Abstract
Background
Multiparameter flow cytometry allows the detection of minor monoclonal B-cell populations. Using this technique combined with morphology, we were struck by the presence of minor populations of small monoclonal B cells in bone marrows of patients with diffuse large B-cell lymphoma in routine diagnostic samples and performed a systematic retrospective study.
Design and Methods
Bone marrows of 165 patients with primary diffuse large B-cell lymphoma without histological evidence of concurrent non-Hodgkin’s lymphoma were studied by routine microscopy of trephines and smears, immunohistochemistry and multiparameter flow cytometry.
Results
Diffuse large B-cell lymphoma infiltration in marrows was documented in 11 of 165 patients. Morphological examination consistently revealed a higher tumor load than evidenced by flow cytometry. Of interest, only 3 of 119 patients with diffuse large B-cell lymphoma not otherwise specified, the largest subtype, showed marrow infiltration. By contrast, flow cytometry revealed a minor monoclonal B-cell population in 24 of 165 patients, none of whom showed diffuse large B-cell lymphoma infiltration by morphology. Of interest, morphological examination revealed the presence of small B cells in the marrows of those patients. Moreover, 11 of 39 (28.2%) of patients with diffuse large B-cell lymphoma not otherwise specified of ABC subtype and only 3 of 80 (3.7%) with the GCB subtype showed these monoclonal small B cells (P=0.0002). In addition 4 of 8 (50%), 4 of 15 (26.7%) and 2 of 3 (66.7%) patients with primary testicular, primary central nervous system and leg-type diffuse large B-cell lymphoma, respectively, showed monoclonal small B cells.
Conclusions
Bone marrow infiltration with diffuse large B-cell lymphoma in patients with diffuse large B-cell lymphoma not otherwise specified is rare at diagnosis. By contrast, a high number of diffuse large B-cell lymphoma not otherwise specified of the ABC subtype but not of GCB subtype is associated with monoclonal small B cells in the marrow. Whether these monoclonal small B cells are precursors of diffuse large B-cell lymphoma of the ABC type or arise in a common background that favors clonal B-cell expansion remains to be demonstrated.
Keywords: lymphoma, B cell, lymphocytosis
Introduction
Primary diffuse large B-cell lymphoma (DLBCL) accounts for about 30% of all adult non-Hodgkin’s lymphomas. Several distinct clinical and genetic entities are now recognized.1 The largest entity is DLBCL not otherwise specified (NOS). This lymphoma type is further divided in two molecular subgroups based on gene expression profiling; a germinal center B-cell (GCB) subtype and an activated B-cell (ABC) subtype, sharing much of the gene expression profile of normal GCBs and ABCs, respectively.2 Importantly, there are other clinical and genetic distinctions between these subgroups: ABC DLBCL has a worse overall and failure free survival compared with the GCB subgroup. In addition, translocation of the BCL2 gene is seen in GCB DLBCL, whereas trisomy 3 and a chromosome 19q amplicon are present in ABC DLBCL.2,3 The ABC DLBCL is further characterized by constitutional activation of the NF-κB pathway, and displays a specific pattern of gene amplifications and deletions leading to upregulation of oncogenes such as the transcription factor SPIB and upregulation of the FOXP1 gene, whereas this is not the case for GCB DLBCL.2,3 The division of DLBCL into those of putative germinal center B-cell origin and those of putative activated B-cell origin may well have an impact on the development of specific therapies for these diseases. In addition to DLBCL not otherwise specified, novel DLBCL entities have also been included in the World Health Organization (WHO) classification.1 Among those, some entities may also share an origin from activated B cells; primary DLBCL of the central nervous system (CNS) frequently shows deletion of major histocompatibility class II genes, unlike most other DLBCL, and interestingly shares also a gene expression pattern with activated B cells.4,5 Primary testicular DLBCL shares these latter two features with primary central nervous system DLBCL, but is as yet not recognized as a special DLBCL type in the recent World Health Organization classification.4,6 By contrast, primary cutaneous DLBCL of leg type is recognized as a novel DLBCL type, and may also have an origin from activated B cells.1,7
In the present study, we studied monoclonal B cells in the bone marrow of DLBCL patients by flow cytometry, morphological and immunohistochemical examination. Apart from different incidences of marrow infiltration by DLBCL according to type and subtype, we report the striking incidence of monoclonal small B cells in patients with ABC DLBCL, but rarely in GCB DLBCL. We also demonstrate the same clonal origin for monoclonal small B cells and DLBCL in the one case in which such a study was possible.
