Abstract
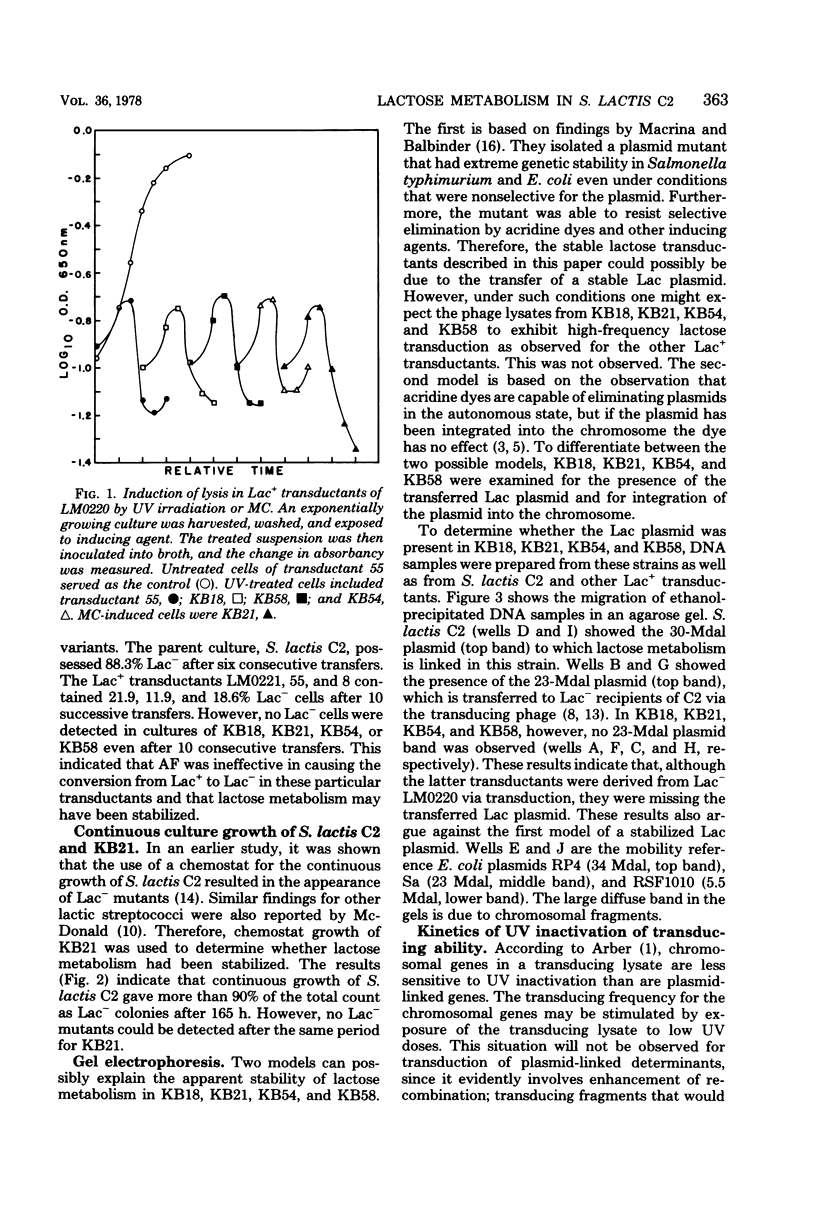
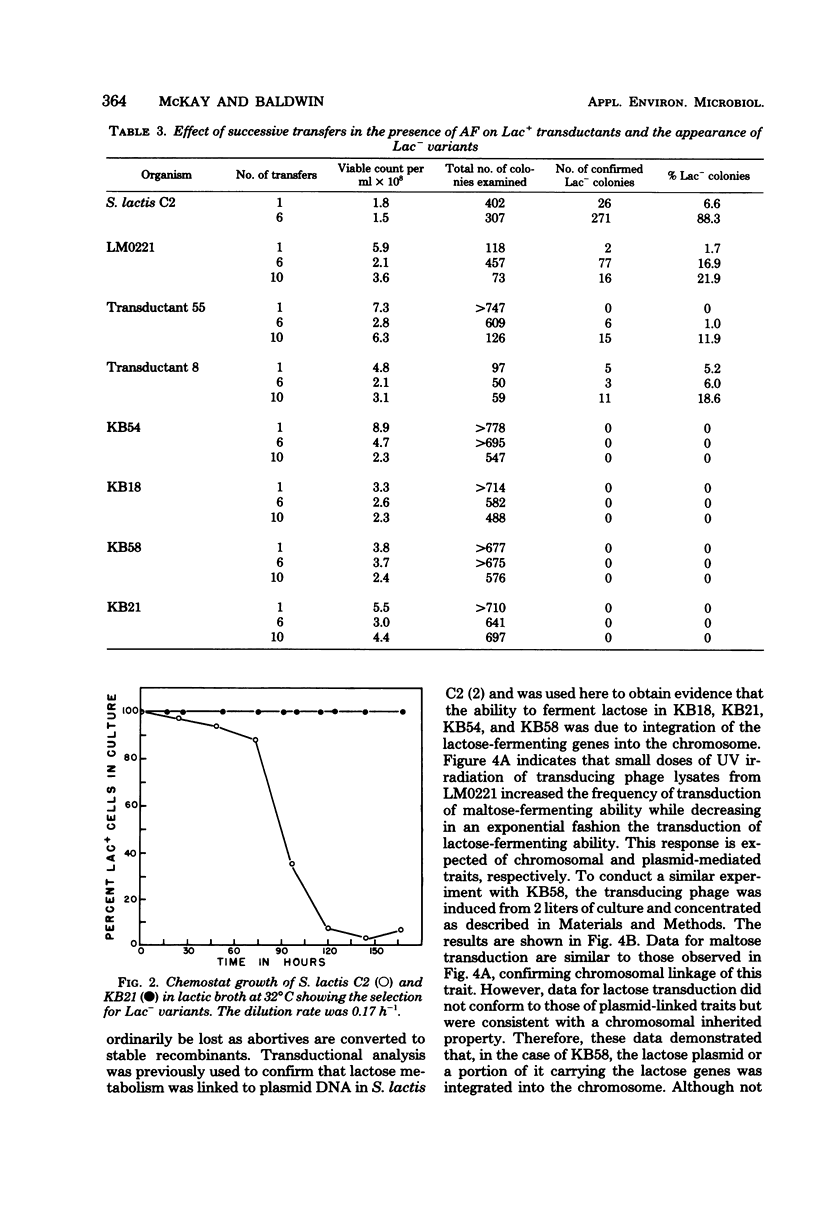
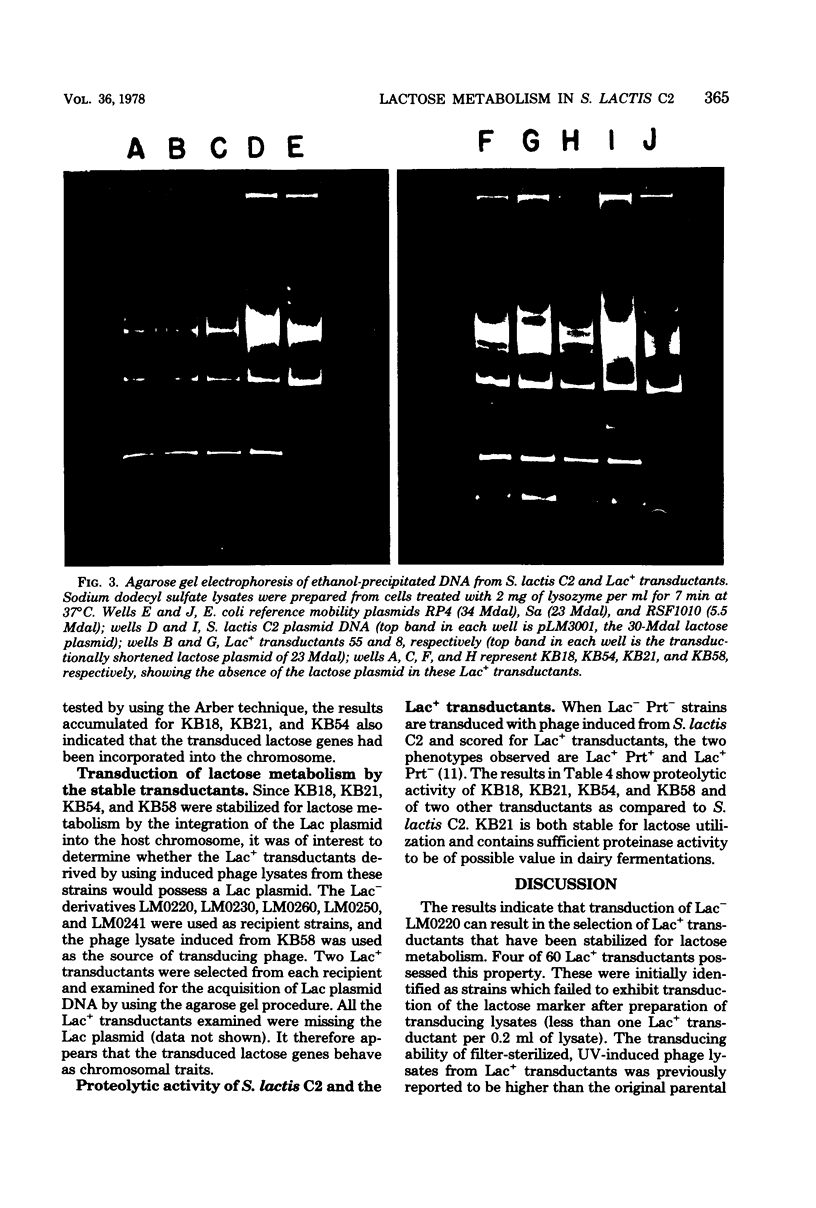
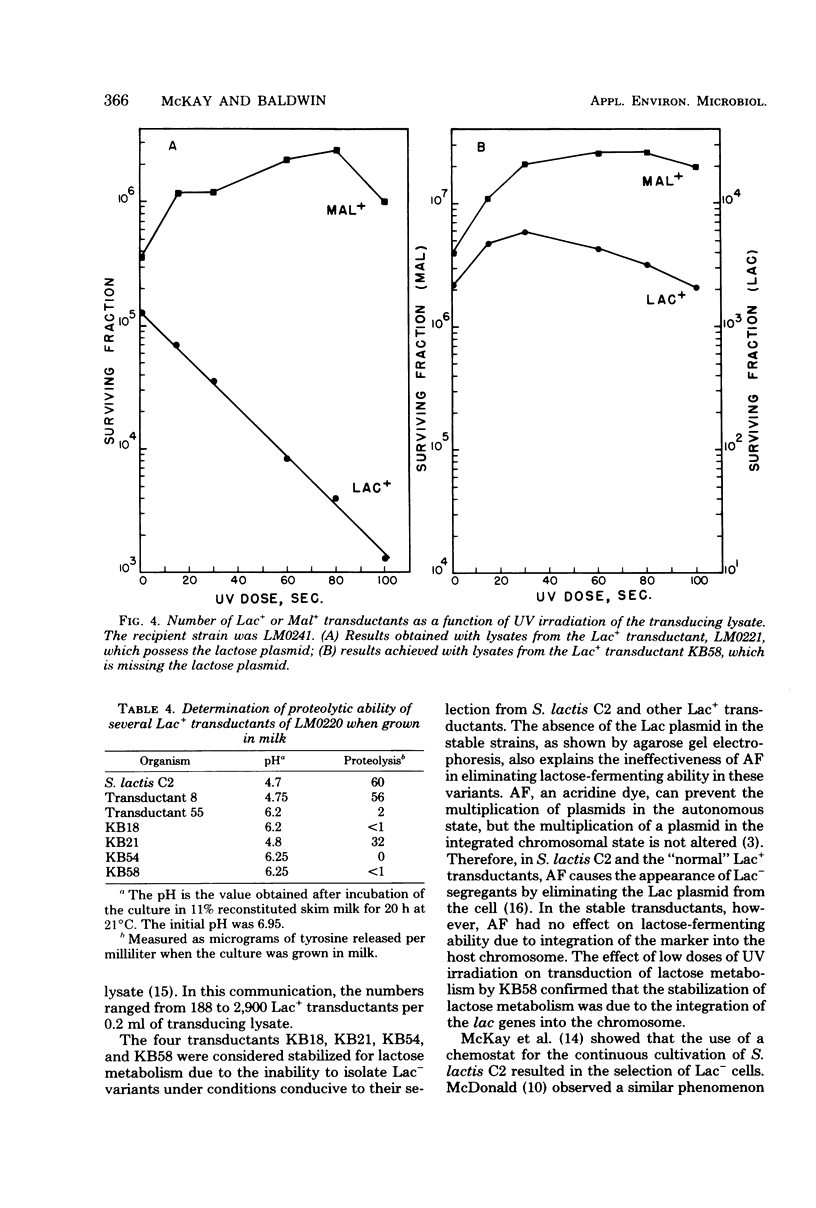
The integration of the lactose plasmid from lactic streptococci into the host chromosome could stabilize this trait for dairy fermentations. Sixty lactose-positive (Lac+) transductants of lactose- and proteinase-negative (Lac− Prt−) LM0220 were induced for temperature phage by UV irradiation or mitomycin C. Four of the transductants, designated KB18, KB21, KB54, and KB58, yielded lysates demonstrating less than one Lac+ transductant per 0.2 ml of phage lysate. Successive transferring in the presence of acriflavine did not yield Lac− segregants from KB18, KB21, KB54, or KB58, whereas Streptococcus