Abstract
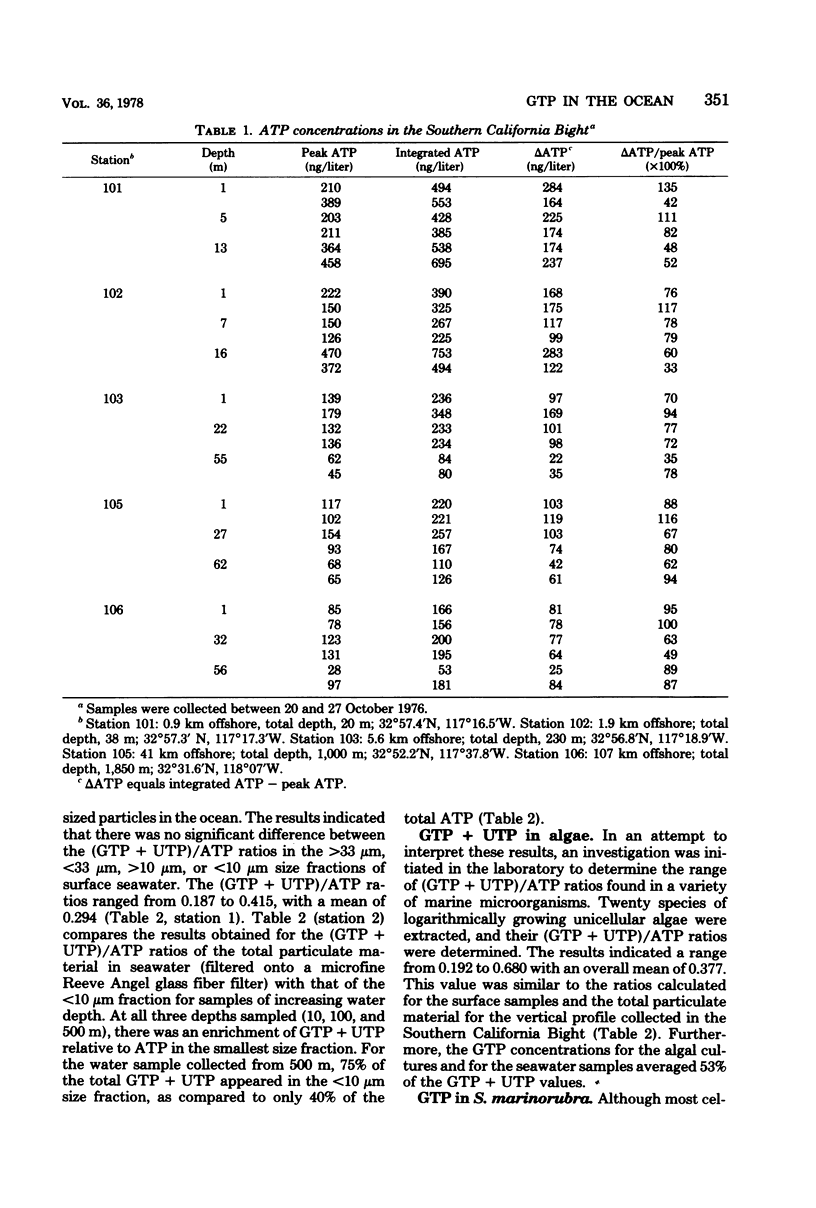
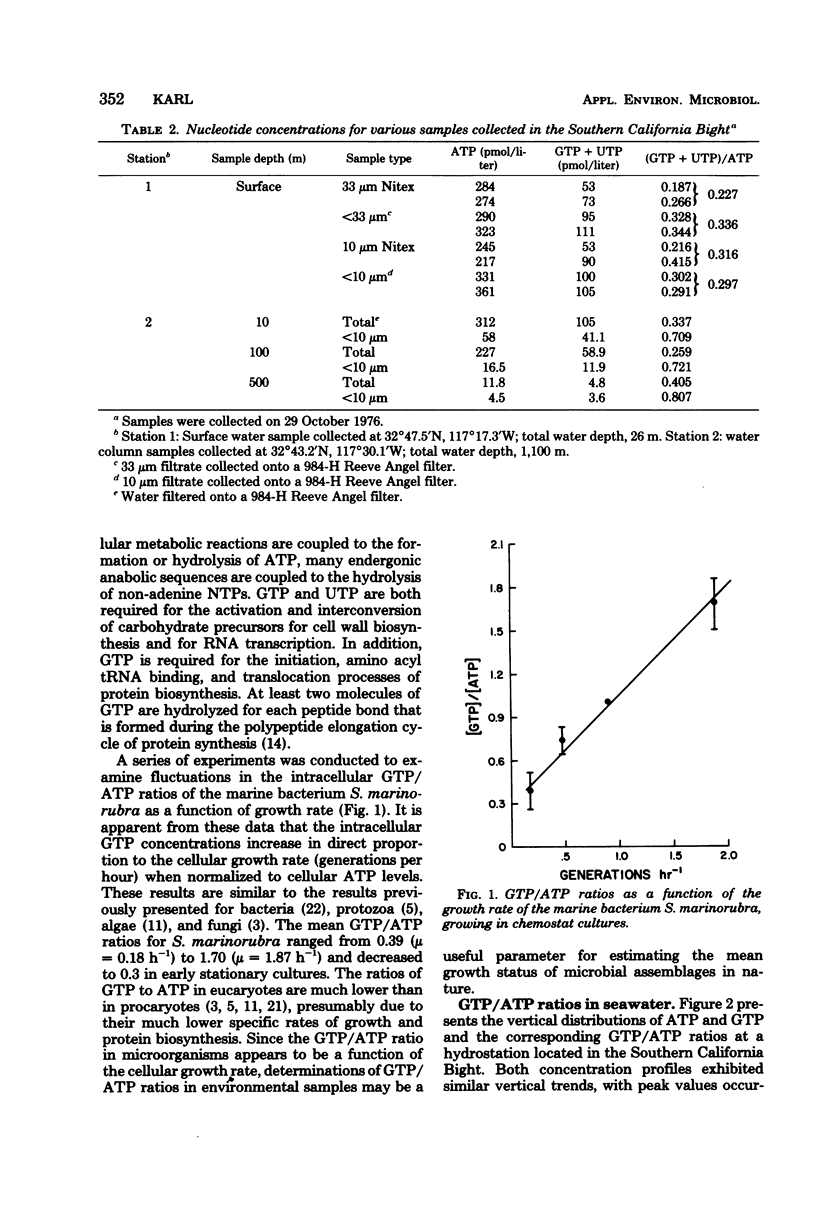
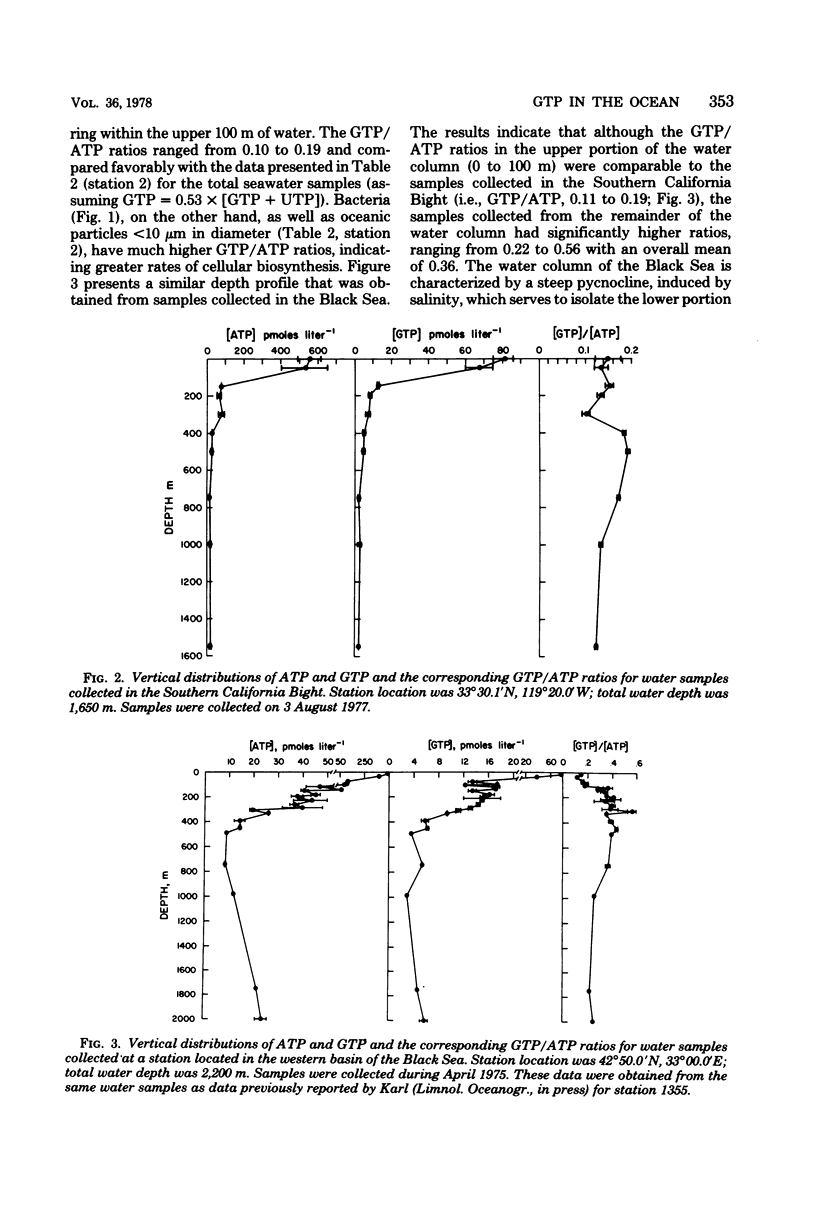
A comparison between the ATP concentrations based on peak height light emission values (0 to 3 s) and integrated light flux determinations (15 to 75 s) for a variety of seawater samples revealed that the integrated method of light detection consistently yielded higher ATP concentrations, ranging from 1.38 to 2.35 times larger than the corresponding peak ATP values. A significant correlation (r = 0.923) was observed for a plot of ΔADP (i.e., integrated ATP - peak ATP) versus GTP + UTP, suggesting that the analytical interference on the ATP assay was the result of the presence of non-adenine nucleotide triphosphates. Size-fractionation studies revealed an enrichment of the non-adenine nucleotide triphosphates, relative to ATP, in the smallest size fraction analyzed (<10 μm). Investigations were conducted with 20 species of unicellular marine algae to determine their intracellular nucleotide concentrations, and these determinations were compared to the levels measured in lab cultures of the marine bacterium Serratia marinorubra. These results indicated that the intracellular GTP/ATP ratios in S. marinorubra increase in direct proportion to the rate of cell growth, and that the GTP/ATP ratios in bacteria are much greater