Design and Methods
Patients
Patients with a primary diagnosis of DLBCL retrieved from the lymphoma registry at the Norwegian Radium Hospital were studied. Only consecutive cases in a given time period, depending on the type of DLBCL, were included under the following conditions: i) the cases were first diagnoses; ii) the patients were not diagnosed with previous non-Hodgkin’s lymphoma nor with composite lymphoma (4 patients were excluded in the time period studied because of concurrent marginal zone lymphoma, chronic lymphocytic leukemia, follicular lymphoma and hairy cell leukemia, respectively); iii) a bone marrow aspirate sample at diagnosis had been submitted for multiparameter flow cytometric analysis. For any of the above inclusion criteria, 75% of the consecutive cases of DLBCL diagnosed at our institute were omitted from this study. The time periods in which DLBCL lymphomas were first diagnosed are as follows: i) for DLBCL, NOS, DLBCL leg-type, PMLBCL and T/HRBCL, January 2006-October 2008; ii) for primary CNS DLBCL, January 2002-December 2007; and iii) for primary testicular DLBCL, January 2002-October 2008. The time periods in which primary central nervous system and primary testicular DLBCL were selected were extended with regard to the other types of DLBCL to allow more cases of these subtypes to be collected. A total of 165 patients were thus included in the study. The availability of blood samples analyzed by flow cytometry was not a selection criterion but the results were recorded when available. Bone marrow trephines and smears of all 165 patients were available for morphological examination. The clinical files were analyzed for a limited set of patients’ characteristics such as age, sex, stage of disease according to the Ann Arbor classification, international prognostic index (IPI) and clinical history.8,9 Patient survival data were not collected in view of the short follow up. The study was approved by the institutional review board.
Histopathology
Hematoxylin and eosin stained sections of diagnostic biopsies and bone marrow trephine sections, as well as Wright-stained bone marrow smears, on all patients were reviewed by 2 of the authors (AMT and JD). Immunohistochemical staining was performed on all diagnostic biopsies of the tumors as well as on the bone marrow trephine biopsies. DLBCL not otherwise specified were divided into GCB and ABC subtype by immunohistochemistry according to the algorithm of Hans et al.10 The following primary antibodies were used for immunohistochemistry: anti-CD5 (Novocastra Laboratories, Newcastle upon Tyne, UK), anti-CD10 (Novocastra), anti-CD20 (Dako, Glostrup, Denmark), anti-CD30 (Dako), anti-CD138 (Dako), anti-MUM1 (Dako), anti-bcl2 (Dako), anti-bcl6 (Dako), and Ki-67 (Dako). Bone marrow trephine sections were stained with anti-CD20, anti-CD5, anti-CD10, anti-CD23 (Novocastra), anti-CD138, anti-cyclin D1 (Lab Vision, Fremont, CA, USA), anti-kappa (Dako), anti-lambda (Dako), anti-IgM (Dako) and anti-IgD (Dako). For most of the immunohisto-chemical stains, rabbit anti-mouse antibody labeled with a peroxidase-conjugated polymer (Dako EnVision System, Dako) was used according to the manufacturer’s instructions. The slides were counterstained with hematoxylin.
Flow cytometry
Bone marrow aspirates and/or blood samples were analyzed by 5-or 6-parameter flow cytometry.11 A stain, lyse and wash method was used as previously described.12 Monoclonal antibodies included: anti-CD19 (clone SJ25C, BD Biosciences, San Jose, CA, USA), anti–CD20 (clone L27, BD Biosciences), anti-CD22 (clone 4KB128, Dako, Glostrup, Denmark), FMC7 (clone FMC7, Dako), anti-CD5 (clone UCHT2, BD Biosciences), anti-CD10 (clone HI10a, BD Biosciences), anti-CD23 (clone MHM6, Dako), anti-CD45 (clone 2D1, BD Biosciences), anti-CD38 (clone HB7, BD Biosciences), anti-CD138 (clone BB4, Serotec, Raleigh, NC, USA), anti-kappa (clone TB28-2, BD Biosciences) and anti-lambda (clone 1-155-2, BD Biosciences) and anti-bcl2 (clone 124, Dako). All antibodies were directly conjugated to fluoresceine thyocyanate (FITC), phycoerythrine (Pe), phycoerythrinecyanine 5 (PeCy5), peridinin chlorophyll protein (PerCP), peridinin chlorophyll protein-cy5.5 (PerCP-Cy5.5) or allophycocyanin (APC). Stained samples were run on a Facscalibur (BD Biosciences) equipped with a 488 nm and 630 nm laser. A two-step acquisition was performed; a total of 15,000 cells were run in list mode, followed by a live gate analysis on CD19+ events until the tube was empty. Data were analyzed using FlowJo 7.1 software (Tree Star Inc., San Francisco, CA, USA). Not all markers have been performed on all samples, due to the number of cells available for the analysis. Monoclonal B cells were identified by either immunoglobulin light chain restriction (defined by a κ/λ ratio >4 or <0.5), an abnormal B-cell immunophenotype or a combination of both as described by Sanchez et al.11 and Nieto et al.13 Abnormal immunophenotypes are defined as aberrant levels of expression of a marker or asynchronous expression of markers with regard to normal mature B cells. In short, monoclonal small B cells were eventually divided into those with a CLL-like immunophenotype and a non-CLL-like immunophenotype as is the convention for monoclonal B-cell lymphocytosis.14 A third category of monoclonal B cells, non-classifiable was created to include cases that showed findings atypical for CLL (bright CD20 expression or FMC7 expression) and cases of which the immunophenotypical analysis was incomplete to prove or exclude the CLL phenotype.