lactis C2 (parent culture) and three other Lac+ transductants showed 12 to 88% conversion from Lac+ to Lac− within 6 to 10 repetitive transfers. When grown in continuous culture, KB21 did not show any Lac− variants in 168 h, while S. lactis C2 had 96% conversion from Lac+ to Lac− in 144 h. Agarose gel electrophoresis of plasmid DNA isolated from KB18, KB21, KB54, and KB58 revealed that the lactose plasmid, pLM2103, normally present in Lac+ transductants, was missing. This suggested integration of the transferred lactose plasmid into the chromosome. In contrast to phage lysates induced from S. lactis C2, which exhibited an exponential decrease in the number of Lac+ transductants after exposure to small doses of UV irradiation, the transduction frequency for lactose metabolism was stimulated by UV irradiation of lysates from KB58. The latter indicated chromosomal linkage for lac and that integration of the lactose genes plasmid into the chromosome had occurred.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L. Isolation and examination of transducing bacteriophage particles from Streptococcus lactis C2. J Dairy Sci. 1976 Mar;59(3):396–404. doi: 10.3168/jds.s0022-0302(76)84219-1. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Balbinder E. Genetic Characterization of a Stable F' lac Plasmid. J Bacteriol. 1972 Oct;112(1):503–512. doi: 10.1128/jb.112.1.503-512.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald I. J. Occurence of lactose-negative mutants in chemostat cultures of lactic streptococci. Can J Microbiol. 1975 Mar;21(3):245–251. doi: 10.1139/m75-035. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Plasmid distribution and evidence for a proteinase plasmid in Streptococcus lactis C2-1. Appl Microbiol. 1975 Apr;29(4):546–548. doi: 10.1128/am.29.4.546-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Simultaneous loss of proteinase- and lactose-utilizing enzyme activities in Streptococcus lactis and reversal of loss by transduction. Appl Microbiol. 1974 Sep;28(3):342–346. doi: 10.1128/am.28.3.342-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness T. A., Sandine W. E., Brown L. R. Characterization of lac+ transductants of Streptococcus lactis. Appl Microbiol. 1974 Nov;28(5):753–758. doi: 10.1128/am.28.5.753-758.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



