triggering Sepia chromatophore activity (9, 11). To compare the effects caused by NO and glutamate, images of the dissected skin, treated with 200 μm DEA/NO and 100 μm glutamate, were acquired at set times, before and during the drug application (Fig. 5B). The NO donor-elicited expansion of the chromatophore occurred in about 1 min. On the contrary, glutamate achieves expansion in much faster times, e.g. 5 s, as previously reported (11). In both cases, the expansion occurs only in the region in which the epidermis was removed. In control experiments, addition of DEA, the exhausted product of DEA/NO action, did not result in detectable effects (supplemental Fig. S4).
NO Signaling in Chromatophore Organs
The mechanism by which NO induces chromatophore expansion was then addressed in a series of experiments performed on isolated juvenile chromatophore organs. Chromatophores were treated by bath application with different substances, and the effects were examined by monitoring their expansion or NO production.
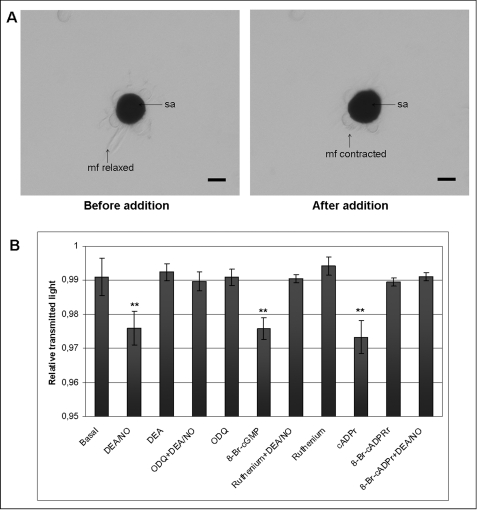
Treatment with DEA/NO induced muscle fibers contraction resulting in chromatophore expansion (Fig. 6A). Chromatophore expansion was measured under different experimental conditions as relative transmitted light, corresponding expansion to a decrease and retraction to an increase in transmitted light (Fig. 6B). The NO donor was able to induce a highly significant expansion of pigmented sacculus with respect to the basal control in the absence of any additive. NO-induced chromatophore expansion was abolished by treatment with the guanylyl cyclase inhibitor, ODQ, and the ryanodine receptor blocker, ruthenium red. Treatment with 8-Br-cGMP, a membrane-permeable, phosphodiesterase-resistant active analog of cGMP, resulted in a significant chromatophore expansion. A similar effect is induced by cADP ribose, the physiological allosteric modulator of the ryanodine receptor, which stimulates calcium-induced calcium release at lower cytosolic concentrations. 8-Br-cADP ribose, an antagonist of cADP ribose, suppressed the expansion induced by DEA/NO. ODQ, ruthenium red, 8-Br-cADP ribose, and the exhausted NO donor, DEA, alone had no significant effect. Chromatophore organs treated with glutamate in ASW showed a strong increase of NO production with concomitant contraction of muscle fibers that initially were in a relaxed state (Fig. 7A).
FIGURE 6.
NO signaling in chromatophore organs. Sepia isolated juvenile chromatophore organs were treated by bath application with different substances, and the effects were examined by monitoring chromatophore expansion. A, addition of DEA/NO (100 μm) on isolated chromatophore organs causes muscle fibers contraction resulting in pigment sacculus expansion. Before NO donor addition, the muscle fibers appear relaxed. Scale bars, 20 μm. B, isolated juvenile chromatophore organs were treated with the NO donor DEA/NO (100 μm), DEA (100 μm), ODQ (10 μm), 8-Br-cGMP (100 μm), ruthenium red (1 μm), cADP ribose (25 μm), or 8-Br-cADP ribose (25 μm). When indicated, organs were preincubated for 5 min with ODQ or ruthenium red or 8-Br-cADP ribose before DEA/NO treatment. The chromatophore expansion was recorded, and changes in transmitted light were measured as reported under “Experimental Procedures.” Data are expressed as means ± S.E. (error bars). Statistical analysis was performed as reported under “Experimental Procedures.” **, p < 0.01 with respect to the basal control, n = 8 experiments. mf, muscle fiber; sa, sacculus.