than in growing algae, presumably due to the differences in rates of cellular biosynthesis. It is concluded that quantitative determinations of GTP/ATP ratios in environmental sample extracts may be useful for measuring microbial growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALFOUR W. M., SAMSON F. E., Jr Transphosphorylases in the firefly lantern. Arch Biochem Biophys. 1959 Sep;84:140–142. doi: 10.1016/0003-9861(59)90562-4. [DOI] [PubMed] [Google Scholar]
- Costantini M. G., Zippel R., Sturani E. Levels of the ribonucleoside triphosphates and rate of RNA synthesis in Neurospora crassa. Biochim Biophys Acta. 1977 Jun 17;476(4):272–278. doi: 10.1016/0005-2787(77)90291-x. [DOI] [PubMed] [Google Scholar]
- Deluca M. Firefly luciferase. Adv Enzymol Relat Areas Mol Biol. 1976;44:37–68. doi: 10.1002/9780470122891.ch2. [DOI] [PubMed] [Google Scholar]
- Echetebu C. O., Plesner P. The pool of ribonucleoside triphosphates in synchronized Tetrahymena pyriformis. J Gen Microbiol. 1977 Dec;103(2):389–392. doi: 10.1099/00221287-103-2-389. [DOI] [PubMed] [Google Scholar]
- GREEN A. A., MCELROY W. D. Crystalline firefly luciferase. Biochim Biophys Acta. 1956 Apr;20(1):170–176. doi: 10.1016/0006-3002(56)90275-x. [DOI] [PubMed] [Google Scholar]
