Abstract
A novel contact printing method utilizing a sacrificial layer of polyacrylic acid (PAA) was developed to selectively modify the upper surfaces of arrayed microstructures. The method was characterized by printing polystyrene onto SU-8 microstructures to create an improved substrate for a cell-based microarray platform. Experiments measuring cell growth SU-8 arrays modified with polystyrene and fibronectin demonstrated improved growth of NIH 3T3 (93% vs. 38%), HeLa (97% vs. 77%), and HT1080 (76% vs. 20%) cells relative to that for the previously used coating method. In addition, use of the PAA sacrificial layer permitted the printing of functionalized polystyrene, carboxylate polystyrene nanospheres, and silica nanospheres onto the arrays in a facile manner. Finally, a high concentration of extracellular matrix materials (ECM), such as collagen (5 mg/mL) and gelatin (0.1%), was contact printed onto the array structures using as little as 5 μL of the ECM reagent and without the formation of a continuous film bridge across the microstructures. Murine embryonic stem cells cultured on arrays printed with this gelatin-hydrogel remained in an undifferentiated state indicating an adequate surface gelatin layer to maintain these cells over time.
Keywords: contact printing, microfabrication, SU-8, cell culture, micropallet
Introduction
Recent advances in microfabrication technologies have created powerful and flexible experimental tools for the cell biologist [1–5]. Chief among these devices are cellular microarrays, miniaturized lab-on-a-chip platforms for culture of living cells in defined locations, that enable parallel screening of large numbers of cells in a high-throughput format [4, 6]. These platforms are proving of value for a variety of assays used in basic cell biology, stem cell research, and drug discovery. Important aspects of these miniaturized systems as a cell-culture substrate are their surface and material properties [7, 8]. It is well-known that both the chemical and mechanical properties of the surface on which cells grow have major influences on cell physiology and survival [8]. These properties include charge, hydrophobicity, elasticity, and roughness among many others; furthermore, their relative importance is often cell-type specific [7]. Thus, the ability to tailor the properties of these miniaturized devices is both pertinent and necessary for producing valid results in biological assays.
One material which is gaining favor for the microfabrication of lab-chip technologies used in biologic applications is the optically clear photoresist SU-8 [9]. This material has found widespread use for creating micron-scale structures and patterned surfaces for a variety of life science applications, including microfluidic cell analysis/culture systems [10–12], single-cell manipulation devices [13, 14], cell-based biosensors [15–18], and cell arrays [19, 20]. Although SU-8 has been shown to be biocompatible [21–23], its extremely low surface roughness, hydrophobicity and resistance to deposition of biological material make it less than ideal as a cell culture substrate [22, 24, 25]. Surface modifications have proven helpful in this regard with plasma oxidation and chemical surface treatments showing improved growth characteristics for cells cultured on SU-8 surfaces [9, 26–28]. Although these methods have proven efficacious, they impose limitations in the types of chemical surface modification that can be performed to tailor the growth surface.
In this paper, we develop a low cost, simple and flexible surface coating method to contact print a broad range of materials on the surface of arrayed SU-8 microstructures used as a cell culture substrate. The top surfaces of individual structures were printed without creating fibrous bridging or intrusion of the printed material into the interstices between structures. Both soft (hydrogel) and hard (polystyrene) layers could be added to the surface of the SU-8 structures to tailor the properties of the culture substrate. The process was also demonstrated with a series of differentially charged polymers to selectively modify the electrostatic charge of the surfaces. The various modifications were shown to improve growth characteristics of a variety of cell types on the cell array platform. Importantly, extracellular matrices could be coated onto the top surfaces of the microstructures at the high concentrations utilized in biomedical research. As an example of the value of the technique, the growth and maintenance of undifferentiated stem cells as clonal colonies was demonstrated on a cell array contact printed with a gelatin hydrogel.
Experimental
Materials and Methods
SU-8-2100 photoresist and SU-8 developer (1-methoxy-2-propyl acetate) were purchased from MicroChem Corp. (Newton, MA, USA). UVI-6976 photoinitiator (triarylsulfonium hexafluoroantimonate salts in propylene carbonate) was purchased from Dow Chemical (Torrance, CA) and poly(dimethylsiloxane) (PDMS) (Sylgard 184 silicone elastomer kit) was purchased from Dow Corning (Midland, MI). Polystyrene cell culture Petri dishes (Falcon 353001) were ordered from Fisher scientific (Pittsburgh, PA). Poly(acrylic acid) (M.W. 50,000) 25% in water was ordered from Polysciences Inc. (Warrington, PA). β-Mercaptoethanol and all organic solvents were ordered from Sigma-Aldrich (St. Louis, MO). (Heptadecafluoro-1,1,2,2-tetrahydrodecyl) trichlorosilane was from Gelest Inc. (Morrisville, PA). To prepare masks for micropallet fabrication, the patterns were first drawn using TurboCAD (IMSI/Design, LLC, Novato, CA) and then sent to Fineline Imaging (Colorado Springs, CO) for printing and fabricating the final chrome mask. Dulbecco’s Modified Eagle Medium (DMEM), RPMI 1640 medium, fetal bovine serum (FBS), bovine serum albumen (BSA), Glasgow minimum essential medium (G-MEM), ES cell qualified fetal bovine serum (FBS), MEM nonessential amino acids, L-glutamine, MEM sodium pyruvate, 1x phosphate buffered saline (PBS, ph = 7.4), 0.05% trypsin-EDTA and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA). ES cell qualified 0.1% gelatin and leukemia inhibitory factor (LIF) at 10 U/mL were purchased from Millipore (Temecula, CA). Fibronectin, collagen-I, glass microscope slides and all other reagents were obtained from Fisher Scientific (Pittsburgh, PA).
