Abstract
2-deoxy-D-glucose (2DG) is known as a synthetic inhibitor of glucose. 2DG regulates various cellular responses including proliferation, apoptosis and differentiation by regulation of glucose metabolism in cancer cells. However, the effects of 2DG in normal cells, including chondrocytes, are not clear yet. We examined the effects of 2DG on dedifferentiation with a focus on the β-catenin pathway in rabbit articular chondrocytes. The rabbit articular chondrocytes were treated with 5 mM 2DG for the indicated time periods or with various concentrations of 2DG for 24 h, and the expression of type II collagen, c-jun and β-catenin was determined by Western blot, RT-PCR, immunofluorescence staining and immunohistochemical staining and reduction of sulfated proteoglycan synthesis detected by Alcain blue staining. Luciferase assay using a TCF (T cell factor)/LEF (lymphoid enhancer factor) reporter construct was used to demonstrate the transcriptional activity of β-catenin. We found that 2DG treatment caused a decrease of type II collagen expression. 2DG induced dedifferentiation was dependent on activation of β-catenin, as the 2DG stimulated accumulation of β-catenin, which is characterized by translocation of β-catenin into the nucleus determined by immunofluorescence staining and luciferase assay. Inhibition of β-catenin degradation by inhibition of glycogen synthase kinase 3-β with lithium chloride (LiCl) or inhibition of proteasome with z-Leu-Leu-Leu-CHO (MG132) accelerated the decrease of type II collagen expression in the chondrocytes. 2DG regulated the post-translational level of β-catenin whereas the transcriptional level of β-catenin was not altered. These results collectively showed that 2DG regulates dedifferentiation via β-catenin pathway in rabbit articular chondrocytes.
Keywords: cartilage, articular; cell dedifferentiation; chondrocytes; collagen type II; deoxyglucose; β-catenin
Introduction
Chondrocytes of articular cartilage develop through differentiation of mesenchymal cells during embryonic development (Barth et al., 1997; Sandell and Adler, 1999; DeLise et al., 2000). The differentiated chondrocytes can then proliferate and undergo hypertrophic maturation. Chondrocytes are composed of a dense extracellular matrix (ECM), such as collagen, fibronectin and sulfated proteoglycan (Eyre, 2002). The phenotype of the differentiated chondrocyte is distinguished by type II collagen expression and synthesis of sulfated proteoglycan including aggrecan. A sufficient number of cartilage-specific matrix molecules were required for the maintenance of homeostasis in normal articular cartilage (Poole, 1999; Sandell and Aigner, 2001). Destruction of cartilage-specific matrix molecules such as type II collagen and proteoglycan leads to arthritis by biochemical changes in chondrocytes (Charni-Ben Tabassi and Garnero, 2007; Rousseau and Delmas, 2007; Dayer et al., 2007; Henrotin et al., 2007). Therefore, synthesis and maintenance of type II collagen and proteoglycan are important for proper function of articular chondrocytes.
Glucose is the main energy source and also known as a precursor of sulfated proteoglycan of chondrocytes (Kim and Conrad, 1976; Sweeney et al., 1993). Destruction of articular cartilage is a hall marker of arthritis (Sandell and Aigner, 2001) and is associated with abnormal glucose metabolism (Dunham et al., 1989, 1992; Nahir et al., 1990). 2-deoxy-D-glucose (2DG) is a synthetic analogue of glucose that is capable of inhibiting glycolysis and glycosylation (Wick et al., 1957; Jain et al., 1985; Kaplan et al., 1990). In comparison with glucose, the 2-hydroxyl group at the second carbon is replaced by a hydrogen group in 2DG, and this change leads to inhibition of glycolysis and glycosylation. 2DG also induces stress in the endoplasmic reticulum (ER) by disturbance of cell homeostasis (Kishi et al., 2010), which causes accumulation of unfolded protein in the ER. Up to now, the effect of 2DG on the cell response was mainly investigated in a variety of cancer cells. In cancer cells, 2DG regulates cell responses such as proliferation (Halicka et al., 1995; Zhang et al., 2006) by inhibiting glycolysis and glycosylation. However, the effects of 2DG on normal cells including chondrocytes are not clear yet.
β-catenin is composed of a 130 amino acid amino-terminal domain, 12 imperfect repeats of 42 amino acids, and a carboxy-terminal domain of amino acids (Willert and Nusse, 1998). The amino terminus of β-catenin is important for stability of β-catenin, and recognized and degradaded by a ubiquitin-proteasome pathway (Munemitsu et al., 1996; Yost et al., 1996; Barth et al., 1997). The β-catenin directly binds to the adenomatous polyposis coli (APC) protein in association with axin/axil, protein phosphatase 2A (PP2A) and glycogen synthase kinase 3 β (GSK3 β). This complex results in the phosphorylation of ser 29, ser 33, ser 37, thr41 and ser 45 at the N-terminal of β-catenin (Orford et al., 1997).