FIGURE 7.
NO production in chromatophore organs. Sepia isolated juvenile chromatophore organs were treated by bath application with different substances, and the effects were examined by monitoring NO production. A, addition of glutamate (250 μm) causes an increase of NO production. Muscle fibers, initially relaxed, contract after glutamate addition. Scale bars, 20 μm. B, isolated juvenile chromatophore organs were treated with glutamate (250 μm) in the absence or presence of EGTA (2 mm), caffeine (5 mm), NMDA (250 μm), NMDA + glycine (NMDA 250 μm + glycine 50 μm). When indicated, chromatophores were preincubated for 4 min with 5-HT (10 μm) or 10 min with d-AP5 (200 μm) or CNQX (250 μm) before glutamate treatment. NO production was monitored in 20 min, and the maximum value was reported as means ± S.E. (error bars). Statistical analysis was performed as reported under “Experimental Procedures.” **, p < 0.01 with respect to the basal control; n = 8 experiments. mf, muscle fiber; sa, sacculus.
Glutamate induces a significant increase of fluorescence with respect to the basal control in which a value of fluorescence, due to incident light, was recorded (Fig. 7B). Likewise in Ca2+-free ASW, e.g. in the presence of EGTA, glutamate induced a similar increase of NO production. 5-HT treatment, to inhibit calcium releases from internal stores, suppressed glutamate-induced NO production. Treatment with caffeine, which induces calcium release from internal stores, caused a notable increase of NO production.
Finally, to identify the ionotropic receptors, involved in glutamate-induced NO production, isolated chromatophore organs were incubated with the selective AMPA antagonist CNQX or the NMDA antagonist d-AP5, and NO production was monitored after the addition of glutamate (Fig. 7B). NO production induced by glutamate was inhibited by d-AP5 and CNQX. Moreover, treatment with NMDA and glycine, in low levels magnesium SW, to activate NMDA receptors, results in an increase in NO production comparable with that observed with glutamate. As expected, NMDA alone is not able to induce a significant increase of NO production with respect to the basal.
DISCUSSION
An increasing body of evidence indicates that NO plays several important biological roles in mollusc cephalopods. These include visual and tactile learning in O. vulgaris (23, 24), symbiosis in the squid Euprymna scolopes (25), ink defense system, neurotransmission, manipulative behavior, regulation of blood flow and pressure, and statocyst activity in the cuttlefish S. officinalis (26–28, 18, 29–31). In this paper, we provide a new addition to this list by showing that the NO signaling pathway is also involved in the regulation of chromatophore expansion underlying the unique and highly complex body patterning behavior in Sepia.
NOS localization by both NADPH diaphorase and immunohistochemistry/cytochemistry and NO detection in muscle fibers from embryo to adult suggest the involvement of NO in chromatophore physiology. Indeed, the most important outcome of the present study is that NO induces chromatophore expansion and acts as a key mediator in the glutamate-dependent pathway of chromatophore organ regulation.
Regarding the NO-induced chromatophore expansion, the dose-response data for DEA/NO would suggest that very high, nonphysiological concentrations of NO are needed to induce chromatophore expansion. But, caution should be exercised before predicting the NO levels actually released by NO donors and reaching the target. In fact, DEA/NO concentration does not provide information as to the actual fluxes of NO that are produced because the specific experimental conditions can affect the parameters that determine the fluxes of NO generation, half-lives, and stoichiometry of NO per donor (32). It follows that even the reported stoichiometry of NO release from DEA/NO is variable, ranging from 1 (33) to 1.5 (34). Additional limitations due to local concentration issues, side reactions in the tissue incubation mixture, and possibly poor membrane permeability may add to this complexity, so use of concentrations as high as 100 μm or more of this donor to elicit sustained and detectable biological effects is not uncommon (35).