Fabrication of the arrays
Arrays composed of square pedestals of 50 or 100 μm on a side and 36 μm gap were fabricated from SU-8 in a manner similar to that described previously [29]. Each pedestal is here after referred to as a micropallet. On these arrays, every 10 × 10 region of micropallets was surrounded by numbered micropallets for ease of tracking the cultured cells. Selected arrays were silanized by vapor-phase deposition of heptadecafluoro-1,1,2,2-tetrahydrodecyl)trichlorosilane to enable creation of the heterogeneous wetted surface needed for cell localization to the micropallets as described previously [30].
Measurement of the Contact Angle of a Water Droplet
The static water contact angle on the SU-8 was measured using standard methods [31].
Polystyrene Source
Polystyrene with a near neutral charge was obtained by dissolving commercial Petri dishes in the organic solvent cyclopentanone (5% – 30% w/w). Positively-charged polystyrene was prepared by crosslinking polystyrene (in cyclopentanone 10% w/w) and 4-vinylpyridine [32]. 4-Vinylpyridine was added to the solution at ratio of 1:9 (4-vinylpyridine: polystyrene, w/w). Dibenzoyl peroxide was also added into the solution as the catalyst at a ratio of 1:100 (dibenzoyl peroxide:[4-vinylpyridine + polystyrene], w/w). The solution was sealed in an amber glass bottle and put in a 65 °C water bath for 16 hr to crosslink the 4-vinylpyridine with polystyrene. After incubation, the solution was allowed to cool to room temperature before contact printing.
Contact Printing
To contact print polystyrene on the arrays, solubilized polystyrene (1 mL) was spun onto a glass slide (1″ × 3″) to form a liquid film using a standard spin coater (WS-400-6NPP, Laurell Technologies, North Wales, PA). A spin speed of 1000 rpm was used. Spin time was 20 s unless stated otherwise. A micropallet array was placed in contact with the film for 2 sec to transfer the polystyrene to the surfaces of the micropallets. After this printing step, the array was lifted from the glass slide and baked on a hot plate at 120 °C for 1 hr to evaporate the solvent from the polystyrene layer.
A similar contact printing process was used to place a PAA sacrificial layer onto micropallet top surfaces. PAA (25% in water, w/w) was spun on a glass slide at 5000 rpm. The PAA was then contact printed on the micropallet array which was then baked at 120 °C for 5 min in a manner similar to that described for polystyrene.
Positively-charged polystyrene was spun on a glass slide at 700 rpm and contact printed as described above. Negatively-charged polystyrene (carboxylate polystyrene beads, 50 nm, 2.5% solids (w/v) aqueous suspension, Polysciences Inc., Warrington, PA, 130 μL) was spread evenly by hand on the surface of a 1″ × 3″ glass slide over a 1 cm2 area. The micropallet array was then gently contact printed on this liquid film, and allowed to dry at room temperature followed by a 2 hr bake at 120 °C.
To create a “glass-like” surface, a suspension of 50 nm silica beads (5% solids in water as provided by the manufacturer, Polysciences Inc., Warrington, PA) was used in the same process conditions as described for the carboxylate polystyrene nanospheres.
Arrays were contact printed with extracellular matrix (ECM) as follows. After the PAA sacrificial layer was removed from the array, 5 μL of collagen (5 mg/mL) or of human plasma fibronectin (1 mg/mL) was pipetted onto the glass slide (1″ × 3″) and spread over a 1 cm2 area. The array and glass slide were brought into contact as described above.
Electron microscopy
The arrays were observed using an environmental scanning electron microscope (ESEM) (Quanta 200, FEI Company, Hillsboro, OR). The ESEM was performed in low vacuum (0.75 Torr) mode and a backscattered electron detector (BSED) was chosen to take images of the microstructures.
Measurement of intrusion length
The intrusion length was defined as the distance from the micropallet top surface to the farthest extension of the polystyrene along the side wall. To measure this distance, the contact printed micropallets were viewed side-on. After contact printing, micropallets were scraped off the glass substrate using a 20 gauge hypodermic needle (BD, Franklin Lakes, NJ, USA) and imaged by brightfield microscopy. The images were analyzed with Irfanview software (www.irfanview.com) to calculate the pixel number of the intrusion length on the images. The dimension represented by the pixel number was calibrated by imaging a 100 μm standard to obtain its pixel number via Irfanview.