β-catenin plays a crucial role in cell to cell adhesion through both cadherin and Wnt signaling and is expressed during the chondrogenesis or cartilage destruction. β-catenin plays a pivotal role in the developmental process through regulating the Wnt signaling pathway (Tufan and Tuan, 2001; Tufan et al., 2002). Wnt signaling leads to the inactivation of GSK-β kinase, which leads to the accumulation of β-catenin in the cytoplasm. β-catenin then translocates into the nucleus to increase transcription of target genes, such as those controlled by the T cell factor (TCF)/lymphoid enhancer factor (LEF) promoter. Previous data showed increased β-catenin by treatment with interleukin (IL)-1 β (Goldring et al., 1994; Demoor-Fossard et al., 2001), retinoic acid (RA) (Cash et al., 1997; Weston et al., 2000), or by monolayer cultured (Lefebvre et al., 1990; Yoon et al., 2002) and this led to dedifferentiation in chondrocytes, which could be redifferentiated by a three-dimentional cultured condition. Therefore, β-catenin is believed to be an essential component of the biological processes and maintenance of chondrocytes phenotypes. Previous studies also indicated that a variety of cytokines induce β-catenin, and that β-catenin inhibits differentiation in chondrocytes. However, the relative roles of β-catenin and glucose deprivation have not been determined. In this study, we investigated the effect of β-catenin on 2DG-regulated dedifferentiation of primary rabbit articular chondrocytes.
Results
2DG induces dedifferentiation in rabbit articular chondrocytes
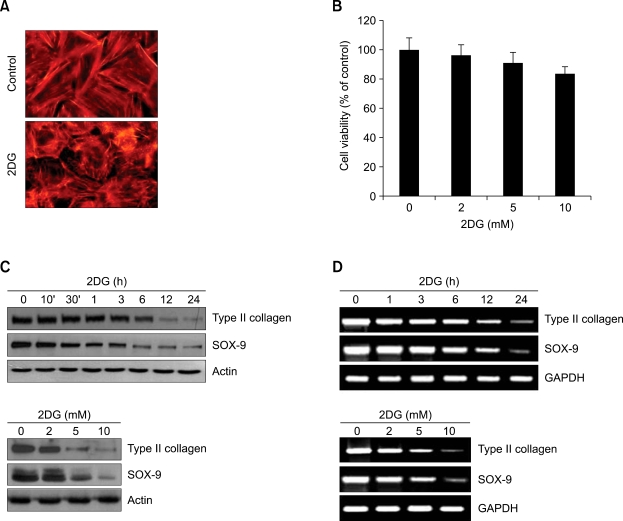
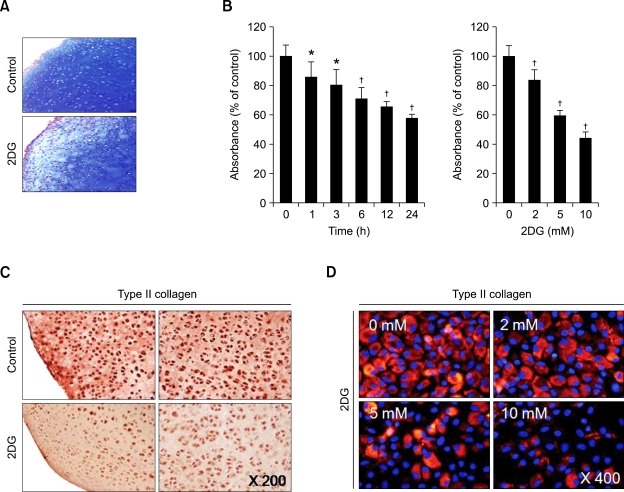
Because glucose serves as a precursor of glycosaminoglycan in chondrocytes, the supply of glucose is important for the formation of cartilage. The differentiated phenotype of chondrocytes is determined by the expression levels of the extracellular matrix, such as glucosaminoglycan and type II collagen. We first investigated the effects of 2DG on the morphology of chondrocytes. Chondrocytes were treated with 5 mM 2DG for 24 h or treated with 5 mM 2DG for the indicated time periods (Figures 1A and 1B). Treatment of 2DG was accompanied by significant changes in the actin cytoskeletal architecture. However, this reorganization of the actin cytoskeleton by 2DG did not induce cytotoxicity at concentrations up to 10 mM in the chondrocytes, as determined by the immunofluorescence staining and MTT assay (Figures 1A and 1B). Next, we tested the effect of 2DG on dedifferentiation of chondrocytes. The primary chondrocytes were treated with 5 mM 2DG for the indicated time periods or treated with specific concentrations of 2DG for the 24 h (Figures 1C and 1D). 2DG significantly decreased the expression of type II collagen (a marker for differentiation of chondrocytes) and SOX-9 (a potent activator of the chondrocyte-specific enhancer of the proalpha 1 (II) collagen gene) in a time- and dose-dependent manner, as determined by Western blot analysis and RT-PCR (Figures 1C and 1D). Consistent with the expression patterns of type II collagen, 2DG reduced production of sulfated proteoglycan in a dose- and time-dependent manner, as determined by immunohistochemical staining (Figure 2A) and Alcian blue staining (Figure 2B). Cells treated with 2DG resulted in a 40% decrease in sulfated proteoglycan production compared to the control cells (Figure 2B). As expected, 2DG treatment of cartilage explants caused a decrease of type II collagen at a dose of from 2 mM to 10 mM, as detected by immunohistochemical staining (Figure 2C) and immunofluorescence staining (Figure 2D), respectively. These results demonstrated that 2DG causes dedifferentiation of articular chondrocytes in both primary cultured cells and cartilage explants.
Figure 1.