Analysis of rearranged immunoglobulin heavy chain genes
In order to establish a possible clonal relationship between the monoclonal B cells in the marrow and the diagnostic DLBCL, rearranged immunoglobulin heavy chain genes were amplified and sequenced, and sequences were compared. This was attempted for 10 patients, of whom paired samples were available. B5-fixed formic-acid decalcified bone marrow trephine biopsies were used for 9 patients whereas for patient 11, a frozen bone marrow sample was available. Frozen left-over bone marrow samples or fixed clots were no longer available in the other patients. DLBCL samples consisted of frozen tissue samples.
The detection of immunoglobulin heavy chain (IgH) rearrangements, a multiplex polymerase chain reaction (PCR) method using a set of primers against the framework one region (FR1), FR2 and FR3 of the IgH variable gene segment (VH) and joining gene segment (JH) was performed as described before.15 The PCR products were analyzed using capillary electrophoresis (ABI 3100 Genetic Analyzer, Applied Biosystems, Witerstad, Germany). Before sequencing, the PCR products were size-fractionated on a 1.5% low-melting agarose gel, and the appropriate bands excised from the gel and extracted using the Qiaquick gel extraction kit (Qiagen) according to the manufacturer’s recommendations. An aliquot of the products was directly sequenced from both ends with the ABI Prism Dye Terminator Cycle Sequencing Kit (Applied Biosystems) using the ABI 3100 Genetic Analyzer (Applied Biosystems). The obtained sequences were analyzed using IgBLAST (www.ncbi.nlm.nih.gov/igblast) and IMGT (www.imgt.org) databases.
Results
Patients’ characteristics
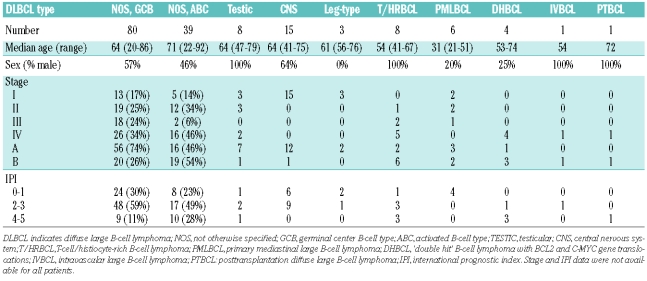
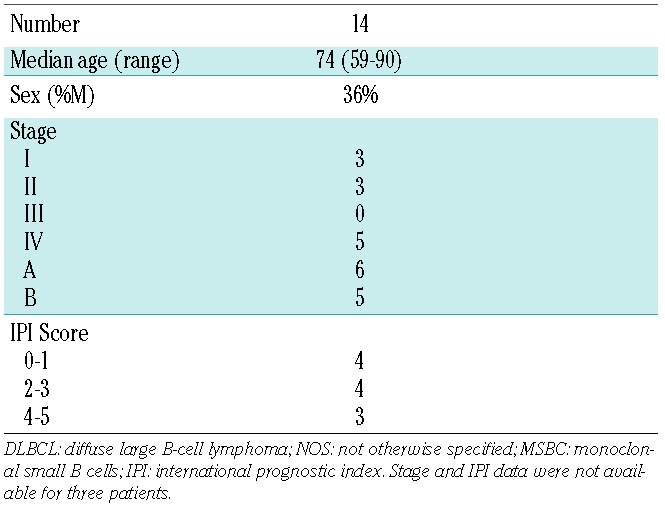
The clinical data for the 165 patients in this study are summarized in Table 1. The median age, sex distribution, distribution of stage and IPI scores of the patients are different between the distinct DLBCL types. These characteristics are within a range of what is typical for those lymphoma types. Patient treatment and survival data have not been included in this study. Table 2 summarizes the clinical data of the 14 of 116 with DLBCL not otherwise specified and monoclonal small B cells in their bone marrow samples. Not unexpectedly in view of the fact that monoclonal small B cells mostly are detected in ABC DLBCL, the clinical data of patients with monoclonal small B cells in their bone marrow resemble closely those of patients with ABC DLBCL with respect to age (with the median age about ten years older than GCB DLBCL) and the predominance of female patients.
Table 1.
Clinical data of all patients in the study according to diffuse large B-cell lymphoma subtype.
Table 2.
Clinical data of patients with diffuse large B-cell lymphoma, NOS with bone-marrow infiltration with MSBC.