Laser-based micropallet release
Release of micropallets was performed as previously described [33, 34]. Briefly, a laser pulse (5 ns, 532 nm) from a Q-switch Nd:YAG laser (Minilite I, Continuum Electro-Optics Inc., Santa Clara, CA) was focused by a microscope objective at the interface of the micropallet base and the glass substrate. The focused pulse formed a plasma and cavitation bubble. The expansion of the cavitation bubble between the micropallet and glass substrate mechanically dislodged the structure [35].
Cell culture
Tumor cell lines and murine embryonic stem (ES) cell lines were employed in these studies. The adherent cell types HeLa, a human ovarian carcinoma cell line, 3T3, a murine fibroblast cell line, and HT1080, a human fibrosarcoma cell line, were employed in comparing cell survival rate on the different surfaces. Feeder-independent murine ES cells (129 strain) were obtained from the University of North Carolina-Animal Models Core Facility (UNC-AMC, Chapel Hill, NC). ES cell medium containing leukemia inhibitory factor (LIF) was prepared as previously described [34]. Before plating cells, the arrays were sterilized by immersion in 75% ethanol for 5 min. The ethanol was removed by aspiration and the arrays were allowed to dry under sterile conditions. To plate cells on the array, cells suspended in the appropriate media were added to the array chamber and allowed to settle and adhere. Arrays were pre-coated with the extracellular matrix fibronectin prior to use unless otherwise noted. Cells plated on the array were cultured in a humidified, 5% CO2 atmosphere at 37 °C.
Measurement of cell survival
Eight hours after plating the cells, the micropallet arrays were imaged by brightfield microscopy using an inverted microscope (Axiovert 135, Carl Zeiss USA, Thornwood, NY) and CCD camera (Qcolor3, Olympus America Inc., Center Valley, PA). On each array, thirty randomly chosen micropallets that contained an attached cell were identified and recorded. The arrays were returned to a humidified, 5% CO2 atmosphere at 37 °C for further culture. After an additional 60 hr, the pallets identified at the start of the experiment were re-imaged to follow colony formation, and therefore, viability over time.
RESULTS AND DISCUSSION
Characterization of contact printing polystyrene on the micropallet array
The micropallet array is a novel platform that enables individual cells or colonies grown on the array to be isolated efficiently and with high viability [19, 20]. The array is made up of microstructures termed micropallets photolithographically defined using a photoresist such as SU-8. To localize cells to the tops of the pallets, an air barrier is created between the microstructures to block cell access to the interpallet regions. Vapor-based silanization of the arrays with a hydrophobic perfluoroalkylsilane generates hydrophobic alley ways between the pallets entrapping air when the array is placed in an aqueous solution [30]. These air bubbles or virtual walls are critical in localizing cells to the top surfaces of the micropallets. The micropallets are individually removable using a pulsed laser to permit isolation of the attached cells. The arrays can be coated with extracellular matrices to improve cell adherence, but optimization of cell culture on the arrays would be enhanced if standard materials used in biomedical research, for example polystyrene, were used as the substrate for cell attachment and growth. To determine whether the top surfaces of the pallets could be coated with polystyrene, arrays (50 μm pallets, 100 μm height, 50 μm interpallet gap) were contact printed with polystyrene. A thin layer of polystyrene was spin coated onto a glass slide and the array placed on the polystyrene film (Fig. 1A). When silanized arrays were contact printed with polystyrene (5%, 10%, 20% and 30%, w/w, polystyrene/cyclopentanone), the polystyrene formed a bead on the micropallet top and did not evenly wet the silanized surface. The silanized array could not be successfully coated with polystyrene likely as a result of the different surface tensions between the silanized SU-8 pallet and the polystyrene/cyclopentanone solution.
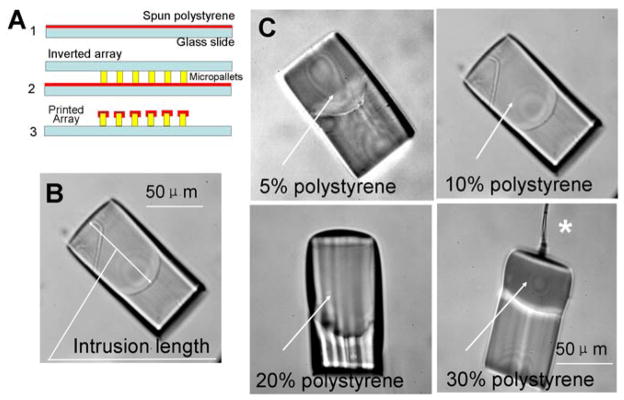
Fig. 1.
(A) Fabrication process flow for the contact printing method: 1. Spin polystyrene on glass slide, 2. Contact the micropallet array with the polystyrene film, 3. Remove the array leaving polystyrene printed on each micropallet. (B) Microscopic image of a micropallet contact printed with polystyrene and viewed side-on to demonstrate the extension of the polystyrene along the sidewall (intrusion length, see text). (C) Microscopic images of individual micropallets seen side-on after removal from an array contact printed with increasing concentrations of polystyrene. The asterisk marks a fiber of polystyrene remaining from the printing process.
