Abstract
Five species of rumen bacteria with overlapping substrate fermentative capabilities were tested for substrate preferences and evidence of catabolite regulatory mechanisms. All five bacteria showed evidence of some type of catabolite regulatory mechanism. In the six-substrate test system that was used, utilization of every substrate was inhibited by another substrate in at least one of the bacteria. Inhibited versus noninhibited substrate data suggest that the five bacteria have different strategies of substrate utilization and thus occupy separate niches in the rumen. The significance of these observations to understanding the rumen ecosystem is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
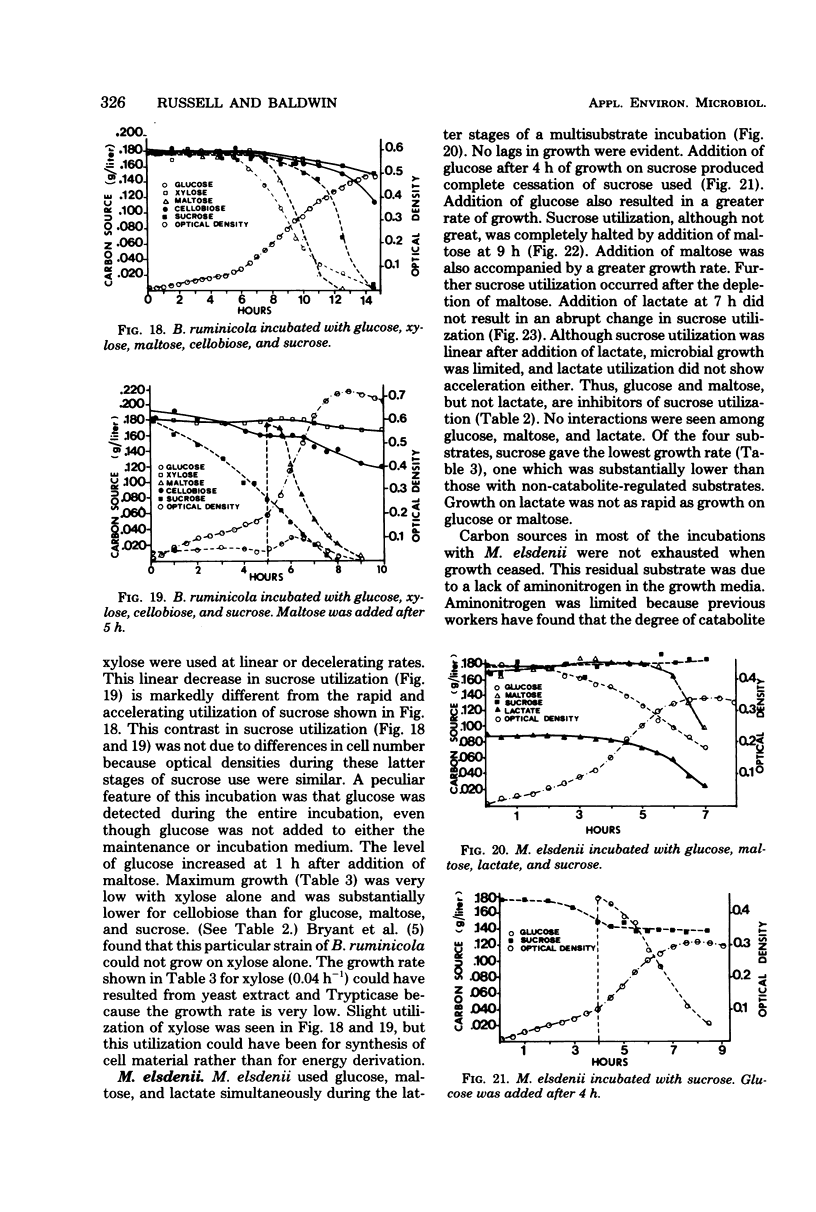
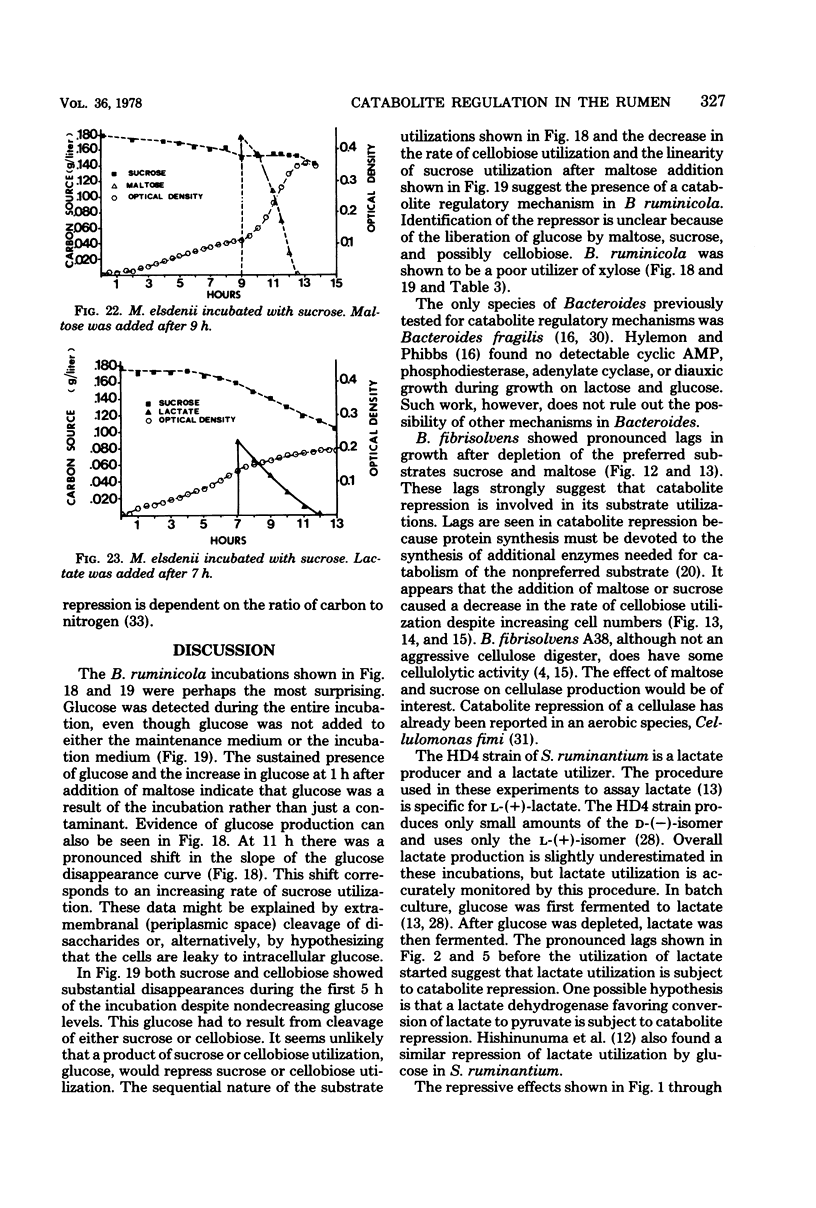
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N. The anaerobic monotrichous butyric acid-producing curved rod-shaped bacteria of the rumen. J Bacteriol. 1956 Jul;72(1):16–21. doi: 10.1128/jb.72.1.16-21.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. O., WINDERMAN S., GORMAN J. Comparison of the alpha-glucosidases of Saccharomyces produced in response to five non-allelic maltose genes. Biochim Biophys Acta. 1963 Jan 8;67:42–53. doi: 10.1016/0006-3002(63)91795-5. [DOI] [PubMed] [Google Scholar]
- Haggerty D. M., Schleif R. F. Kinetics of the onset of catabolite repression in Escherichia coli as determined by lac messenger ribonucleic acid initiations and intracellular cyclic adenosine 3',5'-monophosphate levels. J Bacteriol. 1975 Sep;123(3):946–953. doi: 10.1128/jb.123.3.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan N., Durr I. F. Induction of beta-galactosidase in Lactobacillus plantarum. J Bacteriol. 1974 Oct;120(1):66–73. doi: 10.1128/jb.120.1.66-73.