2DG causes dedifferentiation in rabbit articular chondrocytes. (A) Rabbit articular chondrocytes were untreated or treated with 5 mM 2DG for 24 h. Cells were stained for F-actin with rhodamine-conjugated phalloidin. (B) Cells were untreated or treated with the 5 mM 2DG for the indicated time periods. Cell viability was determined by MTT assay. (C) Rabbit articular chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (upper panel) or with the specific concentrations of 2DG for 24 h (lower panel). Expressions of type II collagen and SOX-9 were analyzed by Western blot analysis. Expressions of β-actin were used as loading controls. (D) Articular chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (upper panel) or with the specific concentrations of 2DG for 24 h (lower panel). Expressions of type II collagen and SOX-9 were detected by RT-PCR. Expressions of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as loading controls. The data represent a typical experiment, whereby similar results were obtained from four independent experiments.
Figure 2.
2DG reduces sulfated proteoglycan accumulation and type II collagen expression in cartilage explants and chondrocytes. (A) Articular cartilage was untreated or treated with 5 mM 2DG for 24 h. Accumulation of sulfated proteoglycan was determined by Alcian blue staining of cross-sections of cartilage. (B) Rabbit articular chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (left panel) or with the specific concentrations of 2DG for 24 h (right panel). (C) Cartilage explants were untreated or treated with 5 mM 2DG for 24 h or with the specific concentrations of 2DG for 24 h. (D) Primary rabbit articular chondrocytes were untreated or treated with 5 mM 2DG for 24 h or with the specific concentrations of 2DG for 24 h. Cells were stained with DAPI. Type II collagen was determined by immunohistochemical staining (C) and immunofluorescence staining (D). Data are presented as results of a typical experiment (A, C, D) and as mean values with standard deviation (B) (n = 4). *, P < 0.05; †, P < 0.01 compared with untreated cells.
2DG causes β-catenin accumulation in rabbit articular chondrocytes
Previous data showed that chondrocytes were dedifferentiated by IL-1 β (Goldring et al., 1994; Demoor-Fossard et al., 2001), retinoic acid (RA) (Cash et al., 1997; Weston et al., 2000; Ryu et al., 2002) and specific culture conditions, such as a monolayer culture (Lefebvre et al., 1990; Yoon et al., 2002). On the other hand, β-catenin is up-regulated by IL-1 β, RA, and monolayer cultured cells in both primary cultured cells and explants cartilage, whereas when the dedifferented cells re-differentiated by 3-dimensional culture in alginate gel, the levels of β-catenin dramatically decreased (Ryu et al., 2002). Thus, β-catenin is a crucial modulator of the phenotypes of chondrocytes during the differentiation and dedifferentiation of chondocytes.
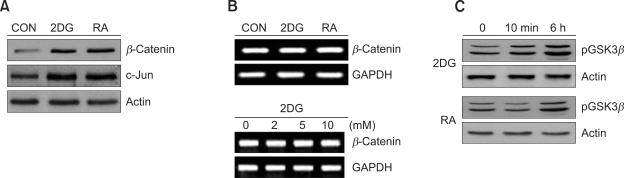
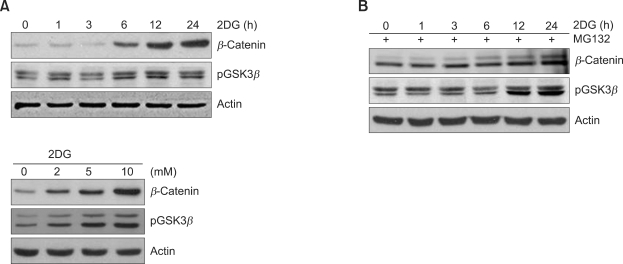
We next investigated whether changes in β-catenin expression with 2DG-induced dedifferentiation in chondrocytes. The β-catenin expression levels were significantly increased by treatment with 5 mM 2DG for the indicated time periods as determined by Western blot analysis (Figure 3A). On the other hand, the transcriptional levels of β-catenin in 2DG treated cells did not alter, as indicated by RT-PCR (Figure 3B). As mentioned above, RA treated cells showed increased β-catenin (Figure 3A), so RA was used as a positive control for our experiments. Because inactivation of GSK-3β is required for the activation of β-catenin, we examined the protein levels of phospho-GSK-3β in 2DG treated cells (Figure 3C). These results indicated that 2DG regulates β-catenin expression through the inactivation of GSK-3 in rabbit articular chondrocytes.
Figure 3.
2DG stimulates β-catenin expression in rabbit articular chondrocytes. (A) Articular chondrocytes were untreated or treated with 5 mM 2DG or with 1 µM RA for 24 h. Expressions of β-catenin, c-jun and actin were determined by Western blot analysis. (B) Primary chondrocytes were untreated or treated with 5 mM 2DG or with 1 µM RA for 24 h or with the specific concentrations of 2DG for 24 h. Expressions of β-catenin and GAPDH were detected by RT-PCR. GAPDH was used as a loading control. (C) Cells were untreated or treated with 5 mM 2DG or with 1 µM RA for the indicated time periods. Expressions of phosphorylated GSK-3β and actin were analyzed by Western blot analysis. (A, C) Expressions of Actin were used as loading controls of blots. The data represent a typical experiment, whereby similar results were obtained from four independent experiments.