Since native SU-8 is significantly less hydrophobic than silanized SU-8, non-silanized arrays were contact printed with polystyrene. After printing, the micropallets were scraped off the glass substrate using steel fine-pointed forceps and observed in side view under a microscope (Fig. 1B & C). At polystyrene concentrations of 30%, the polystyrene solution was excessively viscous and long polystyrene fibrils were created during the contact printing process. When the concentration was 20%, polystyrene could be printed on the upper surface of the micropallet, but formed a dome-shaped structure. This result was undesirable as the dome-shaped coating would interfere with plating of cells on the upper surface of the micropallets. Reduction of the polystyrene concentration to ≤10% provided a homogeneous and flat contact printed surface (Fig. 1C). For subsequent experiments, the concentration of the polystyrene used for contact printing was 10%.
To determine the role of film thickness in the percentage of coated pallets on an array, the spin-coating speed used to create the polystyrene film was varied. The percentage of micropallets that were individually coated with a homogeneous layer of polystyrene was 100% when the spin speed was in the range of 600 – 1300 rpm. At higher speeds the polystyrene layer was very thin and dried before printing could be accomplished.
Prior to use as cell arrays, the polystyrene-printed arrays were silanized and then overlaid with an aqueous solution; however, stable virtual walls did not form on these silanized polystyrene-printed arrays preventing the use of these arrays for cell separations. Close inspection of the polystyrene-printed pallets revealed that the polystyrene tracked along the sides of the pallet during the printing step (Fig. 1B). It is likely that the polystyrene coating on the sides of the pallets prevented effective silanization of the pallet side walls, and thus the side walls remained too hydrophilic to stably entrap air between the pallets. To assess whether the polystyrene tracking or intrusion along the pallet side walls could be minimized during contact printing, the following parameters were varied: film thickness (spin-coating speed), micropallet size, and interpallet gap. The distance that the polystyrene tracked along the side of the pallet was then measured (Fig. 1B). The polystyrene intrusion was independent of pallet size and inter-pallet gap and the magnitude of the intrusion length was inversely related to the spin speed used to prepare the ink for contact printing (Fig. S1A, B & C); however, the reduction of the intrusion length was not apparent until the spin speed increased to more than 1500 rpm at which point the success rate of contact printing the array with polystyrene was less than 50% (n = 20). For spin speeds from 1000-to-1400 rpm, the intrusion length was 50 μm. Since the intrusion could not be reduced significantly compared with the micropallet height, this strategy for polystyrene contact printing was unsatisfactory.
Improved contact printing process
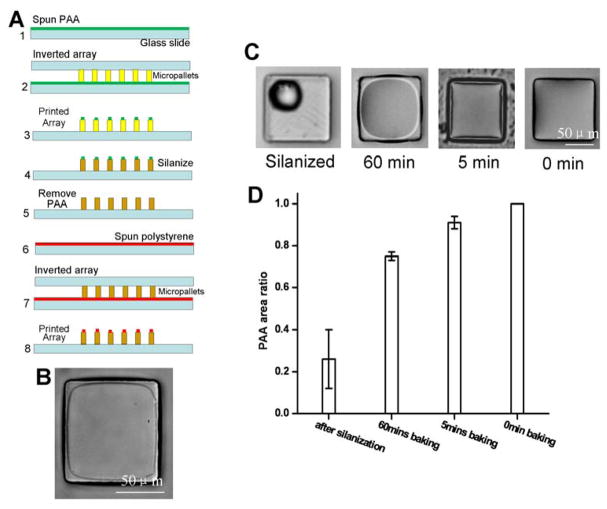
The hydrophobic silane coating was effective in preventing polystyrene wetting of the pallet side walls, but also prevented wetting of the top surface. A strategy was developed to silanize the side walls while protecting the pallet top surfaces with a sacrificial layer. Polyacrylic acid (PAA) is a water soluble polymer that has been previously reported as a sacrificial layer in microfabrication applications [36]. The modified polystyrene printing process incorporated a PAA sacrificial layer as shown in Figure 2A. In the initial step, the PAA solution was contact printed onto a native micropallet array. The extent of surface coverage of the PAA on the micropallet top surface was controllable by varying the SU-8 baking time during array fabrication (Fig. 2C & D). On freshly fabricated SU-8 arrays with no post bake, the PAA solution wetted the top surface well. As bake time increased, the PAA solution showed progressive dewetting so that a smaller area of the micropallet top surface was covered-- from 91% ± 3% after a 5 min bake to 75% ± 2% after 60 min. There was no apparent difference in wetting of the PAA solution on the pallet top surfaces when the arrays were baked 60 min or overnight. Irrespective of the bake time, there was no extension of the PAA onto the side walls of the pallets. The dependence of wetting on bake time was most likely due to changes in the surface hydrophobicity during baking. The contact angle of a water droplet on freshly fabricated arrays, arrays baked at 120 °C (5 min), arrays baked at 120 °C (60 min), and arrays baked at 120 °C (overnight) was 70° ± 1.2°, 77° ± 2.0°, 84° ± 1.3°, and 86° ± 0.6°, respectively. When the pallets were silanized, the PAA formed spherical beads on the silanized surface. This silanized surface possessed a water-droplet, contact angle of 122° ± 4.0°. Thus the more hydrophilic the pallet surface, the more extensive the surface wetting of the hydrophilic PAA solution and the area of the micropallet upper surface protected by the PAA sacrificial layer was readily controlled by the array bake time.
