Abstract
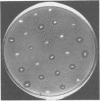
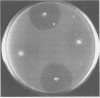
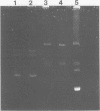
A simple bacteriological technique involving inhibition zone production on a lawn of Escherichia coli was developed to detect antimetabolite toxin production by phytopathogenic species of Pseudomonas. It was established that the mechanism of E. coli inhibition paralleled that of phytotoxin-induced chlorosis of plant tissue. Derivatives of Pseudomonas tabaci and Pseudomonas phaseolicola which did not produce antimetabolite were readily identified by use of this technique. The presence of plasmid DNA in P. tabaci strains was demonstrated, but no physical evidence for plasmid involvement in tabtoxin production was found. The role of antimetabolite production in the pathogenicity of P. phaseolicola was also investigated. The method was extended to show that strains of P. maculicola, P. syringae, and P. coronofaciens also produce antimetabolites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fulbright D. W., Leary J. V. Linkage analysis of Pseudomonas glycinea. J Bacteriol. 1978 Nov;136(2):497–500. doi: 10.1128/jb.136.2.497-500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantotti B. V., Patil S. S., Mandel M. Apparent Involvement of a Plasmid in Phaseotoxin Production by Pseudomonas phaseolicola. Appl Environ Microbiol. 1979 Mar;37(3):511–516. doi: 10.1128/aem.37.3.511-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Extrachromosomally determined antibiotic production. Annu Rev Microbiol. 1978;32:373–392. doi: 10.1146/annurev.mi.32.100178.002105. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Lacy G. H., Leary J. V. Plasmid-mediated transmission of chromosomal genes in Pseudomonas glycinea. Genet Res. 1976 Jun;27(3):363–368. doi: 10.1017/s001667230001658x. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri S., Chakrabarty A. Isolation of plasmid deoxyribonucleic acid from Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):410–416. doi: 10.1128/jb.126.1.410-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. D. Bacteriophage and serological methods for the identification of Pseudomonas phaseolicola (Burkh.) Dowson. Ann Appl Biol. 1970 Dec;66(3):387–395. doi: 10.1111/j.1744-7348.1970.tb04618.x. [DOI] [PubMed] [Google Scholar]