Abstract
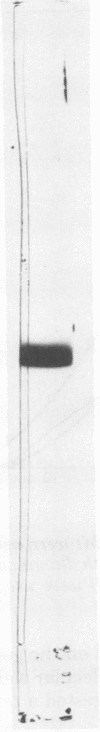
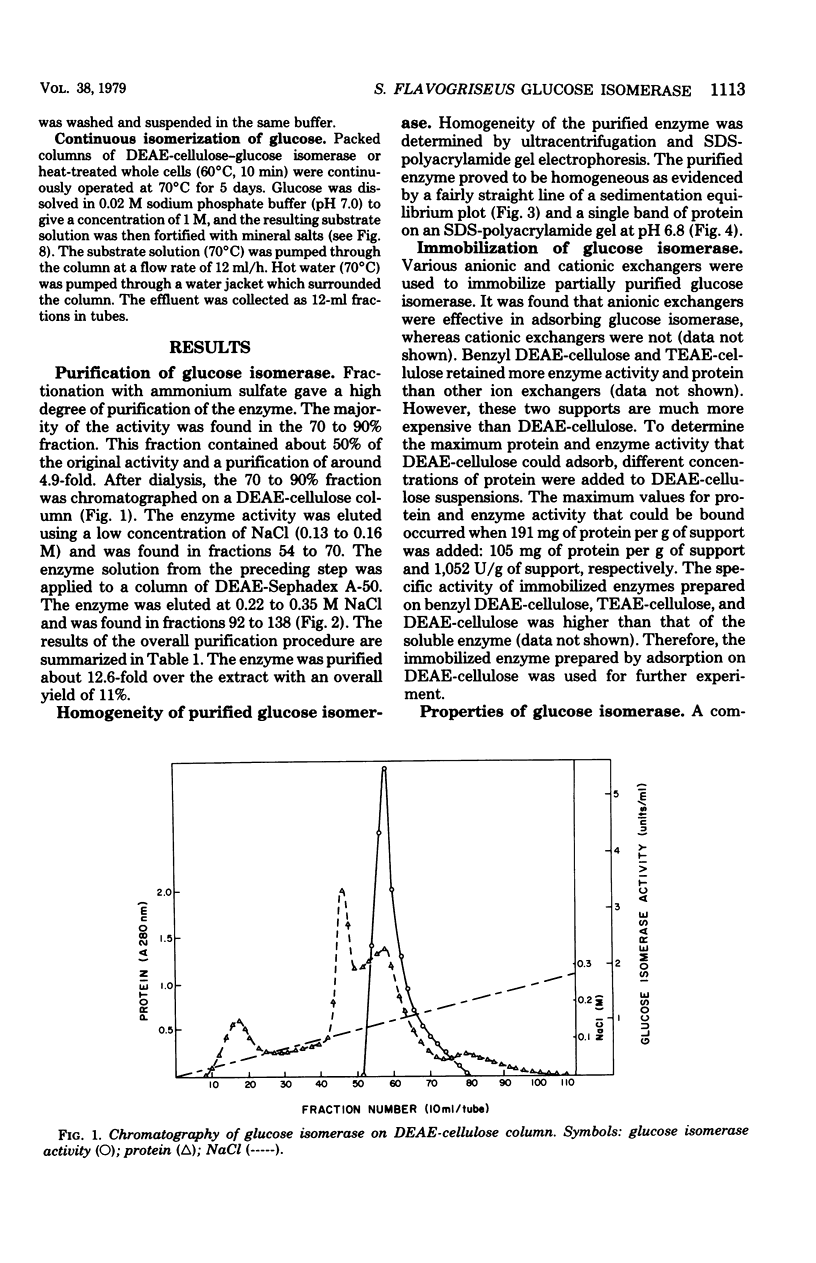
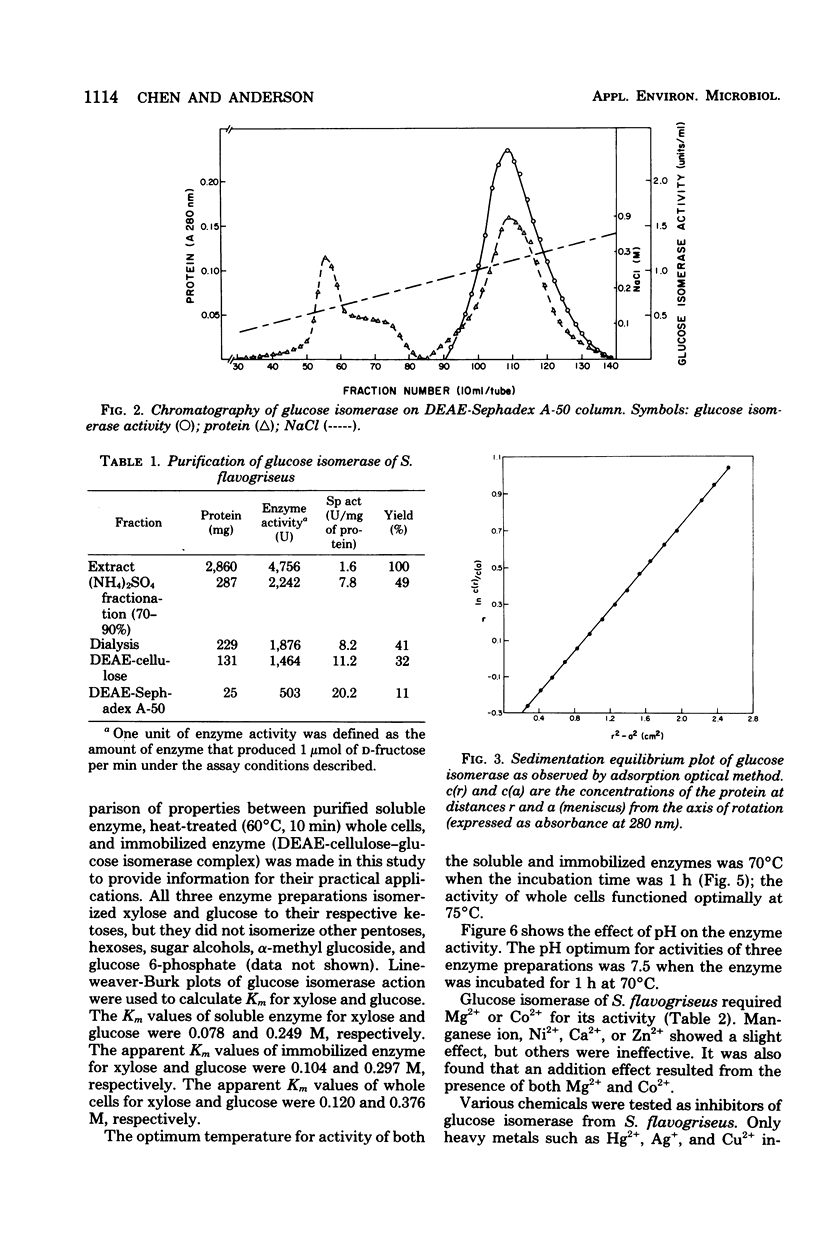
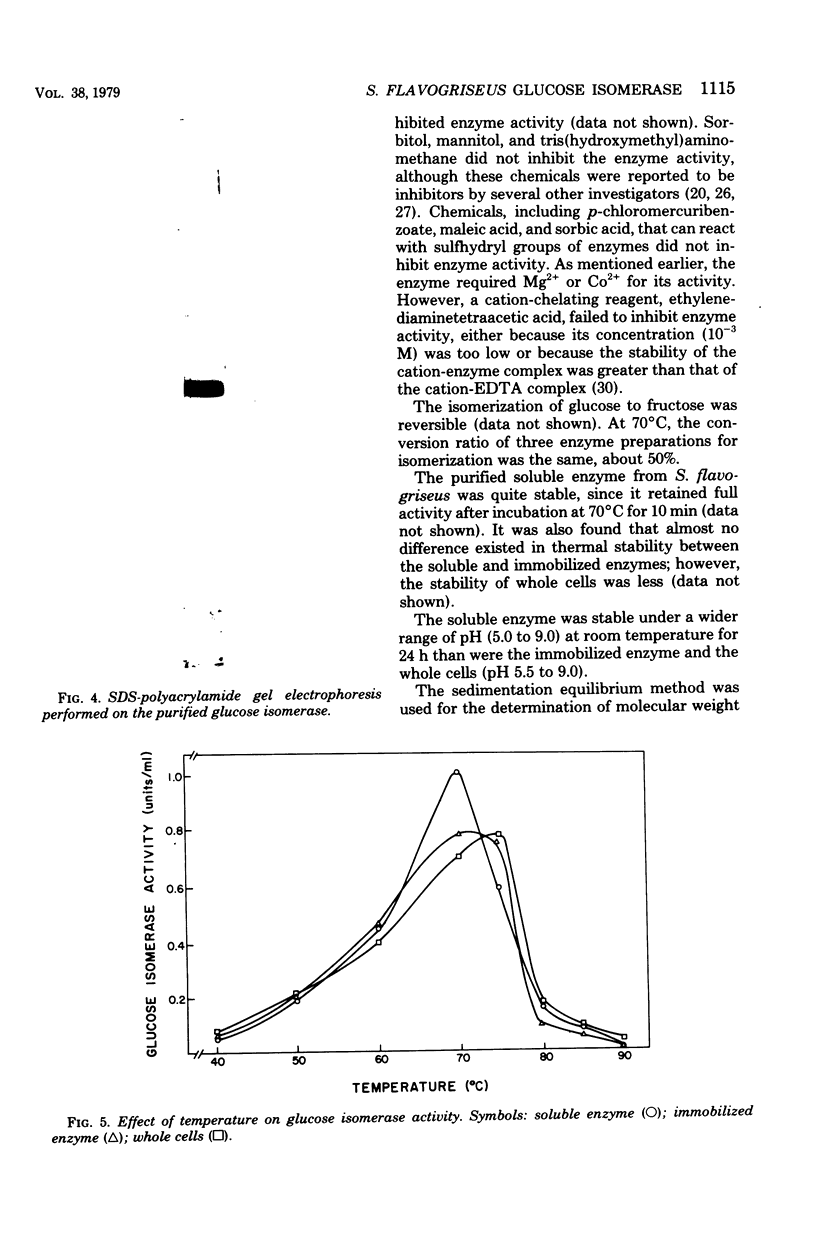
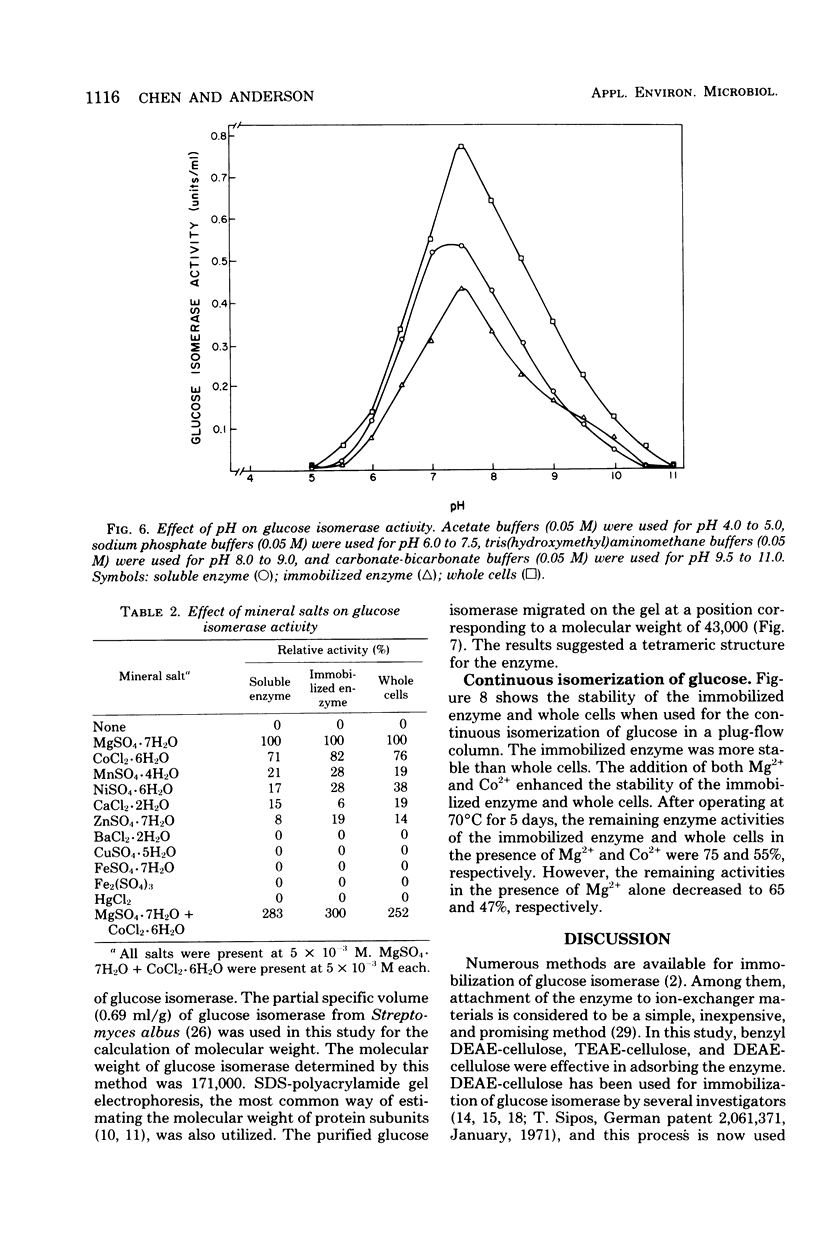
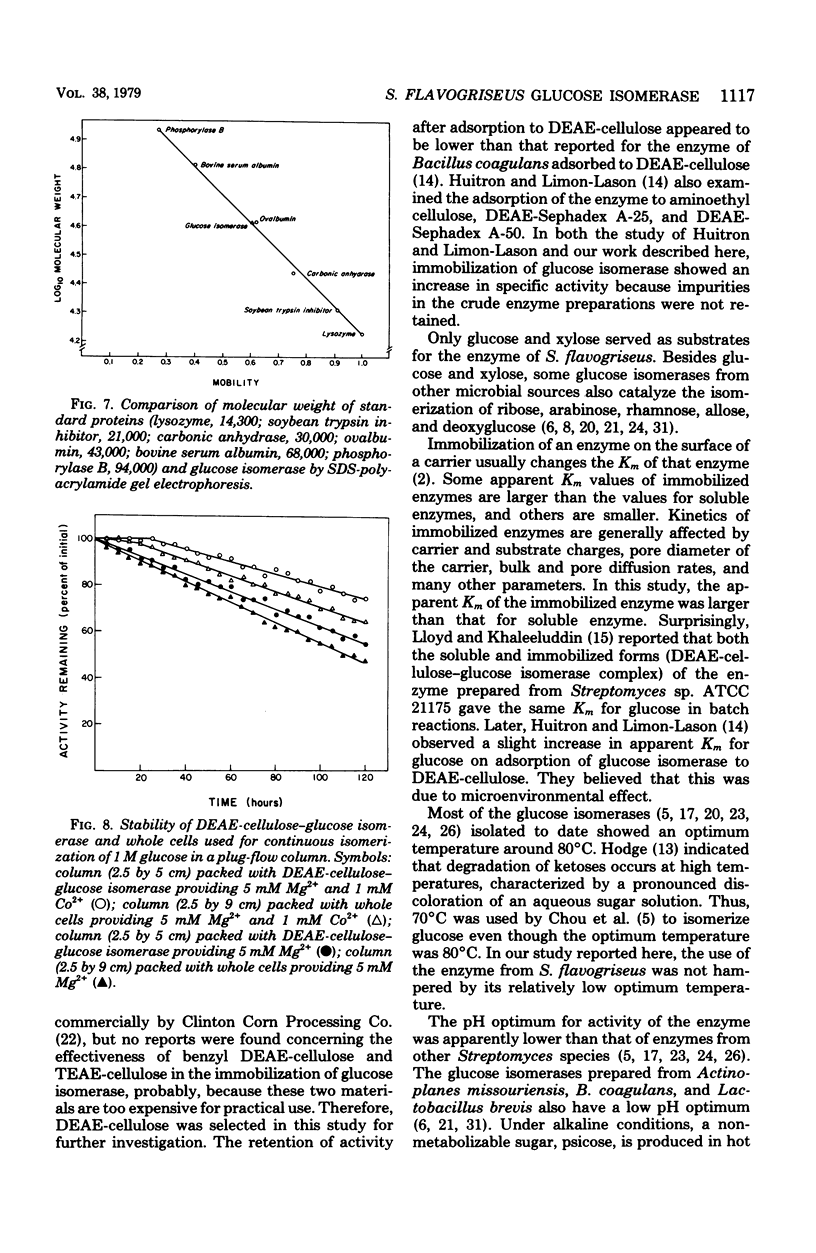
Glucose isomerase (EC 5.3.1.5) produced from Streptomyces flavogriseus was purified by fractionation with (NH4)2SO4 and chromatography on diethylaminoethyl (DEAE)-cellulose and DEAE-Sephadex A-50 columns. The purified enzyme was homogeneous as shown by ultracentrifugation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Benzyl DEAE-cellulose, triethylaminoethyl-cellulose, and DEAE-cellulose were effective in the immobilization of partially purified glucose isomerase. Several differences in