Fig. 2.
(A) Process flow of the contact printing method using the PAA sacrificial layer: 1. Spin PAA on glass slide, 2. Contact the micropallet array with the PAA film, 3. Remove the array leaving PAA printed on the top surface of each micropallet, 4. Silanize the PAA printed array, 5. Remove PAA, 6. Spin polystyrene on glass slide, 6. Contact the array with the polystyrene film, 7. Remove the array leaving polystyrene printed only on the top surface of the micropallets. (B) Image of the upper surface of a micropallet 10 min after contact printing with polystyrene as described in “A”. (C) Micrographs showing PAA coverage on the upper surfaces of individual micropallets under various conditions. (D) Histogram of PAA coverage area on the micropallet surface under varied conditions.
Arrays baked for 60 min and printed with PAA, were silanized. Following silanization, the arrays were incubated in 40% ethanol for 2 hrs to dissolve the PAA layer. This process yielded an array of pallets with silanized side walls, but unmodified top surfaces. Polystyrene was then contact printed on the arrays as described above. When these polystyrene-printed pallets were removed from the array and observed by microscopy, no polystyrene was observed on the pallet sidewalls. Thus, the hydrophobicity of the side walls prevented polystyrene intrusion onto the side wall and limited the polystyrene coating to the top surface of the pallet (Fig. 2B). When an aqueous solution was overlaid onto these arrays, a stable virtual air wall microscopically indistinguishable from that on standard arrays was formed.
Contact printing of charged polystyrene and nanospheres
To demonstrate the versatility of the contact printing method, negatively-charged carboxylate polystyrene nanospheres, positively-charged polystyrene, and silica nanospheres were printed onto arrays. The arrays were first contacted printed with PAA, silanized, and the PAA layer removed. Then the carboxylate polystyrene nanospheres (Fig. 4A), positively-charged polystyrene (Fig. 4B), and silica nanospheres (Fig. 4C) were printed onto the surface of the arrays. In each case the material was transferred to the surface of the pallets without coating the pallet side walls.
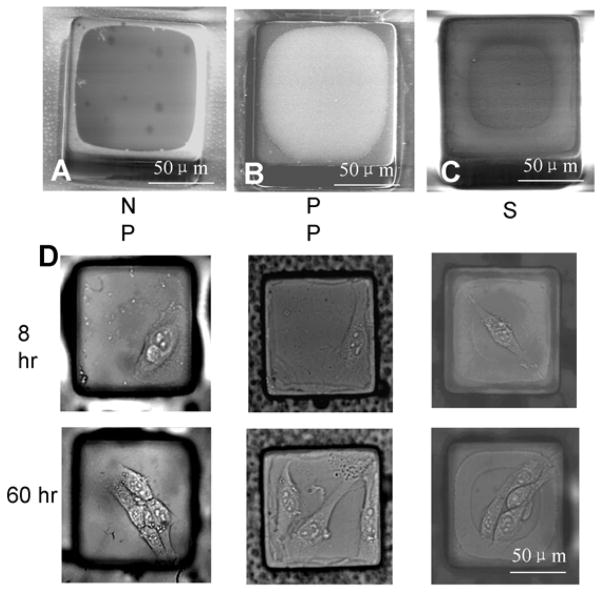
Fig. 4.
SEM images of individual micropallets contact printed with (A) 50 nm carboxylate polystyrene beads, (B) positively charged polystyrene, or (C) 50 nm silica beads using the PAA sacrificial layer method. (D) Microscopic images of 3T3 cells cultured for 8 hr and 60 hr on 50 nm carboxylate polystyrene (negatively charged polystyrene, “NP”), positively charged polystyrene (“PP”), and 50 nm silica beads (“S”) contact printed on micropallets.
Comparison of contact-printed arrays to conventionally-coated arrays
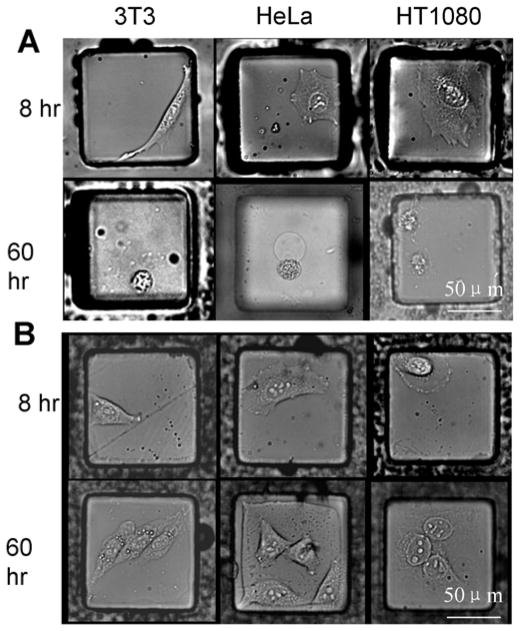
A series of experiments was performed to compare cell growth and survival on the contact-printed arrays to standard SU-8 micropallet arrays. The native SU-8 array was silanized and coated with fibronectin as described previously [30]. The contact-printed arrays were coated with polystyrene and then contact printed a second time with fibronectin (1 mg/mL). Three adherent cell types, 3T3, HeLa and HT1080, were cultured on the standard arrays and the contact-printed arrays (three arrays per experiment). For these experiments, 6,000 cells were loaded onto each array (20,000 micropallets/array) to achieve plating of ≤1 cell on the individual micropallets. It was observed that at 8 hr, the cells attached on both standard arrays as well as contact-printed arrays. After 60 hr only 38% ± 14% 3T3, 77% ± 5% HeLa, and 20% ± 14% HT1080 cells grew into colonies on the standard arrays (Fig. 3A). In contrast, colony formation was substantially higher on contact-printed arrays for 3T3 (93% ± 2%), HeLa (97% ± 3%), and HT1080 (76% ± 4%) cells (Fig. 3B). This result may be due to a shielding of the cells from the SU-8 surface by the polystyrene layer or to a higher quality layer of fibronectin deposited on the polystyrene vs. SU-8 surface.