Pathology data
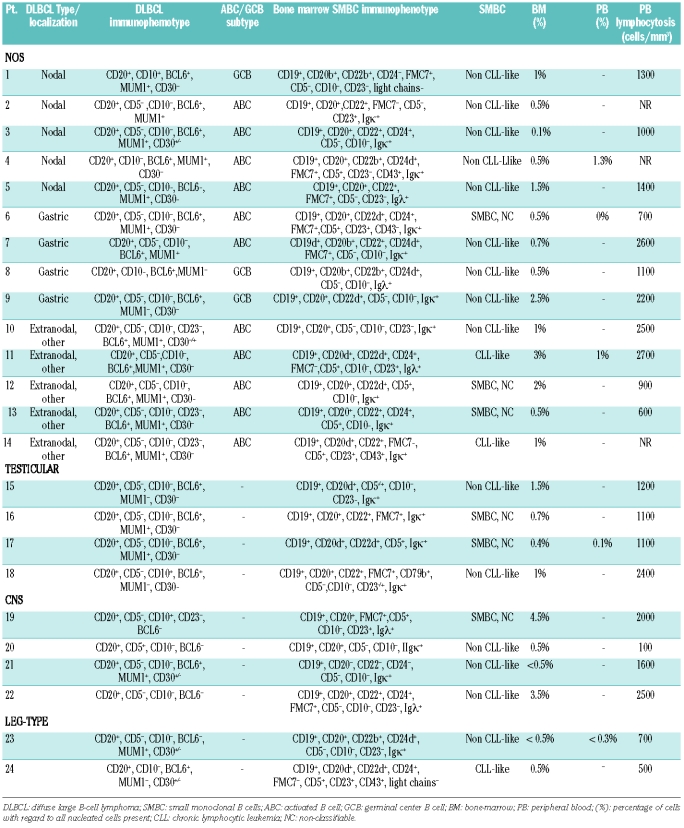
Bone marrow smears and bone marrow trephine biopsies revealed focal infiltration by DLBCL in 11 of 165 (6.7%) cases. Of interest, this incidence is lower for DLBCL not otherwise specified (2.5%) and is more marked for T-cell/histiocyte-rich B-cell lymphoma (25%) and DLBCL with a dual translocation involving C-MYC and BCL2 (100%), although the number of these DLBCL subtypes are low in this study. Flow cytometry detected monoclonal B cells in 7 of 11 of these patients. Of interest, flow cytometry underestimated the degree of marrow involvement in all these cases (Online Supplementary Table S1). Flow cytometry further revealed monoclonal B cells in 24 of 165 cases that did not show infiltration by DLBCL on morphological examination. Surprisingly, a careful morphological and immunohistochemical examination of smears and trephines showed infiltration by small B cells without cytological atypia. The distribution of these small lymphocytes was either diffuse or consisted of focal interstitial small infiltrates of small lymphoid cells (Figure 1). These infiltrates were never seen in the paratrabecular areas; intra-sinusoidal infiltration was also not seen. Combining the flow cytometry data revealing a B-cell population that is monoclonal or immunophenotypically aberrant with the morphology of infiltrating B cells in the marrow allows us to reasonably conclude that the monoclonal B cells detected by flow cytometry are monoclonal small B cells. Table 3 summarizes the incidence of bone marrow DLBCL or monoclonal small B cells in the different DLBCL types and subtypes in our study. Monoclonal small B cells were present with notable differences in the frequency among the different types and subtypes of DLBCL. Surprisingly, monoclonal small B cells are mostly seen in patients with the ABC subtype of DLBCL not otherwise specified (11 of 39 cases), as well as in primary testicular, primary central nervous system and leg-type diffuse large B-cell lymphoma. The incidence of monoclonal small B cells in the GCB subtype of DLBCL not otherwise specified (3 of 80 cases) is limited, despite representing the largest subgroup of DLBCL. This difference in incidence of monoclonal small B cells in ABC versus GCB DLBCL not otherwise specified is significant with the P value being 0.0002 using the Fisher’s Exact Test. No monoclonal small B cells were noted in patients with primary mediastinal B-cell lymphoma (PMLBCL) or T-cell/histiocyte-rich B-cell lymphoma (T/HLBCL). However, the number of those cases in the study is low. The percentage of monoclonal small B cells in the marrow did not exceed 4.5% of all nucleated cells, and did not exceed 1.3% of nucleated cells in the peripheral blood, in the 5 cases in which blood was analyzed (Table 4). None of the patients showed peripheral blood lymphocytosis exceeding 5,000/mm3, the upper limit used for diagnosing monoclonal B-cell lymphocytosis in the blood.14 The highest lymphocyte count recorded was 2,700/mm3 for patient 11. Importantly, clinical records did not mention leukemia in any of the patients. Follow-up bone marrow flow cytometry and morphology were available for 4 patients. Three of those 4 continued to show monoclonal small B cells in the marrow after treatment, but none showed infiltration by DLBCL. The level of monoclonal small B cells at follow up was between 0.2% and 2%, with follow-up staging performed at seven, eight, nine and 14 months, respectively.