properties were found between purified soluble enzyme, immobilized enzyme (DEAE-cellulose-glucose isomerase), and heat-treated whole cells. Glucose and xylose served as substrate for the enzyme. Whole cells had the highest Km values for glucose and xylose; the soluble enzyme had the lowest values. The optimum temperature for activity of the soluble and immobilized enzymes was 70°C; that for whole cells was 75°C. The pH optimum for the three enzyme preparations was 7.5. Magnesium ion or Co2+ was required for enzyme activity; an addition effect resulted from the presence of both Mg2+ and Co2+. The enzyme activity was inhibited by Hg2+, Ag+, or Cu2+. The conversion ratio of the enzyme for isomerization was about 50%. The soluble and immobilized enzymes showed a greater heat stability than whole cells. The soluble enzyme was stable over a slightly wider pH (5.0 to 9.0) range than the immobilized enzyme and whole cells (pH 5.5 to 9.0). The molecular weight of the enzyme determined by the sedimentation equilibrium method was 171,000. A tetrameric structure for the enzyme was also indicated. After operating at 70°C for 5 days, the remaining enzyme activity of the immobilized enzyme and whole cells, which were used for the continuous isomerization of glucose in a plug-flow type of column in the presence of Mg2+ and Co2+, was 75 and 55%, respectively. Elimination of Co2+ decreased operational stability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananichev A. V., Ulezlo I. V., Rezuikov