Fig. 3.
Microscopic images of 3T3, HeLa and HT1080 cells cultured for 8 hr and 60 hr after plating on (A) SU-8 micropallets coated with fibronectin using standard methodology, and (B) micropallets contact printed with polystyrene and then fibronectin.
3T3 cells cultured on arrays printed with charged polystyrene or silica beads
To demonstrate the usage of the contact-printed charged surfaces, 3T3 cells were plated on contact-printed arrays (6,000 cells/array, 20,000 micropallets/array). Since charged polystyrene and silica are known to provide surfaces appropriate for cell attachment and growth [37–39], no ECM coating was used in these experiments. The success rate for growth of colonies was 93% ± 3% on the carboxylate polystyrene, 94% ± 4% on the positively-charged polystyrene, and 95% ± 5% on silica (Fig. 4D). Cells did not attach and grow on uncoated arrays of native SU-8 pallets as has been reported previously [9].
Stem cell culture and isolation on contact printed arrays
Gelatin is a well known ECM used to maintain cultured embryonic stem cells in an undifferentiated state. In a previous report, a very low gelatin concentration (0.025%) was required when using simple adsorption to coat micropallet arrays [34]. This low concentration was necessary to prevent a continuous film of gelatin from forming over the entire array surface as occurred when attempting to coat with a standard concentration (0.1%) of gelatin. However, the procedure resulted in a very thin layer of gelatin compared to that typically used for the growth of these cells. To test if a thicker gelatin layer could be placed on the micropallet surface, 0.1% gelatin was contact printed on an array that had been previously silanized and the sacrificial PAA layer removed. It was found that the process was able to selectively coat the upper surfaces of the individual micropallets without forming a continuous layer of gelatin across the array. Contact printing of gelatin could be performed multiple times to achieve an increasingly thick surface gelatin layer (Fig. S2 B & C).
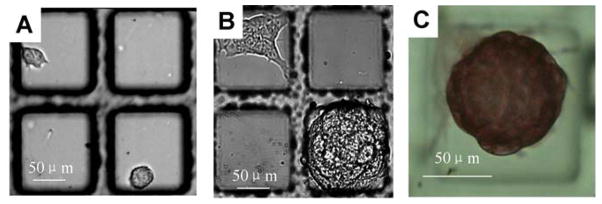
To demonstrate that the contact printed gelatin layer on the micropallet arrays provided an excellent substrate for stem cell culture, murine ES cells (8,000 cells) were plated on an array containing 20,000 micropallets printed with gelatin. After 24 hr, the media was exchanged and the array was observed under brightfield microscopy. Single ES cells were found adherent to the micropallets (Fig. 5A). The array was then cultured for an additional 72 hr with daily media exchange. At 96 hr, dome-shaped colonies consistent with undifferentiated cells were present (Fig. 5B) [40]. Occasional colonies were seen consisting of flattened, well-spread cells suggesting some degree of differentiation. To better determine the presence of undifferentiated and differentiated colonies on the array, alkaline phosphatase staining of the arrayed cells was performed (Fig. 5C) [34, 40]. In these experiments, 70% ± 12% (n = 10 colonies on each of three arrays) of the colonies remained undifferentiated. This finding is consistent with prior studies quantifying the percentages of undifferentiated and differentiated ES cell colonies grown under standard culture conditions on gelatin-coated polystyrene Petri dishes [34]. The release, collection and expansion of ES cell colonies on contact-printed micropallets was performed as described previously [34]. It was observed that of the released and collected colonies, 80% remained undifferentiated at 72 hr after collection (Fig. S3).
Fig. 5.
(A) Single ES cells cultured on 0.1% gelatin contact printed micropallets. (B) Undifferentiated and differentiated ES cell colonies on 0.1% gelatin contact printed micropallets after 96 hr in culture. (C) Alkaline phosphatase staining of an ES cell colony on an array.