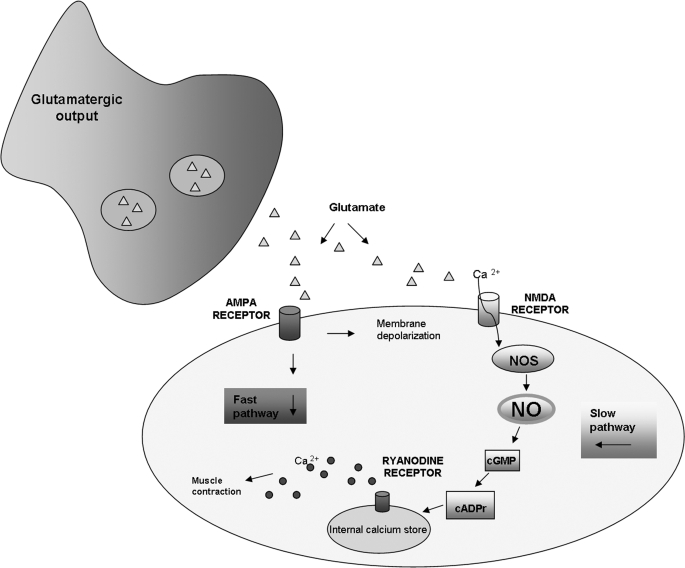
Pharmacological bioassays and NO production experiments have permitted delineation of the signaling pathway by which NO, together with glutamate, regulates the chromatophore activity, providing an important contribution toward an understanding of the complex interplay of messenger molecules, receptors, and pathways that mediate body patterning behavior. The emerging picture of glutamate-NO neuroregulation of chromatophore cell expansion is schematically illustrated in Fig. 8. Two different channels by which glutamate activates body patterning may be envisaged. The fast one, NO-independent, is activated during the production of transient body patterns, e.g. in response to disturbances as well as to the appearance of predators, prey, and conspecifics. The slow one, NO-mediated, is involved in the long term maintenance of the body coloration pattern for effective camouflage. At the neuromuscular junction, glutamate released at presynaptic level is able to activate both AMPA and NMDA receptors. The activation of AMPA receptors induces a membrane depolarization and a rapid increase in cytoplasmic calcium level that is responsible for a fast muscle contraction resulting in the transient chromatophore expansion. On the other hand, the activation of NMDA induces a prolonged calcium elevation as suggested to occur in squid (13). The increased calcium is responsible for NOS activation and consequent NO production. The finding that glutamate-induced NO production is inhibited by AMPA and NMDA receptor antagonists suggests the involvement of both glutamate receptors in NO production. On this basis, it is reasonable to hypothesize that, as well as in squid, NMDA receptors are more calcium-permeable than AMPA receptors, but they depend on AMPA receptors in inducing membrane depolarization, required for activation (13). NO thus produced is able to activate soluble guanylyl cyclase with consequent production of cGMP, as revealed by the significant chromatophore expansion by 8-Br-cGMP and the inhibition of NO-induced process in the presence of the guanylyl cyclase inhibitor, ODQ. The finding that NO-induced chromatophore expansion is abolished by treatment with the ryanodine receptor blocker, ruthenium red, together with NO production increases or decreases with agents inducing or blocking calcium release from internal stores, suggests that chromatophore expansion involves release of calcium from ryanodine stores. The cADP ribose mediation of the effect of cGMP on ryanodine stores is clearly evident from its ability to induce chromatophore expansion as well as from the inhibitory effect of its antagonist, 8-Br-cADP ribose, on NO-induced chromatophore expansion. The ability of NO to induce calcium release from ryanodine stores via cGMP and cADP ribose has been reported to occur in some invertebrate nonmuscle cells, such as pond snail neurons and sea urchin eggs, where this pathway is involved in growth cone filopodial dynamics and calcium mobilization processes during fertilization and anaphase (36, 37). In these systems it has been hypothesized that cGMP, via a cGMP-dependent protein kinase, activates, through phosphorylation, the enzyme that catalyzes the synthesis of cADP ribose, ADP-ribosyl cyclase, or one of its regulators. A similar mechanism could be operative in Sepia chromatophore organs.
FIGURE 8.