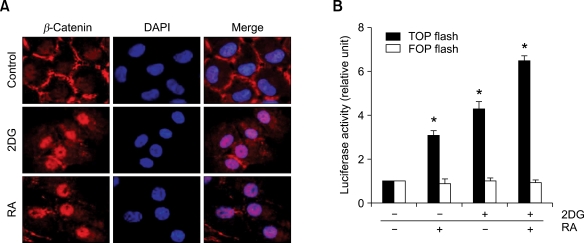
Previous studies showed that most β-catenins are localized in cell-to-cell contacts and RA remarkably increased levels of β-catenin in the nuclear fraction. Therefore, we examined the localization of β-catenin in chondrocytes. As shown in Figure 4, consistent with the treatment of RA, 2DG-treated cells revealed β-catenin distribution mainly distributed in the nucleus, which was demonstrated by immunocytochemical staining (Figure 4A). To establish whether the β-catenin-TCF/LEF complex induces transcriptional activity via translocation of β-catenin, we next proceeded to examine TCF/LEF reporter gene activity using TOPflash (optimal TCF/LEF-binding site) and FOPflash (mutanted TCF/LEF-binding site) (Figure 4A). This reporter gene assay showed a transcriptional activity of β-catenin-TCF/LEF transcriptional activity. Immunofluorescence staining and luciferase activity assay showed significantly increased levels of β-catenin and TCF/LEF promoter activity in 2DG-treated cells (Figures 4A and 4B).
Figure 4.
2DG increases nuclear localization of β-catenin in rabbit articular chondrocytes. (A) Articular chondrocytes were untreated or treated with 5 mM 2DG, 1 µM RA, and 5 mM 2DG in the presence of 1 µM RA for 24 h. Expressions of β-catenin and 4,6-diamidini-2-phenylindole (DAPI) were double stained and analyzed by immunofluorescence microscopy. (B) Chondrocytes were transfected with active (TOPFlash) or inactive (FOPFlash) TCF/LEF reporter gene for β-catenin and untreated or treated with 5 mM 2DG or with 1 µM RA or with 5 mM 2DG in the presence of 1 µM RA for 24 h. The TCF/LEF reporter activity was detected using luminometer. Data are presented as results of a typical experiment (A) and as mean values with standard deviation (B) (n = 4). *, P < 0.01 compared with untreated cells.
We hypothesized that β-catenin might play a crucial role in 2DG-induced dedifferentiation in chondrocytes. To confirm this possibility, we examined the expression level of β-catenin in cells treated with MG132. As shown in Figure 5. The β-catenin expression by 2DG caused increase in a dose- and time-dependent manner, as determined by Western blot analysis (Figure 5A). As expected, there was inhibition of β-catenin degradation via inhibition of ubiquitin proteasome with MG132-induced accumulation of β-catenin in chondrocytes (Figure 5B).
Figure 5.
2DG induces β-catenin expression via pGSK3β in rabbit articular chondrocytes. (A) Articular chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (upper panel) or with the specific concentrations of 2DG for 24 h (lower panel). Expressions of β-catenin, phosphorylated GSK-3β and actin were detected by Western blot analysis. (B) Chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods in the presence of 40 µM proteasome inhibitor Z-Leu-Leu-Leu-al (MG132). Expressions of β-catenin, phosphorylated GSK-3β and actin were detected by Western blot analysis. Data are presented as results of a typical experiment.
2DG regulates dedifferentiation through β-catenin accumulation in rabbit articular chondrocytes
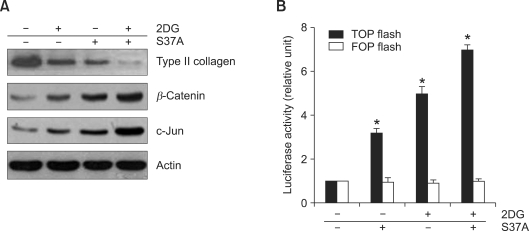
Next, we investigated the effect of LiCl, inhibitor of GSK-3β, on 2DG-induced dedifferentiation of chondrocytes. As shown in Figure 6A, Western blot analysis revealed that LiCl treatment leads to accumulation of β-catenin and a reduction of type II collagen expression. Phosphorylated c-jun was also markedly increased by LiCl treatment, which is similar to what Ryu et al. reported. In addition, MG132, a blocker of β-catenin degradation by the inhibition of 26S proteasome, also resulted in an increase of β-catenin and c-jun, and a decrease of type II collagen expression (Figure 6A). Treatment of LiCl or MG132 also caused enhanced expression of β-catenin and reduced expression of type II collagen, as determined by luciferase assay (Figure 6B). These results indicate that 2DG causes increased expression of β-catenin. Next we examined whether the accumulation of β-catenin regulates 2DG-induced dedifferentiation by using S37A β-catenin (a stable non-ubiquitinatable form of β-catenin). Consistent with the results shown in Figure 6, we found that ectopic expression of S37A β-catenin accelerated a reduction of type II collagen of chondrocytes (Figure 7A). Consistent with the expression levels of β-catenin, c-jun, known as a β-catenin target gene, increased as demonstrated by Western blot analysis (Figure 7A). Transfection of S37A β-catenin dramatically increased TCF/LEF activity (Figure 7B). These results revealed that 2DG induced dedifferentiation by translocation of β-catenin into the nucleus.
Figure 6.