Conclusion
A low cost, simple and flexible method to contact print a broad range of materials on the upper surface of arrayed microstructures has been described. Individual structures were printed without overflow of the printed material along the sidewalls of the structure or bridging of material between structures. The technique was suitable for creating a hard (polystyrene) or soft (gelatin) coatings, and could be used to establish coatings of various electrostatic charge. The utility and flexibility of the method were demonstrated by tailoring the surface properties of SU-8 microstructures for culturing cells using a variety of coatings. The modifications to the microstructures will benefit future studies in which cells with unique substrate requirements for attachment and growth are patterned on the array for analysis and sorting. Culture of primary cells and stem cells from a variety of tissues, such as respiratory epithelia or intestinal crypts may be so enhanced.
Supplementary Material
Acknowledgments
This research was supported by NIH (EB007612 and HG004843). The authors thank Chapel Hill Analytical and Nanofabrication Laboratory (CHANL) for providing access to the facility’s instrumentation. The authors also thank Dr. Yuli Wang for valuable discussions and Colleen Phillips and Jonathan Clark for technical support.
References
- 1.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. PNAS. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paguirigan AL, Beebe DJ. Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays. BioEssays. 2008;30:811–821. doi: 10.1002/bies.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez F. Biological Applications of Microfluidics. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 4.Yarmush ML, King KR. Living-cell microarrays. Annu Rev Biomed Eng. 2009;11:235–257. doi: 10.1146/annurev.bioeng.10.061807.160502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sims CE, Allbritton NL. Analysis of single mammalian cells on-chip. Lab Chip. 2007;7:423–440. doi: 10.1039/b615235j. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes TG, Diogo MM, Clark DS, Dordick JS, Cabral JMS. High-throughput cellular microarray platforms: applications in drug discovery, toxicology and stem cell research. Trends Biotechnol. 2009;27:342–349. doi: 10.1016/j.tibtech.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirone DM, Chen CS. Strategies for engineering the adhesive microenvironment. J Mammary Gland Biol Neoplasia. 2004;9:405–417. doi: 10.1007/s10911-004-1410-z. [DOI] [PubMed] [Google Scholar]
- 8.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment - implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Bachman M, Sims CE, Li GP, Allbritton NL. Simple photografting method to chemically modify and micropattern the surface of SU-8 photoresist. Langmuir. 2006;22:2719–2725. doi: 10.1021/la053188e. [DOI] [PubMed] [Google Scholar]
- 10.Dittami GM, Ayliffe HE, King CS, Rabbitt RD. A Multilayer MEMS Platform for Single-Cell Electric Impedance Spectroscopy and Electrochemical Analysis. J Microelectromech Syst. 2008;17:850–862. doi: 10.1109/JMEMS.2008.921726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakamoto Y, Inoue I, Moriguchi H, Yasuda K. Analysis of single-cell differences by use of an on-chip microculture system and optical trapping. Fresen J Anal Chem. 2001;371:276–281. doi: 10.1007/s002160100999. [DOI] [PubMed] [Google Scholar]
- 12.Wu M-H, Cai H, Xu X, Urban JPG, Cui Z-F, Cui Z. A SU-8/PDMS Hybrid Microfluidic Device with Integrated Optical Fibers for Online Monitoring. Biomed Microdevices. 2005;7:323–329. doi: 10.1007/s10544-005-6074-y. [DOI] [PubMed] [Google Scholar]
- 13.Umehara S, Wakamoto Y, Inoue I, Yasuda K. On-chip single-cell microcultivation assay for monitoring environmental effects on isolated cells. Biochem Biophys Res Commun. 2003;305:534–540. doi: 10.1016/s0006-291x(03)00794-0. [DOI] [PubMed] [Google Scholar]
- 14.Chronis N, Lee LP. Electrothermally Activated SU-8 Microgripper for Single Cell Manipulation in Solution. J Microelectromech Syst. 2004;14:857–863. [Google Scholar]
- 15.Calleja M, Tamayo J, Nordström M, Boisen A. Low-noise polymeric nanomechanical biosensors. Appl Phys Lett. 2006;88:113901–113903. [Google Scholar]
- 16.Johansson A, Calleja M, Rasmussen PA, Boisen A. SU-8 cantilever sensor system with integrated readout. Sens Actuators A Phys. 2005;123–124:111–115. [Google Scholar]
- 17.Shew BY, Kuo CH, Huang YC, Tsai YH. UV-LIGA interferometer biosensor based on the SU-8 optical waveguide. Sens Actuators A Phys. 2005;120:383–389. [Google Scholar]
- 18.Wang L, Wu Z-Z, Xu B, Zhao Y, Kisaalita WS. SU-8 microstructure for quasi-three-dimensional cell-based biosensing. Sens Actuators B Chem. 2009;140:349–355. [Google Scholar]