Figure 1.
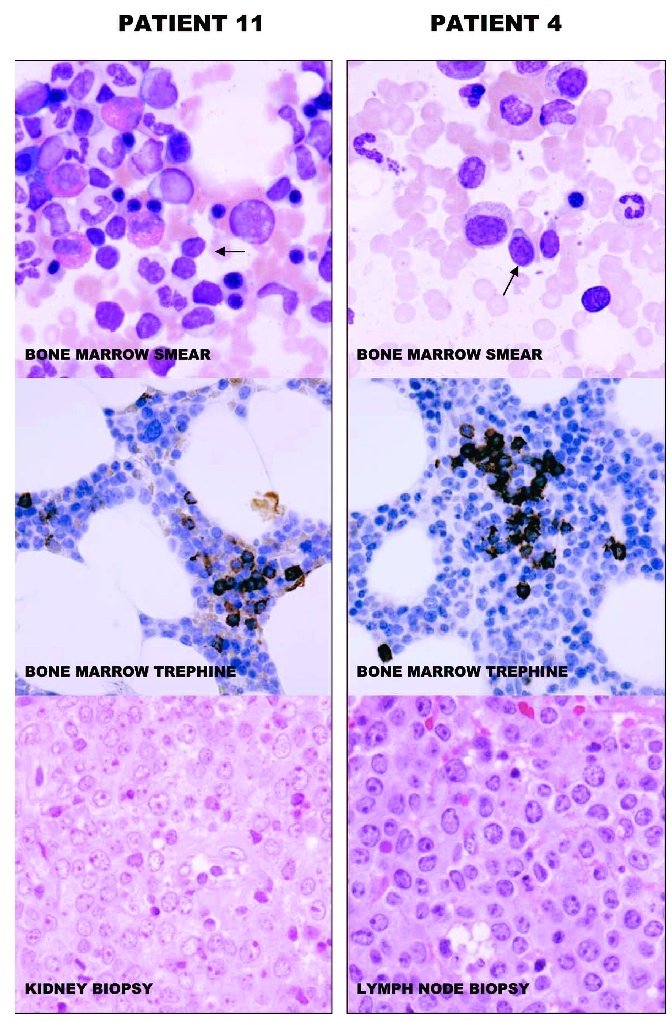
Wright’s-stained bone marrow smears (original x 600) and anti-CD20 stained sections of bone marrow trephines (original x 200) and hematoxylin and eosin stained tissue biopsies (original x 400) of patients 11 and 4 are illustrated. Small lymphoid cells (arrows), likely corresponding to MSBC are seen in the smears. The cells have round nuclei without prominent nucleoli and have varying amounts of cytoplasm. The cells of patient 4, having MSBC with a non-CLL-like phenotype have more cytoplasm than cells of patient 11, having MSBC with a CLL-like phenotype. The bone marrow trephines of both cases show sparse and small collections of small CD20+ B cells. The tissue biopsies show diffuse infiltration with large lymphoid cells with multiple nucleoli, typical of DLBCL. The images were acquired with an Olympus BX50 microscope (Olympus Corporation, Tokyo, Japan) equipped with a Hamamatsu C4742-95-10SC camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan). The acquisition software was Paint Shop Pro 7.02 (Corel Corporation, Ottawa, Canada).
Table 3.
Incidence of bone marrow infiltration with MSBC and DLBCL.
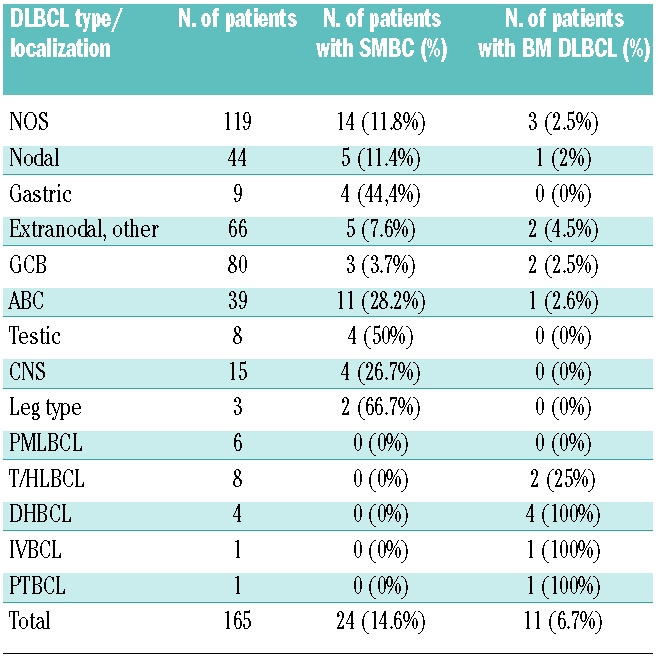
Table 4.