Accumulation of β-catenin induces dedifferentiation in rabbit articular chondrocytes. (A) Chondrocytes were untreated or treated with 5 mM 2DG for 24 h in the presence of 10 mM lithium chloride (LiCl) or 40 µM proteasomal inhibitor Z-Leu-Leu-Leu-CHO (MG132). Expressions of type II collagen, β-catenin, c-jun and actin were detected by Western blot analysis. Actin was used as a loading control. (B) Chondrocytes were transfected with active (TOPFlash) or inactive (FOPFlash) TCF/LEF reporter gene and then treated with the vehicle alone as a control (con), 5 mM 2DG or 5 mM 2DG with 10 mM LiCl or 40 µM MG132, and TCF/LEF reporter activity was monitored by luminometer. Data are presented as results of a typical experiment (A) and as mean values with standard deviation (B) (n = 4). *, P < 0.01 compared with untreated cells.
Figure 7.
Ectopic expression of S37A β-catenin causes phenotype loss of chondrocytes. (A) Chondrocytes were transfected with empty vector or S37A β-catenin for 6 h, and treated with the vehicle alone as a control (con) or 5 mM 2DG for 24 h. After 24 h incubation, expressions of type II collagen, β-catenin, c-Jun and β-actin were detected by Western blot analysis. Actin was used as loading control. (B) Chondrocytes were transfected with active (TOPFlash) or transfected with inactive (FOPFlash) TCF/LEF reporter gene and S37A β-catenin, and treated with the vehicle alone as a control or with 5 mM 2DG, and TCF/LEF reporter activity was monitored by luminometer. Data are presented as results of a typical experiment (A) and as mean values with standard deviation (B) (n = 4). *, P < 0.01 compared with untreated cells.
Dedifferentiation by 2DG occurs via β-catenin accumulation, not by ER-stress
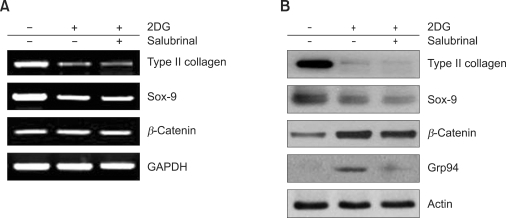
Because our current and previous findings indicated that 2DG caused endoplasmic reticulum stress (ER-stress) response in articular chondrocytes, we next examined the relationship between 2DG-induced dedifferentiation and ER stress. The level of GRP94 (an ER stress inducible gene) was expressed during the up-regulation of β-catenin and down-regulation of type II collagen expression by 2DG. The addition of salubrinal (an inhibitor of ER stress) to the 2DG treated chondrocytes inhibited GRP94 expression. However, expression of β-catenin and type II collagen did not appear to change (Figure 8). These results indicate that 2DG-induced dedifferentiation is regulated by the β-catenin pathway but not by an ER stress response.
Figure 8.
Dedifferentiation by 2DG occurs through β-catenin accumulation in rabbit articular chondrocytes. (A) Chondrocytes were untreated or treated with 5 mM 2DG for 24 h in the presence of 20 µM ER-stress inhibitor salubrinal. Expressions of type II collagen, SOX-9, β-catenin and GAPDH were determined by RT-PCR. GAPDH was used as a loading control. (B) Primary chondrocytes were untreated or treated with 5 mM 2DG for 24 h in the presence of 20 µM salubrinal, ER-stress inhibitor. Expressions of type II collagen, SOX-9, β-catenin, Grp94 and β-actin were determined by Western blot analysis. Data are presented as results of a typical experiment.
Discussion
2DG serves as a competitive inhibitor of glucose. In cancer cells, 2DG regulates cell responses such as proliferation and apoptosis by inhibiting glycolysis and glycosylation (Wick et al., 1957; Jain et al., 1985; Kaplan et al., 1990). Cartilage is a unique tissue, since it functions under normal conditions and uses glucose as the main energy substrate and main precursor for glycosaminoglycan synthesis (Kim and Conrad, 1976; Sweeney et al., 1993; Cho et al., 2003). Despite the importance of an appropriate glucose supply in cartilage homeostasis, the effects and the mechanisms underlying 2DG on various responses, such as differentiation of articular chondrocytes, are still poorly understood. Cartilage and chondrocytes were destroyed under effects of mechanical stress and non-mechanical stress (Kaarniranta et al., 1998; Ragan et al., 2000; Salter et al., 2001; Fermor et al., 2002; Sironen et al., 2002; Oliver et al., 2005). ER stress is involved in various diseases including autoimmune disease such as rheumatoid arthritis and neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (Purcell et al., 2003; Forman et al., 2003; Gow and Sharma, 2003; Zhao et al., 2005; Paschen and Mengesdorf, 2005). Oliver et al (2005) showed that ER stress by tunicamycin decreased aggrecan but not type II collagen expression in the C-28/I2 chondrocytes cell line. Downregulation of β-catenin expression is a critical key for chondrogenic differentiation from mesenchymal cells and a decrease of β-catenin is necessary for phenotypes maintenance of differentiated chondrocytes (Ryu et al., 2002). Yoon et al. (2002) suggested that phorbol 12-myristate 13-acetate (PMA) inhibited chondrogenesis via a decrease of β-catenin in chondrifying mesenchimal cells. Also, Blom et al. (2009) reported that β-catenin was upregulation in osteiarthritic mice. These previous findings suggested that β-catenin might be regulated in dedifferentiated chondrocytes by 2DG. The results of this study show that 2DG causes dedifferentiation through β-catenin in rabbit articular chondrocytes. Ryu et al. (2002) reported that stimulators such as IL-1β and RA caused dedifferentiation via