- 19.Wu ZZ, Zhao Y, Kisaalita WS. Interfacing SH-SY5Y human neuroblastoma cells with SU-8 microstructures. Colloids Surf B Biointerfaces. 2006;52:14–21. doi: 10.1016/j.colsurfb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Young G, Bachman M, Sims CE, Li GP, Allbritton NL. Collection and expansion of single cells and colonies released from a micropallet array. Anal Chem. 2007;79:2359–2366. doi: 10.1021/ac062180m. [DOI] [PubMed] [Google Scholar]
- 21.Kotzar G, Freas M, Abel P, Fleischman A, Roy S, Zorman C, Moran JM, Melzak J. Evaluation of MEMS materials of construction for implantable medical devices. Biomater. 2002;23:2737–2750. doi: 10.1016/s0142-9612(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 22.Voskerician G, Shive MS, Shawgo RS, von Recum H, Anderson JM, Cima MJ, Langer R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomater. 2003;24:1959–1967. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 23.Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Li Y, Cima MJ, Langer RA. BioMEMS review: MEMS technology for physiologically integrated devices. Proc IEEE. 2004;92:6–21. [Google Scholar]
- 24.Weisenberg BA, Mooradian DL. Hemocompatibility of materials used in microelectromechanical systems: platelet adhesion and morphology in vitro. J Biomed Mater Res. 2002;60:283–291. doi: 10.1002/jbm.10076. [DOI] [PubMed] [Google Scholar]
- 25.Stangegaard M, Wang Z, Kutter JP, Dufva M, Wolff A. Whole genome expression profiling using DNA microarray for determining biocompatibility of polymeric surfaces. Mol BioSyst. 2006;2:421–428. doi: 10.1039/b608239d. [DOI] [PubMed] [Google Scholar]
- 26.Hennemeyer M, Walther F, Kerstan S, Schürzinger K, AMG, Stark RW. Cell proliferation assays on plasma activated SU-8. Microelectron Engr. 2008;85:1298–1301. [Google Scholar]
- 27.Tao SL, Popat KC, Norman JJ, Desai TA. Surface modification of SU-8 for enhanced biofunctionality and nonfouling properties. Langmuir. 2008;24:2631–2636. doi: 10.1021/la703066z. [DOI] [PubMed] [Google Scholar]
- 28.Vernekar VN, Cullen DK, Fogleman N, Choi Y, Garcia AJ, Allen MG, Brewer GJ, LaPlaca MC. SU-8 2000 rendered cytocompatible for neuronal bioMEMS applications. J Biomed Mater Res A. 2009;89:138–151. doi: 10.1002/jbm.a.31839. [DOI] [PubMed] [Google Scholar]
- 29.Pai JH, Wang Y, Salazar GT, Sims CE, Bachman M, Li GP, Allbritton NL. Photoresist with low fluorescence for bioanalytical applications. Anal Chem. 2007;79:8774–8780. doi: 10.1021/ac071528q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Sims CE, Marc P, Bachman M, Li GP, Allbritton NL. Micropatterning of living cells on a heterogeneously wetted surface. Langmuir. 2006;22:8257–8262. doi: 10.1021/la061602k. [DOI] [PubMed] [Google Scholar]
- 31.Hu S, Ren X, Bachman M, Sims CE, Li GP, Allbritton NL. Tailoring the surface properties of poly(dimethylsiloxane) microfluidic devices. Langmuir. 2004;20:5569–5574. doi: 10.1021/la049974l. [DOI] [PubMed] [Google Scholar]
- 32.Stevens MP. Polymer chemistry: an introduction. Oxford University Press; 1999. [Google Scholar]
- 33.Salazar GT, Wang Y, Young G, Bachman M, Sims CE, Li GP, Allbritton NL. Micropallet arrays for the separation of single, adherent cells. Anal Chem. 2007;79:682–687. doi: 10.1021/ac0615706. [DOI] [PubMed] [Google Scholar]
- 34.Shadpour H, Sims CE, Thresher RJ, Allbritton NL. Sorting and expansion of murine embryonic stem cell colonies using micropallet arrays. Cytometry A. 2009;75:121–129. doi: 10.1002/cyto.a.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinto-Su PA, Salazar GT, Sims CE, Allbritton NL, Venugopalan V. Mechanism of Pulsed Laser Microbeam Release of SU-8 2100 Polymer Micropallets for the Collection and Separation of Adherent Cells. Anal Chem. 2008;80:4675–4679. doi: 10.1021/ac800129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linder V, Gates BD, Ryan D, Parviz BA, Whitesides GM. Water-soluble sacrificial layers for surface micromachining. Small. 2005;1:730–736. doi: 10.1002/smll.200400159. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey WS, Hertl W, Nowlan ED, Binkowski NJ. Surface treatments and cell attachment. In Vitr. 1984;20:802–808. doi: 10.1007/BF02618296. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto A, Mishima S, Maruyama N, Sumita M. Quantitative evaluation of cell attachment to glass, polystyrene, and fibronectin- or collagen-coated polystyrene by measurement of cell adhesive shear force and cell detachment energy. J Biomed Mater Res. 2000;50:114–124. doi: 10.1002/(sici)1097-4636(200005)50:2<114::aid-jbm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Steele JG, Dalton BA, Johnson G, Underwood PA. Adsorption of fibronectin and vitronectin onto Primaria and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomater. 1995;16:1057–1067. doi: 10.1016/0142-9612(95)98901-p. [DOI] [PubMed] [Google Scholar]
- 40.Heo J, Lee JS, Chu IS, Takahama Y, Thorgeirsson SS. Spontaneous differentiation of mouse embryonic stem cells in vitro: characterization by global gene expression profiles. Biochem Biophys Res Commun. 2005;332:1061–1069. doi: 10.1016/j.bbrc.2005.04.173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.