Model for glutamate-NO-cGMP-induced chromatophore expansion in S. officinalis. Presynaptically released glutamate activates both AMPA and NMDA receptors. The activation of AMPA receptors induces the membrane depolarization and the fast muscle contraction resulting in transient chromatophore expansion. The voltage-dependent activation of NMDA receptors induces a prolonged Ca2+ elevation responsible for NOS activation and consequent NO production. NO activates the soluble guanylyl cyclase resulting in the production of cGMP. cGMP induces the formation of cADP ribose, which interacts with ryanodine-like receptors and causes the release of Ca2+ from the endoplasmic reticulum, resulting in muscle fibers contraction and chromatophore expansion.
An interesting point of data emerging from this study is that glutamate-induced NO production is modulated by calcium availability. In particular, both external and internal calcium contribute to NO production, and the relative roles are dictated by the competition of calcium blockers (e.g. 5-HT) and releasers.
To the best of our knowledge, this is the first report showing that the signaling NO-cGMP-cADP ribose-ryanodine receptors is functionally active in striated muscle contraction. This was unexpected considering that in mammalian skeletal muscles NO has been shown to promote contraction mainly through a cGMP-independent pathway, involving S-nitrosylation of ryanodine receptors (38–40). On the other hand, the NO/cGMP pathway is commonly believed to be involved in relaxation of mammalian skeletal and smooth muscles (38, 41). More recently, however, the signaling NO/cGMP/cADP ribose pathway has been reported to be involved in opossum esophageal longitudinal smooth muscle contraction (42). In this context, our results may be of particular interest in relation to the biological relevance of the NO/cGMP/cADP ribose/ryanodine receptors in mammalian muscle contraction. In Sepia chromatophore organs NO acts as local mediator of chromatophore expansion because NOS has not been reported to be expressed in the posterior chromatophore lobes (17, 18) from which motoneurons, innervating skin mantle chromatophores, originate (8).
An interesting result emerging from this study is the presence of NO in isolated chromatophore organs from adult squid, thus suggesting that our finding is not species-specific, but it is of general significance for decapod cephalopods. The detection of both NO and NOS in the pigment compartment of Sepia chromatophore organs suggests the possible involvement of NO in pigment production. Interestingly, NO and NOS are present in the embryo cytoelastic sacculus, which is characterized by a region devoid of pigment, located in the center of the sacculus. This region will disappear as the development proceeds, resulting in chromatophores fully pigmented at hatching. Noteworthy, NO has been reported to participate in the regulation of the activity of indoleamine 2,3-dioxygenase, the mammalian counterpart of the enzyme that catalyzes the first and rate-limiting step in ommochrome biosynthesis, tryptophan-2,3-dioxygenase (43, 44). The differential solubility in Triton X-100-containing buffers of embryo-juvenile pigments with respect to that of adult chromatophores suggests moreover significant chemical changes in the chromatophore pigments during development, which may deserve further attention.
In conclusion, evidence has been presented for the first time for a critical role of the glutamate-NO-cGMP signaling pathway in the body patterning behavior of the cuttlefish S. officinalis. The effect is apparently associated with specific, relatively long term responses that can be attributed to the need of maintaining the patterns for camouflage or other adaptive purposes. Besides disclosing novel remarkable roles of NO in invertebrates, these results fill a gap in the current understanding of the mechanisms of neuroregulation of body patterning in cephalopods.
Supplementary Material
Acknowledgments
We thank Prof. Maurice Elphick (Queen Mary University, London, UK) for useful advice and suggestions and the Marine Resourches for Research and Marine Supplies Services of the Stazione Zoologica.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- FMRFamide
- Phe-Met-Arg-Phe-NH2
- AMPA
- α-amino-3-hydroxy-5-metil-4-isoxazolone propionate
- d-AP5
- d-(−)-2hy amino-5-phosphonopentanoic acid
- ASW
- artificial seawater
- 8-Br-cADP-ribose
- 8-bromo-cyclic ADP ribose
- 8-Br-cGMP
- 8-bromo-cyclic GMP
- c-PTIO
- [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxy1-3-oxide]
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- DAF-FM-DA
- 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- DEA
- diethylamine
- DEA/NO
- 2-(N,N-diethylamino)-diazenolate-2-oxide
- 5-HT
- 5-hydroxytryptamine
- MOPS
- 4-morpholinepropanesulfonic acid
- NMDA
- N-methyl-d-aspartic acid
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PBS
- phosphate-buffered saline
- SW
- seawater
- TRIM
- 1-(2-trifluormethylphenyl)imidazole.