β-catenin in rabbit articular chondrocytes. Consistent with the above report, in the present study, β-catenin is up regulated at protein level during dedifferentiation by 2DG but not at the transcriptional level. These results suggest that increased β-catenin may be related to dedifferentiation by 2DG in chondrocytes. Therefore, in this study, we focused on the β-catenin pathway and its regulation mechanism by 2DG. Our findings showed that 2DG-induced dedifferentiation in a time- and dose-dependent manner, as demonstrated by the reduction of sulfated proteoglycan accumulation and type II collagen expression. Previous studies have demonstrated that stimulators such as IL-1β-(Goldring et al., 1994; Demoor-Fossard et al., 2001) and RA-induced (Cash et al., 1997; Weston et al., 2000) β-catenin expression in chondrocytes is mediated by decreased activities of GSK3-β. Therefore, we searched whether the phospho-GSK3-β level increased in 2DG treated cells. Our results showed that β-catenin expression and phospho-GSK3-β expression was upregulated by 2DG synchronously with the downregulation of type II collagen. β-catenin expression by post-translational modification was induced by inhibition of GSK3-β with LiCl or 26S proteasome with MG132. Increased β-catenin significantly downregulated type II collagen and sulfated proteoglycan accumulation. Next, we examined the effect of S37A β-catenin, a stable non-ubiquitinatable form of β-catenin, in chondrocytes. Transfection of S37A β-catenin led to the same results. These results were confirmed by transfecting the TOPflash and FOPflash plasmid, the promoter region which contains binding sites for the β-catenin transcription factor. Then we investigated whether the increased activities of the TCF/LEF promoter resulted from the positive effect of 2DG on β-catenin expression. 2DG also caused endoplasmic reticulum (ER) stress in cells (Harding et al., 2002; Zhang and Kaufman, 2004; Schroder and Kaufman, 2005), which suggests that 2DG may induce dedifferentiation as part of the ER stress response of the chondrocytes. Next we examined whether dedifferentiation was regulated by 2DG-induced ER stress. As shown in Figure 8, the expression of grp94, an ER stress marker protein, was increased by 2DG treatment. We also observed that 2DG-induced grp94 expression was blocked with salubrinal, an inhibitor of ER stress, in chondrocytes. However, the expression of type II collagen and SOX-9 did not change by salubrinal treatment. This suggests that dedifferentiation by 2DG is regulated by the β-catenin pathway but not by the ER stress pathway. In summary, our result collectively indicates that 2DG regulates dedifferentiation via the β-catenin pathway in rabbit articular chondrocytes.
Methods
Culture of primary chondrocytes and experimental conditions
Rabbit articular chondrocytes were isolated from joint cartilage of 2-week-old New Zealand White rabbits by enzymatic digestion as described previously. Briefly, cartilage slices were dissociated enzymatically for 6 h in 0.2% collagenase type II (381 units/ml of solid, Sigma, St.Louis, MO) in Dulbecco's modified Eagle's medium (DMEM; Invitrogen). After collecting individual cells by brief centrifugation, the cells were suspended in DMEM supplemented with 10% (v/v) bovine calf serum, 50 µg/ml streptomycin, and 50 units/ml penicillin and were then plated on culture dishes at a density of 5 × 104 cells/cm2. The medium was changed every 2 days after seeding. After 3 days in culture, the medium was changed with glucose-free medium and cells were treated with the indicated pharmacological reagents.
Western blot analysis
For Western blot analysis, whole cell lysates were prepared by extracting protein using a buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% SDS supplemented with protease inhibitors and phosphatase inhibitors. Proteins were size-fractionated by SDS-PAGE and transferred to a nitrocellulose membrane. The nitrocellulose sheet was blocked with 5% non-fat dry milk in Tris-buffered saline. The following antibodies were used: anti-type II collagen (santa cruz, CA), anti-SOX-9 (santacruz, CA), anti-β-catenin (santacruz, CA), anti-pGSK3-β (Cell Signaling, MA), anti-c-jun (santacruz, CA), anti-GRP94 (santacruz, CA), and anti-β-actin (santacruz, CA). Blots were developed using a peroxidase-conjugated secondary antibody and a chemiluminescence system.
Reverse transcriptation polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIZOL (Life Technology, MD) according to the manufacturer's protocol. The following primers and conditions were used for PCR in rabbit articular chondrocytes : for type II collagen (370-bp product), 5'-GACCCCATGCAGTACATGCG-3' (sense) and 5'-AGCCGCCATTGATGGTCTCC-3' (antisnese) with an annealing temperature of 52℃; for SOX-9 (386-bp product), 5'-GCGCGTGCAGCACAAGAAGGACCACCCGGATTACAAGTAC-3' (sense) and 5'-CGAAGGTCTCGATGTTGGAGATGACGTCGCTGCTCAGCTC-3' (antisense) with an annealing temperature of 60℃; for β-catenin (480-bp product), 5'-GAAAATCCAGAGTGGACAATGGCTACT-3' (sense) and 5'-ACCATAACTGCAGCCTTAACC-3' (antisense) with an annealing temperature of 52℃; for glyceraldehydes 3-phosphate dehydrogenase (GAPDH; 299-bp product), 5'-TCACCATCTTCCAGGAGCGA-3' (sense) and 5'-CACAATGCCGAAGTGGTCGT-3' (antisense) with an annealing temperature of 50℃.