- King J. D., White D. C. Muramic acid as a measure of microbial biomass in estuarine and marine samples. Appl Environ Microbiol. 1977 Apr;33(4):777–783. doi: 10.1128/aem.33.4.777-783.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975 May 26;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Nielsen R. An improved analysis of adenosine triphosphate by the luciferase method. Acta Chem Scand. 1968;22(6):1745–1756. doi: 10.3891/acta.chem.scand.22-1745. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Greifner M. I., Chock P. B. Determination of adenosine 5'-triphosphate by the luciferase system with a stopped-flow spectrometer. Anal Biochem. 1975 May 26;66(1):259–264. doi: 10.1016/0003-2697(75)90744-7. [DOI] [PubMed] [Google Scholar]
- SMITH R. C., MAALOE O. EFFECT OF GROWTH RATE ON THE ACID-SOLUBLE NUCLEOTIDE COMPOSITION OF SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 May 11;86:229–234. doi: 10.1016/0304-4165(64)90047-9. [DOI] [PubMed] [Google Scholar]
- Sachsenmaier W., Immich H., Grunst J., Scholz R., Bücher T. Free ribonucleotides of Physarum polycephalum. Eur J Biochem. 1969 Apr;8(4):557–561. doi: 10.1111/j.1432-1033.1969.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe W. J., Bancroft K. Use of the adenylate energy charge ratio to measure growth state of natural microbial communities. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2112–2115. doi: 10.1073/pnas.72.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]