Immunophenotypes of concurrent diffuse large B-cell lymphoma and bone-marrow SMBC.
The complete immunophenotype of the DLBCL as well as that of the associated monoclonal small B cells is given in Table 4. Monoclonal small B cells could be identified by immunoglobulin light chain restriction only (12 cases), by an aberrant immunophenotype only (patients 1 and 24, identified by B cells lacking immunoglobulin light chain expression in both cases, either in combination with bright expression of CD22 and absent CD24 expression or coexpression of CD5, CD23 with absent FMC7 expression, respectively) or by a combination of immunoglobulin light chain restriction with an aberrant phenotype (10 cases). The pathological marker expression of each case has been summarized in Table 4. Of 24 monoclonal small B cells, 15 displayed a non-CLL-like immunophenotype, 3 showed a CLL-like immunophenotype and the other 6 were unclassifiable because they displayed an atypical expression of CD20 or FMC7 for CLL despite expression of CD5 and CD23 or because the data were incomplete (one case). Representative flow cytometric results are illustrated in Online Supplementary Figure S1. It is of note that the expression of CD5 or CD23 by the respective monoclonal small B cells and DLBCL does not correspond. None of the monoclonal small B-cell infiltrates expressed cyclin D1.
Molecular clonality analysis
In 10 cases a diagnostic sample pair was available to investigate whether the DLBCL and monoclonal small B cells in the bone marrow were derived from the same B-cell clone. However, this analysis was only successful for patient 11. DNA degradation in the B5-fixed decalcified bone marrow samples was the reason for failure in 9 cases. B5-fixed decalcified trephine biopsies have previously been reported to yield poor PCR results, likely due to DNA fragmentation16 However, for patient 11, a stored frozen bone marrow sample could be used for the analysis. The rearranged IgH gene of the monoclonal small B cells and the DLBCL of patient 11 showed a similar IgH gene sequence, including the clone-specific CDR3 region (Online Supplementary Figure S2). A somatic mutation frequency of 2.9% with respect to the germ line gene IGHV4-30-4.01 was found, with the same mutations being present in the genes of bone marrow monoclonal small B cells and the DLBCL.
Discussion
A systematic study of bone marrow infiltration in consecutive cases of various subtypes of DLBCL at our institution revealed that infiltration with DLBCL at diagnosis is rather rare for DLBCL not otherwise specified (2.5%) but more frequent for minor subtypes such as T-cell/histiocyte-rich B-cell lymphoma and DLBCL with both C-MYC and BCL2 translocation. By contrast, we found that marrows in DLBCL not otherwise specified were infiltrated by monoclonal small B cells in 11.8% of cases, an unexpected finding. The same clone was also present in the blood in 3 out of the 4 patients from whom blood samples were available. In addition, follow-up samples after treatment revealed persisting monoclonal small B cells in 3 out of 4 patients. Monoclonal small B cells in DLBCL has not previously been reported. However, occult bone marrow involvement by DLBCL has been reported.17–19 By definition, occult marrow infiltration cannot morphologically be detected. However, it is assumed that monoclonal B cells in such cases are DLBCL cells. Little emphasis has been paid on the presence of small B cells in such marrows and it is, therefore, not excluded that some of the cases reported as occult bone marrow infiltration by DLBCL correspond to infiltration by monoclonal small B cells. Hanson et al. reported that 5 out of 75 of their cases with DLBCL showed monoclonal cells in the marrow by flow cytometry, about 0.09–3%, without morphological evidence for DLBCL infiltration.17 Two of those 5 patients revealed a similar monoclonal cell population in the blood. Interestingly, 4 out of these 5 patients had only localized DLBCL. Similarly, Stacchini et al., Gomyo et al. and Talaukar et al. detected monoclonal B cells by flow cytometry in 2 out of 33, 9 out of 57, and 8 out of 21 of their DLBCL cases, respectively, that showed no morphological involvement of the bone marrow with DLBCL.18,19