REFERENCES
- 1.Hanlon R. T., Messenger J. B. (1988) Philos. Trans. R. Soc. Lond. B Biol. Sci. 320, 437–487 [Google Scholar]
- 2.Messenger J. B. (2001) Biol. Rev. Camb. Philos. Soc. 76, 473–528 [DOI] [PubMed] [Google Scholar]
- 3.Hanlon R. T. (2007) Curr. Biol. 17, R400–R404 [DOI] [PubMed] [Google Scholar]
- 4.Florey E. (1969) Am. Zool. 9, 429–442 [DOI] [PubMed] [Google Scholar]
- 5.Packard A., Hochberg F. G. (1977) The Biology of Cephalopods (Nixon M., Messenger J. B. eds) pp. 191–231, Academic Press, London [Google Scholar]
- 6.Fox H. M., Vevers G. (1960) The Nature of Animal Colours, pp. 50–57, Sidgwick and Jackson, London [Google Scholar]
- 7.Han Q., Calvo E., Marinotti O., Fang J., Rizzi M., James A. A., Li J. (2003) Insect Mol. Biol. 12, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubas F., Hanlon R. T., Ferguson G. P., Pinsker H. M. (1986) J. Exp. Biol. 121, 1–25 [DOI] [PubMed] [Google Scholar]
- 9.Loi P., Saunders R., Young D., Tublitz N. (1996) J. Exp. Biol. 199, 1177–1187 [DOI] [PubMed] [Google Scholar]
- 10.Loi P. K., Tublitz N. (1997) J. Exp. Biol. 200, 1483–1489 [DOI] [PubMed] [Google Scholar]
- 11.Loi P. K., Tublitz N. J. (2000) J. Comp. Neurol. 420, 499–511 [DOI] [PubMed] [Google Scholar]
- 12.Messenger J., Cornwell C., Reed C. (1997) J. Exp. Biol. 200, 3043–3054 [DOI] [PubMed] [Google Scholar]
- 13.Lima P. A., Nardi G., Brown E. R. (2003) Eur. J. Neurosci. 17, 507–516 [DOI] [PubMed] [Google Scholar]
- 14.Lima P. A., Messenger J. B., Brown E. R. (1997) J. Physiol. 504.P, 2P [Google Scholar]
- 15.Lima P. A., Messenger J. B., Brown E. R. (1998) J. Physiol. 513.P, 127P9782164 [Google Scholar]
- 16.Palumbo A., d'Ischia M. (2007) Advances in Experimental Biology on Nitric Oxide (Trimmer B., Tota B. eds) pp. 45–64, Elsevier, London [Google Scholar]
- 17.Di Cosmo A., Di Cristo C., Palumbo A., d'Ischia M., Messenger J. B. (2000) J. Comp. Neurol. 428, 411–427 [DOI] [PubMed] [Google Scholar]
- 18.Di Cristo C., Fiore G., Scheinker V., Enikolopov G., d'Ischia M., Palumbo A., Di Cosmo A. (2007) Eur. J. Neurosci. 26, 1599–1610 [DOI] [PubMed] [Google Scholar]
- 19.Keefer L. K., Nims R. W., Davies K. M., Wink D. A. (1996) Methods Enzymol. 268, 281–293 [DOI] [PubMed] [Google Scholar]
- 20.Lemaire J. (1970) Bull. Soc. Zool. France 95, 773–782 [Google Scholar]
- 21.Chao D. S., Hwang P. M., Huang F., Bredt D. S. (1996) Methods Enzymol. 268, 488–496 [DOI] [PubMed] [Google Scholar]
- 22.Kojima H., Urano Y., Kikuchi K., Higuchi T., Hirata Y., Nagano T. (1999) Angew Chem. Int. Ed. Engl. 38, 3209–3212 [DOI] [PubMed] [Google Scholar]
- 23.Robertson J. D., Bonaventura J., Kohm A. P. (1994) Proc. R. Soc. Lond. B Biol. Sci. 256, 269–273 [DOI] [PubMed] [Google Scholar]