Alcian blue staining
The cells were fixed with 95% methanol at -20℃ for 2 min and stained with 0.1% Alcian blue in 0.1 M HCl overnight. The chondrocytes were washed three times with PBS buffer and 6 M guanidine HCl was added for 6 h. Production of sulfated proteoglycan was measured at 620 nm by Enzyme-Linked Immunosorbent Assay (ELISA).
Immunofluorescence staining
Rabbit chondrocytes were fixed in 3.5% paraformaldehyde in PBS for 15 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS. The fixed cells were washed three times with PBS and incubated with for 2 h with antibodies against type II collagen, β-catenin or β-actin. The cells were washed and incubated with secondary antibodies for 1 h, washed with PBS, and observed under a fluorescence microscope.
Immunohistochemical staining
The cartilage explants (125 mm3) were fixed in 4% paraformaldehyde in PBS for 24 h at 4℃, dehydrated with graded ethanol, embedded in paraffin, and sectioned into 4 µm slices as described previously. The sections were stained by standard procedures using Alcian blue or antibody against type II collagen, and visualized by developing with a kit purchased from DAKO (Carpinteria, CA).
Transfection and reporter gene assays
Retroviral vector (5 µg) containing cDNA for S37A β-catenin was transfected to chondrocytes using CarriGene reagent (Kinovate Life Sciences, CA), following the procedure recommended by the manufacturer. The transfected cells, which were cultured in complete medium for 24 h, were used in further assay as indicated in each experiment. To investigate β-catenin-TCF/LEF signaling, cells were transiently transfected with 1 µg TCF/LEF reporters, TOPFlash (optimal LEF-binding site) or FOPFlash (mutated LEF-binding site) (Upstate Biotech., Lake Placid, NY), and 1 µg pCMV-β-galactosidase. Following incubation with the indicated pharmacological reagents for 48 h, luciferase activity was measured and normalized for transfection efficiency using β-galactosidase activity.
Determination of cell viability
Chondrocytes were seeded on 96 well plates at 2 × 104 cells/well. After incubation with the specific concentrations of 2DG (Sigma, Steinheim, Germany), cell viablity (ATCC; LCG Promochem, Zurich, Switzerland) was performed according to the manufacturer's instructions.
Data analysis and statistics
The results are represented as the mean SD and compared using one-factor ANOVA for comparing individual treatments with their respective control values.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST)(2009-0071662, 2010-0003239 & 2009-0084569).
Abbreviations
- 2DG
2-deoxy-D-glucose
References
- 1.Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 3.Cash DE, Bock CB, Schughart K, Linney E, Underhill TM. Retinoic acid receptor alpha function in vertebrate limb skeletogenesis: a modulator of chondrogenesis. J Cell Biol. 1997;136:445–457. doi: 10.1083/jcb.136.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charni-Ben Tabassi N, Garnero P. Monitoring cartilage turnover. Curr Rheumatol Rep. 2007;9:16–24. doi: 10.1007/s11926-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 5.Cho SH, Oh CD, Kim SJ, Kim IC, Chun JS. Retinoic acid inhibits chondrogenesis of mesenchymal cells by sustaining expression of N-cadherin and its associated proteins. J Cell Biochem. 2003;89:837–847. doi: 10.1002/jcb.10553. [DOI] [PubMed] [Google Scholar]
- 6.Dayer E, Dayer JM, Roux-Lombard P. Primer: the practical use of biological markers of rheumatic and systemic inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:512–520. doi: 10.1038/ncprheum0572. [DOI] [PubMed] [Google Scholar]
- 7.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 8.Demoor-Fossard M, Galera P, Santra M, Iozzo RV, Pujol JP, Redini F. A composite element binding the vitamin D receptor and the retinoic X receptor alpha mediates the transforming growth factor-beta inhibition of decorin gene expression in articular chondrocytes. J Biol Chem. 2001;276:36983–36992. doi: 10.1074/jbc.M011442200. [DOI] [PubMed] [Google Scholar]
- 9.Dunham J, Chambers MG, Jasani MK, Bitensky L, Chayen J. Quantitative criteria for evaluating the early development of osteoarthritis and the effect of diclofenac sodium. Agents Actions. 1989;28:93–97. doi: 10.1007/BF02022987. [DOI] [PubMed] [Google Scholar]
- 10.Dunham J, Hoedt-Schmidt S, Kalbhen DA. Structural and metabolic changes in articular cartilage induced by iodoacetate. Int J Exp Pathol. 1992;73:455–464. [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002;10:792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 13.Forman MS, Lee VM, Trojanowski JQ. 'Unfolding' pathways in neurodegenerative disease. Trends Neurosci. 2003;26:407–410. doi: 10.1016/S0166-2236(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 14.Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, Arbiser JL, Apperley JF. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gow A, Sharma R. The unfolded protein response in protein aggregating diseases. Neuromolecular Med. 2003;4:73–94. doi: 10.1385/NMM:4:1-2:73. [DOI] [PubMed] [Google Scholar]
- 16.Halicka HD, Ardelt B, Li X, Melamed MM, Darzynkiewicz Z. 2-Deoxy-D-glucose enhances sensitivity of human histiocytic lymphoma U937 cells to apoptosis induced by tumor necrosis factor. Cancer Res. 1995;55:444–449. [PubMed] [Google Scholar]