Of interest, DLBCL not otherwise specified with an activated B-cell (ABC) immunophenotype showed a much higher infiltration frequency with monoclonal small B cells (28.2%) than DLBCL not otherwise specified with a germinal center B-cell (GCB) immunophenotype (3.7%) (P=0.0002). Other DLBCL types and subtypes with a supposed activated B-cell origin (5–7), such as testicular, central nervous system and leg-type DLBCL showed a similar high infiltration frequency with monoclonal small B cells (50%, 26.7% and 66.7%, respectively). Whether those monoclonal small B cells seen in the bone marrows of our patients are similar to those cells seen in the blood of elderly people, termed monoclonal B-cell lymphocytosis (MBL), remains to be demonstrated.14,20,21 Monoclonal B-cell lymphocytosis has been defined as a minimal clonal small B-cell lymphoproliferation that does not meet the criteria of established leukemia or lymphoma types.14 Although we have no systematic data from flow cytometric analysis of peripheral blood in patients with DLBCL in our study, monoclonal small B cells in the blood was detected in 4 of the 5 patients for whom these data were available and who presented with monoclonal small B cells in the bone marrow. In those patients, monoclonal small B cells in the blood showed a similar immunophenotype as that in the bone marrow (data not shown) which might suggest that monoclonal small B cells are similar to monoclonal B-cell lymphocytosis described in elderly people. However, the immunophenotypes of the monoclonal small B cells are non-CLL-like in 15 of 24 cases and CLL-like in 3 of 24 cases, the remaining being non-classifiable. This contrasts with the high frequency of CLL-like immunophenotypes for monoclonal B-cell lymphocytosis, and may suggest a different origin for monoclonal small B cells and monoclonal B-cell lymphocytosis. The prevalence of monoclonal B-cell lymphocytosis in the peripheral blood is between 0.5% and 1% of the adult population, but increases with age to as much as 5% in the seventh decade of life20–23 or even higher in one recent study using a more sensitive technique.13 Of note, the incidence of bone marrow monoclonal small B cells in patients with DLBCL of the ABC subtype is higher than that of monoclonal B-cell lymphocytosis in a healthy population. Moreover, the incidence of non-CLL-like monoclonal small B cells in our patients with ABC DLBCL (26.7%), is even higher when compared to the incidence of non-CLL-like monoclonal B-cell lymphocytosis in an age-matched population, which is about 1–2%.20–23
Discordant bone marrow involvement in DLBCL has been reported before and its frequency may be as high as 38%.24–27 It is defined as the presence of small cell non-Hodgkin’s lymphoma in the bone marrow of patients with DLBCL at diagnosis. The bone marrow lymphoma can be clonally related as well as be clonally unrelated to the DLBCL.24 Such cases with discordant marrow involvement are rare in our series (n=4) and have been excluded from the study. Whether bone marrow monoclonal small B cells in DLBCL is the lower end of the spectrum of discordant marrow lymphoma cannot be excluded. However, the lymphoma in the bone marrow of cases with discordant extra-medullar DLBCL is mostly follicular lymphoma;24 none of the monoclonal small B cells associated with DLBCL in our study display the immunophenotype of follicular lymphoma.
We could not systematically study the clonal identities of monoclonal small B cells in the marrow because of lack of proper materials in this retrospective study. It is of interest that of the 24 patients with monoclonal small B cells, 10 have discordant immunophenotypes with the paired DLBCL. In 9 of these cases, monoclonal small B cells express CD5 whereas the paired DLBCL does not. In one case the opposite is seen. Discordant immunophenotypes might indicate that monoclonal small B cells and DLBCL are not clonally related. However, the demonstration of identical rearranged immunoglobulin genes in one of these patients, patient 11 in our study, demonstrates that bone marrow monoclonal small B cells with a discordant immunophenotype can progress to DLBCL. This patient has monoclonal small B cells with a CLL immunophenotype, but did not develop CLL. The patient’s DLBCL did not display the CLL immunophenotype, as is the case in Richter’s syndrome. Two similar cases have previously been reported.24
To better characterize monoclonal small B cells in patients with DLBCL, it will be necessary to prospectively collect bone marrow from patients with DLBCL, to isolate the small B cells and perform clonal identity analyses as well as genetic analyses to compare the results with those obtained in the DLBCL of the respective patients. The results from such a study could elucidate further the nature of monoclonal small B cells and perhaps part of the oncogenesis of DLBCL of the ABC subtype.
Acknowledgments
the authors would like to thank Ms. Jennifer Ellen Kerr for secretarial assistance.
Footnotes
Funding: this study was sponsored through a grant by the Norwegian Cancer Society.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
AMT designed and performed research, analyzed and interpreted the data and wrote the manuscript. HH collected the clinical data and helped to write the manuscript; AW performed part of the research and helped to write the manuscript. IMI collected and analyzed part of the data. JW analyzed part of the data. WCC helped in writing the manuscript. JD helped to design research, analyzed and interpreted the data, and participated in writing the manuscript.