A. A., Bezborodov A. M., Egorov A. M. Gliukozonzomeraza iz Actinomyces olivocinereus 154 i ee immobilizatsiia na aminirovannom silokhrome. Biokhimiia. 1978 Jul;43(7):1294–1302. [PubMed] [Google Scholar]
- Chen W. P., Anderson A. W., Han Y. W. Extraction of glucose isomerase from Streptomyces flavogriseus. Appl Environ Microbiol. 1979 Apr;37(4):785–787. doi: 10.1128/aem.37.4.785-787.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. P., Anderson A. W., Han Y. W. Production of Glucose Isomerase by Streptomyces flavogriseus. Appl Environ Microbiol. 1979 Feb;37(2):324–331. doi: 10.1128/aem.37.2.324-331.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. C., Ladisch M. R., Tsao G. T. Studies on glucose isomerase from a Streptomyces species. Appl Environ Microbiol. 1976 Oct;32(4):489–493. doi: 10.1128/aem.32.4.489-493.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sanchez S., Smiley K. L. Properties of D-xylose isomerase from Streptomyces albus. Appl Microbiol. 1975 Jun;29(6):745–750. doi: 10.1128/am.29.6.745-750.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Smiley K. L. Free and immobilized glucose isomerase from Streptomyces phaeochromogenes. Appl Microbiol. 1971 Apr;21(4):588–593. doi: 10.1128/am.21.4.588-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka K. Purification, crystallization and properties of the D-xylose isomerase from Lactobacillus brevis. Biochim Biophys Acta. 1968 Mar 25;151(3):670–680. doi: 10.1016/0005-2744(68)90015-6. [DOI] [PubMed] [Google Scholar]