- 17.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 18.Henrotin Y, Addison S, Kraus V, Deberg M. Type II collagen markers in osteoarthritis: what do they indicate? Curr Opin Rheumatol. 2007;19:444–450. doi: 10.1097/BOR.0b013e32829fb3b5. [DOI] [PubMed] [Google Scholar]
- 19.Jain VK, Kalia VK, Sharma R, Maharajan V, Menon M. Effects of 2-deoxy-D-glucose on glycolysis, proliferation kinetics and radiation response of human cancer cells. Int J Radiat Oncol Biol Phys. 1985;11:943–950. doi: 10.1016/0360-3016(85)90117-8. [DOI] [PubMed] [Google Scholar]
- 20.Kaarniranta K, Elo M, Sironen R, Lammi MJ, Goldring MB, Eriksson JE, Sistonen L, Helminen HJ. Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation. Proc Natl Acad Sci USA. 1998;95:2319–2324. doi: 10.1073/pnas.95.5.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan O, Navon G, Lyon RC, Faustino PJ, Straka EJ, Cohen JS. Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 1990;50:544–551. [PubMed] [Google Scholar]
- 22.Kim JJ, Conrad HE. Kinetics of mucopolysaccharide and glycoprotein synthesis by chick embryo chondrocytes. Effect of D-glucose concentration in the culture medium. J Biol Chem. 1976;251:6210–6217. [PubMed] [Google Scholar]
- 23.Kishi S, Shimoke K, Nakatani Y, Shimada T, Okumura N, Nagai K, Shin-Ya K, Ikeuchi T. Nerve growth factor attenuates 2-deoxy-D-glucose-triggered endoplasmic reticulum stress-mediated apoptosis via enhanced expression of GRP78. Neurosci Res. 2010;66:14–21. doi: 10.1016/j.neures.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre V, Peeters-Joris C, Vaes G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim Biophys Acta. 1990;1051:266–275. doi: 10.1016/0167-4889(90)90132-w. [DOI] [PubMed] [Google Scholar]
- 25.Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahir AM, Vitis N, Silbermann M. Cellular enzymatic activities in the articular cartilage of osteoarthritic and osteoporotic hip joints of humans: a quantitative cytochemical study. Aging (Milano) 1990;2:363–369. doi: 10.1007/BF03323952. [DOI] [PubMed] [Google Scholar]
- 27.Oliver BL, Cronin CG, Zhang-Benoit Y, Goldring MB, Tanzer ML. Divergent stress responses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. J Cell Physiol. 2005;204:45–50. doi: 10.1002/jcp.20261. [DOI] [PubMed] [Google Scholar]
- 28.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 29.Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D662–D670. doi: 10.2741/poole. [DOI] [PubMed] [Google Scholar]
- 31.Purcell AW, Todd A, Kinoshita G, Lynch TA, Keech CL, Gething MJ, Gordon TP. Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity? Clin Exp Immunol. 2003;132:193–200. doi: 10.1046/j.1365-2249.2003.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragan PM, Chin VI, Hung HH, Masuda K, Thonar EJ, Arner EC, Grodzinsky AJ, Sandy JD. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys. 2000;383:256–264. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 33.Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:346–356. doi: 10.1038/ncprheum0508. [DOI] [PubMed] [Google Scholar]
- 34.Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG, Chun CH, Oh SH, Seong JK, Huh TL, Chun JS. Regulation of the chondrocyte phenotype by beta-catenin. Development. 2002;129:5541–5550. doi: 10.1242/dev.129.23.5541. [DOI] [PubMed] [Google Scholar]
- 35.Salter DM, Millward-Sadler SJ, Nuki G, Wright MO. Integrin-interleukin-4 mechanotransduction pathways in human chondrocytes. Clin Orthop Relat Res. 2001:S49–S60. doi: 10.1097/00003086-200110001-00006. [DOI] [PubMed] [Google Scholar]
- 36.Sandell LJ, Adler P. Developmental patterns of cartilage. Front Biosci. 1999;4:D731–D742. doi: 10.2741/sandell. [DOI] [PubMed] [Google Scholar]
- 37.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 39.Sironen RK, Karjalainen HM, Elo MA, Kaarniranta K, Torronen K, Takigawa M, Helminen HJ, Lammi MJ. cDNA array reveals mechanosensitive genes in chondrocytic cells under hydrostatic pressure. Biochim Biophys Acta. 2002;1591:45–54. doi: 10.1016/s0167-4889(02)00247-1. [DOI] [PubMed] [Google Scholar]
- 40.Sweeney C, Mackintosh D, Mason RM. UDP-sugar metabolism in Swarm rat chondrosarcoma chondrocytes. Biochem J. 1993;290(Pt 2):563–570. doi: 10.1042/bj2900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J. 2001;15:1436–1438. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- 42.Tufan AC, Daumer KM, DeLise AM, Tuan RS. AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res. 2002;273:197–203. doi: 10.1006/excr.2001.5448. [DOI] [PubMed] [Google Scholar]
- 43.Weston AD, Rosen V, Chandraratna RA, Underhill TM. Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J Cell Biol. 2000;148:679–690. doi: 10.1083/jcb.148.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224:963–969. [PubMed] [Google Scholar]
- 45.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 46.Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem. 2002;277:8412–8420. doi: 10.1074/jbc.M110608200. [DOI] [PubMed] [Google Scholar]
- 47.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 48.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P, Schwartz L, Icard P. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26:3561–3566. [PubMed] [Google Scholar]
- 50.Zhao L, Longo-Guess C, Harris BS, Lee JW, Ackerman SL. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet. 2005;37:974–979. doi: 10.1038/ng1620. [DOI] [PubMed] [Google Scholar]