The authors reported no potential conflicts of interest.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- 2.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105(36):13520–5. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riemersma SA, Jordanova ES, Schop RF, Philippo K, Looijenga LH, Schuuring E, et al. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B-cell lymphomas arising in immune-privileged sites. Blood. 2000;96(10):3569–77. [PubMed] [Google Scholar]
- 5.Camilleri-Broët S, Crinière E, Broët P, Delwail V, Mokhtari K, Moreau A, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–6. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 6.Booman M, Douwes J, Glas AM, de Jong D, Schuuring E, Kluin PM. Primary testicular diffuse large B-cell lymphomas have activated B-cell-like subtype characteristics. J Pathol. 2006;210(2):163–71. doi: 10.1002/path.2033. [DOI] [PubMed] [Google Scholar]
- 7.Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, Willemze R, et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood. 2005;105(9):3671–8. doi: 10.1182/blood-2004-04-1594. [DOI] [PubMed] [Google Scholar]
- 8.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31(11):1860–1. [PubMed] [Google Scholar]
- 9.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project (no authors listed) A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 10.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez ML, Almeida J, Vidriales B, Lopez-Berges MC, Garcia-Marcos MA, Moro MJ, et al. Incidence of phenotypic aberrations in a series of 467 patients with B chronic lymphoproliferative disorders: basis for the design of specific four-color stainings to be used for minimal residual disease investigation. Leukemia. 2002;16(8):1460–9. doi: 10.1038/sj.leu.2402584. [DOI] [PubMed] [Google Scholar]
- 12.Rothe G, Schmitz G. Consensus protocol for the flow cytometric immunophenotyping of hematopoietic malignancies. Working Group on Flow Cytometry and Image Analysis. Leukemia. 1996;10(5):877–95. [PubMed] [Google Scholar]
- 13.Nieto WG, Almeida J, Romero A, Teodosio C, Lopez A, Henriques AF, Sanchez ML, et al. Increased frequency (12%) of circulating chronic lymphocytic leukaemia-like B-cell clones in healthy subjects using a highly senstitive multicolour flow cytometry approach. Blood. 2009;114(1):33–7. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 14.Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, et al. The International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130(3):325–32. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 15.van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 16.Tbakhi A, Totos G, Pettay JD, Myles J, Tubbs RR. The effect of fixation on detection of B-cell clonality by polymerase chain reaction. Mod Pathol. 1999;12(3):272–8. [PubMed] [Google Scholar]
- 17.Hanson CA, Kurtin PJ, Katzmann JA, Hoyer JD, Li C-Y, Hodnefield JM, et al. Immunophenotypic analysis of peripheral blood and bone marrow in the staging of B-cell malignant lymphoma. Blood. 1999;94(11):3889–96. [PubMed] [Google Scholar]
- 18.Stacchini A, Demurtas A, Godia L, Martine G, Antinoro V, Palestro G. Flow cytometry in the bone marrow staging of mature B-cell neoplasms. Cytometry Part B (Clin Cytometry) 2003;54(1):10–8. doi: 10.1002/cyto.b.10023. [DOI] [PubMed] [Google Scholar]
- 19.Gomyo H, Shimoyama M, Minagawa K, Yakushuin K, Urahama N, Okamura A, et al. Morphologic, flow cytometric and cytogenetic evaluation of bone marrow involvement in B-cell lymphoma. Haematologica. 2003;88(12):1358–65. [PubMed] [Google Scholar]
- 20.Rawstron AC, Green MJ, Kuzmicki A, Kennedy B, Fenton JA, Evans PA, et al. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood. 2002;100(2):635–9. doi: 10.1182/blood.v100.2.635. [DOI] [PubMed] [Google Scholar]
- 21.Ghia P, Prato G, Scielzo C, Stella S, Geuna M, Guida G, et al. Monoclonal CD5+ and CD5- B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103(6):2337–42. doi: 10.1182/blood-2003-09-3277. [DOI] [PubMed] [Google Scholar]
- 22.Rawstron AC, Bennett FL, O'Connor SJ, Kwok M, Fenton JA, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–83. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 23.Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Ghia P, Marti GE, Caporaso NE. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–67. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer M, Spitzer M, Mandl-Weber S, Stecker K, Schmidt B, Hoefler H, Quintanilla-Martinez L, Fend F. Discordant bone marrow involvement in diffuse large B cell lymphoma: comparative molecular analysis reveals a heterogeneous group of disorders. Lab Invest. 2003;83(1):107–14. doi: 10.1097/01.lab.0000050762.61660.27. [DOI] [PubMed] [Google Scholar]
- 25.Kluin PM, van Krieken JH, Kleiverda K, Kluin-Nelemans HC. Discordant morphologic characteristics of B-cell lymphomas in bone marrow and lymph node biopsies. Am J Clin Pathol. 1990;94(1):59–66. doi: 10.1093/ajcp/94.1.59. [DOI] [PubMed] [Google Scholar]
- 26.Robertson LE, Redman JR, Butler JJ, Osborne BM, Velasquez WS, McLaughlin P, et al. Discordant bone marrow involvement in diffuse large-cell lymphoma: a distinct clinical-pathologic entity associated with a continuous risk of relapse. J Clin Oncol. 1991;9(2):236–42. doi: 10.1200/JCO.1991.9.2.236. [DOI] [PubMed] [Google Scholar]
- 27.Hodges GF, Lenhardt TM, Cotelingam JD. Bone marrow involvement in large-cell lymphoma. Prognostic implications of discordant disease. Am J Clin Pathol. 1994;101(3):305–11. doi: 10.1093/ajcp/101.3.305. [DOI] [PubMed] [Google Scholar]