- 24.Robertson J. D., Bonaventura J., Kohm A., Hiscat M. (1996) Proc. R. Soc. Lond. B Biol. Sci. 263, 1739–1743 [DOI] [PubMed] [Google Scholar]
- 25.Davidson S. K., Koropatnick T. A., Kossmehl R., Sycuro L., McFall-Ngai M. J. (2004) Cell. Microbiol. 6, 1139–1151 [DOI] [PubMed] [Google Scholar]
- 26.Palumbo A., Di Cosmo A., Poli A., Di Cristo C., d'Ischia M. (1999) J. Neurochem. 73, 1254–1263 [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A., Poli A., Di Cosmo A., d'Ischia M. (2000) J. Biol. Chem. 275, 16885–16890 [DOI] [PubMed] [Google Scholar]
- 28.Fiore G., Poli A., Di Cosmo A., d'Ischia M., Palumbo A. (2004) Biochem. J. 378, 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halm M. P., Chichery M. P., Chichery R. (2003) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 134, 139–146 [DOI] [PubMed] [Google Scholar]
- 30.Schipp R., Gebauer M. (1999) Invert. Neurosci. 4, 9–15 [DOI] [PubMed] [Google Scholar]
- 31.Tu Y., Budelmann B. U. (2000) Brain Res. 865, 211–220 [DOI] [PubMed] [Google Scholar]
- 32.Li Q., Lancaster J. R., Jr. (2009) Nitric Oxide 21, 69–75 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt K., Desch W., Klatt P., Kukovetz W. R., Mayer B. (1997) Naunyn Schmiedebergs Arch. Pharmacol. 355, 457–462 [DOI] [PubMed] [Google Scholar]
- 34.Ramamurthi A., Lewis R. S. (1997) Chem. Res. Toxicol. 10, 408–413 [DOI] [PubMed] [Google Scholar]
- 35.Shinyashiki M., Chiang K. T., Switzer C. H., Gralla E. B., Valentine J. S., Thiele D. J., Fukuto J. M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welshhans K., Rehder V. (2007) Eur. J. Neurosci. 26, 1537–1547 [DOI] [PubMed] [Google Scholar]
- 37.Willmott N., Sethi J. K., Walseth T. F., Lee H. C., White A. M., Galione A. (1996) J. Biol. Chem. 271, 3699–3705 [DOI] [PubMed] [Google Scholar]
- 38.Kobzik L., Reid M. B., Bredt D. S., Stamler J. S. (1994) Nature 372, 546–548 [DOI] [PubMed] [Google Scholar]
- 39.Stoyanovsky D., Murphy T., Anno P. R., Kim Y. M., Salama G. (1997) Cell Calcium 21, 19–29 [DOI] [PubMed] [Google Scholar]
- 40.Xu L., Eu J. P., Meissner G., Stamler J. S. (1998) Science 279, 234–237 [DOI] [PubMed] [Google Scholar]
- 41.Carvajal J. A., Germain A. M., Huidobro-Toro J. P., Weiner C. P. (2000) J. Cell. Physiol. 184, 409–420 [DOI] [PubMed] [Google Scholar]
- 42.Campbell R. K., Wells R. W., Miller D. V., Paterson W. G. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1543–G1548 [DOI] [PubMed] [Google Scholar]
- 43.López A. S., Alegre E., Díaz A., Mugueta C., González A. (2006) Immunol. Lett. 106, 163–171 [DOI] [PubMed] [Google Scholar]
- 44.Thomas S. R., Terentis A. C., Cai H., Takikawa O., Levina A., Lay P. A., Freewan M., Stocker R. (2007) J. Biol. Chem. 282, 23778–23787 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.