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Evidence against the presence of cyclic AMP and related enzymes in selected strains of Bacteroides fragilis. Biochem Biophys Res Commun. 1974 Sep 9;60(1):88–95. doi: 10.1016/0006-291x(74)90176-4. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Eaton N. R. Purification and characterization of maltase and alpha-methyl glucosidase from yeast. Biochim Biophys Acta. 1967 Sep 12;146(1):173–180. doi: 10.1016/0005-2744(67)90084-8. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Site of catabolite inhibition of carbohydrate metabolism. J Bacteriol. 1973 May;114(2):885–887. doi: 10.1128/jb.114.2.885-887.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2324–2328. doi: 10.1073/pnas.71.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Siegel L. S., Hylemon P. B., Phibbs P. V., Jr Cyclic adenosine 3',5'-monophosphate levels and activities of adenylate cyclase and cyclic adenosine 3',5'-monophosphate phosphodiesterase in Pseudomonas and Bacteroides. J Bacteriol. 1977 Jan;129(1):87–96. doi: 10.1128/jb.129.1.87-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. J., Leatherwood J. M. Derepressed synthesis of cellulase by Cellulomonas. J Bacteriol. 1976 Nov;128(2):609–615. doi: 10.1128/jb.128.2.609-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Tillier F., Monod J. Catabolite modulator factor: a possible mediator of catabolite repression in bacteria. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3476–3479. doi: 10.1073/pnas.73.10.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P. K., Rosen O. M. Cyclic 3':5'-adenosine monophosphate in Escherichia coli during transient and catabolite repression. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1436–1440. doi: 10.1073/pnas.71.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


